- 1Department of Cardiology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China
- 2Department of Hematology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China
Objective: To compare the clinical benefits of rivaroxaban and warfarin in patients with non-valvular atrial fibrillation (NVAF) with high bleeding risk.
Methods: A retrospective study was conducted on patients with high bleeding risk NVAF who were hospitalized at the First Affiliated Hospital of Zhengzhou University between May 31, 2016 and May 31, 2019 and took at least rivaroxaban and warfarin. The clinical benefits of both drugs were assessed by efficacy benefit and safety risk. The primary efficacy benefit was a composite end point for stroke (both ischemic and hemorrhagic) and systemic embolism. The secondary efficacy end points were death and myocardial infarction (MI). The principal safety end point was the composite end point of fatal bleeding and critical organ bleeding.
Results: A total of 1,246 patients with high bleeding risk were enrolled, including 787 patients in the rivaroxaban group and 459 patients in the warfarin group. Results of the primary efficacy benefit endpoint were obtained from 104 patients (13.2%) in the rivaroxaban group and 88 (19.2%) patients in the warfarin group (hazard ratio [HR]: 0.681; 95% confidence interval [CI]: 0.512–0.906; P < 0.001 for non-inferiority). The principal safety end points were observed in 49 (6.23%) patients in the rivaroxaban group and in 55 (11.98%) patients in the warfarin group (HR: 0.469 in the rivaroxaban group; 95% CI: 0.314–0.702; P < 0.001). With respect to secondary efficacy and benefit endpoints, 28 (3.56%) patients in the rivaroxaban group and 22 (4.79%) patients in the warfarin group died, with an HR of 0.760 (95% CI: 0.435–1.329; P = 0.336); 32 (4.07%) patients in the rivaroxaban group; and 26 (5.66%) patients in the warfarin group had MI, with an HR of 1.940 (95% CI: 0.495–1.069, P = 0.254) in the rivaroxaban group.
Conclusions: Rivaroxaban is non-inferior to warfarin in the prevention of stroke and systemic embolism in patients with high blood NVAF. Rivaroxaban is superior to warfarin in reducing fatal bleeding and bleeding in critical organs.
Clinical Trial Registration: Chinese Clinical Trials Registry, identifier ChiCTR2100052454.
Introduction
Patients with atrial fibrillation (AF) have a higher incidence and risk of embolism, with a 4–5-fold increased risk of ischemic stroke. The proportion of stroke caused by AF increases with age; overall, the proportion is about 10–24% in persons aged between 80 and 89 years (1–3). Current professional society guidelines recommend treatment with an oral anticoagulant for patients with AF who are at an increased risk of thromboembolism (4–6). Past use of warfarin has significantly reduced the risk of stroke in patients with AF. Furthermore, warfarin treatment has a narrow therapeutic range, interacts with food and other drugs, and requires regular international normalized ratio (INR) monitoring and frequent dose adjustments (7–9). In recent years, rivaroxaban has been approved for stroke prevention in AF in randomized controlled trials, owing to its non-inferiority in both efficacy and safety when compared to warfarin. In addition, anticoagulant monitoring is not required, and the significant reduction in drug-food interactions also changes the prospects for stroke prevention in AF (10).
