- 1Guang’anmen Hospital, China Academy of Chinese Medical Sciences, Beijing, China
- 2School of Chinese Medicine, LKS Faculty of Medicine, The University of Hong Kong, Hong Kong, Hong Kong SAR, China
- 3Inflammation and Immune Mediated Diseases Laboratory of Anhui, School of Pharmacy, Anhui Institute of Innovative Drugs, Anhui Medical University, Hefei, China
- 4Department of Chinese Medicine, The University of Hong Kong-Shenzhen Hospital, Shenzhen, China
Introduction: Chronic heart failure (CHF) has become an increasing concern with the aging of the population. This study aims to evaluate the effectiveness and safety of Qili Qiangxin capsules (QLQX) for CHF.
Methods: A systematic review and meta-analysis on clinical studies was conducted. The mechanisms of preclinical studies were summarized.
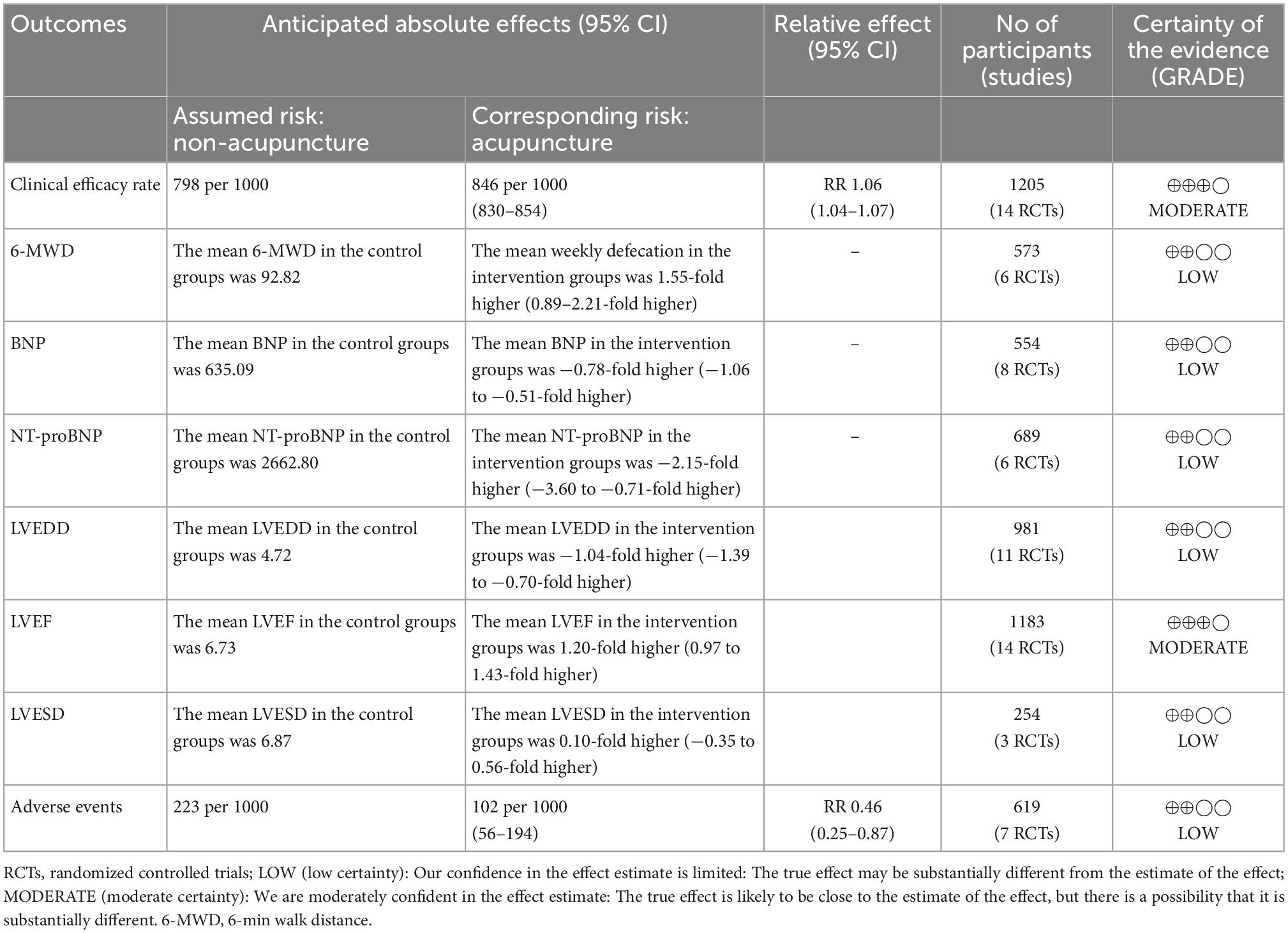
Results: We searched six electronic databases by 20 July 2022, and finally, 7 preclinical experiments (PEs) and 24 randomized controlled trials were included. The risk of bias was accessed by the SYRCLE and RoB 2.0 tool, respectively. PEs indicated that QLQX suppresses myocardial apoptosis, inhibits renin-angiotensin-aldosterone system activation, improves water retention, and enhances cardiocyte remodeling. In clinical studies, compared with routine treatment, QLQX could improve the indicators: clinical efficacy rate (RR = 1.16, 95% CI [1.12, 1.22], GRADE: moderate), left ventricular end-diastolic dimension (SMD = −1.04, 95% CI [−1.39, −0.70], GRADE: low), left ventricular ejection fraction (SMD = 1.20, 95% CI [0.97, 1.43], GRADE: moderate), 6-minute walk distance (SMD = 1.55, 95% CI [0.89, 2.21], GRADE: low), brain natriuretic peptide (SMD = −0.78, 95% CI [−1.06, −0.51], GRADE: low), N-terminal pro-brain natriuretic peptide (SMD = −2.15, 95% CI [−3.60, −0.71], GRADE: low), and adverse events (RR = 0.46, 95% CI [0.25, 0.87], GRADE: low).
Discussion: In summary, QLQX exerts a potential mechanism of utility on myocardial apoptosis and cardiac function and has noteworthy clinical adjuvant efficacy and safety in patients with CHF.
Systematic review registration: https://www.crd.york.ac.uk/prospero/.
1. Introduction
