- Department of Pediatrics, Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University, Wenzhou, China
Background: Kawasaki disease (KD) is an acute febrile systemic vasculitis of unknown etiology that occurs during early childhood, commonly involving the coronary artery, and can lead to coronary artery aneurysms (CAAs).
Methods: The demographic, clinical, and laboratory data of KD patients without coronary artery lesions (N-CAL) and with CAA were collected during 2005–2020 at the Second Affiliated Hospital of Wenzhou Medical University. The patients were divided into the development cohort and the validation cohort. First, we compared the general information, symptoms, and laboratory data of N-CAL and CAA patients in the development cohort and the total cohort and screened out the different indices by logistic regression analysis. Then, we established three models and compared the area under the curve (AUC) values of the receiver operating characteristic (ROC) curves to identify meaningful models for CAA, which were further verified by decision curve analysis (DCA). Second, taking into account previous reports on the importance of gender to CAA, gender stratification was conducted.
Results: The analysis of clinical and blood indices revealed the following novel features: (i) Many factors were found to be related to CAA, including IVIG resistance and the symptoms of rash, oral changes, and cervical lymphadenopathy. (ii) The development cohort was analyzed by logistic regression, and three models were established. The ROC curves showed that Model 2, composed of IVIG resistance, rash, oral changes, and cervical lymphadenopathy, had a better AUC value and easily to evaluate in the prediction of CAA. (iii) The selected model for predicting CAA in the development cohort was further confirmed in the validation cohort through DCAs. (iv)We further compared the items enrolled in the three models above between the N-CAL and CAA groups by sex, and the results indicated that female KD patients without rash, oral changes, and cervical lymphadenopathy were more likely to develop CAA.
Conclusion: The absence of rash, oral changes, and cervical lymphadenopathy are risk factors for CAA, especially in female KD patients. Accurately recognizing symptoms, early diagnosis, and standard treatment for KD are key to reducing the incidence of CAA.
HIGHLIGHTS
- The results are more reliable because of the large numbers of patients and the wide span of years.
- This is the first study to indicate the importance of the three symptoms (rash, oral changes, and cervical lymphadenopathy) in predicting CAA in KD patients. More importantly, this finding can be easily applied to clinical practice.
- This is the first study to demonstrate that the absence of rash, oral changes and cervical lymphadenopathy are more prominent predictors of CAA in female KD patients.
Introduction
Kawasaki disease (KD) is an acute systemic vasculitis disease that mainly occurs in children under 5 years of age and affects small- and medium-sized blood vessels, especially the coronary arteries. Although intravenous immunoglobulin (IVIG) decreases the incidence of coronary artery lesions (CALs) from 25 to 4% (1), KD has become the leading cause of secondary heart damage in children worldwide (2–4).
Studies have shown that CAL in Kawasaki disease may persist and develop into stenosis and occlusion (5). Coronary artery aneurysms (CAAs) cause serious damage to children in particular. In recent years, IVIG resistance and incomplete Kawasaki disease (IC-KD) has been increasingly recognized by researchers and clinicians. IVIG resistance is defined as persistent fever 36–48 h after IVIG or recrudescent fever within 1 week (6). Studies suggest that IVIG resistance reflects the severity of underlying inflammation in children, which leads to an increased risk of CAA (7).
The main clinical manifestations of KD include fever, rash, oral changes, anomalies of the extremities, bilateral non-exudative conjunctivitis, and cervical lymphadenopathy. Fever with four or five clinical symptoms is considered to be complete KD. Fever with less than four clinical symptoms is considered to be incomplete KD (8). Clinical symptoms alone are insufficient, and other laboratory tests play an auxiliary role in the diagnosis of incomplete KD, including increased platelets (PLT), decreased serum albumin (ALB), low serum sodium (Na), and many other indices. Incomplete KD is also considered to be one of the risk factors for CAA (9).
Studies have shown that anemia is an independent risk factor for CAA (10). Other studies found that male sex, delayed treatment with IVIG, increased c-reactive protein (CRP) (11), extreme age (12), and tachycardia (13) were all risk factors for CAA (14, 15). Now, using the data from a children’s hospital in southeastern China over several years, we retrospectively analyzed the characteristics of CAA patients and conducted further research so that the features of CAA patients—one of the most serious complications in KD—can be understood better.
Materials and Methods
Participants and Study Design
Our study was approved by the Ethics Committee of the Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University. We collected data from Kawasaki disease patients who were hospitalized in the Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University from 2005 to 2020.
Admission criteria were as follows: fever for more than 5 days, accompanied by the following 4 or 5 clinical manifestations; bilateral conjunctival injection, oral changes, cervical non-suppurative lymphadenopathy, rash, and extremity changes. Patients with these symptoms were diagnosed with complete Kawasaki disease. Patients with only three or fewer concomitant symptoms were diagnosed with incomplete KD.
Exclusion criteria were as follows: (1) Not in the acute stage; (2) Not treated with IVIG; (3) Uncertain diagnoses that cannot be distinguished from other diseases, such as septicemia and juvenile idiopathic arthritis.