Several studies have comparatively analyzed the clinical benefits of rivaroxaban and warfarin (11–16). Manesh et al. (11) conducted a prospective randomized controlled trial—ROCKET-AF—including 14,264 patients with non-valvular atrial fibrillation (NVAF) from 1,178 medical centers across 45 countries. The results showed that rivaroxaban was non-inferior to warfarin in the prevention of stroke or non-central embolism. Furthermore, the incidences of intracranial hemorrhage and fatal hemorrhage were lower in the rivaroxaban group than the warfarin group; no significant difference in the risk of massive hemorrhage was observed between the two groups. However, their study included only a relatively small number of Asian patients and did not differentiate between high and low bleeding risk. Hence, Lee et al. (12) compared the safety and effectiveness of oral anticoagulants in Asian patients with NVAF, and the results showed that rivaroxaban was associated with a lower incidence of ischemic stroke and major bleeding than warfarin. However, patients with prior stroke, cerebral hemorrhage, or gastrointestinal bleeding were not included in the study, and the results of the study could not be extrapolated to patients with high bleeding risk. The ARISTOPHANES study, the largest retrospective observational study conducted by Gyh et al. (13), showed that rivaroxaban had a lower incidence of stroke and systemic embolism than warfarin. Although the study further analyzed various subgroup indicators, such as heart failure, cerebral infarction, CHADS2-VASc score, and HAS-BLED score, the results were not explained in detail. Further, the study population was entirely from the United States; hence, data on Asian patients with high bleeding risk were not available, which was likely relevant to the final results (17, 18). The Centers for Disease Control and Prevention reported a higher intracranial hemorrhage (ICH) mortality among Asians compared with whites (19). The reason is multifactorial; genetically, Asian patients are more likely to be warfarin sensitive or highly sensitive responders, who seem prone to excessive bleeding (20). Except for the variations in distributions of genetic polymorphisms for warfarin metabolism (21, 22), Asian patients tended to have lower body weight, smaller proportions of prior myocardial infarction (23, 24) etc. However, in clinical practice, patients on Asian with AF with multiple complications tend to have high bleeding risk. In 2010, The European Society of Cardiology (ESC) guidelines for the treatment of AF introduced for the first time the HAS-BLED bleeding risk assessment program (25). Therefore, this study specifically conducted a comparative analysis on Asian patients with AF with high bleeding risk.
Methods
The study data were collected from 146,413 patients hospitalized between May 31, 2016 and May 31, 2019 in 11 wards (including 2 critical care units) of the Department of Cardiology at The First Affiliated Hospital of Zhengzhou University, Heyi, and Zhengdong Hospital. Among these, patients with AF were screened out. The inclusion criteria were as follows: (1) Dynamic electrocardiogram was in line with the diagnostic criteria of AF; (2) imaging diagnosis excluded valvular disease; (3) meeting the diagnostic criteria for high risk of bleeding (HAS-BLED score ≥3 points); (4) Those requiring routine anticoagulant therapy with warfarin or rivaroxaban (risk stratification score ≥1 according to CHADS2-VASC); and (5) age ≥18 years. The following patients were excluded: (1) Those that did not comply to treatment regimen; (2) those lost to telephonic follow-up; and (3) those who switched to other types of oral anticoagulant medication during follow-up.
Study participants were divided into two groups: (i) Rivaroxaban group, rivaroxaban (Bayer Healthcare Co., LTD., National drug approval J20180077, 20 mg × 7 tablets) was used for anticoagulant therapy. Fixed oral doses of 15 mg or 20 mg per session were given at fixed times of the day, depending on the patient's creatinine clearance. (ii) Warfarin group: warfarin (Qilu Pharmaceutical Co., LTD., National drug approval H37021334, 2.5 mg × 60 tablets) was used for anticoagulant therapy, and the initial dose was controlled at 2.5 mg/d. Coagulation indices were monitored before medication, and the INR values were measured. The INR values were tested regularly while the patients were on medication, and the values were controlled between 2.0 and 3.0.
A total of 1,343 patients with NVAF who took at least one anticoagulant drug were enrolled between the above-mentioned dates. Each patient was followed-up for 730 days from the beginning of medication by inquiring about hospitalization case, medical advice, and telephone contact. Endpoints related to all-cause death, stroke, MI, systemic embolism, and fatal and critical organ bleeding were recorded during drug administration. Eighteen cases were lost to follow-up, 48 cases did not adhere to the prescribed medication, and 31 cases changed to other types of anticoagulants that were excluded from the study scope. The number of patients eligible for the study was 1,246.
Study Endpoints
The primary efficacy benefit end point of this retrospective study was defined as the composite end point of stroke (ischemic and hemorrhagic) and systemic embolism, and the secondary efficacy benefit end point was defined as all-cause death and MI. The primary safety risk end point was defined as major bleeding, including composite end point of fatal or critical organ bleeding. Fatal bleeding was defined as whole blood transfusion or erythrocyte concentration ≥2 units or hemoglobin reduction ≥2 g/dL. Bleeding in critical organs was defined as bleeding from any of the following anatomical sites: intracranial, spinal, ocular, pericardial, joint, retroperitoneal, or muscular compartment syndrome. Bleeding events involving the central nervous system that met the definition of stroke were considered as hemorrhagic strokes and included in both the primary efficacy and safety end points.
