- 1Department of Cardiovascular Medicine, First Affiliated Hospital of Xi'an Jiaotong University, Xi'an, China
- 2Key Laboratory of Molecular Cardiology of Shaanxi Province, Xi'an, China
- 3Department of Cardiology, Northwest Women's and Children's Hospital of Xi'an Jiaotong University Health Science Center, Xi'an, China
- 4National Engineering Research Center for Beijing Biochip Technology, Beijing, China
- 5Department of Nephrology, First Affiliated Hospital of Xi'an Jiaotong University, Xi'an, China
- 6Department of Cardiology, Xi'an International Medical Center Hospital, Xi'an, China
- 7Department of Cardiology, Xi'an No.1 Hospital, Xi'an, China
- 8Department of Ophthalmology, Xi'an People's Hospital, Xi'an, China
- 9Department of Cardiology, Xi'an People's Hospital, Xi'an, China
- 10Department of Medicine, Yale University School of Medicine, New Haven, CT, United States
- 11Department of Medicine, Veterans Administration Healthcare System, West Haven, CT, United States
Objective: Renalase, a novel secretory flavoprotein with amine oxidase activity, is secreted into the blood by the kidneys and is hypothesized to participate in blood pressure (BP) regulation. We investigated the associations of renalase with BP and the risk of hypertension by examining renalase single nucleopeptide polymorphism (SNPs), serum renalase levels, and renal expression of renalase in humans.
Methods: ① Subjects (n = 514) from the original Baoji Salt-Sensitive Study cohort were genotyped to investigate the association of renalase SNPs with longitudinal BP changes and the risk of hypertension during 14 years of follow-up. ② Two thousand three hundred and ninety two participants from the Hanzhong Adolescent Hypertension Study cohort were used to examine the association of serum renalase levels with hypertension. Renalase expression in renal biopsy specimens from 193 patients were measured by immunohistochemistry. ③ Renalase expression was compared in hypertensive vs. normotensive patients.
Results: ① SNP rs7922058 was associated with 14-year change in systolic BP, and rs10887800, rs796945, rs1935582, rs2296545, and rs2576178 were significantly associated with 14-year change in diastolic BP while rs1935582 and rs2576178 were associated with mean arterial pressure change over 14 years. In addition, SNPs rs796945, rs1935582, and rs2576178 were significantly associated with hypertension incidence. Gene-based analysis found that renalase gene was significantly associated with hypertension incidence over 14-year follow-up after adjustment for multiple measurements. ② Hypertensive subjects had higher serum renalase levels than normotensive subjects (27.2 ± 0.4 vs. 25.1 ± 0.2 μg/mL). Serum renalase levels and BPs showed a linear correlation. In addition, serum renalase was significantly associated with the risk of hypertension [OR = 1.018 (1.006–1.030)]. ③ The expression of renalase in human renal biopsy specimens significantly decreased in hypertensive patients compared to non-hypertensive patients (0.030 ± 0.001 vs. 0.038 ± 0.004).
Conclusions: These findings indicate that renalase may play an important role in BP progression and development of hypertension.
Introduction
Hypertension is one of the most common diseases, and is also a major risk factor for cardiovascular and cerebrovascular diseases and chronic kidney disease (CKD) (1). Several studies have shown that sympathetic over-activity is crucial in the pathogenesis of hypertension, and interventions of sympathetic deactivation have been shown to lower BP levels (2). Therefore, identifying novel mechanisms of sympathetic regulation would enhance our understanding of biological mechanisms of blood pressure (BP) regulation, and might facilitate the development of specific targeted drugs for hypertension.
Renalase, firstly discovered in 2005, is a 342-amino-acid flavoprotein with monoamine oxidase activity. It is highly expressed in the kidneys and heart, and circulates in the blood to modulate cardiac function and BP (3, 4). In animal studies, renalase deficiency has been shown to increase BP (5), and recombinant renalase has a hypotensive effect (6, 7). In humans, serum renalase levels are higher in hypertensive than normotensive individuals (8, 9). By contrast, Schlaich et al. (10) found that serum renalase was higher in normotensive controls than in patients with resistant hypertension. In addition, it had been showed that plasma renalase was not different between hypertensive and nomotensive groups (11). Previous human and clinical studies examined small cohorts, and yielded conflicting (8–14). Therefore, the relationships of circulating renalase with BP levels and hypertension in the general population are still unclear. In addition, the correlation between serum and kidney renalase in hypertensive patients needs to be further clarified.
Human renalase (gene name: RNLS) is encoded by a 311 Kbp gene with 10 exons located on chromosome 10q23.33 (4). An association between RNLS gene and hypertension was demonstrated in several studies. Zhao et al. (15) discovered for the first time in the Han Chinese population that the renalase encoding gene was a new susceptibility gene for hypertension, and its genetic variations might affect BP. RNLS polymorphisms have also reported to be associated with hypertension in hemodialysis patients and type 2 diabetes patients (16, 17). However, some studies failed to replicate this association (18, 19). Most importantly, all previous studies were cross-sectional. Whether genetic variations in RNLS gene can predict the hypertension incidence over time has not been investigated.
Therefore, in our study, we used both single marker-based and gene-based analyses to examine the relationships of RNLS gene with longitudinal BP changes and incident hypertension in a family-based cohort. In addition, we also used our previously established cohort to assess the association between serum renalase levels and the risk of hypertension. Lastly, we compared kidney renalase expression levels in persons with and without hypertension.
Methods
The entire study was divided into three sections: a longitudinal cohort study, a cross-sectional cohort study and a case-control study.
Section 1: A Longitudinal Cohort Study to Examine the Associations of RNLS Gene With Longitudinal BP Changes and Hypertension Incidence
From April to November 2004, we established the cohort of Baoji Salt-Sensitive Study, which recruited 514 adults from 124 families in seven villages in Baoji city, Shaanxi, China. The detailed study design has been published previously (20–22). To identify the associations of potential genetic variants with longitudinal BP changes and the incidence of hypertension, we followed this cohort in 2009, 2012, and 2018. During each follow-up, the same 3-day examination as that of the baseline period was performed. Data on the development of hypertension and use of antihypertensive drugs was obtained by use of a standard questionnaire. During each of the three 3-day follow-up visits, three BP measurements were obtained. The mean of the nine BP measurements was calculated for the current analysis.
Section 2: A Cross-Sectional Cohort Study to Study the Association of Serum Renalase Levels With the Risk of Hypertension
The study population was derived from the Hanzhong Adolescent Hypertension Study. Established in 1987, this ongoing prospective, population-based cohort study of 4,623 adolescents underwent serial follow-ups to investigate the development of cardiovascular risk factors originating from children and young adults. Details of the study protocol have been published elsewhere (23–25). To explore the association of circulating renalase with the risk of hypertension, we used cross-sectional analysis of the most recent follow-up data in 2017. The selection process of the subjects is described in Supplementary Figure 1. A total of 2,780 subjects were followed up in 2017. Participants were excluded if they had missing BP levels (n = 28), laboratory data on serum or urine (n = 352), height and weight (n = 1), or if they had a self-identified history of cardiovascular disease, stroke or renal failure (n = 7), leaving 2,392 participants for the current analysis.