Chronic heart failure (CHF) is a syndrome of venous blood stasis and arterial blood supply insufficiency due to impaired cardiac function and failure of pumping ventricular blood completely (1). The incidence of CHF has been increasing yearly with the aging of the population, and CHF is the end stage of various cardiac diseases. Routine treatment (RT) of CHF includes the use of diuretics to reduce cardiac load, angiotensin-converting enzyme inhibitors/angiotensin II receptor antagonists to reverse ventricular remodeling, β-blockers to inhibit sympathetic excitation, and digitalis drugs to inhibit Na+/K+-ATPase (1, 2). However, the contraindications of RT limit its use in CHF patients and result in a high risk of death or recurrence (3).
Oxidative stress and myocardial apoptosis are associated with the development and progression of CHF, particularly, the induction of HO-1 in CHF has been validated as an important cardioprotective adaptation against pathological left ventricular remodeling (4–6). QLQX is a Chinese patent medicine and has been commonly used for the treatment of CHF with wide acceptance in Chinese patients (7–9). It consists of Astragali Radix, Ginseng Radix et Rhizoma, Aconiti Lateralis Radix Preparata, Salvia Miltiorrhiza Radix et Rhizoma, Descurainiae Semen Lepidii Semen, Alismatis Rhizoma, Polygonati Odorati Rhizoma, Cinnamomi Ramulus, Carthami Flos, Periplocae Cortex, and Citri Reticulatae Pericarpium in the Chinese pharmacopeia. Notable that QLQX could exert cardioprotective effects by inhibiting the ROS/AMPK/mTOR signaling pathway to attenuate apoptosis and autophagic cell death (10). It has also been included in the Guidelines for Diagnosis and Treatment of Heart Failure in China, as a clinical recommendation for the treatment of CHF. Previous studies (11–13) have shown the promising effect of QLQX on CHF. This study aimed to explore the efficacy of QLQX for CHF and its molecular mechanisms through both preclinical and clinical aspects.
2. Materials and methods
2.1. Formulations and chemical
The inclusion of intervention in the study, QLQX, is based on the Chinese New Drug Certificate (National medicine permission number: Z20040141), and the patent “A pharmaceutical composition and preparation method for the treatment of chronic heart failure” (patent number: ZL02146573.8). The quality control, prescription composition and chemical composition involved in the included studies are shown in Supplementary Table 1.
