
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cardiovasc. Med., 23 February 2022
Sec. Heart Failure and Transplantation
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.787975
Post-transplant lymphoproliferative disorder (PTLD) is a spectrum of lymphoid conditions frequently associated with the Epstein Barr Virus (EBV) and the use of potent immunosuppressive drugs after solid organ transplantation. PTLD remains a major cause of long-term morbidity and mortality following heart transplantation (HT). Epstein-Barr virus (EBV) is a key pathogenic driver in many PTLD cases. In the majority of PTLD cases, the proliferating immune cell is the B-cell, and the impaired T-cell immune surveillance against infected B cells in immunosuppressed transplant patients plays a key role in the pathogenesis of EBV-positive PTLD. Preventive screening strategies have been attempted for PTLD including limiting patient exposure to aggressive immunosuppressive regimens by tailoring or minimizing immunosuppression while preserving graft function, anti-viral prophylaxis, routine EBV monitoring, and avoidance of EBV seromismatch. Our group has also demonstrated that conversion from calcineurin inhibitor to the mammalian target of rapamycin (mTOR) inhibitor, sirolimus, as a primary immunosuppression was associated with a decreased risk of PTLD following HT. The main therapeutic measures consist of immunosuppression reduction, treatment with rituximab and use of immunochemotherapy regimens. The purpose of this article is to review the potential mechanisms underlying PTLD pathogenesis, discuss recent advances, and review potential therapeutic targets to decrease the burden of PTLD after HT.
De novo malignancy is an important cause of long-term morbidity and mortality in solid organ transplant (SOT) recipients. The incidence of de novo malignancy in adults has been reported to be ~20% after 10 years (1–5) and as high as 40–70% during a 20-year period of immunosuppression after transplantation (6–10). Heart transplant (HT) recipients are at particularly increased risk of developing malignancies after transplantation, which is increased up to 4-fold compared with renal transplant recipients (11–16). With the improvement of early survival following HT and the increasing number of older patients receiving HT (17), malignancy becomes relatively more important than other causes of morbidity and mortality post-transplant (18). Indeed, malignancy is the main cause of death at 5 years after HT (2).
Post-transplant lymphoproliferative disorder (PTLD) is a spectrum of lymphoid conditions associated with the use of potent immunosuppressive drugs after SOT or hematopoietic stem-cell (HSC) transplantation (19–21). PTLD is the second most frequent malignancy after skin cancers in HT recipients, representing up to 10% of de novo malignancies post-HT (14) contributing to the overall mortality of HT patients, with a 5-year overall survival rate in the pre-rituximab era of 20% (14). Most PTLD cases are B-cell neoplasms, and up to 35% occur within the 1st year following transplantation (early PTLD), with more than 50% of cases associated with Epstein–Barr virus (EBV) (22). This review describes updated information on PTLD, including diagnosis, prevalence and risk factors, highlights insights into the pathophysiology and examines treatment strategies and future directions of research to treat this devastating complication following HT.
Data from transplant registries during the past two decades have reported an increased incidence of PTLD (2, 23–25). Analysis of data from the U.S. Organ Procurement Transplant Network (OPTN)/United Network for Organ Sharing (UNOS) database on adult transplantation performed in the United States between 1999 and 2008 demonstrated that the incidence of PTLD was highest in lung recipients [5.72 per 1,000 person-years (PY)], intermediate in liver (2.44/1,000 PY) and heart recipients (2.24/1,000 PY), and lowest in kidney recipients (1.58/1,000 PY). In HT recipients, PTLD was previously reported as the third most common malignancy with incidence of 2.24/1,000 PY (23).
A recent national registry of adult and pediatric SOT recipients from the United States with data from 2005 to 2014 reported a 5-year cumulative incidence of PTLD ranging from 0.6 to 9% in adult transplant recipients and from 2 to 15.8% in pediatric transplant recipients, with the highest PTLD rates for intestine transplant (9%) (combined adult and pediatric data). The rates of adult PTLD for heart and kidney transplants were 0.9 and 0.6%, respectively. These rates were found to be lower in patients with EBV positive serology compared to those with negative serology (25). However, an earlier single-center analysis of biopsy-confirmed PTLD in 6,607 HSC and SOT recipients between 1989 and 2010 in Belgium, reported overall incidence of 2.12%, with the highest among HT recipients (5.0%) (26). Pediatric SOT recipients were noted to experience higher incidence of PTLD than adults, which can be attributed in large part to the development of primary EBV infection after transplantation (27, 28). Indeed, pediatric recipients were more commonly EBV mismatched than were adult recipients for all organ types (25).
The incidence of EBV-negative PTLD was reported to be increasing over time in a cohort of 176 SOT recipients (29). In contrast, EBV positive PTLD cases tend to occur early post- transplant whereas EBV-negative PTLD cases have a continued increase in incidence in each year (30). The data on incidence of PTLD after HT are derived from single-center and multicenter reports with incidence rates that range from 0.7 to 6.8% (Table 1) (5, 14, 15, 31–42). Kotton et al. (25) analyzed PLTD rates based on EBV serology and type of organ transplant, the overall rates of PTLD in adult HT subgroup was 0.9% in all serology, 2.1% in EBV-negative serology and 0.6% in EBV-positive serology. Similarly, in adult kidney transplant recipients, PTLD rates were 0.6% in all serology, 1.7% in EBV-negative serology, and 0.5% in EBV-positive serology.
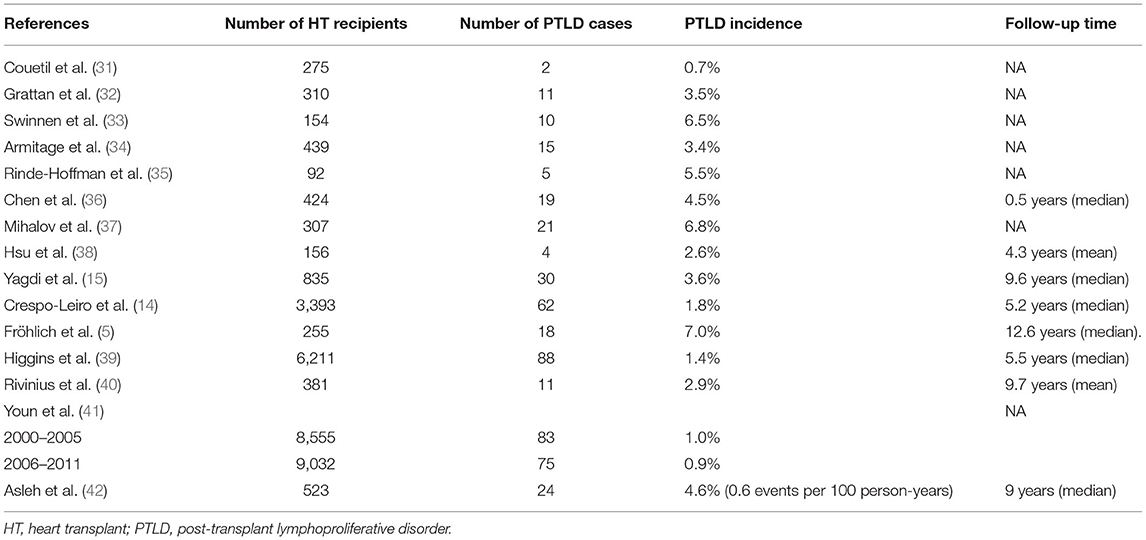
Table 1. Published data on the incidence of post-transplant lymphoproliferative disorder in cardiac transplant recipients.
The risk of PTLD is affected by the type of organ transplanted with the lowest risk observed in kidney transplant recipients compared to heart and lung transplant recipients (23, 26, 27). This may be, at least partially, explained by more intensive use of immunosuppression in recipients of thoracic organs. Although not fully understood, the increased incidence of PTLD after lung and intestinal transplantation may also be attributed to the large number of EBV-infected donor lymphocytes residing within these transplanted organs (27). In a previous study by Opelz and Döhler (43), the risk of lymphoma during the first post-transplant year was reported to be the highest for combined heart-lung recipients followed by lung, heart and kidney with the lowest risk. Moreover, the steepest long-term increase was noted for HT recipients.
The age of the transplant recipient is a factor affecting the risk of PTLD with greatest risk in both age extremes. SOT subjects aged < 10 and > 60 years were reported to be at increased risk of PTLD (43). Data from HT patients revealed that the HT recipient age was not associated with PTLD risk (14, 41, 42). One study found that recipient age < 18 years was strongly associated with increased risk of PTLD in HT recipients, which was independent of recipient EBV seronegative status (44).
Additionally, a higher incidence of PTLD has been reported in Caucasian SOT recipients (45, 46). However, this association has not been established in HT recipients in particular (44). SOT donor and recipient‘s genetic variation has been identified as a factor in the development of PTLD. SOT recipient positivity for HLA DR13 or B38, have been associated with higher risk of the PTLD (47), whereas donor haplotypes HLA-A1, HLA-B8, and HLA-DR3 were identified as protective factors (48). Furthermore, a higher degree of HLA mismatch was also associated with increased risk of PTLD (24, 47).
