- 1Department of Cardiology, The Affiliated Ganzhou Hospital of Nanchang University, Ganzhou, China
- 2Second Clinical Medical College, Nanchang University, Nanchang, China
- 3State Key Laboratory of Ophthalmology, Zhongshan Ophthalmic Center, Sun Yat-sen University, Guangzhou, China
Background: Intraocular bleeding is a devastating adverse event for patients with atrial fibrillation (AF) receiving anticoagulant therapy. It is unknown whether non-vitamin K oral anticoagulants (NOACs) compared with warfarin can reduce the risk of intraocular bleeding in patients with AF. Herein, we conducted a meta-analysis to evaluate the effect of NOACs vs. warfarin on intraocular bleeding in the AF population.
Methods: Studies were systematically searched from the Embase, PubMed, and Cochrane databases until April 2022. We included studies if they enrolled patients with AF and compared the intraocular bleeding risk between NOACs and warfarin and if they were randomized controlled trials (RCTs) or observational cohort studies. The random-effects model was chosen to evaluate the pooled odds ratios (ORs) and 95% confidence intervals (CIs).
Results: A total of 193,980 patients with AF from 5 randomized controlled trials (RCTs) and 1 cohort study were included. The incidence of intraocular bleeding among AF patients treated with warfarin and NOACs was 0.87% (n = 501/57346) and 0.61% (n = 836/136634), respectively. In the pooled analysis with the random-effects model, the use of NOACs was not significantly associated with the risk of intraocular bleeding (OR = 0.74; 95% CI 0.52–1.04, P = 0.08) compared with warfarin use. In addition, the sensitivity analysis with the fixed-effects model suggested that NOAC users had a lower incidence of intraocular bleeding than patients with warfarin (OR = 0.57; 95% CI 0.51–0.63, P < 0.00001).
Conclusions: Our current meta-analysis suggested that the use of NOACs had no increase in the incidence of intraocular bleeding compared with warfarin use in patients with AF. Whether the use of NOACs is superior to warfarin needs more research to confirm.
Introduction
Atrial fibrillation (AF) has become the most common arrhythmia that affects people worldwide. Patients who have been diagnosed with AF are more prone to suffer from thromboembolic events (1, 2). As such, we urgently need to find an appropriate treatment that can prevent the risk of thromboembolism in patients with AF (3, 4). Although warfarin, a kind of vitamin K antagonists, has already proven to be practical for thromboprophylaxis (5), it still has many disadvantages (e.g., interactions with other drugs or food, frequent monitoring of international normalized ratio) (6, 7). Recently, non-vitamin K oral anticoagulants (NOACs; i.e., dabigatran, rivaroxaban, apixaban, and edoxaban) have been widely used in patients with AF. Newly published guidelines consistently recommend the use of NOACs as the criterion for anticoagulant therapy in patients with AF in terms of their effectiveness and safety compared with warfarin (2, 3, 8).
Although NOACs are safer than warfarin for AF-related stroke prevention (9), the concern of bleeding risks still remains in the NOAC users. In patients with AF receiving anticoagulant therapy, a rare but serious complication of NOACs is intraocular bleeding (10, 11), which may cause visual acuity impairment and sometimes require surgical intervention if it deteriorates. Although three prior meta-analyses by Caldeira et al. (12), Sun et al. (13), and Phan et al. (14) have compared the risk of intraocular bleeding caused by NOACs and warfarin, they have yielded different results. Sun et al. (13) included 12 studies with a sample size of 102,627 patients with AF or venous thromboembolism (VTE) and found that NOACs could reduce the incidence of intraocular bleeding by up to 20% compared with warfarin. In contrast, Caldeira et al. (12) included 17 studies with 117,563 patients with AF or VTE, and claimed that there was no difference in intraocular bleeding between NOACs and warfarin. Phan et al. (14) conducted a network meta-analysis by including 102,617 patients with AF or VTE from 12 RCTs (11,746 treated with apixaban, 18,132 with edoxaban, 11,893 with rivaroxaban, 16,074 with dabigatran, 18,389 with switched NOACs and 44,764 with warfarin). They concluded that only edoxaban was associated with a significantly diminished risk of intraocular bleeding compared with warfarin. Moreover, a prospective cohort study conducted by Campello et al. (15), enrolling 275 cases and 322 controls, found that there was a slightly increased incidence of bleeding in thrombophilia patients treated with NOACs. Subsequently, a large observational cohort study by Park et al. (16) enrolled 27,496 patients with warfarin and 93,691 NOAC users and concluded that the risk of intraocular bleeding was lower in the NOAC group. It is still unclear whether the use of NOACs compared with warfarin can reduce the risk of intraocular bleeding in patients with AF. In the present meta-analysis, we re-evaluated the effect of NOACs vs. warfarin on intraocular bleeding in the AF population. Furthermore, we only included patients diagnosed with AF, rather than patients with AF or VTE. Not only RCTs but also observational cohort studies were included in our analysis.
