- 1Department of Cardiology, Guangdong Cardiovascular Institute, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, Guangzhou, China
- 2School of Medicine, South China University of Technology, Guangzhou, China
Background: Red blood cell distribution width (RDW) is associated with cardiovascular mortality. However, the relationship between preoperative RDW and outcomes after thoracic endovascular aortic repair (TEVAR) in type B aortic dissection (TBAD) remains to be determined.
Methods: We review the records of 678 patients with TBAD and treated with TEVAR in three centers. Patients were divided into two groups according to the admission RDW cut-off by receiver operating characteristic curve analysis [≤13.5% (n = 278) and >13.5% (n = 400)]. The association between RDW and long-term mortality was evaluated using Cox survival analysis. Additionally, we used general additive models (GAM) with restricted cubic splines (RCS) to explore non-linear relationships between RDW and outcomes.
Results: Subjects with a high RDW had significantly higher in-hospital mortality rates (1.4 vs. 4.3%, P = 0.038). A total of 70 subjects died after a median follow-up period of 3.3 years. Kaplan–Meier analysis showed that subjects with an RDW >13.5% had worse survival rates than those with lower RDW values (P < 0.001). Multivariate Cox proportional hazard modeling revealed that an RDW >13.5% was an independent predictor of long-term mortality (adjusted HR = 2.27, P = 0.006). Also, we found that there was a non-linear relationship between RDW and mortality from RCS, and RDW of 13.5% might be an inflection point to distinguish the long-term mortality risk of TBAD patients.
Conclusion: As an inexpensive and routinely measured parameter, RDW holds promise as a novel prognostic marker in patients with TBAD receiving TEVAR. We found that an RDW >13.5% on admission was independently associated with increased long-term mortality.
Introduction
Type B aortic dissection (TBAD) is a fatal disease, characterized by acute and subacute forms that present with several complications including a high mortality [(1), p. 2873–926]. Thoracic endovascular aortic repair (TEVAR) is an emerging treatment approach that has rapidly gained acceptance by clinicians for treating acute and sub-acute TBAD [(2, 3); (4), p. 407–16]. Compared to open surgery and medical treatment, TEVAR is considered a superior treatment strategy with lower mortality rates (5). However, postoperative mortality is still high during short- and long-term follow-ups [(3); (6), p. 213–25]. Therefore, early identification of patients at high risk for adverse outcomes is essential for clinicians to make the necessary therapeutic adjustments in a timely manner to improve prognosis.
Red blood cell distribution width (RDW) is a routine laboratory test parameter that reflects variations in the volume of red blood cells (RBC) volume [(7), p. 71–4]. In TBAD patients, blood entering the intima-media space may lead to blood loss. Liu et al. found that RDW was significantly higher in patients with anemia than in those with normal RBC levels [(8), p. 205–9]. In addition, RDW can be influenced by imbalanced physiological conditions, including oxidative stress, tissue hypoxia, neurohumoral hyperactivity, endothelial dysfunction, and chronic inflammation, all of which play an important role in aortic dissection (AD) [(9), p. 72–3]. In several clinical conditions, a high RDW has been associated with adverse outcomes. Therefore, we hypothesized that RDW could be a prognostic marker for patients with TBAD receiving TEVAR. The present study aimed to determine the relationship between RDW and adverse outcomes in patients with TBAD after TEVAR.
Materials and Methods
Patient Selection
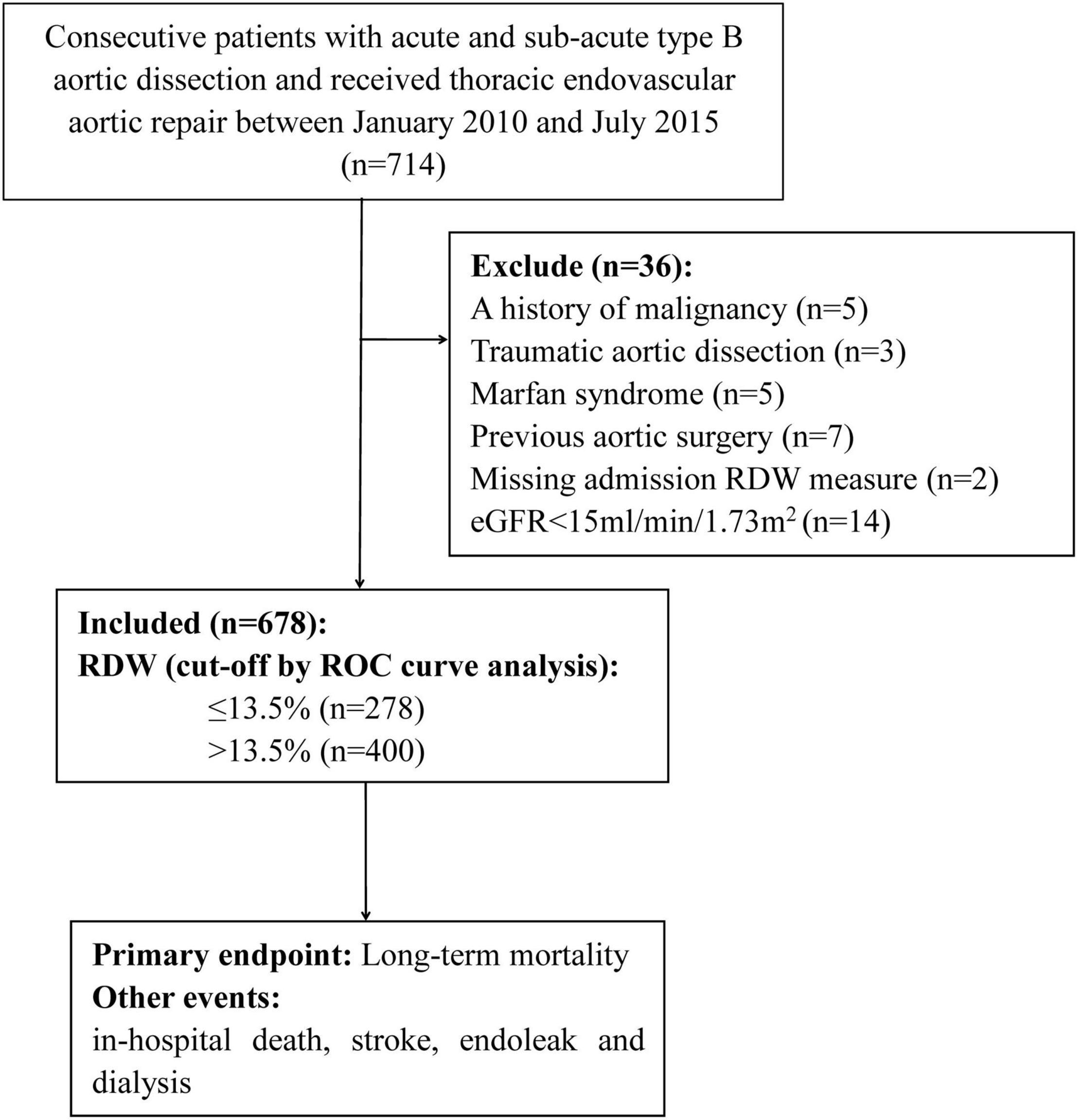
