
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cardiovasc. Med., 16 February 2022
Sec. Hypertension
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.831297
This article is part of the Research TopicPregnancy and Cardiovascular DiseasesView all 15 articles
Gestational diabetes mellitus (GDM) and preeclampsia (PE) are common pregnancy complications with similar risk factors and pathophysiological changes. Evidence from previous studies suggests that the incidence of PE is significantly increased in women with GDM, but whether GDM is independently related to the occurrence of PE has remained controversial. GDM complicated by PE further increases perinatal adverse events with greater impact on the future maternal and offspring health. Identify factors associated with PE in women with GDM women, specifically those that are controllable, is important for improving pregnancy outcomes. This paper provides the findings of a review on the correlation between GDM and PE, factors associated with PE in women with GDM, possible mechanisms, and predictive markers. Most studies concluded that GDM is independently associated with PE in singleton pregnancy, and optimizing the treatment and management of GDM can reduce the incidence of PE, which is very helpful to improve pregnancy outcomes.
Gestational diabetes mellitus (GDM) and preeclampsia (PE) are common complications in pregnancy with similar risk factors, including obesity, advanced age, and multiple pregnancy (1, 2). Moreover, in both GDM and PE, the pathophysiological processes involve oxidative stress, pro-inflammatory factor release, vascular endothelial dysfunction (3, 4), which all increase the risk of future maternal diabetes and cardiovascular disease (5–8); thus, a correlation between GDM and PE may exist.
GDM is defined as glucose intolerance diagnosed for the first time during pregnancy (9). The International Association of Diabetes and Pregnancy Study Groups (IADPSG) recently updated the diagnostic criteria for GDM according to the findings of the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study, an oral glucose tolerance test (OGTT) must be performed in a fasting state using 75 g of glucose at 24–28 weeks (10). In 2013, the World Health Organization (WHO) further defined the diagnostic criteria of GDM. GDM is defined as meeting the above 75 g OGTT diagnostic criteria at any time during pregnancy, and the upper limit levels of fasting and 2-h blood glucose were defined (11). With the increasing prevalence of obesity and changes in people's lifestyle, the prevalence of GDM has also significantly increased two to three times in ~10 years (12–14). The prevalence rate of GDM in the Middle East and some North African countries has reached 15.2% (2), while that of Chinese mainland is 14.8% (15). Increased insulin resistance and pancreatic β-cell dysfunction are the major pathogenesis of GDM, which may already exist before pregnancy, especially in obese populations (2). GDM is associated with adverse pregnancy outcomes. Studies have found that the incidence of PE is significantly increased in GDM (16, 17). However, whether GDM is independently associated with the occurrence of PE or because of the effects of their common risk factors, especially obesity, remains controversial.
PE refers to new hypertension (systolic or diastolic blood pressure ≥140 or ≥90 mmHg, respectively) diagnosed at or after 20 weeks of gestation with proteinuria, or at least one other organ (kidney, liver, nervous system, blood system, and uteroplacenta) dysfunction (18). PE is the main cause of maternal and fetal mortality and morbidity (19, 20). GDM complicated by PE further increases perinatal adverse events (21–24), future maternal risk of chronic hypertension, cardiovascular disease, and diabetes (25–27); offspring body mass index (BMI) also steadily increases over time (28). Identifying factors associated with occurrence of PE in women with GDM, especially those that are controllable, is important for improving pregnancy outcomes. This review describes the relationship between GDM and PE, factors associated with occurrence of PE in women with GDM, and impact of GDM on PE in twin pregnancy and in pregnant women with polycystic ovary syndrome (PCOS). It also explores possible impact mechanisms and predictive markers to improve pregnancy outcomes.
We retrieved studies from the PubMed, Ovid, and Wiley from the inception of the databases to June 2021, with the search terms “gestational diabetes mellitus” and “preeclampsia.” We cross-referenced these terms with “obesity,” “body mass index,” “gestational weight gain,” “early onset,” “blood glucose,” “polycystic ovary syndrome,” “twin pregnancy,” “management,” “mechanism,” “predictive markers,” “risk factors,” “insulin,” “metformin,” “Glibenclamide.” We carefully screened all the articles, and focused on articles covering multivariate analysis to judge the independent correlation.
HAPO is a large international prospective blinded cohort study involving 23,316 pregnant women in 15 centers from nine countries, assessing the relationship between blood glucose below diabetes levels and pregnancy outcomes. The HAPO study found that the occurrence of PE is positively associated with blood glucose level even after adjusting for clinical center, age, BMI, height, smoking status, alcohol consumption, family history of diabetes, gestational age at OGTT, and urinary tract infection (29). Following the IADPSG diagnostic criteria, secondary analysis showed that non-obese women with GDM was also associated with PE after adjusting for the above confounding factors, but the association was lower than obesity (16). Population-based retrospective cohort studies in several countries also showed GDM was independently associated with the occurrence of PE (17, 30–40). According to a retrospective cohort study in Sweden, obesity is the main confounding factor (32); however, another retrospective cohort study in France suggested that obesity is not related with the occurrence of PE in women with GDM (36). Different diagnostic criteria for GDM have had little impact on the occurrence of PE (41). A few studies suggest that GDM is not associated with the occurrence of PE after removing the effect of pre pregnancy BMI and other factors (42–46). A retrospective cohort study in Germany showed that there was no independent correlation between GDM and PE, regardless of obesity before pregnancy, and it was unknown whether it was related to the strict control of blood glucose levels (45). In studies conducted in Australia and Japan (42, 43), cases included those within the diagnostic criteria of IADPSG but not up to their own national standards, so blood glucose levels were relatively low, which may have affect the results. Based on these previous findings (Table 1), most studies support that GDM was independently associated with the occurrence of PE in singleton pregnancy. In addition, GDM is also a major risk factor for recurrent (47) and new postnatal PE in the absence of a PE history (48). The history of GDM in first pregnancy is also a risk factor for PE in the second pregnancy (49).
PE also affects the occurrence of GDM; a retrospective cohort study in Korea showed that a history of PE in first pregnancy is a risk factor for the development of GDM in subsequent pregnancies (50). However, a retrospective study in Chile suggested that the history of PE in previous pregnancies is negatively associated with the occurrence of GDM in the next pregnancy (13). Whether PE associated with the occurrence of GDM should be further confirmed in large sample studies.
BMI is a common index used to evaluate nutritional status, even among pregnant women. The World Health Organization (WHO) categorizes BMI into underweight, normal weight, overweight, and obesity with values <18.50, 18.50–24.99, 25.00–29.99, and ≥30.00 kg/m2, respectively (51). Obesity is a common risk factor for GDM and PE, an individual participant data meta-analysis of European, North American and Australian cohorts showed that obesity increased the risk of GDM by three times and the risk of PE by two times (52). Both obesity and GDM are independent associated with PE (16, 31, 32), and the combination of the two has a greater impact than either one alone (16, 31). A Sweden population-based retrospective cohort study that included the data of 13,057 women with GDM who needed treatment, analyzed the impact of pre pregnancy BMI on PE, and showed the highest risk of GDM with obesity, but without significant interaction between obesity and GDM. In addition, obesity had less effect on PE in women with GDM comparing with women without GDM, which may be the result of insulin resistance in both GDM and obesity (31). Most studies suggested that pre pregnancy BMI was independently associated with the occurrence of PE in women with GDM (23, 53–56). In a retrospective cohort study, maternal obesity, early GDM diagnosis and poor glycemic control were the three independent factors related to PE in women with GDM, of which obesity was the highest risk (56). Only one population-based retrospective study in France suggested that the incidence of PE in women with GDM was not associated with pre pregnancy BMI (36). In a randomized controlled trial evaluating metformin for GDM, obesity was not associated with PE in metformin- and/or insulin-treated women, but the incidence of PE was significantly associated with being overweight. The reason for this result may be related to the drug treatment and blood glucose control; furthermore, aspirin was not excluded as a confounding factor (57). In a prospective observational study, considering the level of blood glucose control and treatment methods, obesity was only related to PE in insulin treatment group with poor blood glucose control, but not in diet treatment group (regardless of blood glucose control) and insulin treatment group with good blood glucose control (58). Thus, the effect of pre pregnancy BMI on PE in women with GDM may be also related to blood glucose level and treatment methods.
GWG, another commonly used indicator for nutritional status during pregnancy, is related to pregnancy complications (59, 60). In 2009, the Institute of Medicine (IOM)/National Academy of Medicine (NAM) recommended that the total weight gain during pregnancy of underweight, normal weight, overweight, and obese women according to the WHO BMI classification should respectively be 12.5–18.0, 11.5–16.0, 7.0–11.5, and 5.0–9.0 kg. The average weekly weight gain in the middle and third stages of gestation should be 0.51 (0.44–0.58), 0.42 (0.35–0.50), 0.28 (0.23–0.33), and 0.22 (0.17–0.27) kg, respectively (61). A meta-analysis reported that 30, 34, and, 37% of women with GDM had insufficient, adequate, and excessive GWG (which occurred more in pre pregnancy overweight or obese women), respectively (62). Although GWG is significantly elevated in women with GDM combined with PE (23, 53), most studies considered that the overall excess GWG had no independent correlation with the occurrence of PE (23, 36, 53, 63–65); the same result was reported for obese women with GDM (66). A recent retrospective cohort study of 1,606 women with GDM in China reported different conclusions, after adjusting for maternal age, pre pregnancy BMI, maternal education, in vitro fertilization, fasting, and 2 h glucose, the risk of the total excess GWG developing to PE is 2.06 times; with 2.28 and 2.17 times in the second and third trimesters, respectively (67). It has also been suggested that weight gain in early pregnancy is associated with the occurrence of PE (57). Since we were unable to determine whether pregnant women would develop GDM at the beginning of pregnancy, the management of weight after GDM diagnosis was more significant. A retrospective cohort study conducted in the US evaluated the effect of GWG on pregnancy outcome after the diagnosis of GDM; however, GWG after the diagnosis of GDM was not related to the occurrence of PE adjusted for black, pre pregnancy BMI, and chronic hypertension. However, in a logistic regression model, the weekly weight gain of pregnant patients after GDM diagnosis was evaluated as a continuous variable, after adjusting the pre pregnancy BMI, mother's age, and weekly weight gain before GDM diagnosis, the probability of PE increased by 83% for every 0.45 kg/week of weight gain (68). Recent prospective cohort study in China suggested that a unit increase in GWG level after GDM diagnosis is not related with the occurrence of PE, but in women with excessive GWG before GDM diagnosis, both adequate and excessive GWG after GDM diagnosis increased the incidence of PE (69). Both insufficient weight gain after diagnosis of GDM and total insufficient GWG are not associated with the occurrence of PE (36, 65, 67–69); however, in obese women with GDM, total insufficient GWG is negatively associated with the occurrence of PE (66). A meta-analysis of GWG and GDM pregnancy outcomes showed that excessive GWG is associated with an increased risk of pregnancy-induced hypertension, but PE was not analyzed separately (62). Further clinical studies are required to evaluate whether GWG affects PE occurrence in women with GDM, especially after the diagnosis of GDM. Furthermore, whether the recommended GWG criteria by the IOM /NAM in 2009 is applicable to women with GDM also requires further validation. A Chinese study found that among women with GDM, with weight gain within the receiver operating characteristic target, the incidence of pregnancy-induced hypertension is lower than that of women with weight gain within the IOM target (70). In an Australian study, for the GWG of women with GDM, 2 kg was subtracted from that of the IOM target, which did not improve the prognostic outcome (71).
