- 1Department of Cardiovascular Medicine, The Second Affiliated Hospital of Nanchang University, Nanchang, China
- 2Department of Cardiothoracic Surgery, The Second Affiliated Hospital of Nanchang University, Nanchang, China
Objective: To investigate the relationship between hypertension and basilar atherosclerosis evaluated by high-resolution magnetic resonance imaging (HR-MRI) in the Chinese Han population.
Methods: High resolution-MRI vessel wall imaging was performed in selected 193 patients for various indications. Multivariable logistic regression models based on odds ratio (OR) with their associated 95% confidence interval (CI) were used to assess the relationship between hypertension and basilar artery (BA) plaque, moderate or severe stenosis of BA plaque, and vulnerable plaque. A linear regression model was used to assess the relationship between hypertension and BA plaque numbers.
Results: Patients with hypertension had a higher proportion of BA plaque and vulnerable plaque as well as more number of enhancements of BA plaque and serious plaque compared with normotensive patients (all values of p < 0.05). Multivariable logistic regression analysis indicated that patients with hypertension had an increased risk for and more number of enhancements of BA plaque (adjusted-OR: 4.32, 95% CI 1.89–9.88, p < 0.001; adjusted-β: 0.55, 95% CI 0.14–0.96, p = 0.009, respectively) and had a higher proportion of moderate or severe stenosis of BA plaque and vulnerable plaque (adjusted-OR: 3.08, 95% CI 0.77–12.32, p = 0.111; adjusted-OR: 4.52, 95% CI 1.50–13.64, p = 0.007, respectively) compared with the normotensive group. Moreover, there was a saturation effect of age on the prevalence of BA plaque and vulnerable plaque.
Conclusion: Hypertension was the independent risk factor of BA plaque and vulnerable plaque assessed by HR-MRI in the Chinese Han population.
Introduction
Intracranial atherosclerosis is the main cause of stroke in the Chinese population, accounting for 46.6% of ischemic stroke cases, but the pathology is not well understood because it cannot be easily studied in living patients (1). When the basilar artery (BA), the most important vessel in the posterior circulation, has insufficient blood flow for the posterior circulation, patients would present with clinical signs and symptoms of brain ischemia (2).
Conventional techniques, such as computerized tomography angiography (CTA) and digital subtraction angiography (DSA), could reveal the abnormalities of the vascular lumen, but were unable to fully reveal the lesion of vascular wall and accurately distinguish the pathological characteristics of atherosclerotic plaque (3–8). In addition, the size, shape, signal intensity, and enhancement characteristics of atherosclerotic vulnerable plaque could not be recognized through luminal imaging (3–8). Interest in intracranial high-resolution magnetic resonance imaging (HR-MRI) is growing (4–8). Compared with conventional cavity imaging methods, HR-MRI could visualize the intracranial arterial disease and identify previously hidden mural lesions (7, 8).
The vulnerability of atherosclerotic plaque is closely related to the occurrence of stroke. There are five main criteria for vulnerable plaques: active inflammation, a thin cap with a macro-lipid core, endothelial denudation with platelet aggregation on the surface, rupture of the fibrous cap, and severe stenosis; The five secondary criteria for vulnerable plaques include superficial calcified nodules, yellow plaque under a vascular microscope, intraplaque hemorrhage (IPH), endothelial dysfunction, and positive remodeling (3–5).
Active inflammation, rupture of the fibrous cap, severe stenosis, intraplaque hemorrhage, and positive remodeling can be identified on HR-MRI (3–5). Inflammation plays a critical role in plaque initiation, progression, and disruption (9, 10). Histological analysis from atherosclerotic rabbits indicated that the degree of macrophage infiltration on the vascular wall was proportional to plaque enhancement on HR-MRI (11). Histological analysis indicated that gadolinium (Gd) enhancement of carotid plaque on HR-MRI was significantly associated with macrophages infiltration, neovascularization, and loose fibrosis in patients undergoing endarterectomy for carotid stenosis (12, 13). Therefore, inflammation could possibly be the underlying mechanism that causes plaque enhancement (3). Plaque contrast enhancement is associated with vascular active inflammation, and the high signal intensity on T1 weighted images (T1WI) strongly indicated the presence of intraplaque hemorrhage (14). When the plaque is large, a thin cap with a macro-lipid core and superficial calcified nodules could also be identified (3).
Based on the above background, this study aimed to assess the atherosclerotic plaque and vulnerable characteristics of BA based on HR-MRI and further analyze the risk factors of BA sclerosis in the Chinese Han population.
Materials and Methods
Participants
This was a retrospective study performed at the Second Affiliated Hospital of Nanchang University. Between January 2018 and January 2021, HR-MRI was performed on 231 individuals of consecutive Chinese Han population for various indications. Eligible participants were adults aged 18 years and older who voluntarily performed the HR-MRI. They had at least one of the following diseases: hypertension, diabetes, impaired cognitive function, ischemic stroke, or transient ischemic attacks. After excluding 38 subjects with confirmed or highly suspected non-atherosclerotic vasculopathy, such as arteritis, moyamoya disease, and dissection, 193 patients were included in this analysis. Written informed consent was obtained from all participants before each HR-MRI examination. The study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Ethics Committees of the Second Affiliated Hospital of Nanchang University.
Clinical Data Collection
Participants’ demographic characteristics [age, sex, and body mass index (BMI)], lifestyles (smoking and alcohol habits), medical history [hypertension, type II diabetes mellitus (T2DM), ischemic stroke, and transient ischemic attacks (TIA)], and medication usage were collected by trained research staff. Cognitive function was assessed using the Mini-Mental State Examination (MMSE) scale by a qualified medical professional (15).