Donor to recipient EBV seromismatch (D+/R-), or (D-/R+) represents one of the strongest risk factors for PTLD development (44, 49–54). Moreover, the incidence of PTD post SOT has a bimodal curve, with an initial spike, mostly involving EBV-positive transplant recipients, during the first 12 months followed by a late spike, mostly involving EBV-negative recipients, 5–15 years after transplantation (26, 55, 56). Both EBV recipient serostatus (negative vs. positive) (41, 44) and EBV infection (42) were found to be strongly associated with increased risk of PTLD in HT patients.
An analysis of the SRTR National Registry Data in the United States was comprised of 112,756 kidney transplants (PTLD cases; 0.51%), 13,937 HT (1.0%), and 40,437 lung transplants (0.95%). EBV seronegative status at the time of transplant was associated with increased risk of PTLD with the highest risk in HT (44).
The risk of PTLD due to immunosuppression therapy is related to different immunosuppression approaches including T-cell depletion strategies. In SOT, the induction therapy with the monoclonal agent antibody, muromonab-CD3 (Orthoclone OKT3), when added to maintenance immunosuppression was found to be associated with higher risk of PTLD (33, 43). There are conflicting data regarding anti-thymocyte globulin (ATG) with some reports of increased risk of PTLD (43, 57) whereas others showed no increased risk of PTLD (58). Data from HT studies have reported an increased risk of PTLD associated with ATG (14, 41) but not with OKT (14). However, we have recently shown that the risk of PTLD was similar between HT recipients who received OKT3 and those received ATG induction therapy (42). Moreover, the associated OKT3 risk with PTLD in HT recipients has been shown to be dose-dependent (33).
Regarding maintenance immunosuppression, the contribution of each immunosuppressive agent is not clear, due to the frequent use of multiple agents in different doses and at different times post transplantation. Calcineurin inhibitors (CNIs; tacrolimus and cyclosporine) have been implicated as potential risk factors for PTLD following SOT (11, 24, 43). The multicenter Collaborative Transplant study (11) found that antithymocyte/antilymphocyte globulin or the monoclonal anti-T-cell antibody OKT3 use and use of a combination of cyclosporine and azathioprine wee independent risk factors of PTLD, but there was no increase in PTLD risk when cyclosporine was used alone.
Analysis of HT recipients from the SRTR National Registry Data in the United States reported that cyclosporine was associated with decreased risk of PTLD when compared to tacrolimus (44). However, data form HT patients showed that tacrolimus as an individual agent was not associated with PTLD risk (14). Although mycophenolate Mofetil (MMF) in standard immunosuppressive regimens after HT was found to be associated with a significantly lower risk of all de novo malignancies in general (59), it was not found to be associated with PTLD risk (14). When compared to MMF, azathioprine was not found to be associated with risk of PTLD post HT (41, 42, 44).
Sirolimus (SRL) has been shown to have antitumor and anti-EBV proliferation effects in vivo (60, 61). Data from kidney transplant patients suggests reduced risk of overall de novo malignancies (62, 63) and skin cancer (62–64). However, SRL use post transplantation was not found to be associated with decreased PTLD risk post kidney transplant (62).
A recent study from our group demonstrated that conversion to SRL as primary immunosuppression, with withdrawal of CNI therapy, was associated with a decreased risk of all de novo malignancies, PTLD, and subsequent primary occurrences of non-melanoma skin cancer after HT (42).
The pathogenesis of PTLD involves one or more of the following mechanisms: (1) impaired immune surveillance of tumor cells due to immunosuppression; (2) decreased anti-viral immune activity and oncogenic effect of EBV; and (3) derangement of molecular signaling/DNA repair mechanisms by direct effect of immunosuppressive agents (Figure 1).
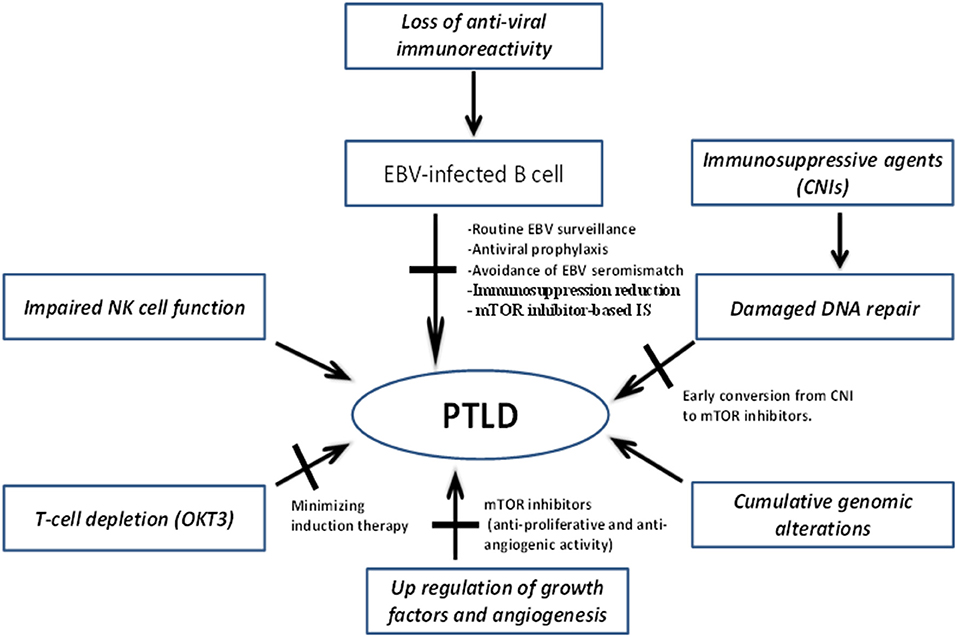
Figure 1. Mechanisms underlying pathogenesis of PTLD and potential targets to mitigate disease development and progression. CNIs, Calcineurin inhibitors; EBV, Epstein–Barr virus; IS, immunosuppression; mTOR, mammalian target of rapamycin; NK, natural killer; PTLD, post-transplant lymphoproliferative disorder.
The abnormal cell proliferation is driven in 50–80% of PTLD cases by EBV (49). The life cycle of EBV is initiated by an infection in immunocompetent hosts followed by lytic cycling and latency in the reticuloendothelial system. After that, EBV changes its viral gene program to express a type III gene latency program, characterized by expression of nine viral proteins including latent membrane protein 1 (LMP-1) and Epstein-Barr nuclear antigens (EBNAs-). To avoid host T lymphocyte recognition of these highly immunogenic latency III proteins, the virus transitions down further to a latency type II gene program, of which some genes provide surrogate co-stimulatory signals to host B cells to promote cell survival and differentiation. The resultant memory B cells have expressing EBV-encoded- RNA genes, concealing itself from host responses (65, 66).
In immunocompetent hosts, EBV-specific CD8+ effector and memory T cells are responsible for control of these EBV-infected B cells from abnormal and uncontrolled proliferation (67). This host T cell control of B cell proliferation is suppressed by immunosuppression (68). Therefore, Impaired T-cell immune surveillance against infected B cells in immunosuppressed transplant patients plays a key role in the pathogenesis of EBV-positive PTLD (69).
The pathogenesis of EBV-negative cases of PTLD is less clear. However, previous genomic studies demonstrated that EBV negative PTLD cases share genomic alterations seen in diffuse large B-cell Lymphoma (70–72) and T-cell lymphomas in immunocompetent patients (73). In contrast, EBV-positive PTLD cases have fewer such genomic abnormalities (74).
The mechanisms of the increased risk for PTLD due to induction therapy with monoclonal induction antibodies are unclear. However, animal models showed that low T cell numbers at the time of transplantation from depleting antibodies increased the risk of PTLD (75). Moreover, impaired T-cell immune surveillance against EBV-infected B cells in immunosuppressed transplant patients plays a key role in the pathogenesis of EBV-positive PTLD (69). By expressing different latent antigens during B-cell development, EBV incorporates the normal B-cell program, thereby promoting proliferation and transformation of these cells. In normal circumstances, these antigens elicit a T-cell response that destroys the majority of EBV-infected B cells. This immunologic response is diminished in transplant recipients, hence increasing the risk of B-cell transformation and development of lymphomas.
CNIs exhibit pro-carcinogenic potential via inducing transforming growth factor-β production, which enhances tumor progression and angiogenesis, and inhibits DNA repair enzymes facilitating accumulation of mutations (76).
The decreased risk of overall post-transplant malignancies including PTLD in patients treated with SRL is related to its additive inhibitory effects on tumor growth, including antiproliferative and antiangiogenic activities beyond its immunosuppressive effect. The mammalian target of rapamycin (mTOR) pathway is a regulatory serine-threonine kinase, activated via the phosphatidylinositol-3-kinase (AKT) which has been implicated in progression of malignancies (77). SRL inhibits the (PI3K) signaling pathway contributing to the regulation of cell proliferation. SRL also inhibits transcription activator3 (STAT3) which mediates gene expression in cell growth and apoptosis and remains unregulated in many tumor types (78–80). Moreover, SRL exerts potent anti-angiogenic activity in vitro and in vivo in established tumors via inhibition of vascular endothelial growth factor (VEGF) production (81). Additionally, SRL has been shown to have anti-EBV proliferation effects in vivo (60, 61) and may avert growth of EBV-transformed B lymphocytes (82).