Section 3: A Case-Control Study to Examine the Renalase Expressions in Human Renal Tissue of Hypertensive Patients
Patients (n = 193) who had undergone renal biopsy for work-up of proteinuria, chronic kidney disease, and acute kidney injury were recruited from December 2018 to November 2019 in the Renal Division of First Affiliated Hospital of Xi'an Jiaotong University. Clinical and anthropometric data were extracted for analysis. The exclusion criteria were as follows: (1) history of infectious diseases, chronic heart disease, stroke, chronic obstructive pulmonary disease and abnormal liver function; (2) estimated glomerular filtration rate (eGFR) <90 mL/min/1.73 m2 on admission and (3) inability or unwillingness to participate in the study. The renal biopsy diagnoses included membranous nephropathy (n = 83), IgA nephropathy (n = 35), mesangial and proliferative nephritis (n = 34), systemic lupus erythematosus (n = 20), purpura nephritis (n = 9), focal segmental glomerulosclerosis (n = 4), renal amyloidosis (n = 3), diabetic nephropathy (n = 3), vasculitis (n = 1), and proliferative endocapillary glomerulonephritis (n = 1).
The Ethics Committee of the First Affiliated Hospital of Xi'an Jiaotong University approved the study protocol (code: 2015–128). Each participant provided written informed consent. This study follows the principles of the Declaration of Helsinki, and all studies procedures were carried out in accordance with institutional guidelines (ClinicalTrials.gov, registration number: NCT02734472).
BP Measurement and Definitions
BP was measured by the trained staff using a standard mercury sphygmomanometer as previously described (24, 26, 27). The mean arterial pressure (MAP) was calculated as DBP + [1/3 × (SBP–DBP)]. Hypertension was defined as SBP of ≥140 mm Hg, DBP ≥ 90 mm Hg or as the use of antihypertensive medications. High-normal blood pressure was SBP 120–139 and/or DBP 80–89. Grade 1 hypertension was SBP 140–159 and/or DBP 90–99. Grade 2 hypertension was SBP ≥ 160 and/or DBP ≥ 100 as recommended by 2020 International Society of Hypertension (ISH) hypertension practice guidelines (28). The subtypes of hypertension were further defined as isolated systolic hypertension (ISH: SBP ≥ 140 mmHg and DBP <90 mmHg), isolated diastolic hypertension (IDH: SBP <140 mmHg and DBP ≥ 90 mmHg), and systolic diastolic hypertension (SDH: SBP ≥ 140 mmHg and DBP ≥ 90 mmHg) in the absence of antihypertensive treatment (28).
Blood Biochemical Analyses
Triglycerides, total cholesterol, high-density lipoprotein (HDL), low-density lipoprotein (LDL), aspartate aminotransferase (AST), alanine aminotransferase (ALT), serum uric acid, serum Na+ and K+, serum phosphate, serum creatinine, blood urea nitrogen, fasting glucose levels, C-reactive protein, glycosylated hemoglobin and urinary albumin-creatinine ratio (uACR) were assessed by an automatic biochemical analyzer (Hitachi, Tokyo, Japan). Details of these assays were described previously (24, 26, 27). Serum renalase levels were measured by using commercially available enzyme-linked immunosorbent assay (ELISA) kits (Cloud-Clone Corp., Houston, TX, USA).
Immunohistochemistry Staining
Immunohistochemical (IHC) staining was performed as previously reported (29–31). Briefly, serial 5 μm paraffin-embedded fixed tissue sections were dewaxed and rehydrated. These sections were incubated with renalase antibody (Product code: ab178700, Abcam, USA 1:200) at 4°C overnight, and with secondary antibody at room temperature for 30 min. The sections were photographed by an upright fluorescence microscopic imaging system (BX51 Olympus, Japan). To evaluate the expression of renalase, 10 non-overlapping fields (magnification × 40) were randomly selected from each kidney section. The immunoperoxidase stain was quantified using the Image Pro Plus 6.0 imaging software (Media Cybernetics, USA). Data were expressed as the mean optical density in each field area. Details of this methods were described before (29, 30).
SNP Selection and Genotyping
Ten tagging SNPs in RNLS: rs10509540, rs7922058, rs999951, rs10887800, rs796945, rs1935582, rs7076491, rs2296545, rs2576178 and rs17109290, were selected from RNLS gene using the National Center for Biotechnology Information database and the Genome Variation Server database and based on the following criteria: tag SNPs in the CHB and Asian database selected by the Haploview 4.2 software (Broad Institute, Cambridge, MA, USA) with Hardy-Weinberg equilibrium (HWE) P ≥ 0.05, a minor allele frequency (MAF) ≥ 0.05, and r2 ≥ 0.8. All the genotyping experiments were done by CapitalBio (CapitalBio Corp, Beijing, China) as described previously (20, 32, 33).
Statistical Analyses
For the analyses in family-based cohort study, quality control, including genotyping call rate, Mendelian consistency, minor allele frequency and Hardy-Weinberg equilibrium on parental SNP data, was conducted by PLINK software (version 1.9). Associations of each SNP with longitudinal BP changes were assessed by mixed-effect linear regression models in three genetic models (dominant, recessive, and additive) (32–34). Regarding the analyses of hypertension incidence, 51 participants with hypertension at baseline were excluded. We examined the additive association between each SNP and incident hypertension by using a generalized linear mixed model, which permits multilevel modeling when the response variable follows a binary distribution (e.g., incident hypertension). All models were adjusted for the fixed effects of age, sex, and BMI, and the random effect for familial correlations using glmer function in lme4 R package.
Gene-based analysis was also performed to evaluate the overall association of a candidate gene with BP changes over time and incident hypertension (32, 34, 35). The truncated product method (TPM), which combines P values from single SNP association analyses, is an approach that would evaluate the association between a candidate gene and a trait. Gene-based analysis was performed using R software (version 3.0.1; http://www.r-project.org).
For the analyses in the cross-sectional cohort study, we performed linear and logistic regression analyses to test associations of serum renalase with BP levels and the risk of hypertension. These analyses were multivariate, adjusting for traditional cardiovascular risk factors and potential confounders. In the observational study, Student's t-test was used to determine statistical differences between two groups. All statistical analyses were conducted using SPSS 16.0 (SPSS, Inc., Chicago, IL). P < 0.05 was considered statistically significant.
Results
Analyses for Associations of Renalase SNPs With Longitudinal BP Changes and Hypertension Incidence in the Longitudinal Cohort Study
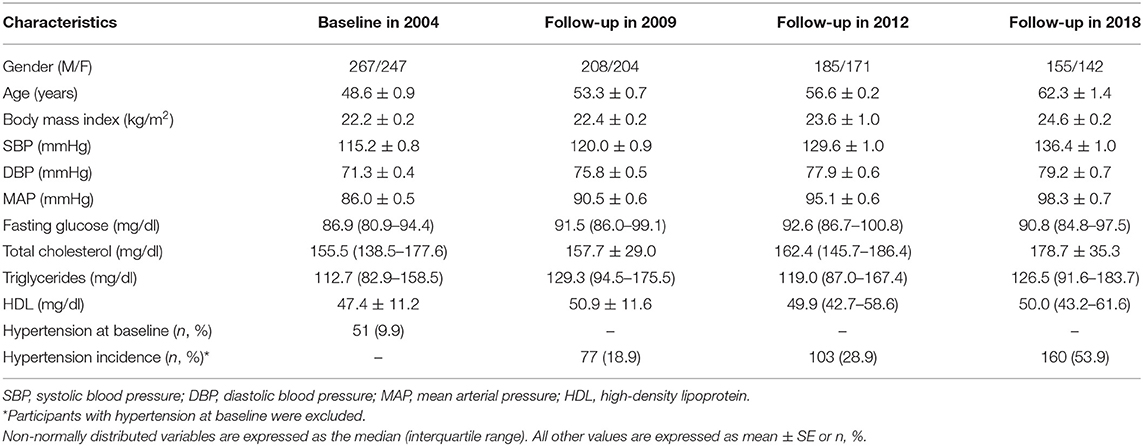
Participants were 49 years old and had had a BMI of 22.2 kg/m2. Their mean baseline SBP, DBP, and MAP were 115.2, 71.3, and 86.0 mmHg, respectively. During 14 years of follow-up, average SBP, DBP, and MAP increased by 21.2, 7.9, and 12.3 mmHg, respectively, and 160 (53.9%) subjects who were normotensive at baseline developed hypertension (Table 1).
