- 1Department of Cardiology, The Affiliated Hospital of Jiaxing University, Jiaxing, China
- 2Department of General Practice, The Affiliated Hospital of Jiaxing University, Jiaxing, China
Background: A pocket hematoma is a well-recognized complication that occurs after pacemaker or defibrillator implantation. It is associated with increased pocket infection and hospital stay. Patients suffering from atrial fibrillation and undergoing cardiovascular electronic implantable device (CIED) surgery are widely prescribed and treated with direct oral anticoagulants (DOACs). In this study, the use of a novel compression device was evaluated to examine its ability to decrease the incidence of pocket hematomas following device implantation with uninterrupted DOACs.
Methods: A total of 204 participants who received DOACs and underwent CIED implantation were randomized into an experimental group (novel compression device) and a control group (elastic adhesive tape with a sandbag). The primary outcome was pocket hematoma, and the secondary outcomes were skin erosions and patient comfort score. Grade 3 hematoma was defined as a hematoma that required anticoagulation therapy interruption, re-operation, or prolonged hospital stay.
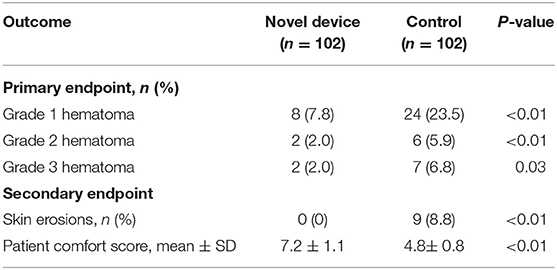
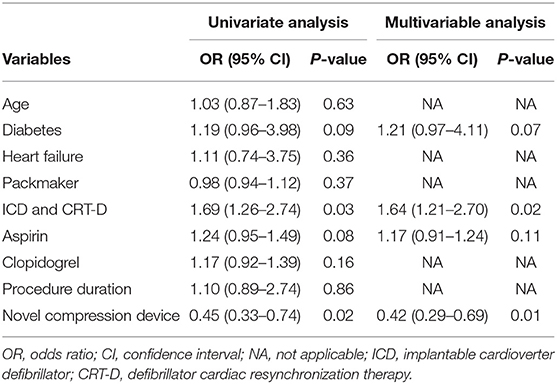
Results: The baseline characteristics of both groups had no significant differences. The incidence of grades 1 and 2 hematomas was significantly lower in the compression device group than in the conventional pressure dressing group (7.8 vs. 23.5 and 2.0 vs. 5.9%, respectively; P < 0.01). Grade 3 hematoma occurred in 2 of 102 patients in the experimental group and 7 of 102 patients in the control group (2.0 vs. 6.9%; P = 0.03). The incidence rates of skin erosion were significantly lower, and the patient comfort score was much higher in the compression device group than in the control group (P < 0.01). Multivariable logistic regression analysis showed that the use of novel compression device was a significant protective factor for pocket hematoma (OR = 0.42; 95% CI, 0.29–0.69, P = 0.01).
Conclusions: The incidence of pocket hematomas and skin erosions significantly decreases when the proposed compression device is used for patients undergoing device implantation with uninterrupted DOACs. Thus, the length of hospital stay and re-operation rate can be reduced, and patient comfort can be improved.
Clinical Trial Registration: http://www.chictr.org.cn, identifier: ChiCTR2100049430.
Introduction
Cardiovascular electronic implantable devices (CIEDs), such as implantable pacemakers, implantable cardioverter–defibrillators (ICDs), and cardiac resynchronization therapy defibrillators, have become the standard therapy utilized in the management of different cardiac conditions (e.g., bradyarrhythmias), primary and secondary preventions against sudden cardiac death, and amelioration of congestive heart failure (1–3). With a consistent increase in human life expectancy and advancement in medical technology, approximately 1.2–1.4 million CIEDs are implanted each year globally (4). However, CIED procedures may cause complications. A well-recognized complication associated with these procedures is pocket hematoma, which is reported in 2–9% of patients. Patients on antithrombotic therapy have an increased risk of having pocket hematoma (5–7).
Antithrombotic management in patients undergoing CIED implantation and requiring long-term oral anticoagulants or antiplatelet therapy is a growing strategic dilemma (8). The number of prescribed direct oral anticoagulants (DOACs) has grown substantially since the publication of the BRUISE CONTROL trial. DOACs are widely used to prevent stroke in patients with non-valvular atrial fibrillation (9, 10). However, approximately 25% of patients undergoing CIED implantation require long-term DOACs, leading to the increased incidence rates of perioperative bleeding (11). Furthermore, the temporary withdrawal of these anticoagulant drugs prior to device implantation poses a three-fold increased risk of ischemic stroke, myocardial infarction, and systemic thromboembolism, especially in moderate-to-high-risk patients (12, 13). The continuation of DOACs during the perioperative period also increases the risk of pocket hematoma (14, 15).
Traditionally, a pressure dressing fixed with elastic adhesive tape that is then covered with a sandbag has been used to prevent pocket hematoma after CIED implantation. However, the sandbag often migrates from its fixed position because of the unique location of the device pocket. It does not provide adequate pressure, resulting in the need for repeated fixation and exacerbating patient discomfort (16). Furthermore, adhesive tapes designed for pressure dressings may lead to skin erosions. No commercially available devices have been shown to be effective in patients with uninterrupted DOACs after CIED implantation. Therefore, we developed a novel compression device to reduce the incidence of postoperative pocket hematomas and improve patient comfort. In this randomized study, we aimed to assess the efficacy of the proposed compression device in preventing pocket hematomas compared with that of conventional techniques in patients on uninterrupted DOACs.