Prevention is an important measure, because the main risk factors for PTLD are EBV and the degree of immunosuppression. Strategies, such as limiting patient exposure to aggressive immunosuppressive regimens with rapid withdrawal or tapering of agents required for maintenance of graft function may decrease the incidence of PTLD. EBV monitoring has been incorporated into the routine evaluation of SOT patients. Avoidance of seropositive donors to seronegative recipients when multiple donor options are available is a measure that can further reduce the risk of PTLD.
While the degree of immunosuppression required and the timing of immunosuppression withdrawal differs, the consensus is that more aggressive withdrawal of immunosuppression to maintenance target concentrations is associated with lower incidence of PTLD. Among pediatric renal allograft recipients, the prevalence of PTLD has decreased with time, and this finding is attributed to policies of tapering CNIs to lower maintenance target trough concentration of 5–9 ng/mL (83). SOT recipients who are EBV-seronegative before transplant are commonly monitored for EBV viremia at regular intervals after transplant. Reduction of immunosuppression in patients with EBV viremia has been shown to reduce the incidence of early PTLD in pediatric SOT recipients (84, 85). The role of antiviral prophylaxis for PTLD prevention remains controversial for SOT recipients who are seronegative for EBV but receiving organs from seropositive donors. Retrospective observational studies have shown conflicting results (85–87), and a recent meta-analysis examining prophylactic or preemptive antiviral agents reported no significant effect on the incidence of PTLD across all types of organ transplants and age groups in high-risk EBV-naïve patients following SOT (88). A previous prospective study involving pediatric liver transplant showed that ganciclovir for 2 weeks immediately after transplant followed by 50 weeks of either acyclovir or placebo resulted in similar rates of PTLD (89). In the absence of convincing evidence, the use of antiviral agents as prophylaxis for PTLD prevention in EBV mismatched patients is not recommended (87).
The approach of preemptive treatment of PTLD at the time of viral reactivation with rituximab has been applied in allogeneic hematopoietic cell transplantation recipients and prevented PTLD without excess infections or mortality (90–92). In a series of 299 cardiac transplant patients, 31 had EBV reactivation and 6 had an EBV primary infection. Thirty-one patients had decreased immunosuppression and 15 had a single dose of rituximab at 375 mg/m2. All patients had a decrease in viral load. There was one possible PTLD and one death secondary to pulmonary PTLD. Unlike in PTLD complicating HSC transplants, the role of preemptive use of rituximab to prevent PTLD in SOT recipients is less clear. In the Swiss Transplant Cohort Study, no significant differences in the incidence of PTLD were found between SOT recipients receiving induction therapy with or without rituximab, although none of the patients (0/191) who received rituximab developed PTLD, while 57 of 4,574 (1.2%) patients without rituximab induction developed PTLD during follow-up (93). Therefore, further studies are warranted to determine the role of preemptive use of rituximab in preventing PTLD among SOT recipients.
In addition to tapering CNIs, our group has studied the effects of mammalian target of rapamycin (mTOR) antagonists on the incidence of post-transplant malignancies including PTLD among HT recipients (42). Sirolimus (SRL) and its derivative, everolimus, are mTOR inhibitors that suppress tumor growth in animal models (94) and have been successfully used in treating selective types of cancers (95). In HT recipients, studies assessing the effect of mTOR inhibition on the development of PTLD are lacking due to the relatively small pool of HT recipients treated with mTOR antagonists. A single-center retrospective analysis from our group showed that early conversion to a maintenance SRL-based immunosuppression, with complete withdrawal of CNIs, was associated with attenuation of cardiac allograft vasculopathy progression and improvement not only in cardiac outcomes but also in late survival after HT compared with continued CNI use over a mean follow-up of ~9 years (96). The improvement in late survival with SRL could not be entirely attributed to attenuation of CAV progression. Therefore, a subsequent analysis of malignancies from our center suggested that conversion to SRL was significantly associated with a decreased risk of PTLD (HR: 0.13; 95% CI: 0.03–0.59; p = 0.009) (42). The effects on PTLD were independent of EBV infection and type of induction therapy. The mechanisms behind which SRL confers protection against PTLD are not entirely clear. A previous study showed that the PI3K/Akt/mTOR pathway was constitutively active in EBV-positive B lymphomas from patients with PTLD, and that SRL combined with PI3K-δ inhibitor synergistically suppressed the proliferation of EBV-positive B lymphoma cells (97).
The World Health Organization (WHO) classified PTLD in four main categories based on morphologic, immunophenotypic, genetic, and clinical features: (i) Early lesions including plasmacytic hyperplasia and infectious mononucleosis-like PTLD, (ii) Polymorphic PTLD, (iii) Monomorphic PTLD, and (iv) Classic Hodgkin lymphoma-like PTLD (87). Initial management depends on the type of PTLD and immunosuppression reduction strategies.
The main strategies of PTLD treatment include reduction of immunosuppression, immunotherapy with the CD20 monoclonal antibody rituximab, chemotherapy, radiation therapy, adoptive immunotherapy with EBV-specific cytotoxic T lymphocytes, or a combination of these. The choice of strategy depends on the PTLD subtype, aggressiveness of PTLD, associated toxicities and the type of transplant. The main goals of therapy are eradication of PTLD and preservation of graft function. Not uncommonly, these goals are conflicting. Reduction of immunosuppression, which is commonly employed for PTLD eradication, increases the risk of graft rejection and it may not be feasible in single-organ transplants of vital organ (such as HT). In these cases, alternative therapies for PTLD must be used.
In accordance with the recommendations by the National Comprehensive Cancer Network (NCCN), the British Committee for Standards in Hematology, and the European Best Practice Guidelines for renal transplantation for most patients with early lesions, reduction of immunosuppression is the first step and additional agents are reserved for those who cannot tolerate reduction in immunosuppression and patient with residual disease (98–100). Data regarding the efficacy of reduction of immunosuppression are derived from observational studies in which patients also received other treatment strategies. Most early lesions either resolve completely or improve significantly within few weeks due to reduction of immunosuppression (101).
The optimal reduction of immunosuppression regimen depends on the histology, stage of PTLD, organ involvement, the presence of dual organ transplantation and the estimated risk related to graft loss or rejection. Steroid only reduction is not effective in most patients. Reduction by at least 50% the CNIs and discontinuation of other immunosuppressive drugs is recommended but not always feasible (53).
Polymorphic PTLD are defined as polyclonal or monoclonal lymphoid infiltrates that demonstrate evidence of malignant transformation but do not meet all of the criteria for one of the B cell or T/NK cell lymphomas (53, 102). Patients with polymorphic CD20-positive PTLD, receive rituximab in addition to reduced immunosuppression as an initial management strategy. Since polymorphic PTLD, by definition, consists of a mixture of monoclonal CD20-positive B-cell and polyclonal T-cell infiltrates, it is commonly treated with rituximab. Complete remission with rituximab monotherapy is relatively low in adult patients (<50%) (103–105) and identifies a population of patients that require additional chemotherapy. Pediatric patients have generally higher response rates to rituximab monotherapy (106, 107). For patients with monomorphic PTLD (those with monoclonal lymphoid proliferations that meet the criteria for one of the B cell or T/NK cell lymphomas), an approach including reduction in immunosuppression, rituximab and combination chemotherapy either concurrently or sequentially is indicated (22, 108). Patients with CD20-positive polymorphic PTLD with poor performance status or minimal symptoms may be candidates for rituximab alone. Combination chemotherapy with cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP), although not studied in randomized clinical trials vs. rituximab single therapy, achieved complete response in over 50% of cases (22, 105). The phase II sequential treatment of CD20-positive PTLD (PTLD-1) trial (22) involving 70 patients recruited from 2003 to 2007 has established sequential treatment with four cycles of weekly rituximab followed by four cycles of CHOP every 21 days (CHOP-21) as a standard of care in CD20-positive PTLD after SOL. Overall, 53 of 59 patients had a complete or partial response (90%) to sequential treatment, of which 40 (68%) were complete responses. The median survival using this regimen has significantly improved compared to the preceding rituximab monotherapy trials (6.6 years vs. 1.2–3.5 years, respectively) (103, 109, 110). Sequential therapy was also associated with less drug toxicity, particularly treatment-related mortality, as compared to the preceding retrospective case series of first-line chemotherapy in PTLD (13% vs. up to 31%, respectively) (22, 111). Importantly, the initial response to rituximab induction was found to be a prognostic factor for overall survival. This observation has led to a subsequent study demonstrating the feasibility, safety, and efficacy of treatment stratification into rituximab or rituximab plus CHOP consolidation according to response to rituximab induction (105). Consolidation therapy with rituximab only for patients who achieved a complete response after rituximab induction [88/126 patients (70%)] was safe (8% treatment-related mortality) and resulted in comparable median overall survival (6.6 years) compared to sequential therapy (PTLD-1) (22, 105). These findings demonstrate that rituximab without the need for chemotherapy is an appropriate therapeutic strategy when complete response is achieved after four cycles of rituximab induction in patients with CD20-positive PTLD complicating SOT. For patients not expressing CD20, chemotherapy without rituximab and surgery (for those with a localized disease) are indicated. T-cell lymphomas do not respond to rituximab and should be treated according to their pathology. Patients with classic Hodgkin lymphoma-like PTLD (the least frequent type of PTLD) should be treated according to the treatment standards of Hodgkin-lymphomas (112–114).