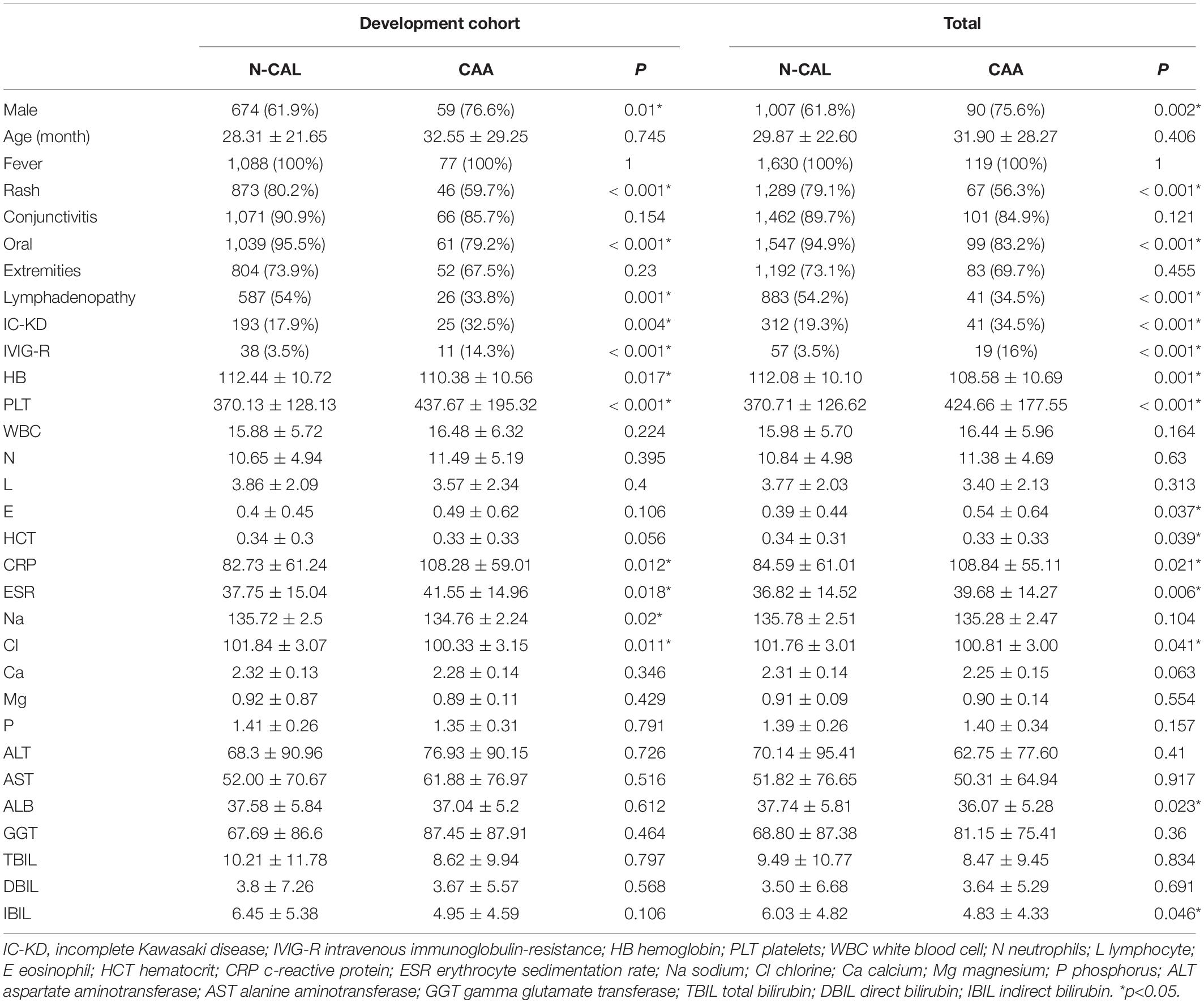
For the purpose of our study, to better characterize the features of CAA patients, we grouped the patients into N-CAL and CAA, and compared them. We included patients who had not developed CAL from the onset of the disease to the end of the follow-up as the N-CAL group. The patients with CAA at any stage from the onset of the disease to the end of the follow-up were classified into the CAA group. To make our results more reliable, the total patients were randomly divided into the development cohort and the validation cohort with the R package caret. A total of 2/3 were included in the development cohort, and 1/3 were included in the validation cohort. By comparing the data of the N-CAL and CAA groups in the development cohort and the total cohort, we found that CAA patients had lower rates in rash, oral changes and cervical lymphadenopathy. Besides, the three symptoms were proved to be significant in predicting CAA by establishing models, which were verified in the validation cohort. Considering that KD is more prevalent in male gender, we conducted gender stratification to further comparison. The results indicated that the absence of rash, oral changes, and cervical lymphadenopathy were better predictors of CAA in female KD patients.
Clinical and Laboratory Data
Considering the subjectivity of the diagnosis of Kawasaki disease, the clinical symptoms evaluation and the final diagnosis of all patients were carried out by experienced pediatric cardiologists. The blood tests and the laboratory examinations after admission were conducted after fasting and prior to IVIG. All demographic data, clinical symptoms and laboratory tests were collected by trained doctors. Echocardiography was performed by senior doctors in the Ultrasound Department of the Second Affiliated Hospital of Wenzhou Medical University to determine the existence of CAA. CAA is diagnosed mainly by echocardiography. In recent years, researchers have found that this is a better method to identify CAA based on Z value calculation. However, because of the large span of our cases and a large number of Z value deletions, we used the following criteria in this study for the definition of CAA: (1) the lumen diameter of children < 5 years old is at least 3 mm or > 5 years old at least 4 mm. (2) The inner diameter of the segment is at least 1.5 times that of the adjacent segment, and (3) the lumen is obviously irregular (16).
Follow-Up
All patients were followed up regularly after discharge, and echocardiography was performed at approximately 1, 3 and 6 months after discharge. All the patients in the group had at least one echocardiographic follow-up result; otherwise, they were regarded as lost to follow-up.
Data Analysis
The classified variables were described by count and percentage, and the chi-squared test was used to compare two groups. Continuous variables are expressed as the mean ± standard deviation, and t tests or Mann–Whitney U tests were used to compare two groups. P < 0.05 indicates that the difference is statistically significant. Logistic regression analysis was used for multivariate analysis. The area under the curve (AUC) value under the receiver operating characteristic (ROC) curve was used to judge the effectiveness of the model, and the decision curve analysis (DCA) was used for further confirmation.
