
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 31 January 2022
Sec. Cardiovascular Imaging
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.816404
This article is part of the Research Topic Advances in Cardiac Imaging and Heart Failure Management View all 42 articles
Background: Various adverse outcomes such as mortality and rehospitalization are associated with left ventricular non-compaction (LVNC). Due to data limitations, prospective risk assessment for LVNC remains challenging. This study aimed to investigate the influence of right ventricular (RV) dysfunction on the clinical outcomes of patients with LVNC through accurate and comprehensive measurements of RV function.
Methods and Results: Overall, 117 patients with LVNC (47.6 ± 18.3 years, 34.2% male) were enrolled, including 53 (45.3%) and 64 (54.7%) patients with and without RV dysfunction, respectively. RV dysfunction was defined as meeting any two of the following criteria: (i) tricuspid annular systolic excursions <17 mm, (ii) tricuspid S′ velocity <10 cm/s, and (iii) RV fractional area change (FAC) <35%. The proportion of biventricular involvement was significantly higher in patients with RV dysfunction than in controls (p = 0.0155). After a follow-up period of 69.0 [33.5, 96.0] months, 18 (15.4%) patients reached the primary endpoint (all-cause mortality), with 14 (26.4%) and 4 (6.3%) from the RV dysfunction group and normal RV function group, respectively. The Kaplan–Meier method and log-rank test revealed that patients with RV dysfunction had a higher risk of all-cause mortality than those in the control group (hazard ratio [HR]: 5.132 [2.003, 13.15], p = 0.0013). Similar results were obtained for patients with left ventricular ejection fraction (LVEF) <50% [HR, 6.582; 95% confidence interval (CI), 2.045–21.19; p = 0.0367]. The relationship between RV dysfunction and heart failure rehospitalization and implantation of implantable cardioverter-defibrillator (ICD)/cardiac resynchronization therapy (CRT) was not statistically significant (both p > 0.05). The multivariable Cox proportional hazard modeling analysis showed that RV dysfunction (HR: 4.950 [1.378, 17.783], p = 0.014) and impaired RV global longitudinal strain (RVGLS) (HR: 1.103 [1.004, 1.212], p = 0.041) were independent predictors of mortality rather than increased RV end-diastolic area and decreased LVEF (both p > 0.05).
Conclusions: RV dysfunction is associated with the prognosis of patients with LVNC.
Left ventricular non-compaction (LVNC) is a rare cardiomyopathy that is characterized by a thin and compacted epicardial layer, trabeculae, and deep intertrabecular recesses in the left ventricular myocardium (1). It is associated with asymptomatic, embolic events, and an inherent risk of malignant arrhythmia. Furthermore, sudden death caused by LVNC can be prevented by inserting an implantable cardioverter-defibrillator (ICD) (2, 3). The increased awareness of LVNC among cardiologists and improved imaging technologies have led to a better understanding of this condition, resulting to it being a widely recognized cardiomyopathy (4). Prospective risk assessment of LNVC is difficult because of the wide variation in its clinical outcomes (5–7). In addition, only a few studies have evaluated prognostic predictors (8).
Right ventricular (RV) dysfunction occurs in a substantial proportion of patients with LVNC (9–11). An accurate and reproducible assessment of RV function is required to assess the prognosis of patients; however, such an assessment remains difficult because of limited data on patients with LVNC, the complex shape of the RV, and a high load dependency. Conventional parameters assessing RV function include tricuspid annular plane systolic excursion (TAPSE), RV fractional area change (FAC), and tricuspid S′ velocity (12). Myocardial functional dynamics can be assessed with good accuracy using a 2D strain imaging technique, such as speckle-tracking echocardiography (STE) (13). Its applicability has been extended to RV function assessments (14) to detect early systolic functional abnormalities during the preclinical stage (15, 16). A recent study evaluated the role of RV function in the clinical outcomes of LVNC and showed that the right ventricular end-diastolic area (RVEDA) index is a strong prognostic marker that independently predicts death or the need for heart transplantation in patients with LVNC and indicates the prognostic value of the RV size (6). However, the prognostic value of factors such as TAPSE and RV FAC remain weak. Moreover, another study on 14 patients with LVNC demonstrated that RV dysfunction is a marker of advanced LVNC and poor prognosis (11); however, its sample size was relatively small. Considering the limited data on RV function with prognostic values of LVNC, the current study aimed to investigate the impact of RV dysfunction on LVNC-related clinical outcomes by accurate and comprehensive measurement of RV function.