Radiation therapy can be used for patients with localized disease (115). In primary CNS PTLD, rituximab and high-dose methotrexate favorably impact survival (116) although CNS PTLD generally has a dismal prognosis. For patients with persistent disease despite reduction of immunosuppression and combined chemotherapy, adoptive immunotherapy with EBV-specific cytotoxic T lymphocytes (EBV-CTLs) can be used as a therapeutic option for high-risk rituximab-refractory cases, including promising results obtained among patients with CNS PTLD treated with EBV-CTLs (117). In an earlier report from 1994, 5 HSC transplant recipients with monoclonal EBV-induced PTLD achieved complete remission (CR) after infusion of lymphocytes (donor lymphocyte infusion) from their EBV-seropositive transplant donors (118). Subsequent small case series have confirmed that donor-derived EBV-CTLs induce clearance of viremia and persistent CR of EBV-associated lymphomas after HSC transplantation in 50–70% of patients (119, 120). In SOT patients, autologous EBV-CTLs have been shown to induce CR or transient partial remission (PR) of EBV-induced PTLD (121–124). However, autologous EBV-CTLs rarely result in clearance of EBV viremia (122, 124, 125). Additionally, their use is time-consuming and also limited in seronegative SOT recipients and in those treated with rituximab. Therefore, partially HLA-matched EBV-CTLs derived from healthy donors other than the transplant donor (third-party donors) have been investigated for treatment of refractory EBV-PTLD cases. Haque et al. (126) first reported the use of such cells in the treatment of 8 SOT recipients with EBV-induced PTLD in 2002. Subsequently, the same group reported on 31 SOT and 2 HCT recipients with EBV-induced PTLD, of whom 14 achieved CR and 3 achieved PR (127). Additional case series including small number of patients have used third-party EBV-CTLs to treat EBV-associated PTLD showing promising results and a favorable safety profile (128–130). A recent study by Prockop et al. (117) involving 46 recipients of allogeneic HSC transplant or SOT with established EBV-induced PTLD who had failed rituximab therapy has demonstrated that third-party EBV-CTLs that are partially HLA-matched and appropriately HLA restricted can induce durable CR or PR in a high proportion of patients without significant toxicity, graft injury, or graft-vs.-host disease (GvHD). Specifically, CR or sustained PR was achieved in 68% of HCT recipients and 54% of SOT recipients, and patients who achieved CR/PR or stable disease after cycle 1 had 1-year survival of 89 and 82%, respectively. These promising findings suggest that off-the-shelf EBV-CTLs can provide multiple immediately accessible options for potentially curative treatment of high-risk rituximab-refractory EBV-associated lymphomas complicating HSC transplantation or SOT.
Old data from retrospective studies reported that mortality of monomorphic PTLD exceeds 80% and all PTLD types are associated with poor overall survival of <50% (131). However, most of these studies report outcomes before the rituximab era, which have improved survival. Predictors of worse outcomes include older age (>55 years), serum creatinine >1.5 mg/dL, elevated LDH, location of disease (central nervous system), and monomorphic or T cell (132), Eastern Cooperative Oncology Group (ECOG) performance status ≥2 and more than one site involvement (133). The introduction of rituximab has improved the outcomes of patients with CD20-positive PTLD. Moreover, response to rituximab induction remained a predictive marker for overall survival despite treatment stratification (22, 105). Patients who survive PTLD and undergo retransplantation, have excellent graft survival (134).
PTLD is a complication of chronic immunosuppression after HT, related to EBV activation resulting in proliferation of EBV-positive B cells in most cases. Prevention of PTLD is particularly important and this can be achieved with tapering immunosuppression, use of mTOR inhibitors in lieu of CNIs, routine surveillance of EBV viral loads, particularly in patients with EBV mismatch. Survival after PTLD has improved. The main therapeutic measures consist of immunosuppression reduction, treatment with rituximab in CD20-positive patients who achieve complete response to rituximab induction, and use of rituximab in combination with CHOP chemotherapy (R-CHOP) as consolidation in patients who do not achieve complete response to rituximab induction. Further studies are warranted to validate the role of mTOR antagonists, tailoring immunosuppression based on the risk of rejection, infection, and malignancies including PTLD. The use of novel types of chemotherapy and immunotherapy in PTLD is under investigation.
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
CNI, calcineurin inhibitor; CR, complete remission; EBV, Epstein-Barr virus; HSC, hematopoietic stem cell; HT, heart transplantation; mTOR, mammalian target of rapamycin; NK, natural killer; OKT3, muromonab-CD3; PR, partial remission; PTLD, post-transplant lymphoproliferative disorder; SOT, solid organ transplant; SRL, sirolimus.
1. Buell JF, Gross TG, Woodle ES. Malignancy after transplantation. Transplantation. (2005) 80:S254–64. doi: 10.1097/01.tp.0000186382.81130.ba
2. Engels EA, Pfeiffer RM, Fraumeni JF, Kasiske BL, Israni AK, Snyder JJ, et al. Spectrum of cancer risk among US solid organ transplant recipients. J Am Med Assoc. (2011) 306:1891. doi: 10.1001/jama.2011.1592
3. Penn I. Incidence and treatment of neoplasia after transplantation. J Heart Lung Transpl. (1993) 12:S328–36.
4. Hunt S. Malignancy in organ transplantation: Transplant Proc. (2002) 34:1874–6. doi: 10.1016/S0041-1345(02)03105-6
5. Fröhlich GM, Rufibach K, Enseleit F, Wolfrum M, von Babo M, Frank M, et al. Statins and the risk of cancer after heart transplantation. Circulation. (2012) 126:440–7. doi: 10.1161/CIRCULATIONAHA.111.081059
6. Kauffman HM, Cherikh WS, McBride MA, Cheng Y, Hanto DW. Post-transplant de novo malignancies in renal transplant recipients: the past and present. Transpl Int. (2006) 19:607–20. doi: 10.1111/j.1432-2277.2006.00330.x
7. Rascente M, Pisani F, Barletta A, D'Angelo M, Giammaria A, Parzanese I, et al. Malignancies after kidney transplantation. Transplant Proc. (2005) 37:2529–31. doi: 10.1016/j.transproceed.2005.06.064
8. Samhan M, Al-Mousawi M, Donia F, Fathi T, Nasim J, Nampoory MRN. Malignancy in renal recipients. Transplant Proc. (2005) 37:3068–70. doi: 10.1016/j.transproceed.2005.07.046
9. Morath C, Mueller M, Goldschmidt H, Schwenger V, Opelz G, Zeier M. Malignancy in renal transplantation. J Am Soc Nephrol. (2004) 15:1582–8. doi: 10.1097/01.ASN.0000126194.77004.9B
10. Nafar M, Einollahi B, Hemati K, Gholi FPR, Firouzan A. Development of malignancy following living donor kidney transplantation. Transplant Proc. (2005) 37:3065–7. doi: 10.1016/j.transproceed.2005.08.011
11. Opelz G, Henderson R. Incidence of non-hodgkin lymphoma in kidney and heart transplant recipients. Lancet. (1993) 342:1514–6. doi: 10.1016/S0140-6736(05)80084-4
12. Fortina AB, Caforio ALP, Piaserico S, Alaibac M, Tona F, Feltrin G, et al. Skin cancer in heart transplant recipients: frequency and risk factor analysis. J Hear Lung Transplant. (2000) 19:249–55. doi: 10.1016/S1053-2498(99)00137-0
13. Kasiske BL, Snyder JJ, Gilbertson DT, Wang C. Cancer after kidney transplantation in the United States. Am J Transplant. (2004) 4:905–13. doi: 10.1111/j.1600-6143.2004.00450.x
14. Crespo-Leiro MG, Alonso-Pulpón L, Vázquez de Prada JA, Almenar L, Arizón JM, Brossa V, et al. Malignancy after heart transplantation: incidence, prognosis and risk factors. Am J Transplant. (2008) 8:1031–9. doi: 10.1111/j.1600-6143.2008.02196.x
15. Yagdi T, Sharples L, Tsui S, Large S, Parameshwar J. Malignancy after heart transplantation: analysis of 24-year experience at a single center. J Card Surg. (2009) 24:572–9. doi: 10.1111/j.1540-8191.2009.00858.x
16. Doesch AO, Müller S, Konstandin M, Celik S, Kristen A, Frankenstein L, et al. Malignancies after heart transplantation: incidence, risk factors, and effects of calcineurin inhibitor withdrawal. Transplant Proc. (2010) 42:3694–9. doi: 10.1016/j.transproceed.2010.07.107
17. Lund LH, Edwards LB, Kucheryavaya AY, Dipchand AI, Benden C, Christie JD, et al. The registry of the international society for heart and lung transplantation: thirtieth official adult heart transplant report-−2013; focus theme: age. J Hear Lung Transplant. (2013) 32:951–64. doi: 10.1016/j.healun.2013.08.006
18. Goldfarb SB, Levvey BJ, Edwards LB, Dipchand AI, Kucheryavaya AY, Lund LH, et al. The registry of the international society for heart and lung transplantation: nineteenth pediatric lung and heart–lung transplantation report-−2016; focus theme: primary diagnostic indications for transplant. J Hear Lung Transplant. (2016) 35:1196–205. doi: 10.1016/j.healun.2016.08.019
19. Penn I, Hammond W, Brettschneider L, Starzl TE. Malignant lymphomas in transplantation patients. Transplant Proc. (1969) 1:106–12.