Laboratory Assay
Blood samples were collected using venipuncture after an overnight fast of at least 12 h. Blood biochemistries for fasting blood glucose (FBG), total cholesterol (TC), total triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), very low-density lipoprotein cholesterol (VLDL), plasma homocysteine (Hcy), serum uric acid and creatinine, blood urea nitrogen (BUN), and creatinine kinase-MB (CK-MB) were measured using automatic clinical analyzers at the clinical laboratory of the Second Affiliated Hospital of Nanchang University. The formula for estimated glomerular filtration rate (eGFR) used the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation (16). Hypertension was defined as a seated systolic blood pressure (SBP) ≥ 140 mmHg and/or diastolic blood pressure (DBP) ≥ 90 mmHg on at least three separate clinic visits or previously diagnosed hypertension. The clinical classification in hypertension (Class I, II, and III) was defined as the 140/90, 160/100, and 180/110 mmHg threshold. The diagnosis of incident diabetes was defined as fasting glucose > 7.0 mmol/L and/or self-reported diabetes.
HRMR Imaging Protocol
A 3.0 T MR scanner (GE Discovery 750W, America) with a 19-channel phased-array head-neck coil was used in this study. All patients underwent conventional T2 weighted imaging, diffusion-weighted imaging (DWI), three-dimensional (3D) time-of-flight (TOF) magnetic resonance angiography (MRA), and BA HR-MRI imaging, which included high resolution-T1 weighted imaging (HR-T1WI) plain and contrast material-enhanced black-blood magnetic resonance images, 3D-multiple overlapping thin slab angiography (MOTSA) sequences, and magnetization-prepared rapid gradient echo (MP-RAGE) sequences. Then, 3D-HRMRI images were acquired in a traversal plane to cover the major intracranial arteries identified on the TOF MRA. The HR-T1WI was obtained using a fast spin echo 3D technique (CUBE T1WI). The parameters were as follows: repetition time (TR) = 1,300 ms, echo time (TE) = 16 ms, field of view (FOV) = 18 cm, matrix = 320 × 320, zero-fill interpolation (ZIP) = 512, slice thickness = 0.6 mm, echo train length = 10, and number of excitation = 2. The display resolution was 0.35 mm × 0.35 mm × 0.6 mm. In total, 120 coronal slices covering the anterior and posterior circulation were acquired with a scan time of 15 min.
Image Analysis of Basilar Artery Plaque
Images were independently analyzed by two experienced HR-MRI specialists blinded to the clinical data using visual inspection. The differences between the two observers were settled by consensus. Picture quality was assessed using a previously developed four-point scale (one = poor quality, two = sufficient quality, three = good quality, and four = excellent) (17). Images were assessed on all cross-sectional image slices with a score greater than or equal to two.
A plaque was defined as an eccentric wall thickening, and the thinnest part was estimated to be lesser than 50% of the thickest point by visual observation (18, 19). In our study, the number of plaques was only counted at the main trunk of BA. We defined the reference sites as the nearest plaque-free segments proximal or distal to the maximal lumen narrowing sites. The stenosis degree of BA was calculated by the ratio between the lumen diameter at the maximal lumen narrowing site and the reference luminal area (19, 20), which was classified as mild stenosis (< 50%), moderate stenosis (50–70%), and severe stenosis (70–99%). Plaque contrast enhancement was categorized based on black-blood MRI (grade 0, enhancement less than or equal to that of normal arterial walls seen elsewhere; grade I, enhancement greater than grade 0 but less than that of the pituitary infundibulum; and grade II, enhancement greater than