Included in the study, were patients with consecutive TBAD who underwent TEVAR between January 2010 and July 2015, they were retrospectively identified at the Guangdong Provincial People’s Hospital (Guangzhou, China), Nanhai Hospital of Guangdong General Hospital (Foshan, China), and Zhuhai Hospital of Guangdong Provincial People’s Hospital (Zhuhai, China). TBAD was diagnosed according to a multi-detector computed tomography scanning, and patients without complications received TEVAR when the true lumen was severely compressed or when the false lumen was >22 mm in diameter [(10), p. 374–81]. The operation was performed in a cardiac catheterization room with a suitable anatomical structure. According to the pathology of the aorta, the aortic stent diameter generally exceeds the standard by 5–10%. In order to eliminate tears at the proximal entrance, all stent grafts were deployed retrogradely through the percutaneous femoral artery approach. The left subclavian artery was covered when necessary to obtain a 1.5–2-cm proximal landing area. The exclusion criteria were as follows: (1) age <18 years; (2) AD caused by trauma, iatrogenic injury, or intramural hematoma; (3) diagnosis of Marfan syndrome; (4) previous TEVAR or surgery for AD; (5) chronic AD (onset >90 days before treatment) (11); (6) no previous TEVAR interventions; (7) history of malignancy or hematological disease, except anemia; and (8) end-stage kidney disease (Figure 1). The study protocol was approved by the central ethics committee of the Guangdong Provincial People’s Hospital (2017-15), with a waiver for informed consent. This approval by the central ethics committee applied to the other two collaborating hospitals as well.
Biochemical Assays and Data Collected
Upon admission of the patients, venous blood was collected to determine specific laboratory parameters. RDW was measured using an automated blood cell counter (LH780, Beckman Coulter, Brea, CA, United States), with a normal range of 11–16%. Clinical data were collected through chart review and electronic case reports entered into EpiData software 3.1 (The EpiData Association, Odense, Denmark) and processed using a consistency check on two copies. The estimated glomerular filtration rate (eGFR) in Chinese patients was evaluated using the 4-variable Modification of Diet in Renal Disease (MDRD) equation [(12), p. 2937–44].
Follow-Up and Endpoints
All patients were followed up by trained researchers via telephone interviews or clinic record visits from 9 August 2016 to 30 February 2017. In addition, the full clinical records of readmitted patients and outpatients were reviewed for adverse events. The primary endpoint was long-term mortality, which was defined as death from all causes after being diagnosed with TBAD. In-hospital death, stroke, type I endoleak, and dialysis events were recorded. Type I endoleak is defined as significant when detected during intraoperative control angiography or primary postoperative CTA control (in-hospital) [(13), p. 1022–33, 1033.e15]. Stroke includes ischemic or hemorrhagic stroke, with the clinical diagnosis supported by brain imaging.
Statistical Analysis
All statistical analyses were performed using SPSS software (version 22.0; SPSS, Inc., Chicago, IL, United States) and R version 3.6.11 (the R Foundation). Mean (±SD) or median (quartile range) values were used to describe continuous variables, which were compared using two independent sample t-tests or non-parametric tests based on the data. Categorical variables were reported as absolute values with percentages and compared using the Chi-squared test. Receiver operator characteristic (ROC) curve analysis was performed to evaluate the predictive value of RDW for in-hospital and long-term mortality. In addition, the optimal cut-off values were calculated. The optimal cut-off values for the ROC curves were established using the Youden index. Univariate Cox survival analysis was conducted to assess the risk factors for long-term mortality, indicators with P-values less than 0.05 were placed in a multivariate Cox proportional hazard model for further analysis. To visually evaluate the functional relationships between continuous variable and outcomes, the way similar with previous studies explored [(14), p. e007054; (15), p. 4322–9], we used general additive models (GAM) with restricted cubic splines (RCS) to assess the non-linear relationships between RDW and outcomes. Statistical significance was set at P < 0.05.