Methods
This meta-analysis and systematic review were performed according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) items (17). Since the results of studies included in this meta-analysis have been published, we did not need to provide ethical approval.
Strategy of Literature Search
In order to find studies comparing the effects of NOACs and warfarin on patients with AF, we conducted a systematic search of articles published in the PubMed, Embase, and Cochrane databases before April 2022. The search terms were included as follows: 1) novel oral anticoagulants, non-vitamin K oral anticoagulants, direct oral anticoagulants, apixaban, edoxaban, dabigatran, rivaroxaban; 2) vitamin K antagonists, warfarin; and 3) atrial fibrillation. The detailed search strategies based on electronic databases were provided in Supplementary Table 1. There were no language restrictions in the search process.
Eligibility Criteria
We included randomized controlled trials (RCTs) or observational cohort studies that reported the effect of NOACs (dabigatran, rivaroxaban, apixaban, or edoxaban) compared with warfarin in non-valvular AF patients. We chose studies that used intraocular bleeding as the outcome, which was defined as major bleeding. In this meta-analysis, only subretinal hemorrhage, vitreous hemorrhage, hyphema, and suprachoroidal hemorrhage were considered the major bleeding event, precluding minor uncomplicated bleedings (e.g., subconjunctival hemorrhages), which met the criteria set by the International Society on Thrombosis and Hemostasis (18). Studies focusing on AF patients with ablation, cardioversion, or left-atrial appendage were excluded. Certain publication types with insufficient data (e.g., comments, reviews, letters, case reports, expert opinions, and editorials) were also excluded.
Data Extraction
After retrieving the literature, two reviewers screened them independently through title and abstract for the potential studies and then did a full-text reading to find the literature that met the requirements. Disagreements were resolved through discussion or consultation with a third researcher. Two reviewers extracted the following data: author, the year of publication, data source, study design, patient information, type and dosage of NOACs, follow-up period, number of events, and sample size.
Quality Assessment
The bias risk of RCTs was assessed using the Cochrane Collaboration's tool on the selection bias, performance bias, detection bias, attrition bias, reporting bias, and other biases. For each domain of the Cochrane Collaboration's tool, the bias risk was scored as “low,” “unclear,” or “high” risk. For observational cohort studies, the Newcastle-Ottawa Scale (NOS) tool was used to assess the study quality. The NOS tool had a total of 9 points from 3 major sections: the selection of cohorts (0-4 points), the comparability of cohorts (0-2 points), and the assessment of the outcome (0–3 points). In this meta-analysis, the study with a NOS score of ≥ 6 points was defined as moderate to high quality, and a NOS score of < 6 points was considered a low quality (19).
Data Analysis
The statistical heterogeneity across the included studies was assessed using the P-value in the Cochrane Q test and the I2 statistic. A P-value of <0.1 or I2 value of > 50% suggested significant heterogeneity. For each included study, we collected the number of events and the sample size in the warfarin- or NOAC- groups, which were pooled by the random-effect model in consideration of the substantial heterogeneity across the included studies. The pooled results were expressed as the odds ratios (ORs) and 95% confidence intervals (CIs). In the sensitivity analysis, we re-performed the above-mentioned analysis using a fixed-effects model. Moreover, we performed the sensitivity analysis by deleting data from a single study to analyze the impact of one single study on the combined effect size. According to the Cochrane handbook, the publication bias was not formally assessed when the number of the included studies was < 10.
All the statistical analyses were performed using the Review Manager version 5.4 software (the Cochrane Collaboration 2014, Nordic Cochrane Centre Copenhagen, Denmark; https://community.cochrane.org/). In this study, a P-value of < 0.05 indicated statistical significance.