GWG not only affects the occurrence of PE in women with GDM, but also perinatal outcomes in those complicated by PE. Although total excessive GWG is a protective factor for preterm birth, middle trimester excessive GWG is a risk factor for large gestational age and late trimester excessive GWG is a risk factor for severe PE and cesarean section (72). In conclusion, although GWG has no greater impact than pre pregnancy BMI on PE in women with GDM, it is a controllable factor during pregnancy.
There is no consensus on the screening and diagnosis of GDM in early pregnancy. Screening is usually recommended in the first trimester or during prenatal care to exclude the presence of diabetes in high-risk women (10, 11, 73). Opinions also differ on whether early- or later-onset GDM affects PE. This may be related to the heterogeneity in diagnostic criteria for early-onset GDM and different time definitions and sample sizes. A large retrospective cohort study in Portugal including 18,518 pregnant women with GDM reported that the incidence of early-onset GDM (≤ 12 weeks) was 34.4% according to the IADPSG diagnostic criteria; there was no difference in the incidence of PE between women with early- and later-onset GDM (74). Furthermore, several studies have reported the same conclusion (55, 75–79). In early-onset GDM, metformin or insulin treatment is more needed (75, 78, 79), it is uncertain whether it will affect the occurrence of PE. However, women with early-onset GDM have more risk factors for PE, such as older age, multiple pregnancy, and higher pre pregnancy BMI (80, 81). Others suggest a significantly increased incidence of PE in women with early-onset GDM (81–84). A retrospective US cohort study of 2,596 diet-treated women with GDM shows a 2-fold incidence of PE in early diagnosis (<24 weeks) compared to women with GDM diagnosed after 24 weeks; the risk was 2.4-fold even after adjusting for maternal age, race, parity, weight, and glycemic control differences (82). In another prospective cohort study evaluating the risk of PE in women with GDM, the risk of PE in GDM diagnosed within 20 weeks of pregnancy was 8-fold, even after adjusting for pre pregnancy BMI, OGTT and control blood glucose levels (56). Whether early- or later- onset GDM affects the occurrence of PE needs to be verified by large sample prospective trials. However, for the high-risk population with GDM, early screening and active treatment may reduce the risk of PE (85, 86).
The HAPO study showed that PE is linearly positively associated with the maternal glucose level, for every 1-standard deviation increase in OGTT blood glucose (fasting, 1 h, and 2 h), with the odds ratio of PE between 1.21 and 1.28 (29). The 5th International Symposium on Gestational Diabetes recommended that the blood glucose control criteria during pregnancy for fasting, 1 h, and 2 h blood glucose levels be <5.3, <7.8, or <6.7 mmol/L, respectively (87). The risk of PE in women with GDM increases with increasing levels of glucose impairment at diagnosis (88, 89). However, optimizing glycemic treatment can reduce the occurrence of PE (23, 90, 91), and poor glycemic control is related to the occurrence of PE (54, 56–58). Whether the blood glucose level at OGTT or the blood glucose control level can independently predict the occurrence of PE is unclear. As previously reported, blood glucose control is an independent risk factor for PE (56, 57), and OGTT blood glucose levels are not associated with the occurrence of PE (56, 57, 92). However, others reported that although optimizing blood glucose treatment can reduce the risk of PE, it is not an independent influencing factor, but the OGTT fasting blood glucose level is independent and significantly correlated with the occurrence of PE (23). Accordingly, two other studies also support the finding that OGTT blood glucose levels are an independent risk factor for the development of PE (93, 94). A Chinese retrospective cohort study reported that the blood glucose level at OGTT and after treatment of GDM did not independently predict the occurrence of PE, while the fasting blood glucose level at OGTT is an important risk factor for such (54). In conclusion, blood glucose levels should be more strictly controlled in women with severely-impaired glucose tolerance. Some prospective cohort studies and meta-analysis showed that glycosylated hemoglobin (HbA1c) ≥ 5.9% in early pregnancy significantly was associated with the risk of PE (95–98). It is controversial whether HbA1c level in the second trimester of pregnancy is related to the occurrence of PE, the secondary analysis of HAPO and a retrospective study showed that the HbA1c level during OGTT was related to the occurrence of PE (99, 100). However, two retrospective studies showed that HbA1c level in the second trimester of pregnancy was not associated with PE (54, 101). A higher HbA1c level (5.5–5.9%) within the normal range during OGTT also is an independent risk factor for preeclampsia in women with GDM in a China retrospective cohort study (102). The risk of PE in women with GDM is also related to blood glucose variability. Women with poor blood glucose monitoring compliance are more susceptible to PE than women with good compliance (103). Continuous blood glucose monitoring is helpful for detecting all postprandial blood glucose peaks and recording the impact of diet. It is conducive for the timely adjustment of the treatment plan and for reducing blood glucose variations. The incidence of PE is significantly lower than that in women whose blood glucose alone is monitored, and the mean amplitude of glycemic excursions is also an independent risk factor for PE (104). Blood glucose variability may affect the occurrence of PE by increasing oxidative stress. However, a prospective study with a small sample size showed that the glycemic variability in the third trimester of non-insulin dependent GDM was not associated with the incidence of PE (105).
Age, parity and race are uncontrollable factors, which are also related to the occurrence of PE in GDM women. More studies suggested that advanced age was not independent associated the occurrence with PE in women with GDM (23, 54, 55). Only a retrospective study considered that advanced age was an independent risk factor for PE in women with GDM (53). In a randomized controlled trial, nulliparity was independently associated with the occurrence of PE in women with GDM (57), another retrospective study reached the same conclusion (53). Other studies showed that parity was not associated with PE in women with GDM (23, 54, 55). Ethnicity also has an impact on the occurrence of PE in women with GDM. In a randomized controlled trial in New Zealand/Australia, 724 multi-ethnic subjects were included, the risk of PE in Polynesian is twice that in European/Caucasian/mixed (57). In the retrospective study in US, there was no difference in the incidence of PE among different ethnicity, including Mexican American, Caucasian and African American (53). The same conclusion was reached in the retrospective study of Fiji, that is ethnicity had no effect on the occurrence of PE in women with GDM (55).
Table 2 summarizes whether the above factors are independently associated with the occurrence of PE in women with GDM.
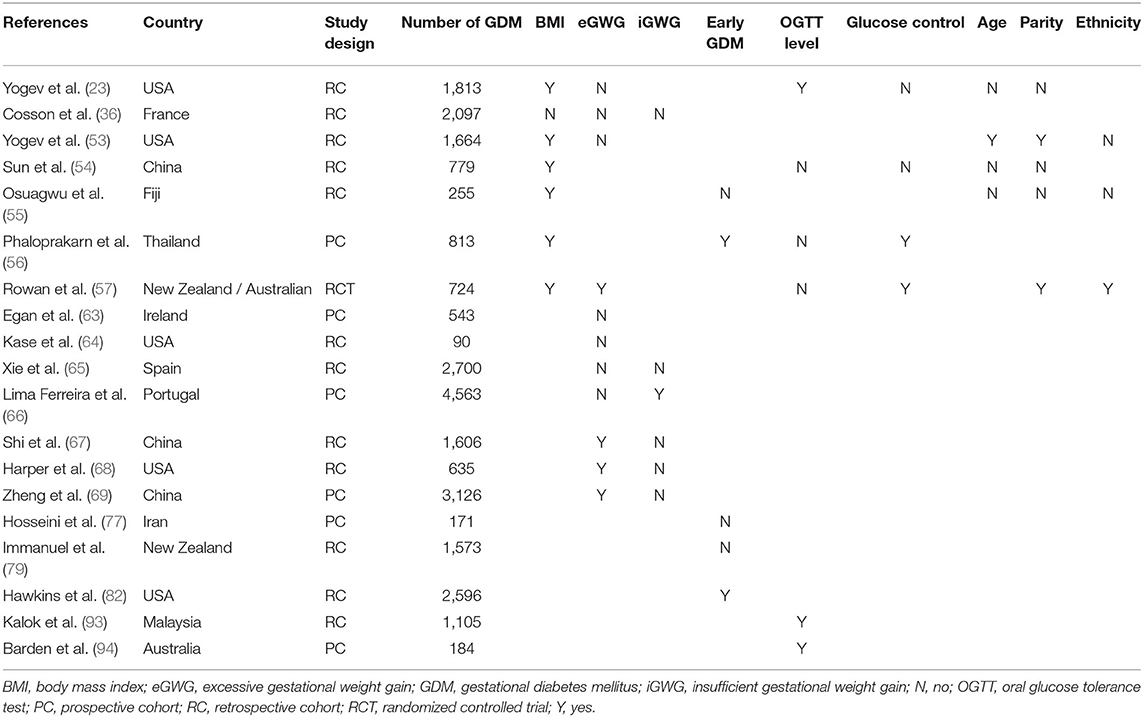
Table 2. Factors independent affecting the incidence of preeclampsia in women with gestational diabetes mellitus.
The first treatment of GDM is lifestyle intervention, including diet and daily activities. When hyperglycemia is evident after ≥1–2 weeks of lifestyle interventions, daily glucose testing should be continued, and pharmacological treatment should be initiated. Insulin is the most traditionally-preferred drug; oral hypoglycemic drugs, glibenclamide and metformin, are also used in some countries (2). Considering that these drugs can cross the placenta, the American Diabetes Association does not recommend them as first-line drugs for GDM (106). Two randomized controlled trials showed that intervention with GDM (including dietary recommendations, blood glucose monitoring, and insulin treatment) significantly reduced the risk of PE (90, 91), and a meta-analyses revealed similar conclusions (107).
Lifestyle intervention mainly includes diet and exercise, the diet should contain sufficient macronutrients and micronutrients, carbohydrates with low glycemic index are recommended (2). A randomized controlled trial showed that a Mediterranean Diet, supplemented with extra-virgin olive oil and pistachios, can reduce the incidence of adverse pregnancy events of GDM, the incidence of PE in women with GDM was not different from that without GDM (108). However, omega-3 fatty acids supplementation had no effect on the incidence of PE in women with GDM (109). Inositol is considered as a food supplement, randomized controlled trials and meta-analysis believe that it can prevent the occurrence of GDM, but it cannot prevent the occurrence of pregnancy induced hypertension in high-risk groups of GDM (110–112). For women diagnosed with GDM, inositol supplementation also cannot reduce the risk of pregnancy induced hypertension/PE (113–115). Meta-analysis showed that there was a significant negative association between smoking during pregnancy and incidence of PE (116), but smoking during pregnancy did not reduce the incidence of PE in women with GDM in a retrospective cohort study (117). Moderate exercises during pregnancy are helpful to control pregnancy weight and blood glucose for women with GDM, but it has no effect on the occurrence of PE (118, 119).
