
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med., 04 March 2022
Sec. Heart Valve Disease
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.799253
 Qiang Li1†
Qiang Li1† Yifei Zhang2†
Yifei Zhang2† Haozhang Huang1,3†
Haozhang Huang1,3† Weihua Chen4,5†
Weihua Chen4,5† Shanshan Shi4,5†
Shanshan Shi4,5† Shiqun Chen1
Shiqun Chen1 Bo Wang1
Bo Wang1 Wenguang Lai1,6
Wenguang Lai1,6 Zhidong Huang1
Zhidong Huang1 Zhiling Luo2
Zhiling Luo2 Jiyan Chen1,3,6
Jiyan Chen1,3,6 Ning Tan1,3,6
Ning Tan1,3,6 Jin Liu1*
Jin Liu1* Yong Liu1,3,6*
Yong Liu1,3,6*Background: Left ventricular ejection fraction (LVEF) is a vital variable to describe left ventricle systolic function and contractility of left ventricle. However, the association between LVEF and the prognostic effect in patients with moderate or severe mitral regurgitation (MR) is still controversial.
Methods: This study comprised 30,775 coronary artery disease (CAD) patients who underwent coronary arteriography (CAG) in the Cardiorenal ImprovemeNt (CIN) registry from January 2007 to December 2018. Patients were divided into none or mild MR group and moderate or severe MR group, and 3 levels of LVEF ≥50, 40–50%, and <40% were further distinguished according to hospital baseline. Univariate and multivariate Cox proportional analyses were used to investigate the association between LVEF levels and long-term all-cause mortality in patients with different MR severities.
Results: Of 30,775 CAD patients (62.9 ± 10.6 years, females 23.8%), 26,474 (86.0%) patients had none or mild MR. Compared with none or mild MR patients, patients with moderate or severe MR were older and had worse cardio-renal function. In multivariable Cox proportional analysis, LVEF < 40% was independently associated with higher mortality compared with LVEF ≥ 50% in all kinds of MR severity {none or mild MR [adjusted hazard ratio (HR): 1.79; 95% CI: 1.56–2.05, p < 0.001], moderate or severe MR [adjusted HR: 1.57; 95% CI: 1.29–1.91, p < 0.001]}.
Conclusions: LVEF is a reliable prognostic index in CAD patients, even in those with moderate or severe MR. LVEF monitoring would still be clinically useful in CAD patients with moderate or severe MR. Clinical trials are needed to prospectively evaluate the optimal threshold for LVEF in patients with moderate or severe MR.
Atherosclerotic coronary artery disease (CAD) remains a major health burden globally and is a leading cause of morbidity and mortality worldwide (1). Myocardial ischemia affects patient's cardiac function and leads to a decrease in left ventricular ejection fraction (LVEF). Therefore, LVEF is considered to be an important indicator of cardiac function and prognosis in patients with CAD (2).
Mitral regurgitation (MR) is a growing public health problem, which generally progresses insidiously, and causes left-ventricular overload and dysfunction (3). Some studies have shown that nearly one in five CAD patients have MR (4), and MR significantly increases the risk of mortality in patients (5–7). However, moderate or severe MR increases the actual measurement of LVEF and overestimates patient cardiac function. In patients with CAD combined with moderate or severe MR, the relationship between LVEF and prognosis is unclear.
Our primary objective was to investigate the association between LVEF and long-term prognosis in CAD patients with different severities of MR.
The Cardiorenal ImprovemeNt (CIN) Registry is a single-center, observational cohort study. During the period from January 2007 to December 2018, a total of 88,938 patients underwent coronary arteriography (CAG) and 59,667 patients were diagnosed with CAD according to the 10th Revision Codes of the International Classification of Diseases (ICD-10; I20.xx–I25.xx, I50.00001, and I91.40001) in Guangdong Provincial People's Hospital, China (ClinicalTrials.gov NCT04407936).
We excluded participants aged <18 years (n = 19), with cancer (n = 879), with missing echocardiographic data (n = 12,988), who underwent CAG followed by mitral valve operation (n = 481), and who lacked follow-up LVEF data (n = 14,525). Finally, 30,775 CAD patients were included in our study (Supplementary Figure 1).
