
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 23 March 2022
Sec. Cardiovascular Genetics and Systems Medicine
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.816847
Type 2 diabetes (T2D) is characterized by increased levels of blood glucose but is increasingly recognized as a heterogeneous disease, especially its multiple discrete cardiovascular phenotypes. Genetic variations play key roles in the heterogeneity of diabetic cardiovascular phenotypes. This study investigates possible associations of ATP-sensitive potassium channel (KATP) variants with cardiovascular phenotypes among the Chinese patients with T2D. Six hundred thirty-six patients with T2D and 634 non-diabetic individuals were analyzed in the study. Nine KATP variants were determined by MassARRAY. The KATP rs2285676 (AA + GA, OR = 1.43, 95% CI: 1.13–1.81, P = 0.003), rs1799858 (CC, OR = 1.42, 95% CI: 1.12–1.78, P = 0.004), and rs141294036 (CC, OR = 1.45, 95% CI: 1.15–1.83, P = 0.002) are associated with increased T2D risk. A follow-up of at least 45.8-months (median) indicates further association between the 3 variants and risks of diabetic-related cardiovascular conditions. The associations are categorized as follows: new-onset/recurrent acute coronary syndrome (ACS) (rs2285676/AA + GA, HR = 1.37, 95% CI: 1.10–1.70, P = 0.005; rs141294036/TT + CT, HR = 1.59, 95% CI: 1.28–1.99, P < 0.001), new-onset stroke (rs1799858/CC, HR = 2.58, 95% CI: 1.22–5.43, P = 0.013; rs141294036/CC, HR = 2.30, 95% CI: 1.16–4.55, P = 0.017), new-onset of heart failure (HF) (rs1799858/TT + CT, HR = 2.78, 95% CI: 2.07–3.74, P < 0.001; rs141294036/TT + CT, HR = 1.45, 95% CI: 1.07–1.96, P = 0.015), and new-onset atrial fibrillation (AF) (rs1799858/TT + CT, HR = 2.05, 95% CI: 1.25–3.37, P = 0.004; rs141294036/CC, HR = 2.31, 95% CI: 1.40–3.82, P = 0.001). In particular, the CC genotype of rs1799858 (OR = 2.38, 95% CI: 1.11–5.10, P = 0.025) and rs141294036 (OR = 1.95, 95% CI: 1.04–3.66, P = 0.037) are only associated with the risk of ischemic stroke while its counterpart genotype (TT + CT) is associated with the risks of HF with preserved ejection fraction (HFpEF) (rs1799858, OR = 3.46, 95% CI: 2.31–5.18, P < 0.001) and HF with mildly reduced ejection fraction (HFmrEF) (rs141294036, OR = 2.74, 95% CI: 1.05–7.15, P = 0.039). Furthermore, the 3 variants are associated with increased risks of abnormal serum levels of triglyceride (TIRG) (≥ 1.70 mmol/L), low-density lipoprotein cholesterol (LDL-C) (≥ 1.40 mmol/L), apolipoprotein B (ApoB) (≥ 80 mg/dL), apolipoprotein A-I (ApoA-I) level (< 120 mg/dL), lipoprotein(a) Lp(a) (≥ 300 mg/dL) and high-sensitivity C-reactive protein (HsCRP) (≥ 3.0 mg/L) but exhibited heterogeneity (all P < 0.05). The KATP rs2285676, rs1799858, and rs141294036 are associated with increased risks of T2D and its related cardiovascular phenotypes (ACS, stroke, HF, and AF), but show heterogeneity. The 3 KATP variants may be promising markers for diabetic cardiovascular events favoring “genotype-phenotype” oriented prevention and treatment strategies.
Type 2 diabetes (T2D) is characterized by increased levels of blood glucose owing to the combination of insulin resistance (IR), impaired insulin secretion, or (and) glucagon excess (1). The T2D is a serious public health hazard worldwide, especially in the present day China. Cardiovascular diseases (CVDs), including arteriosclerosis cardiovascular disease (ASCVD), heart failure (HF), and atrial fibrillation (AF), are the prominent causes of death and disability in patients with T2D. T2D and its related CVDs result in a complex phenotype profile that is caused by many individual genetic events and environmental factors (2). Besides the environmental factors, evidences indicate that genetic variants may play a pivotal role in the initiation and progression of diabetic cardiovascular phenotypes. The complex genetic background poses more challenges in the prevention and treatment of T2D and its related CVDs. Therefore, early assessments of populations at greater genetic predisposition for different cardiovascular phenotypes are important and necessary for early and comprehensive management of T2D and its associated comorbidities.
Adenosine Triphosphate-sensitive potassium channels (KATP) are important mediators as they couple high-fidelity metabolic sensors (e.g., glucose homeostasis) to end effectors for cardiovascular protection (e.g., ASCVD, HF, and AF) (3). Their key function makes them a novel promising target to improve diabetes and its cardiovascular complications. The KATP is a protein complex with four Kir6.x subunits (inwardly-rectifying potassium channel, Kir6.1 and Kir6.2) and four SURx subunits (sulfonylurea receptor, SUR1 and SUR2). Different combinations of the two subunits and different isoforms produce channels with diverse physiological and pathological properties, exhibiting a high degree of genetic polymorphism.
As vital high-fidelity metabolic sensors, KATP single nucleotide polymorphisms (SNPs) are relevant to T2D risk in the global population (4, 5), but the relationship presents characteristics with genetic heterogeneity in different regions and races of the world. In Caucasian populations, the KATP variants are associated with increased T2D risk in the Europeans (e.g., rs5219) (4), the French (e.g., rs1799859) (6), the Turks (e.g., rs1799854 and rs1799859) (7), and the Iranians (e.g., rs757110) (8). In contrast, the KATP rs757110 is associated with decreased T2D risk in the British (9). However, some KATP variants are not associated with T2D risk, such as rs1799845 and rs1801261 in the British (10), rs5219 in the Arabs (11), rs5219 and rs757110 in the Siberians (5). In non-Caucasian populations, the KATP variants are associated with increased T2D risk in the Japanese (e.g., rs5219 and rs757110) (12), the Mongolians (e.g., rs1799858 and rs2074308) (13), the Indians (e.g., chr11:17417205C > T and rs5210) (14) and the Nigerians (e.g., rs1799854) (15) rather than the Punjab population of India (e.g., rs1799854 and rs1801261) (16) and the Sub-Saharan Africans (e.g., rs5219 in the Ghanaians and the Nigerians) (17). Previous meta-analysis shows that KATP rs5219 is a common variant for T2D risk in the world population, and has similar effect on the susceptibility risk to T2D in the Europeans and the East Asian (e.g., the Japanese and the Koreans), but the loci are not associated with T2D risk in the Chinese Han population (4).
As an important end effector of cardiac protection, only few studies report the relationships between KATP mutations and cardio-cerebrovascular disease risk. The KATP variants are associated with T2D-related stroke in the Poles (e.g., rs1799854) (18), coronary atherosclerotic heart disease (CAD) in the British (e.g., rs757110 and rs61688134) (9, 19), HF in the Ukrainians (e.g., rs5210, rs5219 and rs757110) (20) and the Americans (e.g., rs5219) (21), and AF in the Americans (e.g., rs72554071) (22). Importantly, these cardiovascular risks may be higher under the crosstalk between KATP mutation (e.g., rs757110) and traditional risk factors (e.g., smoking) (23).
These findings suggest an ethnic-specific/geographical genetic pleiotropy of KATP variants manifested as different clinical phenotypes of diabetes. However, the genotype-phenotype correlation of KATP mutations on T2D and its related CVDs in the Chinese population are rarely reported. This study characterizes the genotype-phenotype correlation between KATP variants and T2D/associated CVDs in China.
A total of 1,270 participants from South China enrolled in this study, starting on January 1, 2013 and ending on December 31, 2020, were divided into two groups: non-T2D group (N = 634) and T2D group (N = 636). The inclusion criteria for the case group were as follows: (1) individuals aged 18 years or older who were newly diagnosed with T2D referring to the 2012 clinical practice guidelines and its update of the American Diabetic Association (24, 25); (2) patients with T2D who had not received therapy; and (3) patients who signed the consent form. Inclusion criteria for the control group were as follows: (1) individuals aged 18 years or older, (2) in healthy conditions based on anamnesis and physical examination results, (3) no evidence of T2D or family history of T2D, and (4) patients who signed the consent form. The non-T2D participants, got a health examination at the same hospital during the same period, and were enrolled as the control group. Subjects were excluded from enrollment in the study upon presenting (1) a history of hypertension (HTN), CAD, HF, and stroke. However, the history of lacunar cerebral infarcts (LCIs) or (and) atrial fibrillation (AF) at enrollment, the new onset of HTN, CAD, HF, and stroke during the follow-up will be not considered as exclusion criteria. The subjects who had a history of LCI or/and AF but no history of HTN, CAD, HF, and stroke at the time of enrollment were not considered as exclusion criteria. Stroke and LCI were evaluated by magnetic resonance imaging (MRI) or (and) computed tomography (CT). Stroke was evaluated at the time of enrollment by MRI or (and) CT; (2) high levels of aspartate aminotransferase (AST) and alanine transaminase (ALT) (more than 3 times the upper limit of the normal); (3) low estimated glomerular filtration rate (less than 90 ml/min•1.73 m2); (4) or (and) any other factors that contribute to serum lipid abnormalities, such as medical disorders (thyroid disease, Cushing’s syndrome, and pheochromocytoma) and drugs [thiazide diuretics, beta-receptor blockers (BBs), steroid, estrogen, progesterone, immunosuppressants, or retinoids]. Dyslipidemia is defined as an increase in triglyceride levels (TIRG) ≥ 1.7 mmol/L, total cholesterol (TC) ≥ 4.0 mmol/L, low-density lipoprotein cholesterol (LDL-C) ≥ 1.4 mmol/L, high-density lipoprotein cholesterol (HDL-C) < 1.0 mmol/L, apolipoprotein B (ApoB) ≥ 80 mg/dL, apolipoprotein A-I (ApoA-I) < 120 mg/dL or lipoprotein (a) [Lp(a) ≥ 300 mg/dL], and any their combinations. All patients with dyslipidemia were newly diagnosed at the time of enrollment in this study. Dyslipidemia diagnosis is in accordance with the recommendations on lipid disorders management of ESC/EAS (26). Echocardiography is evaluated using the standard sections at enrollment according to the recommendations of the American Society of Echocardiography (26). Blood biochemistry analyses were carried out at the time of enrollment according to standard analytical methods.