Methods
Study Population and Design
This single-center randomized controlled clinical trial was conducted in the heart center affiliated with Jiaxing University from July 2020 to May 2021. Approximately 1,000 devices were implanted each year in this center. All the patients admitted for the permanent CIED implantation of a pacemaker, ICD, or CRT-D were considered in this study. They were included if they were ≥18 years old, undergoing CIED implantation, and on long-term DOAC (dabigatran and rivaroxaban) therapy. The exclusion criteria were as follows: (1) without anticoagulation, (2) anticoagulation with warfarin, (3) history of any psychiatric illness, (4) coagulation disorder or severe anemia, and (5) refusal to participate. Patients with a body mass index of >35 kg/m2 were also excluded from this trial because the novel compression device was designed for individuals with normal body sizes. A total of 204 participants screened for eligibility were randomized at 1:1 into an experimental group and a control group by using a random number table and stratified by device type. Patients in both groups were instructed to continue taking aspirin, clopidogrel, or ticagrelor because of high-risk clinical features at their cardiologist's discretion.
This study was approved by the Human Ethics Research Committee of the Affiliated Hospital of Jiaxing University. All the participants were asked to sign their informed consent before enrollment. The trial was registered at the Chinese Clinical Trial Registry (ChiCTR; http://www.chictr.org.cn; ChiCTR2100049430).
Device Implantation and Postprocedure Management
All procedures were performed by a team of experienced cardiologists, who each had experience of at least 300 device implantations. After prophylactic antibiotics (first-generation cephalosporin or macrolides to patients with a penicillin allergy) and local anesthesia were administered, a pectoral incision was made. Venous access was obtained by puncturing the subclavian vein, and leads were implanted under fluoroscopic guidance in a cath lab. Active fixation was applied to all of the atrial leads and the majority of the ventricular leads. All devices were implanted without the use of electrocautery, and the wound was closed with absorbable suture.
The patients were advised to remain in a supine position for 8 h during the immediate postoperative period. Then, they were gradually moved from a 30° semireclining position to a sitting position. They were encouraged to get out of bed and walk around 24 h after their operation, but they were instructed to avoid extending their ipsilateral shoulder during physical activities. Standard device interrogation and chest radiograph were conducted at an appropriate time after CIED implantation. The pocket was assessed on postoperative days 1, 3, and 7.
Novel Compression Device and Conventional Pressure Dressing
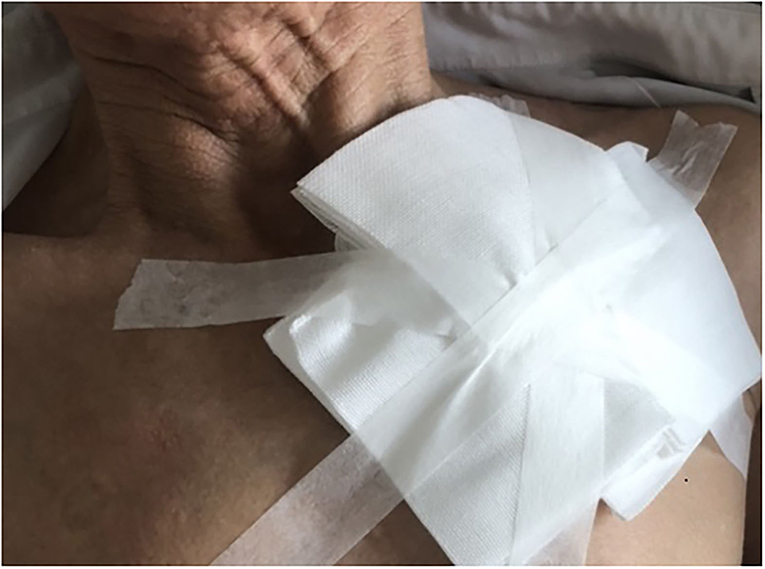
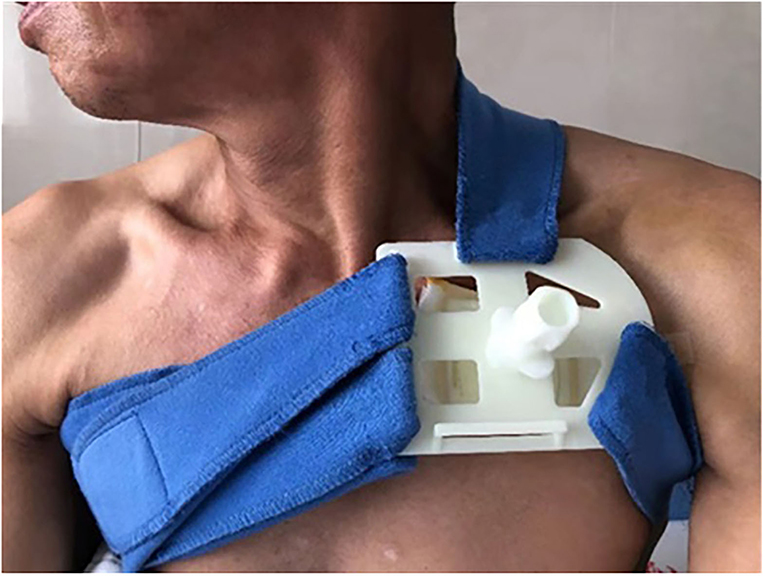
All the participants were asked to stay in bed for 24 h after CIED implantation. A conventional pressure dressing or the novel compression device was employed immediately, and vital signs were monitored. Conventional pressure dressing, which is commonly used, was applied to cover the surgical incision. The gauze was kept in place with adhesive tape, and the sandbag was adjusted repeatedly to maintain its location for adequate pressure and ensure the patient's comfort (Figure 1). Granted with a national patent (Chinese patent number: ZL 2014 1 0037276.7), the self-design novel pocket compression device was composed of a support plate, a shoulder band, a pressure-adjusting knob, and a pressure-adjusting screw. It was constructed from cotton fabric, medical plastic, and silica gel (Figures 2, 3). The pressure-adjusting knob was manually adjusted by medical staff based on previous experience and patient comfort. The novel compression device did not require adhesive tape, which can cause skin erosion. For the novel compression device group, the wound was locally pressurized for 8 h, and the screw was reversely adjusted by two to three turns every 2 h to reduce pressure. In the control group, a traditional sandbag was used after the procedure, placed directly above the dressing, and relaxed for 10 min every 2 h. The sandbags were removed after 8 h.