The presence of MR and the levels of LVEF were confirmed by the first examination of echocardiography. All CAD patients were stratified into 2 groups based on MR severity (none or mild MR vs. moderate or severe MR). MR severity was mainly evaluated by visual assessment integrating Doppler data from multiple acoustic windows, incorporating qualitative and semi-quantitative methods (8, 9).
The calculations for LVEF used the biplane-Simpson method by the end diastolic and end systolic apical 4- and 2-chamber views. Patients were divided based on MR severity into 3 groups according to the classification of the American College of Cardiology (ACC) as follows: normal: LVEF ≥ 50%; mild dysfunction: 40% ≤ LVEF <50%; moderate or severe dysfunction: LVEF <40%. In addition, the data quality control and periodical database verification were controlled by senior echocardiography physicians.
The endpoint of the study was long-term all-cause mortality. Patient's follow-up information was obtained from the Guangdong Provincial Public Security, which was matched with the electronic Clinical Management System of the Guangdong Provincial People's Hospital records according to the unique ID number of patients.
The comorbidities involved: hypertension (HT); diabetes mellitus (DM); acute myocardial infarction (AMI); congestive heart failure (CHF), defined as New York Heart Association class >2 or Killip class >1 (10); atrial fibrillation (AF); stroke; chronic kidney disease (CKD), defined as eGFR ≤ 60 ml/min/1.73 m2; anemia, defined as hematocrit <36% for women and <39% for men (11); hyperlipidemia, defined according to 2016 ESC guidelines for treating dyslipidemias (12).
All results were summarized and stratified into 2 groups according to MR severity (none or mild MR vs. moderate or severe MR). Descriptive statistics are reported as the mean [standard deviation (SD)], median [interquartile range (IQR)], or number and percentage when appropriate. Continuous variables were tested for differences between groups using t-test and ANOVA, and Pearson chi-squared tests for dichotomous variables, using Fisher's exact test when needed. Time-to-event data were presented graphically using Kaplan–Meier curves. Log-rank tests were used to compare the survival rate among LVEF subgroups.
The association between LVEF and log-term mortality was assessed by univariate and multivariate Cox proportional analyses in different models. Model 1 involved univariate Cox analysis, model 2 involved adjustment of age (as a continuous variable) and gender, and model 3 involved adjustment of demographic characteristics (age and gender), complications [HT, DM, AMI, CHF, CKD, AF, stroke, anemia, percutaneous coronary intervention (PCI), and hyperlipidemia], and medications [angiotensin-converting enzyme inhibitor/angiotensin receptor blocker (ACEI/ARB), beta-blockers, calcium channel blocker (CCB), statins, antiplatelet, mineralocorticoid receptor antagonist (MRA), loop diuretics, and oral anticoagulants (OAC)]. A value of p < 0.05 was considered statistically significant, and all statistical tests were two-sided. All statistical analyses were undertaken using R 4.0.3 (R Institute for Statistical Computing, Vienna, Austria).
We analyzed 30,775 patients with CAD, who were diagnosed from January 2007 to December 2018 [mean age 62.9 ± 10.6 years, 7,328 (23.8%) females]. In total, 6,340 (20.6%) patients had AMI, 3,057 (9.9%) had CHF, 17,350 (56.4%) had HT, and 973 (3.2%) had AF. Patients were divided into 2 groups according to MR severity; 26,474 (86.0%) patients had none or mild MR, and 4,301 (14.0%) patients had moderate or severe MR.
Compared to patients with none or mild MR, those with moderate or severe MR were older, had higher pro-brain natriuretic peptide (pro-BNP), a larger left ventricular end-diastolic dimension (LVEDD), and lower LVEF. In contrast, patients in moderate or severe MR were more likely to combine with AMI, CHF, AF, DM, CKD, anemia, hyperlipidemia, and were less likely to use HT, beta-blockers and CCB. The detailed clinical characteristics of patients are listed in Table 1.
We examined the prognostic effects of different levels of LVEF in patients with CAD combined with different degrees of MR. In none or mild MR group, Kaplan–Meier curves showed that patients in the group with lower baseline mean LVEF had a higher risk of all-cause death than patients in the other groups during the follow-up period (log-rank test, p < 0.001; Figure 1A). Similar results were obtained in patients with moderate or severe MR (log-rank test, p < 0.001; Figure 1B).
