- Department of Cardiology, The First Affiliated Hospital of Dalian Medical University, Dalian, China
Objective: This study aimed to explore the outcomes of His-Purkinje conduction system pacing (HPCSP) and to screen the predictors of left ventricular (LV) complete reverse remodeling in patients with true left bundle branch block (LBBB) and heart failure with reduced ejection fraction (HFrEF).
Methods: Patients who underwent HPCSP for true LBBB and HFrEF from April 2018 to August 2020 were consecutively enrolled. All participants were followed up for at least 1 year. Thrombosis, infection, lead dislodgement, perforation, and other complications were observed after HPCSP. Clinical data, including echocardiographic parameters, electrocardiogram measurements, and cardiac function, were assessed before and after the procedure.
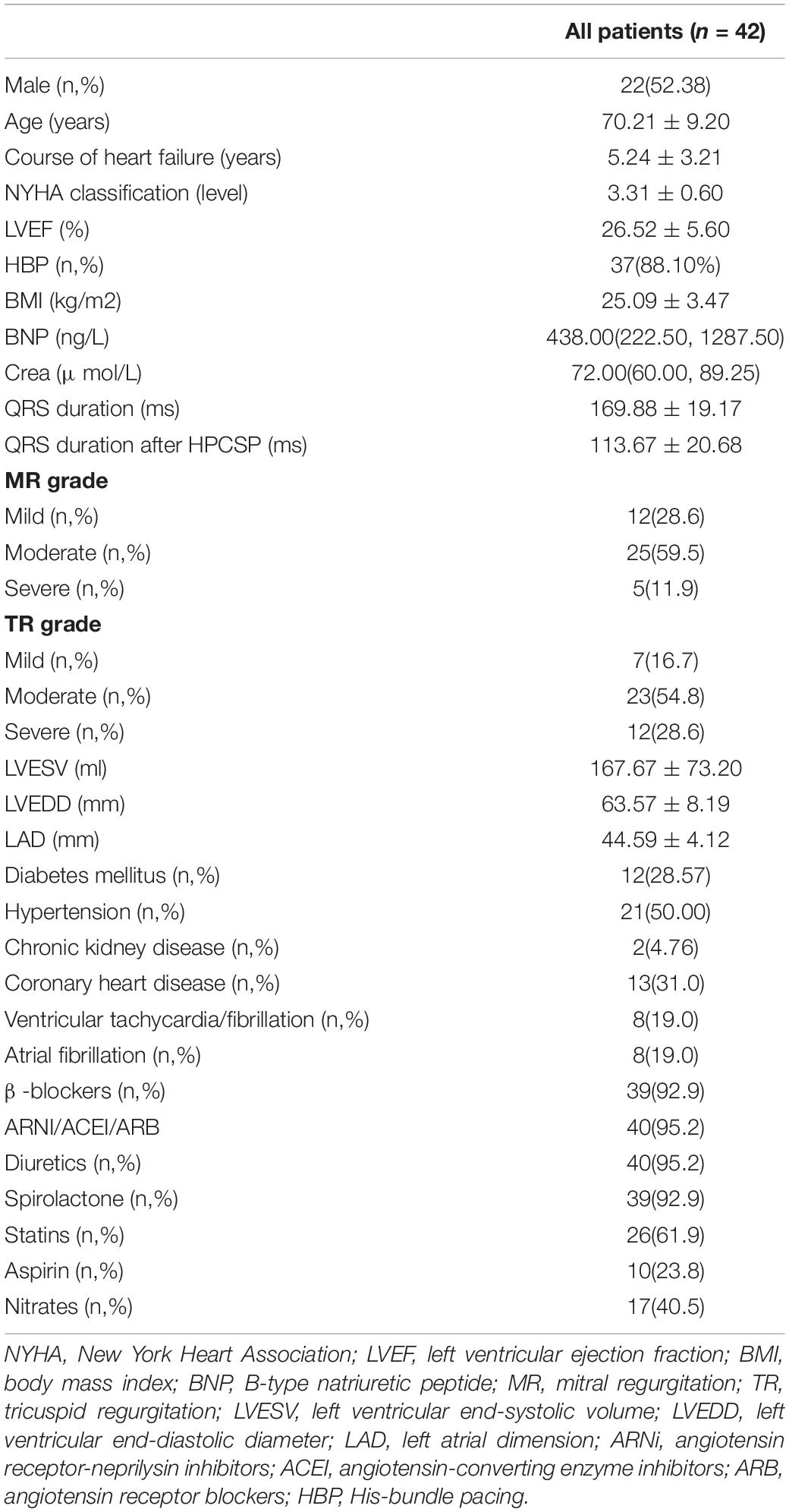
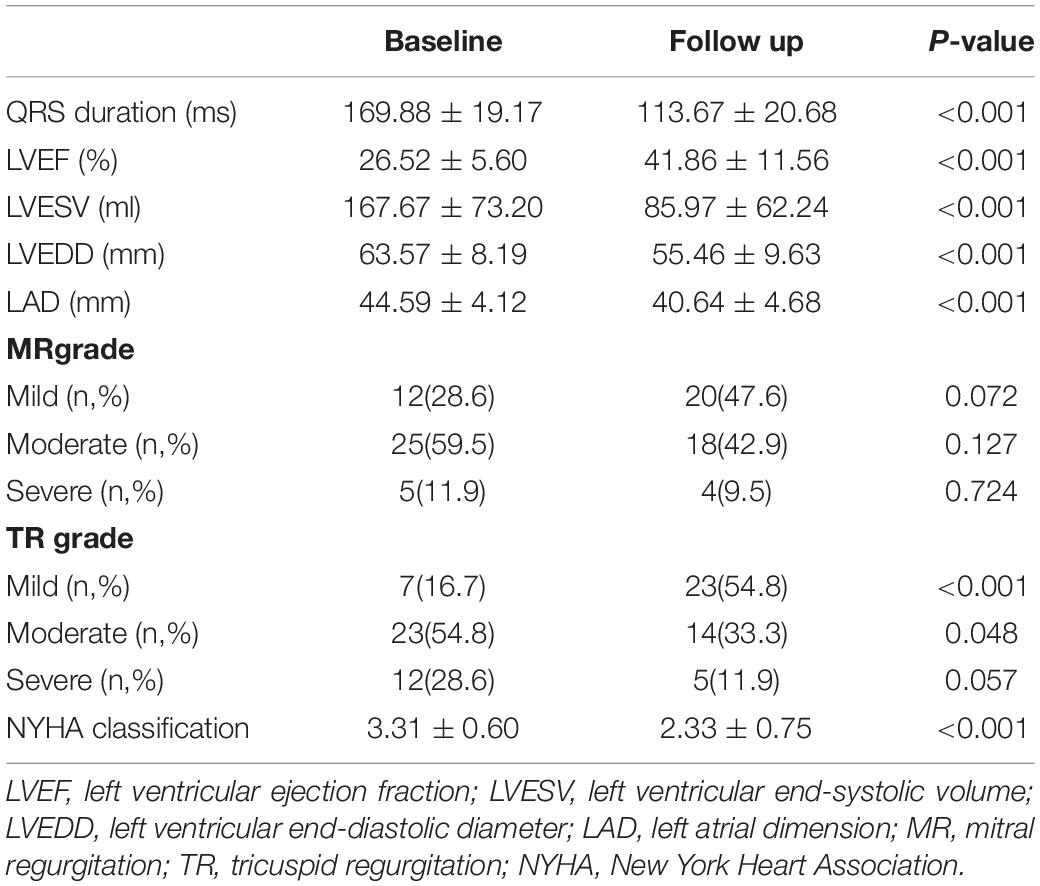
Results: A total of 46 patients were enrolled. HPCSP was successfully deployed in 42 cases (91.30%), which included 37 cases with His bundle pacing (HBP) and 5 cases with left bundle branch pacing (LBBP). The QRS duration decreased significantly (169.88 ± 19.17 ms vs. 113.67 ± 20.68 ms, P < 0.001). Left ventricular end-systolic volume (LVESV) (167.67 ± 73.20 ml vs. 85.97 ± 62.24 ml, P < 0.001), left ventricular end-diastolic diameter (LVEDD) (63.57 ± 8.19 mm vs. 55.46 ± 9.63 mm, P = 0.003) and left ventricular ejection fraction (LVEF) (26.52 ± 5.60% vs. 41.86 ± 11.56%, P < 0.001) improved dramatically. Complete reverse remodeling of