Statistical Analysis
Normality test was performed on the measurement data. Normally distributed data were represented as mean ± SD; non-normally distributed data were represented as medians with interquartile ranges (IQR). Classified data were expressed as percentages. Independent-sample t-test or Mann–Whitney U test was used for pairwise comparison, and the chi-square test was used for comparison of rates. The primary efficacy end point was determined to be a non-inferiority margin of 1.46 by the non-inferiority test. The risk ratios, confident intervals (CIs), and P-values of efficacy end points and safety end points were calculated using the multivariate Cox proportional risk model, and treatment drugs were considered the only co-variable. P ≤ 0.05 indicated statistical difference. By plotting the cumulative rate of events over time, the difference between the occurrence of two sets of data events was apparent.
Results
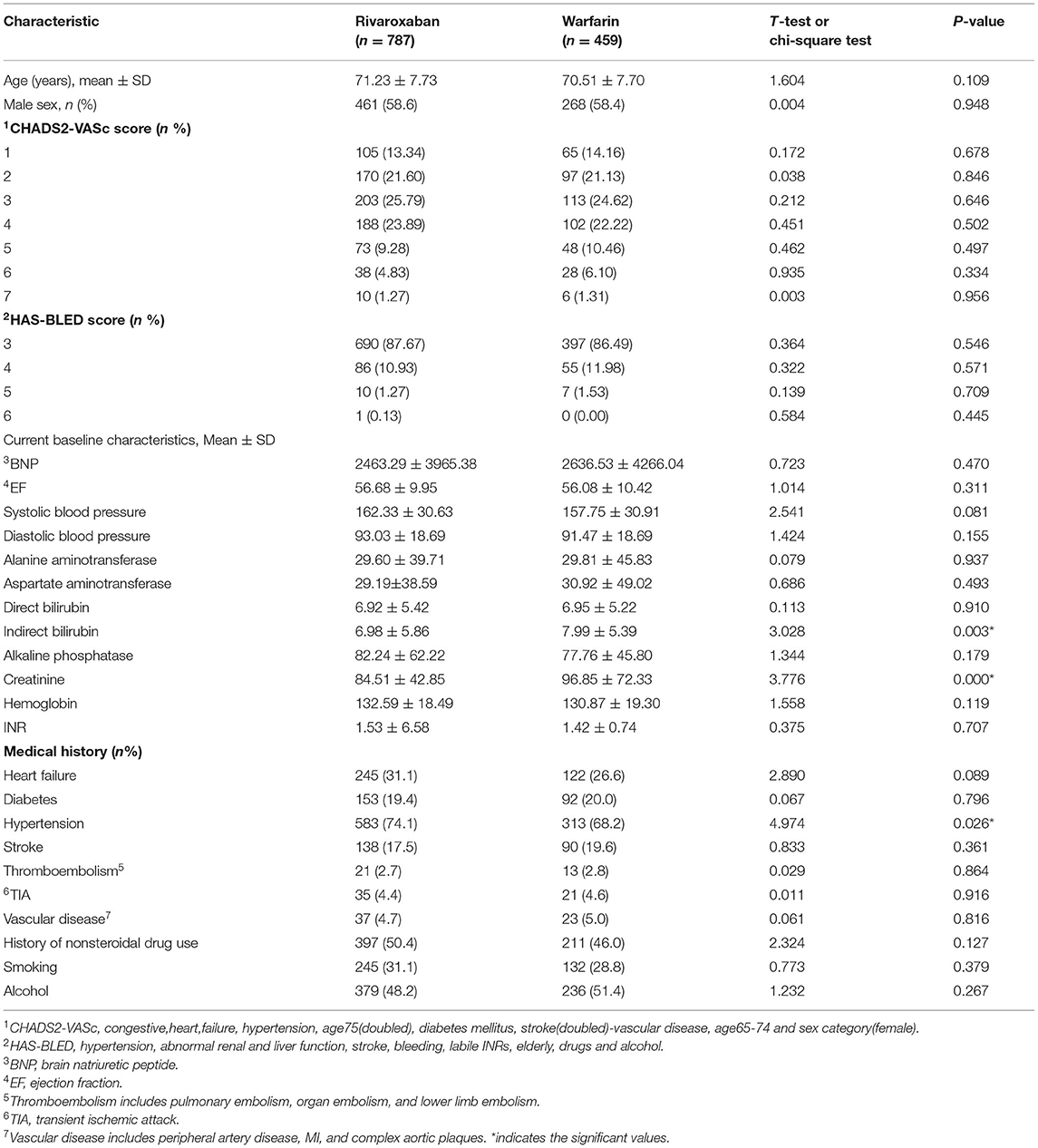
The main clinical characteristics of the patients included in the analysis are shown in Table 1. Corresponding statistical methods were used to compare the baseline data of the two groups. Most clinical characteristics were similar between the two groups.