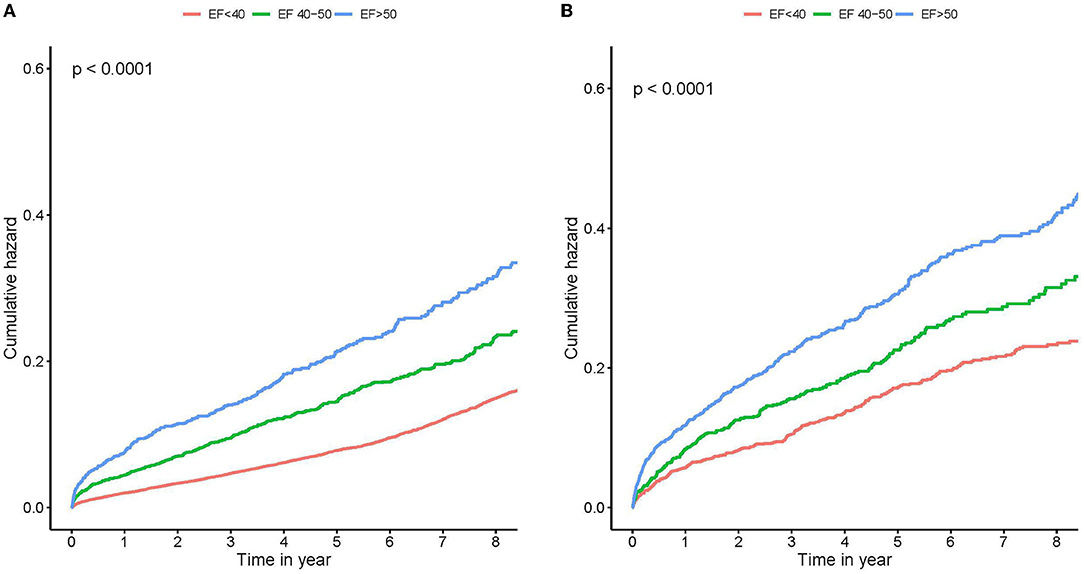
Figure 1. Kaplan–Meier survival curves stratified by left ventricular ejection fraction (LVEF). Survival stratified by LVEF <40, 40 to 50%, and ≥50% throughout none or mild MR (A), and moderate or severe MR (B). Note the large mortality difference between different LVEF groups. MR, mitral regurgitation.
In the univariate Cox proportional risk analysis, both the none or mild MR group and the moderate or severe MR group produced results that LVEF <40% was associated with an adverse event rate of all-cause death [Hazard ratio (HR): 2.38; 95% CI: 2.13–2.66, p < 0.001; HR: 2.23; 95% CI: 1.91–2.60, p < 0.001). After adjusting for age and gender, LVEF <40% remained significantly associated with all-cause death (HR: 2.27; 95% CI: 2.03–2.54, p < 0.001; HR: 2.27; 95% CI: 1.94–2.65, p < 0.001). On multivariable Cox proportional risk analysis, after adjustment for confusion factors (age, gender, HT, DM, AMI, CHF, CKD, AF, stroke, anemia, PCI, hyperlipidemia, ACEI/ARB, beta-blockers, CCB, statins, antiplatelet, MRA, loop diuretics, and OAC), LVEF <40% remained significantly associated with all-cause death regardless of MR severity—None or mild MR (HR: 1.79; 95% CI: 1.56–2.05, p < 0.001), and moderate or severe MR (HR: 1.57; 95% CI: 1.29–1.91, p < 0.001) (Table 2, Figure 2). Patients with LVEF <40% had a higher risk of death compared to patients with none or mild MR and LVEF ≥ 50%, after adjusting for confounding factors (Figure 3).