20. Nourse JP, Jones K, Gandhi MK. Epstein-barr virus-related post-transplant lymphoproliferative disorders: pathogenetic insights for targeted therapy. Am J Transplant. (2011) 11:888–95. doi: 10.1111/j.1600-6143.2011.03499.x
21. Dierickx D, Habermann TM. Post-transplantation lymphoproliferative disorders in adults. N Engl J Med. (2018) 378:549–62. doi: 10.1056/NEJMra1702693
22. Trappe R, Oertel S, Leblond V, Mollee P, Sender M, Reinke P, et al. Sequential treatment with rituximab followed by CHOP chemotherapy in adult B-cell post-transplant lymphoproliferative disorder (PTLD): the prospective international multicentre phase 2 PTLD-1 trial. Lancet Oncol. (2012) 13:196–206. doi: 10.1016/S1470-2045(11)70300-X
23. Sampaio MS, Cho YW, Qazi Y, Bunnapradist S, Hutchinson IV, Shah T. Posttransplant malignancies in solid organ adult recipients. Transplantation. (2012) 94:990–8. doi: 10.1097/TP.0b013e318270bc7b
24. Caillard S, Lamy FX, Quelen C, Dantal J, Lebranchu Y, Lang P, et al. Epidemiology of posttransplant lymphoproliferative disorders in adult kidney and kidney pancreas recipients: report of the French registry and analysis of subgroups of lymphomas. Am J Transplant. (2012) 12:682–93. doi: 10.1111/j.1600-6143.2011.03896.x
25. Kotton CN, Huprikar S, Kumar D. Transplant infectious diseases: a review of the scientific registry of transplant recipients published data. Am J Transplant. (2017) 17:1439–46. doi: 10.1111/ajt.14195
26. Dierickx D, Tousseyn T, Sagaert X, Fieuws S, Wlodarska I, Morscio J, et al. Single-center analysis of biopsy-confirmed posttransplant lymphoproliferative disorder: incidence, clinicopathological characteristics and prognostic factors. Leuk Lymphoma. (2013) 54:2433–40. doi: 10.3109/10428194.2013.780655
27. Taylor AL, Marcus R, Bradley JA. Post-transplant lymphoproliferative disorders (PTLD) after solid organ transplantation. Crit Rev Oncol Hematol. (2005) 56:155–67. doi: 10.1016/j.critrevonc.2005.03.015
28. Newell KA, Alonso EM, Whitington PF, Bruce DS, Millis JM, Piper JB, et al. Posttransplant lymphoproliferative disease in pediatric liver transplantation. Transplantation. (1996) 62:370–5. doi: 10.1097/00007890-199608150-00012
29. Luskin MR, Heil DS, Tan KS, Choi S, Stadtmauer EA, Schuster SJ, et al. The impact of EBV status on characteristics and outcomes of posttransplantation lymphoproliferative disorder. Am J Transplant. (2015) 15:2665–73. doi: 10.1111/ajt.13324
30. Morton M, Coupes B, Ritchie J, Roberts SA, Klapper PE, Byers RJ, et al. Post-transplant lymphoproliferative disorder in adult renal transplant recipients: survival and prognosis. Leuk Lymphoma. (2016) 57:299–305. doi: 10.3109/10428194.2015.1050391
31. Couetil JP, McGoldrick JP, Wallwork J, English TAH. Malignant tumors after heart transplantation. J Heart Transplant. (1990) 9:622–6.
32. Grattan MT, Moreno-Cabral CE, Starnes VA, Oyer PE, Stinson EB, Shumway NE. Eight-year results of cyclosporine-treated patients with cardiac transplants. J Thorac Cardiovasc Surg. (1990) 99:500–9. doi: 10.1016/S0022-5223(19)36981-8
33. Swinnen LJ, Costanzo-Nordin MR, Fisher SG, O'Sullivan EJ, Johnson MR, Heroux AL, et al. Increased incidence of lymphoproliferative disorder after immunosuppression with the monoclonal antibody OKT3 in cardiac-transplant recipients. N Engl J Med. (1990) 323:1723–8. doi: 10.1056/NEJM199012203232502
34. Armitage JM, Kormos RL, Stuart RS, Fricker FJ, Griffith BP, Nalesnik M, et al. Posttransplant lymphoproliferative disease in thoracic organ transplant patients: ten years of cyclosporine-based immunosuppression. J Hear Lung Transplant. (1991) 10:877–87.
35. Rinde-Hoffman D, Cintron GB, Ferguson JE, Toole JC, Bugni WB. Lymphoproliferative disorder early after cardiac transplantation. Am J Cardiol. (1991) 68:1724–5. doi: 10.1016/0002-9149(91)90340-Q
36. Chen JM, Barr ML, Chadburn A, Frizzera G, Schenkel FA, Sciacca RR, et al. Management of lymphoproliferative disorders after cardiac transplantation. Ann Thorac Surg. (1993) 56:527–38. doi: 10.1016/0003-4975(93)90893-M
37. Mihalov ML, Gattuso P, Abraham K, Holmes EW, Reddy V. Incidence of post-transplant malignancy among 674 solid-organ-transplant recipients at a single center. Clin Transplant. (1996) 10:248–55.
38. Hsu R-B, Chen RJ, Chou N-K, Ko W-J, Wang S-S, Chu S-H. Low incidence of malignancy after transplantation in Chinese heart allograft recipients. Transpl Int. (2005) 18:283–8. doi: 10.1111/j.1432-2277.2004.00029.x
39. Higgins RS, Brown RN, Chang PP, Starling RC, Ewald GA, Tallaj JA, et al. A multi-institutional study of malignancies after heart transplantation and a comparison with the general United States population. J Hear Lung Transplant. (2014) 33:478–85. doi: 10.1016/j.healun.2014.01.862
40. Doesch A, Rivinius R, Helmschrott M, Ruhparwar A, Schmack B, Klein B, et al. Analysis of malignancies in patients after heart transplantation with subsequent immunosuppressive therapy. Drug Des Devel Ther. (2014) 9:93. doi: 10.2147/DDDT.S75464
41. Youn J-C, Stehlik J, Wilk AR, Cherikh W, Kim I-C, Park G-H, et al. Temporal trends of de novo malignancy development after heart transplantation. J Am Coll Cardiol. (2018) 71:40–9. doi: 10.1016/j.jacc.2017.10.077
42. Asleh R, Clavell AL, Pereira NL, Smith B, Briasoulis A, Alnsasra H, et al. Incidence of malignancies in patients treated with sirolimus following heart transplantation. J Am Coll Cardiol. (2019) 73:499. doi: 10.1016/j.jacc.2019.03.499
43. Opelz G, Döhler B. Lymphomas after solid organ transplantation: a collaborative transplant study report. Am J Transplant. (2004) 4:222–30. doi: 10.1046/j.1600-6143.2003.00325.x
44. Dharnidharka VR, Lamb KE, Gregg JA, Meier-Kriesche H-U. Associations between EBV serostatus and organ transplant type in PTLD risk: an analysis of the SRTR National Registry Data in the United States. Am J Transplant. (2012) 12:976–83. doi: 10.1111/j.1600-6143.2011.03893.x
45. Dharnidharka VR, Tejani AH, Ho P-L, Harmon WE. Post-transplant lymphoproliferative disorder in the United States: young Caucasian males are at highest risk. Am J Transplant. (2002) 2:993–8. doi: 10.1034/j.1600-6143.2002.21019.x
46. Nee R, Hurst FP, Dharnidharka VR, Jindal RM, Agodoa LY, Abbott KC. Racial variation in the development of posttransplant lymphoproliferative disorders after renal transplantation. Transplantation. (2011) 92:190–5. doi: 10.1097/TP.0b013e3182200e8a
47. Hussain SK, Makgoeng SB, Everly MJ, Goodman MT, Martínez-Maza O, Morton LM, et al. HLA and risk of diffuse large B cell lymphoma after solid organ transplantation. Transplantation. (2016) 100:2453–60. doi: 10.1097/TP.0000000000001025
48. Reshef R, Luskin MR, Kamoun M, Vardhanabhuti S, Tomaszewski JE, Stadtmauer EA, et al. Association of HLA polymorphisms with post-transplant lymphoproliferative disorder in solid-organ transplant recipients. Am J Transplant. (2011) 11:817–25. doi: 10.1111/j.1600-6143.2011.03454.x
49. Green M, Michaels MG. Epstein-barr virus infection and posttransplant lymphoproliferative disorder. Am J Transplant. (2013) 13:41–54. doi: 10.1111/ajt.12004
50. Allen UD, Preiksaitis JK. Epstein-barr virus and posttransplant lymphoproliferative disorder in solid organ transplantation. Am J Transplant. (2013) 13:107–20. doi: 10.1111/ajt.12104
51. Walker RC, Paya CV, Marshall WF, Strickler JG, Wiesner RH, Velosa JA, et al. Pretransplantation seronegative Epstein-Barr virus status is the primary risk factor for posttransplantation lymphoproliferative disorder in adult heart, lung, and other solid organ transplantations. J Hear Lung Transplant. (1995) 14:214–21.