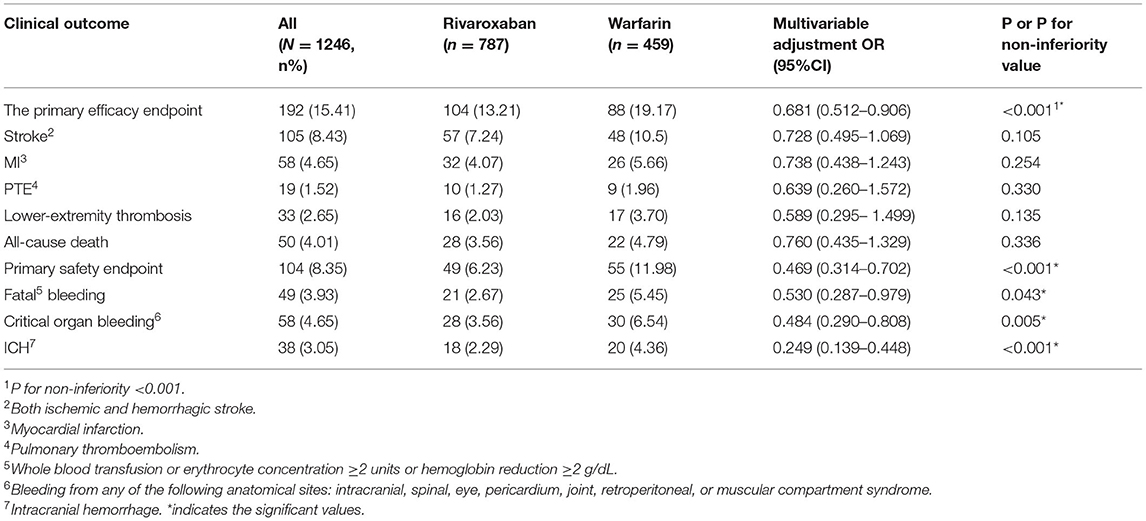
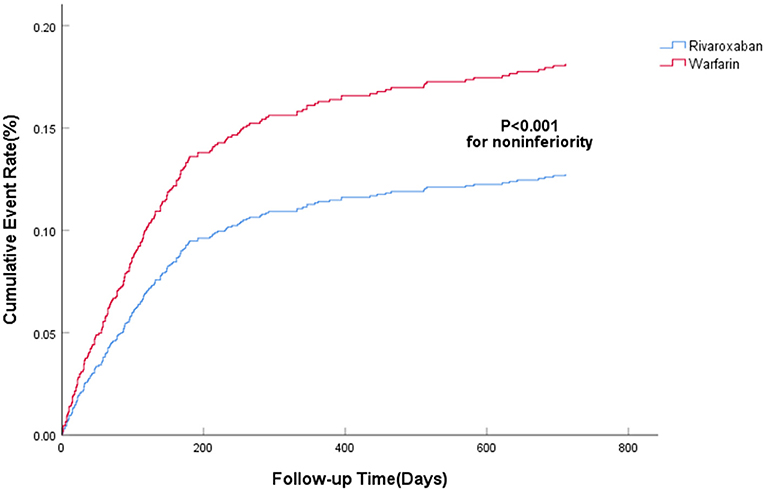
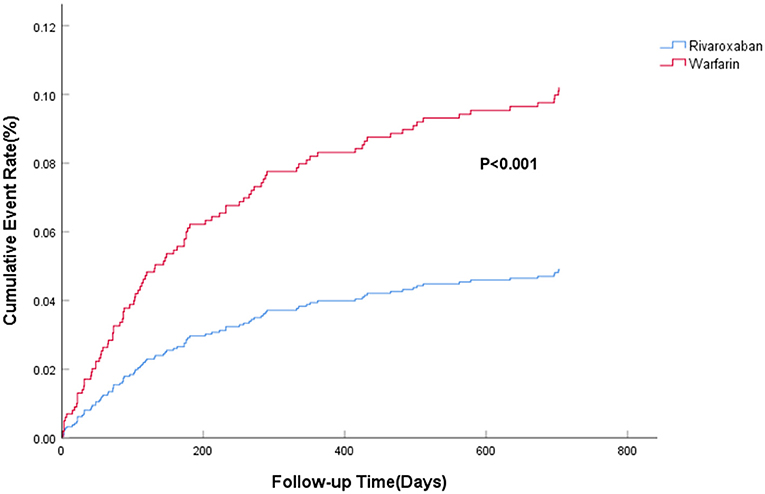
The efficacy benefit and safety end points in this study are shown in Table 2. The principal efficacy benefit end points were obtained from 104 cases (13.2%) in the rivaroxaban group and 88 cases (19.2%) in the warfarin group, and the hazard ratio (HR) of the rivaroxaban group was 0.681 (95% CI: 0.512–0.906; P for non-inferiority <0.001), suggesting that rivaroxaban was non-inferior to warfarin in preventing stroke and non-central embolism in this population with high bleeding risk, as shown in Figure 1. The principal safety end points were observed in 49 (6.23%) patients in the rivaroxaban group and 55 (11.98%) patients in the warfarin group, with an HR of 0.469 (95%CI: 0.314–0.702; P < 0.001), as shown in Figure 2. Fatal bleeding occurred in 21 patients in the rivaroxaban group (2.67%) and 25 patients in the warfarin group (5.45%), with an HR of 0.530 (95% CI: 0.287–0.979; P = 0.043). Furthermore, 28 (3.56%) patients in the rivaroxaban group and 20 (6.54%) patients in the warfarin group had critical organ bleeding (HR: 0.484 in the rivaroxaban group, 95% CI: 0.290–0.808; P = 0.005). Analysis of ICH events showed that 18 (2.29%) ICH events were recorded in the rivaroxaban group and 20 (4.36%) ICH events in the warfarin group, and the HR of the rivaroxaban group was 0.249 (95% CI: 0.139–0.448; P < 0.001), indicating that rivaroxaban was more advantageous than warfarin in reducing fatal bleeding and critical organ bleeding. The difference in critical organ bleeding was mainly due to ICH.