Results
Study Selection
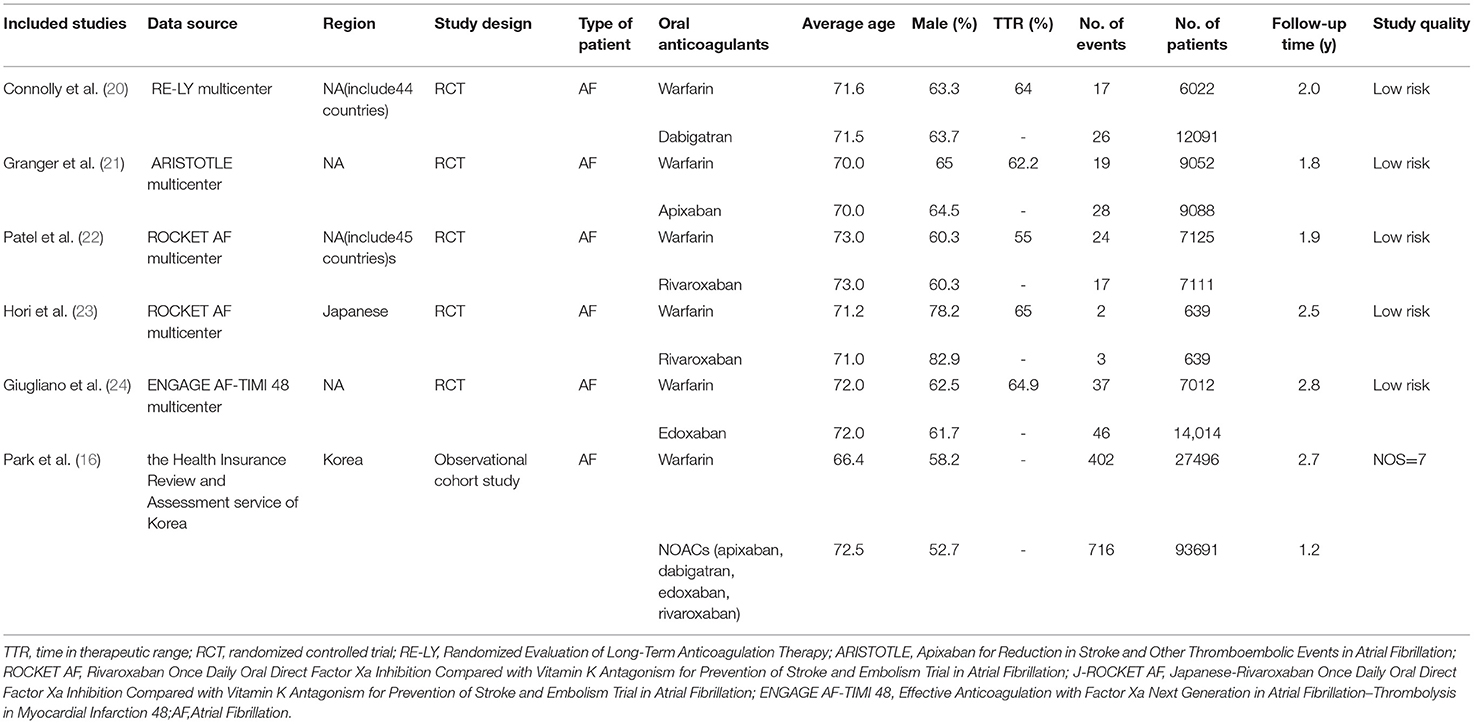
After a careful literature search, a total of 11,678 articles were initially selected from the electronic database. Among them, 2,943 articles were excluded due to the repeated selection, and 8652 were eliminated after the screenings of the titles and abstracts. Subsequently, 77 articles were precluded after the full-text screenings because (1) studies were not RCTs or observational cohorts (n = 65); (2) no specific data were given (n = 5); (3) warfarin was not used as the reference (n = 7). Finally, a total of 6 studies [5 RCTs (20–24) and 1 observational cohort study (16)] were selected in this meta-analysis (Figure 1).