The genomic location, minor allele frequency, and Hardy-Weinberg test for each SNP are shown in Supplementary Table 1. No SNPs deviated statistically significantly from Hardy-Weinberg equilibrium.
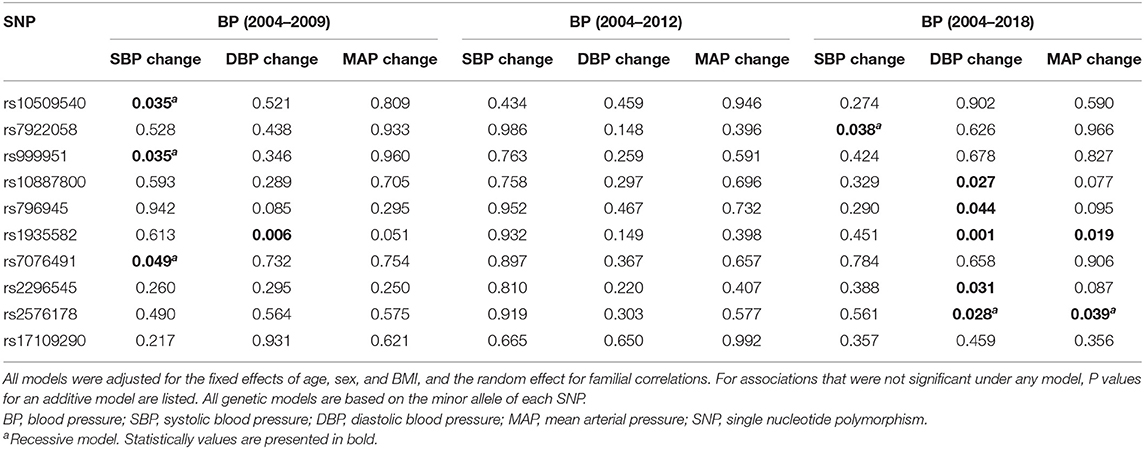
Table 2 shows the associations of each SNP in RNLS gene with 5-year change (2004–2009), 8-year change (2004–2012), and 14-year change (2004–2018) in BP levels. SNPs rs10509540, rs999951, and rs7076491 were significantly associated with 5-year change in SBP while SNP rs1935582 was associated with 5-year change in DBP. SNP rs7922058 was associated with 14-year change in SBP, and rs10887800, rs796945, rs1935582, rs2296545, and rs2576178 were significantly associated with 14-year change in DBP while rs1935582 and rs2576178 were associated with MAP change over 14 years after Bonferroni correction. In addition, gene-based analyses found that RNLS was significantly associated with longitudinal DBP change (PTPM = 1.48e−5) and MAP change (PTPM = 0.0085) over a 14-year follow-up (Table 3).
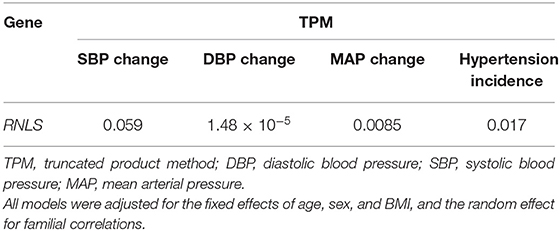
Table 3. Gene-based associations of RNLS with longitudinal BP changes and hypertension incidence over 14 years of follow-up.
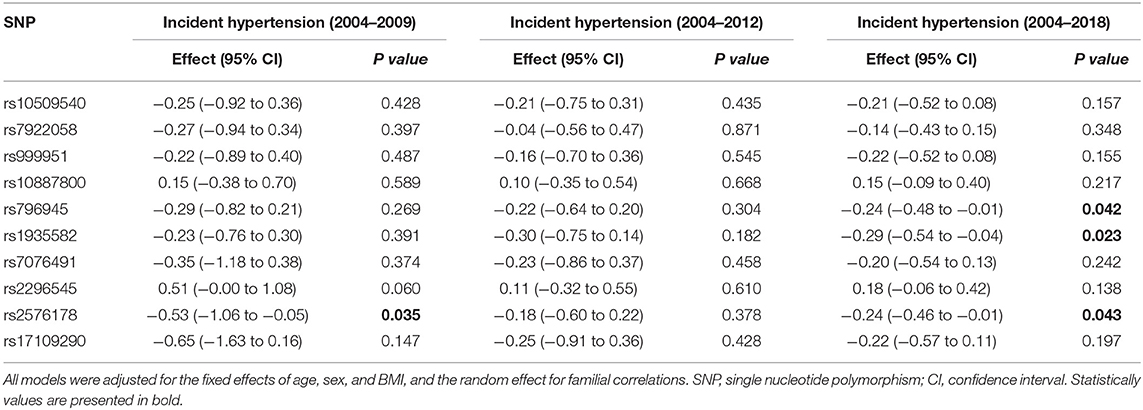
Next, we examined the relationships of RNLS SNPs with 5-year (2004–2009), 8-year (2004–2012), and 14-year (2004–2018) incidences of hypertension, which are shown in Table 4. SNP rs2576178 was significantly associated with the incidence of hypertension during 5 years of follow-up. SNPs rs796945, rs1935582, and rs2576178 were significantly associated with hypertension incidence over 14 years. Furthermore, RNLS was found to be significantly associated with hypertension incidence from gene-based analysis over 14-year follow-up (PTPM = 0.017, Table 3) after adjustment for multiple testing.
Associations of Serum Renalase With BP Levels and the Risk of Hypertension in the Cross-Sectional Cohort Study
Participants with hypertension were older, and had higher prevalence of men, alcohol drinking, smoking, diabetes, family history of hypertension, and higher levels of BMI, heart rate, serum uric acid, fasting glucose, total cholesterol, triglycerides, LDL, serum creatinine, urinary albumin/creatinine, and lower levels of eGFR and HDL compared with those with normotension (Table 5).