or equal to that of the pituitary infundibulum) (21). IPH was defined as high signal intensity (150% intensity compared with the normal gray matter) on MP-RAGE images (20). The rupture of the fibrous cap was identified as the absence of the dark band between the lumen and the plaque core as well as the presence of a bright gray region adjacent to the lumen, corresponding to recent plaque hemorrhage or mural thrombus on 3D-MOTSA images (22). In our study, vulnerable plaque of the BA was defined as having one of the following characteristics (6): Severe stenosis, enhancement greater than or equal to grade I, IPH, or the rupture of the fibrous cap.
Statistical Analysis
Continuous variables were expressed as means ± standard deviation (SD) or median (Q1–Q3). For the variables in both groups that exhibit a normal distribution, an independent t-test was used. For the variables that do not show a normal distribution, a non-parametric test was used. Categorical variables were expressed as count (percentage) and differences between groups were measured by the chi-square test.
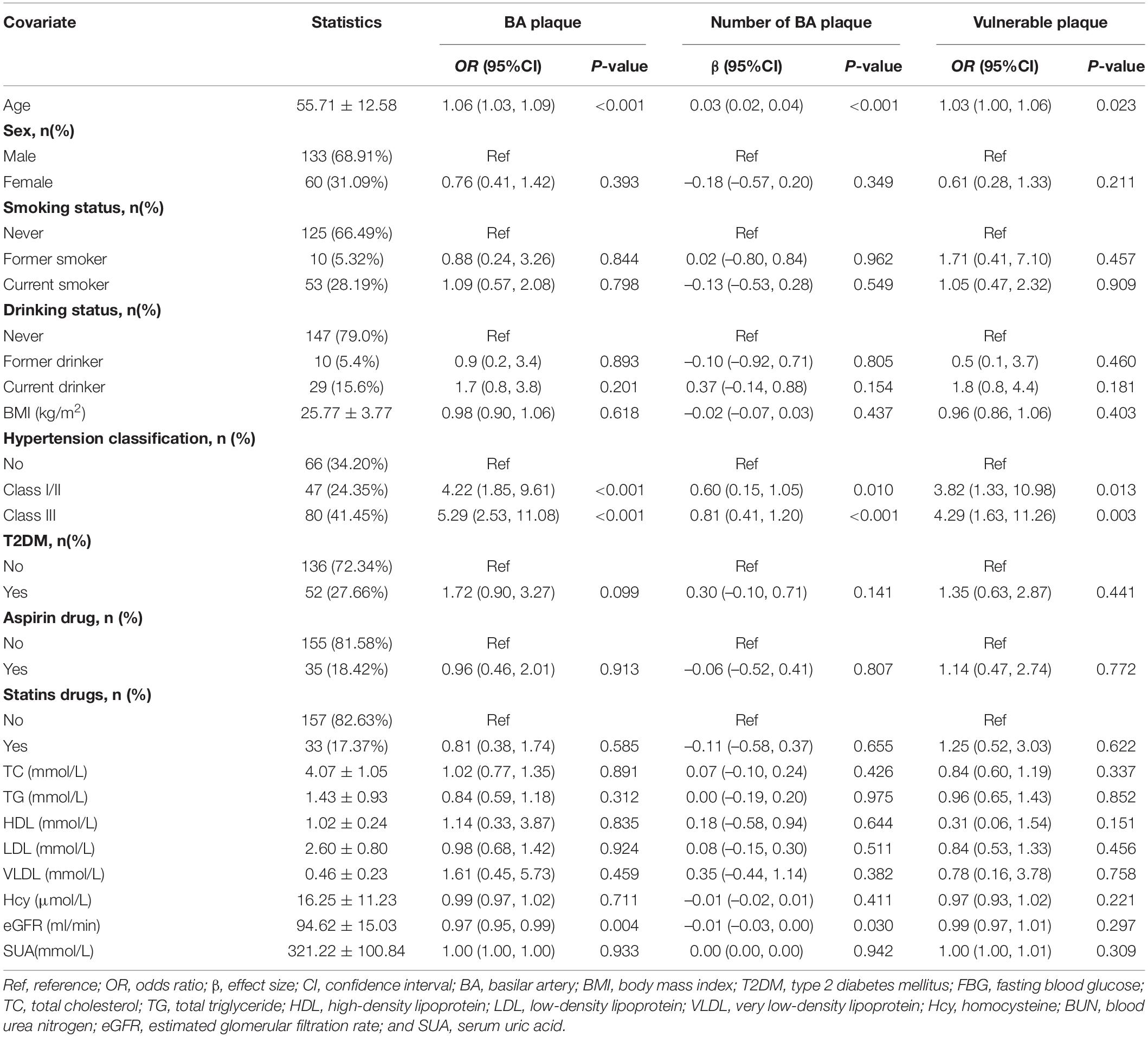
Univariate analyses were performed to identify variables predictive of BA plaque and its numbers, as well as vulnerable plaque. Multivariable logistic regression models based on odds ratio (OR) with their associated 95% confidence interval (CI) were used to assess the relationship between hypertension and BA plaque, moderate or severe stenosis of BA plaque, and vulnerable plaque. A linear regression model was used to assess the relationship between hypertension and BA plaque numbers. The crude model was not adjusted for any confounder. The model I was only adjusted for age and sex. The model II was a confounder model. This confounder model screened covariates, such as age, sex, BMI, smoking and drinking status, T2DM, aspirin, statin, TC, TG, HDL, LDL, VLDL, Hcy, serum uric acid, and eGFR. We selected these confounders based on the fact that when they were included in this model, they changed the matched effect size by at least 10%. Table 1 shows the association of each confounder with the outcomes of interest. We considered the confounder model to be the main model.
To ensure the robustness of data analysis, we also did the subgroup analyses that were performed using stratified multivariate regression and interaction analyses. Considering that there was a saturation effect of age on the prevalence of BA plaque or vulnerable plaque, the generalized additive model and smooth curve fitting (penalized spline method) were used to visually show the relationship between age and BA plaque and vulnerable plaque (Supplementary Figures 1, 2). If non-linearity was detected, we first use a recursive algorithm to calculate the inflection points and then construct a two-segment binary logistic regression model on both sides of the inflection points.
All statistical analyses were performed using the statistical package R (The R Foundation, version 3.4.3)1 and the Empower (R; X&Y Solutions, Inc., Boston, MA, United States).2 All values of p are two-tailed, and p < 0.05 was considered statistically significant.
Results
Clinical Characteristics of the Study Population
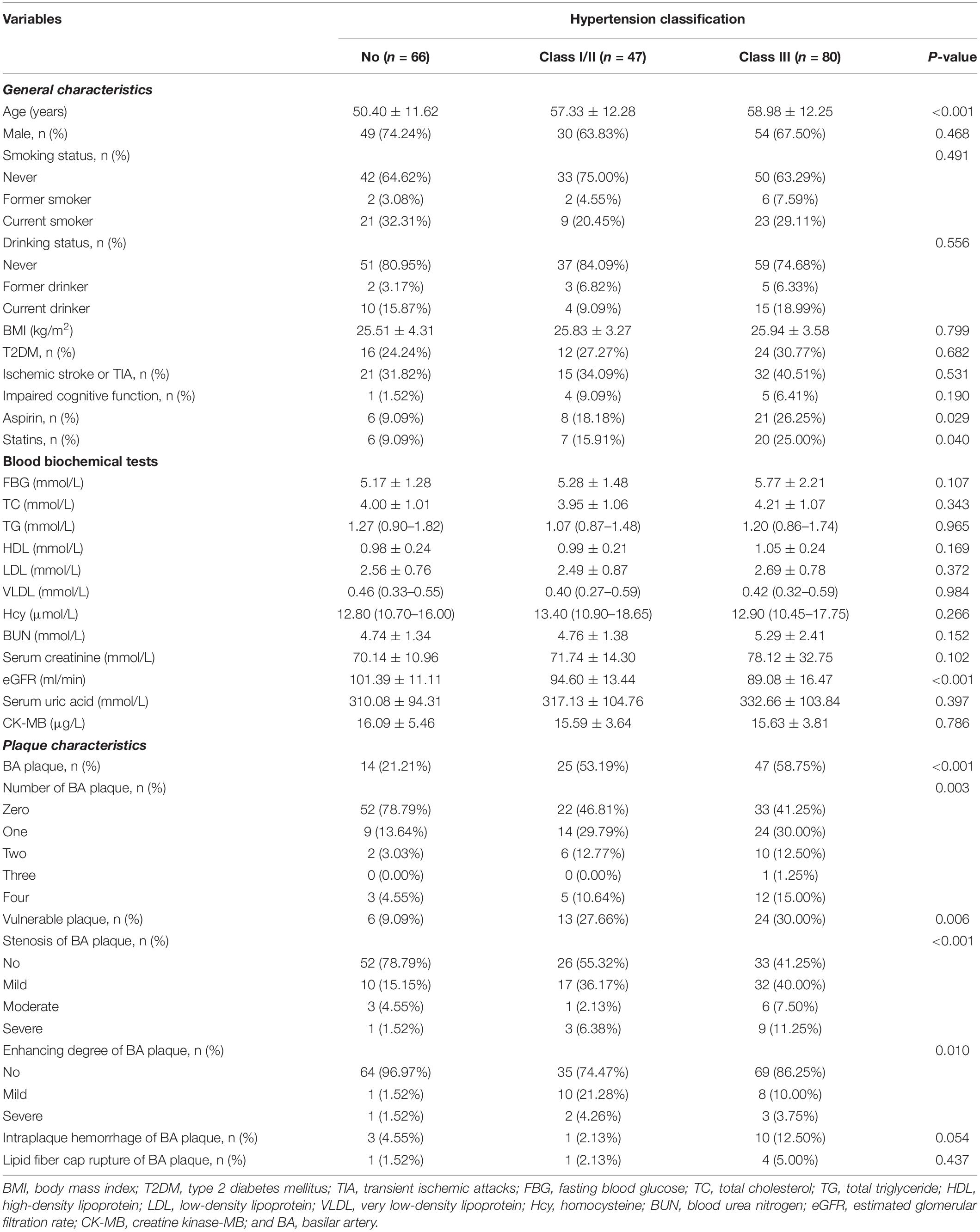
This study included 193 subjects (age: 55.71 ± 12.58 years, range 26–87 years; men, 68.91%), and the prevalence of hypertension was 65.80%. The clinical characteristics of participants are presented in Table 2 by the categories of hypertension classification. Compared with the normotensive group, patients with hypertension had a higher proportion of older adults, of aspirin and statins usage, and of BA plaque and vulnerable plaque, lower extents of eGFR, and more number of enhancements of BA plaques and serious plaque (all p < 0.05).