In this study, the primary endpoints are defined as the risks of new-onset/recurrent acute coronary syndrome (ACS), new-onset of stroke, new-onset of HF, and new-onset of AF during the follow-up. The secondary endpoints are defined as the risks of LCI at enrollment, AF at enrollment, and the total AF risk at the end of the follow-up period. These endpoint events were identified from three information sources, including the Center for Disease Control and Prevention, medical records, and participants and their families. Subjects were enrolled in the study on their first day of T2D diagnosis. December 31, 2020 was the final follow-up date. During the follow-up, events were diagnosed with reference to the following guidelines: (1) new-onset/recurrent ACS is defined as ST-elevation of myocardial infarction (MI), non-ST-elevation of MI or unstable angina, confirmed by cardiac biomarkers and coronary angiography. (2) LCIs are small infarcts (2–20 mm in diameter) caused by the occlusion of small penetrating end-arteries. New-onset of stroke is defined as the presence of a focal/global cerebral event including hemorrhagic stroke (HS), ischemic stroke (IS), and cardiogenic stroke (CS). Both LCI and strokes were evaluated by MRI or (and) CT at enrollment. (3) New-onset of HF is defined as the first occurrence of a HF admission (including at least one overnight hospitalization to emergency) (27), including HF with reduced ejection fraction [HFrEF, left ventricular ejection fraction (LVEF) ≤ 40%], HF with mildly reduced ejection fraction (HFmrEF, LVEF = 41–49%), and HF with preserved ejection fraction (HFpEF, LVEF ≥ 50%). (4) The AF is assessed according to prior medical records or electrocardiogram records from the enrollment to follow-up, including AF at enrollment and new-onset of AF. The new-onset of AF is defined as the newly detected AF in participants with no previous diagnosis of this condition before enrollment.
Nine KATP SNPs (rs2285676, rs11046182, rs1799858, rs4148671, rs78148713, rs145456027, rs147265929, rs61928479, and rs141294036) were identified using the MassARRAY (Sequenom) system as previously described (26). Specific primers for the 9 KATP SNPs are shown in Supplementary Table 1. The 9 KATP SNPs have 100% accurate genotypes for further analyses.
All analyses were performed using PASS 15.0 (Statistical Solution Ltd, Cork, Ireland) and SPSS 20.0 (SPSS, Chicago, IL). LDlink (LDmatrix) online tool1 was used to calculate the linkage disequilibrium (LD) for the 9 KATP variants in the Chinese populations as shown in Supplementary Figure 1. The Hardy-Weinberg equilibrium was investigated for both non-diabetic and diabetic subjects as shown in Supplementary Table 2. Data of categorical variables are presented as numbers (percentages) while continuous variables are presented as mean ± standard deviation. The difference among the subjects without or with T2D was analyzed by χ2-test for the categorical variables, or by independent sample t-test for the continuous variables. The dominant models for the minor allele of the 9 KATP SNPs were tested to obtain odds ratios (ORs)/hazard ratios (HRs), 95% confidence intervals (CIs), and P-values. Associations of the 9 KATP SNPs with T2D were assessed using binary logistic regression model as well as LCI, different types of dyslipidemia, and low-grade inflammation levels [high-sensitivity C-reactive protein (HsCRP) ≥ 3.0 mg/L]. Associations of the 9 KATP SNPs with new-onset/recurrent ACS were assessed using Cox proportional hazards regression model; the new-onset of stroke, HF, and AF were assessed similar to ACS. In particular, the relationships between KATP variants and different stroke subtypes (HS, IS and CS) were assessed using multinomial logistic regression model; the different HF subtypes (HFpEF, HFmrEF, and HFrEF) were assessed similarly. Bonferroni correction was conducted to protect from Type I error. Further, the heritability (explained variance,%) of KATP variants for events was calculated according to the method reported by Yang et al. (28). All probabilities are two-tailed. A P-value < 0.05 is considered statistically significant.
Among all the study subjects (N = 1,270), 4.1% were presented with a history of LCI (52 patients), and 3.5% with a history of AF (44 patients) at the time of enrollment, as shown in Table 1. Subjects with or without T2D at enrollment indicate obvious differences in LCI incidence rates (P = 0.024), levels of systolic blood pressure (SBP, P < 0.001) and diastolic blood pressure (DBP, P < 0.001), white blood cell count (WBC, P < 0.001), serum levels of TRIG (P < 0.001), HDL-C (P < 0.001), ApoA-I (P = 0.018), uric acid (UA, P < 0.001), HsCRP (P = 0.030), left atrial end-diastolic dimension (LAD, P = 0.012), LVEF (P = 0.028), left ventricular mass index (LVMI, P = 0.001) and E/e’ ratio (P = 0.003). During the follow-up period, none of the study subjects in the control group progressed to T2D, none dropped out of the study, and the subjects suffered from cardiovascular conditions are as follows: new-onset/recurrent ACS (416 patients, 32.8%), new-onset of stroke (58 patients, 4.7%), new-onset of HF (282 patients, 22.2%) and new-onset of AF (94 patients, 7.4%), as shown in Supplementary Table 3. Subjects with or without T2D present obvious differences for new-onset of HF (P = 0.005) and HF subtypes (P = 0.013); the differences remain significant upon factoring the medications in the analyses, such as mineralocorticoid receptor antagonist (MRA, P = 0.047), BBs (P = 0.015), and renin-angiotensin system inhibitors (RSIs, P < 0.001) at the end of follow-up.
As shown in Figure 1 and Supplementary Table 4, KATP SNPs rs2285676 (AA + GA, adjusted OR = 1.43, 95% CI: 1.13–1.81, P = 0.003), rs1799858 (CC, adjusted OR = 1.42, 95% CI: 1.12–1.78, P = 0.004), rs145456027 (TT, adjusted OR = 2.94, 95% CI: 1.39–6.22, P = 0.005), rs61928479 (AA, adjusted OR = 1.71 95% CI: 1.18–2.47, P = 0.005), and rs141294036 (CC, adjusted OR = 1.45, 95% CI: 1.15–1.83, P = 0.002) significantly correlate with moderate to high risk T2D except for rs11046182, rs4148671, rs78148713, and rs147265929 (all adjusted P > 0.05).
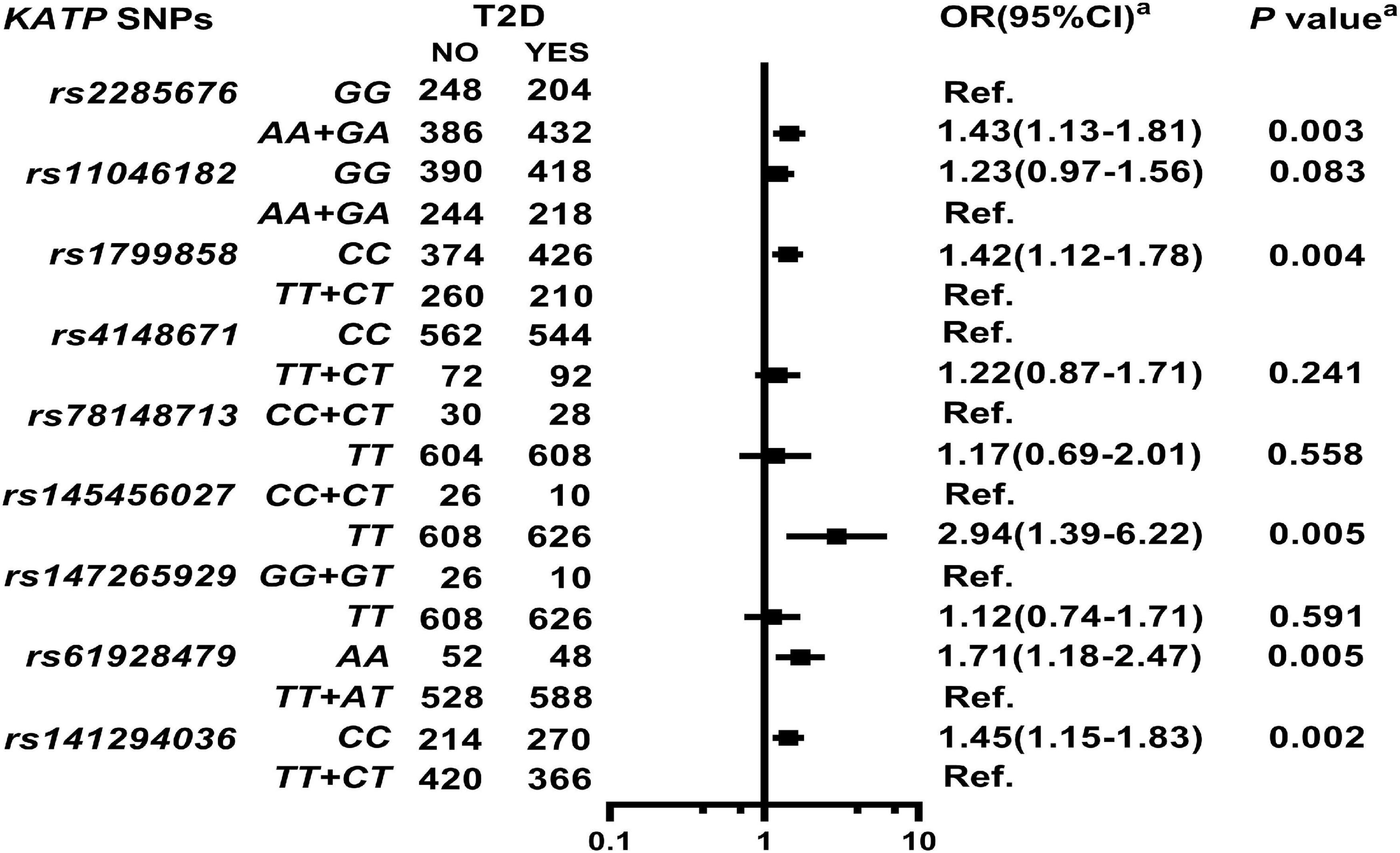
Figure 1. Association of ATP-sensitive potassium channels (KATP) single nucleotide polymorphisms (SNPs) with Type 2 diabetes (T2D) in the study participants. aModel: After adjustment for gender, age, smoking, alcohol consumption, body mass index (BMI), systolic blood pressure (SBP), diastolic blood pressure (DBP), high-sensitivity C-reactive protein (HsCRP), and renin-angiotensin-aldosterone system (RAAS activity) [angiotensin converting enzyme (ACE), renin, angiotensin I (Ang I), angiotensin II (Ang II), and aldosterone (ALD)].