52. Walker RC, Marshall WF, Strickler JG, Wiesner RH, Velosa JA, Habermann TM, et al. Pretransplantation assessment of the risk of lymphoproliferative disorder. Clin Infect Dis. (1995) 20:1346–53. doi: 10.1093/clinids/20.5.1346
53. Paya CV, Fung JJ, Nalesnik MA, Kieff E, Green M, Gores G, et al. Epstein-Barr virus-induced posttransplant lymphoproliferative disorders. ASTS/ASTP EBV-PTLD task force and the mayo clinic organized international consensus development meeting. Transplantation. (1999) 68:1517–25. doi: 10.1097/00007890-199911270-00015
54. Kremers WK, Devarbhavi HC, Wiesner RH, Krom RAF, Macon WR, Habermann TM. Post-transplant lymphoproliferative disorders following liver transplantation: incidence, risk factors and survival. Am J Transplant. (2006) 6:1017–24. doi: 10.1111/j.1600-6143.2006.01294.x
55. Quinlan SC, Pfeiffer RM, Morton LM, Engels EA. Risk factors for early-onset and late-onset post-transplant lymphoproliferative disorder in kidney recipients in the United States. Am J Hematol. (2011) 86:206–9. doi: 10.1002/ajh.21911
56. Morton M, Coupes B, Roberts SA, Klapper PE, Byers RJ, Vallely PJ, et al. Epidemiology of posttransplantation lymphoproliferative disorder in adult renal transplant recipients. Transplantation. (2013) 95:470–8. doi: 10.1097/TP.0b013e318276a237
57. Fernberg P, Edgren G, Adami J, Ingvar Å, Bellocco R, Tufveson G, et al. Time trends in risk and risk determinants of non-hodgkin lymphoma in solid organ transplant recipients. Am J Transplant. (2011) 11:2472–82. doi: 10.1111/j.1600-6143.2011.03704.x
58. Dharnidharka VR, Stevens G. Risk for post-transplant lymphoproliferative disorder after polyclonal antibody induction in kidney transplantation. Pediatr Transplant. (2005) 9:622–6. doi: 10.1111/j.1399-3046.2005.00361.x
59. Oneill J, Edwards L, Taylor D. Mycophenolate mofetil and risk of developing malignancy after orthotopic heart transplantation: analysis of the transplant registry of the international society for heart and lung transplantation. J Hear Lung Transplant. (2006) 25:1186–91. doi: 10.1016/j.healun.2006.06.010
60. Nepomuceno RR, Balatoni CE, Natkunam Y, Snow AL, Krams SM, Martinez OM. Rapamycin inhibits the interleukin 10 signal transduction pathway and the growth of Epstein Barr virus B-cell lymphomas. Cancer Res. (2003) 63:4472–80.
61. Majewski M, Korecka M, Kossev P, Li S, Goldman J, Moore J, et al. The immunosuppressive macrolide RAD inhibits growth of human Epstein-Barr virus-transformed B lymphocytes in vitro and in vivo: a potential approach to prevention and treatment of posttransplant lymphoproliferative disorders. Proc Natl Acad Sci USA. (2000) 97:4285–90. doi: 10.1073/pnas.080068597
62. Yanik EL, Gustafson SK, Kasiske BL, Israni AK, Snyder JJ, Hess GP, et al. Sirolimus use and cancer incidence among US kidney transplant recipients. Am J Transplant. (2015) 15:129–36. doi: 10.1111/ajt.12969
63. Euvrard S, Morelon E, Rostaing L, Goffin E, Brocard A, Tromme I, et al. Sirolimus and secondary skin-cancer prevention in kidney transplantation. N Engl J Med. (2012) 367:329–39. doi: 10.1056/NEJMoa1204166
64. Dharnidharka VR, Schnitzler MA, Chen J, Brennan DC, Axelrod D, Segev DL, et al. Differential risks for adverse outcomes 3 years after kidney transplantation based on initial immunosuppression regimen: a national study. Transpl Int. (2016) 29:1226–36. doi: 10.1111/tri.12850
65. Babcock GJ, Decker LL, Volk M, Thorley-Lawson DA. EBV persistence in memory B cells in vivo. Immunity. (1998) 9:395–404. doi: 10.1016/S1074-7613(00)80622-6
66. Babcock GJ, Hochberg D, Thorley-Lawson DA. The expression pattern of epstein-barr virus latent genes in vivo is dependent upon the differentiation stage of the infected B cell. Immunity. (2000) 13:497–506. doi: 10.1016/S1074-7613(00)00049-2
67. Macedo C, Donnenberg A, Popescu I, Reyes J, Abu-Elmagd K, Shapiro R, et al. EBV-specific memory CD8+ T cell phenotype and function in stable solid organ transplant patients. Transpl Immunol. (2005) 14:109–16. doi: 10.1016/j.trim.2005.02.001
68. Cohen JI, Bollard CM, Khanna R, Pittaluga S. Current understanding of the role of Epstein–Barr virus in lymphomagenesis and therapeutic approaches to EBV-associated lymphomas. Leuk Lymphoma. (2008) 49:27–34. doi: 10.1080/10428190802311417
69. Thorley-Lawson DA, Gross A. Persistence of the Epstein–Barr virus and the origins of associated lymphomas. N Engl J Med. (2004) 350:1328–37. doi: 10.1056/NEJMra032015
70. Fink SEK, Gandhi MK, Nourse JP, Keane C, Jones K, Crooks P, et al. A comprehensive analysis of the cellular and EBV-specific MicroRNAome in primary CNS PTLD identifies different patterns among EBV-associated tumors. Am J Transplant. (2014) 14:2577–87. doi: 10.1111/ajt.12858
71. Finalet Ferreiro J, Morscio J, Dierickx D, Vandenberghe P, Gheysens O, Verhoef G, et al. EBV-positive and EBV-negative posttransplant diffuse large B cell lymphomas have distinct genomic and transcriptomic features. Am J Transplant. (2016) 16:414–25. doi: 10.1111/ajt.13558
72. Menter T, Juskevicius D, Alikian M, Steiger J, Dirnhofer S, Tzankov A, et al. Mutational landscape of B-cell post-transplant lymphoproliferative disorders. Br J Haematol. (2017) 178:48–56. doi: 10.1111/bjh.14633
73. Margolskee E, Jobanputra V, Jain P, Chen J, Ganapathi K, Nahum O, et al. Genetic landscape of T- and NK-cell post-transplant lymphoproliferative disorders. Oncotarget. (2016) 7:37636–48. doi: 10.18632/oncotarget.9400
74. Morscio J, Dierickx D, Ferreiro JF, Herreman A, Van Loo P, Bittoun E, et al. Gene expression profiling reveals clear differences between EBV-positive and EBV-negative posttransplant lymphoproliferative disorders. Am J Transplant. (2013) 13:1305–16. doi: 10.1111/ajt.12196
75. Cho PS, Mueller NJ, Cameron AM, Cina RA, Coburn RC, Hettiaratchy S, et al. Risk factors for the development of post-transplant lymphoproliferative disorder in a large animal model. Am J Transplant. (2004) 4:1274–82. doi: 10.1111/j.1600-6143.2004.00506.x
76. Hojo M, Morimoto T, Maluccio M, Asano T, Morimoto K, Lagman M, et al. Cyclosporine induces cancer progression by a cell-autonomous mechanism. Nature. (1999) 397:530–4. doi: 10.1038/17401
77. Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. (2012) 149:274–93. doi: 10.1016/j.cell.2012.03.017
78. Bjornsti M-A, Houghton PJ. The tor pathway: a target for cancer therapy. Nat Rev Cancer. (2004) 4:335–48. doi: 10.1038/nrc1362
79. Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. (2002) 2:489–501. doi: 10.1038/nrc839
80. Yokogami K, Wakisaka S, Avruch J, Reeves SA. Serine phosphorylation and maximal activation of STAT3 during CNTF signaling is mediated by the rapamycin target mTOR. Curr Biol. (2000) 10:47–50. doi: 10.1016/S0960-9822(99)00268-7
81. Sodhi A, Chaisuparat R, Hu J, Ramsdell AK, Manning BD, Sausville EA, et al. The TSC2/mTOR pathway drives endothelial cell transformation induced by the Kaposi's sarcoma-associated herpesvirus G protein-coupled receptor. Cancer Cell. (2006) 10:133–43. doi: 10.1016/j.ccr.2006.05.026
82. Khalpey Z, Miller DV, Schmitto JD, Kushwaha SS. Long-term maintenance therapy for post–cardiac transplant monoclonal lymphoproliferative disorder: caveat mammalian target of rapamycin. Transplant Proc. (2011) 43:1893–9. doi: 10.1016/j.transproceed.2011.03.033
83. Shapiro R, Scantlebury VP, Jordan ML, Vivas C, Ellis D, Lombardozzi-Lane S, et al. Pediatric renal transplantation under tacrolimus-based immunosuppression. Transplantation. (1999) 67:299–303. doi: 10.1097/00007890-199901270-00020