Univariate Analyses for Basilar Artery Plaque and Vulnerable Plaque
Univariate analyses found that older adults, patients with hypertension, and those with lower eGFR increased the risk for BA plaque and its numbers, while older adults and patients with hypertension were associated with the risk of vulnerable plaque (Table 1).
Relationship Between Hypertension and Basilar Artery Plaque and Vulnerable Plaque
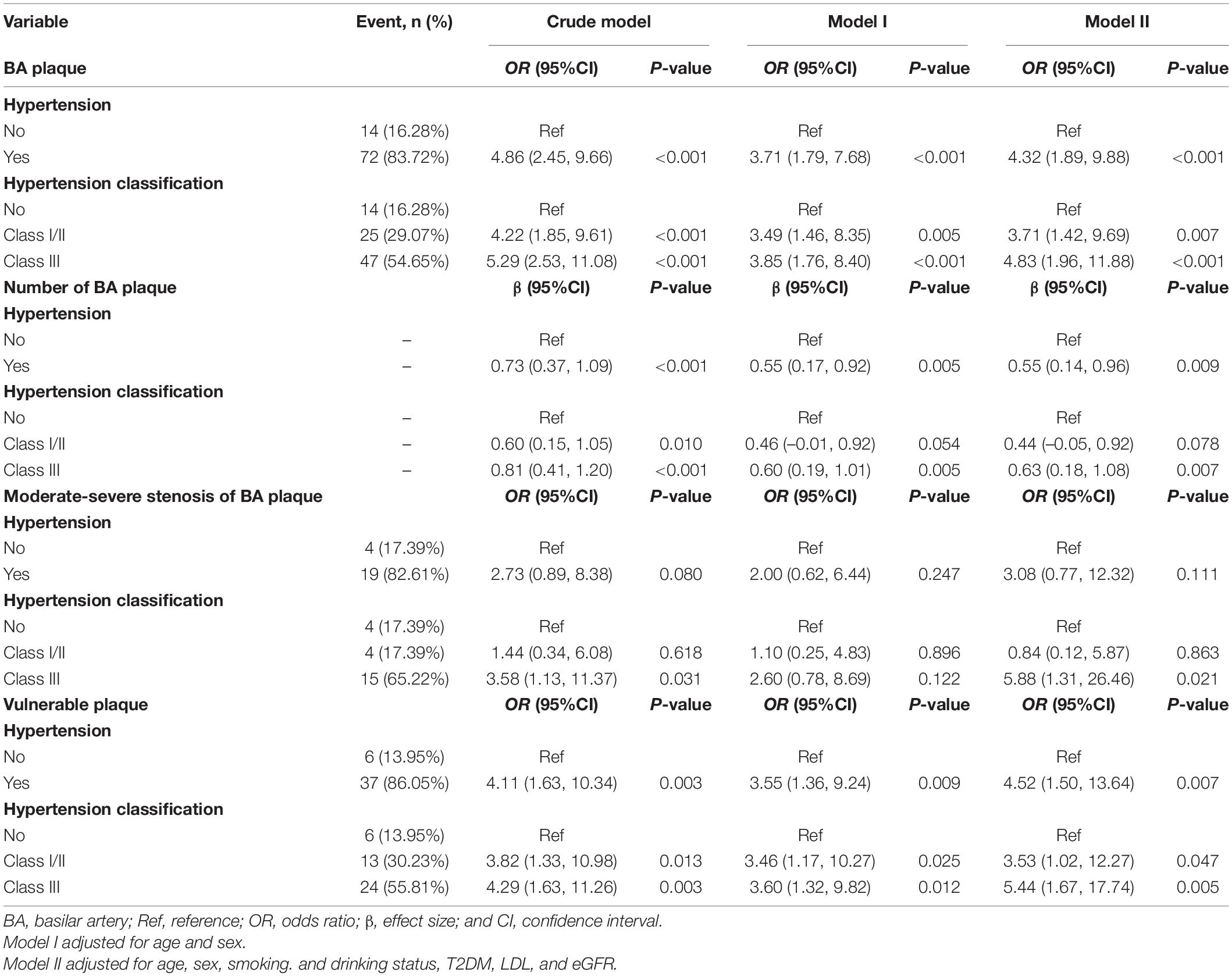
Multivariable analyses indicated that patients with hypertension had an increased risk for BA plaque and its numbers (adjusted-OR: 4.32, 95% CI 1.89–9.88, p < 0.001; adjusted-β: 0.55, 95% CI 0.14–0.96, p = 0.009, respectively) and had a higher proportion of moderate or severe stenosis of BA plaque and vulnerable plaque (adjusted-OR: 3.08, 95% CI 0.77–12.32, p = 0.111; adjusted-OR: 4.52, 95% CI 1.50–13.64, p = 0.007, respectively; Table 3) compared with the normotensive group. When the normotensive group was used as reference, individuals with tertiary hypertension had an increased risk for BA plaque and its numbers (adjusted-OR: 4.83, 95% CI 1.96–11.88, p < 0.001; adjusted-β: 0.63, 95% CI 0.18–1.08, p = 0.007, respectively) and had a higher proportion of moderate or severe stenosis of BA plaque and vulnerable plaque (adjusted-OR: 5.88, 95% CI 1.31–26.46, p = 0.021; adjusted-OR: 5.44, 95% CI 1.67–17.74, p = 0.005, respectively).