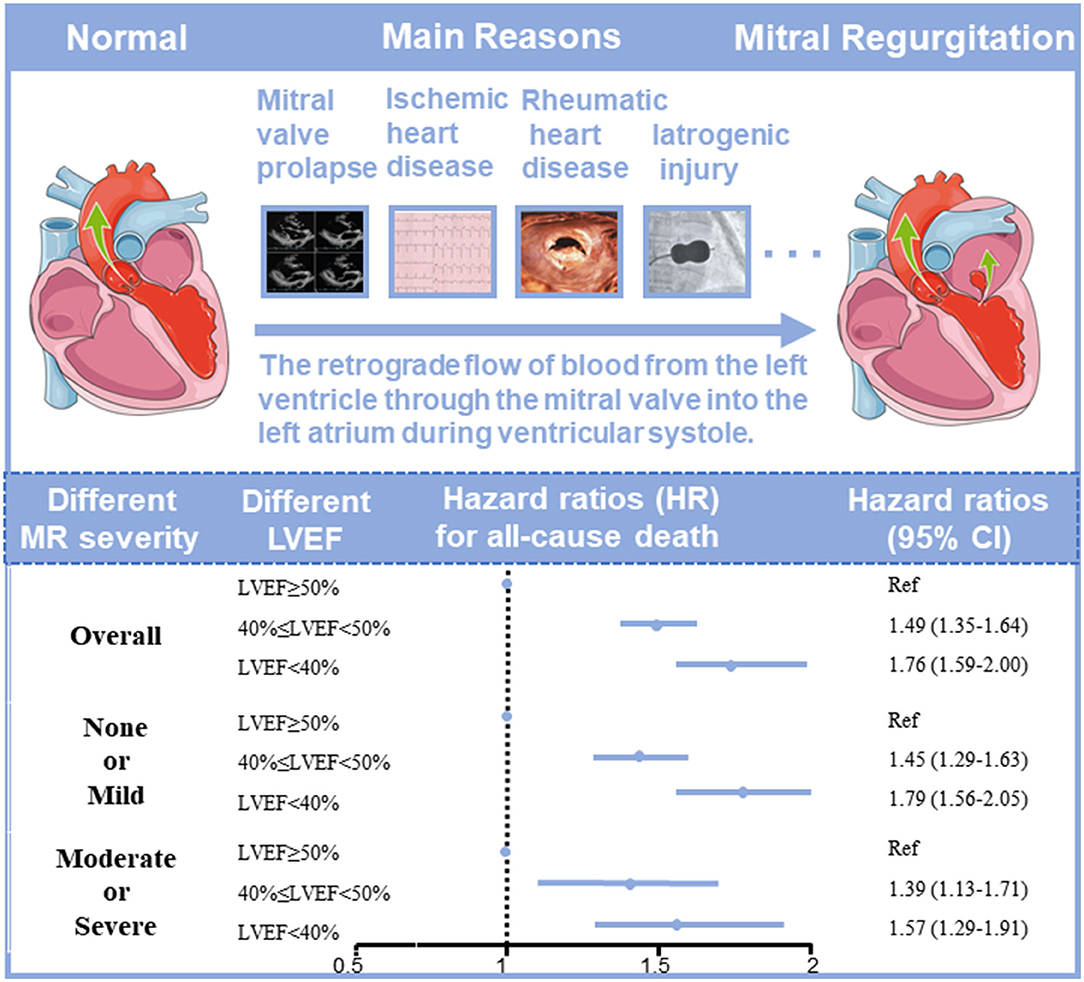
Figure 2. The association between left ventricular ejection fraction (LVEF) and long-term prognosis in coronary artery disease (CAD) patients with different severities of mitral regurgitation (MR).
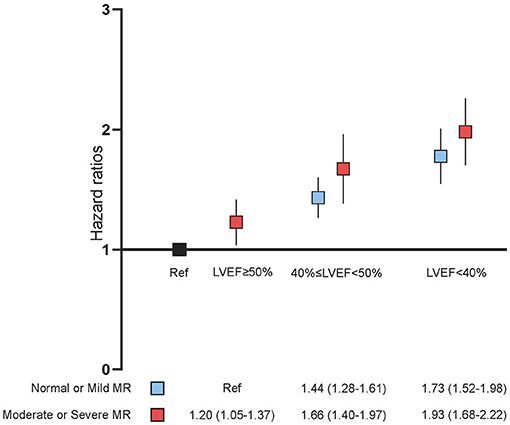
Figure 3. Multivariate Cox proportional analyses. Hazard ratios (HR) for all-cause death (95% CI) adjusted for age, gender, hypertension, diabetes mellitus, acute myocardial infarction, congestive heart failure, chronic kidney diseases, atrial fibrillation (AF), stroke, anemia, percutaneous coronary intervention (PCI), hyperlipidemia, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker (ACEI/ARB), beta-blockers, calcium channel blocker (CCB), statins, antiplatelet, mineralocorticoid receptor antagonist (MRA), loop diuretics, and oral anticoagulants (OAC). MR, mitral regurgitation; LVEF, left ventricular ejection fraction.