As shown in Figure 2, Supplementary Table 5, and Supplementary Figure 2, KATP rs2285676 (AA + GA, adjusted HR = 1.37, 95% CI: 1.10–1.70, P = 0.005) and rs141294036 (TT + CT, adjusted HR = 1.59, 95% CI: 1.28–1.99, P < 0.001) are associated with a moderate risk of new-onset/recurrent ACS after a median follow-up of 45.8-months (IQR: 27.4–55.3 months).
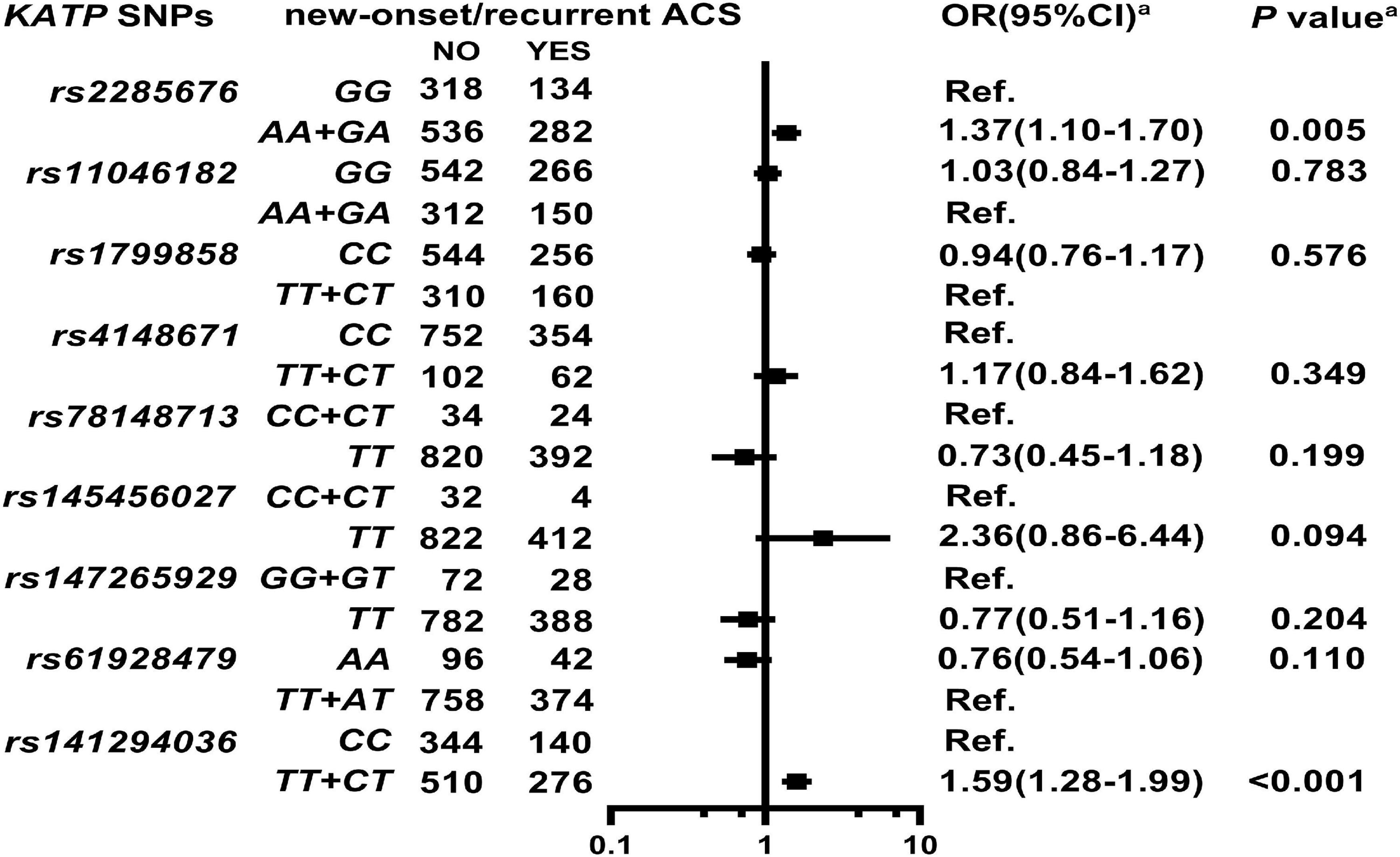
Figure 2. Association of KATP SNPs with new onset/recurrent ACS in the study participants. aModel: After adjustment for gender, age, smoking, alcohol consumption, BMI, white blood cell count (WBC), blood glucose levels [fasting blood sugar (FBS), postprandial blood glucose 2 h (P2hBS) and glycosylated hemoglobin (HbA1C), liver function [alanine aminotransferase (ALT), aspartate aminotransferase (AST), and albumin (Alb)], renal function [serum creatinine (Scr), blood urea nitrogen (BUN) and uric acid (UA)], serum sodium and potassium levels, HsCRP, HbA1C, RAAS activity (ACE, renin, Ang I, Ang II, and ALD), dyslipidemia {triglyceride (TRIG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), apolipoproteinB (ApoB), high-density lipoprotein cholesterol (HDL-C), apolipoproteinA-I (ApoA-I), and lipoprotein(a) [Lp(a)]}, medical condition [type 2 diabetes (T2D), EH, heart failure (HF) and atrial fibrillation (AF)], NYHA functional classification, and echocardiography index [right ventricular end-diastolic diameter (RVD), right atrial end-diastolic dimension (RAD), left ventricular end-diastolic diameter (LVD), left atrial end-diastolic dimension (LAD), left ventricular mass index (LVMI), and left ventricular ejection fraction (LVEF)], and combined medication [antiplatelet drugs, warfarin, statins, renin-angiotensin system inhibitors (RSIs), beta-receptor blockers (BBs), mineralocorticoid receptor antagonist (MRA), calcium-channel blockers (CCBs), diuretics, digoxin, nitrates, and hypoglycemic agents].
As shown in Figure 3A, Supplementary Table 6, and Supplementary Figure 3, KATP rs1799858 (CC, adjusted HR = 2.58, 95% CI: 1.22–5.43, P = 0.013) and rs141294036 (CC, adjusted HR = 2.30, 95% CI: 1.16–4.55, P = 0.017) correlate with increased risk of new-onset stroke based on the median follow-up period of 50.8-months (IQR: 44.0–58.7 months). As shown in Figure 3B and Supplementary Table 7, further analyses of stroke subtype indicate that rs1799858 (CC, adjusted OR = 2.38, 95% CI: 1.11–5.10, P = 0.025) and rs141294036 (CC, adjusted OR = 1.95, 95% CI: 1.04–3.66, P = 0.037) are associated only with IS.
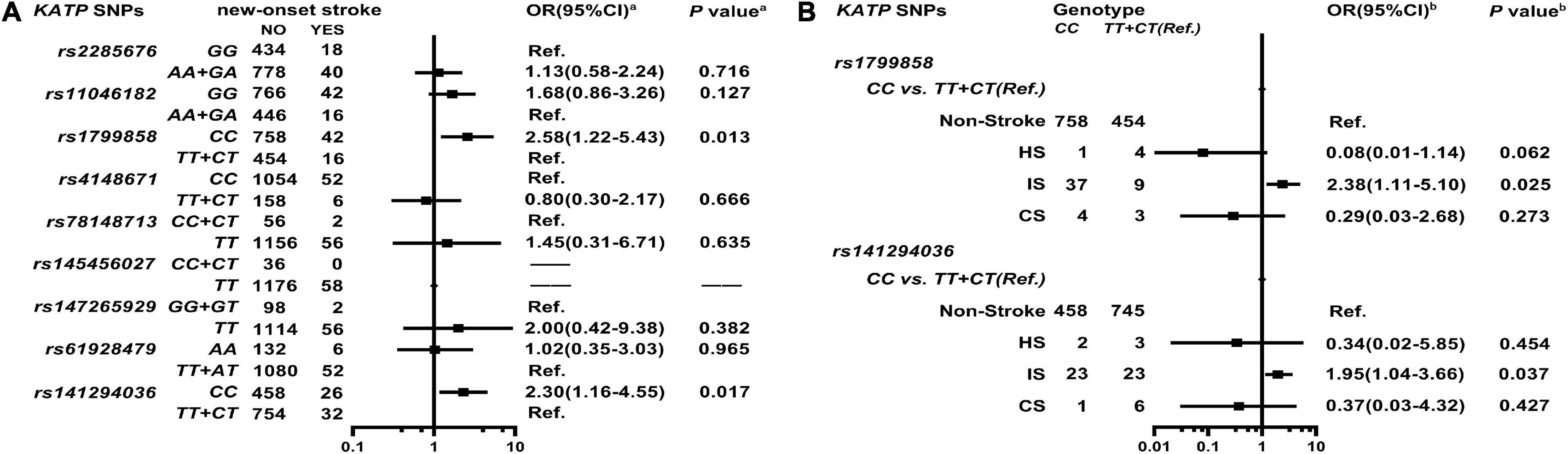
Figure 3. Association of KATP SNPs with the new-onset of stroke in the study participants. aModel (A) After adjustment for gender, age, smoking, alcohol consumption, BMI, WBC, blood glucose levels (FBS, P2hBS and HbA1C), liver function (ALT, AST and Alb), renal function (Scr, BUN and UA), serum sodium and potassium levels, HsCRP, RAAS activity (ACE, renin, Ang I, Ang II, and ALD), dyslipidemia [TRIG, TC, LDL-C, ApoB, HDL-C, ApoA-I and Lp(a)], medical condition [T2D, EH, CAD(ACS), AF, and LCI], NYHA functional classification, and echocardiography index (RVD, RAD, LVD, LAD, and LVEF), and combined medication (antiplatelet drugs, warfarin, statins, RSIs, BBs, MRA, CCBs, diuretics, digoxin, nitrates, and hypoglycemic agents). bModel (B) It is the same as Model (A).