Results
Many Factors Were Found to Be Related to CAA in the Development Cohort and the Total Cohort
All subjects (n = 1749) were randomly divided into two groups using the R package caret: the development cohort (67%, n = 1165) and the validation cohort (33%, n = 584). It has been proven suitable to include 2/3–4/5 patients as the development cohort (17, 18). The baseline values of the total cohort and the development cohort are displayed in Table 1, according to whether CAA was present. In the development cohort, more male patients (p = 0.01) and incomplete Kawasaki disease patients (p = 0.004) were found in the CAA group. No significant difference in age (p = 0.745) was found between the two groups. In terms of clinical symptoms, rash, oral changes, and cervical lymphadenopathy were considered to be less common in the CAA group, and the p values were < 0.001, < 0.001, and 0.001, respectively. Regarding the therapeutic response, the rate of IVIG resistance (p < 0.001) was found to be higher in the CAA group. In addition, lower hemoglobin (p = 0.017) and serum chloride levels (p = 0.011), and higher platelets (p < 0.001), CRP (p = 0.012), and ESR (p = 0.018) were identified in the CAA group. Similar differences in the above indicators were observed in the total cohort, and the results are shown in detail in Table 1.
The Significance of the Absence of Rash, Oral Changes and Cervical Lymphadenopathy in Predicting CAA
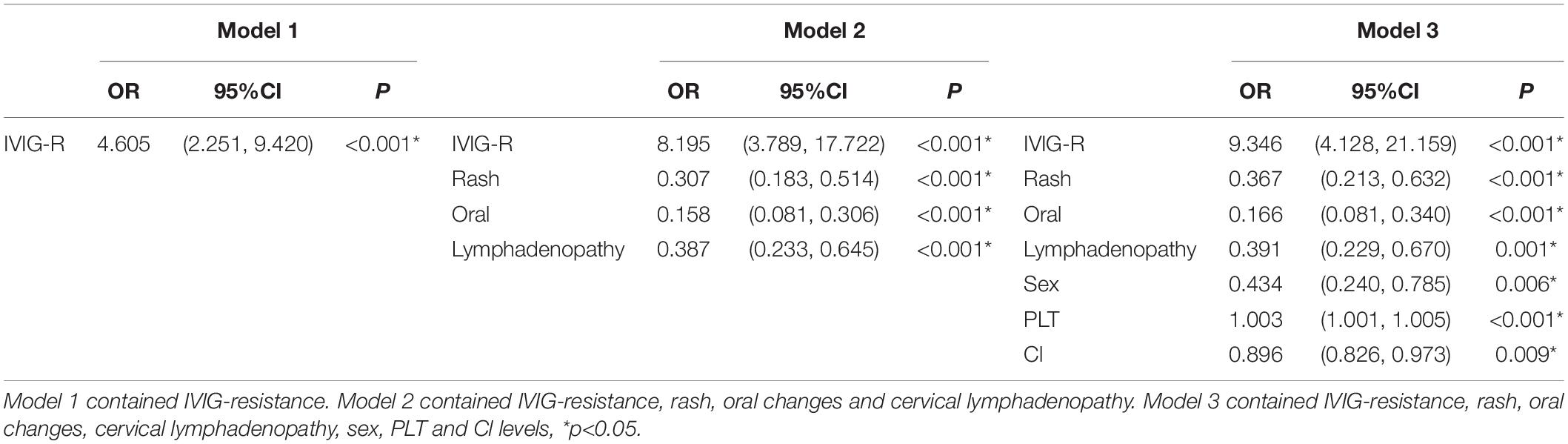
To better identify the risk factors for CAA, logistic regression analysis was further carried out. The results are shown in Table 2. It revealed that sex (female to male) (OR:0.385, 95%CI:0.193, 0.769), rash (OR:0.446, 95%CI:0.222, 0.895), oral changes (OR:0.208, 95%CI:0.08, 0.540), cervical lymphadenopathy (OR:0.409, 95%CI:0.215, 0.776), IVIG-resistance (OR:11.154, 95%CI:4.594, 27.08), PLT (OR:1.003, 95%CI:1.001, 1.004) and Cl levels (OR:0.894, 95%CI:0.811, 0.986) were all relative factors for CAA, which means that the male patients, patients without rash, oral changes, or cervical lymphadenopathy, patients with IVIG-resistance, high PLT levels, and low Cl levels were at higher risk for developing CAA. The results are shown in the form of a forest map in Figure 1, and because of the difference in scale, we divided the forest map into two parts.

Figure 1. Multivariable-adjusted forest plot for the risks of CAA. IVIG-R was separated from other indices because of the great difference in the abscissa scale. *p < 0.05.
Combined with the results of our statistical analysis and considering the important role of IVIG resistance in predicting CAA, which has been generally accepted by researchers and proven in our study, three models were established. Model 1 contained only IVIG resistance. Model 2 added three indicators of clinical symptoms (rash, oral changes, and cervical lymphadenopathy). In Model 3, sex and PLT and Cl levels were also added. The specific p values and OR values are shown in Table 3. The ROC curves of the three models are plotted in Figure 2. The AUC of Model 3 (AUC = 0.791) was the best, but it was also the most complex model. The AUC of Model 2 (AUC = 0.740) was slightly lower than that of Model 3, but it was simpler and neater than Model 3. Both models were better than Model 1 (AUC = 0.575). Therefore, Model 2 was chosen for further study.