the LV with normalized LVEF and LVEDD was found in nearly half of the patients (45.24%). A short QRS duration after HPCSP was a strong predictor of normalized LVEF and LVEDD (P < 0.001). The thresholds increased markedly in two patients approximately 6 months after HBP. No patients died during the total follow-up period of 20.07 ± 6.45 months.
Conclusion: Complete reverse remodeling of the LV could be found in nearly half of the patients with HFrEF and true LBBB after HPCSP, and the short QRS duration after HPCSP was a strong predictor.
Introduction
Approximately 30% of patients with heart failure and left ventricular (LV) desynchronization showed no response to cardiac resynchronization therapy (CRT) via conventional biventricular pacing (BiVP) (1, 2). A greater response to BiVP was found in patients with true left bundle branch block (LBBB) (3).
Several studies have illustrated that His-Purkinje conduction system pacing (HPCSP), including His bundle pacing (HBP) and left bundle branch pacing (LBBP), could be a better option for CRT (4–8). Singh et al. demonstrated that normalized LVEF was found in 71.43% of patients with LBBB-induced cardiomyopathy after HPCSP (9). How can the proportion of LV complete reverse remodeling with normalized LVEF and LV end-diastolic diameter (LVEDD) be maximized? Obviously, the predictors are still unclear. This study aimed to evaluate the efficacy of the HPCSP and explore the predictors of LV complete reverse remodeling in patients with true LBBB and heart failure with reduced ejection fraction (HFrEF).