Secondary efficacy and benefit endpoints: Overall, 28 (3.56%) patients in the rivaroxaban group and 22 (4.79%) in the warfarin group died, with an HR of 0.760 (95% CI: 0.435–1.329; P = 0.336); 32 (4.07%) patients in the rivaroxaban group and 26 (5.66%) patients in the warfarin group had MI, with an HR of 1.940 (95% CI: 0.495–1.069, P = 0.254) in the rivaroxaban group. This showed no significant difference between the rivaroxaban and warfarin groups in the prevention of all-cause death and MI.
Discussion
In this retrospective study, we evaluated the clinical benefits of rivaroxaban and warfarin by comparing efficacy benefits and safety risks. Rivaroxaban was non-inferior to warfarin in preventing stroke and non-central embolism. The most worrisome complication of anticoagulation is bleeding, and the low incidence of fatal or critical anatomic bleeding in the rivaroxaban group supports its clinical use. ROCKET-AF and other related large studies concluded that rivaroxaban is non-inferior to warfarin in terms of efficacy and benefit in patients with AF (11). Rivaroxaban has a similar overall risk of major bleeding as warfarin. However, in the subcategory of major bleeding, rivaroxaban was associated with a lower incidence of fatal bleeding and intracranial hemorrhage, but no significant difference in the risk of major bleeding was observed between the two groups (13, 26, 27). However, ROCKET-AF data were collected from 45 countries and did not differentiate between high and low risk of bleeding. The results obtained in our study show that these conclusions are applicable to Asian populations with higher risk of bleeding, which provides additional evidence for clinical use.