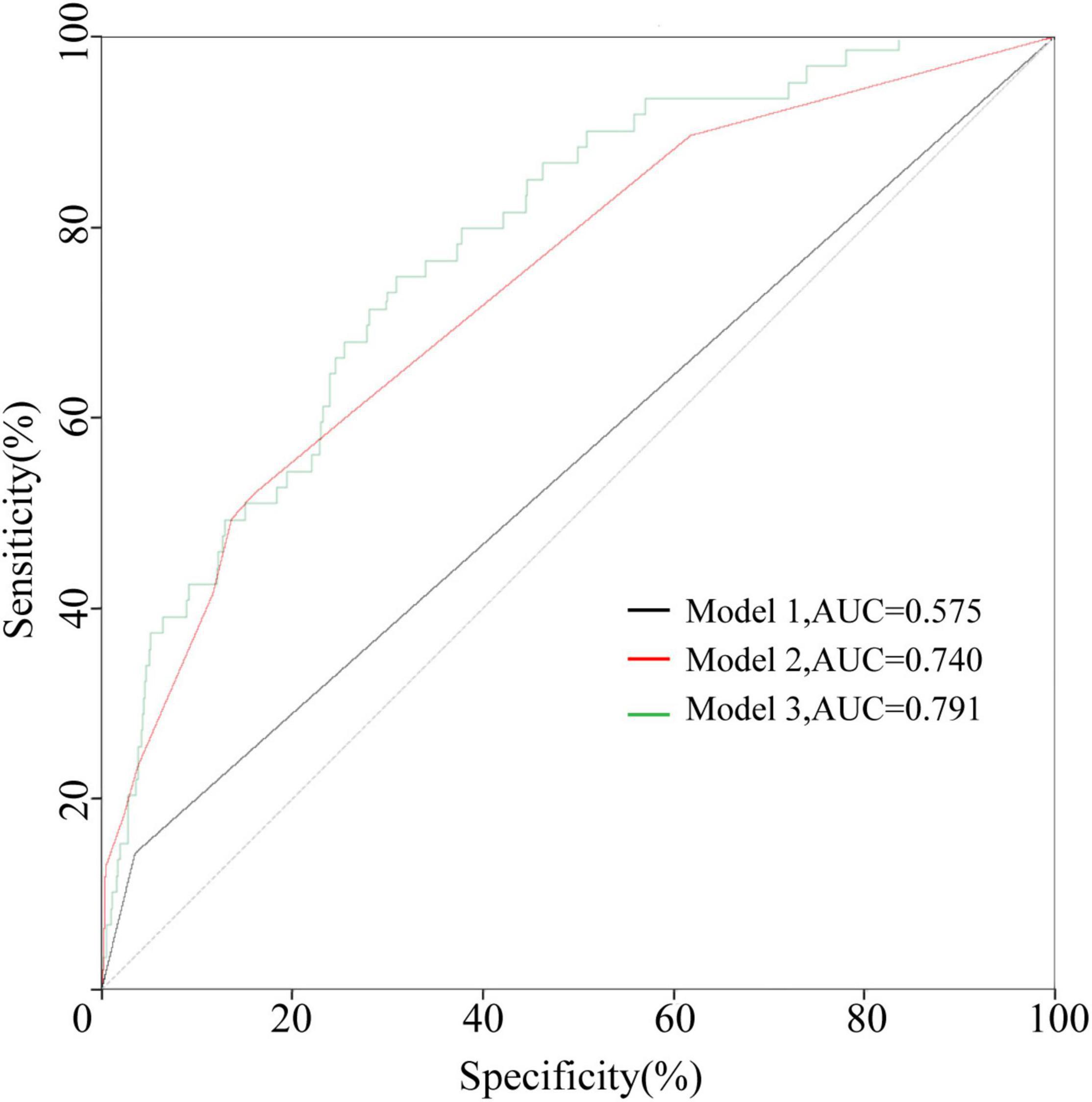
Figure 2. The ROC curves of the three models in the development cohort. Model 1 contained IVIG resistance. Model 2 contained IVIG resistance, rash, oral changes, and cervical lymphadenopathy. Model 3 contained IVIG resistance, rash, oral changes, cervical lymphadenopathy, sex, PLT, and Cl levels.
The Significance of the Absence of Rash, Oral Changes and Cervical Lymphadenopathy in Predicting CAA Was Confirmed in the Validation Cohort
The ROC curve of Model 2 was carried out on the validation cohort, and the AUC of Model 2 in the validation cohort was greater than 0.7 (Figure 3). To study further, the DCAs for the development cohort and the validation cohort of the three models were performed, and are shown in Figure 4. There was no significant difference between Model 2 and Model 3, and both Model 2 and Model 3 were superior to Model 1; thus, we discovered the importance of the absence of three clinical symptoms (rash, oral changes, and cervical lymphadenopathy) in addition to IVIG resistance in predicting CAA.

Figure 3. The ROC curve of Model 2 in the validation cohort. Model 2 contained IVIG resistance, rash, oral changes, and cervical lymphadenopathy.
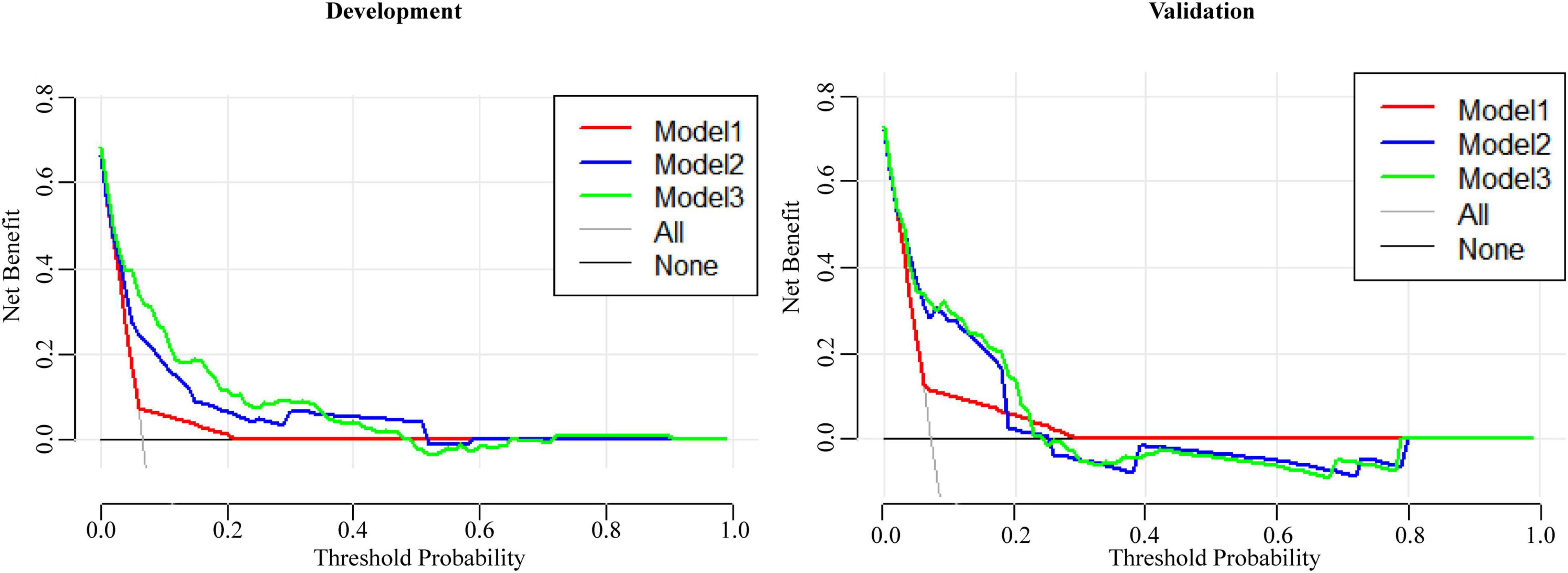
Figure 4. The DCAs of three models for predicting CAA in the development cohort and the validation cohort.