Although women with GDM who require insulin treatment tend to have higher blood glucose at OGTT (120–122), there was no significant difference in the incidence of PE compared to that in women on diet treatment alone (23, 39, 120–122). This may be related to the better management in the insulin treatment group (121); even the incidence of PE in women with GDM treated with insulin is consistent with those with normal glucose tolerance (123). If insulin treatment reaches the established blood glucose control level, the risk of PE in GDM with obesity is not different from that in normal weight (58).
For the use of glibenclamide and insulin in GDM, more comparisons were reported on blood glucose control and neonatal outcomes, and less on PE. A retrospective cohort study in California showed that the incidence of PE in a glibenclamide-treated group was twice that in an insulin-treated group, and the risk was still 2.32 times higher after adjusting for confounding factors (124). Another randomized controlled trial study found that there was no difference in the incidence of PE between glibenclamide- and insulin-treated groups (125). The same result was found even among women with GDM with significantly increased oral glucose stimulation test and fasting hyperglycemia (126).
A randomized controlled trial of 733 pregnant women in 10 hospitals in New Zealand and Australia compared the pregnancy outcomes between the administration of metformin and insulin for GDM. Although the incidence of PE in the metformin-treated group was lower than that in the insulin-treated group, the difference was not statistically significant (127). In other studies, metformin also had no effect on the incidence of PE (128–131). However, the metformin-treated group had less weight gain after treatment (127, 129). No relevant data exist on whether metformin is advantageous in obese women with GDM. A recent meta-analysis evaluated the efficacy of metformin, glibenclamide, and insulin in the treatment of GDM. Metformin showed a trend of reducing PE compared with insulin, but there was no significant difference. The incidence of PE in the glibenclamide-treated group was slightly higher than that in insulin-treated group; however, there was also no significant difference (132). Metformin may prevent PE by reducing the production of anti angiogenic factors, improving endothelial dysfunction and changing cell homeostasis and energy allocation (133), it is expected to become an ideal drug for preventing PE in women with GDM.
With the increase in maternal age and application of assisted reproductive technology, the incidence of twin pregnancy has been increasing (134, 135). Twin pregnancy is a common risk factor of GDM and PE (1, 2), the correlation between twin pregnancy and hypertensive disease/PE was higher than that of GDM (34, 136). Population-based retrospective cohort studies across different time periods (2005–2011 and 2012–2016) in Canadians show a higher incidence of PE in twin pregnancies than in singletons, with or without GDM (35, 40). Retrospective case control studies in China and Australia also show a significantly higher incidence of PE in women with GDM with twin pregnancies than that in women with singletons (137, 138). The two population-based cohort studies in Canadians derived different conclusions on whether GDM is associated with PE in twin pregnancy. Early studies show that the risk of PE in twin pregnancy women with GDM is still 1.54-fold after adjusting for maternal age, ethnicity, parity, and prior hypertension (40). However, recent studies suggest that GDM was not associated with PE in twin pregnancy after adjusting for maternal age, smoking, parity, race, pre pregnancy BMI, and auxiliary factors (35). This difference may be related to the fact that early studies did not adjust for pre pregnancy BMI, because obesity significantly increases the risk of PE (52). The conclusions of other studies revealed inconsistencies; some suggested that the incidence of PE in twin pregnant women with GDM was significantly higher than that without GDM (135, 138, 139), and GDM was independent associated with PE in twin pregnancy (135, 139), while others suggested no significant difference between the two groups (140, 141). Whether GDM is independent associated with PE in twin pregnancy requires further validation; overall, GDM has less impact on PE in twin pregnancy than in singleton pregnancy (35, 40, 135).
Women with PCOS have increased insulin resistance and hyperandrogenemia (142, 143). PCOS increases the incidence of GDM and PE, and the results are independent of obesity and assisted reproductive technology (144–146). A meta-analysis showed that pregnant women with PCOS had a 2.89- and 1.87-fold risk of GDM and PE, respectively (145). The incidence of PE is significantly higher in women with GDM combined with PCOS than in those without PCOS (147–150). The risk was 2-3-fold after adjusting for factors such as age, pre pregnancy BMI, and parity (148–150). Only one study believes that there was no correlation between PCOS and PE in women with GDM after adjusting for confounding factors (147). A Chinese prospective study found no difference in the incidence of PE between women with GDM with PCOS and those without PCOS, although the result was affected by factors such as small sample size and early intervention (151). Pregnant women with PCOS combined with GDM tend to be older and have higher pre pregnancy BMI (152–154). It is necessary to determine whether GDM associated with PE in pregnant women with PCOS. A prospective, double-blind, multicenter trial including 228 pregnant women with PCOS in Norway revealed that there was no statistical correlation between early GDM and PE occurrence (152). Similarly, two other studies were added to the earlier one, which increased the number of pregnant women with PCOS to 722. The results still show that GDM did not increase the incidence of PE in pregnant women with PCOS, regardless of whether GDM occurred early or later (153). Another prospective study in China found that the incidence of PE in PCOS pregnant women with GDM is significantly higher than that in pregnant women without GDM but did not analyze whether it was independent associated with PE (154). Thus, the effect of GDM on PE in pregnant women with PCOS is less than the effect of PCOS on PE in women with GDM.
Although GDM is associated with PE, the exact mechanism underlying the two disease associations is unclear. The pathophysiological process of PE involves two stages, early insufficient trophoblast invasion leads to incomplete spiral artery remodeling, which causes placental ischaemia and oxidative stress. The diseased placenta progressively secretes elevated amounts of anti-angiogenic factors [soluble fms-like tyrosine kinase-1 (sFlt1) and soluble endoglin] that cause maternal inflammation and vascular endothelial dysfunction, and finally lead to systemic diseases (1, 155). Hyperglycemia can induce trophoblast inflammation and autophagy, inhibit trophoblast migration and invasion (156–158). Neutrophils in GDM are over-activated and release excessive neutrophil extracellular traps (NETs) (159, 160). Excessive NETs hinder the blood circulation in the villous space, resulting in placental ischemia, which is related to the occurrence of PE (161–163). Oxidative stress increases in women with GDM (164–166), hyperglycemia can induce oxidative stress through a variety of ways, including the formation of advanced glycation end products (AGEs) (166). The production of reactive oxygen species increases during oxidative stress, resulting in a decrease in circulating nitric oxide (NO) level and bioavailability (166), which leads to vasodilation dysfunction. AGEs are significantly increased in women with GDM (167), and can promote the occurrence of PE by inducing oxidative stress and inflammation (168–170). Moreover, the pro-inflammatory cytokines serum tumor necrosis factor-α(TNF-α) and Interleukin-6 (IL-6) have been found increased in the circulation of women with GDM (171, 172), which are associated with endothelial dysfunction (172), and also increased in women with PE (173, 174). Some studies suggest that TNF-α, IL-6, and C-reactive protein (CRP) are independent risk factors for PE in women with GDM (94, 175, 176), and others suggest that in addition to the increased level of CRP, the imbalance of Interleukin-17 /Interleukin-35 may also be involved in the pathogenesis of GDM complicated with PE (177). Genetic variants are also associated with PE in women with GDM, the MIR146Ars2910164CC genotype, HNF1αgene p. I27L TT genotype, and ACE I / D polymorphism DD genotype was significantly higher in women with GDM complicated with PE (178–180). Obesity is the main influencing factor of PE in women with GDM in this paper. There are many of same pathophysiological changes between obesity and GDM, but obesity was concluded to be associated with greater oxidative stress and inflammation including the imbalance of fat factors (181), which are related to the occurrence of PE. Hyperinsulinemia and insulin resistance caused by obesity before pregnancy are related to the migration of cytotrophoblast and the reduction of uterine spiral artery remodeling, which is more likely to lead to placental ischemia (182). The mechanism of gestational diabetes mellitus affecting the occurrence of preeclampsia is shown in Figure 1.
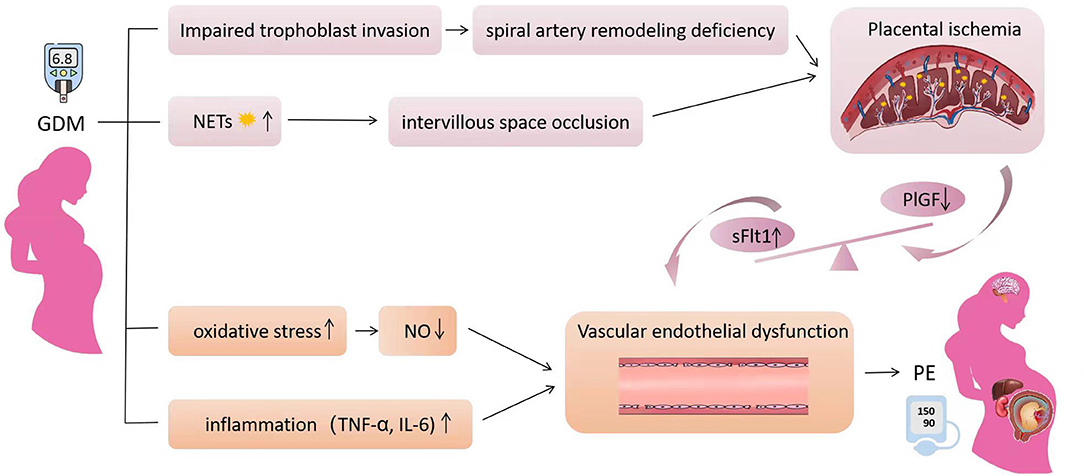
Figure 1. The mechanism of gestational diabetes mellitus affecting the occurrence of preeclampsia. Hyperglycemia inhibits trophoblast migration and invasion by inducing trophoblast inflammation and autophagy, which can lead to uterine spiral artery remodeling deficiency. Neutrophils in GDM are over-activated and release excessive neutrophil extracellular traps resulting intervillous space occlusion. The two factors cause placental ischemia and hypoxia, resulting in the imbalance of anti-angiogenic factors, which can lead to vascular endothelial injury. In addition, increased oxidative stress in GDM leads to decreased nitric oxide synthesis and activity, resulting in vasodilation dysfunction. The increased inflammatory factors further aggravate the vascular endothelial injury. The clinical symptoms of preeclampsia eventually appeared, including hypertension and multiple organ injury. Obesity exaggerates all pathways affecting PE.
Multiple biochemical markers have been studied to predict the occurrence of GDM and PE, and CRP, TNF-α, IL-6, and B-type natriuretic peptides are common predictive markers (183, 184), but none are used as practical clinical markers. Serum sFlt1 / placental growth factor (PlGF) is a valid marker for predicting and diagnosing PE (185). It is also significantly elevated in the blood of women with GDM complicated with PE (186). However, whether it can early identify the risk of PE in women with GDM needs further research. In conclusion, there are no practical markers to predict the occurrence of PE in women with GDM, and we need to explore the pathophysiology of GDM and PE further.