Patients diagnosed with LVNC at the Peking Union Medical College Hospital based on the criteria described by Jenni (17) (a non-compacted/compacted ratio >2.0 in end-systole) and had at least one transthoracic echocardiography (TTE) at baseline between January 1, 2006, to June 30, 2021, were enrolled in the study. Patients with other cardiovascular conditions, including ischemic cardiomyopathy, primary valvular illness, congenital heart disease, cancer, or severe multi-system failure, and those who could not complete the follow-up period were excluded. This study was approved by the local ethics committee, and informed consent was obtained from all participants. The patients were categorized into two groups according to RV function, with RV dysfunction defined as meeting any two of the following criteria: (i) TAPSE <17 mm, (ii) tricuspid S′ velocity <10 cm/s, and (iii) RV FAC <35% (Figure 1) (12, 18).
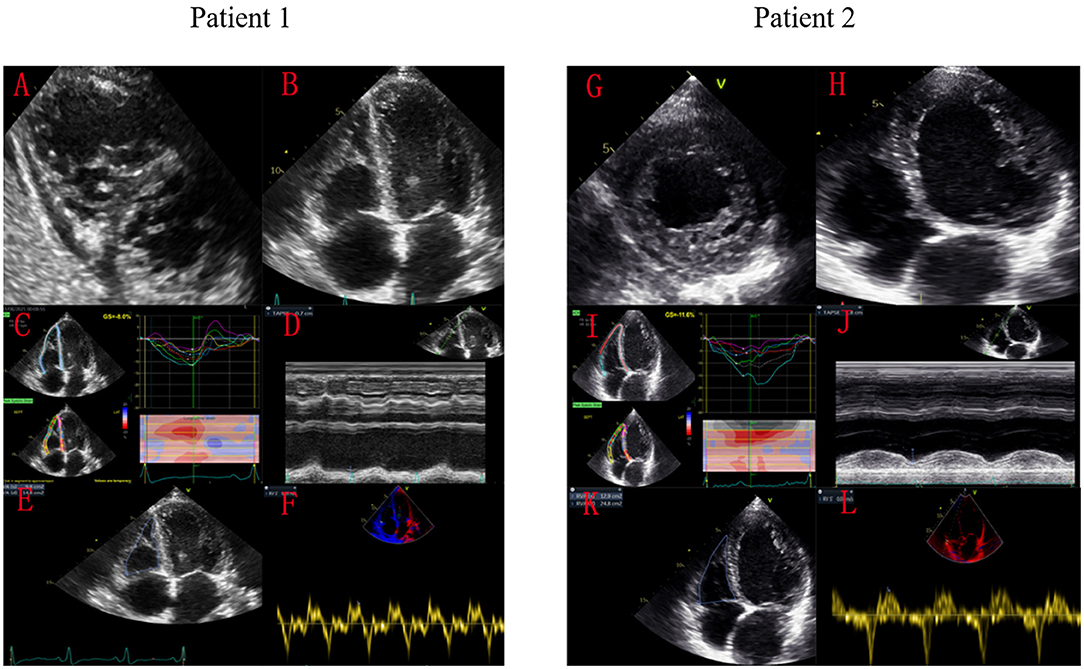
Figure 1. Speckle-tracking echocardiography images of two patients with LVNC and the same LVGLS. Patient 1 with significant RV dysfunction died of cardiogenic shock, while patient 2 survived for long-term after treatment. Patient 1 had significant echocardiographic manifestations of RV non-compaction (A,B). His RVGLS was −8.0% (C), TAPSE was 7mm (D), RV FAC was 24% (E), and tricuspid S′ was 0.05 m/s (F). Patient 2 only had LV echocardiography manifestations of ventricular non-compaction (G,H). His RVGLS was −11.6% (I), TAPSE was 18 mm (J), RV FAC was 47% (K), and tricuspid S′ was 0.07 m/s (L).
The clinical and demographic characteristics of the patients were collected from chart reviews, laboratory data, and auxiliary examinations at the time of enrollment. Baseline data, including age, sex, systolic blood pressure (SBP), diastolic blood pressure (DBP), heart rate (HR), and the levels of N-terminal fragment of pro-hormone brain natriuretic peptide (NT-proBNP), cardiac troponin I (cTnI), albumin (Alb), hemoglobin (Hb), and creatinine (Cr) were collected. The estimated glomerular filtration rate (eGFR) was calculated according to the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation (19). Transthoracic echocardiography was performed using commercially available equipment (Vivid 7 and Vivid E9, GE Medical Systems, Horten, Norway). Right ventricular involvement was diagnosed based on the criteria described by Jenni et al. applied to the right ventricle (17). The RVEDA, RVESA, tricuspid S′ velocity, and TAPSE were assessed according to current guidelines (12). RV FAC, expressed as a percentage, was calculated as (RVEDA-RVESA)/RVEDA. LVEDV, LVESV, and left ventricular ejection fraction (LVEF) were measured using Simpson's biplane method. Speckle tracking was automatically validated using advanced quantification software (EchoPAC Clinical Workstation Software, GE Healthcare) and confirmed visually from 2D images in the apical four-chamber, two-chamber, and three-chamber views. Global and segmental measurements of longitudinal strain were performed by assessing the peak longitudinal strain of the RV free wall. This was calculated as the arithmetic mean of the strain values in the three segments of the ventricular free wall strain obtained from a six-segment region of interest (20, 21).