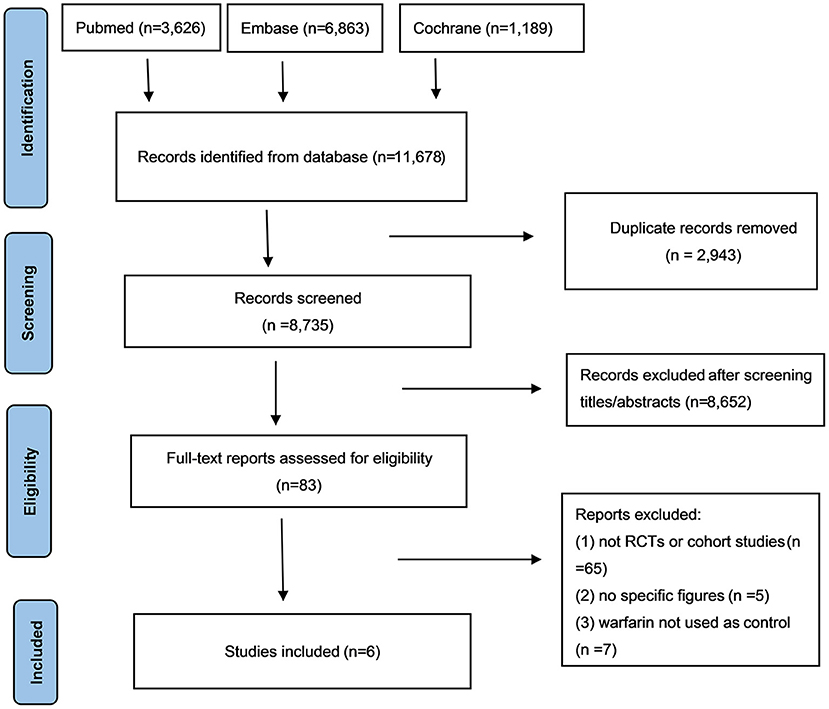
Figure 1. The process of literature search of this meta-analysis. NOACs, novel oral anticoagulants; CI, confidence interval.
Study Characteristics
The baseline characteristics of the included studies are listed in Table 1. The publication date of these articles ranged from 2009 to 2020, and the sample size ranged from 1,278 to 121,187. Specifically, Connolly et al. (20) included a total of 18,113 patients diagnosed with AF (6,022 patients on warfarin and 12,091 patients on NOACs). Granger et al. (21) enrolled 18,201 patients with AF, but 9,052 patients with warfarin and 9,088 patients with NOACs were included in this meta-analysis. Patel et al. (22) studied 14,264 patients with AF, including 7,111 patients treated with NOACs only and 7,125 patients treated with warfarin only. A total of 1,278 patients with AF were included in the study by Hori et al. (23), half of whom were treated with NOACs and half with warfarin. Giugliano et al. (24) studied 21,105 patients diagnosed with AF, including 7,012 patients who received warfarin and 14,014 patients who received NOACs. The study by Park et al. (16) included 121,187 patients with AF (27,496 on warfarin and 93,681 on NOACs). Among the various outcome, subretinal hemorrhage, vitreous hemorrhage, hyphema, and suprachoroidal hemorrhage were considered intraocular bleeding (18), precluding minor uncomplicated bleedings.
For the study quality assessment, all the 5 RCTs (20–24) had a low risk of bias, and the observational cohort by Park et al. (16) had an acceptable quality with a NOS of 7 points.
Incidence of Intraocular Bleeding Between NOACs vs. Warfarin
In the pooled analysis, the incidence of intraocular bleeding in AF patients treated with warfarin and NOACs was 0.87% (n = 501/57,346) and 0.61% (n = 836/136,634), respectively. Our pooled results based on the random-effects model showed that the use of NOACs was not significantly associated with the risk of intraocular bleeding (OR = 0.74, 95% CI 0.52–1.04) compared with warfarin use (Figure 2). Of note, there was high heterogeneity across the selected studies (I2 = 66%).