In most studies, GDM is independently associated with PE in singleton pregnancy, and pre pregnancy BMI and blood glucose levels are closely related with the occurrence of PE. Therefore, optimizing the treatment and management of GDM can reduce the incidence of PE. Oral hypoglycemic drugs, including metformin and glibenclamide, showed no significant difference in the occurrence of PE compared with insulin, despite a decreasing trend for metformin. The effects of GWG on PE, especially after the diagnosis of GDM and early-onset GDM, are controversial, and thus warrant further prospective studies. Twin pregnancy and PCOS significantly increased the occurrence of PE in women with GDM. However, GDM has less effect on PE in twin pregnancy and pregnant women with PCOS. The prevalence of GDM is significantly increased, which also increases the incidence of PE. Therefore, identifying the controllable factors affecting PE of GDM is important for improving pregnancy outcomes. GDM may affecting the occurrence of PE by inducing placental ischemia, increasing oxidative stress and inflammation. Understanding the pathophysiological mechanism of GDM affecting the occurrence of PE is helpful to find effective markers and preventive measures, which needs further studies.
YY collected material and wrote the first draft. NW contributed to design of the study and provided critical feedback. All authors contributed to the article and agree to be accountable for the content of the work.
This study was funded by the National Natural Science Foundation of China (No. 81700706), the 345 Talent Project of ShengJing Hospital, the Clinical Research Project of Liaoning Diabetes Medical Nutrition Prevention Society (No. LNSTNBYXYYFZXH-RS01B), Natural Science Foundation of Liaoning Province (No. 2021-MS-182), the Science Foundation of Liaoning Education Department (No. LK201603), and the Virtual Simulation Experiment Teaching Project of China Medical University (No.2020-47).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
AGEs, advanced glycation end products; BMI, body mass index; CRP, C-reactive protein; GDM, gestational diabetes mellitus; GWG, gestational weight gain; HAPO, Hyperglycemia and Adverse Pregnancy Outcome; HbA1c, glycosylated hemoglobin; IADPSG, International Association of Diabetes and Pregnancy Study Groups; IL-6, interleukin-6; IOM, Institute of Medicine; NAM, National Academy of Medicine; NETs, sneutrophil extracellular traps; NO, nitric oxide; OGTT, oral glucose tolerance test; PCOS, polycystic ovary syndrome; PE, preeclampsia; PlGF, placental growth factor; sFlt1, soluble fms-like tyrosine kinase-1; TNF-α, tumor necrosis factor; WHO, World Health Organization.
1. Rana S, Lemoine E, Granger JP, Karumanchi SA. Preeclampsia: pathophysiology, challenges, and perspectives. Circ Res. (2019) 124:1094–112. doi: 10.1161/CIRCRESAHA.118.313276
2. McIntyre HD, Catalano P, Zhang C, Desoye G, Mathiesen ER, Damm P. Gestational diabetes mellitus. Nat Rev Dis Primers. (2019) 5:47. doi: 10.1038/s41572-019-0098-8
3. Phoswa WN, Khaliq OP. The role of oxidative stress in hypertensive disorders of pregnancy (preeclampsia, gestational hypertension) and metabolic disorder of pregnancy (gestational diabetes mellitus). Oxid Med Cell Longev. (2021) 2021:5581570. doi: 10.1155/2021/5581570
4. McElwain CJ, Tuboly E, McCarthy FP, McCarthy CM. Mechanisms of endothelial dysfunction in pre-eclampsia and gestational diabetes mellitus: windows into future cardiometabolic health? Front Endocrinol. (2020) 11:655. doi: 10.3389/fendo.2020.00655
5. Vounzoulaki E, Khunti K, Abner SC, Tan BK, Davies MJ, Gillies CL. Progression to type 2 diabetes in women with a known history of gestational diabetes: systematic review and meta-analysis. BMJ. (2020) 369:m1361. doi: 10.1136/bmj.m1361
6. Kramer CK, Campbell S, Retnakaran R. Gestational diabetes and the risk of cardiovascular disease in women: a systematic review and meta-analysis. Diabetologia. (2019) 62:905–14. doi: 10.1007/s00125-019-4840-2
7. Wu P, Kwok CS, Haththotuwa R, Kotronias RA, Babu A, Fryer AA, et al. Pre-eclampsia is associated with a twofold increase in diabetes: a systematic review and meta-analysis. Diabetologia. (2016) 59:2518–26. doi: 10.1007/s00125-016-4098-x
8. Mongraw-Chaffin ML, Cirillo PM, Cohn BA. Preeclampsia and cardiovascular disease death: prospective evidence from the child health and development studies cohort. Hypertension. (2010) 56:166–71. doi: 10.1161/HYPERTENSIONAHA.110.150078
9. Metzger BE, Coustan DR. Summary and recommendations of the Fourth International Workshop-Conference on Gestational Diabetes Mellitus. The Organizing Committee. Diabetes Care. (1998) 21 Suppl 2:B161–7.
10. International International Association of D, Pregnancy Study Groups Consensus P,, Metzger BE, Gabbe SG, Persson B, Buchanan TA, et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. (2010) 33:676–82. doi: 10.2337/dc09-1848
11. WHO. Diagnostic Criteria and Classification of Hyperglycaemia First Detected in Pregnancy. WHO Guidelines Approved by the Guidelines Review Committee. Geneva: WHO (2013).
12. Gortazar L, Flores-Le Roux JA, Benaiges D, Sarsanedas E, Paya A, Mane L, et al. Trends in prevalence of gestational diabetes and perinatal outcomes in Catalonia, Spain, 2006 to 2015: the Diagestcat Study. Diabetes Metab Res Rev. (2019) 35:e3151. doi: 10.1002/dmrr.3151
13. Garmendia ML, Mondschein S, Montiel B, Kusanovic JP. Trends and predictors of gestational diabetes mellitus in Chile. Int J Gynaecol Obstet. (2020) 148:210–8. doi: 10.1002/ijgo.13023
14. Brown J, Kapurubandara S, McGee TM. Confounding effect of ethnic diversity on booking-in body mass index and prevalence of gestational diabetes and hypertensive disorders in pregnant women in western Sydney 1997-2016. Aust N Z J Obstet Gynaecol. (2020) 60:369–75. doi: 10.1111/ajo.13077
15. Gao C, Sun X, Lu L, Liu F, Yuan J. Prevalence of gestational diabetes mellitus in mainland China: a systematic review and meta-analysis. J Diabetes Investig. (2019) 10:154–62. doi: 10.1111/jdi.12854
16. Catalano PM, McIntyre HD, Cruickshank JK, McCance DR, Dyer AR, Metzger BE, et al. The hyperglycemia and adverse pregnancy outcome study: associations of GDM and obesity with pregnancy outcomes. Diabetes Care. (2012) 35:780–6. doi: 10.2337/dc11-1790
17. Conde-Agudelo A, Belizan JM. Risk factors for pre-eclampsia in a large cohort of Latin American and Caribbean women. BJOG. (2000) 107:75–83. doi: 10.1111/j.1471-0528.2000.tb11582.x
18. Brown MA, Magee LA, Kenny LC, Karumanchi SA, McCarthy FP, Saito S, et al. Hypertensive disorders of pregnancy: ISSHP classification, diagnosis, and management recommendations for international practice. Hypertension. (2018) 72:24–43. doi: 10.1161/HYPERTENSIONAHA.117.10803
19. Duley L. The global impact of pre-eclampsia and eclampsia. Semin Perinatol. (2009) 33:130–7. doi: 10.1053/j.semperi.2009.02.010
20. Bauserman M, Thorsten VR, Nolen TL, Patterson J, Lokangaka A, Tshefu A, et al. Maternal mortality in six low and lower-middle income countries from 2010 to 2018: risk factors and trends. Reprod Health. (2020) 17:173. doi: 10.1186/s12978-020-00990-z
21. Nunes JS, Ladeiras R, Machado L, Coelho D, Duarte C, Furtado JM. The influence of preeclampsia, advanced maternal age and maternal obesity in neonatal outcomes among women with gestational diabetes. Rev Bras Ginecol Obstet. (2020) 42:607–13. doi: 10.1055/s-0040-1710300
22. Fan ZT, Yang HX, Gao XL, Lintu H, Sun WJ. Pregnancy outcome in gestational diabetes. Int J Gynaecol Obstet. (2006) 94:12–6. doi: 10.1016/j.ijgo.2006.03.021
23. Yogev Y, Xenakis EM, Langer O. The association between preeclampsia and the severity of gestational diabetes: the impact of glycemic control. Am J Obstet Gynecol. (2004) 191:1655–60. doi: 10.1016/j.ajog.2004.03.074
24. Xu F, Yang S, Liu Y, Zheng X, Yang H, Zhang J, et al. Placental pathology and neonatal outcomes in pre-eclampsia with gestational diabetes mellitus. J Matern Fetal Neonatal Med. (2021) 34:1149–54. doi: 10.1080/14767058.2020.1786513
25. Chen KH, Chen LR. Provoking factors for postpartum chronic hypertension in women with preceding gestational hypertension/preeclampsia: a longitudinal cohort study of 22,798 pregnancies. Int J Med Sci. (2020) 17:543–8. doi: 10.7150/ijms.39432