This study is the first largest cohort study to evaluate the association between LVEF and long-term prognosis in CAD patients with different MR severities. In our study, low LVEF in the patient with none and mild MR increased the mortality risk by four-fifths compared to the patient with normal LVEF. In the patient with moderate and severe MR, this risk remained nearly three-fifths. Our study suggests that LVEF remains a reliable assessment of prognosis in patients with variable degrees of MR.
MR is a growing public health burden, whose prevalence increases with increasing age, and the incidence of MR will increase due to population aging and growth (13). MR generally progresses insidiously, and causes left-ventricular overload and dysfunction due to the heart compensating for increasing regurgitant volume by left-atrial enlargement. LVEF, the cornerstone of contemporary clinical practice, is defined as the stroke volume indexed to the end-diastolic volume (EDV). It is one of the most important measured variables in clinical practice and regularly used by clinicians to describe systolic function and contractility (13), guiding the therapies and clinical decision of a serious of cardiovascular disease (13, 14). Especially in patients with MR, LVEF is particularly important as an important prognostic factor in the evaluation of optimal timing for surgery (15–18). However, MR being the most common valvular abnormality worldwide in patients with CAD, it is caused by retrograde flow of blood from the left ventricle (LV) through the mitral valve into the left atrium (LA) during cardiac systole (19–21), and contributes to the confounding effect of MR volume (22). Therefore, the assessment of cardiac function in MR patients using LVEF may underestimate the degree of intrinsic myocardial systolic dysfunction. However, it remains controversial whether LVEF is an accurate assessment of the prognosis of patients with MR. LVEF is a cornerstone of contemporary clinical practice, guiding the use of treatments and interventions for a range of cardiovascular diseases. However, the value of LVEF in guiding patients with MR remains unclear. Rosenhek et al. (23) suggested that LVEF may remain in the normal range for a long time, which makes LVEF not an accurate assessment of cardiac function in patients with MR. Similarly, in patients with significant primary MR, cardiac magnetic resonance study recently showed that LV dilatation generally occurs in the mid apical section of the ventricle and only later, at an advanced stage of disease process, occurs at the LV base (24). Therefore, a number of studies have concluded that LVEF does not accurately assess the prognosis of patients with MR and have begun to try to find new and more accurate metrics (25, 26). However, Enriquez-Sarano et al. (15) concluded that among the predictors of mortality after mitral valve closure insufficiency surgery, preoperative echocardiographic LVEF remains the best predictor of late survival. Other studies have similarly found that low preoperative LVEF (<60%) predicts postoperative LV dysfunction and is independently associated with increased postoperative mortality (16, 27). This is consistent with our study, which confirms that LVEF remains consistently effective for patient prognosis, regardless of the degree of regurgitation in patients with mitral valve insufficiency. Furthermore, in patients with moderate to severe MR, a decrease in LVEF also suggests a poor prognosis.
The following mechanism may explain why the overestimated LVEF can remain effective in indicating prognosis. Although LV dysfunction may be hidden behind normal LVEF due to altered loading conditions (28), MR causes LV and atria to expand due to volume overload and increased preload, resulting in a series of compensatory myocardial remodeling, which may lead to irreversible depression of ventricular and atrial function, and LV dilation increases ventricular wall stress leading to deterioration of LV function (29). Subsequent LV dilatation in turn affects mitral leaflet engagement, and LV systolic dysfunction reduces the strength of mitral valve closure, leading to worsening of MR and further worsening LV dysfunction (30). Finally, over time, patients with severe MR develop irreversible impairment of LV systolic function (31) and exhibit a progressive decrease in LVEF.