84. Lee TC, Savoldo B, Rooney CM, Heslop HE, Gee AP, Caldwell Y, et al. Quantitative EBV viral loads and immunosuppression alterations can decrease PTLD incidence in pediatric liver transplant recipients. Am J Transplant. (2005) 5:2222–8. doi: 10.1111/j.1600-6143.2005.01002.x
85. McDiarmid SV, Jordan S, Lee GS, Toyoda M, Goss JA, Vargas JH, et al. Prevention and preemptive therapy of posttransplant lymphoproliferative disease in pediatric liver recipients. Transplantation. (1998) 66:1604–11. doi: 10.1097/00007890-199812270-00006
86. Styczynski J, van der Velden W, Fox CP, Engelhard D., de la Camara R, Cordonnier C, Ljungman P. Management of Epstein-Barr Virus infections and post-transplant lymphoproliferative disorders in patients after allogeneic hematopoietic stem cell transplantation: Sixth European Conference on Infections in Leukemia (ECIL-6) guidelines. Haematologica. (2016) 101:803–11. doi: 10.3324/haematol.2016.144428
87. Allen UD, Preiksaitis JK. Post-transplant lymphoproliferative disorders, Epstein-Barr virus infection, and disease in solid organ transplantation: guidelines from the American Society of transplantation infectious diseases community of practice. Clin Transplant. (2019) 33:e13652. doi: 10.1111/ctr.13652
88. AlDabbagh MA, Gitman MR, Kumar D, Humar A, Rotstein C, Husain S. The role of antiviral prophylaxis for the prevention of Epstein-Barr virus-associated posttransplant lymphoproliferative disease in solid organ transplant recipients: a systematic review. Am J Transplant. (2017) 17:770–81. doi: 10.1111/ajt.14020
89. Green M, Kaufmann M, Wilson J, Reyes J. Comparison of intravenous ganciclovir followed by oral acyclovir with intravenous ganciclovir alone for prevention of cytomegalovirus and Epstein-Barr virus disease after liver transplantation in children. Clin Infect Dis. (1997) 25:1344–9. doi: 10.1086/516139
90. Worth A, Conyers R, Cohen J, Jagani M, Chiesa R, Rao K, et al. Pre-emptive rituximab based on viraemia and T cell reconstitution: a highly effective strategy for the prevention of Epstein–Barr virus-associated lymphoproliferative disease following stem cell transplantation. Br J Haematol. (2011) 155:377–85. doi: 10.1111/j.1365-2141.2011.08855.x
91. Cesaro S, Murrone A, Mengoli C, Pillon M, Biasolo MA, Calore E, et al. The real-time polymerase chain reaction-guided modulation of immunosuppression enables the pre-emptive management of Epstein-Barr virus reactivation after allogeneic haematopoietic stem cell transplantation. Br J Haematol. (2005) 128:224–33. doi: 10.1111/j.1365-2141.2004.05287.x
92. Choquet S, Varnous S, Deback C, Golmard JL, Leblond V. Adapted treatment of Epstein-Barr virus infection to prevent posttransplant lymphoproliferative disorder after heart transplantation. Am J Transplant. (2014) 14:857–66. doi: 10.1111/ajt.12640
93. Walti LN, Mugglin C, Sidler D, Mombelli M, Manuel O, Hirsch HH, et al. Association of antiviral prophylaxis and rituximab use with posttransplant lymphoproliferative disorders (PTLDs): a nationwide cohort study. Am J Transplant. (2021) 21:2532–42. doi: 10.1111/ajt.16423
94. Guba M, von Breitenbuch P, Steinbauer M, Koehl G, Flegel S, Hornung M, et al. Rapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: involvement of vascular endothelial growth factor. Nat Med. (2002) 8:128–35. doi: 10.1038/nm0202-128
95. Geissler EK, Schnitzbauer AA, Zülke C, Lamby PE, Proneth A, Duvoux C, et al. Sirolimus use in liver transplant recipients with hepatocellular carcinoma. Transplantation. (2016) 100:116–25. doi: 10.1097/TP.0000000000000965
96. Asleh R, Briasoulis A, Kremers WK, Adigun R, Boilson BA, Pereira NL, et al. Long-term sirolimus for primary immunosuppression in heart transplant recipients. J Am Coll Cardiol. (2018) 71:636–50. doi: 10.1016/j.jacc.2017.12.005
97. Furukawa S, Wei L, Krams SM, Esquivel CO, Martinez OM. PI3Kδ inhibition augments the efficacy of rapamycin in suppressing proliferation of Epstein–Barr virus (EBV)+ B cell lymphomas. Am J Transplant. (2013) 13:2035–43. doi: 10.1111/ajt.12328
98. Parker A, Bowles K, Bradley JA, Emery V, Featherstone C, Gupte G, et al. Management of post-transplant lymphoproliferative disorder in adult solid organ transplant recipients - BCSH and BTS guidelines. Br J Haematol. (2010) 149:693–705. doi: 10.1111/j.1365-2141.2010.08160.x
99. EBPG Expert Group on Renal Transplantation. European best practice guidelines for renal transplantation. Section IV: long-term management of the transplant recipient. IV.6.1. Cancer risk after renal transplantation. Post-transplant lymphoproliferative disease (PTLD): prevention and treatment. Nephrol Dial Transplant. (2002) 17(Suppl.4):31–3. doi: 10.1093/ndt/17.suppl_4.31-b
100. Horwitz SM, Zelenetz AD, Gordon LI, Wierda WG, Abramson JS, Advani RH, et al. NCCN guidelines insights: non-Hodgkin's lymphomas, version 3.2016. J Natl Compr Canc Netw. (2016) 14:1067–79. doi: 10.6004/jnccn.2016.0117
101. Tsai DE, Hardy CL, Tomaszewski JE, Kotloff RM, Oltoff KM, Somer BG, et al. Reduction in immunosuppression as initial therapy for posttransplant lymphoproliferative disorder: analysis of prognostic variables and long-term follow-up of 42 adult patients. Transplantation. (2001) 71:1076–88. doi: 10.1097/00007890-200104270-00012
102. Jaffe ES, Campo E, Harris NL, Pileri SA, Stein H, Swerdlow SH. Introduction and overview of the classification of the Lymphoid neoplasms. In: WHO 4th edn, WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Geneva: WHO Press (2008). p. 190–8. Available online at: http://apps.who.int/bookorders/anglais/detart1.jsp?codlan=1&codcol=70&codcch=4002
103. Choquet S. Efficacy and safety of rituximab in B-cell post-transplantation lymphoproliferative disorders: results of a prospective multicenter phase 2 study. Blood. (2006) 107:3053–7. doi: 10.1182/blood-2005-01-0377
104. Choquet S, Oertel S, LeBlond V, Riess H, Varoqueaux N, Dörken B, et al. Rituximab in the management of post-transplantation lymphoproliferative disorder after solid organ transplantation: proceed with caution. Ann Hematol. (2007) 86:599–607. doi: 10.1007/s00277-007-0298-2
105. Trappe RU, Dierickx D, Zimmermann H, Morschhauser F, Mollee P, Zaucha JM, et al. Response to rituximab induction is a predictive marker in B-cell post-transplant lymphoproliferative disorder and allows successful stratification into rituximab or R-CHOP consolidation in an International, Prospective, Multicenter Phase II Trial. J Clin Oncol. (2017) 35:536–43. doi: 10.1200/JCO.2016.69.3564
106. Zierhut H, Kanzelmeyer N, Buescher A, Höcker B, Mauz-Körholz C, Tönshoff B, et al. Course of renal allograft function after diagnosis and treatment of post-transplant lymphoproliferative disorders in pediatric kidney transplant recipients. Pediatr Transplant. (2021) 25:e14042. doi: 10.1111/petr.14042
107. Webber SA, Naftel DC, Fricker FJ, Olesnevich P, Blume ED, Addonizio L, et al. Lymphoproliferative disorders after paediatric heart transplantation: a multi-institutional study. Lancet. (2006) 367:233–9. doi: 10.1016/S0140-6736(06)67933-6
108. Trappe RU, Choquet S, Reinke P, Dreyling M, Mergenthaler H-G, Jäger U, et al. Salvage therapy for relapsed posttransplant lymphoproliferative disorders (PTLD) with a second progression of PTLD after upfront chemotherapy: the role of single-agent rituximab. Transplantation. (2007) 84:1708–12. doi: 10.1097/01.tp.0000295987.12996.19
109. Oertel SHK, Verschuuren E, Reinke P, Zeidler K, Papp-Váry M, Babel N, et al. Effect of anti-CD 20 antibody rituximab in patients with post-transplant lymphoproliferative disorder (PTLD). Am J Transplant. (2005) 5:2901–6. doi: 10.1111/j.1600-6143.2005.01098.x