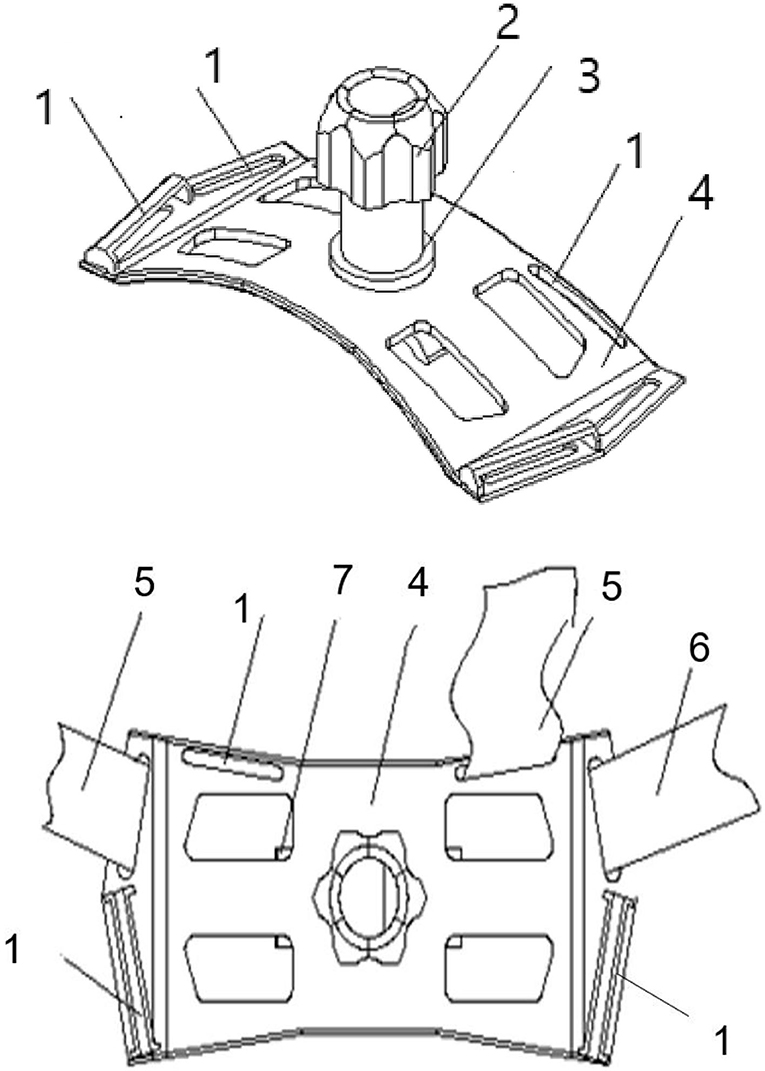
Figure 3. Structural diagram of the new compression device. 1, Shoulder strap fixing hole; 2, pressure-adjusting knob; 3, pressure-adjusting screw; 4, support plate; 5, shoulder band; 6, chest band; and 7, breather hole.
Outcome Measures
The primary outcomes were grade 1, 2, and 3 pocket hematomas between the groups after CIED procedures. The grading scale of hematoma types was as follows: grade 1 hematoma if a patient experienced ecchymosis or mild effusion around the surgical incision, no swelling, or pain in the device pocket; grade 2 hematoma if a patient suffered from large effusion in the pocket leading to swelling and causing functional impairment or pain to the device pocket; and grade 3 hematoma, also known as clinically significant hematoma, was defined according to the BRUISE CONTROL trial as any pocket hematoma requiring re-operation, resulting in the prolonged hospital stay (defined as extended hospitalization for >24 h primarily due to hematoma), and requiring interruption or reversal of DOACs (defined as intentional withholding, or an antidote indicated for the reversal of DOACs was used in response to pocket hematoma, resulting in subtherapeutic anticoagulation for >24 hours) [17. The decision for the interruption of anticoagulation therapy or requiring a second operation or prolonged hospital stay was made by two cardiologists who independently evaluated the wound without any information about group assignment.