Our study found that LVEF remains valid for assessing the prognosis of patients with MR, and lower LVEF value is associated with worse prognosis. Patients with MR should be actively followed up with echocardiography to assess the prognosis and adjust the treatment plan and means by focusing on the patient's LVEF. Strict LVEF monitoring should be performed in patients with normal LVEF in order to predict the patient's prognosis and propose reasonable therapeutic measures to improve the prognosis of MR patients in a timely manner. The prognosis of patients with moderate to severe MR with decreased LVEF should be actively improved by mitral valve surgery, which is the only way to improve left ventricular systolic dysfunction due to MR (32).
There are several limitations in our study. Firstly, the data was extracted from a single-center retrospective study, which hampered us from controlling a variety of confounders in analyses, whereas sizeable data extracted from medical records were allowed to control a variety of confounders and selection bias in analyses. Secondly, there existed population selection bias, and we lacked patients from primary hospitals. However, more than half of our population came from non-teaching hospitals and community hospitals, in urban and rural areas. Third, we lacked regular monitoring of dynamic changes in LVEF, which may be more important. However, the ultrasound on admission was performed by professional cardiac ultrasound experts with a small measurement bias. We cannot exclude the influence of other confounding factors on the results, including coronary artery bypass grafting (CABG) following CAG, the number of diseased vessels, and the incidence of full revascularization. The above variables are very meaningful for the analysis and interpretation of the results, and we will further collect and analyze the above variables in future studies. Finally, information about cause-specific death was not available in this study, and it is difficult to examine the significant correlation between LVEF and cause-specific death.
LVEF is a reliable prognostic index in CAD patients regardless of MR severity, and helpful for risk stratification. Reduced LVEF is associated with poor outcome in CAD patients with MR. Therefore, follow-up cardiac ultrasound for these patients is highly warranted.
The original contributions presented in the study are included in the article/Supplementary Files, further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by Guangdong Provincial People's Hospital Ethics Committee. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
YL, JL, NT, and JC: research idea and study design. QL, YZ, HH, WC, SS, SC, BW, WL, ZH, and ZL: data acquisition. JL and YL: data analysis/interpretation. SC and QL: statistical analysis. YL, JL, and JC: supervision and mentorship. All authors contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved. All authors contributed to the article and approved the submitted version.
This study was supported by grants from the National Science Foundation of China (Nos. 81970311 and 82070360), Study on the function and mechanism of the potential target for early warning of cardiorenal syndrome after acute myocardial infarction based on transformism (DFJH201919), Beijing Lisheng Cardiovascular Health Foundation (LHJJ20141751), and Clinical Medicine Research Fund of Guangdong Province (2019ZX01). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.799253/full#supplementary-material
Supplementary Figure 1. Flow chart of the study population. CAD, coronary artery disease; CAG, coronary arteriography; LVEF, left ventricular ejection fraction; MR, mitral regurgitation.
1. Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke statistics-2019 update: a report from the American heart association. Circulation. (2019) 139:e56–528. doi: 10.1161/CIR.0000000000000659