Subgroup Analyses Between Hypertension and Basilar Artery Plaque and Vulnerable Plaque
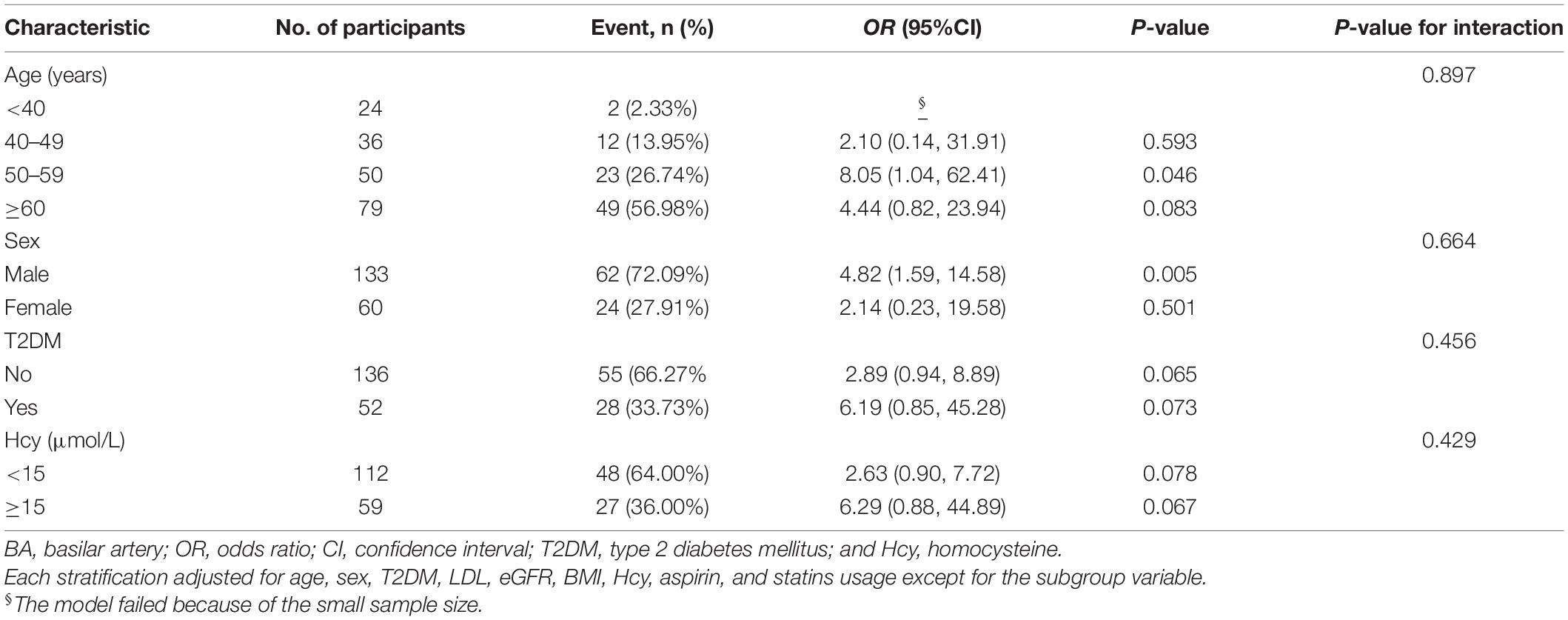
To explore whether this positive association between hypertension and BA plaque was still stable in different subgroups, we conducted stratified and interaction analyses. Subgroup analyses showed that older adults, men, patients with T2DM, and those with hyper-homocysteinemia (HHcy was defined as Hcy ≥ 15 μmol/L) increased the ORs of hypertension on the risk for BA plaque (Table 4) and vulnerable plaque (Table 5). In terms of sex differences, we found that male participants were more likely to smoke and drink and had lower extents of HDL and higher levels of Hcy, serum uric acid, and creatinine compared with female subjects (all p < 0.05, Supplementary Table 1). Furthermore, there were no significant interactions between hypertension and BA plaque as well as vulnerable plaque in any of the subgroups, such as age (< 40, 40–49, 50–59, and ≥ 60 years old), sex (male vs. female), T2DM (no vs. yes), HHcy (no vs. yes) (all p for interactions > 0.05).