Materials and Methods
Patient Enrollment and Follow-Up
Patients with true LBBB and HFrEF who underwent HPCSP from April 2018 to August 2020 were consecutively enrolled in our center. The exclusion criteria were recent myocardial infarction or cardiac surgery (<3 months). All patients consented to their treatment, which was approved by the hospital ethics board. LBBP would be the alternative therapy for those patients whose first choice of HBP failed, and BiVP would be the rescue therapy if HPCSP failed. All patients received guideline-directed medical therapy for at least 3 months before implantation.
Regular follow-up was conducted 1, 3, 6, 12, 18, and 24 months after the operation. During the follow-up, the 12-lead electrocardiogram (ECG), echocardiography, postoperative complications, and pacemaker parameters were monitored. The events of thrombosis, infection, lead dislodgement, perforation, stroke, rehospitalization due to heart failure, or death were recorded.
The left ventricular end-systolic volume (LVESV), LVEDD, and left atrial diameter (LAD) were measured following the guidelines of the American Society of Echocardiography. LVEF was measured using the biplane Simpson’s method, and the maximum mitral regurgitation (MR) and tricuspid regurgitation (TR) were measured by the vena contracta width with color-flow Doppler.
Criteria and Definition
True LBBB was defined as QRS duration > 140 ms in men (>130 ms in women) and the presence of at least 2 mid-QRS notches or slurs in leads I, aVL, V1, V2, V5, and V6 (10). An LVEF higher than 50% and an LVEDD less than 50 mm were considered LV complete reverse remodeling.
His bundle pacing usually had two independent capture thresholds, including a His-bundle capture threshold and an LBBB correcting threshold in those patients. An abrupt decrease in the Stim-LV active time (LVAT) of more than 10 ms and the morphologies of Qr, qR, or rSR’ in lead V1 were the simple criteria for left bundle branch capture.
Implantation Procedure and Device Programme
The HBP and LBBP were performed using the Select Secure pacing lead (Model 3830, 69 cm, Medtronic Inc., Minneapolis, MN, United States) and a fixed-curve sheath (C315 HIS, Medtronic Inc., Dublin, Ireland). His bundle electrograms were mapped in a unipolar configuration and recorded in the system (Prucka Cardiolab, GE Healthcare, Waukesha, WI, United States). As described in our previous publications, LBBB correcting thresholds lower than 3.0 V/0.4 ms were accepted (11).
The LBBP was further performed when HBP failed to correct LBBB or when the corrected threshold was above 3.0 V/0.4 ms. The sheath and lead were advanced approximately 1–2 cm from the His bundle region. The unipolar-tip paced QRS configuration and pacing impedance were monitored along with the measurement of peak LV activation times in lead V5 for LBBP. All patients accepted a CRT defibrillator (D) or CRT pacemaker (P) device according to the guidelines. The leads were then connected to the left atrium (LA), right ventricle (RV), and LV ports. The LV-RV delay was programmed to ensure the shortest QRS duration. The 3,830 lead was connected to the LV port, and the longer interventricular delay was programmed to ensure ventricular activation via conduction system pacing.
If HPCSP was unsuccessful, an LV lead was implanted via the traditional coronary venous approach. The LV lead was positioned with a standard technique in the lateral or posterolateral LV vein on patients with BiVP if possible. The RV lead was implanted in the right ventricular septum.
Statistical Analysis
Statistical analyses were performed using SPSS 23.0. Continuous variables were expressed as the mean ± SD or median and were compared with independent two-samples, paired t-test, or Wilcoxon test. Categorical variables were expressed as numbers (%) and were compared using the Fisher’s exact test. Univariate and multivariate analyses were performed using logistic regression to determine the independent predictors of LV complete reverse remodeling after HPCSP. The optimal cutoff of QRS duration was shown on the receiver operator characteristic (ROC) curve with the maximized sensitivity and specificity. P < 0.05 (two-tailed) was considered to be statistically significant.
Results
Baseline Patient Characteristics and Clinical Events
A total of 46 patients were enrolled in this study. The HPCSP was successfully deployed in 42 cases (91.30%), which included 37 cases (80.43%) with HBP and 5 cases (10.87%) with LBBP, and the other 4 patients for whom HPCSP failed accepted BiVP. All patients were implanted with CRT defibrillator (D) (30, 65.22%) or CRT pacemaker (P) devices. All patients were followed up for at least 12 months, and the follow-up duration was 20.07 ± 6.45 months. The LBBB was corrected in all 42 patients after HBCSP with a correcting threshold of 2.13 ± 0.65 V/0.4 ms, and the His-bundle capture threshold was 1.71 ± 0.87.
The baseline characteristics of the patients are shown in Table 1. The average age of the patients was 70.21 ± 9.20 years, the average LVEF value was 26.52 ± 5.60%, and the average QRS duration was 169.88 ± 19.17 ms. During the follow-up, one patient was rehospitalized due to heart failure, and no patients died. Complications such as thrombosis, infection, lead dislodgement, perforation, and stroke were not found in any of the patients. The thresholds increased markedly (3.0 V/1.0 ms) in two patients approximately 6 months after HBP, and then the thresholds decreased to 1.5 V/0.4 ms after resetting the lead.