110. González-Barca E, Domingo-Domenech E, Capote FJ, Gómez-Codina J, Salar A, Bailen A, et al. Prospective phase II trial of extended treatment with rituximab in patients with B-cell post-transplant lymphoproliferative disease. Haematologica. (2007) 92:1489–94. doi: 10.3324/haematol.11360
111. Elstrom RL, Andreadis C, Aqui NA, Ahya VN, Bloom RD, Brozena SC, et al. Treatment of PTLD with rituximab or chemotherapy. Am J Transplant. (2006) 6:569–76. doi: 10.1111/j.1600-6143.2005.01211.x
112. Rosenberg AS, Klein AK, Ruthazer R, Evens AM. Hodgkin lymphoma post-transplant lymphoproliferative disorder: a comparative analysis of clinical characteristics, prognosis, and survival. Am J Hematol. (2016) 91:560–5. doi: 10.1002/ajh.24346
113. Kampers J, Orjuela-Grimm M, Schober T, Schulz TF, Stiefel M, Klein C, et al. Classical Hodgkin lymphoma-type PTLD after solid organ transplantation in children: a report on 17 patients treated according to subsequent GPOH-HD treatment schedules. Leuk Lymphoma. (2017) 58:633–8. doi: 10.1080/10428194.2016.1205742
114. Twist CJ, Hiniker SM, Gratzinger D, Gutkin PM, Merriott DJ, Iagaru A, et al. Treatment and outcomes in classic Hodgkin lymphoma post-transplant lymphoproliferative disorder in children. Pediatr Blood Cancer. (2019) 66:pbc.27803. doi: 10.1002/pbc.27803
115. Orlandi E, Iorio GC, Bartoncini S, Gallio E, Cavallo F, Santoro F, et al. Role of radiotherapy in post-transplant lymphoproliferative disorders: three case reports and review of the literature. Clin Lymphoma Myeloma Leuk. (2021) 21:e309–16. doi: 10.1016/j.clml.2020.11.006
116. Evens AM, Choquet S, Kroll-Desrosiers AR, Jagadeesh D, Smith SM, Morschhauser F, et al. Primary CNS posttransplant lymphoproliferative disease (PTLD): an international report of 84 cases in the modern era. Am J Transplant. (2013) 13:1512–22. doi: 10.1111/ajt.12211
117. Prockop S, Doubrovina E, Suser S, Heller G, Barker J, Dahi P, et al. Off-the-shelf EBV-specific T cell immunotherapy for rituximab-refractory EBV-associated lymphoma following transplantation. J Clin Invest. (2020) 130:733–47. doi: 10.1172/JCI121127
118. Papadopoulos EB, Ladanyi M, Emanuel D, Mackinnon S, Boulad F, Carabasi MH, et al. Infusions of donor leukocytes to treat Epstein-Barr virus-associated lymphoproliferative disorders after allogeneic bone marrow transplantation. N Engl J Med. (1994) 330:1185–91. doi: 10.1056/NEJM199404283301703
119. Doubrovina E, Oflaz-Sozmen B, Prockop SE, Kernan NA, Abramson S, Teruya-Feldstein J, et al. Adoptive immunotherapy with unselected or EBV-specific T cells for biopsy-proven EBV+ lymphomas after allogeneic hematopoietic cell transplantation. Blood. (2012) 119:2644–56. doi: 10.1182/blood-2011-08-371971
120. Icheva V, Kayser S, Wolff D, Tuve S, Kyzirakos C, Bethge W, et al. Adoptive transfer of epstein-barr virus (EBV) nuclear antigen 1-specific t cells as treatment for EBV reactivation and lymphoproliferative disorders after allogeneic stem-cell transplantation. J Clin Oncol. (2013) 31:39–48. doi: 10.1200/JCO.2011.39.8495
121. Savoldo B, Goss JA, Hammer MM, Zhang L, Lopez T, Gee AP, et al. Treatment of solid organ transplant recipients with autologous Epstein Barr virus–specific cytotoxic T lymphocytes (CTLs). Blood. (2006) 108:2942–9. doi: 10.1182/blood-2006-05-021782
122. Khanna R, Bell S, Sherritt M, Galbraith A, Burrows SR, Rafter L, et al. Activation and adoptive transfer of Epstein-Barr virus-specific cytotoxic T cells in solid organ transplant patients with posttransplant lymphoproliferative disease. Proc Natl Acad Sci USA. (1999) 96:10391–6. doi: 10.1073/pnas.96.18.10391
123. Comoli P, Maccario R, Locatelli F, Valente U, Basso S, Garaventa A, et al. Treatment of EBV-related post-renal transplant lymphoproliferative disease with a tailored regimen including EBV-specific T cells. Am J Transplant. (2005) 5:1415–22. doi: 10.1111/j.1600-6143.2005.00854.x
124. Sherritt MA, Bharadwaj M, Burrows JM, Morrison LE, Elliott SL, Davis JE, et al. Reconstitution of the latent T-lymphocyte response to Epstein-Barr virus is coincident with long-term recovery from posttransplant lymphoma after adoptive immunotherapy. Transplantation. (2003) 75:1556–60. doi: 10.1097/01.TP.0000058745.02123.6F
125. Comoli P, Labirio M, Basso S, Baldanti F, Grossi P, Furione M, et al. Infusion of autologous Epstein-Barr virus (EBV)-specific cytotoxic T cells for prevention of EBV-related lymphoproliferative disorder in solid organ transplant recipients with evidence of active virus replication. Blood. (2002) 99:2592–8. doi: 10.1182/blood.V99.7.2592
126. Haque T, Wilkie GM, Taylor C, Amlot PL, Murad P, Iley A, et al. Treatment of Epstein-Barr-virus-positive post-transplantation lymphoproliferative disease with partly HLA-matched allogeneic cytotoxic T cells. Lancet. (2002) 360:436–42. doi: 10.1016/S0140-6736(02)09672-1
127. Haque T, Wilkie GM, Jones MM, Higgins CD, Urquhart G, Wingate P, et al. Allogeneic cytotoxic T-cell therapy for EBV-positive posttransplantation lymphoproliferative disease: results of a phase 2 multicenter clinical trial. Blood. (2007) 110:1123–31. doi: 10.1182/blood-2006-12-063008
128. Barker JN, Doubrovina E, Sauter C, Jaroscak JJ, Perales MA, Doubrovin M, et al. Successful treatment of EBV-associated posttransplantation lymphoma after cord blood transplantation using third-party EBV-specific cytotoxic T lymphocytes. Blood. (2010) 116:5045–9. doi: 10.1182/blood-2010-04-281873
129. Sun Q, Burton R, Reddy V, Lucas KG. Safety of allogeneic Epstein-Barr virus (EBV)-specific cytotoxic T lymphocytes for patients with refractory EBV-related lymphoma. Br J Haematol. (2002) 118:799–808. doi: 10.1046/j.1365-2141.2002.03683.x
130. Chiou FK, Beath S V, Wilkie GM, Vickers MA, Morland B, Gupte GL. Cytotoxic T-lymphocyte therapy for post-transplant lymphoproliferative disorder after solid organ transplantation in children. Pediatr Transplant. (2018) 22:e13133. doi: 10.1111/petr.13133
131. Humar A, Hébert D, Davies HD, Humar A, Stephens D, O'Doherty B, et al. Randomized trial of ganciclovir vs. ganciclovir plus immune globulin for prophylaxis against epstein-barr virus related posttransplant lymphoproliferative disorder. Transplantation. (2006) 81:856–61. doi: 10.1097/01.tp.0000202724.07714.a2
132. Caillard S, Porcher R, Provot F, Dantal J, Choquet S, Durrbach A, et al. Post-transplantation lymphoproliferative disorder after kidney transplantation: report of a nationwide french registry and the development of a new prognostic score. J Clin Oncol. (2013) 31:1302–9. doi: 10.1200/JCO.2012.43.2344
133. Leblond V, Dhedin N, Bruneel M-FM, Choquet S, Hermine O, Porcher R, et al. Identification of prognostic factors in 61 patients with posttransplantation lymphoproliferative disorders. J Clin Oncol. (2001) 19:772–8. doi: 10.1200/JCO.2001.19.3.772
Keywords: PTLD, heart transplantation, Epstein-Barr virus, immunosuppression, mTOR inhibitors, rituximab
Citation: Asleh R, Alnsasra H, Habermann TM, Briasoulis A and Kushwaha SS (2022) Post-transplant Lymphoproliferative Disorder Following Cardiac Transplantation. Front. Cardiovasc. Med. 9:787975. doi: 10.3389/fcvm.2022.787975
Received: 01 October 2021; Accepted: 01 February 2022;
Published: 23 February 2022.
Edited by:
Gaurang Vaidya, University of Kentucky, United StatesReviewed by:
Zeinab Afify, The University of Utah, United StatesCopyright © 2022 Asleh, Alnsasra, Habermann, Briasoulis and Kushwaha. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sudhir S. Kushwaha, a3VzaHdhaGEuc3VkaGlyQG1heW8uZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.