2.2. Search strategy
This study was pre-registered (PROSPERO, No. CRD42021248464) and conducted following the PRISMA statement (14). The checklist was shown in Supplementary Table 2. The literature search was conducted in the databases of PubMed, Cochrane, Embase, Chinese National Knowledge Infrastructure (CNKI), China Science and Technology Journal (VIP), and Wanfang. The retrieval time was from the database establishment to 20 July 2021. MeSH terms combined with free search words were used for the literature search. The search strategy of the PubMed database was shown in Supplementary Table 3.
2.3. Eligible criteria
Inclusion criteria met the following requirements: (1) study type: preclinical experiments (PEs) or randomized controlled trials (RCTs); (2) cellular or animal models preclinically compatible with CHF, and patients clinically diagnosed with CHF; (3) QLQX alone or combined with RT as the intervention, and in preclinical experiments, the mechanism of QLQX was explored; (4) control group received RT or placebo; (5) there was no restriction on the outcome indicators of PEs, and the outcome indicators of RCTs were included as follows: clinical efficacy rate, cardiac function, 6-min walk distance (6-MWD), brain natriuretic peptide (BNP), N-terminal pro-brain natriuretic peptide (NT-proBNP), and adverse events. The exclusion criteria were as follows: (1) studies that included non-CHF patients; (2) repetitive studies, review, protocol, comment, case report, etc.; (3) PE or RCT included other Chinese medicine or related interventions in addition to QLQX; (4) data of studies was not available even though contacted with original authors.
2.4. Literature quality assessment
According to the Systematic Review Centre for Laboratory animal Experimentation (SYRCLE) (15), a risk of bias tool, PEs were assessed according to the following items: (1) sequence generation; (2) baseline characteristics; (3) allocation concealment; (4) random housing; (5) blinding of performance; (6) random outcome assessment; (7) blinding of detection; (8) incomplete outcome data; (9) selective outcome reporting; (10) other sources of bias. According to the Cochrane criteria, we assessed the quality of the included RCTs using the Risk of Bias 2.0 (RoB 2.0) (16) in six aspects: (1) bias arising from the randomization process; (2) bias due to deviations from intended intervention; (3) bias due to missing outcome data; (4) bias in measurement of the outcome; (5) bias in selection of the reported result; (6) overall. Under the supervision and coordination of a researcher (QH), two researchers (BS and YW) assessed the risk of the included PEs, and another two researchers (WY and YJ) assessed the risk of the included RCTs according to “low risk,” “high risk,” or “some concerns,” respectively.
2.5. Data extraction and analyses
The following data was extracted: (1) basic information of the included PEs (the first author, publication year, animal species, sex, number of animals, weight, intervention, experiment duration) and RCTs (the first author, publication year, sample size, age information of the patients, intervention, trial duration); (2) all outcome measures of experiments; (3) outcome measures including clinical efficacy rate, cardiac function, 6-MWD, BNP, NT-proBNP, and adverse events. Under the supervision of a researcher (HC), two researchers (HSL and JW) extracted the data of the PEs, and the other two researchers (CL and HZ) extracted the data of the RCTs.
The Stata 17.0 software (Stata Corp., College Station, TX, USA) was applied to statistical analysis: (1) random-effect model was employed, as different RT medicine or dosage may affect the therapeutic effect; (2) Cohen’s d and 95% CI were used for continuous variables; (3) relative risk (Relative Risk) and 95% CI were used for categorical variables; (4) A p-value < 0.05 indicated statistically significant; (5) Q statistics and I2 were used to evaluate the heterogeneity of each pooled analysis, and the p-value in Q statistics < 0.05 or I2 > 50% meant high heterogeneity; (6) the sensitivity analysis was carried out when any meta-analysis presented high heterogeneity; (7) as for the difference of interventions or classification of outcome measures, subgroup analyses were inclined to carry on.
2.6. GRADE assessment
This study used GRADE (Grades of Recommendation, Assessment, Development and Evaluation) (17), a transparent and structured quality rating system, to grade each of the outcome indicators in meta-analysis with four levels of evidence: high, moderate, low, and very low. The accessment followed the GRADE handbook. The reasons for downgrading levels of evidence certainty in the included studies were shown below: high risk of bias in results (18), high level of heterogeneity (19), low generalizability of the study (20), no statistical significance of effect size (21), and publication bias in the study (22).