The Absence of Rash, Oral Changes, and Cervical Lymphadenopathy Were Better Predictors of CAA in Female Patients
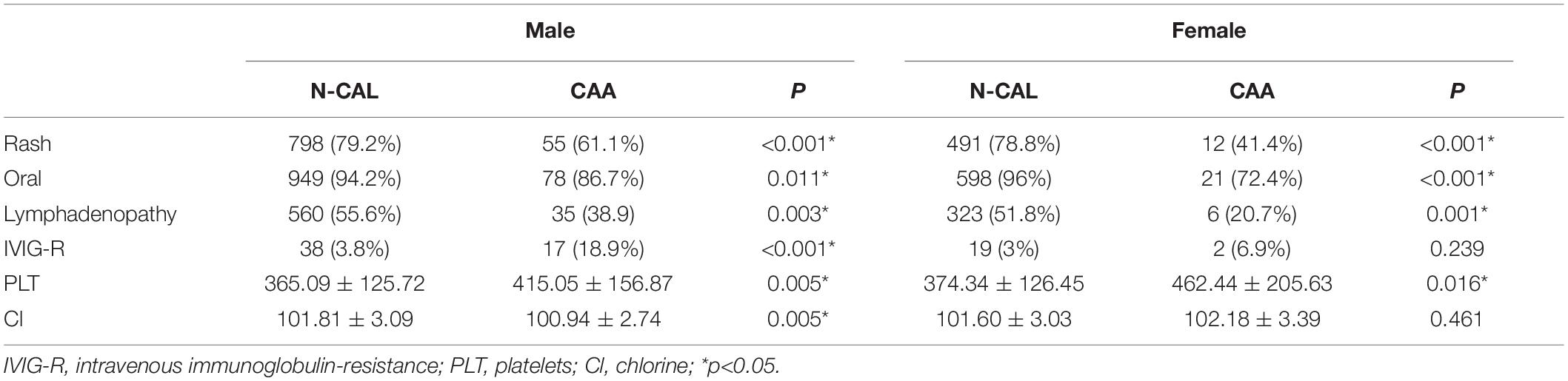
Previous studies have suggested the importance of sex in predicting CAA. Considering this, we grouped the total cohort according to sex and CAA status and focused on the distribution of rash, oral changes, and cervical lymphadenopathy between groups. The description is shown in Figure 5. We compared the items enrolled in the three models above between the N-CAL and CAA groups in different sexes, and the results are shown in Table 4. The results show that the symptoms of rash, oral changes, and cervical lymphadenopathy, which we were interested in, all had statistically significant differences between N-CAL and CAA patients in both male and female patients. Then, we compared the three symptoms through the ROC curves in predicting CAA with different genders and found an interesting phenomenon. In female patients with KD, the absence of rash, oral changes, and cervical lymphadenopathy had a greater effectiveness in predicting CAA than in male patients, which was demonstrated by the superior AUC in females (AUC = 0.786) than in males (AUC = 0.639). The specific p values are displayed in Table 5, and the ROC curves are shown in Figure 6.

Figure 5. Bar charts of the total cohort classified by sex were drawn to describe (A) the distributions of CAA and (B) the symptoms of rash, oral changes, and cervical lymphadenopathy.
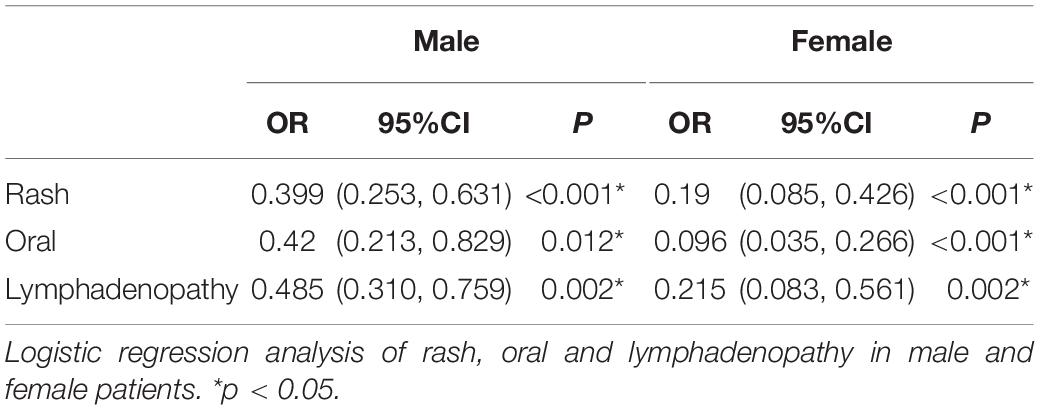
Table 5. Logistic regression analysis of rash, oral and lymphadenopathy in male and female patients.