Lead Outcome of His-Purkinje Conduction System Pacing
There was a slight trend of increment in the correct threshold after follow-up in patients with HBCSP (from 2.13 ± 0.65 V/0.4 ms to 2.52 ± 0.42 V/0.4 ms, P = 0.051). The impedance decreased slightly after the follow-up (from 621.82 ± 135.80 Ω to 462.46 ± 109.95 Ω, P = 0.022). The correct threshold of the LBBB in patients with HPCSP was not different from that in patients with BiVP (2.13 V ± 0.65/0.4 ms vs. 2.36 V ± 0.45/0.4 ms, P = 0.351). All the changes are shown in Table 2. The pacing percentage at the final follow-up was 95.14 ± 4.17%.
Clinical Outcomes of His-Purkinje Conduction System Pacing
Complete LV reverse remodeling was found in nearly half of the patients (45.24%) approximately 6.03 ± 3.50 months after the operation. Approximately 97.62% of patients responded to HPCSP. The LVEF value was higher than 50% in 23 patients (54.76%) soon after the operation (5.21 ± 3.10 months), and the LVEDD decreased to less than 50 mm in 21 patients (50.00%) approximately 6.84 ± 3.72 months after the operation. The changes in values such as QRS duration, cardiac structure, and cardiac function are shown in Table 3. The continuous changes in LVEF, LVESV, and LVEDD after HPCSP are shown in Figure 1.
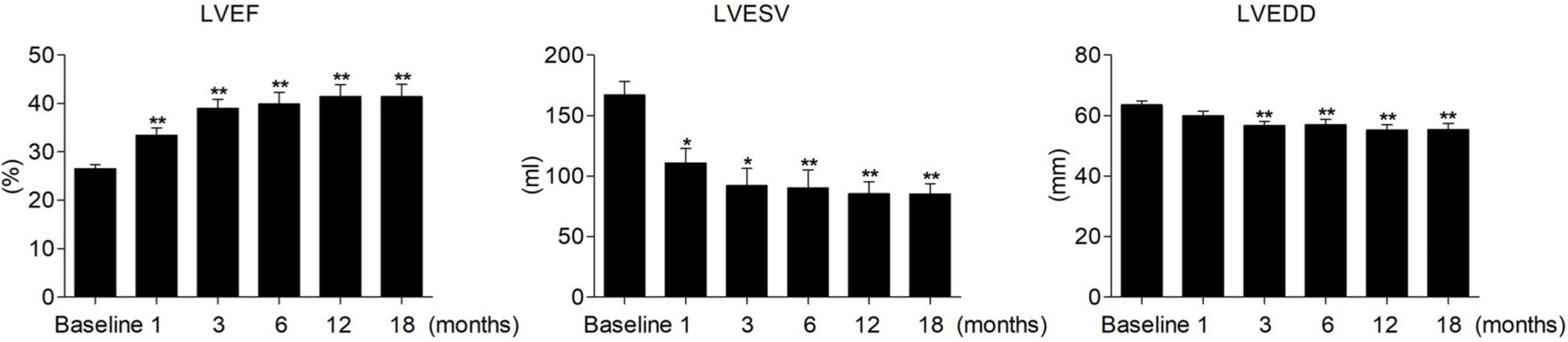
Figure 1. Continuous changes in LVEF, LVESV, and LVEDD after HPCSP. LVEF, left ventricular ejection fraction; LVESV, left ventricular end-systolic volume; LVEDD, left ventricular end-diastolic diameter. *vs baseline P < 0.05, **vs baseline P < 0.001.
Clinical Features of Patients With Left Ventricular Complete Reverse Remodeling
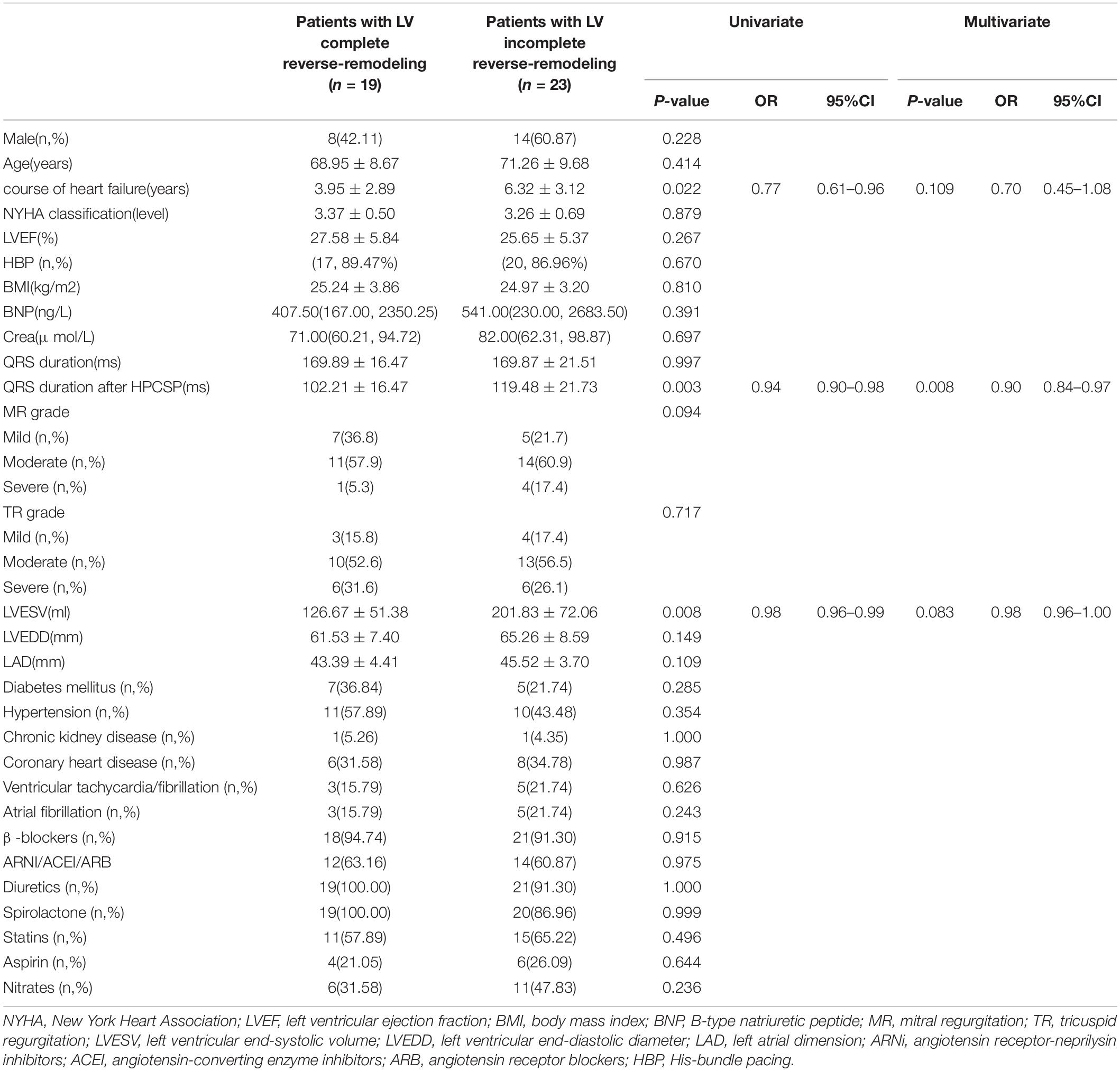
Univariate analysis showed that a short course of heart failure (P = 0.022), small LVESV before HPCSP (P = 0.008), and short QRS duration after pacing (P = 0.003) were related to LV complete reverse remodeling. Further multivariate regression analysis demonstrated that a short QRS duration was an independent predictor of normalized LVEF and LVEDD in patients with true LBBB and heart failure after HPCSP (OR 0.90, 95% CI: 0.84–0.97, P = 0.008), which is shown in Table 4. The area under the ROC curve was 0.819. The cutoff point was 107 ms with a sensitivity of 78.3% and a specificity of 77.9%.
Discussion
We proved that HBP and LBBP could dramatically improve heart function, and complete LV reverse remodeling was demonstrated in nearly half of the patients (45.24%) with true CLBBB and HFrEF. To the best of our knowledge, this was the first study to demonstrate that a short QRS duration after HPCSP was a strong independent predictor of LV complete reverse remodeling.