The patients were regularly followed up at the outpatient cardiomyopathy clinic. Data including current status, medication use, and re-examination (if necessary), were obtained from clinical visits made regularly or telephone calls to ascertain readmission for worsening. All-cause death was assigned as the primary endpoint and recorded by chart review, telephone contact, and inspection of electronic files for death certificates. For patients without events, the date of the last contact was used for survival analysis. The secondary endpoints were re-hospitalization for cardiac reasons and ICD/cardiac resynchronization therapy (CRT) implantation.
One-sample Kolmogorov–Smirnov tests and histograms were used to check the normality of the continuous data. Continuous variables were expressed as mean ± SD (for normally distributed variables) or median [interquartile range (IQR)] (for non-normally distributed variables). Levene's test was performed to test the homogeneity of the variances. Normally distributed variables were compared using an unpaired t-test (homoscedasticity) or Welch's correction (non-homoscedasticity). Non-normally distributed variables were compared using the Mann–Whitney U test. Categorical data were expressed as percentages and compared using Pearson's χ2-test or Fisher's exact test, as appropriate. Survival analysis was performed using the Kaplan-Meier method by defining the time-to-event as the interval from the baseline to the primary endpoint. Kaplan-Meier survival curves were compared using the log-rank test. A univariable Cox proportional hazard model was used to analyze the relationship between the primary endpoint and baseline variables, such as echocardiographic parameters, blood pressure, and serum biochemical parameters. Results were reported as hazard ratios (HRs) with 95% confidence intervals (CIs). Variables with p < 0.05, at the univariable analysis or with a prior given clinical relevance were further tested using multivariable Cox regression analysis. Statistical significance was defined as a two-tailed p-value of < 0.05. Statistical analysis was performed using GraphPad Prism (Version 8.4.2; GraphPad Software Inc., USA) and SPSS (Version 24.0; SPSS Inc., Chicago, IL, USA).
A total of 117 patients with LVNC (mean age: 47.6 ± 18.3 years, 34.2% men) were finally enrolled after excluding two patients with a single ventricle and six with unavailable data. The demographic and baseline characteristics of all subjects are summarized in Table 1. The study population included 64 (54.7%) patients without RV dysfunction and 53 (45.3%) patients with RV dysfunction. Patients with RV dysfunction had lower SBP than the controls (112.3 ± 16.6mmHg vs. 119.2 ± 17.6mmHg, p = 0.0437). The RV dysfunction group had higher NT-proBNP (4,506 [1,692, 9,155]pg/ml vs. 603 [98, 2,184]pg/ml, p < 0.0001) and Cr (85.0 [72.8, 100.8]μmol/l vs. 72.5 [62.3, 89.8]μmol/l, p = 0.0160) than the control group, and no significant difference in the eGFR values was found between the two groups. The baseline echocardiographic parameters are presented in Table 1. The proportion of biventricular involvement was significantly higher in patients with RV dysfunction than in controls (p = 0.0155). Patients with RV dysfunction had higher RVEDA than those in the control group (18.6 [15.1, 25.5]cm2 vs. 14.8 [11.6, 18.2]cm2, p < 0.0001) and significantly impaired RV global longitudinal strain (RVGLS) (−8.8 ± 3.8% vs. −17.8 ± 6.0%, p < 0.0001). Furthermore, the RV dysfunction group had relatively high LVEDV (170.0 [132.0, 255.5]ml vs. 122.0 [85.0, 155.5]ml, p < 0.0001), low LVEF (29.4 ± 13.8% vs. 50.6 ± 14.0%, p < 0.0001), and impaired LV global longitudinal strain (LVGLS) (−5.3 [−9.0, −3.2]% vs. −13.9 [−19.6, −10.5]%, p < 0.0001). Heart failure medications, including β-blockers, angiotensin-converting enzyme (ACE) inhibitors/angiotensin receptor blockers (ARBs), spironolactone, diuretics, and digoxin, were more frequently used during the follow-up period in the RV dysfunction group than in the controls (all p < 0.05).
During a median follow-up time of 69.0 months [33.5, 96.0], 18 patients (15.4%) reached the primary endpoint, including 14 (26.4%) and 4 patients (6.3%) from the RV dysfunction group and the control group, respectively. Patients with RV dysfunction had a higher risk of all-cause mortality than those in the control group (HR, 5.132; 95%CI, 2.003–13.15; p = 0.0013). The Kaplan–Meier curve is shown in Figure 2. The relationship between RV dysfunction and heart failure rehospitalization and ICD/CRT implantation was not statistically significant (both p > 0.05).