The secondary outcomes were skin erosions and patient comfort scores. Skin erosions were defined as skin damage observed during the removal of the adhesive tape or dressing (17). The comfort level of the patients was assessed on a visual analog scale (VAS) during the application of a sandbag or compression. For comfort level assessment, the patients were required to describe how they felt on a 100 mm VAS ranging from “0” as “very uncomfortable” to “10” as “very comfortable” (18).
Statistical Analysis
The sample size calculation was based on our pilot study, a minimum sample of 200 participants were required to have 90 power to detect a 40% relative reduction in incidence of pocket hematoma in compression device group with an α of 0.05. Data were statistically analyzed with SPSS v.23 (SPSS Inc., Chicago, IL, USA). Continuous variables were expressed as means ± SD for continuous variables and percentages for qualitative variables. They were then compared using a T-test or a Mann–Whitney U test. Categorical variables were summarized as numbers and percentages and compared via chi-square or Fisher's exact test. Logistic regression model was applied to identify the predictors of pocket hematomas. Age, diabetes, heart failure, packmaker, ICD/CRT-D, aspirin, clopidogrel, procedure duration, and novel compression device were considered as covariates in the univariate analysis. For the multivariate models, clinical risk factors which were univariate predictors (P < 0.01) were regarded as covariates. Data with P < 0.05 were considered statistically significant.
Results
Comparison of Patient Characteristics
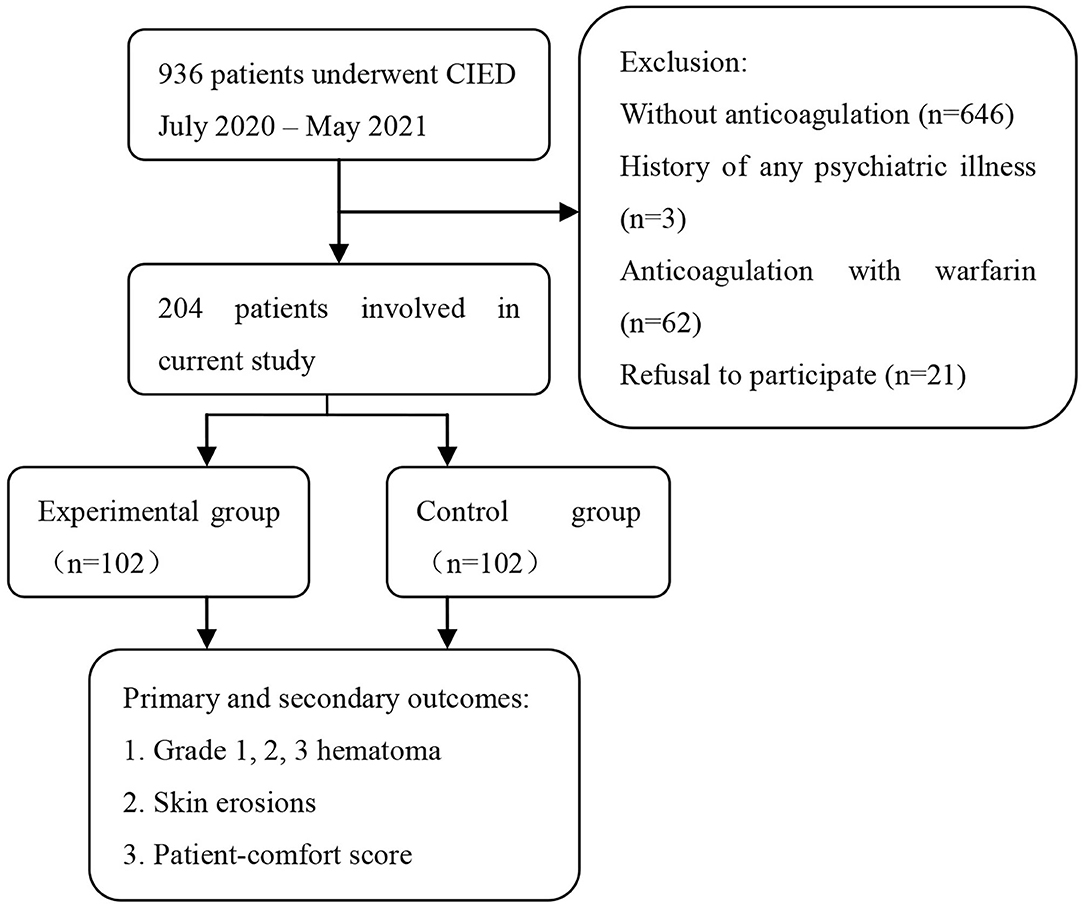
The demographics and clinical characteristics of the study population are displayed in Table 1. Of the 936 patients included in the study, 204 were recruited because they were on DOAC therapy at the time of device implantation. A total of 732 patients were excluded from the present study because of the following: 646 did not take any anticoagulant therapy, 62 received anticoagulation with warfarin, three had a history of psychiatric illness, and 21 refused to participate in the study (Figure 4).