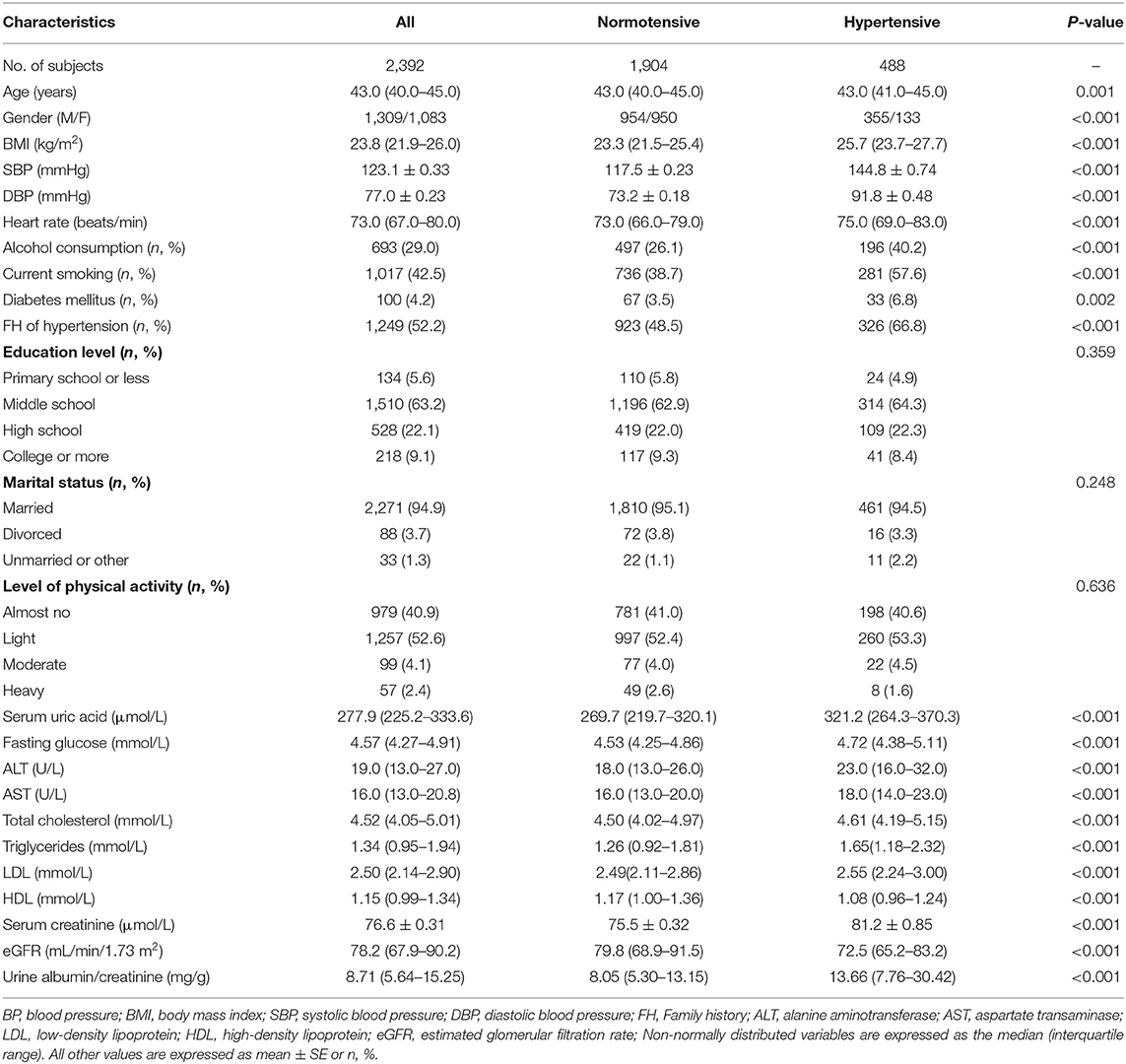
Table 5. Characteristics of participants categorized by BP status in the cross-sectional cohort study (n = 2,392).
Serum renalase levels were higher in hypertensive subjects when compared with normotensive subjects (27.2 ± 0.4 vs. 25.1 ± 0.2 μg/mL, P < 0.001; Figure 1A). Furthermore, there was a stepwise increase in renalase levels from normotension, to high-normal, to grade 1 hypertension, and finally to grade 2 hypertension (24.8, 25.6, 27.1, and 27.7 μg/mL respectively, Pfortrend < 0.001; Figure 1B). We further examine serum renalase in different groups with normotensive and hypertensive subtypes. Participants with ISH had higher serum renalase levels than IDH group (27.4 ± 0.9 vs. 25.2 ± 1.0 μg/mL, P = 0.025]. Participants with SDH had higher serum renalase compared with IDH participants (28.2 ± 0.7 vs. 25.2 ± 0.9 μg/mL, P = 0.014; Figure 1C). Serum renalase was 25.2, 27.4, 25.2, and 28.2 μg/mL for subjects with normotension, ISH, IDH, and SDH, respectively (Pfortrend < 0.001).
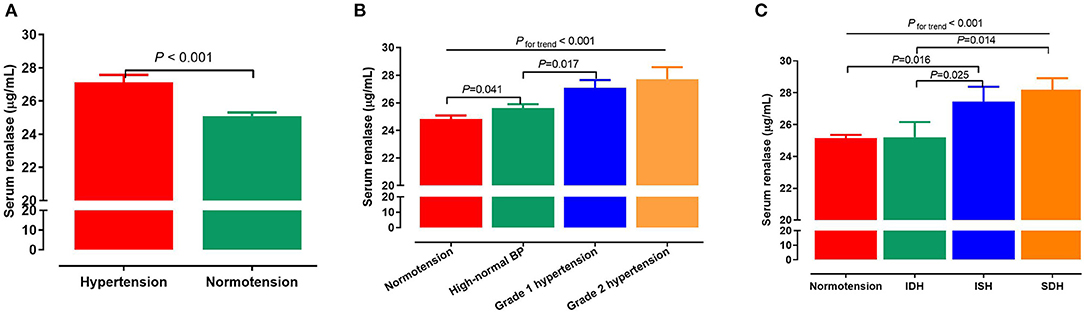
Figure 1. Associations of serum renalase with BP levels and hypertension. (A) Serum renalase levels in subjects with normotension and hypertension. (B) Serum renalase levels in subjects with different grades of BP, including normotension, high-normal, grade 1 and grade 2 hypertension. (C) Serum renalase levels in subjects with normotension and different hypertensive subtypes, including ISH, IDH, SDH. The differences between two groups were analyzed by Student's t-test, and analysis of variance (ANOVA) was used to test the linearity across different grades and subtypes of hypertension. BP, blood pressure; ISH, isolated systolic hypertension; IDH, isolated diastolic hypertension; SDH, systolic diastolic hypertension.
SBP levels were positively correlated with serum renalase (β = 0.056, P = 0.003), age, BMI, total cholesterol, eGFR, but negatively correlated with sex. In addition, DBP was positively correlated with serum renalase (β = 0.046, P = 0.015), age, BMI, total cholesterol, triglycerides, and negatively correlated with sex in all subjects (Supplementary Table 2). Furthermore, serum renalase [OR = 1.018 (1.006–1.030), P = 0.003] was significantly associated with the risk of hypertension after adjusting for multiple confounders (Figure 2).
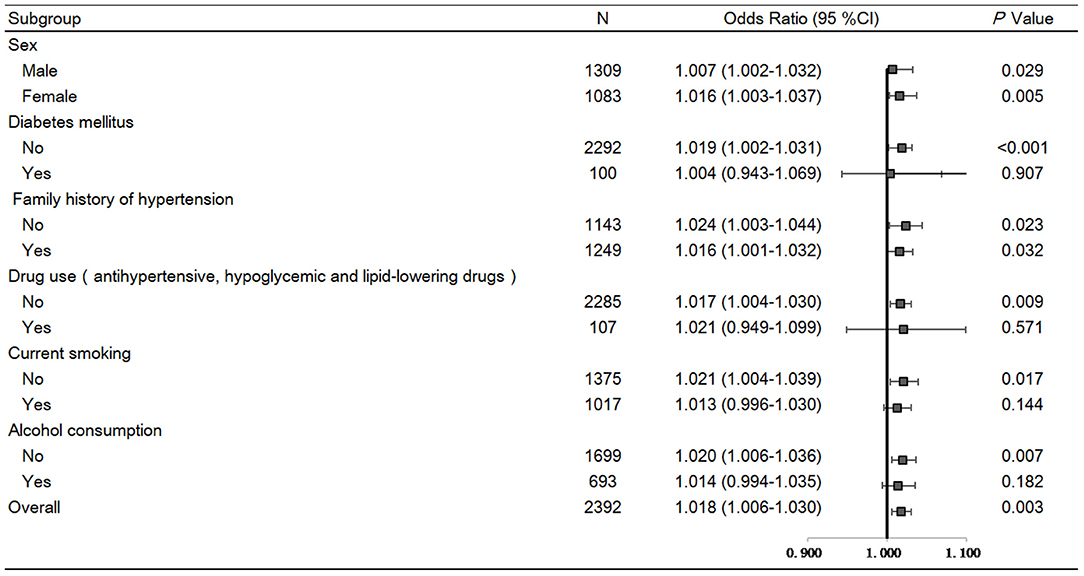
Figure 2. Forest plots of odds ratios (ORs) for serum renalase and risk of hypertension in a total of 2,392 subjects stratified by sex, BMI, diabetes, family history of hypertension, and drug use. Logistic regression analyses to test associations of serum renalase with hypertension risk after adjustment for age, smoke, BMI, eGFR, total cholesterol, triglycerides, fasting glucose. Values are the OR (95% confidence interval [95% CI]).