As shown in Figure 4A, Supplementary Table 8, and Supplementary Figure 4, KATP rs1799858 (TT + CT, adjusted HR = 2.78, 95% CI: 2.07–3.74, P < 0.001) and rs141294036 (TT + CT, adjusted HR = 1.45, 95% CI: 1.07–1.96, P = 0.015) are associated with high HF risk based on a median follow-up of 48.1-months (IQR: 39.1–56.8 months). As shown in Figure 4B and Supplementary Table 9, further analyses of HF subtypes show that rs1799858 is associated only with high HFpEF risk (TT + CT, adjusted OR = 3.46, 95% CI: 2.31–5.18, P < 0.001) while rs141294036 is associated with high HFmrEF risk (TT + CT, adjusted OR = 2.74, 95% CI: 1.05–7.15, P = 0.039).
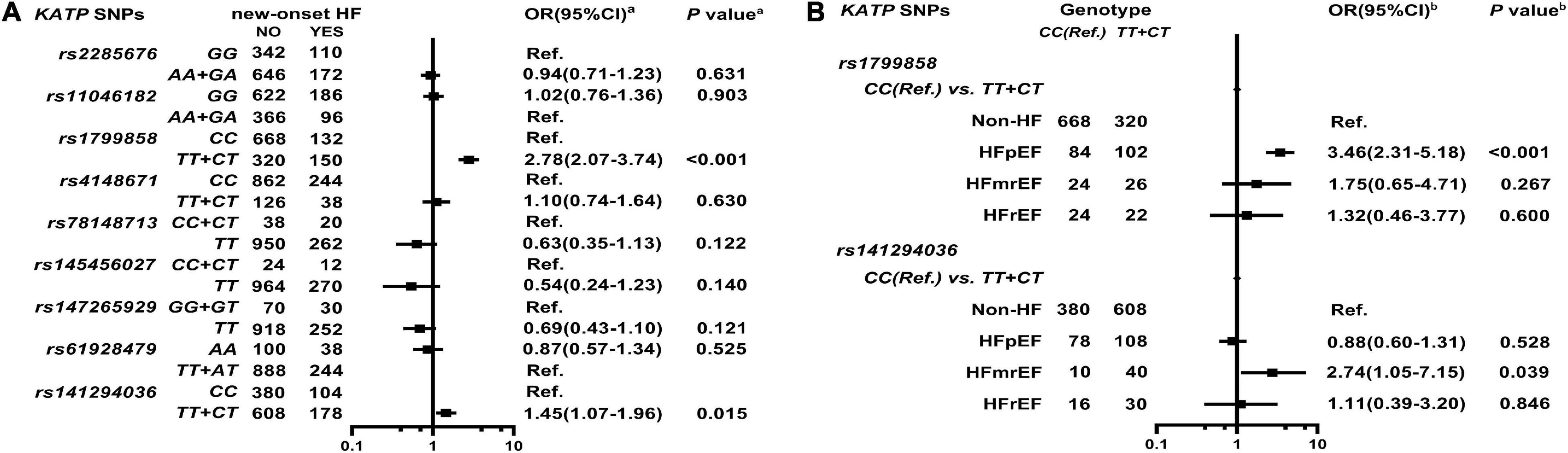
Figure 4. Association of KATP SNPs with incidence of HF in the study participants. aModel (A) After adjustment for gender, age, smoking, alcohol consumption, BMI, WBC, blood glucose levels (FBS, P2hBS, and HbA1C), liver function (ALT, AST, and Alb), renal function (Scr, BUN, and UA), serum sodium and potassium levels, HsCRP, RAAS activity (ACE, renin, Ang I, Ang II, and ALD), dyslipidemia [TRIG, TC, LDL-C, ApoB, HDL-C, ApoA-I, and Lp(a)], medical condition [T2D, HTN, CAD(ACS), and AF], echocardiography index (RVD, RAD, LVD, LAD, and LVEF), and combined medication (antiplatelet drugs, warfarin, statins, RSIs, BBs, MRA, CCBs, diuretics, digoxin, nitrates, and hypoglycemic agents). bModel (B) It is the same as Model (A).
As shown in Supplementary Table 10, two KATP variants are associated with high AF risk at enrollment as follows: rs1799858 (TT + CT, adjusted OR = 2.15, 95% CI: 1.06–4.39, P = 0.035) and rs141294036 (CC, adjusted OR = 2.42, 95% CI: 1.15–5.07, P = 0.020). As shown in Figure 5, Supplementary Table 11, and Supplementary Figure 5, rs1799858 (TT + CT, adjusted HR = 2.05, 95% CI: 1.25–3.37, P = 0.004) and rs141294036 (CC, adjusted HR = 2.31, 95% CI: 1.40–3.82, P = 0.001) are associated with high risk of new-onset of AF based on a median follow-up of 50.1-months (IQR: 42.7–58.4 months).
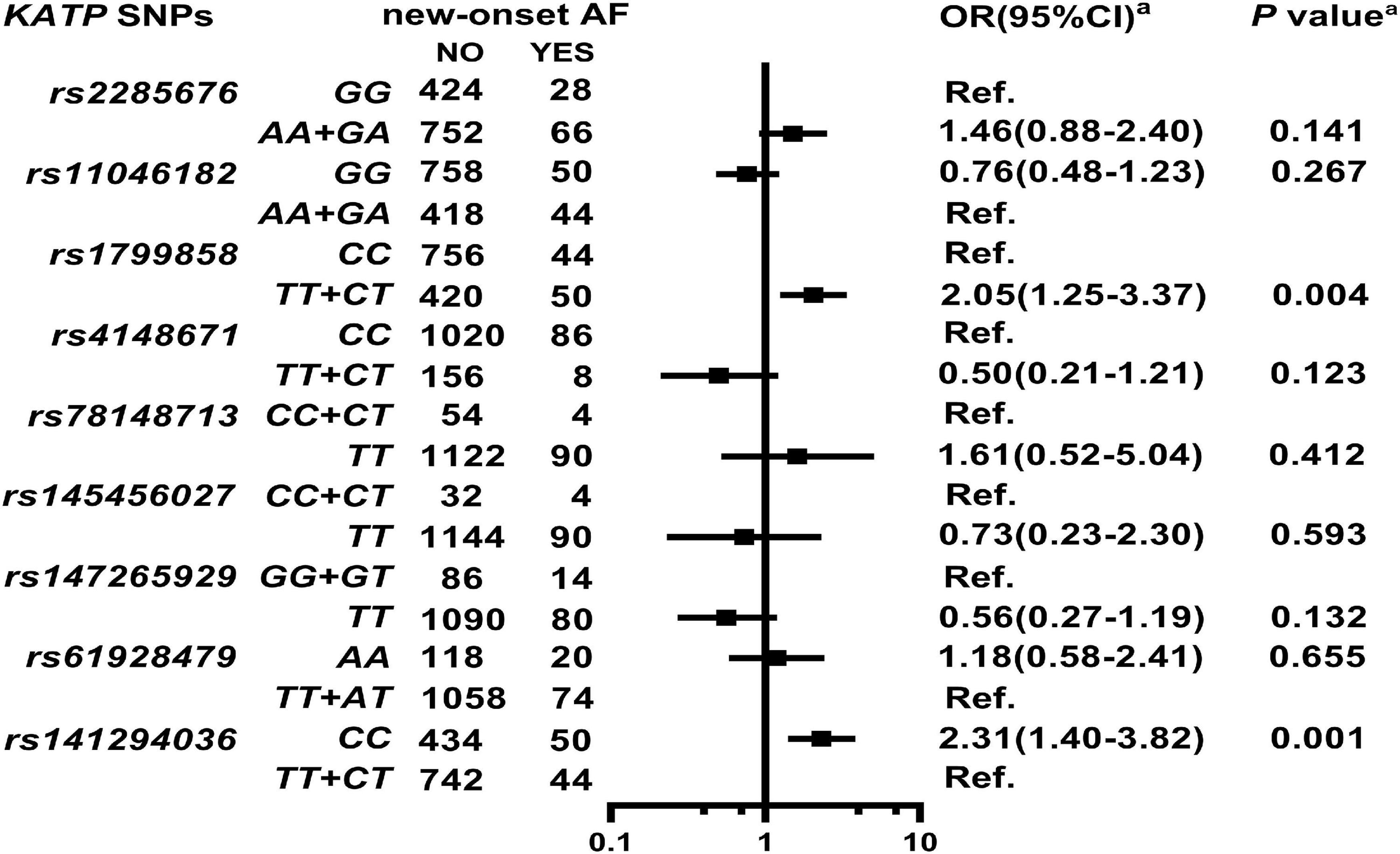
Figure 5. Association of KATP SNPs with the new-onset of AF in the study participants. aModel: After adjustment for gender, age, smoking, alcohol consumption, BMI, WBC, blood glucose levels (FBS, P2hBS and HbA1C), liver function (ALT, AST and Alb), renal function (Scr, BUN, and UA), serum sodium and potassium levels, HsCRP, RAAS activity (ACE, renin, Ang I, Ang II and ALD), dyslipidemia [TRIG, TC, LDL-C, ApoB, HDL-C, ApoA-I and Lp(a)], medical condition [T2D, HTN, CAD(ACS), and HF], echocardiography index (RVD, RAD, LVD, LAD, and LVEF), and combined medication (antiplatelet drugs, warfarin, statins, RSIs, BBs, MRA, CCBs, diuretics, digoxin, nitrates, and hypoglycemic agents).