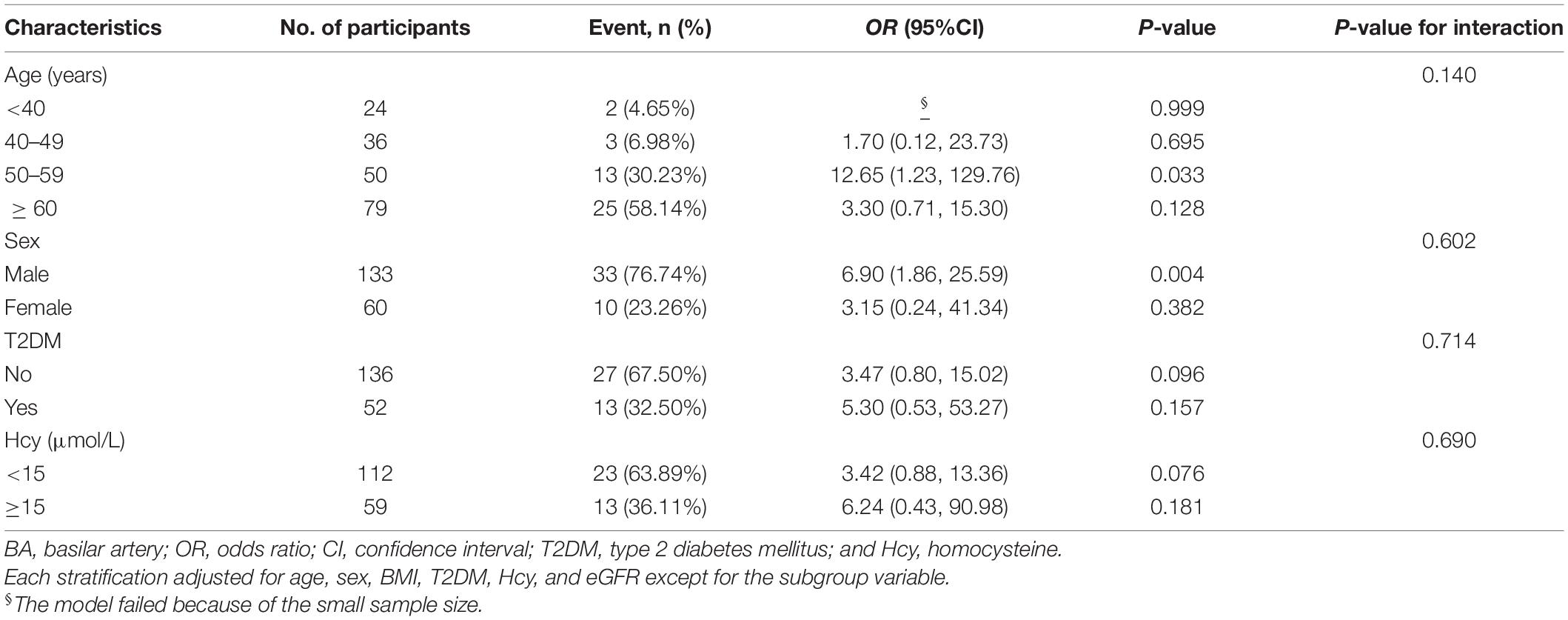
Table 5. Odds ratios of hypertension on BA vulnerable plaque in the prespecified and exploratory subgroups.
Saturation Effect Analysis of Age on the Prevalence of Basilar Artery Plaque or Vulnerable Plaque
Stratified analyses found that compared with the age group of 50–59 years old, subjects with age older than 60 years old had a reduced effect size of hypertension on the risk for BA vulnerable plaque (adjusted-OR: 8.05, 95% CI 1.04–62.41, p = 0.046 vs. adjusted-OR: 4.44, 95% CI 0.82–23.94, p = 0.083; Table 4) and vulnerable plaque (adjusted-OR: 12.65, 95% CI 1.23–129.76, p = 0.033 vs. adjusted-OR: 3.30, 95% CI 0.71–15.30, p = 0.128; Table 5). The clinical characteristics of participants grouped by age are presented in Supplementary Table 2.
Therefore, we used the generalized additive model and penalized spline method to assess whether there was a non-linear relationship between age and the prevalence of BA plaque (Supplementary Figure 1) or vulnerable plaque (Supplementary Figure 2). In the adjusted smoothing curve, the relationship between age and the prevalence of BA plaque and vulnerable plaque was not linear. With the increase of age, the prevalence of BA plaque and vulnerable plaque increased first and then leveled off. Visual inspection shows that the inflection point is approximately 60 years old. We further fitted the association between age and the prevalence of BA plaque and vulnerable plaque using the two-piecewise binary logistic regression model. The inflection points of age were 61 (Supplementary Table 3) and 60 (Supplementary Table 4) years old. The effect size [OR (95% CI)] of age on the prevalence of BA plaque was 1.10 (1.05, 1.16) on the left side and 0.99 (0.92, 1.06) on the right of the inflection point, meanwhile the effect size [OR (95% CI)] of age on the prevalence of vulnerable plaque was 1.09 (1.02, 1.15) on the left side and 0.97 (0.90, 1.04) on the right of the inflection point. These results suggested that there was a saturation effect of age on the prevalence of BA plaque and vulnerable plaque.
Discussion
Digital subtraction angiography is the most accurate and reliable diagnostic method currently, but it is an invasive procedure and cannot reflect the pathological characteristics of the intracranial artery wall. In recent years, HR-MRI imaging has been developed rapidly and has become a useful auxiliary tool of imaging technology. HR-MRI has an outstanding effect in differentiating atherosclerotic plaque, vasculitis, reversible cerebral vasoconstriction syndrome, and arterial dissection (3–8). Compared with other traditional lumen imaging techniques, HR-MRI has been found to be more accurate in determining the extent of atherosclerotic lesions (23, 24). Klein et al. (25) studied 41 patients with pontine infarction and found that HR-MRI was superior to TOF MRA in detecting basilar atherosclerotic lesions. For example, 77% of patients with paramedian wave infarction had evidence of BA plaque on HR-MRI, while 65% of these patients had normal TOF MRA. In ischemic stroke patients with symptomatic BA stenosis, HR-MRI was used to identify IPH or dissection (26). HR-MRI results enabled underlying symptomatic branch atheromatous disease to be detected in patients with lacunar infarction using normal angiography (27). Since basilar stenosis may be underestimated by MRA, HR-MRI may provide additional information for predicting progressive motor deficits and evaluating BA stenosis in patients with acute unilateral pontine infarction (28).
This study found that hypertension was the independent risk factor for BA plaque and vulnerable plaque evaluated by HR-MRI in the Chinese Han population. HR-MRI is an effective tool for identifying stroke etiology in patients with non-stenotic intracranial arteries. Detection of the vessel wall of arteries, such as the middle cerebral artery (MCA) and BA, might improve the ability to identify advanced but unrecognized intracranial atherosclerotic lesions (6). In 15 patients with an acute lacunar infarct but normal angiography findings without apparent cause of stroke, HR-MRI demonstrated enhancing atherosclerotic plaque in the MCA or BA in 9 (60%) patients (27). Similar studies have shown HR-MRI evidence of atheromatous plaque in the MCA of 52 or 46.9% of patients with MCA territory lacunar infarcts or single lenticulostriate infarction and in the BA of 42% of patients with pontine infarcts but normal MRA findings (25, 29, 30). Patients with HR-MRI identified plaque presented larger infarction lesions and more proximal lesions than patients without plaque (25).