26. Kul S, Guvenc TS, Baycan OF, Celik FB, Caliskan Z, Cetin Guvenc R, et al. Combined past preeclampsia and gestational diabetes is associated with a very high frequency of coronary microvascular dysfunction. Microvasc Res. (2021) 134:104104. doi: 10.1016/j.mvr.2020.104104
27. Engeland A, Bjorge T, Daltveit AK, Skurtveit S, Vangen S, Vollset SE, et al. Risk of diabetes after gestational diabetes and preeclampsia. A registry-based study of 230,000 women in Norway. Eur J Epidemiol. (2011) 26:157–63. doi: 10.1007/s10654-010-9527-4
28. Huang Y, Zhang W, Go K, Tsuchiya KJ, Hu J, Skupski DW, et al. Altered growth trajectory in children born to mothers with gestational diabetes mellitus and preeclampsia. Arch Gynecol Obstet. (2020) 301:151–9. doi: 10.1007/s00404-020-05436-2
29. Group HSCR, Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. (2008) 358:1991–2002. doi: 10.1056/NEJMoa0707943
30. Ovesen PG, Jensen DM, Damm P, Rasmussen S, Kesmodel US. Maternal and neonatal outcomes in pregnancies complicated by gestational diabetes. A nation-wide study. J Matern Fetal Neonatal Med. (2015) 28:1720–4. doi: 10.3109/14767058.2014.966677
31. Hilden K, Hanson U, Persson M, Fadl H. Overweight and obesity: a remaining problem in women treated for severe gestational diabetes. Diabet Med. (2016) 33:1045–51. doi: 10.1111/dme.13156
32. Ostlund I, Haglund B, Hanson U. Gestational diabetes and preeclampsia. Eur J Obstet Gynecol Reprod Biol. (2004) 113:12–6. doi: 10.1016/j.ejogrb.2003.07.001
33. Fadl HE, Ostlund IK, Magnuson AF, Hanson US. Maternal and neonatal outcomes and time trends of gestational diabetes mellitus in Sweden from 1991 to 2003. Diabet Med. (2010) 27:436–41. doi: 10.1111/j.1464-5491.2010.02978.x
34. Nerenberg KA, Johnson JA, Leung B, Savu A, Ryan EA, Chik CL, et al. Risks of gestational diabetes and preeclampsia over the last decade in a cohort of Alberta women. J Obstet Gynaecol Can. (2013) 35:986–94. doi: 10.1016/S1701-2163(15)30786-6
35. Hiersch L, Berger H, Okby R, Ray JG, Geary M, McDonald SD, et al. Gestational diabetes mellitus is associated with adverse outcomes in twin pregnancies. Am J Obstet Gynecol. (2019) 220:102 e1–e8. doi: 10.1016/j.ajog.2018.10.027
36. Cosson E, Cussac-Pillegand C, Benbara A, Pharisien I, Nguyen MT, Chiheb S, et al. Pregnancy adverse outcomes related to pregravid body mass index and gestational weight gain, according to the presence or not of gestational diabetes mellitus: a retrospective observational study. Diabetes Metab. (2016) 42:38–46. doi: 10.1016/j.diabet.2015.06.001
37. Stone CA, McLachlan KA, Halliday JL, Wein P, Tippett C. Gestational diabetes in Victoria in 1996: incidence, risk factors and outcomes. Med J Aust. (2002) 177:486–91. doi: 10.5694/j.1326-5377.2002.tb04916.x
38. Bryson CL, Ioannou GN, Rulyak SJ, Critchlow C. Association between gestational diabetes and pregnancy-induced hypertension. Am J Epidemiol. (2003) 158:1148–53. doi: 10.1093/aje/kwg273
39. Billionnet C, Mitanchez D, Weill A, Nizard J, Alla F, Hartemann A, et al. Gestational diabetes and adverse perinatal outcomes from 716,152 births in France in 2012. Diabetologia. (2017) 60:636–44. doi: 10.1007/s00125-017-4206-6
40. Lai FY, Johnson JA, Dover D, Kaul P. Outcomes of singleton and twin pregnancies complicated by pre-existing diabetes and gestational diabetes: a population-based study in Alberta, Canada, 2005-11. J Diabetes. (2016) 8:45–55. doi: 10.1111/1753-0407.12255
41. Schmidt MI, Duncan BB, Reichelt AJ, Branchtein L, Matos MC, Costa e Forti A, et al. Gestational diabetes mellitus diagnosed with a 2-h 75-g oral glucose tolerance test and adverse pregnancy outcomes. Diabetes Care. (2001) 24:1151–5. doi: 10.2337/diacare.24.7.1151
42. Cheung NW, Jiang S, Athayde N. Impact of the IADPSG criteria for gestational diabetes, and of obesity, on pregnancy outcomes. Aust N Z J Obstet Gynaecol. (2018) 58:553–9. doi: 10.1111/ajo.12772
43. Shindo R, Aoki S, Kasai J, Saigusa Y, Nakanishi S, Miyagi E. Impact of introducing the International Association of Diabetes and Pregnancy Study Groups (IADPSG) criteria on pregnancy outcomes in Japan. Endocr J. (2020) 67:15–20. doi: 10.1507/endocrj.EJ19-0279
44. Joffe GM, Esterlitz JR, Levine RJ, Clemens JD, Ewell MG, Sibai BM, et al. The relationship between abnormal glucose tolerance and hypertensive disorders of pregnancy in healthy nulliparous women. Calcium for Preeclampsia Prevention (CPEP) Study Group. Am J Obstet Gynecol. (1998) 179:1032–7. doi: 10.1016/S0002-9378(98)70210-8
45. Weschenfelder F, Hein F, Lehmann T, Schleussner E, Groten T. Contributing factors to perinatal outcome in pregnancies with gestational diabetes-what matters most? a retrospective analysis. J Clin Med. (2021) 10:348. doi: 10.3390/jcm10020348
46. Kosir Pogacnik R, Trojner Bregar A, Lucovnik M, Krajec M, Verdenik I, Blickstein I, et al. The effect of interaction between parity, gestational diabetes, and pregravid obesity on the incidence of preeclampsia. J Matern Fetal Neonatal Med. (2020) 33:931–4. doi: 10.1080/14767058.2018.1509311
47. Emanuel M, Butt S. Frequency and factors leading to recurrent pre-eclampsia. J Pak Med Assoc. (2015) 65:1173–7.
48. Bigelow CA, Pereira GA, Warmsley A, Cohen J, Getrajdman C, Moshier E, et al. Risk factors for new-onset late postpartum preeclampsia in women without a history of preeclampsia. Am J Obstet Gynecol. (2014) 210:338 e1–8. doi: 10.1016/j.ajog.2013.11.004
49. Wainstock T, Sergienko R, Sheiner E. Who is at risk for preeclampsia? risk factors for developing initial preeclampsia in a subsequent pregnancy. J Clin Med. (2020) 9:41103. doi: 10.3390/jcm9041103
50. Lee J, Ouh YT, Ahn KH, Hong SC, Oh MJ, Kim HJ, et al. Preeclampsia: a risk factor for gestational diabetes mellitus in subsequent pregnancy. PLoS ONE. (2017) 12:e0178150. doi: 10.1371/journal.pone.0178150
51. WHO. Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. (2000) 894:1–253. doi: 10.1002/jps.3080150106
52. Santos S, Voerman E, Amiano P, Barros H, Beilin LJ, Bergstrom A, et al. Impact of maternal body mass index and gestational weight gain on pregnancy complications: an individual participant data meta-analysis of European, North American and Australian cohorts. BJOG. (2019) 126:984–95. doi: 10.1111/1471-0528.15661
53. Yogev Y, Langer O, Brustman L, Rosenn B. Pre-eclampsia and gestational diabetes mellitus: does a correlation exist early in pregnancy? J Matern Fetal Neonatal Med. (2004) 15:39–43. doi: 10.1080/14767050310001650707
54. Sun Y, Yang H, Sun WJ. Risk factors for pre-eclampsia in pregnant Chinese women with abnormal glucose metabolism. Int J Gynaecol Obstet. (2008) 101:74–6. doi: 10.1016/j.ijgo.2007.10.008
55. Osuagwu UL, Fuka F, Agho K, Khan A, Simmons D. Adverse maternal outcomes of Fijian women with gestational diabetes mellitus and the associated risk factors. Reprod Sci. (2020) 27:2029–37. doi: 10.1007/s43032-020-00222-6
56. Phaloprakarn C, Tangjitgamol S. Risk assessment for preeclampsia in women with gestational diabetes mellitus. J Perinat Med. (2009) 37:617–21. doi: 10.1515/JPM.2009.108
57. Rowan JA, Gao W, Hague WM, McIntyre HD. Glycemia and its relationship to outcomes in the metformin in gestational diabetes trial. Diabetes Care. (2010) 33:9–16. doi: 10.2337/dc09-1407
58. Langer O, Yogev Y, Xenakis EM, Brustman L. Overweight and obese in gestational diabetes: the impact on pregnancy outcome. Am J Obstet Gynecol. (2005) 192:1768–76. doi: 10.1016/j.ajog.2004.12.049
59. Simko M, Totka A, Vondrova D, Samohyl M, Jurkovicova J, Trnka M, et al. Maternal body mass index and gestational weight gain and their association with pregnancy complications and perinatal conditions. Int J Environ Res Public Health. (2019) 16:1751. doi: 10.3390/ijerph16101751
60. Lewandowska M, Wieckowska B, Sajdak S. Pre-pregnancy obesity, excessive gestational weight gain, and the risk of pregnancy-induced hypertension and gestational diabetes mellitus. J Clin Med. (2020) 9:1980. doi: 10.3390/jcm9061980
61. Rasmussen KM, Yaktine AL. Weight Gain During Pregnancy: Reexamining the Guidelines. The National Academies Collection: Reports funded by National Institutes of Health. Washington, DC: National Institutes of Health (2009).