Several sensitivity analyses were performed. First, removing subjects with diabetes or those taking antihypertensive, hypoglycemic and lipid-lowering drugs produced similar results (Figure 2). In addition, stratifying all participants by sex, BMI, family history of hypertension, did not change the results (Figure 2).
Expression of Renalase in Human Renal Tissue in the Case-Control Study
Patients with hypertension were older, and higher levels of fasting glucose, total cholesterol, LDL, uACR, and lower levels of HDL compared with those with normotension. Serum creatinine, blood urea nitrogen and eGFR were similar between two groups (Table 6).

Table 6. Clinical characteristics of patients categorized by BP status in the case-control study (n = 193).
We assessed the renalase expression levels and its locations in human renal biopsy by IHC. Renalase was strongly expressed in the renal tubules, particularly distal tubules, and scattered in the glomeruli of the normotensive patients (Figure 3A). In the hypertensive renal tissue, the number and intensity of renalase positive-stained area significantly decreased (Figure 3A). Quantification of renalase positive areas in the kidneys, as summarized in Figure 3B, indicated hypertensive group had lower expression of renalase as compared to the normotension group (0.030 ± 0.001 vs. 0.038 ± 0.004, P < 0.001).
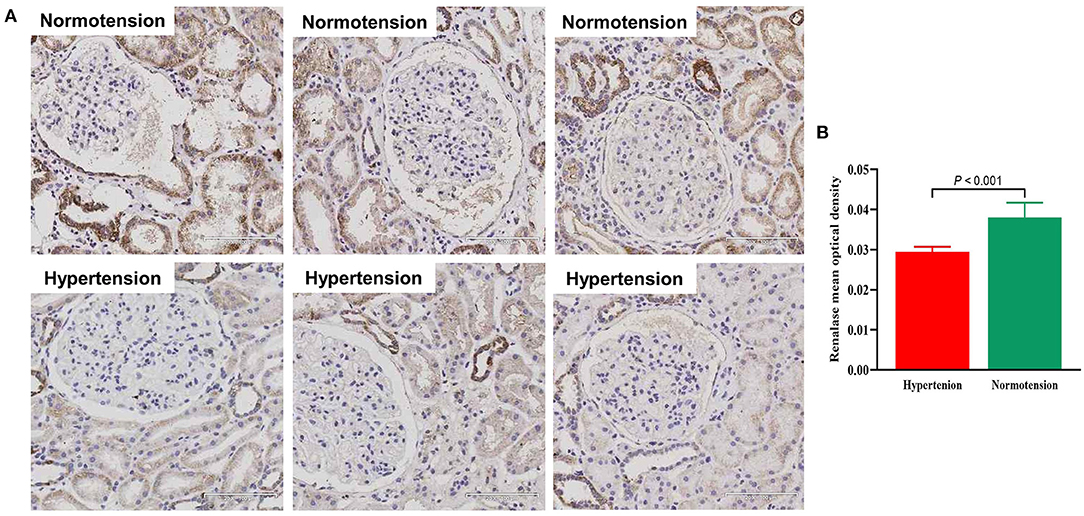
Figure 3. (A) Representative photomicrographs of renalase expression in human renal biopsy visualized by immunochemistry staining in normotensive group and hypertensive group (x200). (B) Bar graph showing quantitative analysis of renalase. Student's t-test was used to determine statistical differences between two groups.
Discussion
To the best of our knowledge, this is the first study to investigate the associations of RNLS gene with BP levels, longitudinal BP changes and hypertension incidence in a Chinese cohort. We identified several novel RNLS SNPs that were significantly associated with BP levels, longitudinal BP changes and hypertension incidence after correcting for multiple testing. The gene-based analysis revealed that RNLS gene was significantly associated with longitudinal BP changes and hypertension incidence over time. These findings underscore potentially important contributions of renalase gene to long-term BP regulation. Moreover, it enhances our understanding of the genetic architecture of BP progression and hypertension.
The association between genetic variants of RNLS gene and hypertension was observed in several cross-sectional studies. For example, Zhao et al. (15) enrolled 2,586 Chinese subjects (1,317 with essential hypertension and 1,269 healthy controls), and showed a higher incidence of hypertension in subjects with G-allele rs2576178. Stec et al. (17) observed that the C-allele of rs2576178 was associated with a higher hypertension in hemodialyzed patients. However, the previous studies were cross-sectional, and the findings were inconsistent. Abdallah et al. (19) revealed that the frequencies of rs2576178 genotypes and alleles were not significantly different between normotensive and hypertensive subjects. In the present study, we identified 3 RNLS variants (rs2576178, rs796945, and rs1935582) that were significantly associated with incident hypertension over 14 years of follow-up. A significant gene-based association of RNLS and incident hypertension was also identified. SNP rs2576178 is located at the 5' flanking regions, and this polymorphism may influence the initiation of transcription or differential splicing (15). SNPs rs796945 and rs1935582 are located at the intronic region and have no inferred functional implication, based on the analysis by Fast SNP. However, evidence has suggested that intronic polymorphisms may be etiologically involved in the development of complex disorders (36). However, we cannot exclude that other SNPs in the same gene in variable grade of linkage disequilibrium with our SNPs could be implicated in BP/hypertension incidence. Future functional studies are needed to elucidate how the identified risk loci contribute to hypertension at the molecular and cellular level.
Renalase, mainly synthesized in the kidney and heart, is secreted into the blood (4, 6, 37). Several studies examining the relationship between circulating renalase and hypertension have yielded discordant results (Supplementary Table 3). For instance, hypertensive subjects showed significantly lower levels of serum renalase than their normotensive counterparts in 34 hemodialyzed patients (14) and 50 patients after surgical repair of aortic coarctation (12). Schlaich et al. (10) demonstrated that serum renalase was lower in 22 patients with resistant hypertension than in 4 normotensive controls. By contrast, some studies have shown opposed observation. Maciorkowska et al. (8) serum renalase was significantly higher in patients with hypertension (n = 121). These data were confirmed by Lemiesz et al. in 88 adolescents (9). In our previously established cohort of 2,392 subjects, we found that serum renalase were significantly higher in hypertensive subjects when compared with the normotensive ones. In this study, serum renalase was significantly associated with the risk of hypertension. The discrepant results of these studies may be attributed to their different renalase measurements in blood. Most studies estimated renalase levels with the use of commercially available enzyme-linked immunosorbent assay (ELISA) (8, 9, 11, 12, 14, 38, 39), whereas few studies used western blots (3, 10). The different study populations, sample sizes, and racial differences among these various studies may be other causes of the discrepant results (Supplementary Table 3).