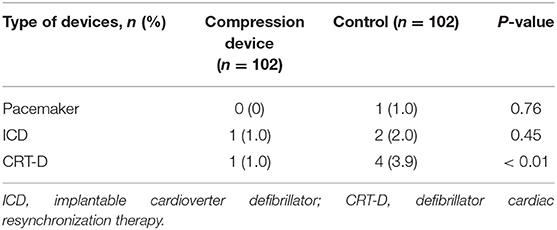
The baseline characteristics had no significant differences between the groups, although the patients in the novel compression device group (73.5 ± 7.4 years) were older than those in the control group (71.1 ± 7.2 years, P = 0.04). Of the patients on DOAC medication, 86/204 (42.2%) were on dabigatran, and 118/204 (57.8%) were on rivaroxaban. Notably, 70 (34.3%) were concomitant on antiplatelet therapy: aspirin (17.6%), clopidogrel (12.7%), and aspirin plus clopidogrel (3.9%). As expected, both groups had a prolonged prothrombin time (23.5 ± 1.3 and 23.8 ± 1.4) and activated partial thromboplastin time (40.9 ± 2.8 and 41.2 ± 2.9). All the patients underwent CIED procedures consisting of 117 permanent pacemaker implantations, 21 CRT and CRT-D, 15 ICD implantations and 51 generator exchange (Table 1).
Primary and Secondary Outcomes
The primary outcome of pocket hematomas occurred in 12/102 (11.8%) patients in the experimental group and 37/102 (36.3%) in the control group. Grade 3 hematoma was detected in nine cases, which required the interruption of anticoagulation therapy, re-operation or prolonged hospital stay, grade 2 hematoma was found in eight cases, and grade 1 hematoma was observed in the remaining cases (Table 2). The application of the novel pocket compression device resulted in a statistically significant reduction in the overall incidence of hematomas (11.8 vs. 36.3%, P < 0.01). Grades 1, 2, and 3 hematomas were observed in 8, 2, and 2 patients in the compression device group, respectively. In the control group, these hematomas were found in 24, 6, and 7 patients, respectively. The incidence of grades 1, 2, and 3 pocket hematomas significantly decreased in the experimental group compared with that in the control group (7.8 vs. 23.5% [P < 0.01], 2.0 vs. 5.9% [P < 0.01], 2.0 vs. 6.9% [P = 0.03], respectively). In experimental group, there was only 1 case of grade 3 hematoma in CRT-D and generator exchange procedures, respectively. In the control group, the incidence of grade 3 hematoma in pacemaker, generator exchange, CRT-D procedure were 1, 2, and 4, respectively. The incidence of grade 3 hematoma in CRT-D procedure was significantly higher in the compression device group than in the control group (1.0 vs. 3.9%, P < 0.01, Table 3). In multivariable analysis demonstrated no significant association between age, diabetes, heart failure, type of CIEDs implanted, antiplatelet agent, or procedure duration and pocket hematoma (Table 4). The use of novel compression device was a significant protective factor for pocket hematoma (OR = 0.42; 95% CI, 0.29–0.69, P = 0.01).
For secondary outcomes, none of the participants in the novel compression device group experienced skin erosion, but 9 of the participants in the control group had skin erosion (0 vs. 8.8%, P < 0.01). The patient comfort score in the experimental group was significantly higher than that in the control group (7.2 ± 1.1 vs. 4.8 ± 0.8, P < 0.01).
Discussion
Pocket hematoma is a major complication after CIED implantations, and it increases the risk of device-related infections (6, 19). In this prospective randomized trial, the efficacy of the proposed novel compression device was evaluated and compared with conventional techniques in patients undergoing electronic device implantations. We found that our self-design pocket compression device was associated with a significantly lower rate of pocket hematoma and skin erosions than that of traditional methods. It could also simultaneously improve patient comfort compared with traditional techniques involving elastic adhesive tape and sandbags. These results also suggested that continued DOAC strategy might be reasonable for patients during the perioperative period. Our results were consistent with the findings of some small cohort studies that continued DOACs during device surgery (20, 21).
Several studies have assessed the occurrence of pocket hematoma, from 0.9% to 28.7%. (16, 22–26). In BRUISE CONTROL trial, the clinically significant device-pocket hematoma occurred in 12 of 343 patients (3.5%) in the continued-warfarin group, as compared with 54 of 338 (16.0%) in the heparin bridging group (27). The frequency of grade 3 hematoma in this study (4.4%) was higher than the 3.5% reported in the BRUISE CONTROL trial despite compression devices and the exclusion of warfarin users. Different anticoagulants used in the trials, usage of electrocautery, and difference of investigator adjudication on pocket hematoma maybe the main interpretations to this discrepancy. The incidence of hematoma in Hu et al.'s study was 13.0 and 44.4% in experimental group and control group (16). In the present study, the incidence of hematoma was 24.0% upon discharge, while the incidence of grade 3 hematoma was only 4.4%. This discrepancy could be attributed to some factors, including differences in study design, baseline characteristics of patients, inconsistencies in the definition of pocket hematoma and non-use of electrocautery (9, 22). In our trial, electrocautery was not employed since the operators do not use electrocautery routinely and the hematoma was defined in accordance with the classification of De Sensi et al. who categorized pocket hematomas into three grades based on clinical phenomena and required interventions. All the clinical findings around the pocket and those leading to therapeutic interventions were recorded in our study, whereas a number of previous studies only included clinically significant hematomas equivalent to grade 3 hematoma in our trial (17, 22, 26).