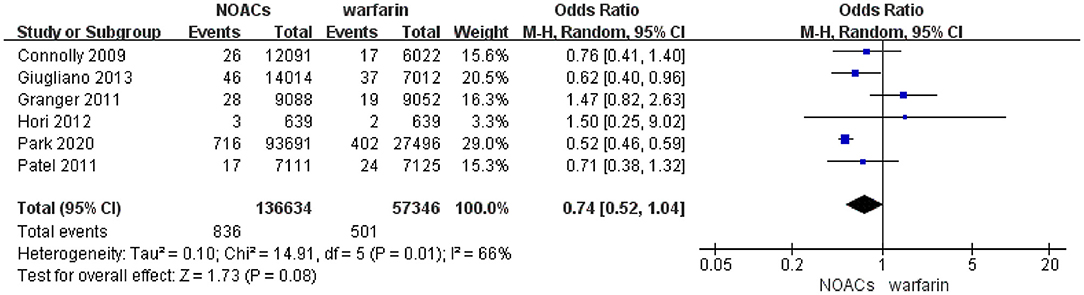
Figure 2. Forest plot of intraocular bleeding events in patients with AF with NOACs vs. warfarin using the random-effects model. NOACs, novel oral anticoagulants; CI, confidence interval.
After excluding the study by Granger et al., we found that the I2 value was reduced from 66% to 0%. The re-analysis after exclusion of the study by Granger et al. showed that NOACs distinctly diminished the rate of intraocular bleeding in patients with AF in comparison with warfarin (OR 0.54; 95% CI 0.48–0.61) (Supplementary Figure 1). In the sensitivity analysis with the fixed-effects model, the pooled results suggested that NOAC users had a lower incidence of intraocular bleeding compared with those patients with warfarin (OR = 0.57; 95% CI 0.51–0.63) (Supplementary Figure 2).
Discussion
Our study was the first meta-analysis to investigate the effect of NOACs on the risk of intraocular bleeding in patients with AF compared with warfarin. According to our study based on the random-effects model, we found that there was no significant difference in the risk of intraocular bleeding in patients with AF between NOACs and warfarin. However, the pooled results changed in the sensitivity analysis when we used the fixed-effects model or excluded the study by Granger et al., and both suggested that NOAC users had a lower risk of intraocular bleeding than warfarin users.
In spite of the low incidence of intraocular bleeding in patients with AF after receiving anticoagulant therapy, it could lead to serious consequences once occurred. Massive intraocular bleeding was often associated with poor vision, and in some cases even required surgical intervention. Three previous predominant meta-analyses on this topic, conducted by Sun et al. (13), Caldeira et al. (12) and Phan et al. (14) respectively, carried out different conclusions. With an analysis involving 57,863 patients with AF or VTE from 12 studies, Sun et al. (13) found that novel oral anticoagulants could reduce the incidence of intraocular bleeding by up to 20% compared with warfarin, but Caldeira et al. (12) including 17 studies with a sample size of 117,563 patients with AF or TVE reported that NOACs couldn't diminish the incidence of intraocular bleeding in comparison with warfarin. However, although the risk estimates of Caldeira et al. cross unity, which indicated an absence of statistical significance, the wide 95% CIs and actual point estimates suggested that NOACs may still have a slight benefit (a decline by 16% in intraocular bleeding) compared with warfarin. Moreover, Phan et al. (14) conducted a network meta-analysis by including 102,617 patients with AF or VTE from 12 RCTs (11,746 of them were treated with apixaban, 18,132 with edoxaban, 11,893 with rivaroxaban, 16,074 with dabigatran, 18,389 with switched NOACs and 44,764 with warfarin). They concluded that edoxaban was associated with a significantly diminished risk of intraocular bleeding compared with warfarin, while Apixaban was the only NOAC associated with an increased risk of intraocular bleeding. Other NOACs were not different from warfarin. We believed that such a result may be caused by the small number of included studies, so we conducted an updated meta-analysis on this topic. In order to further investigate whether NOACs could reduce the incidence of intraocular bleeding in patients with AF compared with warfarin, we included data from newly published studies and then reperformed a meta-analysis on this topic. We did not include patients diagnosed with VTE because the effect of NOACs on patients with VTE and AF may be different, leading to the misestimation of the role of NOACs in the AF population. Unfortunately, it was shown that no significant difference was found between NOACs and warfarin in reducing the intraocular bleeding in patients with AF, according to the result of our meta-analysis.When in a sensitivity analysis, After excluding the study by Granger et al., we found that the I2 value was reduced from 66% to 0%. The re-analysis after exclusion of the study by Granger et al. showed that NOACs distinctly diminished the rate of intraocular bleeding in patients with AF in comparison with warfarin.It seems to suggest that NOACS is superior to warfarin, but due to the large heterogeneity across studies, we cannot draw this conclusion and more studies are needed to confirm.