The etiology of stenosis or occlusion was unclear until the development of HR-MRI. With HR-MRI, stroke etiology is better understood, and factors affecting each etiology can be identified. In Chinese patients with ischemic stroke, hypertension is a risk factor for intracranial artery stenosis and posterior circulation artery stenosis (31). Patients with BA plaque with IPH were older and had a higher prevalence of hypertension and hyperlipidemia than the other patients (26). Metabolic syndrome (MetS) and diabetes mellitus along with hypertension are associated with more extensive ICAS than MetS and hypertension or MetS alone (32). Lifestyle changes and aggressive management of atherosclerotic risk factors, such as hypertension, are vital for preventing the occurrence of stroke.
There were some limitations in our study. A relatively small sample size would affect the generalization ability of the model. Second, our research lacked the dynamic and longitudinal HR-MRI evaluation.
In conclusion, we found that hypertension was the independent risk factor of BA plaque and vulnerable plaque evaluated by HR-MRI in the Chinese Han population.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The studies involving human participants were reviewed and approved by the Institutional Ethics Committees of the Second Affiliated Hospital of Nanchang University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
FH: conceptualization, methodology, research investigation, and writing–original draft preparation. FL: data curation. HX: software. MD: visualization. YX: supervision, writing–reviewing and editing. All authors read and approved the final version of the manuscript.
Funding
This study was supported by grants from the National Nature Science Foundation of China (No. 81760086).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.830664/full#supplementary-material
Supplementary Figure 1 | The smooth curve of correlation between age and BA plaque. Adjust for drinking status, hypertension, and type 2 diabetes mellitus (T2DM). BA, basilar artery.
Supplementary Figure 2 | The smooth curve of correlation between age and BA vulnerable plaque. Adjust for body mass index (BMI), drinking status, hypertension, T2DM, and serum uric acid. BA, basilar artery.
Footnotes
References
1. Wang Y, Zhao X, Liu L, Soo YO, Pu Y, Pan Y, et al. Prevalence and outcomes of symptomatic intracranial large artery stenoses and occlusions in china: the Chinese intracranial atherosclerosis (cicas) study. Stroke. (2014) 45:663–9. doi: 10.1161/STROKEAHA.113.003508
2. Yu J, Li ML, Xu YY, Wu SW, Lou M, Mu XT, et al. Plaque distribution of low-grade basilar artery atherosclerosis and its clinical relevance. BMC Neurol. (2017) 17:8. doi: 10.1186/s12883-016-0785-y
3. Yang WJ, Wong KS, Chen XY. Intracranial atherosclerosis: from microscopy to high-resolution magnetic resonance imaging. J Stroke. (2017) 19:249–60. doi: 10.5853/jos.2016.01956
4. Xu Z, Li M, Lyu J, Hou Z, He J, Mo D, et al. Different risk factors in identical features of intracranial atherosclerosis plaques in the posterior and anterior circulation in high-resolution MRI. Ther Adv Neurol Disord. (2020) 13:1756286420909991. doi: 10.1177/1756286420909991
5. Zhu C, Tian X, Degnan AJ, Shi Z, Zhang X, Chen L, et al. Clinical significance of intraplaque hemorrhage in low- and high-grade basilar artery stenosis on high-resolution MRI. Am J Neuroradiol. (2018) 39:1286–92. doi: 10.3174/ajnr.A5676
6. Mandell DM, Mossa-Basha M, Qiao Y, Hess CP, Hui F, Matouk C, et al. Intracranial vessel wall MRI: principles and expert consensus recommendations of the American society of neuroradiology. Am J Neuroradiol. (2017) 38:218–29. doi: 10.3174/ajnr.A4893
7. Shu JE, Ying ML, Chen XR, Hua JJ, Fu JT, Xia XM, et al. Prognostic value of high-resolution magnetic resonance imaging in evaluating carotid atherosclerotic plaque in patients with ischemic stroke. Medicine. (2017) 96:e8515. doi: 10.1097/MD.0000000000008515
8. Clarke SE, Beletsky V, Hammond RR, Hegele RA, Rutt BK. Validation of automatically classified magnetic resonance images for carotid plaque compositional analysis. Stroke. (2006) 37:93–7. doi: 10.1161/01.STR.0000196985.38701.0c
9. Doodnauth SA, Grinstein S, Maxson ME. Constitutive and stimulated macropinocytosis in macrophages: roles in immunity and in the pathogenesis of atherosclerosis. Philos Trans R Soc Lond B Biol Sci. (2019) 374:20180147. doi: 10.1098/rstb.2018.0147
10. Bäck M, Yurdagul A Jr, Tabas I, Öörni K, Kovanen PT. Inflammation and its resolution in atherosclerosis: mediators and therapeutic opportunities. Nat Rev Cardiol. (2019) 16:389–406. doi: 10.1038/s41569-019-0169-2
11. Hur J, Park J, Kim YJ, Lee HJ, Shim HS, Choe KO, et al. Use of contrast enhancement and high-resolution 3D black-blood MRI to identify inflammation in atherosclerosis. JACC Cardiovasc Imaging. (2010) 3:1127–35. doi: 10.1016/j.jcmg.2010.08.012
12. Cai J, Hatsukami TS, Ferguson MS, Kerwin WS, Saam T, Chu B, et al. In vivo quantitative measurement of intact fibrous cap and lipid-rich necrotic core size in atherosclerotic carotid plaque: comparison of high-resolution, contrast-enhanced magnetic resonance imaging and histology. Circulation. (2005) 12:3437–44. doi: 10.1161/CIRCULATIONAHA.104.528174