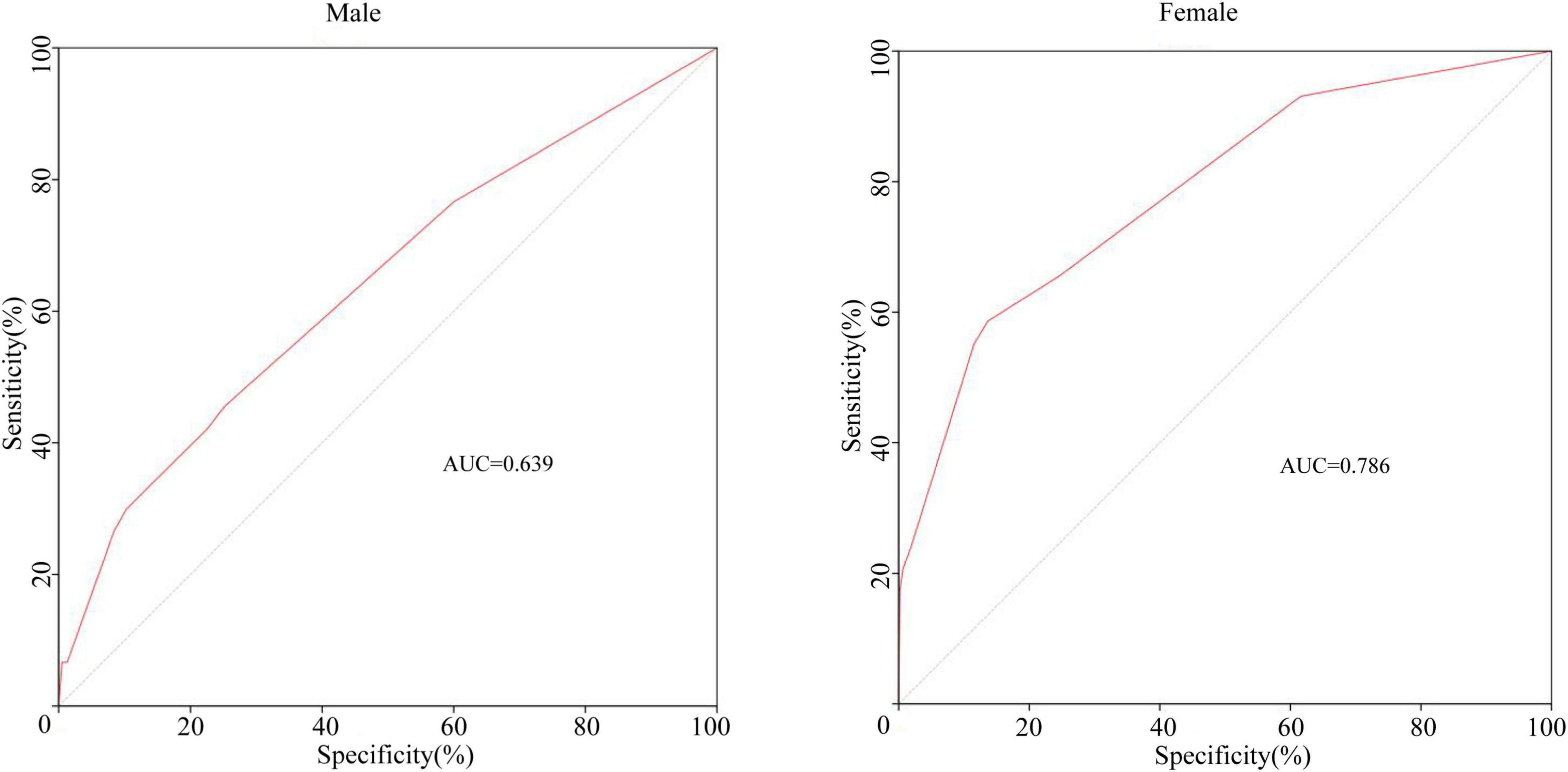
Figure 6. The ROC curves of the combining symptoms (rash, oral changes, and cervical lymphadenopathy) classified by sex for predicting CAA.
Discussion
KD is an acute systemic vasculitis affecting young children, and its incidence is rising sharply (19–21). The main clinical manifestations are fever, rash, oral changes, anomalies of the extremities, bilateral non-exudative conjunctivitis, and cervical lymphadenopathy. KD also affects other systems and leads to symptoms such as vomiting, aseptic pyuria (22), and even aseptic encephalitis (23).
Coronary artery involvement is a frequent complication of KD. According to statistics, 20–25% of KD patients develop severe complications, such as CAA (24), myocardial infarction, and heart failure (25), and a giant aneurysm can occur particularly in untreated patients during the subacute phase. Studies have shown either persistence or stenosis without regression as a long-term consequence of giant coronary aneurysm (GCA). Only some cases reported a regression of GCA in KD (26, 27).
Researchers have done much to identify the risk factors for CAA. Huang et al. (28) found that CAA was significantly related to younger age of onset, higher platelet count, and lower serum albumin levels. A large CAA in patients with KD was related to lower serum sodium levels. Researchers also explained that low levels of serum albumin and immunoglobulin M were risk factors for CAA (29). In addition, male sex, young age (under 1 year old) and high PLT count were considered to be risk factors for CAA in KD patients by Downie (30).
In our study, we also found that the CAA group had lower hemoglobin, higher platelet count, and elevated CRP levels, and that CAA occurred more commonly in male patients. However, age, serum sodium, and serum albumin were not found to be different between the two groups. After regression statistical analysis, hemoglobin, platelet count, and CRP were not found to be independent risk factors for CAA. According to our statistical analysis, the existence of IVIG resistance and the absence of rash, oral changes, and cervical lymphadenopathy play important roles in the occurrence of CAA events, and this result has been well verified in the validation cohort.
Previous studies have shown that sex plays an important role in the occurrence of CAA (31, 32). Therefore, a separate statistical analysis was carried out on different sexes. The results showed that the absence of the three clinical symptoms (rash, oral changes, and cervical lymphadenopathy) were better predictors for CAA, especially in female patients. The sensitivity of the combination of the three symptoms in predicting CAA was higher than that in male patients. This result has not been reported in other studies.