2. Burns RJ, Gibbons RJ, Yi Q, Roberts RS, Miller TD, Schaer GL, et al. The relationships of left ventricular ejection fraction, end-systolic volume index and infarct size to six-month mortality after hospital discharge following myocardial infarction treated by thrombolysis. J Am Coll Cardiol. (2002) 39:30–6. doi: 10.1016/S0735-1097(01)01711-9
3. Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet. (2006) 368:1005–11. doi: 10.1016/S0140-6736(06)69208-8
4. Tcheng JE, Jackman JD, Nelson CL, Gardner LH, Smith LR, Rankin JS, et al. Outcome of patients sustaining acute ischemic mitral regurgitation during myocardial infarction. Ann Intern Med. (1992) 117:18–24. doi: 10.7326/0003-4819-117-1-18
5. Messika-Zeitoun D, Candolfi P, Vahanian A, Chan V, Burwash IG, Philippon JF, et al. Dismal outcomes and high societal burden of mitral valve regurgitation in france in the recent era: a nationwide perspective. J Am Heart Assoc. (2020) 9:e016086. doi: 10.1161/JAHA.120.016086
6. Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, et al. Global burden of cardiovascular diseases and risk factors, 1990-2019: update from the GBD 2019 Study. J Am Coll Cardiol. (2020) 76:2982–3021. doi: 10.1016/j.jacc.2020.11.010
7. Samad Z, Shaw LK, Phelan M, Glower DD, Ersboll M, Toptine JH, et al. Long-term outcomes of mitral regurgitation by type and severity. Am Heart J. (2018) 203:39–48. doi: 10.1016/j.ahj.2018.05.001
8. Enriquez-Sarano M, Tajik AJ, Bailey KR, Seward JB. Color flow imaging compared with quantitative doppler assessment of severity of mitral regurgitation: influence of eccentricity of jet and mechanism of regurgitation. J Am Coll Cardiol. (1993) 21:1211–9. doi: 10.1016/0735-1097(93)90248-Y
9. Spain MG, Smith MD, Grayburn PA, Harlamert EA, DeMaria AN. Quantitative assessment of mitral regurgitation by doppler color flow imaging: angiographic and hemodynamic correlations. J Am Coll Cardiol. (1989) 13:585–90. doi: 10.1016/0735-1097(89)90597-4
10. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. (2021) 42:3599–726. doi: 10.1093/eurheartj/ehab368
11. Aguiar-Souto P, Ferrante G, Del Furia F, Barlis P, Khurana R, Di Mario C. Frequency and predictors of contrast-induced nephropathy after angioplasty for chronic total occlusions. Int J Cardiol. (2010) 139:68–74. doi: 10.1016/j.ijcard.2008.10.006
12. Catapano AL, Graham I, De Backer G, Wiklund O, Chapman MJ, Drexel H, et al. 2016 ESC/EAS guidelines for the management of dyslipidaemias. Rev Esp Cardiol. (2017) 70:115. doi: 10.1016/j.rec.2017.01.002
13. Yusuf S, Pitt B, Davis CE, Hood WB, Cohn JN. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. (1991) 325:293–302. doi: 10.1056/NEJM199108013250501
14. Folse R, Braunwald E. Determination of fraction of left ventricular volume ejected per beat and of ventricular end-diastolic and residual volumes. experimental and clinical observations with a precordial dilution technic. Circulation. (1962) 25:674–85. doi: 10.1161/01.CIR.25.4.674
15. Enriquez-Sarano M, Tajik AJ, Schaff HV, Orszulak TA, Bailey KR, Frye RL. Echocardiographic prediction of survival after surgical correction of organic mitral regurgitation. Circulation. (1994) 90:830–7. doi: 10.1161/01.CIR.90.2.830
16. Enriquez-Sarano M, Tajik AJ, Schaff HV, Orszulak TA, McGoon MD, Bailey KR, et al. Echocardiographic prediction of left ventricular function after correction of mitral regurgitation: results and clinical implications. J Am Coll Cardiol. (1994) 24:1536–43. doi: 10.1016/0735-1097(94)90151-1
17. Matsumura T, Ohtaki E, Tanaka K, Misu K, Tobaru T, Asano R, et al. Echocardiographic prediction of left ventricular dysfunction after mitral valve repair for mitral regurgitation as an indicator to decide the optimal timing of repair. J Am Coll Cardiol. (2003) 42:458–63. doi: 10.1016/S0735-1097(03)00649-1
18. Tribouilloy CM, Enriquez-Sarano M, Schaff HV, Orszulak TA, Bailey KR, Tajik AJ, et al. Impact of preoperative symptoms on survival after surgical correction of organic mitral regurgitation: rationale for optimizing surgical indications. Circulation. (1999) 99:400–5. doi: 10.1161/01.CIR.99.3.400