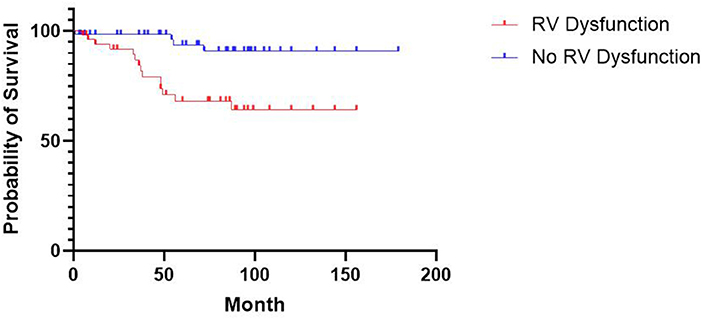
Figure 2. Kaplan-Meier Estimates of Survival According to RV Dysfunction. The difference in survival between patients with and without RV dysfunction was statistically significant (P = 0.0013 by the log-rank test).
To assess the interaction of RV dysfunction with LV systolic dysfunction on patient prognosis, baseline characteristics and outcomes were analyzed in 72 patients with LVEF < 50%, including 45 (62.5%) and 27 (37.5%) patients with and without RV dysfunction, respectively (Table 2). Higher NT-proBNP (3,070 [1,053, 7,724]pg/ml vs. 1,150 [496, 2,622]pg/ml, p = 0.0173), more biventricular involvement (44.4 vs. 7.4%, p = 0.0012), and higher RVEDA (18.6 [14.7, 24.9]cm2 vs. 15.1 [12.2, 19.3]cm2, p = 0.0191) were also found in the RV dysfunction group than in the controls. Differences in LVEF also existed between the two groups (27.0 [15.0, 37.0]% vs. 42.0 [32.0, 45.0]%, p < 0.0001). However, no significant difference was found in LVEDV (170.0 [132.5, 262.0]ml vs. 152.0 [122.0, 192.0]ml, p = 0.1395). In the LV dysfunction population, 12 patients (16.7%) reached the primary endpoint, including 11 (24.4%) from the RV dysfunction group and one patient (3.7%) from the control group. The same results were observed for higher risk of all-cause mortality (HR: 6.582; 95%CI: 2.045–21.19; p = 0.0367) in the RV dysfunction group, and no significant difference was observed for heart failure rehospitalization and implantation of ICD/CRT (both P > 0.05). Furthermore, Table 3 shows the baseline characteristics of the patients who reached the primary endpoint compared to the rest of the population. The proportion of RV dysfunction was significantly higher in the cardiovascular mortality group than in the control group (77.8% vs. 39.4%, p = 0.0039). However, the difference of LVEF (32.4 ± 18.6% vs. 41.6 ± 17.0%, p = 0.0629) and LVGLS (−6.6 [−13.0, −4.6]% vs. −10.5 [−15.0, −5.6]%, p = 0.1939) between two groups were not statistically significant.
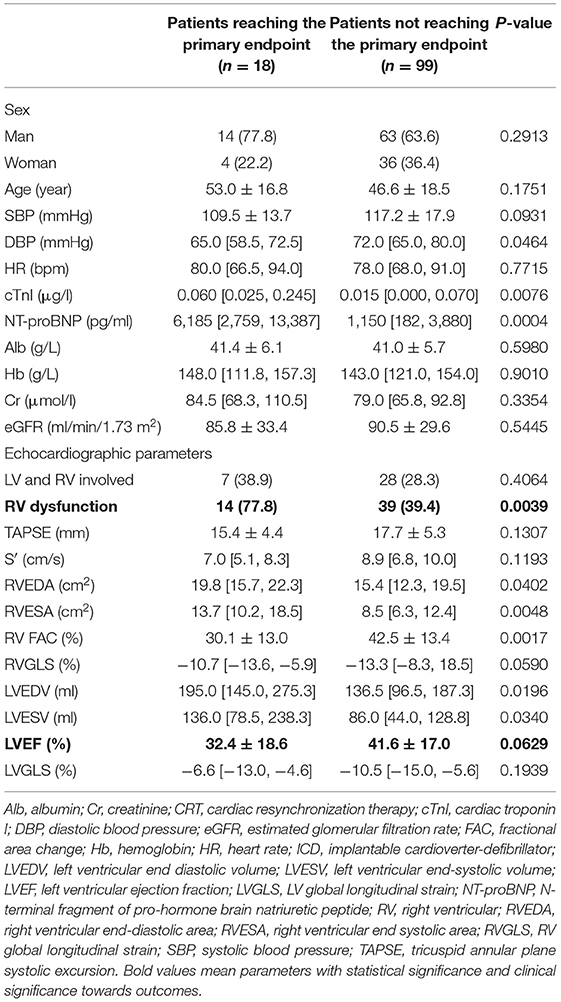
Table 3. Baseline characteristics of patients reaching the primary endpoint and not reaching the primary endpoint.