Analysis of these events individually showed no significant difference between the two anticoagulants in terms of death and MI. This observation may be due to the small sample size, which was not enough to show a difference. In addition, according to the ROCKET-AF test, most deaths (72%) were due to cardiovascular disease, whereas only 6% of deaths were due to non-hemorrhagic stroke or systemic embolism. Therefore, although more fatal or critical organ bleeding events were recorded in the rivaroxaban group, no significant increase in all-cause deaths was observed.
Rivaroxaban showed obvious advantages regarding fatal bleeding, likely because warfarin dose is easily affected by food and other drugs. Rivaroxaban, on the contrary, is taken orally in a fixed daily dose and is not affected by food or other drugs. Moreover, the drug levels of rivaroxaban do not fluctuate with the effect of external factors; hence, it shows a significant advantage over warfarin (28). In addition, possibly, some patients on warfarin therapy did not regularly test their INR values, as recommended after discharge (29), which led to poor control of INR values and increased fatal bleeding events. In terms of critical organ bleeding events, the incidence of events in the rivaroxaban group was significantly different from that in the warfarin group, which was mainly due to the significant reduction in ICH events. These findings were consistent with the results of previous randomized clinical trials.
Our research participants comprised all inpatients treated in our hospital, and all the data were obtained from the hospital medical records system and patient follow-up, which is very similar to the actual situation of clinical treatment. This study provides supplementary evidence for clinical trial results, and provides reference for the clinical practice of drug use.
This retrospective observational study had several limitations. First, our study was constrained by the inherent limitations of a retrospective evaluation of previous data. Hence, only statistical associations could be drawn; causation could not be determined. Second, although possible influencing factors and several comorbidities were adjusted for by multivariate COX regression, potential residual confounders remained. In clinical practice, systemic differences in patients receiving different oral anticoagulants may be present, and if such differences are not observed, the study results may be biased. Therefore, the possibility that the observed correlations could be attributed to factors not considered in our model cannot be ruled out. Third, our data sources were from inpatients in the Department of Cardiology of the First Affiliated Hospital of Zhengzhou University, and the conclusions were most common only to patients in Asians and further research is needed to confirm whether this conclusion is applicable to non-Asian populations.
Finally, given the large number of statistical tests, especially the cross-tests, the possibility of type I errors cannot be ruled out.
Conclusion
Overall, rivaroxaban was non-inferior to warfarin in preventing stroke and systemic embolism in patients with NVAF with high bleeding risk in this study. Rivaroxaban is superior to warfarin in reducing fatal bleeding and bleeding in critical organs.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
Ethical review and approval was not required for this study with human participants, in accordance with the local legislation and institutional requirements. Written informed consent was not required for this study, in accordance with the local legislation and institutional requirements.
Author Contributions
FH and P-HL conceived, designed, and planned the study. P-HL analyzed the data. P-HL and RG interpreted results. P-HL, FH, and RG drafted the report. RG, Z-HL, M-HN, PC, and Y-BS were involved in the critical revision of the manuscript. All authors contributed to the article, data acquisition, and approved the submitted version.
Funding
This work was supported by Science and Technology Plan of Henan Province (No. 182102310160), Medical Science and Technology Research Project of Henan Province (2018020118).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Dr. FH and RG the First Affiliated Hospital of Zhengzhou University for their help, and we thank the patients who participated in this study and their families.