19. Apostolidou E, Maslow AD, Poppas A. Primary mitral valve regurgitation: update and review. Glob Cardiol Sci Pract. (2017) 2017:e201703. doi: 10.21542/gcsp.2017.3
20. Coleman W, Weidman-Evans E, Clawson R. Diagnosing and managing mitral regurgitation. JAAPA. (2017) 30:11–4. doi: 10.1097/01.JAA.0000516342.41351.6d
21. Wu S, Chai A, Arimie S, Mehra A, Clavijo L, Matthews RV, et al. Incidence and treatment of severe primary mitral regurgitation in contemporary clinical practice. Cardiovasc Revasc Med. (2018) 19:960–3. doi: 10.1016/j.carrev.2018.07.021
22. Gaasch WH, John RM, Aurigemma GP. Managing asymptomatic patients with chronic mitral regurgitation. Chest. (1995) 108:842–7. doi: 10.1378/chest.108.3.842
23. Rosenhek R, Rader F, Klaar U, Gabriel H, Krejc M, Kalbeck D, et al. Outcome of watchful waiting in asymptomatic severe mitral regurgitation. Circulation. (2006) 113:2238–44. doi: 10.1161/CIRCULATIONAHA.105.599175
24. Schiros CG, Dell'Italia LJ, Gladden JD, Clark D, Aban I, Gupta H, et al. Magnetic resonance imaging with 3-dimensional analysis of left ventricular remodeling in isolated mitral regurgitation: implications beyond dimensions. Circulation. (2012) 125:2334–42. doi: 10.1161/CIRCULATIONAHA.111.073239
25. Magne J, Szymanski C, Fournier A, Malaquin D, Avierinos JF, Tribouilloy C. Clinical and prognostic impact of a new left ventricular ejection index in primary mitral regurgitation because of mitral valve prolapse. Circ Cardiovasc Imaging. (2015) 8:e003036. doi: 10.1161/CIRCIMAGING.114.003036
26. Witkowski TG, Thomas JD, Debonnaire PJ, Delgado V, Hoke U, Ewe SH, et al. Global longitudinal strain predicts left ventricular dysfunction after mitral valve repair. Eur Heart J Cardiovasc Imaging. (2013) 14:69–76. doi: 10.1093/ehjci/jes155
27. Tribouilloy C, Grigioni F, Avierinos JF, Barbieri A, Rusinaru D, Szymanski C, et al. Survival implication of left ventricular end-systolic diameter in mitral regurgitation due to flail leaflets a long-term follow-up multicenter study. J Am Coll Cardiol. (2009) 54:1961–8. doi: 10.1016/j.jacc.2009.06.047
28. Flemming MA, Oral H, Rothman ED, Briesmiester K, Petrusha JA, Starling MR. Echocardiographic markers for mitral valve surgery to preserve left ventricular performance in mitral regurgitation. Am Heart J. (2000) 140:476–82. doi: 10.1067/mhj.2000.108242
29. Carabello BA. Ischemic mitral regurgitation and ventricular remodeling. J Am Coll Cardiol. (2004) 43:384–5. doi: 10.1016/j.jacc.2003.11.009
30. Asgar AW, Mack MJ, Stone GW. Secondary mitral regurgitation in heart failure: pathophysiology, prognosis, and therapeutic considerations. J Am Coll Cardiol. (2015) 65:1231–48. doi: 10.1016/j.jacc.2015.02.009
31. Crawford MH, Souchek J, Oprian CA, Miller DC, Rahimtoola S, Giacomini JC, et al. Determinants of survival and left ventricular performance after mitral valve replacement. department of veterans affairs cooperative study on valvular heart disease. Circulation. (1990) 81:1173–81. doi: 10.1161/01.CIR.81.4.1173
Keywords: left ventricular ejection fraction, prognosis, coronary artery disease, mitral regurgitation, indicator
Citation: Li Q, Zhang Y, Huang H, Chen W, Shi S, Chen S, Wang B, Lai W, Huang Z, Luo Z, Chen J, Tan N, Liu J and Liu Y (2022) Are There Any Differences in the Prognostic Value of Left Ventricular Ejection Fraction in Coronary Artery Disease Patients With or Without Moderate and Severe Mitral Regurgitation? Front. Cardiovasc. Med. 9:799253. doi: 10.3389/fcvm.2022.799253
Received: 21 October 2021; Accepted: 01 February 2022;
Published: 04 March 2022.
Edited by:
Mao Chen, Sichuan University, ChinaReviewed by:
Özlem Yildirimtürk, Dr. Siyami Ersek Chest Cardiovascular Surgery Training and Research Hospital, TurkeyCopyright © 2022 Li, Zhang, Huang, Chen, Shi, Chen, Wang, Lai, Huang, Luo, Chen, Tan, Liu and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yong Liu, bGl1eW9uZ0BnZHBoLm9yZy5jbg==; Jin Liu, bGphdzM5NzAxNzU2OEAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.