Table 4 shows the results of Cox regression analysis of the predictors of the primary endpoint. In the univariable analysis, the primary endpoint was significantly predicted by increased RVEDA, RV dysfunction, impaired RVGLS, and decreased LVEF (all p <0.05). However, when variables were introduced into the multivariable Cox regression models, only RV dysfunction (HR: 4.950 [1.378, 17.783], p = 0.014) and impaired RVGLS (HR: 1.103 [1.004, 1.212], p = 0.041) were identified as independent predictors of mortality, whereas decreased LVEF and increased RVEDA were not (both p > 0.05).
This study confirmed the prognostic value of RV parameters in a relatively large population of patients with LVNC (117 patients) over a median follow-up period of more than 5 years. RV dysfunction is independently associated with all-cause mortality in patients with LVNC, even after correction for LV function. The impaired RVGLS measured by 2D strain imaging indicates early RV systolic function abnormalities and can also predict outcomes in patients with LVNC.
Impaired RV systolic function (defined as RVEF <35% on cardiac MRI) was identified in 50% and 16% of the population, respectively, in two previous researches (10, 11). In this study, RV dysfunction was identified in 45.3% of the patients with LVNC, which is a relatively large proportion.
Regardless of LV failure, RV dysfunction was identified as an independent prognostic marker for LVNC, which might be due to the following reasons.
First, a remarkable RV non-compaction manifestation may indicate serious pathological changes in the myocardium. A substantial relationship between the non-compacted/compacted ratio and changes in global ventricular function has been reported, which may be not just in the LV (22). In our study, morphological biventricular involvement was significantly higher in the RV dysfunction group, accounting for 41.5% of cases. Along with primary myocardial disease, small vessel “dysfunction” with impaired coronary flow reserve and microcirculatory defects causes functional abnormalities (23). Changes in coronary microcirculation affect the development of myocardial fibrosis, which is associated with a poor prognosis (24).
Second, RV dysfunction alone could be an indicator of a poor prognosis in heart failure. Aside from pathological changes, RV dysfunction may be secondary to severe LV failure. In this study, low LVEF, large ventricular size, elevated NT-proBNP, and more frequently use of heart failure medications were observed in the RV dysfunction group. These LV alterations can lead to RV pressure overload (pulmonary arterial hypertension secondary to chronic pulmonary venous hypertension), ventricular interdependence associated with septal dysfunction and limited pericardial flexibility (25, 26), neuro-hormonal interactions, and reduced RV coronary perfusion secondary to decreased systolic driving pressure (27, 28). Further development of RV remodeling and myocardial fibrosis (29) may lead to right heart impairment, thus forming a vicious circle. In a previous cohort of individuals with heart failure with preserved LVEF, significant fluid overload and lower cardiac output were observed in the RV dysfunction subgroup (30). This could result in severe venous congestion and lower SBP, which allows greater requirements for vasoactive medications, all of which contribute to not only increased mortality due to rapid hemodynamic deterioration but also higher rates of acute kidney injury (31, 32). Moreover, lower input (oral uptake) due to gastrointestinal congestion might lead to difficulty in strategies for congestion relief (including monitoring of diuretic administration and/or improvement of organ perfusion), further causing increased mortality (33). Indeed, RV dysfunction is a critical determinant of prognosis in heart failure, regardless of the degree of LV dysfunction (16, 34, 35). This was consistent with the previous finding that decreasing RVEF is independently associated with clinical events including heart failure and death in LVNC, even after adjustment for LVEF (6, 9).
Third, management strategies for right heart failure remain limited. In addition to the use of diuretics to relieve symptoms, effective ways to improve the histological changes of the right ventricle are lacking. Simultaneously, the influence of left heart failure on prognosis has not been observed in this study, mainly because of the good management of left heart failure. Patients in the present cohort were regularly followed up in our clinical center, and medications including β-blockers, ACE inhibitors/ARBs, spironolactone, diuretics, and digoxin were used following current international guidelines (36). This is the reason why RV dysfunction was associated with mortality in LVNC after correction of LV function, for better management of LV dysfunction in clinical cases.
In this study, the evidence obtained for heart failure rehospitalization and implantation of ICD/CRT was weak. Readmissions were mainly due to worsening symptoms including dyspnea. It is common for left-sided heart failure to receive more clinical attention, whereas signs of systemic congestion (such as edema) are prone to be ignored unless the condition is severe. ICD implantation mainly targets malignant arrhythmia to prevent sudden death. Likewise, CRT mainly indicates non-synchronized ventricular contraction caused by the left bundle branch block, which may not be relevant to RV dysfunction. Additionally, few patients choose to accept device therapies for financial reasons.