Prospective studies have been conducted to explore the application of pocket compression after procedures to prevent hematomas (16, 22, 23, 28). For example, Awada et al. performed a prospective registry study and showed that the use of a marketed compression device approved in Germany can significantly reduce the incidence of pocket hematomas and subsequent infections compared with the control device in patients receiving anticoagulation or dual antiplatelet therapy and undergoing CIED implantation (23). Similarly, another study evaluated a postsurgical vest among 40 patients with the interruption of warfarin and antiplatelet agents during procedures and demonstrated a significant reduction in the occurrence of pocket hematomas (28). Notably, these studies included only the patients on therapeutic warfarin and excluded those receiving DOACs. Therefore, their results might not be applicable to patients on DOACs. Hu et al. conducted a randomized controlled trial involving 108 patients and revealed that a pocket compression device can significantly reduce the occurrence of hematomas and adverse skin reactions after device implantation (16). However, only a small proportion of patients continued antithrombotic medication in their trial. Furthermore, our novel compression tool has a great advantage in terms of clinical practical use since none of their compression devices have a pressure regulator function.
DOACs are easier to administer and obviate the need for regular international normalized ratio monitoring required for warfarin therapy. The number of patients treated with DOACs has increased significantly in clinical practice (29). Hence, there is also an increased risk of bleeding complications especially in patients on multiple antithrombotic agents such as coronary heart disease or receiving valve surgery (30). To our knowledge, this research was the first prospective cohort study with a relatively large sample to evaluate the use of a novel pocket compression device for decreasing the incidence of pocket hematomas following CIED implantation with uninterrupted DOACs. Some studies have indicated various hematoma predisposing procedural and patient factors, including aging, antiplatelet usage, heart failure, and device implantation type (26, 31). Our findings showed that antiplatelet drugs, device implantation type, and pocket hematoma incidence had no significant association, possibly because of the small sample size from one heart center.
The proposed novel pocket compression device has several advantages over the use of elastic adhesive tape and sandbags. First, the specially designed shoulder band and chest band can certainly fix the device over the pocket and sustain the pressure on it when patients change their body position. Second, the pressure-adjusting knob and screw can be manually adjusted to control the tightness of the compression device based on the physician's experience and the patient's comfort while applying the appropriate pressure to the pocket. So far, we did not find any potential complication and the novel compression device were applicable to the patients in present trial. Besides, a more sophisticated compression device with measurement of pressure value is needed in the future. At present, no pressure-adjustable compression device is commercially available. Third, the proposed device is made of cotton fabric and medical silica gel, so it unlikely causes skin erosion, which is especially considerable for the elderly who have sensitive and delicate skin.
Despite these advantages, several limitations should be considered. First, all the participants were recruited from a single center, so our findings might not be generalizable to other populations. Second, the endpoint events regarding the assessment of pocket hematomas and skin erosions were subjective because they relied on the observers' judgment. Nevertheless, these discrepancies were minimized by adopting the standard pocket hematoma definition, and all the events were adjudicated by two physicians (26). Furthermore, the control groups were significantly older than the experimental group, but this fact did not seem to influence the observed incidence of hematoma. Lastly, the incidence of pocket hematomas was analyzed at the time of hospital discharge only. As such, potential bias in the interpretation of the results might occur.
Conclusions
The incidence of pocket hematomas and skin erosions significantly decreases when the proposed pocket compression device is used for patients undergoing CIED implantation with uninterrupted DOACs. Thus, the length of hospital stay and re-operation rate can be decreased, and patient comfort can be improved. Further large multicenter studies should be performed to validate the effectiveness of the novel compression device in this population.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Human Ethics Research Committee of the Affiliated Hospital of Jiaxing University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
Y-PF: conceptualization, methodology, software, investigation, and writing—original draft. LW: data curation, formal analysis, and writing—original draft preparation. C-YZ: resourses, visualization, and investigation. J-CS: software. H-LH: formal analysis, validation, and resourses. C-LZ: project administration and writing—reviewing and editing. C-JH: conceptualization, methodology, writing—reviewing and editing, and supervision. All authors contributed to the article and approved the submitted version.
Funding
This research was funded by Jiaxing Science and Technology Program under Grant Nos. (2021AD30148, 2021AD30132, and 2020AY30006), Provincial-Municipal Joint Construction of Key Medical Disciplines in Zhejiang Province (2019-ss-xxgbx), Zhejiang Provincial Health Science and Technology Program under Grant Nos. (2022ZH011, 2021KY1105), Pioneer Innovation Team of Jiaxing Arteriosclerotic Diseases Research Institute (XFCX–DMYH), Jiaxing Institute of Arteriosclerotic Diseases (2020-dmzdsys), and Peak Discipline Established by the First Hospital of Jiaxing (GFXK-XXGNK).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors would like to express sincere gratitude to these participants and their families.