Although our study did not directly demonstrate a benefit of NOACs, the actual point estimates and 95% CI of our results still suggested a potential benefit of NOACs in reducing the risk of intraocular bleeding. These results had clinical implications for ophthalmologists to correctly manage the patients receiving anticoagulant therapy, especially those with a high probability of intraocular bleeding. Unluckily, our work cannot answer the question of whether patients with AF treated with NOACs are at a lower risk of intraocular bleeding. This meta-analysis needs to be updated in the future with new research data. Current studies support that NOACs at least do not cause more harm than warfarin (25–27), and we predict that NOACs will become more popular among patients since there is no need to frequently draw blood to monitor the international normalized ratio. Nonetheless, clinicians still need to make sure appropriate doses of medication and always be aware of the possibility of bleeding symptoms.
Current studies did not explicitly explain the specific mechanisms of intraocular bleeding in patients receiving anticoagulation therapy. It has been speculated in the literature that NOACs only targeted one site in the coagulation, while warfarin targeted multiple sites (28). Due to the different mechanisms of action, it was also potentially suggested that the risk of intraocular bleeding may be different between NOACs and warfarin. In addition, the effects of different types (apixaban, edoxaban, dabigatran, rivaroxaban) (14, 29, 30) and doses (low, standard, high) (31–33) of NOACs may vary. Further detailed research is needed to confirm this hypothesis.
Limitations of the Study
Overall, there are still some limitations in our study. First of all, no matter what kind of antithrombotic therapy was, intraocular bleeding was uncommon. As a result, the number of studies we included was limited and small. Similarly, the number of intraocular bleeding events in each trial was quite low. Second, the long-term effect of NOACs on intraocular bleeding could not be evaluated due to the short follow-up time of the included studies. Third, our study found no significant difference between NOACs and warfarin in influencing the risk of intraocular bleeding in patients with AF, but the actual point estimates and 95% CI suggested that NOACs still have a potential benefit. Fourth, data from both RCTs and observational studies were combined simultaneously, which may reduce the reliability of the results. Last but not the least, we did not differentiate between specific NOACs, which may have contributed to our lack of statistically significant results. After all, there are pharmacokinetic and pharmacodynamic differences among them, such as bioavailability, protein binding, and metabolism.
Conclusions
Our current meta-analysis suggested that the use of NOACs had no increase in the incidence of intraocular bleeding compared with warfarin use in patients with AF. Whether the use of NOACs is superior to warfarin needs more research to confirm.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We sincerely thank Wengen Zhu (Department of Cardiology, the First Affiliated Hospital of Sun Yat-sen University, Guangzhou of Guangdong, China) for the directions in data interpretation and language polishing.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.813419/full#supplementary-material
References
1. Patel NJ, Atti V, Mitrani RD, Viles-Gonzalez JF, Goldberger JJ. Global rising trends of atrial fibrillation: a major public health concern. Heart. (2018) 104:1989–90. doi: 10.1136/heartjnl-2018-313350
2. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. (2021) 42:373–498. doi: 10.1093/eurheartj/ehaa798
3. January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC Jr., et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines and the heart rhythm society in collaboration with the society of thoracic surgeons. Circulation. (2019) 140:e125–51. doi: 10.1161/CIR.0000000000000665