IVIG resistance has been shown to be the most important risk factor for CAA. Our study indicated that as well. In addition, our study also found that without rash, oral changes, and cervical lymphadenopathy, the risk of CAA was higher than that of patients with the above symptoms, especially in female patients. We found that some of the clinical indicators currently investigated were less sensitive to CAA than the clinical symptoms themselves. This suggested that in addition to searching for biomarkers that can recognize CAA early, we should not forget the importance of recognizing clinical symptoms for the disease. At the same time, the research reminds us to pay more attention to the incidence of CAA in female patients.
The absence of rash, oral changes, and cervical lymphadenopathy at the same time is consistent with the incomplete KD diagnosis. Taking this into account, we included incomplete Kawasaki disease as an index in the statistics. A statistically significant difference was observed between the two groups, but the index was not included after the regression analysis. This may be related to the difficulty in identifying incomplete KD. Although the diagnoses of our patients are all conducted by experienced physicians, with the rapid development of understanding of incomplete KD, older cases may have not recognized this diagnosis. Meanwhile, the number of cases in the CAA group was still not high, and more patients need to be included. Multicenter studies will be helpful. Additionally, long-term complications and the regression of CAA, especially large CAA, should be given more attention.
In conclusion, the absence of rash, oral changes, and cervical lymphadenopathy are risk factors for CAA, especially in female patients. Accurate recognition of symptoms, early diagnosis, and standard treatment for KD are key to reducing the incidence of CAA.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethical Decision Committee of the Research Administration at Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin. Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author Contributions
HY was responsible for data statistics and writing manuscript. WS was responsible for data collection and manuscript revising. HW was responsible for data collection and part of writing. SZ, ZX, RW, XR, and HQ collected the data. JZ, CZ, and MC provided the resources and designed the study. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (Grant Number 81970435).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. McCrindle BW, Rowley AH, Newburger JW, Burns JC, Bolger AF, Gewitz M, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a scientific statement for health professionals from the American heart association. Circulation. (2017) 135:e927–99. doi: 10.1161/cir.0000000000000484
2. Dietz SM, van Stijn D, Burgner D, Levin M, Kuipers IM, Hutten BA, et al. Dissecting Kawasaki disease: a state-of-the-art review. Eur J Pediatr. (2017) 176:995–1009. doi: 10.1007/s00431-017-2937-5
3. Singh S, Bhattad S, Gupta A, Suri D, Rawat A, Rohit M. Mortality in children with Kawasaki disease: 20 years of experience from a tertiary care centre in North India. Clin Exp Rheumatol. (2016) 34(3 Suppl. 97):S129–33.
4. Singh S, Vignesh P, Burgner D. The epidemiology of Kawasaki disease: a global update. Arch Dis Childhood. (2015) 100:1084–8. doi: 10.1136/archdischild-2014-307536
5. Jcs Joint Working Group. Guidelines for diagnosis and management of cardiovascular sequelae in Kawasaki disease (JCS 2013). Digest version. Circ J. (2014) 78:2521–62. doi: 10.1253/circj.cj-66-0096
6. Newburger JW, Takahashi M, Burns JC, Beiser AS, Chung KJ, Duffy CE, et al. The treatment of Kawasaki syndrome with intravenous gamma globulin. N Engl J Med. (1986) 315:341–7. doi: 10.1056/nejm198608073150601
7. Marchesi A, Rigante D, Cimaz R, Ravelli A, Tarissi de Jacobis I, Rimini A, et al. Revised recommendations of the Italian society of pediatrics about the general management of Kawasaki disease. Ital J Pediatr. (2021) 47:16. doi: 10.1186/s13052-021-00962-4
8. Eleftheriou D, Levin M, Shingadia D, Tulloh R, Klein NJ, Brogan PA. Management of Kawasaki disease. Arch Dis Childhood. (2014) 99:74–83. doi: 10.1136/archdischild-2012-302841
9. de Graeff N, Groot N, Ozen S, Eleftheriou D, Avcin T, Bader-Meunier B, et al. European consensus-based recommendations for the diagnosis and treatment of Kawasaki disease – the SHARE initiative. Rheumatology (Oxford England). (2019) 58:672–82. doi: 10.1093/rheumatology/key344
10. Chen JJ, Ma XJ, Liu F, Yan WL, Huang MR, Huang M, et al. Epidemiologic features of Kawasaki disease in Shanghai from 2008 through 2012. Pediatr Infect Dis J. (2016) 35:7–12. doi: 10.1097/inf.0000000000000914
11. Yan F, Pan B, Sun H, Tian J, Li M. Risk factors of coronary artery abnormality in children with Kawasaki disease: a systematic review and meta-analysis. Front Pediatr. (2019) 7:374. doi: 10.3389/fped.2019.00374
12. Cameron SA, Carr M, Pahl E, DeMarais N, Shulman ST, Rowley AH. Coronary artery aneurysms are more severe in infants than in older children with Kawasaki disease. Arch Dis Childhood. (2019) 104:451–5. doi: 10.1136/archdischild-2018-314967
13. Suzuki Y, Iijima M, Sasaki H, Muto T, Tanaka H, Kaneko K, et al. Tachycardia as a potential risk indicator for coronary arterial lesions in Kawasaki disease. Eur J Pediatr. (1999) 158:207–9. doi: 10.1007/s004310051050
14. Belay ED, Maddox RA, Holman RC, Curns AT, Ballah K, Schonberger LB. Kawasaki syndrome and risk factors for coronary artery abnormalities: United States, 1994-2003. Pediatr Infect Dis J. (2006) 25:245–9. doi: 10.1097/01.inf.0000202068.30956.16
15. Han RK, Sinclair B, Newman A, Silverman ED, Taylor GW, Walsh P, et al. Recognition and management of Kawasaki disease. CMAJ. (2000) 162:807–12.