References
1. Hussein AA, Wilkoff BL. Cardiac implantable electronic device therapy in heart failure. Circ Res. (2019) 124:1584–97. doi: 10.1161/CIRCRESAHA.118.313571
2. Writing Group Members, Tracy CM, Epstein AE, Darbar D, DiMarco JP, Dunbar SB, et al. 2012 ACCF/AHA/HRS focused update of the 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American college of cardiology foundation/American heart association task force on practice guidelines. J Thorac Cardiovasc Surg. (2012) 144:e127–45. doi: 10.1016/j.jtcvs.2012.08.032
3. Al-Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis AB, et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American college of cardiology/American heart association task force on clinical practice guidelines and the heart rhythm society. J Am Coll Cardiol. (2018) 72:e91–e220. doi: 10.1016/j.jacc.2017.10.054
4. Calkins H, Hindricks G, Cappato R, Kim YH, Saad EB, Aguinaga L, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Europace. (2018) 20:e1–e160. doi: 10.1093/europace/eux274
5. Sridhar AR, Yarlagadda V, Yeruva MR, Kanmanthareddy A, Vallakati A, Dawn B, et al. Impact of haematoma after pacemaker and CRT device implantation on hospitalization costs, length of stay, and mortality: a population-based study. Europace. (2015) 17:1548–54. doi: 10.1093/europace/euv075
6. Notaristefano F, Angeli F, Verdecchia P, Zingarini G, Spighi L, Annunziata R, et al. Device-pocket hematoma after cardiac implantable electronic devices. Circ Arrhythm Electrophysiol. (2020) 13:e008372. doi: 10.1161/CIRCEP.120.008372
7. Essebag V, Healey JS, Joza J, Nery PB, Kalfon E, Leiria TLL, et al. Effect of direct oral anticoagulants, warfarin, and antiplatelet agents on risk of device pocket hematoma: combined analysis of bruise control 1 and 2. Circ Arrhythm Electrophysiol. (2019) 12:e007545. doi: 10.1161/CIRCEP.119.007545
8. Sticherling C, Marin F, Birnie D, Boriani G, Calkins H, Dan GA, et al. Antithrombotic management in patients undergoing electrophysiological procedures: a European heart rhythm association (EHRA) position document endorsed by the ESC working group thrombosis, heart rhythm society (HRS), and Asia pacific heart rhythm society (APHRS). Europace. (2015) 17:1197–214. doi: 10.1093/europace/euv190
9. Essebag V, Verma A, Healey JS, Krahn AD, Kalfon E, Coutu B, et al. Clinically significant pocket hematoma increases long-term risk of device infection: bruise control infection study. J Am Coll Cardiol. (2016) 67:1300–8. doi: 10.1016/j.jacc.2016.01.009
10. López-López JA, Sterne JAC, Thom HHZ, Higgins JPT, Hingorani AD, Okoli GN, et al. Oral anticoagulants for prevention of stroke in atrial fibrillation: systematic review, network meta-analysis, and cost effectiveness analysis. BMJ. (2017) 359:j5058. doi: 10.1136/bmj.j5058
11. Flaker GC, Theriot P, Binder LG, Dobesh PP, Cuker A, Doherty JU. Management of periprocedural anticoagulation: a survey of contemporary practice. J Am Coll Cardiol. (2016) 68:217–26. doi: 10.1016/j.jacc.2016.04.042
12. Douketis JD, Spyropoulos AC, Spencer FA, Mayr M, Jaffer AK, Eckman MH, et al. Perioperative management of antithrombotic therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American college of chest physicians evidence-based clinical practice guidelines. Chest. (2012) 141: e326S–350S. doi: 10.1378/chest.11-2298
13. Healey JS, Eikelboom J, Douketis J, Wallentin L, Oldgren J, Yang S, et al. Periprocedural bleeding and thromboembolic events with dabigatran compared with warfarin: results from the randomized evaluation of long-term anticoagulation therapy (RE-LY) randomized trial. Circ. (2012) 126:343–8. doi: 10.1161/CIRCULATIONAHA.111.090464
14. Creta A, Finlay M, Hunter RJ, Chow A, Sporton S, Muthumala A, et al. Non-vitamin K oral anticoagulants at the time of cardiac rhythm device surgery: a systematic review and meta-analysis. Thromb Res. (2020) 188:90–6. doi: 10.1016/j.thromres.2020.02.007
15. Heidbuchel H, Verhamme P, Alings M, Antz M, Diener HC, Hacke W, et al. Updated European heart rhythm association practical guide on the use of non-vitamin-K antagonist anticoagulants in patients with non-valvular atrial fibrillation: executive summary. Eur Heart J. (2017) 38:2137–49. doi: 10.1093/eurheartj/ehw058
16. Hu J, Zheng J, Liu X, Li G, Xiao X. Effect of a pocket compression device on hematomas, skin reactions, and comfort in patients receiving a cardiovascular implantable electronic device: a randomized controlled trial. J Interv Card Electrophysiol. (2021). doi: 10.1007/s10840-021-00973-5. [Epub ahead of print].