References
1. Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham study. Stroke. (1991) 22:983–8. doi: 10.1161/01.STR.22.8.983
2. Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, et al. Heart disease and stroke statistics-2017 update: a report from the American heart association. Circ. (2017) 135:e146–603. doi: 10.1161/CIR.0000000000000485
3. Meschia JF, Bushnell C, Boden-Albala B, Braun LT, Bravata DM, Chaturvedi S, et al. Guidelines for the primary prevention of stroke: a statement for healthcare professionals from the American heart association/American stroke association. Stroke. (2014) 45:3754–832. doi: 10.1161/STR.0000000000000046
4. January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American college of cardiology/American heart association task force on practice guidelines and the heart rhythm society. Circ. (2014) 130:2071–104. doi: 10.1161/cir.0000000000000040
5. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. (2016) 37:2893–962. doi: 10.1093/eurheartj/ehw210
6. Macle L, Cairns J, Leblanc K, Tsang T, Skanes A, Cox JL, et al. 2016 Focused update of the canadian cardiovascular society guidelines for the management of atrial fibrillation. Can J Cardiol. (2016) 32:1170–85. doi: 10.1016/j.cjca.2016.07.591
7. Albers GW, Yim JM, Belew KM, Bittar N, Hattemer CR, Phillips BG, et al. Status of antithrombotic therapy for patients with atrial fibrillation in university hospitals. Arch Intern Med. (1996) 156:2311–6. doi: 10.1001/archinte.1996.00440190053006
8. Go AS, Hylek EM, Borowsky LH, Phillips KA, Selby JV, Singer DE. Warfarin use among ambulatory patients with nonvalvular atrial fibrillation: the anticoagulation and risk factors in atrial fibrillation (ATRIA) study. Ann Intern Med. (1999) 131:927–34. doi: 10.7326/0003-4819-131-12-199912210-00004
9. Piccini JP, Hernandez AF, Zhao X, Patel MR, Lewis WR, Peterson ED, et al. Quality of care for atrial fibrillation among patients hospitalized for heart failure. J Am Coll Cardiol. (2009) 54:1280–9. doi: 10.1016/j.jacc.2009.04.091
10. Mazurek M, Huisman M, Rothman K, Paquette M, Teutsch C, Diener HC, et al. Regional differences in antithrombotic treatment for atrial fibrillation: insights from the GLORIA-AF phase II rgistry. Thromb Haemost. (2017) 117:2376–88. doi: 10.1160/TH17-08-0555
11. Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, et al. Rivaroxaban vs. warfarin in non-valvular atrial fibrillation. N Engl J Med. (2011) 365:883–91. doi: 10.1056/NEJMoa1009638
12. Lee SR, Choi EK, Kwon S, Han KD, Jung JH, Cha MJ, et al. Effectiveness and safety of contemporary oral anticoagulants among Asians with non-valvular atrial fibrillation. Stroke. (2019) 50:2245–9. doi: 10.1161/STROKEAHA.119.025536
13. Gyh L, Keshishian A, Li X, Hamilton M, Masseria C, Gupta K., et al. Effectiveness and safety of oral anticoagulants among non-valvular atrial fibrillation patients. Aristophanes Study. (2018) 49:2933–44. doi: 10.1161/STROKEAHA.118.020232
14. Wise JJRO. The BMJ awards 2020: stroke and cardiovascular team of the year. BMJ. (2020) 368: m844 -. doi: 10.1136/bmj.m844
15. Patrick Blin Laurent Fauchier Caroline Dureau-Pournin . Effectiveness and safety of rivaroxaban 15 or 20 mg vs. vitamin K antagonists in non valvular atrial fibrillation. Stroke. (2019)50:2469–76. doi: 10.1161/STROKEAHA.119.025824
16. Coleman CI, Baker WL, Anna-Katharina M, Daniel E, Martinez BK, Bunz TJ, et al. Effectiveness and safety of rivaroxaban vs. warfarin in patients with non-valvular atrial fibrillation and coronary or peripheral artery disease Eur Heart J Cardiovasc Pharmacother. (2020) 3:159–166. doi: 10.1093/ehjcvp/pvz047
17. Yoon CH, Park YK, Kim SJ, Lee MJ, Ryoo S, Kim GM, et al. Eligibility and preference of new oral anticoagulants in patients with atrial fibrillation: comparison between patients with vs. without stroke. Stroke. (2014) 45:2983–8. doi: 10.1161/STROKEAHA.114.005599