Results
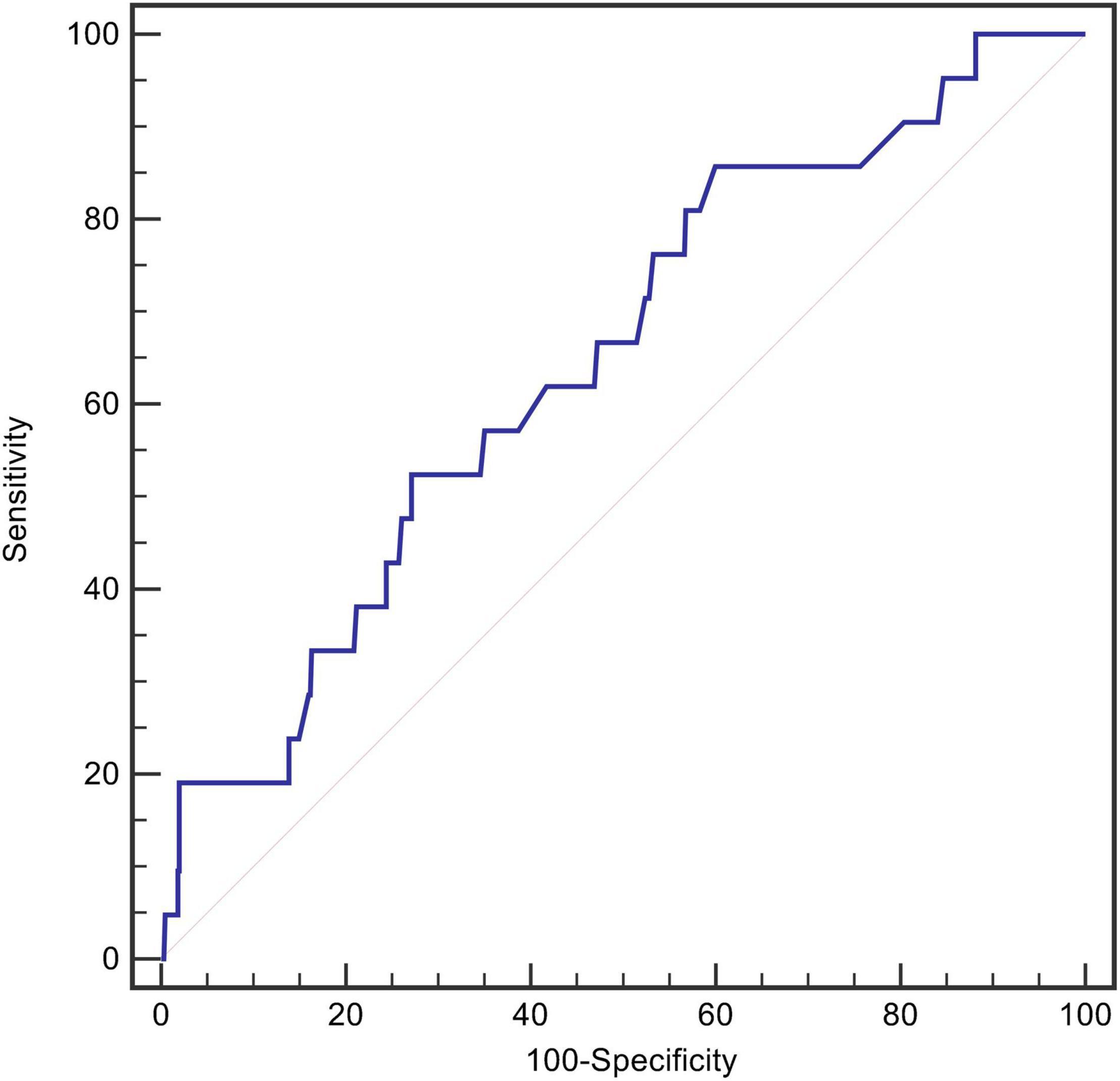
After screening, data from 678 patients were included in the final analysis. The mean age of the patients was 55 ± 11 years, 86.6% were men, and 21 (3.1%) died during the initial hospitalization. An ROC curve analysis was conducted to determine the predictive value of RDW at admission for in-hospital mortality. The optimal cut-off value was 13.5%, with relatively high sensitivity and specificity (AUC = 0.646, 95% CI, 0.530–0.763, P = 0.023, Figure 2).
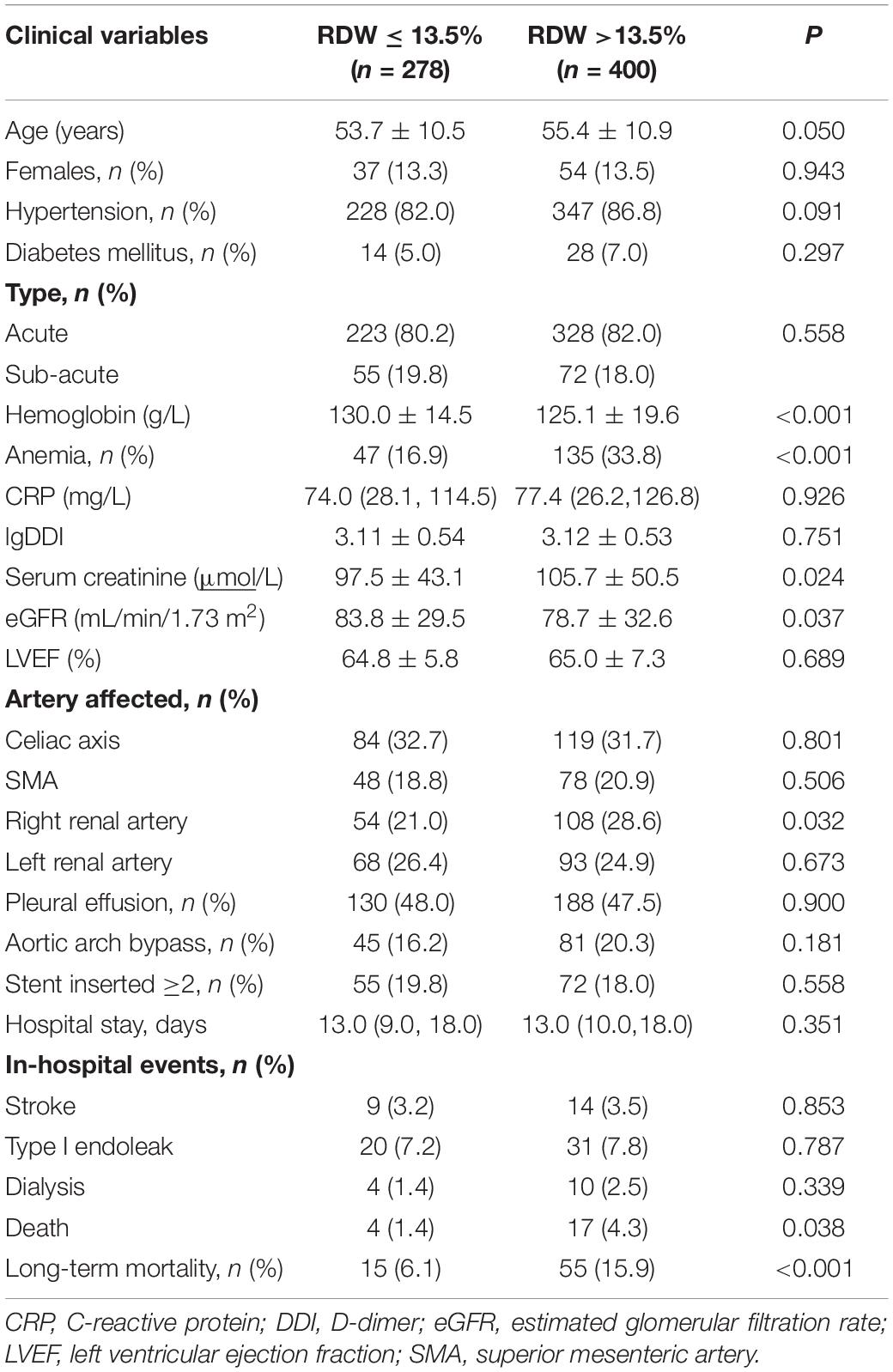
Patients were classified into two groups according to the threshold value of RDW, with RDW ≤13.5% (n = 278) and RDW >13.5% (n = 400). At admission, 26.8% of patients developed anemia, with a higher percentage of subjects with a high RDW (33.3 vs. 16.9%, P < 0.001). Clinical information was compared between the two groups (Table 1). Hemoglobin and eGFR were lower in the group with a RDW >13.5%, while the serum creatinine was higher.