17. Kiuchi K, Okajima K, Tanaka N, Yamamoto Y, Sakai N, Kanda G, et al. Novel compression tool to prevent hematomas and skin erosions after device implantation. Circ J. (2015) 79:1727–32. doi: 10.1253/circj.CJ-15-0341
18. Sung YT, Wu JS. The visual analogue scale for rating, ranking and paired-comparison (VAS-RRP): a new technique for psychological measurement. Behav Res Methods. (2018) 50:1694–715. doi: 10.3758/s13428-018-1041-8
19. Song J, Tark A, Larson EL. The relationship between pocket hematoma and risk of wound infection among patients with a cardiovascular implantable electronic device: an integrative review. Heart Lung. (2020) 49:92–8. doi: 10.1016/j.hrtlng.2019.09.009
20. Kosiuk J, Koutalas E, Doering M, Sommer P, Rolf S, Breithardt OA, et al. Treatment with novel oral anticoagulants in a real-world cohort of patients undergoing cardiac rhythm device implantations. Europace. (2014) 16:1028–32. doi: 10.1093/europace/eut423
21. Jennings JM, Robichaux R, McElderry HT, Plumb VJ, Gunter A, Doppalapudi H, et al. Cardiovascular implantable electronic device implantation with uninterrupted dabigatran: comparison to uninterrupted warfarin. J Cardiovasc Electrophysiol. (2013) 24:1125–9. doi: 10.1111/jce.12214
22. De Sensi F, Miracapillo G, Cresti A, Severi S, Airaksinen KE. Pocket hematoma: a call for definition. Pacing Clin Electrophysiol. (2015) 38:909–13. doi: 10.1111/pace.12665
23. Wiegand UK, LeJeune D, Boguschewski F, Bonnemeier H, Eberhardt F, Schunkert H, et al. Pocket hematoma after pacemaker or implantable cardioverter defibrillator surgery: influence of patient morbidity, operation strategy, and perioperative antiplatelet/anticoagulation therapy. Chest. (2004) 126:1177–86. doi: 10.1378/chest.126.4.1177
24. Awada H, Geller JC, Brunelli M, Ohlow MA. Pocket related complications following cardiac electronic device implantation in patients receiving anticoagulation and/or dual antiplatelet therapy: prospective evaluation of different preventive strategies. J Interv Card Electrophysiol. (2019) 54:247–55. doi: 10.1007/s10840-018-0488-y
25. Ferretto S, Mattesi G, Migliore F, Susana A, De Lazzari M, Iliceto S, et al. Clinical predictors of pocket hematoma after cardiac device implantation and replacement. J Cardiovasc Med. (2020) 21:123–7. doi: 10.2459/JCM.0000000000000914
26. Black-Maier E, Lewis RK, Loungani R, Rehorn M, Friedman DJ, Bishawi M, et al. Cardiovascular implantable electronic device surgery following left ventricular assist device implantation. JACC Clin Electrophysiol. (2020) 6:1131–9. doi: 10.1016/j.jacep.2020.04.030
27. Birnie DH, Healey JS, Wells GA, Verma A, Tang AS, Krahn AD, et al. Pacemaker or defibrillator surgery without interruption of anticoagulation. N Engl J Med. (2013) 368:2084–93. doi: 10.1056/NEJMoa1302946
28. Turagam MK, Nagarajan DV, Bartus K, Makkar A, Swarup V. Use of a pocket compression device for the prevention and treatment of pocket hematoma after pacemaker and defibrillator implantation (STOP-HEMATOMA-I). J Interv Card Electrophysiol. (2017) 49:197–204. doi: 10.1007/s10840-017-0235-9
29. Steffel J, Verhamme P, Potpara TS, Albaladejo P, Antz M, Desteghe L, et al. The 2018 European heart rhythm association practical guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur Heart J. (2018) 39:1330–93. doi: 10.1093/eurheartj/ehy136
30. Chaudhary R, Sharma T, Garg J, Sukhi A, Bliden K, Tantry U, et al. Direct oral anticoagulants: a review on the current role and scope of reversal agents. J Thromb Thrombolysis. (2020) 49:271–86. doi: 10.1007/s11239-019-01954-2
31. Thal S, Moukabary T, Boyella R, Shanmugasundaram M, Pierce MK, Thai H, et al. The relationship between warfarin, aspirin, and clopidogrel continuation in the peri-procedural period and the incidence of hematoma formation after device implantation. Pacing Clin Electrophysiol. (2010) 33:385–8. doi: 10.1111/j.1540-8159.2009.02674.x
Keywords: cardiovascular electronic implantable device, pocket compression device, pocket hematoma, direct oral anticoagulants, skin erosion
Citation: Fei Y-P, Wang L, Zhu C-Y, Sun J-C, Hu H-L, Zhai C-L and He C-J (2022) Effect of a Novel Pocket Compression Device on Hematomas Following Cardiac Electronic Device Implantation in Patients Receiving Direct Oral Anticoagulants. Front. Cardiovasc. Med. 9:817453. doi: 10.3389/fcvm.2022.817453
Received: 18 November 2021; Accepted: 02 February 2022;
Published: 24 February 2022.
Edited by:
Per Morten Sandset, University of Oslo, NorwayReviewed by:
Mohit Turagam, Mount Sinai Hospital, United StatesMichael Nagler, Institute of Clinical Chemistry, Bern University Hospital, Switzerland
Copyright © 2022 Fei, Wang, Zhu, Sun, Hu, Zhai and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chao-Jie He, aGVjaGFvamllODI0QDE2My5jb20=
†These authors share first authorship
 Ye-Ping Fei1†
Ye-Ping Fei1† Jing-Chao Sun
Jing-Chao Sun Hui-Lin Hu
Hui-Lin Hu Chao-Jie He
Chao-Jie He