Feasibility and Safety of His-Purkinje Conduction System Pacing
Although the report showed that the failure rate of BiVP was only 3.6%, it was unfortunate that the suboptimal position was accepted in approximately 20% of patients, which might impair the performance of CRT (12). We proved that the success rate of permanent HPCSP, including LBBP, reached approximately 90% in this study, which might be related to the combined application of HBP and LBBP (13, 14).
Complications such as thrombosis, infection, lead dislodgement and perforation, and other implant-related events were not found. Recently, Bhatt et al. reported that 8% of 101 patients with successful HBP implantation required electrode adjustment (15). In our study, the thresholds in most patients remained stable, with only two patients undergoing electrode adjustment 6 months after the operation. Consistent with our previous study, this study also demonstrated acceptable and stable thresholds for HBP 1 year after the operation (16).
The distal HBP lead helix, by virtue of being in the septal myocardium, played an important role in the favorable capture threshold and amplitude of the R wave (17). However, the failure of HBP was sometimes shown to be a non-negligible issue (18). For patients with a high threshold or failure of HBP, LBBP worked as a promising alternative for delivering physiological pacing to achieve electrical and mechanical synchrony.
Clinical Performance After His-Purkinje Conduction System Pacing
Although BiVP was effective in reducing desynchronization, it was difficult to achieve complete reverse remodeling for the impaired conduction defect (19). This dilemma was somewhat circumvented with HPCSP (20). A series of publications suggested that HPCSP was a favorable choice for patients with CRT indications (21, 22). Li et al. reported that the response rate and super response rate in heart failure patients with LBBB were 88.9 and 44.4%, respectively, which were greater than those of BiVP (66.7 and 16.7%) (23). We showed that the response ratio was 97.62%, and the LV complete reverse remodeling ratio was 45.24% after HPCSP. For those patients with a CRT indication, would HPCSP be the best choice? We hope that an increasing number of studies will explore this issue in the future.