During hospitalization, 51 (7.5%) patients experienced endovascular leaks after the TEVAR procedure, 23 (3.4%) developed stroke, and 14 (2.1%) required dialysis. There was no statistically significant difference between the patients with an RDW above or below 13.5%. However, the in-hospital mortality was higher in patients with an RDW >13.5% (4.3 vs 1.4%, P = 0.038, Table 1).
After a median follow-up of 3.3 years, 70 (10.3%) patients died, and 87 (12.8%) were lost to follow-up. Kaplan–Meier curve analysis showed that patients with an RDW >13.5% had a worse survival than those with a lower RDW (15.9 vs 6.1%, log-rank test = 13.37, P < 0.001, Figure 3). The results of the Cox proportional hazard modeling analysis are presented in Table 2. Univariate survival analysis indicated that RDW >13.5% was associated with long-term mortality (HR = 2.78; 95% CI, 1.57–4.92; P < 0.001). After the adjustment for these variables, RDW >13.5% remained an independent predictor of long-term mortality (adjusted HR = 2.27; 95% CI, 1.27–4.07; P = 0.006).
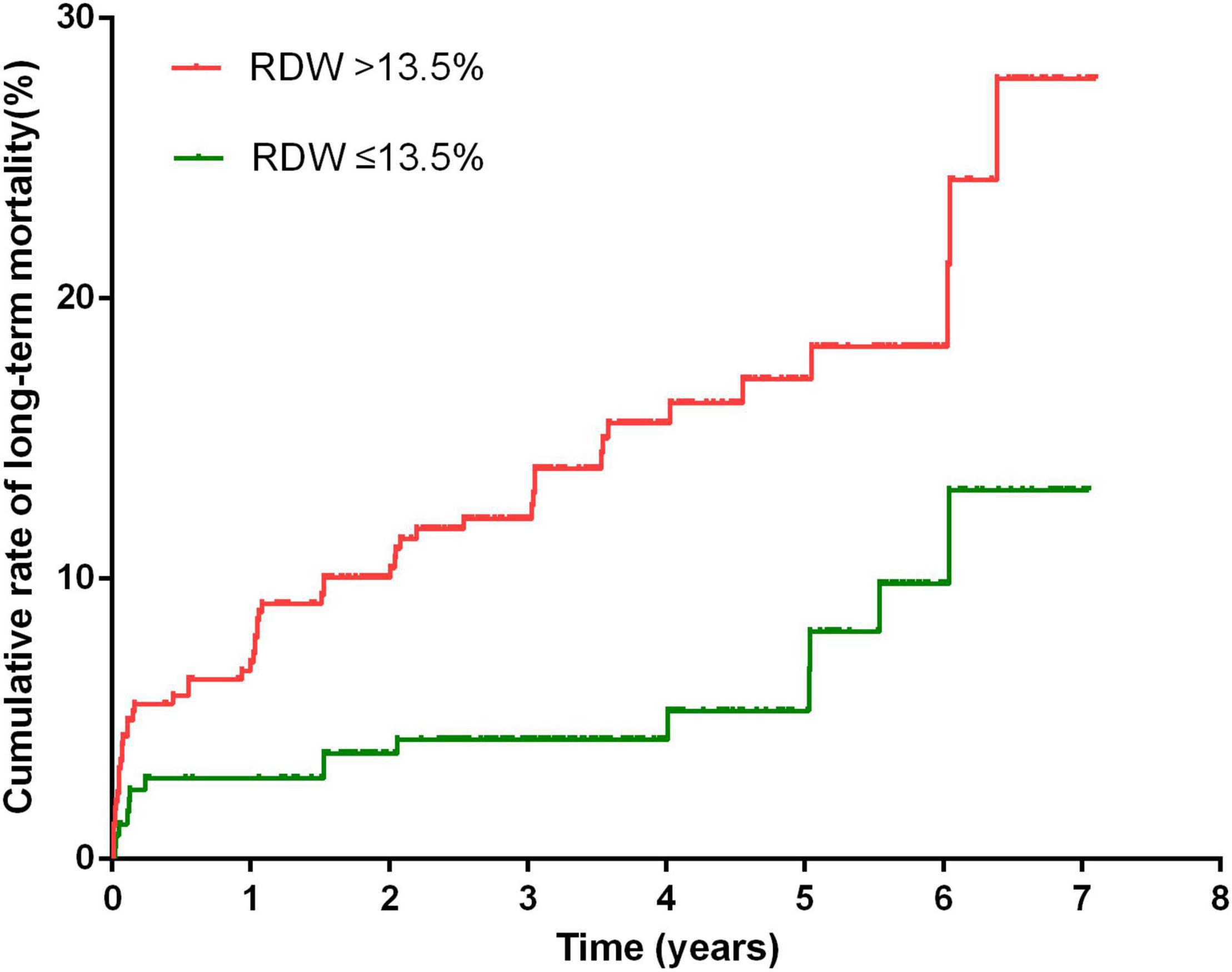
Figure 3. Cumulative rate of long-term mortality in patients with RDW >13.5% and patients with RDW <13.5%.