In contrast to previous studies that only focused on dichotomous definitions of hypertension, our study reports stage-specific hypertension pursuant to the ISH global hypertension practice, including high-normal, grade 1 and grade 2 hypertension. To the best of our knowledge, this is the first study to quantify multi-state levels in the light of these criteria. We found that serum renalase showed a linear increase from normotension to grade 2 hypertension, indicating that circulating renalase may be a novel marker for identifying stages of hypertension. In addition, our study is the first to explore serum renalase levels in different hypertension subtypes. ISH was reported to be associated with an increased incidence of cardiovascular complications. IDH is associated with a low risk of cardiovascular disease mortality, while SDH carries a similarly high risk to ISH (40). Studies have established elevated SBP as a more reliable predictor for adverse cardiovascular outcomes compared to elevated DBP (41, 42). In the present study, we observed serum renalase levels in ISH group were significantly higher when compared with the IDH group and the normotension group. The serum renalase levels in the SDH group were higher than in the IDH group, but were similar to the ISH group. These data indicate that SBP is more closely related to serum renalase levels than DBP.
Renalase is extensively expressed in the kidneys, heart, intestines, liver, skeletal muscles, blood vessels, and central nervous system (4, 6). In the kidneys, renalase is widely expressed in renal tubule epithelial cells, glomeruli, mesangial cells, and podocytes (37, 43). However, few studies have explored the expression level of renalase in human kidney specimens. Huang et al. (44) reported that renalase expression detected by IHC was significantly lower in renal biopsy specimens with kidney disease than in normal kidney tissues, and it inversely correlated with renal injury and apoptosis. To our best knowledge, this is the first study aimed to compare the expression of renalase in human renal tissue from hypertension and normotension. Our results suggested that renalase was strongly expressed in the renal tubules, particularly distal tubules, and scattered in the glomeruli, which is consistent with the study of Wang et al. who showed that renalase was wildly expressed in kidney, and was secreted by tubule epithelial cells primarily (43). However, the previous study showed different renalase expression pattern (3), and different methods of these studies may be causes of discrepant results. Further, the expression in tubular epithelial cells was significantly lower in hypertensive group than in the normotensive group. Previous studies suggested that renalase levels were greatly affected by decreased renal function (45, 46). Our result was convincing because we excluded patients with renal dysfunction and focused on human samples rather than animal tissue. Interestingly, we observed that hypertensive subjects have lower expression of renalase in human renal tissue, but has higher levels of renalase in circulation. The mechanisms underlying this phenomenon remains to be explored. One could speculate that the increase in serum renalase reflect a counter-regulatory response to the increased inflammation and oxidative stress observed in hypertensive subjects (47). Renalase is expressed in macrophages and downregulates the inflammatory response (48). It would be informative but a large task to determine the expression of renalase in different tissues under hypertension.
Although the molecular mechanisms linking renalase to hypertension are not fully understood, several hypotheses have been proposed. Firstly, recombinant renalase exerts powerful and rapid hypotensive effects in rodents; this effect was suggested to be mediated by the degradation of circulating catecholamines, which could decrease cardiac contractility and heart rate (3). Wang et al. (49) further showed that serum renalase levels were correlated with catecholamine levels and SBP in CKD patients. In addition, BP is regulated by inhibiting the activation of the renal dopamine (DA) system. Sizova et al. (50) showed that renalase regulated the dopamine-dependent natriuresis and phosphaturia in 5/6 nephrectomized rats. Another study reported that the DA D1-like receptor agonist fenoldopam increased renalase expression in renal proximal tubule cells from Wistar Kyoto (WKY) rats, which was mainly attributable to D5 receptor (51). Finally, renal sympathetic nerve activity may be involved in the regulation of BP. Jiang et al. (52) found that renal denervation lowered BP, and upregulated the plasma renalase and renalase expression in the kidneys. Future studies are need to investigate mechanisms underlying this association.
The current study has several strengths. It provides the first evidence for the associations of renalase with hypertension and BP levels in humans from three different perspectives: genetic variations, plasma levels, and renal expression. Furthermore, our family-based cohort study was the first to examine the relationship between renalase and hypertension incidence longitudinally. In addition, this cross-sectional cohort study is the largest to date to detect serum renalase levels, and comprehensively explore its relationships with different BP levels, hypertensive grades, and subtypes. However, this study also has its limitations. The novel findings in our study need to be replicated in other cohorts with different genetic background. Moreover, due to the limited number of genotyped SNPs in RNLS gene, less frequent genetic variants may have been left out of the current study. Future research will be needed to explore their associations with longitudinal BP phenotypes and hypertension incidence.
Based on single-marker and gene-based analyses, the present study provides evidence for a role of the RNLS gene in longitudinal BP phenotypes and hypertension incidence. In addition, we reported for the first time that serum renalase level was significantly associated with different BP levels, hypertension grades, and subtypes. We also showed that renalase expression in human renal tissue significantly decreased in hypertensive patients. Findings from the current study indicate that renalase may play an important role in BP progression and development of hypertension. This work provides a basis for potential prevention and a possible therapeutic target for hypertension in the future.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by First Affiliated Hospital of Xi'an Jiaotong University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
YW and J-JM: conceived and designed the experiments. J-JM and W-HL: responsible for subject recruitment. G-LH, CChu, X-YZ, M-FD, TZ, Y-YL, QM, K-KW, YS, DW, YY, HJ, Z-JN, XZ, LW, Z-YM, C-HL, JZ, KG, and H-XL: performed the experiments. G-LH, CChe, QZ, and YW: analyzed the data. YW and CChe: drafted the paper. W-HL, JC, GD, and J-JM: edited and revised manuscript. All authors have read, critically revised, and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81600327) (YW) and (Nos. 82070437 and 81870319) (J-JM), Natural Science Basic Research Program of Shaanxi Province (2021JM-257 and 2021JM-588), Institutional Foundation of the First Affiliated Hospital of Xi'an Jiaotong University (Nos. 2019QN-06 and 2021ZXY-14) (YW), the Clinical Research Award of the First Affiliated Hospital of Xi'an Jiaotong University of China [Nos. XJTU1AF-CRF-2019-004 (J-JM) and XJTU1AF2021CRF-021 (YW)], Research Incubation Fund of Xi'an People's Hospital (No. FZ-61). Grants from the Major Chronic Non-communicable Disease Prevention and Control Research Key Project of the Ministry of Science and Technology of China (2017YFC1307604 and 2016YFC1300104).