16. Song D, Yeo Y, Ha K, Jang G, Lee J, Lee K, et al. Risk factors for Kawasaki disease-associated coronary abnormalities differ depending on age. Eur J Pediatr. (2009) 168:1315–21. doi: 10.1007/s00431-009-0925-0
17. Gao Z, Zhou S, Song H, Wang Y, He X. Nomograms for predicting overall survival and cancer-specific survival of chondroblastic osteosarcoma patients. J Surg Oncol. (2020) 122:1676–84. doi: 10.1002/jso.26185
18. Wang Z, Xu J, Pang M, Guo B, Xu X, Wang H, et al. Nomogram analysis and internal validation to predict the risk of cystobiliary communication in patients undergoing hydatid liver cyst surgery. World J Surg. (2020) 44:3884–92. doi: 10.1007/s00268-020-05661-5
19. Ae R, Makino N, Kosami K, Kuwabara M, Matsubara Y, Nakamura Y. Epidemiology, treatments, and cardiac complications in patients with kawasaki disease: the nationwide survey in Japan, 2017-2018. J Pediatr. (2020) 225:23–9.e2. doi: 10.1016/j.jpeds.2020.05.034
20. Fukazawa R, Kobayashi J, Ayusawa M, Hamada H, Miura M, Mitani Y, et al. JCS/JSCS 2020 guideline on diagnosis and management of cardiovascular sequelae in Kawasaki disease. Circ J. (2020) 84:1348–407. doi: 10.1253/circj.CJ-19-1094
21. Xie LP, Yan WL, Huang M, Huang MR, Chen S, Huang GY, et al. Epidemiologic features of Kawasaki disease in Shanghai from 2013 through 2017. J Epidemiol. (2020) 30:429–35. doi: 10.2188/jea.JE20190065
22. Ohta K, Seno A, Shintani N, Kato E, Yachie A, Seki H, et al. Increased levels of urinary interleukin-6 in Kawasaki disease. Eur J Pediatr. (1993) 152:647–9. doi: 10.1007/bf01955240
23. Burgner D, Curtis N. Kawasaki disease as a cause of encephalitis. Arch Dis Childhood. (2011) 96:988–9. doi: 10.1136/archdischild-2011-300613
24. Liu YC, Lin MT, Wang JK, Wu MH. State-of-the-art acute phase management of Kawasaki disease after 2017 scientific statement from the American heart association. Pediatr Neonatol. (2018) 59:543–52. doi: 10.1016/j.pedneo.2018.03.005
25. Dahlem PG, von Rosenstiel IA, Lam J, Kuijpers TW. Pulse methylprednisolone therapy for impending cardiac tamponade in immunoglobulin-resistant Kawasaki disease. Intensive Care Med. (1999) 25:1137–9. doi: 10.1007/s001340051025
26. Tai IH, Hsieh KS, Liao CC, Kuo HC. Regression of giant coronary aneurysm validated by echocardiography in Kawasaki disease. Circ Cardiovasc Imaging. (2021) 14:e012153. doi: 10.1161/circimaging.120.012153
27. Vergara A, Monda E, Mautone C, Renon F, Di Masi A, Giordano M, et al. Rare case of Kawasaki disease with cardiac tamponade and giant coronary artery aneurysms. Cardiol Young. (2021) 31:865–6. doi: 10.1017/s1047951120004989
28. Huang GY, Ma XJ, Huang M, Chen SB, Huang MR, Gui YH, et al. Epidemiologic pictures of Kawasaki disease in Shanghai from 1998 through 2002. J Epidemiol. (2006) 16:9–14. doi: 10.2188/jea.16.9
29. McCrindle BW, Li JS, Minich LL, Colan SD, Atz AM, Takahashi M, et al. Coronary artery involvement in children with Kawasaki disease: risk factors from analysis of serial normalized measurements. Circulation. (2007) 116:174–9. doi: 10.1161/circulationaha.107.690875
30. Downie ML, Manlhiot C, Collins TH, Chahal N, Yeung RSM, McCrindle BW. Factors associated with development of coronary artery aneurysms after Kawasaki disease are similar for those treated promptly and those with delayed or no treatment. Int J Cardiol. (2017) 236:157–61. doi: 10.1016/j.ijcard.2017.01.068
31. Dietz SM, Kuipers IM, Tacke CEA, Koole JCD, Hutten BA, Kuijpers TW. Giant aneurysms: a gender-specific complication of Kawasaki disease? J Cardiol. (2017) 70:359–65. doi: 10.1016/j.jjcc.2016.12.014
Keywords: Kawasaki, coronary artery aneurysms, rash, oral changes, cervical lymphadenopathy, female
Citation: Yu H, Sun W, Wu H, Zhang S, Xu Z, Wu R, Rong X, Qiu H, Zhu J, Zhang C and Chu M (2022) The Significance of Symptoms in Predicting Coronary Artery Aneurysms of Kawasaki Disease, Especially in Female Patients. Front. Cardiovasc. Med. 9:823862. doi: 10.3389/fcvm.2022.823862
Received: 28 November 2021; Accepted: 28 March 2022;
Published: 28 April 2022.
Edited by:
Emanuele Monda, University of Campania Luigi Vanvitelli, ItalyReviewed by:
Gönül Tanır, Dr. Sami Ulus Child Health and Diseases Training and Research Hospital, TurkeyMario Francesco Iasevoli, University of Salerno, Italy
Copyright © 2022 Yu, Sun, Wu, Zhang, Xu, Wu, Rong, Qiu, Zhu, Zhang and Chu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinshun Zhu, emh1amluc2h1bjAyQDE2My5jb20=; Chunxiang Zhang, emhhbmdjaHg5OTlAMTYzLmNvbQ==; Maoping Chu, Y2htcGluZ0Bob3RtYWlsLmNvbQ==
 Huan Yu
Huan Yu Weiyue Sun
Weiyue Sun Rongzhou Wu
Rongzhou Wu Xing Rong
Xing Rong Maoping Chu
Maoping Chu