Huang et al. found that HBP obviously improved LVEF, LVESV, and NYHA classification in 74 patients with heart failure and LBBB (24). In our study, we also found that the LVESV, LVEDD, MR, and TR significantly improved after HPCSP. Furthermore, an improvement in LA remodeling after HPCSP was shown, which might predict the possibility of rhythm management in patients with atrial fibrillation during long-term follow-up.
The dramatic shortening of QRS duration after HPCSP was also demonstrated in our study (169.88 ± 19.17 ms vs. 113.67 ± 20.68 ms, P < 0.001). It has been proved that the shortening of QRS duration after HPCSP was more obvious compared to BiVP (mean QRS reduction of 20 ms) (25). But the shortening of QRS duration by LBBP was not as obvious as that by HBP (56 vs. 69 ms, P = 0.007) (26). It suggests that we should distinguish LV septal myocardial pacing (LVSP) from HPCSP due to its limited value on LV synchronization and physiological conduction system pacing (27). One of the differences is that LBBP can be fused with intrinsic RV activation for normal ventricular synchronization, whereas LVSP cannot.
Patient Characteristics of Left Ventricular Complete Reverse Remodeling
Quite different from BiVP, HPCSP completely corrected the LBBB and resulted in super electrical resynchronization. This means that all heart failures originating from LBBB without other heart troubles would be cured. However, approximately 30% of the patients still suffered from heart failure, indicating that some other factor plays a role in LV reverse remodeling.
The course of heart failure was an important factor for LV reverse remodeling (28, 29). Similar to those studies, we also found that a longer course of heart failure was more common in patients with LV incomplete reverse remodeling, even though it was not an independent predictor in our study. This result suggests that the early correction of LBBB might be necessary to halt the progression of the cardiomyopathic process.
Current trials demonstrate that factors such as non-ischemic etiology, QRS duration, and morphology can predict BiVP response (30). It was also found that not all the cardiac complete reverse remodeling could be detected in patients with corrected LBBB in our study, which indicated that other etiologies might play a role in heart failure in one patient. Some patients’ conduction bundle lesions were not located at the proximal end of the trunk, which played a role in the normalized cardiac function. Some patients might be complicated by more myocardial lesions, and some patients might suffer from more scar burden.
One of the reasons for the failure of CRT via classical technology might be that too many “true LBBB” cases were contained, which did not meet the strict physiology-based criteria for “true LBBB” after all. It was reasonable to critically evaluate the definition of “true LBBB” and the physiology behind its ECG signature (31). However, QRS shortening plays a central role in the CRT response (32). In our study, we also proved that the short QRS duration after HPCSP was a strong independent predictor of LV complete reverse remodeling. The more synchrony there is after HPCSP, the higher the likelihood of a favorable outcome (33). Whether the difference between HBP and LBBP resulted in different QRS duration and cardiac functions will require further exploration in future studies. QRS duration and morphology reflect the electrical timing and activation sequence of the ventricles; thus, reversal of the electrical pathology indicates a potentially favorable effect of the therapy (34).
Limitations
This was a single-center retrospective study with small sample size. More large-sample and randomized control multicenter studies might be necessary to confirm these results.
Conclusion
His-Purkinje conduction system pacing dramatically reversed cardiac remodeling and cardiac function in patients suffering from HFrEF and true LBBB. To the best of our knowledge, this is the first study to prove that a short QRS duration after HPCSP is a powerful predictor of LV complete reverse remodeling after HPCSP.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Author Contributions
X-MG, D-NL, and F-LZ contributed to the conception and design of the study. F-LZ organized the database. D-NL, Y-NZ, and Y-HY performed the statistical analysis. B-LD and S-YD followed up with the patients. X-MG and D-NL wrote the first draft of the manuscript. L-JG, Y-XD, and Y-LX wrote the sections and proofread the manuscript. All authors contributed to manuscript revision and read and approved the submitted version.
Funding
This study was supported by the Scientific and Technological Innovation Foundation of Dalian City (2020JJ26SN055).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We greatly appreciate all of the participants in the study.