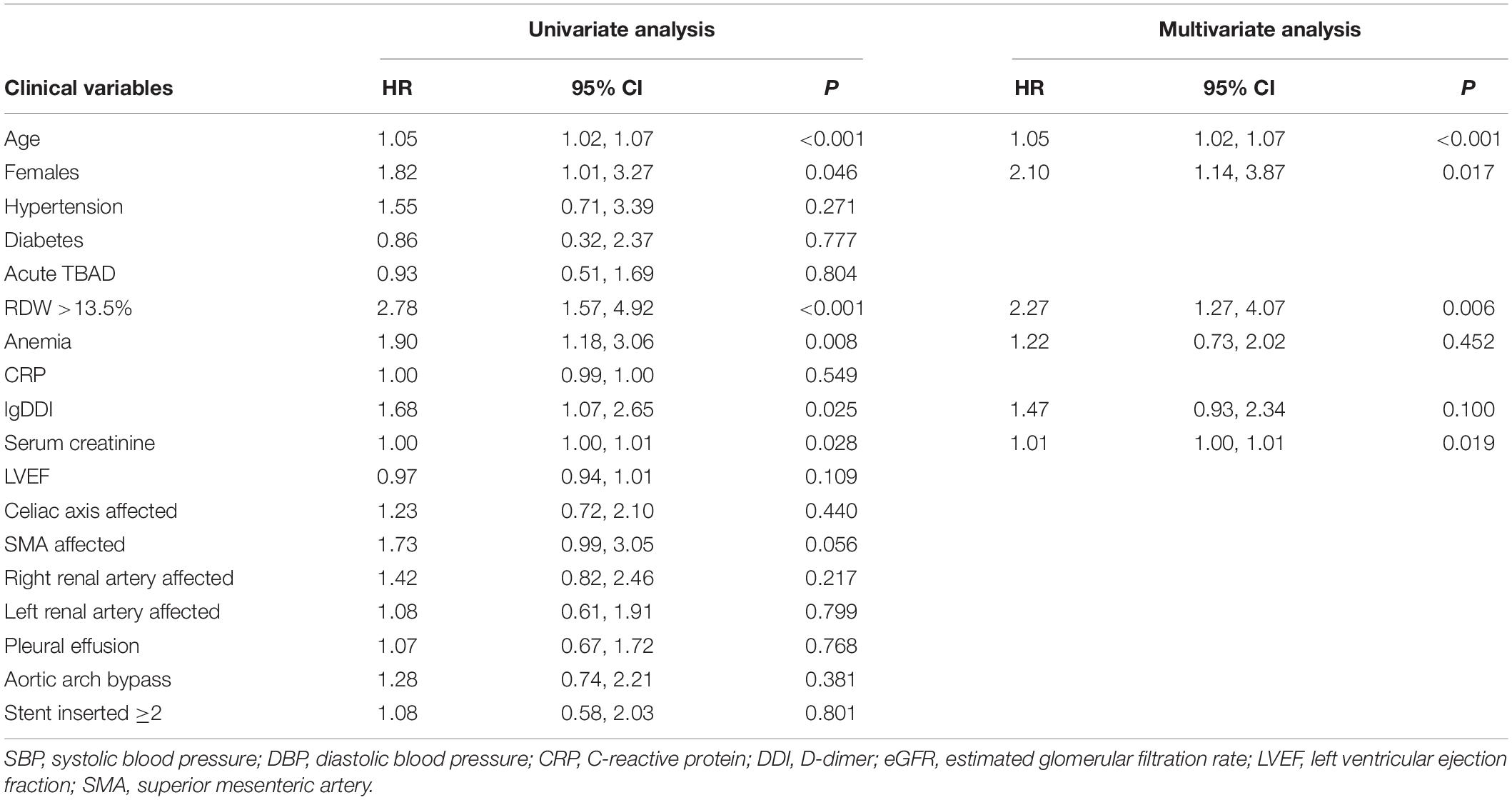
Table 2. Univariate and multivariate Cox proportional hazard modeling analysis for long-term mortality.
The RCS suggested a non-linear association between RDW and mortality, while this significance was not observed in RDW-MACE relationship (Figure 4). There were increasing relationships between RDW and outcomes. The difference was that, RDW-mortality relation first showed a plateau phase and then increased, while RDW-MACE relation was the opposite. Notably, for RDW-mortality relation (Figure 4A), when RDW (x-axis) was 13.5%, the Log RR (relative risk) for mortality (y-axis) was approximately 0, suggesting that RR = 1, which implied RDW at this cutoff value had no impact on the probability of mortality. When RDW >13.5%, the Log RR of mortality increased monotonically, reflecting that the increased long-term mortality risk of TBAD patients with the increase of RDW.