As shown in Figures 6A–E and Supplementary Tables 14–18, KATP rs2285676 (AA + GA genotype) is associated with the elevated risk of higher TRIG serum levels (adjusted OR = 1.37, 95% CI: 1.02–1.84, P = 0.035), LDL-C (adjusted OR = 1.82, 95% CI: 1.13–2.92, P = 0.014), ApoB (adjusted OR = 1.34, 95% CI: 1.03–1.74, P = 0.031), and Lp(a) (adjusted OR = 1.48, 95% CI: 1.13–1.95, P = 0.004). The rs1799858 (TT + CT) is associated with a higher risk of elevated LDL-C serum levels (adjusted OR = 2.26, 95% CI: 1.39–3.69, P = 0.001) and lower ApoA-I serum levels (adjusted OR = 1.57, 95% CI: 1.13–2.19, P = 0.008) while CC genotype is associated with higher Lp(a) serum levels (adjusted OR = 1.52, 95% CI: 1.15–2.01, P = 0.003). Similarly, rs141294036 (TT + CT) is associated with the elevated risk of lower serum ApoA-I levels (adjusted OR = 1.72, 95% CI: 1.28–2.30, P < 0.001) while CC genotype is associated with higher Lp(a) serum levels (adjusted OR = 1.56, 95% CI: 1.19–2.03, P = 0.001). KATP rs4148671 (TT + CT) only correlates with increased risk of higher serum TRIG levels (adjusted OR = 1.62, 95% CI: 1.01–2.60, P = 0.045) while rs61928479 (AA) correlates with the elevated risk of lower serum ApoA-I levels only (adjusted OR = 2.05, 95% CI: 1.22–3.46, P = 0.007). Three KATP SNPs (rs11046182, rs78148713 and rs145456027) do not associate with any type of lipid disorder [HDL-C, ApoB, ApoA-I, and Lp(a), all P > 0.05]. As shown in Figure 6F and Supplementary Table 21, KATP rs1799858 (TT + CT, adjusted OR = 1.47, 95% CI: 1.10–1.96, P = 0.009), rs2285676 (AA + GA, adjusted OR = 1.42, 95% CI: 1.05–1.91, P = 0.023), and rs141294036 (CC, adjusted OR = 1.49, 95% CI: 1.11–2.00, P = 0.008) correlate with the increased risk of higher serum HsCRP levels.
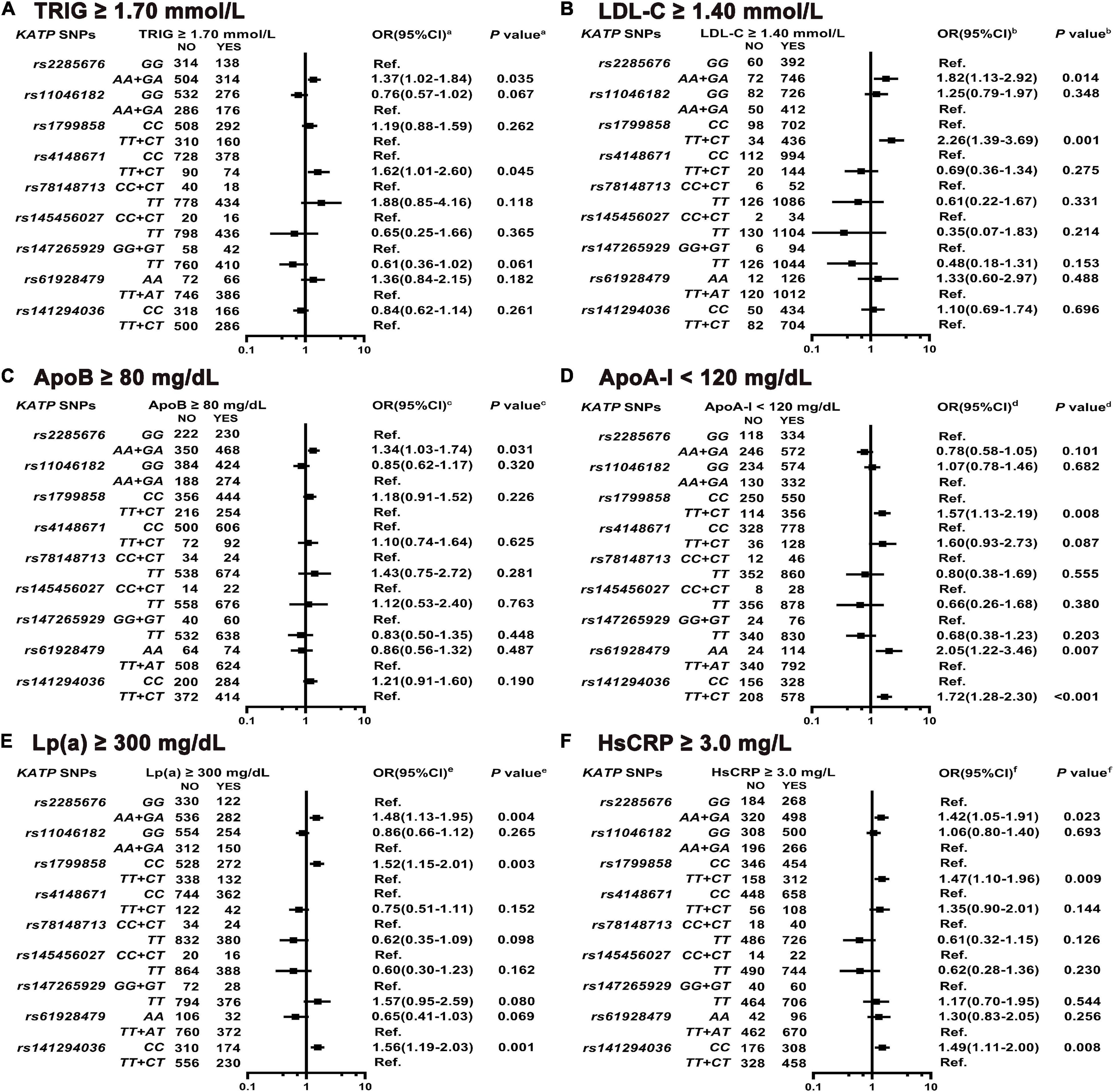
Figure 6. Association of KATP SNPs with abnormal serum levels of blood lipid and HsCRP in the study participants. aModel (A) After adjustment for gender, age, smoking, alcohol consumption, BMI, WBC, T2D, SBP, DBP, blood glucose levels (FBS, P2hBS, and HbA1C), liver function (ALT, AST, and Alb), renal function (Scr, BUN, and UA), serum sodium and potassium levels, HsCRP, RAAS activity (ACE, renin, Ang I, Ang II, and ALD), and dyslipidemia [TC, LDL-C, ApoB, HDL-C, ApoA-I, and Lp(a)]. bModel (B) Is the same as Model 6a except LDL-C, and including TRIG. cModel (C) Is the same as Model 6a except ApoB, and including TRIG. dModel (D) Is the same as Model 6a except ApoA-I, and including TRIG. eModel (E) Is the same as Model 6a except Lp(a), and including TRIG. fModel (F) After adjustment for gender, age, smoking, alcohol consumption, WBC, BMI, T2D, SBP, DBP, blood glucose levels (FBS, P2hBS, and HbA1C), liver function (ALT, AST, and Alb), renal function (Scr, BUN and UA), serum sodium and potassium levels, HsCRP, dyslipidemia (TRIG, TC, LDL-C, HDL-C, ApoA-I, and ApoB) and RAAS activity (ACE, renin, Ang I, Ang II, and ALD).
This study demonstrates that the Chinese participants with the genotypes of rs2285676 (AA + GA), rs1799858 (CC), and rs141294036 (TT + CT) have a 42–45% increased risk of T2D, and the total heritability of the 3 KATP variants for T2D risk is 2.1% (Supplementary Table 4). These findings are in controversy with the previous studies by Baier et al. (14) who reported no association between KATP rs2285676 and T2D in specific Amerindian-derived population, and by Odgerel et al. (13) who reported an association between the T-allele of rs1799858 and T2D in diabetic Mongolians rather than the CC genotype. The subjects with high diabetic-risk genotypes with different KATP variants correlate with increased T2D risk, but the effects show the characteristics of ethnic heterogeneity as follows: The French (rs1799859/AA + AG, by 69%) (6), the Turks (rs1799859/AA + AG, by 3.8-fold) (7), the Finns (rs1799859/AA + AG, by 2-fold), the Japanese (rs5219/KK + EK, by 32%) (12), the Mongolians (TT + CT of rs1799858 and rs2074308, approximately by 80%) (13) and the Pima Indians (chr11:17417205/T-allele, by 1-fold) (14). Two meta-analysis studies confirm that KATP mutations correlate with increased T2D risk (about by 15–16%) in both the Caucasians (e.g., the Europeans) and the non-Caucasians (e.g., the Japanese) (4, 5). Furthermore, KATP variants are associated with antidiabetic efficacy of sulfonylurea hypoglycemic agents (e.g., gliclazide) as well as non-sulfonylureas (e.g., repaglinide), especially secondary sulfonylurea failure (29). This phenomenon is also seen in the Chinese Han subjects with T2D (e.g., rs1801261) (30). These findings suggest that the 3 KATP variants can be targeted to evaluate the effectiveness of hypoglycemic medications in the Chinese in addition to being latent genetic predisposition markers for T2D risk.
Currently, the mechanism by which KATP variants induce high diabetes risk remains unclear, possibly involving inadequate insulin secretion, resistance to insulin, or (and) impaired glucagon secretion. First, the KATP mutations are associated with impaired glucose-induced insulin secretion in the Turks (e.g., rs1799854, rs1799859, and rs5219) (7), the Finns (e.g., rs5219) (31), and the French-Canadians (e.g., rs2285676 and rs11024273) (32). Secondly, KATP variants associate with decreased insulin sensitivity in the Poles (e.g., rs80356622 and rs80356624) (33), the Finns and the Americans (e.g., rs5219) (31). Lastly, KATP rs5219 correlates with impaired glucose-induced suppression of glucagon secretion (34). What is more important is that both animal and clinical studies indicate that KATP variants manifest their deleterious effects at early stages in the progression of T2D (e.g., during the progression from normal blood glucose levels to impaired glucose tolerance) (35), especially under the influence of environmental factors, such as diet (e.g., rs5219 and alcohol consumption in the Koreans) (36) and lifestyle (e.g., rs3758947 and physical inactivity in the Finns) (37). Indeed, R1420H (chr11:17417205C > T) correlates with increased risk of T2D (by 2-fold) with a 7-year earlier onset age (14). A recent study shows that the Chinese Han subjects with KATP rs5219 orrs1801261 are more susceptible to the development of prediabetes (38).