62. Viecceli C, Remonti LR, Hirakata VN, Mastella LS, Gnielka V, Oppermann ML, et al. Weight gain adequacy and pregnancy outcomes in gestational diabetes: a meta-analysis. Obes Rev. (2017) 18:567–80. doi: 10.1111/obr.12521
63. Egan AM, Dennedy MC, Al-Ramli W, Heerey A, Avalos G, Dunne F, et al. excessive gestational weight gain and pregnancy outcomes in women with gestational or pregestational diabetes mellitus. J Clin Endocrinol Metab. (2014) 99:212–9. doi: 10.1210/jc.2013-2684
64. Kase BA, Cormier CM, Costantine MM, Hutchinson M, Ramin SM, Saade GR, et al. Excessive gestational weight gain in women with gestational and pregestational diabetes. Am J Perinatol. (2011) 28:761–6. doi: 10.1055/s-0031-1280857
65. Xie X, Liu J, Pujol I, Lopez A, Martinez MJ, Garcia-Patterson A, et al. Inadequate weight gain according to the institute of medicine 2009 guidelines in women with gestational diabetes: frequency, clinical predictors, and the association with pregnancy outcomes. J Clin Med. (2020) 9:3343. doi: 10.3390/jcm9103343
66. Lima Ferreira J, Voss G, Doria M, Sa Couto A, Principe RM. Benefit of insufficient gestational weight gain in obese women with gestational diabetes mellitus: a multicenter study in Portugal. Diabetes Metab Syndr. (2021) 15:419–24. doi: 10.1016/j.dsx.2021.01.020
67. Shi P, Liu A, Yin X. Association between gestational weight gain in women with gestational diabetes mellitus and adverse pregnancy outcomes: a retrospective cohort study. BMC Pregnancy Childbirth. (2021) 21:508. doi: 10.1186/s12884-021-03982-4
68. Harper LM, Tita A, Biggio JR. The institute of medicine guidelines for gestational weight gain after a diagnosis of gestational diabetes and pregnancy outcomes. Am J Perinatol. (2015) 32:239–46. doi: 10.1055/s-0034-1383846
69. Zheng W, Huang W, Liu C, Yan Q, Zhang L, Tian Z, et al. Weight gain after diagnosis of gestational diabetes mellitus and its association with adverse pregnancy outcomes: a cohort study. BMC Pregnancy Childbirth. (2021) 21:216. doi: 10.1186/s12884-021-03690-z
70. Wu JN, Gu WR, Xiao XR, Zhang Y, Li XT, Yin CM. Gestational weight gain targets during the second and third trimesters of pregnancy for women with gestational diabetes mellitus in China. Eur J Clin Nutr. (2019) 73:1155–63. doi: 10.1038/s41430-018-0358-9
71. Wong T, Barnes RA, Ross GP, Cheung NW, Flack JR. Are the Institute of Medicine weight gain targets applicable in women with gestational diabetes mellitus? Diabetologia. (2017) 60:416–23. doi: 10.1007/s00125-016-4173-3
72. Zhang X, Xiao Y. The association between trimester-specific weight gain and severe preeclampsia/adverse perinatal outcome in gestational diabetes mellitus complicated by preeclampsia: a retrospective case study. Diabetes Ther. (2019) 10:725–34. doi: 10.1007/s13300-019-0589-3
73. American Diabetes A. Classification and diagnosis of diabetes. Diabetes Care. (2015) 38(Suppl.):S8–16. doi: 10.2337/dc15-S005
74. Saraiva M, Fonseca L, Santos T, Vilaverde J, Pereira MT, Pichel F, et al. Mild periconceptional hyperglycemia: predictor of adverse fetomaternal outcomes in gestational diabetes? Acta Diabetol. (2021) 58:1209–15. doi: 10.1007/s00592-021-01714-w
75. Berkowitz GS, Roman SH, Lapinski RH, Alvarez M. Maternal characteristics, neonatal outcome, and the time of diagnosis of gestational diabetes. Am J Obstet Gynecol. (1992) 167:976–82. doi: 10.1016/S0002-9378(12)80023-8
76. Schaffir JA, Lockwood CJ, Lapinski R, Yoon L, Alvarez M. Incidence of pregnancy-induced hypertension among gestational diabetics. Am J Perinatol. (1995) 12:252–4. doi: 10.1055/s-2007-994466
77. Hosseini E, Janghorbani M, Shahshahan Z. Comparison of risk factors and pregnancy outcomes of gestational diabetes mellitus diagnosed during early and late pregnancy. Midwifery. (2018) 66:64–9. doi: 10.1016/j.midw.2018.07.017
78. Glaharn P, Chumworathayi B, Kongwattanakul K, Sutthasri N, Wiangyot P. Proportion of abnormal second 50-g glucose challenge test in gestational diabetes mellitus screening using the two-step method in high-risk pregnant women. J Obstet Gynaecol Res. (2020) 46:229–36. doi: 10.1111/jog.14172
79. Immanuel J, Eagleton C, Baker J, Simmons D. Pregnancy outcomes among multi-ethnic women with different degrees of hyperglycaemia during pregnancy in an urban New Zealand population and their association with postnatal HbA1c uptake. Aust N Z J Obstet Gynaecol. (2021) 61:69–77. doi: 10.1111/ajo.13231
80. Immanuel J, Simmons D. Screening and treatment for early-onset gestational diabetes mellitus: a systematic review and meta-analysis. Curr Diab Rep. (2017) 17:115. doi: 10.1007/s11892-017-0943-7
81. Boriboonhirunsarn D, Sunsaneevithayakul P, Pannin C, Wamuk T. Prevalence of early-onset GDM and associated risk factors in a university hospital in Thailand. J Obstet Gynaecol. (2021) 41:915–9. doi: 10.1080/01443615.2020.1820469
82. Hawkins JS, Lo JY, Casey BM, McIntire DD, Leveno KJ. Diet-treated gestational diabetes mellitus: comparison of early vs. routine diagnosis. Am J Obstet Gynecol. (2008) 198:287e1–6. doi: 10.1016/j.ajog.2007.11.049
83. Mustafa M, Bogdanet D, Khattak A, Carmody LA, Kirwan B, Gaffney G, et al. Early gestational diabetes mellitus (GDM) is associated with worse pregnancy outcomes compared with GDM diagnosed at 24-28 weeks gestation despite early treatment. QJM. (2021) 114:17–24. doi: 10.1093/qjmed/hcaa167
84. Bartha JL, Martinez-Del-Fresno P, Comino-Delgado R. Gestational diabetes mellitus diagnosed during early pregnancy. Am J Obstet Gynecol. (2000) 182:346–50. doi: 10.1016/S0002-9378(00)70222-5
85. Rowan JA, Budden A, Ivanova V, Hughes RC, Sadler LC. Women with an HbA1c of 41-49 mmol/mol (59-66%): a higher risk subgroup that may benefit from early pregnancy intervention. Diabet Med. (2016) 33:25–31. doi: 10.1111/dme.12812
86. Cosson E, Vicaut E, Berkane N, Cianganu TL, Baudry C, Portal JJ, et al. Prognosis associated with initial care of increased fasting glucose in early pregnancy: a retrospective study. Diabetes Metab. (2021) 47:101197. doi: 10.1016/j.diabet.2020.08.007
87. Metzger BE, Buchanan TA, Coustan DR, de Leiva A, Dunger DB, Hadden DR, et al. Summary and recommendations of the Fifth International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care. (2007) 30(Suppl.2):S251–60. doi: 10.2337/dc07-s225
88. Dodd JM, Crowther CA, Antoniou G, Baghurst P, Robinson JS. Screening for gestational diabetes: the effect of varying blood glucose definitions in the prediction of adverse maternal and infant health outcomes. Aust N Z J Obstet Gynaecol. (2007) 47:307–12. doi: 10.1111/j.1479-828X.2007.00743.x
89. Carr DB, Newton KM, Utzschneider KM, Faulenbach MV, Kahn SE, Easterling TR, et al. Gestational diabetes or lesser degrees of glucose intolerance and risk of preeclampsia. Hypertens Pregnancy. (2011) 30:153–63. doi: 10.3109/10641950903115012
90. Crowther CA, Hiller JE, Moss JR, McPhee AJ, Jeffries WS, Robinson JS, et al. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med. (2005) 352:2477–86. doi: 10.1056/NEJMoa042973
91. Landon MB, Spong CY, Thom E, Carpenter MW, Ramin SM, Casey B, et al. A multicenter, randomized trial of treatment for mild gestational diabetes. N Engl J Med. (2009) 361:1339–48. doi: 10.1056/NEJMoa0902430
92. Dennedy MC, Avalos G, O'Reilly MW, O'Sullivan EP, Gaffney G, Dunne F, et al. raised maternal body mass index (BMI) adversely affects maternal and fetal outcomes in glucose-tolerant women according to International Association of Diabetes and Pregnancy Study Groups (IADPSG) criteria. J Clin Endocrinol Metab. (2012) 97:E608–12. doi: 10.1210/jc.2011-2674
93. Kalok A, Ong MY, Hasrori A, Chiang KS, Yazim F, Baharuddin S, et al. Correlation between oral glucose tolerance test abnormalities and adverse pregnancy outcomes in gestational diabetes: a cross-sectional study. Int J Environ Res Public Health. (2020) 17:6990. doi: 10.3390/ijerph17196990
94. Barden A, Singh R, Walters BN, Ritchie J, Roberman B, Beilin LJ. Factors predisposing to pre-eclampsia in women with gestational diabetes. J Hypertens. (2004) 22:2371–8. doi: 10.1097/00004872-200412000-00020
95. Mane L, Flores-Le Roux JA, Benaiges D, Rodriguez M, Marcelo I, Chillaron JJ, et al. Role of first-trimester HbA1c as a predictor of adverse obstetric outcomes in a multiethnic cohort. J Clin Endocrinol Metab. (2017) 102:390–7. doi: 10.1210/jc.2016-2581
96. Mane L, Flores-Le Roux JA, Pedro-Botet J, Gortazar L, Chillaron JJ, Llaurado G, et al. Is fasting plasma glucose in early pregnancy a better predictor of adverse obstetric outcomes than glycated haemoglobin? Eur J Obstet Gynecol Reprod Biol. (2019) 234:79–84. doi: 10.1016/j.ejogrb.2018.12.036
97. Hughes RC, Moore MP, Gullam JE, Mohamed K, Rowan J. An early pregnancy HbA1c >/=59% (41 mmol/mol) is optimal for detecting diabetes and identifies women at increased risk of adverse pregnancy outcomes. Diabetes Care. (2014) 37:2953–9. doi: 10.2337/dc14-1312
98. Kattini R, Hummelen R, Kelly L. Early gestational diabetes mellitus screening with glycated hemoglobin: a systematic review. J Obstet Gynaecol Can. (2020) 42:1379–84. doi: 10.1016/j.jogc.2019.12.015
99. Lowe LP, Metzger BE, Dyer AR, Lowe J, McCance DR, Lappin TR, et al. Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study: associations of maternal A1C and glucose with pregnancy outcomes. Diabetes Care. (2012) 35:574–80. doi: 10.2337/dc11-1687
100. Ho YR, Wang P, Lu MC, Tseng ST, Yang CP, Yan YH. Associations of mid-pregnancy HbA1c with gestational diabetes and risk of adverse pregnancy outcomes in high-risk Taiwanese women. PLoS One. (2017) 12:e0177563. doi: 10.1371/journal.pone.0177563
101. Odsaeter IH, Asberg A, Vanky E, Morkved S, Stafne SN, Salvesen KA, et al. Hemoglobin A1c as screening for gestational diabetes mellitus in Nordic Caucasian women. Diabetol Metab Syndr. (2016) 8:43. doi: 10.1186/s13098-016-0168-y
102. Yin B, Hu L, Meng X, Wu K, Zhang L, Zhu Y, et al. Association of higher HbA1c within the normal range with adverse pregnancy outcomes: a cross-sectional study. Acta Diabetol. (2021) 58:1081–9. doi: 10.1007/s00592-021-01691-0
103. Cosson E, Baz B, Gary F, Pharisien I, Nguyen MT, Sandre-Banon D, et al. Poor reliability and poor adherence to self-monitoring of blood glucose are common in women with gestational diabetes mellitus and may be associated with poor pregnancy outcomes. Diabetes Care. (2017) 40:1181–6. doi: 10.2337/dc17-0369
104. Yu F, Lv L, Liang Z, Wang Y, Wen J, Lin X, et al. Continuous glucose monitoring effects on maternal glycemic control and pregnancy outcomes in patients with gestational diabetes mellitus: a prospective cohort study. J Clin Endocrinol Metab. (2014) 99:4674–82. doi: 10.1210/jc.2013-4332
105. Panyakat WS, Phatihattakorn C, Sriwijitkamol A, Sunsaneevithayakul P, Phaophan A, Phichitkanka A. Correlation between third trimester glycemic variability in non-insulin-dependent gestational diabetes mellitus and adverse pregnancy and fetal outcomes. J Diabetes Sci Technol. (2018) 12:622–9. doi: 10.1177/1932296817752374