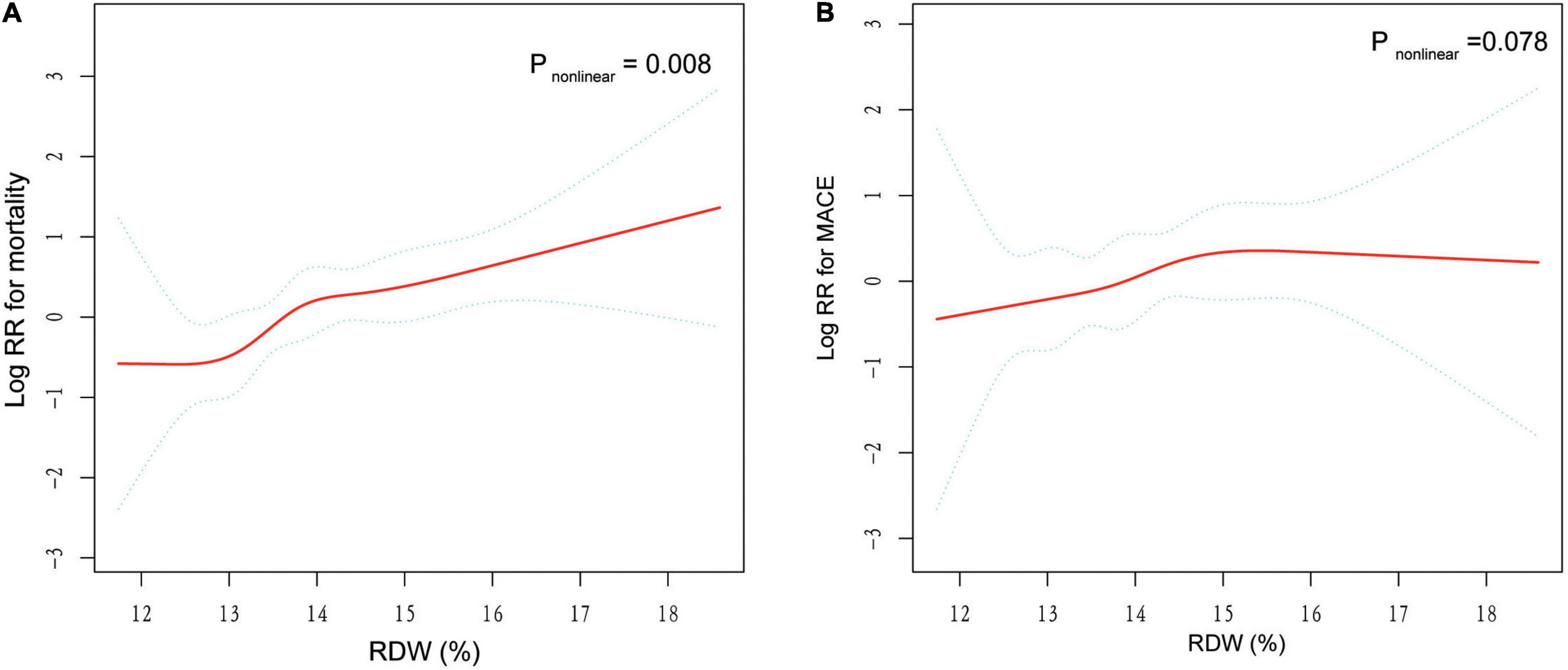
Figure 4. General additive models (GAM) with restricted cubic splines (RCS) demonstrate the relationship between RDW and the risk of mortality and MACE. The resulting figures show the predicted log RR (relative risk) in the y-axis and the RDW in the x-axis. Log RR can be converted to a relative risk by taking antilog. For example, a log RR of 0 implies the relative risk of 1 (no impact on the probability of prognosis), whereas a log RR of 1 implies the relative risk of 2.71 (i.e., 2.71-fold increase in the probability of MACE or mortality). (A) Association between RDW and log RR for mortality. (B) Association between RDW and log RR for MACE. The results were adjusted for covariates that statistically significant in multivariate analysis in Table 2, including age, gender, and serum creatinine.
Discussion
To the best of our knowledge, this study is the first to investigate the prognostic value of RDW in patients with TBAD receiving TEVAR. The results showed that RDW could be a predictor of in-hospital mortality. In addition, an RDW >13.5% was independently associated with an increased risk of long-term mortality.
Besides, we found a non-linear RDW-mortality relationship by GAM with RCS, suggesting that there was a threshold effect in the trend of mortality with RDW, and the two-piecewise linear regression models on both sides of the inflection point were statistically significant. As can be seen from the Figure 4A, the RDW of 13.5% might be the inflection point for distinguishing the mortality risk of TBAD patients, corresponding to the RDW cut-off value determined by the ROC curve.
Type B aortic dissection of the descending aorta or arch of the aorta is a life-threatening disease. Open surgical treatment has a high mortality and significant late complications, which have gradually been replaced by TEVAR [(13, 16), p. 1022–33, 1033.e15]. The epidemiological data indicate that the in-hospital mortality rate is 32% for patients with TBAD and treated with surgery, 7% for those treated with TEVAR, and 10% for those treated with medicine only [(17), p. 800–11]. In our study, the long-term mortality rate was 10.3%, which is similar to previous data [(4), p. 407–16]. Despite the decrease in TEVAR mortality, a significant number of patients still die after this intervention. Therefore, there is an urgent need to identify patients with a high mortality risk.
Red blood cell distribution width quantifies the heterogeneity of circulating RBCs and has been used to differentiate the causes of anemia. Subsequently, RDW is a well-established predictor of short- and long-term outcomes in various clinical conditions, including coronary artery disease and infectious diseases, as well as in the general population [(18), p. 515–23; (19), p. 163–8; (20), 123–7]. However, its potential to provide prognostic information for patients with TBAD remains unclear. In this study, we found that increased RDW was associated with in-hospital and long-term mortality after TEVAR for TBAD.
The following features can explain this effect. First, blood could enter the false lumen through the endovascular tear in patients with TBAD, leading to a decrease in circulating blood volume. This phenomenon can be detected by routine blood tests and may present as anemia. In our analysis, 26.8% of the subjects had anemia on admission. A previous study demonstrated a significant increase in RDW in patients with anemia [(8), p. 205–9]. Anemia is a predictor of adverse outcomes in several cardiovascular diseases [(21), p. 610–20; (22), p. 818–27]. However, this result was not the only explanation, as the RDW significance could not be eliminated by including anemia in our multivariate analysis. Second, increased oxidative stress has been found in patients with AD (23). Oxidative stress can directly damage RBCs and decrease their survival, resulting in increased anisocytosis (24, 25) and increased RDW. Selenium was negatively correlated with RDW as a component of the antioxidant defense system (26). A higher RDW may reflect severe oxidative stress, leading to adverse outcomes. Third, stress on the endoplasmic reticulum might occur, altering the regulation of apoptosis and inflammation. Excessive apoptosis could subsequently promote inflammation and degeneration of the vascular wall, which is an important mechanism for the formation and progression of AD (27). In response to this inflammatory process, pro-inflammatory cytokines are released, resulting in an increase in RDW [(28), p. 1011–23]. Finally, shear stress may be another factor leading to a high RDW and adverse outcomes. Taguchi et al. found that a high shear stress could induce intimal tears, which can progress to overt AD [(29), p. 78–82]. In addition, increased shear stress was associated with the development of retrograde aortic type A dissection, which is a severe complication of TBAD [(30), p. 324–330]. Shear stress can also impair RBC deformation and precipitate hemolysis, promoting increased RDW [(31), p. 1017–25].