Arteriosclerosis cardiovascular disease, such as ACS and stroke are the major macro-vascular complications in patients with T2D (39), and are the leading cause of increased mortality among diabetics, even in people who optimally control the common risk factors for ASCVD. Among the patients with ACS, those with T2D are particularly at an increased risk for recurrent cardiovascular events and premature death, especially in China (40). Our results show that the Chinese participants with high diabetic risk genotype (A-allele) of rs2285676 present 37% increased the risk of new-onset/recurrent ACS after a median follow-up of 45.8-months. The T-allele of rs141294036 is not a diabetic risk predisposition allele, but the Chinese subjects with this mutation are at the highest risk for new-onset/recurrent ACS (increased by 59%). On the other hand, the new-onset of stroke risk increases by 1.58-fold and 1.30-fold among the Chinese subjects expressing the high diabetic risk genotype (CC) of rs1799858 and rs141294036, respectively, after a median follow-up of 50.8-months. However, the CC genotype of rs1799858 and rs141294036 correlates only with moderate and high risk of IS. In addition to being related to the occurrence risk, a recent study further showed that the KATP variants (e.g., rs2237982, rs2283261, rs3819521, and rs8192695) are also linked to the progression of vascular events, and are involved in abnormal transcription in the target-tissue and promoter/enhancer markers (41). Compared to the heritabilityofrs141294036 for new-onset stroke risk (0.44%, Supplementary Table 6), the heritability of the loci for new-onset/recurrent ACS risk (1.34%, Supplementary Table 5) are much higher. These results suggest that the genotypes, rs2285676 (AA + AG), rs1799858 (CC), and rs141294036 (CC) are optimal genetic markers for macro-vascular events in diabetic patients, especially rs141294036 for a new-onset/recurrent ACS risk.
Type 2 diabetes is a very important independent risk factor for HF. Previous studies show that KATP rs5219 prevalence is increased in the American patients with HF (21) while 3 KATP variants are associated with left ventricular remodeling in ischemic HF subjects in Ukraine (e.g., rs5215, rs5219 and rs757110), suggesting that the KATP mutations may correlate with increased HF risk. This study shows a 1.78-fold increase in HF among the Chinese subjects with TT + CT genotype of rs1799858 after a median follow-up of 48.1-months. Compared to the association between rs1799858 and HF, rs141293036 (TT + CT) correlates with weaker HF incidence risk (only increased by 45%). The heritability of HF is heterogeneous, and the genetic characteristics of different HF subtypes are still poorly understood. Further analysis of HF subtypes first indicates that rs1799858 (TT + CT) only correlates with a high risk of HFpEF while rs141294036 (TT + CT) correlates with a moderate risk of HFmrEF. The heritability of rs1799858 for a new-onset of HF risk is up to 3.48% (Supplementary Table 8), especially 2.78% for HFpEF (Supplementary Table 9). As far as the current HFpEF and HFmrEF prevention and treatment strategy are concerned, the “phenotype”- based targeted therapy is a more feasible priority strategy (42). Hence, these findings indicate that the TT + CT genotype of rs1799858 and rs141294036 can serve as a potential phenotypic marker for HFpEF and HFmrEF phenotypes, especially, rs1799858.
Type 2 diabetes is also one of the strongest independent risk factors for AF. However, the relationships between KATP variants and AF risk in the Chinese diabetic subjects remain unclear. This study shows that the Chinese subjects with high diabetic risk genotype (CC) of rs141294036 are at 1.42-fold more risk of having AF at the time of enrollment. The T-allele of rs1799858 does not correlate with diabetes risk, but the Chinese subjects with the mutation are at a higher risk of AF (approximately increased by 1.15-fold). After a median follow-up of 50.1-months, a similar association between the two variants and the risk of a new-onset of AF was found, where the AF risk was more than doubled in the presence of any of these variants (Supplementary Table 11). In this study, the total heritability of rs1799858 and rs141294036 for total AF risk reaches up to 1.85% (Supplementary Table 12). Similarly, an isolated study indicates that the KATP variant, rs387906805 correlates with AF originating from the vein of Marshall (43). A large sample study also indicates an association of KATP rs72554071 with increased AF susceptibility among the Americans based on 8-years follow-up (22). The pathogenic mechanism involves shortening of the action potential duration mediated by a transient outward current (22). These results suggest that rs141294036 (CC) may be a preferred predisposition marker for diabetic AF in the Chinese subjects.
Glucose and lipid metabolism disorders, along with chronic low-grade inflammation, are essential in the initiation and progression of diabetic cardiovascular events. Our study and many other studies, demonstrates the association of KATP variants with glucose metabolism disorders; however, lipid metabolism disorders and inflammation are rarely highlighted. This study indicates that the three diabetic-risk related KATP variants are indeed correlated with dyslipidemia but exhibited heterogeneity as follows: none of the 3 KATP variants correlates with increased serum TC levels (Supplementary Table 19), and decreased serum HDL-L level (Supplementary Table 20). KATP rs2285676 (A-allele) correlates with a higher risk of all subtypes [e.g., TRIG, LDL-C, ApoB and Lp(a)] of dyslipidemia except HDL-C and ApoA-I. The high diabetic risk genotype (CC) of rs1799858 and rs141294036only correlates with moderate risk of increased serum Lp(a) levels while its counterpart genotype (TT + CT) correlates with a moderate risk of decreased serum ApoA-I levels. Besides ApoA-I, the KATP rs1799858 (TT + CT) correlates with a high risk of increased serum LDL-C levels. On the other hand, the three diabetic-risk related KATP variants are associated with a moderate risk of low-grade inflammation levels (HsCRP ≥ 3.0 mg/L).
The clinical heterogeneity of diabetic cardiovascular phenotypes may relate to the combinations of hyperglycemia, different subtypes of dyslipidemia, and chronic low-grade inflammation (44). First, the CC genotype of rs1799858 and rs141294036 correlate with a high IS risk. These two variants correlate with a high risk of higher serum Lp(a) levels. In addition, two recent large sample studies confirm that high serum concentration of Lp(a) is associated with an increased risk of IS, both observationally and causally based on human genetics (45, 46). Second, the TT + CT genotype of rs1799858 and rs141294036 correlate with HFpEF and HFmrEF risks. Both the variants correlate with a high risk of decreased serum in the ApoA-I level. Among the lipoprotein components related to HDL-C, ApoA-I presents the greatest independent risk factor for cardiovascular risk events and is a better predictive marker for HF (47). In mouse models of HF, a reconstituted HDL-C improves the outcomes, especially HFpEF (48). Importantly, under the same adverse effect of HF incidence rate (e.g., 10%), HF occurs earlier (about 1 year; Supplementary Figure 4) in subjects with TT + CT genotype of rs1799858 compared to those with TT + CT genotype of rs141294036. Besides the high risk of decreased serum ApoA-I level, rs1799858 is also related to the coexisting elevated risk of high serum levels of LDL-C and HsCRP. Third, rs2285676 (AA + AG) and rs141294036 (TT + CT) are associated with ACS risk. The difference between them is that the former almost correlates with a higher risk of all subtypes of dyslipidemia except HDL-C and ApoA-I, where the latter shows the complete opposite. A consensus is established on the relationship between arteriosclerotic cholesterol [e.g., TRIG, LDL-C, ApoB and Lp(a)] and ACS risk, but little is known about its relationship with ApoA-I. Lower serum ApoA-I levels are associated with increased ACS risk and is manifested in individuals with a high cardiovascular risk (49). However, coronary atherosclerosis is not reduced by ApoA-I mimetic infusions in patients with ACS and high plaque burden (50). Lastly, rs1799858 (TT + CT) and rs141294036 (CC) are associated with AF risk. Both of them are associated with a high risk of low-grade inflammation (HsCRP ≥ 3.0 mg/L). A recent study shows that chronic low-grade inflammation correlates with increased AF risk in the Chinese participants having a metabolic syndrome (51).
This study is the first long follow-up assessment of the genotype-cardiovascular phenotype profiles of KATP variants among diabetic subjects in China. Several limitations worth mentioning are as follows: First, the study determines the above associations successfully, but the direct evidence with regards to its pathophysiology is yet to be investigated. Second, two potential factors may influence the associations, including the sample size and cardiovascular events (e.g., stroke and HF) during the follow-up. Importantly, it is necessary to explore the association of glycemic parameters, such as HbA1C and FBG with the new onset of cardiovascular phenotypes, given there is a strong association between the glycemic control and macro-vascular complications. Initially, the current work only demonstrates the association between KATP and diabetic cardiovascular phenotypes. These results and plans should be further validated by large-scale, prospective cohort studies with large-scale sample size. Third, there is still a risk of potential Type I error so that Bonferroni adjustment is carried out to correct the significant thresholds. Fourth, microvascular dysfunction (MVD) in T2D is present in many tissues or organs and it manifests itself as a spectrum of specific phenotypes: LCI in the brain (52), HFpEF in the heart (53), and microalbuminuria in the kidney (54). We observed a potential clinical association between KATP genetic variants and MVD-related phenotypes (e.g., LCI and HFpEF), but the direct evidence based on MVD as the pathophysiological entity is still lacking and needs further investigation. Lastly, the crosstalk between the environmental and genetic factors results in the development of diabetes and its associated CVDs. Additionally, the effect of non-coding RNA on the pathophysiological processes from elevated serum glucose levels to different diabetic cardiovascular phenotypes needs further investigation.
The KATP variants, rs2285676, rs1799858, and rs141294036 are associated with increased risks of T2D and their related cardiovascular events (ACS, IS, HF, and AF) but show heterogeneity. These novel findings suggest that the three variants may serve as promising phenotypic markers of diabetic cardiovascular events. These markers are potentially valuable for early and “genotype-phenotype”-oriented prevention and treatment strategies.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s
The study received ethics approval from the Institutional Review Board of Guangzhou First People’s Hospital, South China University of Technology (K-2017-043-02). All procedures performed in studies involving human participants were in accordance with the ethics guidelines of the institutional and with the principles of the Declaration of Helsinki. Written consent has been obtained from each patient or subject after full explanation of the purpose and nature of all procedures used. The patients/participants provided their written informed consent to participate in this study.
CL: literature search, study format, writing protocol, collecting data, processing data, data interpretation, analyzing data, and writing manuscript. YL and JP: study format, writing protocol, recruiting patients, data interpretation, and carrying out the molecular genetics. TG, JZ, SY, and YS: recruiting patients, following up patients, collecting data. DW: echocardiography testing. All authors read and approved the final manuscript.