RV function is not easily obtained and is a time-consuming procedure because of the complex geometry of the RV and the lack of specific right-sided anatomic landmarks to be used as reference points (12). To date, RV assessment is not as systematic as left heart evaluation and is prone to be ignored by clinicians. In this study, the prognostic value of RV dysfunction in LVNC has been highlighted, which indicates the need to emphasize the evaluation of RV function. Moreover, RVGLS was also found to have prognostic value since it detects subtle changes in RV function in several populations (37, 38) and provides early hints during the preclinical stage. In line with the current guidelines, RV function is considered to be of general prognostic importance in heart failure and quantitative RV assessment appears mandatory (39).
This study has some limitations. This was a single-center study with 117 patients with LVNC included and the right ventricle was not optimally visualized in all cases, resulting in missing data on RV function parameters, such as tricuspid S′. Speckle tracking requires user experience and high-quality images, which are not currently recommended for routine RV assessment. Therefore, large sample-sized studies with long-term follow-up are required to confirm this association in patients with LVNC.
This study demonstrated that RV dysfunction is a strong independent and incremental risk factor for all-cause mortality in patients with LVNC. Two-dimensional strain imaging by STE seems to be a quantitative tool for early RV systolic function abnormalities and is associated with outcomes in patients with LVNC. This finding may have implications for the risk assessment of patients with LVNC, suggesting a regular and quantitative assessment of RV function in patients with LVNC.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Ethical Committee of Peking Union Medical College Hospital, Chinese Academy of Medical Science. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
WW was responsible for data screening, data extraction and analysis, and writing of the manuscript. WC, XL, and LF were responsible for the echocardiography data and checked and reviewed the final manuscript. All authors contributed to the article and approved the submitted version.
This work was sponsored by the Chinese National Natural Science Foundation (Grant Number 81670349) and the Beijing Natural Science Foundation (Grant Number 7172166).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; Cr, creatinine; CRT, cardiac resynchronization therapy; cTnI, cardiac troponin I; DBP, diastolic blood pressure; NT-proBNP, N-terminal fragment of pro-hormone brain natriuretic peptide; FAC, fractional area change; eGFR, estimated glomerular filtration rate; HR, heart rate; ICD, implantable cardioverter-defibrillator; LV, left ventricle; LVEDV, left ventricular end diastolic volume; LVESV, left ventricular end systolic volume; LVEF, left ventricular ejection fraction; LVGLS, left ventricular global longitudinal strain; LVNC, left ventricular non-compaction cardiomyopathy; RV, right ventricular; RVEDA, right ventricular end diastolic area; RVESA, right ventricular end systolic area; RVGLS, right ventricular global longitudinal strain; SBP, systolic blood pressure; TAPSE, tricuspid annular plane systolic excursion; 2D, two-dimensional.
1. Maron BJ, Towbin JA, Thiene G, Antzelevitch C, Corrado D, Arnett D, et al. Contemporary definitions and classification of the cardiomyopathies: an American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation. (2006) 113:1807–16. doi: 10.1161/CIRCULATIONAHA.106.174287
2. Oechslin EN, Attenhofer Jost CH, Rojas JR, Kaufmann PA, Jenni R. Long-term follow-up of 34 adults with isolated left ventricular noncompaction: a distinct cardiomyopathy with poor prognosis. J Am Coll Cardiol. (2000) 36:493–500. doi: 10.1016/S0735-1097(00)00755-5
3. Kobza R, Steffel J, Erne P, Schoenenberger AW, Hürlimann D, Lüscher TF, et al. Implantable cardioverter-defibrillator and cardiac resynchronization therapy in patients with left ventricular noncompaction. Heart Rhythm. (2010) 7:1545–9. doi: 10.1016/j.hrthm.2010.05.025
4. Paterick TE, Umland MM, Jan MF, Ammar KA, Kramer C, Khandheria BK, et al. Left ventricular noncompaction: a 25-year odyssey. J Am Soc Echocardiogr. (2012) 25:363–75. doi: 10.1016/j.echo.2011.12.023
5. Vaidya VR, Lyle M, Miranda WR, Farwati M, Isath A, Patlolla SH, et al. Long-term survival of patients with left ventricular noncompaction. J Am Heart Assoc. (2021) 10:e015563. doi: 10.1161/JAHA.119.015563