Conflict of Interest
GD is a named inventor on several issued patents related to the discovery and therapeutic use of renalase. Renalase is licensed to Bessor Pharma, and GD holds an equity position in Bessor and its subsidiary Personal Therapeutics.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors are grateful to the grassroots health staff in Hanzhong and Baoji for providing administrative and technical support during the follow-up. The abstract of this study has been presented at the Joint Meeting of the European Society of Hypertension (ESH) and the International Society of Hypertension (ISH) in 2021.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.800427/full#supplementary-material
References
1. Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. (2005) 365:217–23. doi: 10.1016/S0140-6736(05)17741-1
2. Grassi G, Mark A, Esler M. The sympathetic nervous system alterations in human hypertension. Circ Res. (2015) 116:976–90. doi: 10.1161/CIRCRESAHA.116.303604
3. Xu J, Li G, Wang P, Velazquez H, Yao X, Li Y, et al. Renalase is a novel, soluble monoamine oxidase that regulates cardiac function and blood pressure. J Clin Invest. (2005) 115:1275–80. doi: 10.1172/JCI24066
4. Desir GV. Regulation of blood pressure and cardiovascular function by renalase. Kidney Int. (2009) 76:366–70. doi: 10.1038/ki.2009.169
5. Wu Y, Xu J, Velazquez H, Wang P, Li G, Liu D, et al. Renalase deficiency aggravates ischemic myocardial damage. Kidney Int. (2011) 79:853–60. doi: 10.1038/ki.2010.488
6. Desir GV, Wang L, Peixoto AJ. Human renalase: a review of its biology, function, and implications for hypertension. J Am Soc Hypertens. (2012) 6:417–26. doi: 10.1016/j.jash.2012.09.002
7. Desir GV, Tang L, Wang P, Li G, Sampaio-Maia B, Quelhas-Santos J, et al. Renalase lowers ambulatory blood pressure by metabolizing circulating adrenaline. J Am Heart Assoc. (2012) 1:e002634. doi: 10.1161/JAHA.112.002634
8. Maciorkowska D, Zbroch E, Malyszko J. Circulating renalase, catecholamines, and vascular adhesion protein 1 in hypertensive patients. J Am Soc Hypertens. (2015) 9:855–64. doi: 10.1016/j.jash.2015.08.002
9. Lemiesz M, Tenderenda-Banasiuk E, Sosnowska D, Taranta-Janusz K, Wasilewska A. Serum renalase levels in adolescents with primary hypertension. Pediatr Cardiol. (2018) 39:1258–64. doi: 10.1007/s00246-018-1891-y
10. Schlaich M, Socratous F, Eikelis N, Chopra R, Lambert G, Hennebry S. Renalase plasma levels are associated with systolic blood pressure in patients with resistant hypertension. J Hypertens. (2010) 28:e437. doi: 10.1097/01.hjh.0000379519.82971.02
11. Wang Y, Lv YB, Chu C, Wang M, Xie BQ, Wang L, et al. Plasma renalase is not associated with blood pressure and brachial-ankle pulse wave velocity in chinese adults with normal renal function. Kidney Blood Press Res. (2016) 41:837–47. doi: 10.1159/000452587
12. Wybraniec MT, Mizia-Stec K, Trojnarska O, Chudek J, Czerwienska B, Wikarek M, et al. Low plasma renalase concentration in hypertensive patients after surgical repair of coarctation of aorta. J Am Soc Hypertens. (2014) 8:464–74. doi: 10.1016/j.jash.2014.04.009
13. Yilmaz ZV, Akkas E, Yildirim T, Yilmaz R, Erdem Y. A novel marker in pregnant with preeclampsia: renalase. J Matern Fetal Neonatal Med. (2017) 30:808–13. doi: 10.1080/14767058.2016.1186637
14. Malyszko J, Koc-Zorawska E, Malyszko JS, Kozminski P, Zbroch E, Mysliwiec M. Renalase, stroke, and hypertension in hemodialyzed patients. Ren Fail. (2012) 34:727–31. doi: 10.3109/0886022X.2012.681534
15. Zhao Q, Fan Z, He J, Chen S, Li H, Zhang P, et al. Renalase gene is a novel susceptibility gene for essential hypertension: a two-stage association study in northern Han Chinese population. J Mol Med. (2007) 85:877–85. doi: 10.1007/978-1-59745-442-1
16. Buraczynska M, Zukowski P, Buraczynska K, Mozul S, Ksiazek A. Renalase gene polymorphisms in patients with type 2 diabetes, hypertension and stroke. Neuromolecular Med. (2011) 13:321–7. doi: 10.1007/s12017-011-8158-6
17. Stec A, Semczuk A, Furmaga J, Ksiazek A, Buraczynska M. Polymorphism of the renalase gene in end-stage renal disease patients affected by hypertension. Nephrol Dial Transplant. (2012) 27:4162–6. doi: 10.1093/ndt/gfr293
18. Fava C, Montagnana M, Danese E, Sjogren M, Almgren P, Engstrom G, et al. The Renalase Asp37Glu polymorphism is not associated with hypertension and cardiovascular events in an urban-based prospective cohort: the Malmo diet and cancer study. BMC Med Genet. (2012) 13:57. doi: 10.1186/1471-2350-13-57
19. Abdallah E, Sabry D. Renalase gene polymorphisms in end-stage renal disease patients: an Egyptian study. J Am Sci. (2013) 9:346–9. Available online at: https://www.researchgate.net/publication/303232468_Renalase_gene_polymorphisms_in_end-stage_renal_disease_patients_An_Egyptian_study
20. Wang Y, Du MF, Yao S, Zou T, Zhang XY, Hu GL, et al. Associations of serum uromodulin and its genetic variants with blood pressure and hypertension in chinese adults. Front Cardiovasc Med. (2021) 8:710023. doi: 10.3389/fcvm.2021.710023
21. Zou T, Yao S, Du MF, Mu JJ, Chu C, Hu GL, et al. Associations of corin genetic polymorphisms with salt sensitivity, blood pressure changes, and hypertension incidence in Chinese adults. J Clin Hypertens. (2021) 23:2115–23. doi: 10.1111/jch.14401
22. Du MF, Yao S, Zou T, Mu JJ, Zhang XY, Hu GL, et al. Associations of plasma uromodulin and genetic variants with blood pressure responses to dietary salt interventions. J Clin Hypertens. (2021) 23:1897–906. doi: 10.1111/jch.14347
23. Zheng W, Mu J, Chu C, Hu J, Yan Y, Ma Q, et al. Association of blood pressure trajectories in early life with subclinical renal damage in middle age. J Am Soc Nephrol. (2018) 29:2835–46. doi: 10.1681/ASN.2018030263
24. Wang Y, Hu JW, Qu PF, Wang KK, Yan Y, Chu C, et al. Association between urinary sodium excretion and uric acid, and its interaction on the risk of prehypertension among Chinese young adults. Sci Rep. (2018) 8:7749. doi: 10.1038/s41598-018-26148-3
25. Wang Y, Chen C, Yan Y, Yuan Y, Wang KK, Chu C, et al. Association of uric acid in serum and urine with subclinical renal damage: Hanzhong Adolescent Hypertension Study. PLoS ONE. (2019) 14:e0224680. doi: 10.1371/journal.pone.0224680
26. Wang Y, Chu C, Wang KK, Hu JW, Yan Y, Lv YB, et al. Effect of salt intake on plasma and urinary uric acid levels in Chinese adults: an interventional trial. Sci Rep. (2018) 8:1434. doi: 10.1097/01.hjh.0000539339.99882.e6
27. Wang Y, Yuan Y, Gao WH, Yan Y, Wang KK, Qu PF, et al. Predictors for progressions of brachial-ankle pulse wave velocity and carotid intima-media thickness over a 12-year follow-up: Hanzhong Adolescent Hypertension Study. J Hypertens. (2019) 37:1167–75. doi: 10.1097/HJH.0000000000002020