This study was supported by grants from the Guangzhou Science and Technology Project of China (2012J4100035 and 201804010214), the National Natural Science Foundation of China (81100235), the Natural Science Foundation of Guangdong province (S2011040004458), and the Guangdong Science and Technology Planning Project of China (2014A020212372).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We wish to thank all the study participants from the South China Cardiovascular Related Disease Cohort (SCCDC), research staff, and students who participated in this work.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.816847/full#supplementary-material
ACE, angiotensin converting enzyme; ACS, acute coronary syndrome; AF, atrial fibrillation; Alb, albumin; ALD, aldosterone; ALT, alanine aminotransferase; Ang I/II, angiotensin I/II; ApoA-I, apolipoproteinA-I; ApoB, apolipoproteinB; ASCVD, arteriosclerosis cardiovascular disease; AST, aspartate aminotransferase; BMI, body mass index; BUN, blood urea nitrogen; CAD, coronary atherosclerotic heart disease; Ce-MVD, cerebral microvascular dysfunction; CI, confidence interval; Co-MVD, coronary microvascular dysfunction; CS, cardiogenic stroke; DBP, diastolic blood pressure; HTN, Hypertension; FBG, fasting blood glucose; HbA1C, glycosylated haemoglobin; HDL-C, high-density lipoprotein cholesterol; HF, Heart failure; HFpEF, HF with preserved ejection fraction; HFmrEF, HF with mildly reduced ejection fraction; HFrEF, HF with reduced ejection fraction; HGB, hemoglobin concentration; HS, hemorrhagic stroke; HR, hazard ratio; HsCRP, high-sensitivity C-reactive protein; IS, ischemic stroke; K+, serum potassium; KATP, ATP-sensitive potassium channels; Kir6.x, inward-rectifier potassium channel; LAD, left atrial end-diastolic dimension; LCI, lacunar cerebral infarct; LD, linkage disequilibrium; LDL-C, low-density lipoprotein cholesterol; Lp(a), lipoprotein(a); LVD, left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction; LVMI, left ventricular mass index; MAF, minor allele frequency; MVD, microvascular dysfunction; Na+, serum sodium; OR, odds ratio; P2hBS, postprandial blood glucose 2 h; PLT, platelet count; RAAS, renin-angiotensin-aldosterone system; RAD, right atrial end-diastolic dimension; RVD, right ventricular end-diastolic diameter; SBP, systolic blood pressure; Scr, serum creatinine; SNP, single nucleotide polymorphism; SUR, sulfonylurea receptor; TC, total cholesterol; T2D, type 2 diabetes; TRIG, triglyceride; UA, serum uric acid; WBC, white blood cell count.
1. Knudsen JG, Hamilton A, Ramracheya R, Tarasov AI, Brereton M, Haythorne E, et al. Dysregulation of glucagon secretion by hyperglycemia-induced sodium-dependent reduction of ATP production. Cell Metab. (2019) 29:430–442.e4. doi: 10.1016/j.cmet.2018.10.003
2. Said MA, Verweij N, Van Der Harst P. Associations of combined genetic and lifestyle risks with incident cardiovascular disease and diabetes in the UK biobank study. JAMA Cardiol. (2018) 3:693–702. doi: 10.1001/jamacardio.2018.1717
3. Liu C, Guan T, Lai Y, Zhan J, Shen Y. Genetic predisposition and bioinformatics analysis of ATP-sensitive potassium channels polymorphisms with the risks of elevated apolipoprotein B serum levels and its related arteriosclerosis cardiovascular disease. Aging (Albany NY). (2021) 13:8177–203. doi: 10.18632/aging.202628
4. Qin LJ, Lv Y, Huang QY. Meta-analysis of association of common variants in the KCNJ11-ABCC8 region with type 2 diabetes. Genet Mol Res. (2013) 12:2990–3002. doi: 10.4238/2013.August.20.1
5. Sokolova EA, Bondar IA, Shabelnikova OY, Pyankova OV, Filipenko ML. Replication of KCNJ11 (p.E23K) and ABCC8 (p.S1369A) association in Russian diabetes mellitus 2 type cohort and meta-analysis. PLoS One. (2015) 10:e0124662. doi: 10.1371/journal.pone.0124662
6. Reis AF, Ye WZ, Dubois-Laforgue D, Bellanne-Chantelot C, Timsit J, Velho G. Association of a variant in exon 31 of the sulfonylurea receptor 1 (SUR1) gene with type 2 diabetes mellitus in French Caucasians. Hum Genet. (2000) 107:138–44. doi: 10.1007/s004390000345
7. Gonen MS, Arikoglu H, Erkoc Kaya D, Ozdemir H, Ipekci SH, Arslan A, et al. Effects of single nucleotide polymorphisms in K(ATP) channel genes on type 2 diabetes in a Turkish population. Arch Med Res. (2012) 43:317–23. doi: 10.1016/j.arcmed.2012.06.001
8. Bakhtiyari A, Haghani K, Bakhtiyari S, Zaimy MA, Noori-Zadeh A, Gheysarzadeh A, et al. Association between ABCC8 Ala1369Ser polymorphism (rs757110 T/G) and type 2 diabetes risk in an Iranian population: a case-control study. Endocr Metab Immune Disord Drug Targets. (2021) 21:441–7. doi: 10.2174/1871530320666200713091827
9. Emdin CA, Klarin D, Natarajan P, Consortium CE, Florez JC, Kathiresan S, et al. Genetic variation at the sulfonylurea receptor, type 2 diabetes, and coronary heart disease. Diabetes. (2017) 66:2310–5. doi: 10.2337/db17-0149
10. Gloyn AL, Weedon MN, Owen KR, Turner MJ, Knight BA, Hitman G, et al. Large-scale association studies of variants in genes encoding the pancreatic beta-cell KATP channel subunits Kir6.2 (KCNJ11) and SUR1 (ABCC8) confirm that the KCNJ11 E23K variant is associated with type 2 diabetes. Diabetes. (2003) 52:568–72. doi: 10.2337/diabetes.52.2.568
11. Al-Sinani S, Woodhouse N, Al-Mamari A, Al-Shafie O, Al-Shafaee M, Al-Yahyaee S, et al. Association of gene variants with susceptibility to type 2 diabetes among Omanis. World J Diabetes. (2015) 6:358–66. doi: 10.4239/wjd.v6.i2.358
12. Sakamoto Y, Inoue H, Keshavarz P, Miyawaki K, Yamaguchi Y, Moritani M, et al. SNPs in the KCNJ11-ABCC8 gene locus are associated with type 2 diabetes and blood pressure levels in the Japanese population. J Hum Genet. (2007) 52:781–93. doi: 10.1007/s10038-007-0190-x
13. Odgerel Z, Lee HS, Erdenebileg N, Gandbold S, Luvsanjamba M, Sambuughin N, et al. Genetic variants in potassium channels are associated with type 2 diabetes in a Mongolian population. J Diabetes. (2012) 4:238–42. doi: 10.1111/j.1753-0407.2011.00177.x
14. Baier LJ, Muller YL, Remedi MS, Traurig M, Piaggi P, Wiessner G, et al. ABCC8 R1420H loss-of-function variant in a Southwest American Indian community: association with increased birth weight and doubled risk of type 2 diabetes. Diabetes. (2015) 64:4322–32. doi: 10.2337/db15-0459
15. Engwa GA, Nwalo FN, Chikezie CC, Onyia CO, Ojo OO, Mbacham WF, et al. Possible association between ABCC8 C49620T polymorphism and type 2 diabetes in a Nigerian population. BMC Med Genet. (2018) 19:78. doi: 10.1186/s12881-018-0601-1
16. Matharoo K, Arora P, Bhanwer AJ. Association of adiponectin (AdipoQ) and sulphonylurea receptor (ABCC8) gene polymorphisms with type 2 diabetes in north Indian population of Punjab. Gene. (2013) 527:228–34. doi: 10.1016/j.gene.2013.05.075
17. Asamoah EA, Obirikorang C, Acheampong E, Annani-Akollor ME, Laing EF, Owiredu EW, et al. Heritability and genetics of type 2 diabetes mellitus in sub-Saharan Africa: a systematic review and meta-analysis. J Diabetes Res. (2020) 2020:3198671. doi: 10.1155/2020/3198671
18. Stefanski A, Majkowska L, Ciechanowicz A, Frankow M, Safranow K, Parczewski M, et al. The common C49620T polymorphism in the sulfonylurea receptor gene (ABCC8), pancreatic beta cell function and long-term diabetic complications in obese patients with long-lasting type 2 diabetes mellitus. Exp Clin Endocrinol Diabetes. (2007) 115:317–21. doi: 10.1055/s-2007-967086
19. Smith KJ, Chadburn AJ, Adomaviciene A, Minoretti P, Vignali L, Emanuele E, et al. Coronary spasm and acute myocardial infarction due to a mutation (V734I) in the nucleotide binding domain 1 of ABCC9. Int J Cardiol. (2013) 168:3506–13. doi: 10.1016/j.ijcard.2013.04.210
20. Strutynskyi RB, Voronkov LG, Nagibin VS, Mazur ID, Stroy D, Dosenko VE. Changes of the echocardiographic parameters in chronic heart failure patients with Ile337val, Glu23lys, and Ser1369ala polymorphisms of genes encoding the ATP-sensitive potassium channels subunits in the Ukrainian population. Ann Hum Genet. (2018) 82:272–9. doi: 10.1111/ahg.12250
21. Reyes S, Park S, Johnson BD, Terzic A, Olson TM. KATP channel Kir6.2 E23K variant overrepresented in human heart failure is associated with impaired exercise stress response. Hum Genet. (2009) 126:779–89. doi: 10.1007/s00439-009-0731-9
22. Delaney JT, Muhammad R, Blair MA, Kor K, Fish FA, Roden DM, et al. A KCNJ8 mutation associated with early repolarization and atrial fibrillation. Europace. (2012) 14:1428–32. doi: 10.1093/europace/eus150
23. Rehman K, Tahir A, Niaz S, Shabbir S, Jabeen K, Faheem A, et al. Frequency of PPAR-gamma, FTO and ABCC8 genetic variation in Pakistani cardiovascular smokers. Environ Sci Pollut Res Int. (2020) 27:42611–20. doi: 10.1007/s11356-020-10226-z
24. American Diabetes Association. Standards of medical care in diabetes–2012. Diabetes Care. (2012) 35(Suppl. 1):S11–63. doi: 10.2337/dc12-s011
25. American Diabetes Association. Classification and diagnosis of diabetes: standards of medical care in diabetes-2020. Diabetes Care. (2020) 43(Suppl. 1):S14–31. doi: 10.2337/dc20-S002
26. Liu C, Guan T, Lai Y, Zhu J, Kuang J, Shen Y. ATP-sensitive potassium channels gene polymorphism rs1799858 affects the risk of macro-/micro-vascular arteriosclerotic event in patients with increased low-density lipoprotein cholesterol levels. Lipids Health Dis. (2020) 19:147. doi: 10.1186/s12944-020-01315-6
27. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Bohm M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. (2021) 42:3599–726.