18. Shen AY, Yao JF, Brar SS, Jorgensen MB, Chen W. Racial/ethnic differences in the risk of intracranial hemorrhage among patients with atrial fibrillation. J Am Coll Cardiol. (2007) 50:309–15. doi: 10.1016/j.jacc.2007.01.098
19. Ayala C, Greenlund KJ, Croft JB, Keenan NL, Donehoo RS, Giles WH, et al. Racial/ethnic disparities in mortality by stroke subtype in the United States, 1995–1998. Am J Epidemiol. (2001) 154:1057–63. doi: 10.1093/aje/154.11.1057
20. Mega JL, Walker JR, Ruff CT, Vandell AG, Nordio F, Deenadayalu N, et al. Genetics and the clinical response to warfarin and edoxaban: findings from the randomised, double-blind ENGAGE AF-TIMI 48 trial. Lancet. (2015) 385:2280–7. doi: 10.1016/S0140-6736(14)61994-2
21. Takahashi H, Wilkinson GR, Nutescu EA, Morita T, Ritchie MD, Scordo MG, et al. Different contributions of polymorphisms in VKORC1 and CYP2C9 to intra- and inter-population differences in maintenance dose of warfarin in Japanese, Caucasians, and African-Americans. Pharmacogenet Genomics. (2006) 16:101–10. doi: 10.1097/01.fpc.0000184955.08453.a8
22. Gaikwad T, Ghosh K, Shetty S. VKORC1 and CYP2C9 genotype distribution in Asian countries. Thromb Res. (2014) 134:537–44. doi: 10.1016/j.thromres.2014.05.028
23. Hori M, Connolly SJ, Zhu J, Liu LS, Lau CP, Pais P, et al. Dabigatran versus warfarin: effects on ischemic and hemorrhagic strokes and bleeding in Asians and non-Asians with atrial fibrillation. Stroke. (2013) 44:1891–6. doi: 10.1161/STROKEAHA.113.000990
24. Wong KS, Hu DY, Oomman A, Tan RS, Patel MR, Singer DE, et al. Rivaroxaban for stroke prevention in East Asian patients from the ROCKET AF trial. Stroke. (2014) 45:1739–47. doi: 10.1161/STROKEAHA.113.002968
25. Camm AJ, Kirchhof P, Lip GY, Schotten U, Savelieva I, Ernst S, et al. Guidelines for the management of atrial fibrillation: the task force for the management of atrial fibrillation of the European society of cardiology (ESC). Eur Heart J. (2010) 31:2369–429.
26. Guimaraes HP, Lopes RD, Silva PGMdBe, Liporace IL, Berwanger OJNEJoM. Rivaroxaban in Patients with Atrial Fibrillation and a Bioprosthetic Mitral Valve. 2020. N Engl J Med. 383:2117–2126. doi: 10.1056/NEJMoa2029603
27. Sherwood MW, Nessel CC, Hellkamp AS, Mahaffey KW, Piccini JP, Suh EY, et al. Gastrointestinal bleeding in patients with atrial fibrillation treated with rivaroxaban or warfarin: rocket AF trial. J Am Coll Cardiol. (2015) 66:2271–81. doi: 10.1016/j.jacc.2015.09.024
28. Alberts M, Chen YW, Lin JH, Kogan E, Milentijevic DJS. Risks of stroke and mortality in atrial fibrillation patients treated with rivaroxaban and warfarin. Stroke. (2019) 51: 549–555. doi: 10.1161/STROKEAHA.119.025554
Keywords: warfarin, non-valvular atrial fibrillation, clinical benefits, high bleeding risk, rivaroxaban
Citation: Liu P-H, Liu Z-H, Niu M-H, Chen P, Shi Y-B, He F and Guo R (2022) A Comparative Study of the Clinical Benefits of Rivaroxaban and Warfarin in Patients With Non-valvular Atrial Fibrillation With High Bleeding Risk. Front. Cardiovasc. Med. 9:803233. doi: 10.3389/fcvm.2022.803233
Received: 27 October 2021; Accepted: 24 January 2022;
Published: 16 February 2022.
Edited by:
Xiaofeng Yang, Temple University, United StatesReviewed by:
Suowen Xu, University of Science and Technology of China, ChinaAmr Ghanam, Augusta University, United States
Zsolt Bagi, Augusta University, United States
Copyright © 2022 Liu, Liu, Niu, Chen, Shi, He and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fei He, ZmNjaGVmMiYjeDAwMDQwO3p6dS5lZHUuY24=; Rong Guo, ZmNjZ3VvciYjeDAwMDQwO3p6dS5lZHUuY24=
 Peng-Hui Liu
Peng-Hui Liu Ze-Hua Liu1
Ze-Hua Liu1 Rong Guo
Rong Guo