References
1. Curtis AB. Will His bundle pacing make cardiac resynchronization therapy obsolete? Circulation. (2018) 137:1546–8. doi: 10.1161/CIRCULATIONAHA.117.031787
2. Daubert C, Behar N, Martins RP, Mabo P, Leclercq C. Avoiding non-responders to cardiac resynchronization therapy: a practical guide. Eur Heart J. (2016) 38:1463. doi: 10.1093/eurheartj/ehw270
3. Perrin MJ, Green MS, Redpath CJ, Nery PB, Keren A, Beanlands RS. Greater response to cardiac resynchronization therapy in patients with true complete left bundle branch block: a PREDICT substudy. Europace. (2012) 14:690–5. doi: 10.1093/europace/eur381
4. Wang Z, Wu Y, Zhang J. Cardiac resynchronization therapy in heart failure patients: tough road but clear future. Heart Fail Rev. (2021) 26:735–45. doi: 10.1007/s10741-020-10040-2
5. Sharma PS, Dandamudi G, Herweg B, Wilson D, Singh R, Naperkowski A, et al. Permanent His-bundle pacing as an alternative to biventricular pacing for cardiac resynchronization therapy: a multicenter experience. Heart Rhythm. (2018) 15:413–20. doi: 10.1016/j.hrthm.2017.10.014
6. Upadhyay GA, Vijayaraman P, Nayak HM, Verma N, Dandamudi G, Sharma PS, et al. His corrective pacing or biventricular pacing for cardiac resynchronization inheart failure. J Am Coll Cardiol. (2019) 74:157–9. doi: 10.1016/j.jacc.2019.04.026
7. Barba-Pichardo R, Manovel Sánchez A, Fernández-Gómez JM, Moriña-Vázquez P, Venegas-Gamero J, Herrera-Carranza M. Ventricular resynchronization therapy by direct His-bundle pacing using an internal cardioverter defibrillator. Europace. (2013) 15:83–8. doi: 10.1093/europace/eus228
8. Arnold A, Shun-Shin M, Keene D, Howard J, Sohaib S, Wright I, et al. His resynchronization versus biventricular pacing in patients with heart failure and left bundle branch block. J Am Coll Cardiol. (2018) 72:3112–22. doi: 10.1016/j.jacc.2018.09.073
9. Singh R, Devabhaktuni S, Ezzeddine F, Simon J, Khaira K, Dandamudi G. His-bundle pacing: a novel treatment for left bundle branch block-mediated cardiomyopathy. J Cardiovasc Electrophysiol. (2020) 31:2730–6. doi: 10.1111/jce.14692
10. Strauss DG, Selvester RH, Wagner GS. Defining left bundle branch block in the era of cardiac resynchronization therapy. Rev Portuguesa De Cardiol. (2011) 107:927. doi: 10.1016/j.amjcard.2010.11.010
11. Yang Y, Wang K, Ma P, Zhang R, Waleed K, Yin X, et al. His-purkinje system pacing upgrade improve the heart performances in patients suffering from pacing-induced cardiomyopathy with or without permanent atrial fibrillation. Int J Cardiol. (2021) 335:47–51. doi: 10.1016/j.ijcard.2021.04.012
12. Gamble J, Herring N, Ginks M, Rajappan K, Bashir Y, Betts T. Procedural Success of left ventricular lead placement for cardiac resynchronization therapy: a meta-analysis. JACC Clin Electrophysiol. (2016) 2:69–77. doi: 10.1016/j.jacep.2015.08.009
13. Moriña-Vázquez P, Moraleda-Salas M, Manovel-Sánchez A, Fernández-Gómez J, Arce-Léon Á, Venegas-Gamero J, et al. Early improvement of left ventricular ejection fraction by cardiac resynchronization through His bundle pacing in patients with heart failure. Europace. (2020) 22:125–32. doi: 10.1093/europace/euz296
14. Ponnusamy S, Vijayaraman P. Left bundle branch block-induced cardiomyopathy: insights from left bundle branch pacing. JACC Clin Electrophysiol. (2021) 7:1155–65. doi: 10.1016/j.jacep.2021.02.004
15. Bhatt A, Musat D, Milstein N, Pimienta J, Flynn L, Sichrovsky T, et al. The Efficacy of His bundle pacing: lessons learned from implementation for the first time at an experienced electrophysiology center. JACC Clin Electrophysiol. (2018) 4:1397–406. doi: 10.1016/j.jacep.2018.07.013
16. Ma P, Yang Y, Dai B, Zhang R, Wang N, Li D, et al. Brady-arrhythmias in patients with atrial fibrillation and heart failure of reduced ejection fraction: is his-bundle pacing superior to biventricular pacing? Pacing Clin Electrophysiol PACE. (2021) 44:1193–9. doi: 10.1111/pace.14289
17. Muthumala A, Vijayaraman P. Clinical outcomes of His-Purkinje conduction system pacing. Pacing Clin Electrophysiol PACE. (2021) 44:5–14. doi: 10.1111/pace.14050
18. Vijayaraman P, Chung M, Dandamudi G, Upadhyay G, Krishnan K, Crossley G, et al. His bundle pacing. J Am Coll Cardiol. (2018) 72:927–47.