28. Yang J, Zeng J, Goddard ME, Wray NR, Visscher PM. Concepts, estimation and interpretation of SNP-based heritability. Nat Genet. (2017) 49:1304–10. doi: 10.1038/ng.3941
29. Loganadan NK, Huri HZ, Vethakkan SR, Hussein Z. Clinical and genetic predictors of secondary sulfonylurea failure in type 2 diabetes patients: the SUCLINGEN study. Pharmacogenomics. (2020) 21:587–600. doi: 10.2217/pgs-2019-0171
30. Zhou X, Chen C, Yin D, Zhao F, Bao Z, Zhao Y, et al. A Variation in the ABCC8 gene is associated with type 2 diabetes mellitus and repaglinide efficacy in Chinese type 2 diabetes mellitus patients. Intern Med. (2019) 58:2341–7. doi: 10.2169/internalmedicine.2133-18
31. Stancakova A, Kuulasmaa T, Paananen J, Jackson AU, Bonnycastle LL, Collins FS, et al. Association of 18 confirmed susceptibility loci for type 2 diabetes with indices of insulin release, proinsulin conversion, and insulin sensitivity in 5,327 nondiabetic Finnish men. Diabetes. (2009) 58:2129–36. doi: 10.2337/db09-0117
32. Ruchat SM, Elks CE, Loos RJ, Vohl MC, Weisnagel SJ, Rankinen T, et al. Association between insulin secretion, insulin sensitivity and type 2 diabetes susceptibility variants identified in genome-wide association studies. Acta Diabetol. (2009) 46:217–26. doi: 10.1007/s00592-008-0080-5
33. Malecki MT, Skupien J, Klupa T, Wanic K, Mlynarski W, Gach A, et al. Transfer to sulphonylurea therapy in adult subjects with permanent neonatal diabetes due to KCNJ11-activating [corrected] mutations: evidence for improvement in insulin sensitivity. Diabetes Care. (2007) 30:147–9. doi: 10.2337/dc06-1628
34. Jonsson A, Ladenvall C, Ahluwalia TS, Kravic J, Krus U, Taneera J, et al. Effects of common genetic variants associated with type 2 diabetes and glycemic traits on alpha- and beta-cell function and insulin action in humans. Diabetes. (2013) 62:2978–83. doi: 10.2337/db12-1627
35. Sachse G, Haythorne E, Hill T, Proks P, Joynson R, Terron-Exposito R, et al. The KCNJ11-E23K gene variant hastens diabetes progression by impairing glucose-induced insulin secretion. Diabetes. (2021) 70:1145–56. doi: 10.2337/db20-0691
36. Yun JH, Yoo MG, Park JY, Lee HJ, Park SI. Association between KCNJ11 rs5219 variant and alcohol consumption on the effect of insulin secretion in a community-based Korean cohort: a 12-year follow-up study. Sci Rep. (2021) 11:4729. doi: 10.1038/s41598-021-84179-9
37. Kilpelainen TO, Lakka TA, Laaksonen DE, Laukkanen O, Lindstrom J, Eriksson JG, et al. Physical activity modifies the effect of SNPs in the SLC2A2 (GLUT2) and ABCC8 (SUR1) genes on the risk of developing type 2 diabetes. Physiol Genomics. (2007) 31:264–72. doi: 10.1152/physiolgenomics.00036.2007
38. Xu M, Hu H, Deng D, Chen M, Xu Z, Wang Y. Prediabetes is associated with genetic variations in the gene encoding the Kir6.2 subunit of the pancreatic ATP-sensitive potassium channel (KCNJ11): a case-control study in a Han Chinese youth population. J Diabetes. (2018) 10:121–9. doi: 10.1111/1753-0407.12565
39. Einarson TR, Acs A, Ludwig C, Panton UH. Prevalence of cardiovascular disease in type 2 diabetes: a systematic literature review of scientific evidence from across the world in 2007-2017. Cardiovasc Diabetol. (2018) 17:83. doi: 10.1186/s12933-018-0728-6
40. Zhou M, Liu J, Hao Y, Liu J, Huo Y, Smith SC Jr, et al. Prevalence and in-hospital outcomes of diabetes among patients with acute coronary syndrome in China: findings from the improving care for cardiovascular disease in china-acute coronary syndrome project. Cardiovasc Diabetol. (2018) 17:147. doi: 10.1186/s12933-018-0793-x
41. Jha RM, Zusman BE, Puccio AM, Okonkwo DO, Pease M, Desai SM, et al. Genetic variants associated with intraparenchymal hemorrhage progression after traumatic brain injury. JAMA Netw Open. (2021) 4:e2116839. doi: 10.1001/jamanetworkopen.2021.16839
42. Paulus WJ. Unfolding discoveries in heart failure. N Engl J Med. (2020) 382:679–82. doi: 10.1056/NEJMcibr1913825
43. Olson TM, Alekseev AE, Moreau C, Liu XK, Zingman LV, Miki T, et al. KATP channel mutation confers risk for vein of Marshall adrenergic atrial fibrillation. Nat Clin Pract Cardiovasc Med. (2007) 4:110–6. doi: 10.1038/ncpcardio0792
44. Feingold KR, Grunfeld C. Role of glucose and lipids in the cardiovascular disease of patients with diabetes. In: Feingold KR, Anawalt B, Boyce A, Chrousos G, Dungan K, Grossman A editors et al. Endotext. South Dartmouth, MA: MDText.com, Inc (2000).
45. Arora P, Kalra R, Callas PW, Alexander KS, Zakai NA, Wadley V, et al. Lipoprotein(a) and risk of ischemic stroke in the REGARDS study. Arterioscler Thromb Vasc Biol. (2019) 39:810–8. doi: 10.1161/ATVBAHA.118.311857
46. Arnold M, Schweizer J, Nakas CT, Schutz V, Westphal LP, Inauen C, et al. Lipoprotein(a) is associated with large artery atherosclerosis stroke Aetiology and stroke recurrence among patients below the age of 60 years: results from the BIOSIGNAL study. Eur Heart J. (2021) 42:2186–96. doi: 10.1093/eurheartj/ehab081
47. Liu C, Lai Y, Pei J, Huang H, Zhan J, Ying S, et al. Clinical and genetic analysis of KATP variants with heart failure risk in patients with decreased serum ApoA-I levels. J Clin Endocrinol Metab. (2021) 106:2264–78. doi: 10.1210/clinem/dgab336
48. Mishra M, Muthuramu I, Kempen H, De Geest B. Administration of apo A-I (Milano) nanoparticles reverses pathological remodelling, cardiac dysfunction, and heart failure in a murine model of HFpEF associated with hypertension. Sci Rep. (2020) 10:8382. doi: 10.1038/s41598-020-65255-y
49. Soria-Florido MT, Castaner O, Lassale C, Estruch R, Salas-Salvado J, Martinez-Gonzalez MA, et al. Dysfunctional high-density lipoproteins are associated with a greater incidence of acute coronary syndrome in a population at high cardiovascular risk: a nested case-control study. Circulation. (2020) 141:444–53. doi: 10.1161/CIRCULATIONAHA.119.041658
50. Nicholls SJ, Puri R, Ballantyne CM, Jukema JW, Kastelein JJP, Koenig W, et al. Effect of infusion of high-density lipoprotein mimetic containing recombinant apolipoprotein A-I milano on coronary disease in patients with an acute coronary syndrome in the MILANO-PILOT trial: a randomized clinical trial. JAMA Cardiol. (2018) 3:806–14. doi: 10.1001/jamacardio.2018.2112
51. Wang Z, Wang B, Li X, Zhang S, Wu S, Xia Y. Metabolic syndrome, high-sensitivity C-reactive protein levels and the risk of new-onset atrial fibrillation: results from the Kailuan study. Nutr Metab Cardiovasc Dis. (2021) 31:102–9. doi: 10.1016/j.numecd.2020.06.026
52. Koton S, Schneider ALC, Windham BG, Mosley TH, Gottesman RF, Coresh J. Microvascular brain disease progression and risk of stroke: the ARIC study. Stroke. (2020) 51:3264–70. doi: 10.1161/STROKEAHA.120.030063
53. Taqueti VR. Coronary microvascular dysfunction in heart failure with preserved ejection fraction-common, unrecognized, and prevalent in patients with or without epicardial CAD. JAMA Cardiol. (2021) 6:1118–20. doi: 10.1001/jamacardio.2021.1832
Keywords: type 2 diabetes, ATP-sensitive potassium channels, gene polymorphism, genotype, cardiovascular phenotype
Citation: Liu C, Lai Y, Guan T, Zhan J, Pei J, Wu D, Ying S and Shen Y (2022) Associations of ATP-Sensitive Potassium Channel’s Gene Polymorphisms With Type 2 Diabetes and Related Cardiovascular Phenotypes. Front. Cardiovasc. Med. 9:816847. doi: 10.3389/fcvm.2022.816847
Received: 17 November 2021; Accepted: 21 February 2022;
Published: 23 March 2022.
Edited by:
Nicolle Kraenkel, Charité Universitätsmedizin Berlin, GermanyReviewed by:
Maddalena Trombetta, University of Verona, ItalyCopyright © 2022 Liu, Lai, Guan, Zhan, Pei, Wu, Ying and Shen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cheng Liu, ZXlsaXVjaGVuZ0BzY3V0LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.