28. Unger T, Borghi C, Charchar F, Khan NA, Poulter NR, Prabhakaran D, et al. 2020 international society of hypertension global hypertension practice guidelines. J Hypertens. (2020) 38:982–1004. doi: 10.1097/HJH.0000000000002453
29. Wang Y, Mu JJ, Liu FQ, Ren KY, Xiao HY, Yang Z, et al. Salt-induced epithelial-to-mesenchymal transition in Dahl salt-sensitive rats is dependent on elevated blood pressure. Braz J Med Biol Res. (2014) 47:223–30. doi: 10.1590/1414-431X20133554
30. Wang Y, Xie BQ, Gao WH, Yan DY, Zheng WL, Lv YB, et al. Effects of renin-angiotensin system inhibitors on renal expression of renalase in sprague-dawley rats fed with high salt diet. Kidney Blood Press Res. (2015) 40:605–13. doi: 10.1159/000368536
31. Guo TS, Zhang J, Mu JJ, Liu FQ, Yuan ZY, Ren KY, et al. High-salt intake suppressed microRNA-133a expression in Dahl SS rat myocardium. Int J Mol Sci. (2014) 15:10794–805. doi: 10.3390/ijms150610794
32. Wang Y, Jia H, Gao WH, Zou T, Yao S, Du MF, et al. Associations of plasma PAPP-A2 and genetic variations with salt sensitivity, blood pressure changes and hypertension incidence in Chinese adults. J Hypertens. (2021) 39:1817–25. doi: 10.1097/HJH.0000000000002846
33. Wang Y, Zhou Q, Gao WH, Yan Y, Chu C, Chen C, et al. Association of plasma cyclooxygenase-2 levels and genetic polymorphisms with salt sensitivity, blood pressure changes and hypertension incidence in Chinese adults. J Hypertens. (2020) 38:1745–54. doi: 10.1097/HJH.0000000000002473
34. Shi G, Rice TK, Gu CC, Rao DC. Application of three-level linear mixed-effects model incorporating gene-age interactions for association analysis of longitudinal family data. BMC Proc. (2009) 3:S89. doi: 10.1186/1753-6561-3-S7-S89
35. Zaykin DV, Zhivotovsky LA, Westfall PH, Weir BS. Truncated product method for combining P-values. Genet Epidemiol. (2002) 22:170–85. doi: 10.1002/gepi.0042
36. Tokuhiro S, Yamada R, Chang X, Suzuki A, Kochi Y, Sawada T, et al. An intronic SNP in a RUNX1 binding site of SLC22A4, encoding an organic cation transporter, is associated with rheumatoid arthritis. Nat Genet. (2003) 35:341–8. doi: 10.1038/ng1267
37. Wang Y, Safirstein R, Velazquez H, Guo XJ, Hollander L, Chang J, et al. Extracellular renalase protects cells and organs by outside-in signalling. J Cell Mol Med. (2017) 21:1260–5. doi: 10.1111/jcmm.13062
38. Koc-Zorawska E, Malyszko J, Zbroch E, Malyszko J, Mysliwiec M. Vascular adhesion protein-1 and renalase in regard to diabetes in hemodialysis patients. Arch Med Sci. (2012) 8:1048–52. doi: 10.5114/aoms.2012.32413
39. Li X, Huang Q, Xu J. Renalase gene polymorphisms and plasma levels are associated with preeclampsia: a hospital-based study in the Chinese cohort. Women Health. (2021) 61:957–67. doi: 10.1080/03630242.2021.1994512
40. Hozawa A, Ohkubo T, Nagai K, Kikuya M, Matsubara M, Tsuji I, et al. Prognosis of isolated systolic and isolated diastolic hypertension as assessed by self-measurement of blood pressure at home: the Ohasama study. Arch Intern Med. (2000) 160:3301–6. doi: 10.1001/archinte.160.21.3301
41. Port S, Demer L, Jennrich R, Walter D, Garfinkel A. Systolic blood pressure and mortality. Lancet. (2000) 355:175–80. doi: 10.1016/S0140-6736(99)07051-8
42. Antikainen R, Jousilahti P, Tuomilehto J. Systolic blood pressure, isolated systolic hypertension and risk of coronary heart disease, strokes, cardiovascular disease and all-cause mortality in the middle-aged population. J Hypertens. (1998) 16:577–83. doi: 10.1097/00004872-199816050-00004
43. Wang F, Xing T, Li J, Bai M, Hu R, Zhao Z, et al. Renalase's expression and distribution in renal tissue and cells. PLoS ONE. (2012) 7:e46442. doi: 10.1371/journal.pone.0046442
44. Huang YS, Lai JB, Li SF, Wang T, Liu YN, Zhang QX, et al. Relationship between renalase expression and kidney disease: an observational study in 72 patients undergoing renal biopsy. Curr Med Sci. (2018) 38:268–76. doi: 10.1007/s11596-018-1875-4
45. Malyszko J, Malyszko JS, Rysz J, Mysliwiec M, Tesar V, Levin-Iaina N, et al. Renalase, hypertension, and kidney - the discussion continues. Angiology. (2013) 64:181–7. doi: 10.1177/0003319712459212
46. Desir GV, Peixoto AJ. Renalase in hypertension and kidney disease. Nephrol Dial Transplant. (2014) 29:22–8. doi: 10.1093/ndt/gft083
47. Vaziri ND, Rodríguez-Iturbe B. Mechanisms of disease: oxidative stress and inflammation in the pathogenesis of hypertension. Nat Clin Pract Nephrol. (2006) 2:582–93. doi: 10.1038/ncpneph0283
48. Hollander L, Guo X, Velazquez H, Chang J, Safirstein R, Kluger HM, et al. Renalase expression by melanoma and tumor associated-macrophages promotes tumor growth through a STAT3-mediated mechanism. Cancer Res. (2016) 76:3884–94. doi: 10.1158/0008-5472.CAN-15-1524
49. Wang F, Li J, Xing T, Xie Y, Wang N. Serum renalase is related to catecholamine levels and renal function. Clin Exp Nephrol. (2015) 19:92–8. doi: 10.1007/s10157-014-0951-8
50. Sizova D, Velazquez H, Sampaio-Maia B, Quelhas-Santos J, Pestana M, Desir GV. Renalase regulates renal dopamine and phosphate metabolism. Am J Physiol Renal Physiol. (2013) 305:F839–44. doi: 10.1152/ajprenal.00616.2012
51. Wang S, Lu X, Yang J, Wang H, Chen C, Han Y, et al. Regulation of renalase expression by D5 dopamine receptors in rat renal proximal tubule cells. Am J Physiol Renal Physiol. (2014) 306:F588–96. doi: 10.1152/ajprenal.00196.2013
Keywords: renalase, hypertension, gene polymorphism, blood pressure, renal puncture biopsy
Citation: Wang Y, Chen C, Hu G-L, Chu C, Zhang X-Y, Du M-F, Zou T, Zhou Q, Liao Y-Y, Ma Q, Wang K-K, Sun Y, Wang D, Yan Y, Li Y, Jia H, Niu Z-J, Zhang X, Wang L, Man Z-Y, Gao W-H, Li C-H, Zhang J, Gao K, Li H-X, Chang J, Desir GV, Lu W-H and Mu J-J (2022) Associations of Renalase With Blood Pressure and Hypertension in Chinese Adults. Front. Cardiovasc. Med. 9:800427. doi: 10.3389/fcvm.2022.800427
Received: 23 October 2021; Accepted: 31 January 2022;
Published: 24 February 2022.
Edited by:
Chao-Ling Yang, Oregon Health and Science University, United StatesReviewed by:
Ryan J. Cornelius, Oregon Health and Science University, United StatesYujiro Maeoka, Oregon Health and Science University, United States
Copyright © 2022 Wang, Chen, Hu, Chu, Zhang, Du, Zou, Zhou, Liao, Ma, Wang, Sun, Wang, Yan, Li, Jia, Niu, Zhang, Wang, Man, Gao, Li, Zhang, Gao, Li, Chang, Desir, Lu and Mu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wan-Hong Lu, bHVfd2FuaG9uZ0AxMjYuY29t; Jian-Jun Mu, bXVqanVuQDE2My5jb20=
†These authors have contributed equally to this work
 Yang Wang
Yang Wang