19. Hawkins N, Petrie M, MacDonald M, Hogg K, McMurray J. Selecting patients for cardiac resynchronization therapy: electrical or mechanical dyssynchrony? Eur Heart J. (2006) 27:1270–81. doi: 10.1093/eurheartj/ehi826
20. Vijayaraman P, Bordachar P, Ellenbogen K. The continued search for physiological pacing: where are we now? J Am Coll Cardiol. (2017) 69:3099–114. doi: 10.1016/j.jacc.2017.05.005
21. Shan P, Su L, Zhou X, Wu S, Xu L, Xiao F, et al. Beneficial effects of upgrading to His bundle pacing in chronically paced patients with left ventricular ejection fraction <50. Heart Rhythm. (2018) 15:405–12. doi: 10.1016/j.hrthm.2017.10.031
22. Upadhyay G, Vijayaraman P, Nayak H, Verma N, Dandamudi G, Sharma P, et al. On-treatment comparison between corrective His bundle pacing and biventricular pacing for cardiac resynchronization: a secondary analysis of the His-SYNC Pilot trial. Heart Rhythm. (2019) 16:1797–807. doi: 10.1016/j.hrthm.2019.05.009
23. Li X, Qiu C, Xie R, Ma W, Wang Z, Li H, et al. Left bundle branch area pacing delivery of cardiac resynchronization therapy and comparison with biventricular pacing. ESC Heart Fail. (2020) 7:1711–22. doi: 10.1002/ehf2.12731
24. Huang W, Su L, Wu S, Xu L, Xiao F, Zhou X, et al. Long-term outcomes of His bundle pacing in patients with heart failure with left bundle branch block. Heart. (2019) 105:137–43. doi: 10.1136/heartjnl-2018-313415
25. Adamson P, Abraham W. Cardiac resynchronization therapy for advanced heart failure. Curr Treat Options Cardiovasc Med. (2003) 5:301–9.
26. Wu S, Su L, Vijayaraman P, Zheng R, Cai M, Xu L, et al. Left bundle branch pacing for cardiac resynchronization therapy: nonrandomized on-treatment comparison with His bundle pacing and biventricular pacing. Can J Cardiol. (2021) 37:319–28. doi: 10.1016/j.cjca.2020.04.037
27. Huang W, Zhou X, Ellenbogen K. Pursue physiological pacing therapy: A better understanding of left bundle branch pacing and left ventricular septal myocardial pacing. Heart Rhythm. (2021) 18:1290–1. doi: 10.1016/j.hrthm.2021.05.013
28. Sze E, Samad Z, Dunning A, Campbell K, Loring Z, Atwater B, et al. Impaired Recovery of left ventricular function in patients with cardiomyopathy and left bundle branch block. J Am Coll Cardiol. (2018) 71:306–17. doi: 10.1016/j.jacc.2017.11.020
29. Wang N, Hussain A, Adelstein E, Althouse A, Sharbaugh M, Jain S, et al. Myocardial recovery after cardiac resynchronization therapy in left bundle branch block-associated idiopathic nonischemic cardiomyopathy: A NEOLITH II substudy. Ann Noninvas Electrocardiol. (2019) 24:e12603. doi: 10.1111/anec.12603
30. Kandala J, Altman R, Park M, Singh J. Clinical, laboratory, and pacing predictors of CRT response. J Cardiovasc Transl Res. (2012) 5:196–212. doi: 10.1007/s12265-012-9352-0
31. Calle S, Kamoen V, De Buyzere M, De Pooter J, Timmermans FA. Strain-based staging classification of left bundle branch block-induced cardiac remodeling. JACC Cardiovasc Imaging. (2021) 14:1691–702. doi: 10.1016/j.jcmg.2021.02.019
32. Bazoukis G, Naka K, Alsheikh-Ali A, Tse G, Letsas K, Korantzopoulos P, et al. Association of QRS narrowing with response to cardiac resynchronization therapy-a systematic review and meta-analysis of observational studies. Heart Fail Rev. (2020) 25:745–56. doi: 10.1007/s10741-019-09839-5
33. Ajijola O, Upadhyay G, Macias C, Shivkumar K, Tung R. Permanent His-bundle pacing for cardiac resynchronization therapy: Initial feasibility study in lieu of left ventricular lead. Heart Rhythm. (2017) 14:1353–61. doi: 10.1016/j.hrthm.2017.04.003
Keywords: His-Purkinje conduction system pacing, left bundle branch block, heart failure, QRS duration, predictors
Citation: Guan XM, Li DN, Zhao FL, Zhao YN, Yang YH, Dai BL, Dai SY, Gao LJ, Xia YL and Dong YX (2022) Short QRS Duration After His-Purkinje Conduction System Pacing Predicts Left Ventricular Complete Reverse Remodeling in Patients With True Left Bundle Branch Block and Heart Failure. Front. Cardiovasc. Med. 9:824194. doi: 10.3389/fcvm.2022.824194
Received: 29 November 2021; Accepted: 08 April 2022;
Published: 06 May 2022.
Edited by:
Dragos Cretoiu, Carol Davila University of Medicine and Pharmacy, RomaniaReviewed by:
Hui Li, Anesthesia Center, Fuwai Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, ChinaGiuseppe Damiano Sanna, Azienda Ospedaliero Universitaria Sassari, Italy
Xiaohan Fan, Fuwai Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, China
Copyright © 2022 Guan, Li, Zhao, Zhao, Yang, Dai, Dai, Gao, Xia and Dong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yun-Long Xia, eXVubG9uZ194aWFAMTI2LmNvbQ==; Ying-Xue Dong, ZGxzdXNhbkAxMjYuY29t
†These authors have contributed equally to this work and share first authorship
 Xu-Min Guan
Xu-Min Guan Dan-Na Li†
Dan-Na Li† Yi-Heng Yang
Yi-Heng Yang Yun-Long Xia
Yun-Long Xia Ying-Xue Dong
Ying-Xue Dong