The current study found that an increase in RDW levels upon admission is independently associated with an increase in long-term mortality in patients with TBAD. As an easily available indicator in routine blood testing, RDW can be used as an important factor in prognostic risk stratification, selecting high-risk patients, guiding treatment strategies, and reducing the risk of all-cause death in patients with TBAD. In view of the common prognostic effect of high RDW in several cardiovascular and infectious diseases, a high RDW is likely to represent secondary damage related to inflammatory responses, and oxidative stress. Monitoring changes in RDW may reflect the effects of controlling cardiovascular risk factors. It may be possible to base future follow-up plans on patients’ RDW and improve the long-term survival of AD patients, since many patients with TBAD may not strictly follow the annual follow-up recommendation.
Study Limitation
This study had several limitations. First, due to its retrospective design, confounding factors may have affected the results. However, a multivariate analysis was performed to reduce these. Second, the predictive value of RDW was not compared with that of other inflammatory markers. Third, the RDW was not detected dynamically. Therefore, it is not clear whether a continuous measurement of the dynamic changes in RDW rather than the measurement of the baseline RDW would have a better predictive value for the outcomes of the patient. Finally, the lost to follow-up rate (12.8%) in the present study was relatively high and could lead to selection bias.
Conclusion
As an inexpensive and routinely measured RBC parameter, RDW has been proven to be a useful prognostic marker in patients with TBAD receiving TEVAR. Increased RDW was significantly associated with in-hospital and long-term mortality. This relationship should be investigated in prospective studies with larger sample sizes.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics Statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
QG, YL, and CJ contributed to the conception and design of the study. CJ, AL, LH, and QL contributed to the acquisition, analysis, and interpretation of data. CJ and AL drafted the manuscript. QG and YL critically revised the manuscript. All authors gave final approval and agreed to be accountable for all aspects of work ensuring integrity and accuracy.
Funding
This work was supported by the grants of Natural Science Foundation of Guangdong Province (2019A1515011224) and the High-Level Hospital Construction Project of Guangdong Provincial People’s Hospital (DFJH201811, DFJH201922, and DFJH2020003).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
References
1. Erbel R, Aboyans V, Boileau C, Bossone E, Bartolomeo RD, Eggebrecht H, et al. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The task force for the diagnosis and treatment of aortic diseases of the European society of cardiology (ESC). Eur Heart J. (2014) 35:2873–926. doi: 10.1093/eurheartj/ehu281
2. Brunkwall J, Lammer J, Verhoeven E, Taylor P. ADSORB: a study on the efficacy of endovascular grafting in uncomplicated acute dissection of the descending aorta. J Vasc Surg. (2012) 56:31–6. doi: 10.1016/j.ejvs.2012.03.023
3. Fattori R, Montgomery D, Lovato L, Kische S, Di Eusanio M, Ince H, et al. Survival after endovascular therapy in patients with type B aortic dissection: a report from the International registry of acute aortic dissection (IRAD). JACC Cardiovasc Interv. (2013) 6:876–82. doi: 10.1016/j.jcin.2013.05.003
4. Nienaber CA, Kische S, Rousseau H, Eggebrecht H, Rehders TC, Kundt G, et al. Endovascular repair of type B aortic dissection: long-term results of the randomized investigation of stent grafts in aortic dissection trial. Circ Cardiovasc Interv. (2013) 6:407–16. doi: 10.1161/CIRCINTERVENTIONS.113.000463
5. Fattori R, Tsai TT, Myrmel T, Evangelista A, Cooper JV, Trimarchi S, et al. Complicated acute type B dissection: is surgery still the best option?: a report from the International registry of acute aortic dissection. JACC Cardiovasc Interv. (2008) 1:395–402. doi: 10.1016/j.jcin.2008.04.009
6. Heijmen RH, Thompson MM, Fattori R, Goktay Y, Teebken OE, Orend KH. Valiant thoracic stent-graft deployed with the new captivia delivery system: procedural and 30-day results of the valiant captivia registry. J Endovasc Ther. (2012) 19:213–25. doi: 10.1583/11-3652MR.1
7. Evans TC, Jehle D. The red blood cell distribution width. J Emerg Med. (1991) 9:71–4. doi: 10.1016/0736-4679(91)90592-4
8. Liu Q, Dang AM, Chen BW, Lv NQ, Wang X, Zheng DY. The association of red blood cell distribution width with anemia and inflammation in patients with Takayasu arteritis. Clin Chim Acta. (2015) 438:205–9. doi: 10.1016/j.cca.2014.08.025
9. Loprinzi PD. Comparative evaluation of red blood cell distribution width and high sensitivity C-reactive protein in predicting all-cause mortality and coronary heart disease mortality. Int J Cardiol. (2016) 223:72–3. doi: 10.1016/j.ijcard.2016.08.156
10. Ding H, Liu Y, Xie N, Fan R, Luo S, Huang W, et al. Outcomes of chimney technique for preservation of the left subclavian artery in type B aortic dissection. Eur J Vasc Endovasc Surg. (2019) 57:374–81. doi: 10.1016/j.ejvs.2018.09.005