6. Stämpfli SF, Donati TG, Hellermann J, Anwer S, Erhart L, Gruner C, et al. Right ventricle and outcome in left ventricular non-compaction cardiomyopathy. J Cardiol. (2020) 75:20–6. doi: 10.1016/j.jjcc.2019.09.003
7. Ivanov A, Dabiesingh DS, Bhumireddy GP, Mohamed A, Asfour A, Briggs WM, et al. Prevalence and prognostic significance of left ventricular noncompaction in patients referred for cardiac magnetic resonance imaging. Circ Cardiovasc Imaging. (2017) 10:e006174. doi: 10.1161/CIRCIMAGING.117.006174
8. Greutmann M, Mah ML, Silversides CK, Klaassen S, Attenhofer Jost CH, Jenni R, et al. Predictors of adverse outcome in adolescents and adults with isolated left ventricular noncompaction. Am J Cardiol. (2012) 109:276–81. doi: 10.1016/j.amjcard.2011.08.043
9. Stacey RB, Andersen M, Haag J, Hall ME, McLeod G, Upadhya B, et al. Right ventricular morphology and systolic function in left ventricular noncompaction cardiomyopathy. Am J Cardiol. (2014) 113:1018–23. doi: 10.1016/j.amjcard.2013.12.008
10. Nucifora G, Aquaro GD, Masci PG, Pingitore A, Lombardi M. Magnetic resonance assessment of prevalence and correlates of right ventricular abnormalities in isolated left ventricular noncompaction. Am J Cardiol. (2014) 113:142–6. doi: 10.1016/j.amjcard.2013.08.049
11. Leung SW, Elayi CS, Charnigo RJ Jr, Syed MA. Clinical significance of right ventricular dysfunction in left ventricular non-compaction cardiomyopathy. Int J Cardiovasc Imaging. (2012) 28:1123–31. doi: 10.1007/s10554-011-9925-z
12. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. (2015) 28:1–39.e14. doi: 10.1016/j.echo.2014.10.003
13. Mor-Avi V, Lang RM, Badano LP, Belohlavek M, Cardim NM, Derumeaux G, et al. Current and evolving echocardiographic techniques for the quantitative evaluation of cardiac mechanics: ASE/EAE consensus statement on methodology and indications endorsed by the Japanese Society of Echocardiography. Eur J Echocardiogr. (2011) 12:167–205. doi: 10.1093/ejechocard/jer021
14. Werther Evaldsson A, Ingvarsson A, Smith JG, Rådegran G, Roijer A, Waktare J, et al. Echocardiographic right ventricular strain from multiple apical views is superior for assessment of right ventricular systolic function. Clin Physiol Funct Imaging. (2019) 39:168–76. doi: 10.1111/cpf.12552
15. Tops LF, Delgado V, Marsan NA, Bax JJ. Myocardial strain to detect subtle left ventricular systolic dysfunction. Eur J Heart Fail. (2017) 19:307–13. doi: 10.1002/ejhf.694
16. Carluccio E, Biagioli P, Alunni G, Murrone A, Zuchi C, Coiro S, et al. Prognostic value of right ventricular dysfunction in heart failure with reduced ejection fraction: superiority of longitudinal strain over tricuspid annular plane systolic excursion. Circ Cardiovasc Imaging. (2018) 11:e006894. doi: 10.1161/CIRCIMAGING.117.006894
17. Jenni R, Oechslin E, Schneider J, Attenhofer Jost C, Kaufmann PA. Echocardiographic and pathoanatomical characteristics of isolated left ventricular non-compaction: a step towards classification as a distinct cardiomyopathy. Heart (Br Card Soc). (2001) 86:666–71. doi: 10.1136/heart.86.6.666
18. Gorter TM, van Melle JP, Rienstra M, Borlaug BA, Hummel YM, van Gelder IC, et al. Right heart dysfunction in heart failure with preserved ejection fraction: the impact of atrial fibrillation. J Card Fail. (2018) 24:177–85. doi: 10.1016/j.cardfail.2017.11.005
19. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF III, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. (2009) 150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006
20. Muraru D, Onciul S, Peluso D, Soriani N, Cucchini U, Aruta P, et al. Sex- and method-specific reference values for right ventricular strain by 2-dimensional speckle-tracking echocardiography. Circ Cardiovasc Imaging. (2016) 9:e003866. doi: 10.1161/CIRCIMAGING.115.003866
21. Voigt JU, Pedrizzetti G, Lysyansky P, Marwick TH, Houle H, Baumann R, et al. Definitions for a common standard for 2D speckle tracking echocardiography: consensus document of the EACVI/Ase/Industry Task Force to standardize deformation imaging. J Am Soc Echocardiogr. (2015) 28:183–93. doi: 10.1016/j.echo.2014.11.003
22. Choudhary P, Hsu CJ, Grieve S, Smillie C, Singarayar S, Semsarian C, et al. Improving the diagnosis of LV non-compaction with cardiac magnetic resonance imaging. Int J Cardiol. (2015) 181:430–6. doi: 10.1016/j.ijcard.2014.12.053