106. American Diabetes A. Management of diabetes in pregnancy: standards of medical care in diabetes-2021. Diabetes Care. (2021) 44(Suppl.1):S200–10. doi: 10.2337/dc21-S014
107. Hartling L, Dryden DM, Guthrie A, Muise M, Vandermeer B, Donovan L. Benefits and harms of treating gestational diabetes mellitus: a systematic review and meta-analysis for the US Preventive Services Task Force and the National Institutes of Health Office of Medical Applications of Research. Ann Intern Med. (2013) 159:123–9. doi: 10.7326/0003-4819-159-2-201307160-00661
108. de la Torre NG, Assaf-Balut C, Jimenez Varas I, Del Valle L, Duran A, Fuentes M, et al. Effectiveness of following mediterranean diet recommendations in the real world in the incidence of gestational diabetes mellitus (GDM) and adverse maternal-foetal outcomes: a prospective, Universal, Interventional Study with a Single Group. The St Carlos Study. Nutrients. (2019) 11:1210. doi: 10.3390/nu11061210
109. Gao L, Lin L, Shan N, Ren CY, Long X, Sun YH, et al. The impact of omega-3 fatty acid supplementation on glycemic control in patients with gestational diabetes: a systematic review and meta-analysis of randomized controlled studies. J Matern Fetal Neonatal Med. (2020) 33:1767–73. doi: 10.1080/14767058.2018.1526916
110. D'Anna R, Scilipoti A, Giordano D, Caruso C, Cannata ML, Interdonato ML, et al. Myo-Inositol supplementation and onset of gestational diabetes mellitus in pregnant women with a family history of type 2 diabetes: a prospective, randomized, placebo-controlled study. Diabetes Care. (2013) 36:854–7. doi: 10.2337/dc12-1371
111. Vitagliano A, Saccone G, Cosmi E, Visentin S, Dessole F, Ambrosini G, et al. Inositol for the prevention of gestational diabetes: a systematic review and meta-analysis of randomized controlled trials. Arch Gynecol Obstet. (2019) 299:55–68. doi: 10.1007/s00404-018-5005-0
112. Celentano C, Matarrelli B, Pavone G, Vitacolonna E, Mattei PA, Berghella V, et al. The influence of different inositol stereoisomers supplementation in pregnancy on maternal gestational diabetes mellitus and fetal outcomes in high-risk patients: a randomized controlled trial. J Matern Fetal Neonatal Med. (2020) 33:743–51. doi: 10.1080/14767058.2018.1500545
113. Lubin V, Shojai R, Darmon P, Cosson E. A pilot study of gestational diabetes mellitus not controlled by diet alone: first-line medical treatment with myoinositol may limit the need for insulin. Diabetes Metab. (2016) 42:192–5. doi: 10.1016/j.diabet.2016.01.005
114. Kulshrestha V, Balani S, Kachhawa G, Vanamail P, Kumari R, Sharma JB, et al. Efficacy of myoinositol in treatment of gestational diabetes mellitus in Asian Indian women: a pilot randomized clinical trial. Eur J Obstet Gynecol Reprod Biol. (2021) 260:42–7. doi: 10.1016/j.ejogrb.2021.02.017
115. D'Anna R, Corrado F, Loddo S, Gullo G, Giunta L, Di Benedetto A. Myoinositol plus alpha-lactalbumin supplementation, insulin resistance and birth outcomes in women with gestational diabetes mellitus: a randomized, controlled study. Sci Rep. (2021) 11:8866. doi: 10.1038/s41598-021-88329-x
116. Wei J, Liu CX, Gong TT, Wu QJ, Wu L. Cigarette smoking during pregnancy and preeclampsia risk: a systematic review and meta-analysis of prospective studies. Oncotarget. (2015) 6:43667–78. doi: 10.18632/oncotarget.6190
117. Contreras KR, Kominiarek MA, Zollinger TW. The impact of tobacco smoking on perinatal outcome among patients with gestational diabetes. J Perinatol. (2010) 30:319–23. doi: 10.1038/jp.2009.175
118. Yang X, Tian H, Zhang F, Zhang C, Li Y, Leng J, et al. A randomised translational trial of lifestyle intervention using a 3-tier shared care approach on pregnancy outcomes in Chinese women with gestational diabetes mellitus but without diabetes. J Transl Med. (2014) 12:290. doi: 10.1186/s12967-014-0290-2
119. Brown J, Ceysens G, Boulvain M. Exercise for pregnant women with gestational diabetes for improving maternal and fetal outcomes. Cochrane Database Syst Rev. (2017) 6:CD012202. doi: 10.1002/14651858.CD012696.pub2
120. Koning SH, Hoogenberg K, Scheuneman KA, Baas MG, Korteweg FJ, Sollie KM, et al. Neonatal and obstetric outcomes in diet- and insulin-treated women with gestational diabetes mellitus: a retrospective study. BMC Endocr Disord. (2016) 16:52. doi: 10.1186/s12902-016-0136-4
121. Todorova K, Palaveev O, Petkova VB, Stefanova M, Dimitrova Z. A pharmacoeconomical model for choice of a treatment for pregnant women with gestational diabetes. Acta Diabetol. (2007) 44:144–8. doi: 10.1007/s00592-007-0255-5
122. Huhtala MS, Ronnemaa T, Pellonpera O, Tertti K. Cord serum metabolome and birth weight in patients with gestational diabetes treated with metformin, insulin, or diet alone. BMJ Open Diabetes Res Care. (2021) 9:2022. doi: 10.1136/bmjdrc-2020-002022
123. Bogdanet D, Egan AM, Reddin C, Kgosidialwa O, Kirwan B, Carmody L, et al. ATLANTIC DIP: insulin therapy for women with IADPSG-diagnosed gestational diabetes mellitus. Does it work? J Clin Endocrinol Metab. (2017) 102:849–57. doi: 10.1210/jc.2016-2911
124. Jacobson GF, Ramos GA, Ching JY, Kirby RS, Ferrara A, Field DR. Comparison of glyburide and insulin for the management of gestational diabetes in a large managed care organization. Am J Obstet Gynecol. (2005) 193:118–24. doi: 10.1016/j.ajog.2005.03.018
125. Tempe A, Mayanglambam RD. Glyburide as treatment option for gestational diabetes mellitus. J Obstet Gynaecol Res. (2013) 39:1147–52. doi: 10.1111/jog.12042
126. Ramos GA, Jacobson GF, Kirby RS, Ching JY, Field DR. Comparison of glyburide and insulin for the management of gestational diabetics with markedly elevated oral glucose challenge test and fasting hyperglycemia. J Perinatol. (2007) 27:262–7. doi: 10.1038/sj.jp.7211683
127. Rowan JA, Hague WM, Gao W, Battin MR, Moore MP, Mi GTI. Metformin vs. insulin for the treatment of gestational diabetes. N Engl J Med. (2008) 358:2003–15. doi: 10.1056/NEJMoa0707193
128. Tertti K, Ekblad U, Vahlberg T, Ronnemaa T. Comparison of metformin and insulin in the treatment of gestational diabetes: a retrospective, case-control study. Rev Diabet Stud. (2008) 5:95–101. doi: 10.1900/RDS.2008.5.95
129. Balani J, Hyer SL, Rodin DA, Shehata H. Pregnancy outcomes in women with gestational diabetes treated with metformin or insulin: a case-control study. Diabet Med. (2009) 26:798–802. doi: 10.1111/j.1464-5491.2009.02780.x
130. Ainuddin J, Karim N, Hasan AA, Naqvi SA. Metformin vs. insulin treatment in gestational diabetes in pregnancy in a developing country: a randomized control trial. Diabetes Res Clin Pract. (2015) 107:290–9. doi: 10.1016/j.diabres.2014.10.001
131. Marques P, Carvalho MR, Pinto L, Guerra S. Metformin safety in the management of gestational diabetes. Endocr Pract. (2014) 20:1022–31. doi: 10.4158/EP14018.OR
132. Musa OAH, Syed A, Mohamed AM, Chivese T, Clark J, Furuya-Kanamori L, et al. Metformin is comparable to insulin for pharmacotherapy in gestational diabetes mellitus: a network meta-analysis evaluating 6,046 women. Pharmacol Res. (2021) 167:105546. doi: 10.1016/j.phrs.2021.105546
133. Romero R, Erez O, Huttemann M, Maymon E, Panaitescu B, Conde-Agudelo A, et al. Metformin, the aspirin of the 21st century: its role in gestational diabetes mellitus, prevention of preeclampsia and cancer, and the promotion of longevity. Am J Obstet Gynecol. (2017) 217:282–302. doi: 10.1016/j.ajog.2017.06.003
134. Ananth CV, Chauhan SP. Epidemiology of twinning in developed countries. Semin Perinatol. (2012) 36:156–61. doi: 10.1053/j.semperi.2012.02.001
135. Gortazar L, Flores-Le Roux JA, Benaiges D, Sarsanedas E, Navarro H, Paya A, et al. Trends in prevalence of diabetes among twin pregnancies and perinatal outcomes in catalonia between 2006 and 2015: the DIAGESTCAT study. J Clin Med. (2021) 10:1937. doi: 10.3390/jcm10091937
136. Sheehan ACM, Umstad MP, Cole S, Cade TJ. Does gestational diabetes cause additional risk in twin pregnancy? Twin Res Hum Genet. (2019) 22:62–9. doi: 10.1017/thg.2018.72
137. Xue CY, Su RN, Yang HX. Analysis of the maternal glucolipid metabolism in twin pregnancies complicated by gestational diabetes mellitus. Zhonghua Fu Chan Ke Za Zhi. (2019) 54:741–6. doi: 10.3760/cma.j.issn.0529-567x.2019.11.005
138. Ooi S, Wong VW. Twin pregnancy with gestational diabetes mellitus: a double whammy? Diabetes Care. (2018) 41:e15–e6. doi: 10.2337/dc17-2227
139. Dave ED, Bodnar LM, Vani K, Himes KP. Perinatal outcomes in twin pregnancies complicated by gestational diabetes. Am J Obstet Gynecol MFM. (2021) 3:100396. doi: 10.1016/j.ajogmf.2021.100396
140. Guillen MA, Herranz L, Barquiel B, Hillman N, Burgos MA, Pallardo LF. Influence of gestational diabetes mellitus on neonatal weight outcome in twin pregnancies. Diabet Med. (2014) 31:1651–6. doi: 10.1111/dme.12523
141. Gonzalez Gonzalez NL, Goya M, Bellart J, Lopez J, Sancho MA, Mozas J, et al. Obstetric and perinatal outcome in women with twin pregnancy and gestational diabetes. J Matern Fetal Neonatal Med. (2012) 25:1084–9. doi: 10.3109/14767058.2011.622009
142. Diamanti-Kandarakis E, Dunaif A. Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocr Rev. (2012) 33:981–1030. doi: 10.1210/er.2011-1034
143. Rotterdam EA-SPCWG. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. (2004) 81:19–25. doi: 10.1016/j.fertnstert.2003.10.004
144. Mills G, Badeghiesh A, Suarthana E, Baghlaf H, Dahan MH. Polycystic ovary syndrome as an independent risk factor for gestational diabetes and hypertensive disorders of pregnancy: a population-based study on 91 million pregnancies. Hum Reprod. (2020) 35:1666–74. doi: 10.1093/humrep/deaa099
145. Bahri Khomami M, Joham AE, Boyle JA, Piltonen T, Silagy M, Arora C, et al. Increased maternal pregnancy complications in polycystic ovary syndrome appear to be independent of obesity-a systematic review, meta-analysis, and meta-regression. Obes Rev. (2019) 20:659–74. doi: 10.1111/obr.12829
146. Roos N, Kieler H, Sahlin L, Ekman-Ordeberg G, Falconer H, Stephansson O. Risk of adverse pregnancy outcomes in women with polycystic ovary syndrome: population based cohort study. BMJ. (2011) 343:d6309. doi: 10.1136/bmj.d6309
147. Aktun HL, Yorgunlar B, Acet M, Aygun BK, Karaca N. The effects of polycystic ovary syndrome on gestational diabetes mellitus. Gynecol Endocrinol. (2016) 32:139–42. doi: 10.3109/09513590.2015.1101438
148. Manoharan V, Wong VW. Impact of comorbid polycystic ovarian syndrome and gestational diabetes mellitus on pregnancy outcomes: a retrospective cohort study. BMC Pregnancy Childbirth. (2020) 20:484. doi: 10.1186/s12884-020-03175-5
149. Foroozanfard F, Moosavi SG, Mansouri F, Bazarganipour F. Obstetric and neonatal outcome in PCOS with gestational diabetes mellitus. J Family Reprod Health. (2014) 8:7–12.