4. Steffel J, Verhamme P, Potpara TS, Albaladejo P, Antz M, Desteghe L, et al. The 2018 European heart rhythm association practical guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur Heart J. (2018) 39:1330–93. doi: 10.1093/eurheartj/ehy136
5. Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. (2007) 146:857–67. doi: 10.7326/0003-4819-146-12-200706190-00007
6. Barrios V, Escobar C, Calderón A, Rodríguez Roca GC, Llisterri JL, Polo García J. Use of antithrombotic therapy according to CHA2DS2-VASc score in patients with atrial fibrillation in primary care. Revista espanola de cardiologia (English ed). (2014) 67:150–1. doi: 10.1016/j.rec.2013.07.009
7. Reiffel JA. Will direct thrombin inhibitors replace warfarin for preventing embolic events in atrial fibrillation? Curr Opin Cardiol. (2004) 19:58–63. doi: 10.1097/00001573-200401000-00012
8. Lip GYH, Banerjee A, Boriani G, Chiang CE, Fargo R, Freedman B, et al. Antithrombotic therapy for atrial fibrillation: CHEST guideline and expert panel report. Chest. (2018) 154:1121–201. doi: 10.1016/j.chest.2018.07.040
9. Steffel J, Collins R, Antz M, Cornu P, Desteghe L, Haeusler KG, et al. 2021 European heart rhythm association practical guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Europace. (2021) 23:1612–76. doi: 10.1093/europace/euab157
10. Heidbuchel H, Verhamme P, Alings M, Antz M, Hacke W, Oldgren J, et al. European heart rhythm association practical guide on the use of new oral anticoagulants in patients with non-valvular atrial fibrillation. Europace. (2013) 15:625–51. doi: 10.1093/europace/eut083
11. Mason JO. 3rd, Frederick PA, Neimkin MG, White MF Jr., Feist RM, Thomley ML, et al. Incidence of hemorrhagic complications after intravitreal bevacizumab (avastin) or ranibizumab (lucentis) injections on systemically anticoagulated patients. Retina (Philadelphia, Pa). (2010) 30:1386–9. doi: 10.1097/IAE.0b013e3181e09739
12. Caldeira D, Canastro M, Barra M, Ferreira A, Costa J, Pinto FJ, et al. Risk of substantial Intraocular bleeding with novel oral anticoagulants: systematic review and meta-analysis. JAMA Ophthalmol. (2015) 133:834–9. doi: 10.1001/jamaophthalmol.2015.0985
13. Sun MT, Wood MK, Chan W, Selva D, Sanders P, Casson RJ, et al. Risk of intraocular bleeding with novel oral anticoagulants compared with warfarin: a systematic review and meta-analysis. JAMA Ophthalmol. (2017) 135:864–70. doi: 10.1001/jamaophthalmol.2017.2199
14. Phan K, Lloyd D, Wilson-Smith A, Leung V, Andric M. Intraocular bleeding in patients managed with novel oral anticoagulation and traditional anticoagulation: a network meta-analysis and systematic review. Br J Ophthalmol. (2018). doi: 10.1136/bjophthalmol-2018-312198
15. Park SJ, Lee E, Lee K, Park B, Chung YR. Efficacy and safety of non-vitamin K-antagonist oral anticoagulants for retinal vascular diseases in patients with atrial fibrillation: Korean cohort study. Sci Rep. (2020) 10:4577. doi: 10.1038/s41598-020-61609-8
16. Campello E, Spiezia L, Simion C, Tormene D, Camporese G, Dalla Valle F, et al. Direct oral anticoagulants in patients with inherited thrombophilia and venous thromboembolism: a prospective cohort study. J Am Heart Assoc. (2020) 9:e018917. doi: 10.1161/JAHA.120.018917
17. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ (Clinical Research ed). (2009) 339:b2700. doi: 10.1136/bmj.b2700
18. Schulman S, Kearon C. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. (2005) 3:692–4. doi: 10.1111/j.1538-7836.2005.01204.x
19. Zhou Y, Ma J, Zhu W. Efficacy and safety of direct oral anticoagulants versus warfarin in patients with atrial fibrillation across BMI categories: a systematic review and meta-analysis. Am J Cardiovasc Drugs. (2020) 20:51–60. doi: 10.1007/s40256-019-00362-4
20. Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. (2009) 361:1139–51. doi: 10.1056/NEJMoa0905561
21. Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. (2011) 365:981–92.
22. Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. (2011) 365:883–91. doi: 10.1056/NEJMoa1009638
23. Hori M, Matsumoto M, Tanahashi N, Momomura S, Uchiyama S, Goto S, et al. Rivaroxaban vs. warfarin in Japanese patients with atrial fibrillation – the J-ROCKET AF study. Circ J. (2012) 76:2104–11. doi: 10.1253/circj.CJ-12-0454
24. Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. (2013) 369:2093–104. doi: 10.1056/NEJMoa1310907
25. Zhou Y, He W, Zhou Y, Zhu W. Non-vitamin K antagonist oral anticoagulants in patients with hypertrophic cardiomyopathy and atrial fibrillation: a systematic review and meta-analysis. J Thromb Thrombolysis. (2020) 50:311–7. doi: 10.1007/s11239-019-02008-3
26. Liao XZ, Fu YH, Ma JY, Zhu WG, Yuan P. Non-Vitamin K Antagonist oral anticoagulants vs. warfarin in patients with atrial fibrillation and peripheral artery disease: a systematic review and meta-analysis. Cardiovasc Drugs Ther. (2020) 34:391–9. doi: 10.1007/s10557-020-06962-6
27. Kim IS, Kim HJ, Kim TH, Uhm JS, Joung B, Lee MH, et al. Non-vitamin K antagonist oral anticoagulants have better efficacy and equivalent safety compared to warfarin in elderly patients with atrial fibrillation: a systematic review and meta-analysis. J Cardiol. (2018) 72:105–12. doi: 10.1016/j.jjcc.2018.01.015
28. Chatterjee S, Sardar P, Biondi-Zoccai G, Kumbhani DJ. New oral anticoagulants and the risk of intracranial hemorrhage: traditional and Bayesian meta-analysis and mixed treatment comparison of randomized trials of new oral anticoagulants in atrial fibrillation. JAMA Neurol. (2013) 70:1486–90. doi: 10.1001/jamaneurol.2013.4021
29. Zhu W, Ye Z, Chen S, Wu D, He J, Dong Y, et al. Comparative effectiveness and safety of non-vitamin K antagonist oral anticoagulants in atrial fibrillation patients. Stroke. (2021) 52:1225–33. doi: 10.1161/STROKEAHA.120.031007
30. Rutherford OW, Jonasson C, Ghanima W, Söderdahl F, Halvorsen S. Comparison of dabigatran, rivaroxaban, and apixaban for effectiveness and safety in atrial fibrillation: a nationwide cohort study. Eur Heart J Cardiovasc Pharmacother. (2020) 6:75–85. doi: 10.1093/ehjcvp/pvz086
31. Bang OY, On YK, Lee MY, Jang SW, Han S, Han S, et al. The risk of stroke/systemic embolism and major bleeding in Asian patients with non-valvular atrial fibrillation treated with non-vitamin K oral anticoagulants compared to warfarin: results from a real-world data analysis. PLoS ONE. (2020) 15:e0242922. doi: 10.1371/journal.pone.0242922
32. Jin H, Zhu K, Wang L, Zhou W, Zhi H. Efficacy and safety of non-vitamin K anticoagulants and warfarin in patients with atrial fibrillation and heart failure: A network meta-analysis. Thromb Res. (2020) 196:109–19. doi: 10.1016/j.thromres.2020.08.021
33. Wang KL, Lopes RD, Patel MR, Büller HR, Tan DS, Chiang CE, et al. Efficacy and safety of reduced-dose non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation: a meta-analysis of randomized controlled trials. Eur Heart J. (2019) 40:1492–500. doi: 10.1093/eurheartj/ehy802
Keywords: atrial fibrillation, non-vitamin K oral anticoagulants, warfarin, intraocular bleeding, meta-analysis
Citation: Liu F, Zhang Y, Luo J and Zhou Y (2022) Intraocular Bleeding in Patients With Atrial Fibrillation Treated With NOACs VS. Warfarin: A Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 9:813419. doi: 10.3389/fcvm.2022.813419
Received: 11 November 2021; Accepted: 02 May 2022;
Published: 01 June 2022.
Edited by:
Antonino Tuttolomondo, University of Palermo, ItalyReviewed by:
Elena Campello, University of Padua, ItalyOlga Germanova, Samara State Medical University, Russia
Copyright © 2022 Liu, Zhang, Luo and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fuwei Liu, Z3psaXVmdXdlaUAxNjMuY29t; Jun Luo, bHVvanVuMTk2NkAxNjMuY29t; Yue Zhou, amF5MDQxNkAxMjYuY29t
†These authors share first authorship
 Fuwei Liu
Fuwei Liu Yupei Zhang2†
Yupei Zhang2† Jun Luo
Jun Luo Yue Zhou
Yue Zhou