13. Millon A, Boussel L, Brevet M, Mathevet JL, Canet-Soulas E, Mory C, et al. Clinical and histological significance of gadolinium enhancement in carotid atherosclerotic plaque. Stroke. (2012) 43:3023–8. doi: 10.1161/STROKEAHA.112.662692
14. Klein IF, Labreuche J, Lavallée PC, Mazighi M, Duyckaerts C, Hauw JJ, et al. Is moderate atherosclerotic stenosis in the middle cerebral artery a cause of or a coincidental finding in ischemic stroke? Cerebrovasc Dis. (2010) 29:140–5. doi: 10.1159/000262310
15. Li H, Jia J, Yang Z. Mini-mental state examination in elderly Chinese: a population-based normative study. J Alzheimers Dis. (2016) 53:487–96. doi: 10.3233/JAD-160119
16. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF III, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. (2009) 150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006
17. Zavodni AE, Wasserman BA, McClelland RL, Gomes AS, Folsom AR, Polak JF, et al. Carotid artery plaque morphology and composition in relation to incident cardiovascular events: the multi-ethnic study of atherosclerosis (MESA). Radiology. (2014) 271:381–9. doi: 10.1148/radiol.14131020
18. Xu WH, Li ML, Gao S, Ni J, Zhou LX, Yao M, et al. Plaque distribution of stenotic middle cerebral artery and its clinical relevance. Stroke. (2011) 42:2957–9. doi: 10.1161/STROKEAHA.111.618132
19. Xu YY, Li ML, Gao S, Jin ZY, Sun ZY, Chen J, et al. Etiology of intracranial stenosis in young patients: a high-resolution magnetic resonance imaging study. Ann Transl Med. (2017) 5:319. doi: 10.21037/atm.2017.06.31
20. Sui B, Gao P, Lin Y, Jing L, Qin H. Distribution and features of middle cerebral artery atherosclerotic plaques in symptomatic patients: a 3.0T high-resolution MRI study. Neurol Res. (2015) 37:391–6. doi: 10.1179/1743132815Y.0000000023
21. Qiao Y, Zeiler SR, Mirbagheri S, Leigh R, Urrutia V, Wityk R, et al. Intracranial plaque enhancement in patients with cerebrovascular events on high-spatial-resolution MR images. Radiology. (2014) 271:534–42. doi: 10.1148/radiol.13122812
22. Hatsukami TS, Ross R, Polissar NL, Yuan C. Visualization of fibrous cap thickness and rupture in human atherosclerotic carotid plaque in vivo with high-resolution magnetic resonance imaging. Circulation. (2000) 102:959–64. doi: 10.1161/01.cir.102.9.959
23. Natori T, Sasaki M, Miyoshi M, Ohba H, Katsura N, Yamaguchi M, et al. Evaluating middle cerebral artery atherosclerotic lesions in acute ischemic stroke using magnetic resonance T1-weighted 3-dimensional vessel wall imaging. J Stroke Cerebrovasc Dis. (2014) 23:706–11. doi: 10.1016/j.jstrokecerebrovasdis.2013.06.025
24. Yang H, Zhu Y, Geng Z, Li C, Zhou L, Liu QI. Clinical value of black-blood high-resolution magnetic resonance imaging for intracranial atherosclerotic plaques. Exp Ther Med. (2015) 10:231–6. doi: 10.3892/etm.2015.2469
25. Klein IF, Lavallée PC, Mazighi M, Schouman-Claeys E, Labreuche J, Amarenco P. Basilar artery atherosclerotic plaques in paramedian and lacunar pontine infarctions: a high-resolution MRI study. Stroke. (2010) 41:1405–9. doi: 10.1161/STROKEAHA.110.583534
26. Kang HG, Lee CH, Shin BS, Chung GH, Kwak HS. Characteristics of symptomatic basilar artery stenosis using high-resolution magnetic resonance imaging in ischemic stroke patients. J Atheroscler Thromb. (2021) 28:1063–70. doi: 10.5551/jat.58214
27. Chung JW, Kim BJ, Sohn CH, Yoon BW, Lee SH. Branch atheromatous plaque: a major cause of lacunar infarction (high-resolution MRI study). Cerebrovasc Dis Extra. (2012) 2:36–44. doi: 10.1159/000341399
28. Lim SH, Choi H, Kim HT, Kim J, Heo SH, Chang DI, et al. Basilar plaque on high-resolution MRI predicts progressive motor deficits after pontine infarction. Atherosclerosis. (2015) 240:278–83. doi: 10.1016/j.atherosclerosis.2015.03.029
29. Yoon Y, Lee DH, Kang DW, Kwon SU, Kim JS. Single subcortical infarction and atherosclerotic plaques in the middle cerebral artery: high-resolution magnetic resonance imaging findings. Stroke. (2013) 44:2462–7. doi: 10.1161/STROKEAHA.113.001467
30. Sun LL, Li ZH, Tang WX, Liu L, Chang FY, Zhang XB, et al. High resolution magnetic resonance imaging in pathogenesis diagnosis of single lenticulostriate infarction with nonstenotic middle cerebral artery, a retrospective study. BMC Neurol. (2018) 18:51. doi: 10.1186/s12883-018-1054-z
31. Hua Y, Jia L, Xing Y, Hui P, Meng X, Yu D, et al. Distribution Pattern of atherosclerotic stenosis in Chinese patients with stroke: a multicenter registry study. Aging Dis. (2019) 10:62–70. doi: 10.14336/AD.2018.0602
Keywords: high-resolution magnetic resonance imaging, hypertension, basilar atherosclerosis, vulnerable plaque, Chinese
Citation: Hu F, Lu F, Xiao H, Dong M and Xu Y (2022) Relationship Between Hypertension and Basilar Atherosclerosis in Chinese Han Population: A High-Resolution Magnetic Resonance Imaging Study. Front. Cardiovasc. Med. 9:830664. doi: 10.3389/fcvm.2022.830664
Received: 07 December 2021; Accepted: 29 March 2022;
Published: 27 April 2022.
Edited by:
Seong-Eun Kim, The University of Utah, United StatesReviewed by:
David Saloner, University of California, San Francisco, United StatesNatallia Maroz-Vadalazhskaya, Minsk, Belarus
Copyright © 2022 Hu, Lu, Xiao, Dong and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan Xu, eGlhbzg2eHVAMTYzLmNvbQ==
 Feng Hu
Feng Hu Feng Lu
Feng Lu Huiling Xiao
Huiling Xiao Meixue Dong
Meixue Dong Yan Xu
Yan Xu