150. Alshammari A, Hanley A, Ni A, Tomlinson G, Feig DS. Does the presence of polycystic ovary syndrome increase the risk of obstetrical complications in women with gestational diabetes? J Matern Fetal Neonatal Med. (2010) 23:545–9. doi: 10.3109/14767050903214566
151. Li G, Fan L, Zhang L, Zhang W, Huang X. Metabolic parameters and perinatal outcomes of gestational diabetes mellitus in women with polycystic ovary syndrome. J Perinat Med. (2010) 38:141–6. doi: 10.1515/jpm.2010.034
152. Odsaeter IH, Asberg A, Vanky E, Carlsen SM. HbA1c as screening for gestational diabetes mellitus in women with polycystic ovary syndrome. BMC Endocr Disord. (2015) 15:38. doi: 10.1186/s12902-015-0039-9
153. Fougner SL, Vanky E, Lovvik TS, Carlsen SM. No impact of gestational diabetes mellitus on pregnancy complications in women with PCOS, regardless of GDM criteria used. PLoS ONE. (2021) 16:e0254895. doi: 10.1371/journal.pone.0254895
154. Li X, Liu X, Zuo Y, Gao J, Liu Y, Zheng W. The risk factors of gestational diabetes mellitus in patients with polycystic ovary syndrome: what should we care. Medicine. (2021) 100:e26521. doi: 10.1097/MD.0000000000026521
155. Chappell LC, Cluver CA, Kingdom J, Tong S. Pre-eclampsia. Lancet. (2021) 398:341–54. doi: 10.1016/S0140-6736(20)32335-7
156. Han CS, Herrin MA, Pitruzzello MC, Mulla MJ, Werner EF, Pettker CM, et al. Glucose and metformin modulate human first trimester trophoblast function: a model and potential therapy for diabetes-associated uteroplacental insufficiency. Am J Reprod Immunol. (2015) 73:362–71. doi: 10.1111/aji.12339
157. Heim KR, Mulla MJ, Potter JA, Han CS, Guller S, Abrahams VM. Excess glucose induce trophoblast inflammation and limit cell migration through HMGB1 activation of Toll-Like receptor 4. Am J Reprod Immunol. (2018) 80:e13044. doi: 10.1111/aji.13044
158. Ji L, Chen Z, Xu Y, Xiong G, Liu R, Wu C, et al. Systematic characterization of autophagy in gestational diabetes mellitus. Endocrinology. (2017) 158:2522–32. doi: 10.1210/en.2016-1922
159. Stoikou M, Grimolizzi F, Giaglis S, Schafer G, van Breda SV, Hoesli IM, et al. Gestational diabetes mellitus is associated with altered neutrophil activity. Front Immunol. (2017) 8:702. doi: 10.3389/fimmu.2017.00702
160. Shen D, Lu Y, Li G, Hu M, Li S, Ju H, et al. Mechanism of neutrophil extracellular traps generation and their role in trophoblasts apoptosis in gestational diabetes mellitus. Cell Signal. (2021) 88:110168. doi: 10.1016/j.cellsig.2021.110168
161. Gupta AK, Hasler P, Holzgreve W, Gebhardt S, Hahn S. Induction of neutrophil extracellular DNA lattices by placental microparticles and IL-8 and their presence in preeclampsia. Hum Immunol. (2005) 66:1146–54. doi: 10.1016/j.humimm.2005.11.003
162. Gupta AK, Hasler P, Holzgreve W, Hahn S. Neutrophil NETs: a novel contributor to preeclampsia-associated placental hypoxia? Semin Immunopathol. (2007) 29:163–7. doi: 10.1007/s00281-007-0073-4
163. Vokalova L, van Breda SV, Ye XL, Huhn EA, Than NG, Hasler P, et al. Excessive neutrophil activity in gestational diabetes mellitus: could it contribute to the development of preeclampsia? Front Endocrinol. (2018) 9:542. doi: 10.3389/fendo.2018.00542
164. Kapustin R, Chepanov S, Kopteeva E, Arzhanova O. Maternal serum nitrotyrosine, 8-isoprostane and total antioxidant capacity levels in pre-gestational or gestational diabetes mellitus. Gynecol Endocrinol. (2020) 36:36–42. doi: 10.1080/09513590.2020.1816727
165. Coughlan MT, Vervaart PP, Permezel M, Georgiou HM, Rice GE. Altered placental oxidative stress status in gestational diabetes mellitus. Placenta. (2004) 25:78–84. doi: 10.1016/S0143-4004(03)00183-8
166. Lappas M, Hiden U, Desoye G, Froehlich J, Hauguel-de Mouzon S, Jawerbaum A. The role of oxidative stress in the pathophysiology of gestational diabetes mellitus. Antioxid Redox Signal. (2011) 15:3061–100. doi: 10.1089/ars.2010.3765
167. Sisay M, Edessa D, Ali T, Mekuria AN, Gebrie A. The relationship between advanced glycation end products and gestational diabetes: a systematic review and meta-analysis. PLoS ONE. (2020) 15:e0240382. doi: 10.1371/journal.pone.0240382
168. Jiang L, Yan J, Wu L. Study of the relationship between AGEs and oxidative stress damage to trophoblast cell mitochondria. Ginekol Pol. (2017) 88:372–8. doi: 10.5603/GP.a2017.0070
169. Chen W, Zhang Y, Yue C, Ye Y, Chen P, Peng W, et al. Accumulation of advanced glycation end products involved in inflammation and contributing to severe preeclampsia, in maternal blood, umbilical blood and placental tissues. Gynecol Obstet Invest. (2017) 82:388–97. doi: 10.1159/000448141
170. Guedes-Martins L, Matos L, Soares A, Silva E, Almeida H. AGEs, contributors to placental bed vascular changes leading to preeclampsia. Free Radic Res. (2013) 47(Suppl.1):70–80. doi: 10.3109/10715762.2013.815347
171. Lekva T, Norwitz ER, Aukrust P, Ueland T. Impact of systemic inflammation on the progression of gestational diabetes mellitus. Curr Diab Rep. (2016) 16:26. doi: 10.1007/s11892-016-0715-9
172. Nguyen-Ngo C, Jayabalan N, Salomon C, Lappas M. Molecular pathways disrupted by gestational diabetes mellitus. J Mol Endocrinol. (2019) 63:R51–72. doi: 10.1530/JME-18-0274
173. Ferguson KK, Meeker JD, McElrath TF, Mukherjee B, Cantonwine DE. Repeated measures of inflammation and oxidative stress biomarkers in preeclamptic and normotensive pregnancies. Am J Obstet Gynecol. (2017) 216:527 e1–9. doi: 10.1016/j.ajog.2016.12.174
174. Lau SY, Guild SJ, Barrett CJ, Chen Q, McCowan L, Jordan V, et al. Tumor necrosis factor-alpha, interleukin-6, and interleukin-10 levels are altered in preeclampsia: a systematic review and meta-analysis. Am J Reprod Immunol. (2013) 70:412–27. doi: 10.1111/aji.12138
175. Vieira MC, Begum S, Seed PT, Badran D, Briley AL, Gill C, et al. Gestational diabetes modifies the association between PlGF in early pregnancy and preeclampsia in women with obesity. Pregnancy Hypertens. (2018) 13:267–72. doi: 10.1016/j.preghy.2018.07.003
176. Zak P, Soucek M. Correlation of tumor necrosis factor alpha, interleukin 6 and interleukin 10 with blood pressure, risk of preeclampsia and low birth weight in gestational diabetes. Physiol Res. (2019) 68:395–408. doi: 10.33549/physiolres.934002
177. Cao W, Wang X, Chen T, Xu W, Feng F, Zhao S, et al. Maternal lipids, BMI and IL-17/IL-35 imbalance in concurrent gestational diabetes mellitus and preeclampsia. Exp Ther Med. (2018) 16:427–35. doi: 10.3892/etm.2018.6144
178. Abo-Elmatty DM, Mehanna ET. MIR146A rs2910164 (G/C) polymorphism is associated with incidence of preeclampsia in gestational diabetes patients. Biochem Genet. (2019) 57:222–33. doi: 10.1007/s10528-018-9886-1
179. Beysel S, Pinarli FA, Eyerci N, Kizilgul M, Hepsen S, Alhan A, et al. HNF1A gene pI27L is associated with co-existing preeclampsia in gestational diabetes mellitus. Gynecol Endocrinol. (2020) 36:530–4. doi: 10.1080/09513590.2019.1698023
180. Dmitrenko OP, Karpova NS, Nurbekov MK, Papysheva OV. I/D polymorphism gene ACE and risk of preeclampsia in women with gestational diabetes mellitus. Dis Markers. (2020) 2020:8875230. doi: 10.1155/2020/8875230
181. Zehravi M, Maqbool M, Ara I. Correlation between obesity, gestational diabetes mellitus, and pregnancy outcomes: an overview. Int J Adolesc Med Health. (2021) 33:339–45. doi: 10.1515/ijamh-2021-0058
182. Lopez-Jaramillo P, Barajas J, Rueda-Quijano SM, Lopez-Lopez C, Felix C. Obesity and preeclampsia: common pathophysiological mechanisms. Front Physiol. (2018) 9:1838. doi: 10.3389/fphys.2018.01838
183. Townsend R, Khalil A, Premakumar Y, Allotey J, Snell KIE, Chan C, et al. Prediction of pre-eclampsia: review of reviews. Ultrasound Obstet Gynecol. (2019) 54:16–27. doi: 10.1002/uog.20117
184. Omazic J, Viljetic B, Ivic V, Kadivnik M, Zibar L, Muller A, et al. Early markers of gestational diabetes mellitus: what we know and which way forward? Biochem Med. (2021) 31:030502. doi: 10.11613/BM.2021.030502
185. Agrawal S, Cerdeira AS, Redman C, Vatish M. Meta-analysis and systematic review to assess the role of soluble FMS-like tyrosine kinase-1 and placenta growth factor ratio in prediction of preeclampsia: the SaPPPhirE study. Hypertension. (2018) 71:306–16. doi: 10.1161/HYPERTENSIONAHA.117.10182
Keywords: gestational diabetes, preeclampsia, pregnancy, polycystic ovary syndrome, obesity
Citation: Yang Y and Wu N (2022) Gestational Diabetes Mellitus and Preeclampsia: Correlation and Influencing Factors. Front. Cardiovasc. Med. 9:831297. doi: 10.3389/fcvm.2022.831297
Received: 08 December 2021; Accepted: 20 January 2022;
Published: 16 February 2022.
Edited by:
Laura Sarno, Federico II University Hospital, ItalyReviewed by:
Anamaria Savu, University of Alberta, CanadaCopyright © 2022 Yang and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Na Wu, MzQ0MTUzNTIyM0BxcS5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.