23. Jenni R, Wyss CA, Oechslin EN, Kaufmann PA. Isolated ventricular noncompaction is associated with coronary microcirculatory dysfunction. J Am Coll Cardiol. (2002) 39:450–4. doi: 10.1016/S0735-1097(01)01765-X
24. Nucifora G, Aquaro GD, Pingitore A, Masci PG, Lombardi M. Myocardial fibrosis in isolated left ventricular non-compaction and its relation to disease severity. Eur J Heart Fail. (2011) 13:170–6. doi: 10.1093/eurjhf/hfq222
25. Santamore WP. Dell'Italia LJ. Ventricular interdependence: significant left ventricular contributions to right ventricular systolic function. Prog Cardiovasc Dis. (1998) 40:289–308. doi: 10.1016/S0033-0620(98)80049-2
26. Taylor RR, Covell JW, Sonnenblick EH, Ross JJr. Dependence of ventricular distensibility on filling of the opposite ventricle. Am J Physiol. (1967) 213:711–8. doi: 10.1152/ajplegacy.1967.213.3.711
27. Haddad F, Doyle R, Murphy DJ, Hunt SA. Right ventricular function in cardiovascular disease, part II: pathophysiology, clinical importance, and management of right ventricular failure. Circulation. (2008) 117:1717–31. doi: 10.1161/CIRCULATIONAHA.107.653584
28. Chin KM, Coghlan G. Characterizing the right ventricle: advancing our knowledge. Am J Cardiol. (2012) 110(Suppl):3S−8S. doi: 10.1016/j.amjcard.2012.06.010
29. Farha S, Lundgrin EL, Erzurum SC. Novel therapeutic approaches to preserve the right ventricle. Curr Heart Fail Rep. (2013) 10:12–7. doi: 10.1007/s11897-012-0119-3
30. Kanagala P, Arnold JR, Singh A, Khan JN, Gulsin GS, Gupta P, et al. Prevalence of right ventricular dysfunction and prognostic significance in heart failure with preserved ejection fraction. Int J Cardiovasc Imaging. (2021) 37:255–66. doi: 10.1007/s10554-020-01953-y
31. Sanz J, Sánchez-Quintana D, Bossone E, Bogaard HJ, Naeije R. Anatomy, function, and dysfunction of the right ventricle: JACC State-of-the-Art Review. J Am Coll Cardiol. (2019) 73:1463–82. doi: 10.1016/j.jacc.2018.12.076
32. Vallabhajosyula S, Sakhuja A, Geske JB, Kumar M, Kashyap R, Kashani K, et al. Clinical profile and outcomes of acute cardiorenal syndrome type-5 in sepsis: an eight-year cohort study. PLoS ONE. (2018) 13:e0190965. doi: 10.1371/journal.pone.0190965
33. Mullens W, Damman K, Harjola VP, Mebazaa A, Brunner-La Rocca HP, Martens P, et al. The use of diuretics in heart failure with congestion - a position statement from the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. (2019) 21:137–55. doi: 10.1002/ejhf.1369
34. Ghio S, Guazzi M, Scardovi AB, Klersy C, Clemenza F, Carluccio E, et al. Different correlates but similar prognostic implications for right ventricular dysfunction in heart failure patients with reduced or preserved ejection fraction. Eur J Heart Fail. (2017) 19:873–9. doi: 10.1002/ejhf.664
35. Gorter TM, Hoendermis ES, van Veldhuisen DJ, Voors AA, Lam CS, Geelhoed B, et al. Right ventricular dysfunction in heart failure with preserved ejection fraction: a systematic review and meta-analysis. Eur J Heart Fail. (2016) 18:1472–87. doi: 10.1002/ejhf.630
36. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. (2021) 42:3599–726. doi: 10.1093/eurheartj/ehab368
37. Antoni ML, Scherptong RW, Atary JZ, Boersma E, Holman ER, van der Wall EE, et al. Prognostic value of right ventricular function in patients after acute myocardial infarction treated with primary percutaneous coronary intervention. Circ Cardiovasc Imaging. (2010) 3:264–71. doi: 10.1161/CIRCIMAGING.109.914366
38. van Kessel M, Seaton D, Chan J, Yamada A, Kermeen F, Hamilton-Craig C, et al. Prognostic value of right ventricular free wall strain in pulmonary hypertension patients with pseudo-normalized tricuspid annular plane systolic excursion values. Int J Cardiovasc Imaging. (2016) 32:905–12. doi: 10.1007/s10554-016-0862-8
39. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. (2016) 18:891–975. doi: 10.1002/ejhf.592
Keywords: right ventricular dysfunction, left ventricular non-compaction, mortality, rehospitalization, strain
Citation: Wang W, Chen W, Lin X and Fang L (2022) Influence of Right Ventricular Dysfunction on Outcomes of Left Ventricular Non-compaction Cardiomyopathy. Front. Cardiovasc. Med. 9:816404. doi: 10.3389/fcvm.2022.816404
Received: 16 November 2021; Accepted: 11 January 2022;
Published: 31 January 2022.
Edited by:
Giovanni Benfari, University of Verona, ItalyReviewed by:
Diego Fanti, Integrated University Hospital Verona, ItalyCopyright © 2022 Wang, Chen, Lin and Fang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xue Lin, bGlueHVlcHVtY2hAcXEuY29t; Ligang Fang, ZmFuZ2xncHVtY2hAc2luYS5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.