11. Steuer J, Björck M, Mayer D, Wanhainen A, Pfammatter T, Lachat M. Distinction between acute and chronic type B aortic dissection: is there a sub-acute phase? J Vasc Surg. (2013) 57:627–31. doi: 10.1016/j.ejvs.2013.03.013
12. Ma YC, Zuo L, Chen JH, Luo Q, Yu XQ, Li Y, et al. Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol. (2006) 17:2937–44. doi: 10.1681/ASN.2006040368
13. Fillinger MF, Greenberg RK, McKinsey JF, Chaikof EL. Reporting standards for thoracic endovascular aortic repair (TEVAR). J Vasc Surg. (2010) 52:1022–33. doi: 10.1016/j.jvs.2010.07.008
14. Wu J, Zheng H, Liu X, Chen P, Zhang Y, Luo J, et al. Prognostic value of secreted frizzled-related protein 5 in heart failure patients with and without type 2 diabetes mellitus. Circ Heart Fail. (2020) 13:e007054. doi: 10.1161/CIRCHEARTFAILURE.120.007054
15. Garzotto M, Beer TM, Hudson RG, Peters L, Hsieh YC, Barrera E, et al. Improved detection of prostate cancer using classification and regression tree analysis. J Clin Oncol. (2005) 23:4322–9. doi: 10.1200/JCO.2005.11.136
16. Hagan PG, Nienaber CA, Isselbacher EM, Bruckman D, Karavite DJ, Russman PL, et al. The International registry of acute aortic dissection (IRAD): new insights into an old disease. JAMA. (2000) 283:897–903. doi: 10.1001/jama.283.7.897
17. Nienaber CA, Clough RE. Management of acute aortic dissection. Lancet. (2015) 385:800–11. doi: 10.1016/S0140-6736(14)61005-9
18. Patel KV, Ferrucci L, Ershler WB, Longo DL, Guralnik JM. Red blood cell distribution width and the risk of death in middle-aged and older adults. Arch Intern Med. (2009) 169:515–23. doi: 10.1001/archinternmed.2009.11
19. Tonelli M, Sacks F, Arnold M, Moye L, Davis B, Pfeffer M. Relation between red blood cell distribution width and cardiovascular event rate in people with coronary disease. Circulation. (2008) 117:163–8. doi: 10.1161/CIRCULATIONAHA.107.727545
20. Ku NS, Kim HW, Oh HJ, Kim YC, Kim MH, Song JE, et al. Red blood cell distribution width is an independent predictor of mortality in patients with gram-negative bacteremia. Shock. (2012) 38:123–7. doi: 10.1097/SHK.0b013e31825e2a85
21. Kwok CS, Tiong D, Pradhan A, Andreou AY, Nolan J, Bertrand OF, et al. Meta-analysis of the prognostic impact of anemia in patients undergoing percutaneous coronary intervention. Am J Cardiol. (2016) 118:610–20. doi: 10.1016/j.amjcard.2016.05.059
22. Groenveld HF, Januzzi JL, Damman K, van Wijngaarden J, Hillege HL, van Veldhuisen DJ, et al. Anemia and mortality in heart failure patients a systematic review and meta-analysis. J Am Coll Cardiol. (2008) 52:818–27. doi: 10.1016/j.jacc.2008.04.061
23. Liao M, Liu Z, Bao J, Zhao Z, Hu J, Feng X, et al. A proteomic study of the aortic media in human thoracic aortic dissection: implication for oxidative stress. J Thorac Cardiovasc Surg. (2008) 136:65–72. doi: 10.1016/j.jtcvs.2007.11.017
24. Kiefer CR, Snyder ML. Oxidation and erythrocyte senescence. Curr Opin Hematol. (2000) 7:113–6. doi: 10.1097/00062752-200003000-00007
25. Friedman JS, Lopez MF, Fleming MD, Rivera A, Martin FM, Welsh ML, et al. SOD2-deficiency anemia: protein oxidation and altered protein expression reveal targets of damage, stress response, and antioxidant responsiveness. Blood. (2004) 104:2565–73. doi: 10.1182/blood-2003-11-3858
26. Semba RD, Patel KV, Ferrucci L, Sun K, Roy CN, Guralnik JM, et al. Serum antioxidants and inflammation predict red cell distribution width in older women: the women’s health and aging study I. Clin Nutr. (2010) 29:600–4. doi: 10.1016/j.clnu.2010.03.001
27. Jia LX, Zhang WM, Zhang HJ, Li TT, Wang YL, Qin YW, et al. Mechanical stretch-induced endoplasmic reticulum stress, apoptosis and inflammation contribute to thoracic aortic aneurysm and dissection. J Pathol. (2015) 236:373–83. doi: 10.1002/path.4534
28. Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med. (2005) 352:1011–23. doi: 10.1056/NEJMra041809
29. Taguchi E, Nishigami K, Miyamoto S, Sakamotoand T, Nakao K. Impact of shear stress and atherosclerosis on entrance-tear formation in patients with acute aortic syndromes. Heart Vessels. (2014) 29:78–82. doi: 10.1007/s00380-013-0328-z
30. Osswald A, Karmonik C, Anderson JR, Rengier F, Karck M, Engelke J, et al. Elevated wall shear stress in aortic type B dissection may relate to retrograde aortic type A dissection: a computational fluid dynamics pilot study. Eur J Vasc Endovasc Surg. (2017) 54:324–30. doi: 10.1016/j.ejvs.2017.06.012
Keywords: aortic dissection, red blood cell distribution width, thoracic endovascular aortic repair, prognostic marker, in-hospital mortality
Citation: Jiang C, Liu A, Huang L, Liu Q, Liu Y and Geng Q (2022) Red Blood Cell Distribution Width: A Prognostic Marker in Patients With Type B Aortic Dissection Undergoing Endovascular Aortic Repair. Front. Cardiovasc. Med. 9:788476. doi: 10.3389/fcvm.2022.788476
Received: 02 October 2021; Accepted: 31 March 2022;
Published: 02 May 2022.
Edited by:
Yuli Huang, Southern Medical University, ChinaReviewed by:
Zhihui Dong, Fudan University, ChinaDan Rudic, Augusta University, United States
Lin Xiao, Southern Medical University, China
Copyright © 2022 Jiang, Liu, Huang, Liu, Liu and Geng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qingshan Geng, Z2VuZ3FpbmdzaGFuQGdkcGgub3JnLmNu; Yuan Liu, MTM3MTE2NTI3MzBAMTM5LmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Cheng Jiang
Cheng Jiang Anbang Liu
Anbang Liu Lei Huang1
Lei Huang1 Quanjun Liu
Quanjun Liu Qingshan Geng
Qingshan Geng