3. Results
3.1. Eligible studies
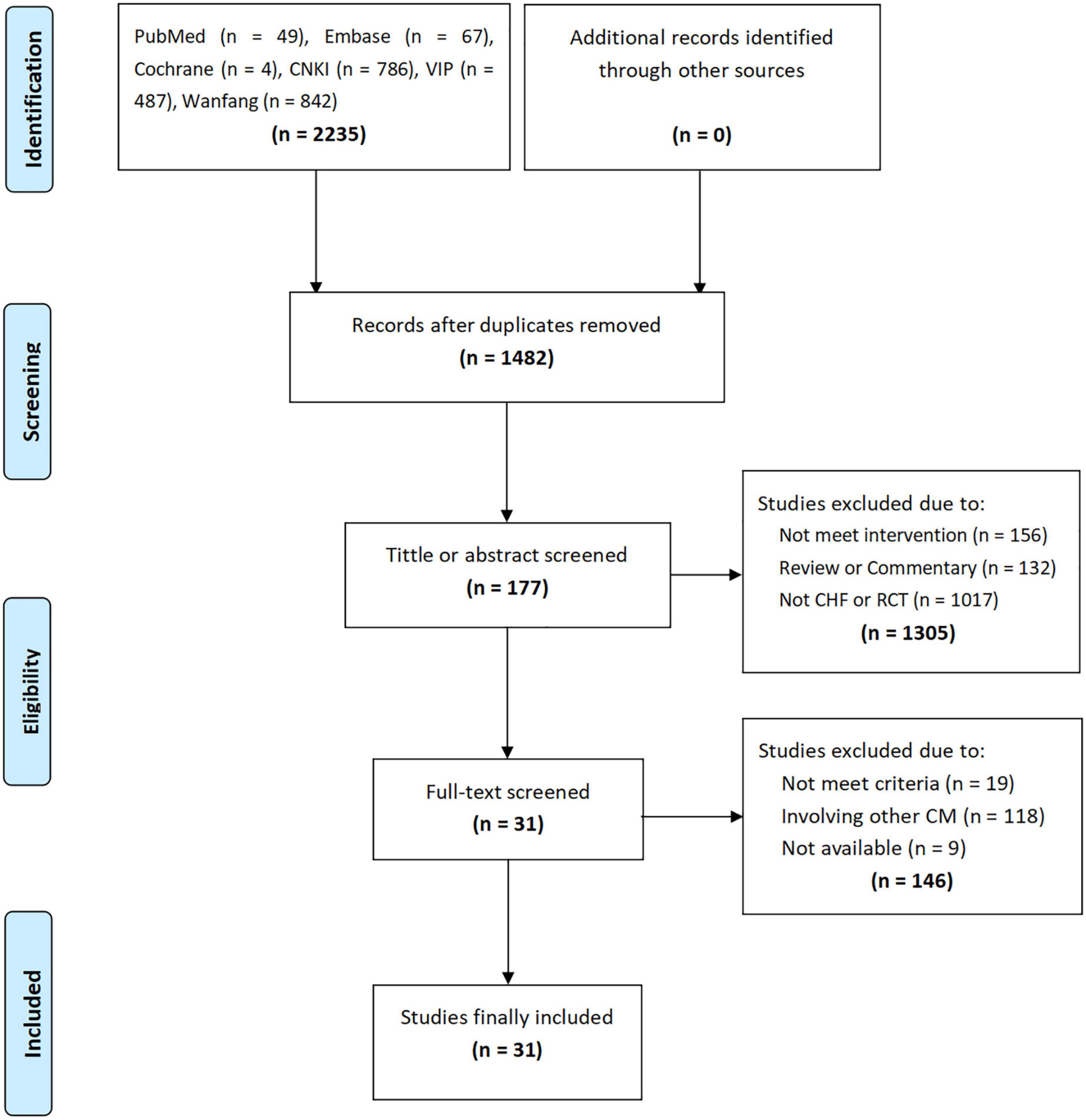
A total of 2,235 studies were retrieved through the initial search, and 1,482 studies were obtained after removing duplicate studies. By screening the titles or abstracts, 177 studies were obtained, followed by the full-text accessment, and 7 PEs and 24 RCTs were included. The flow chart of the study screening is shown in Figure 1.
3.2. Literature characteristics
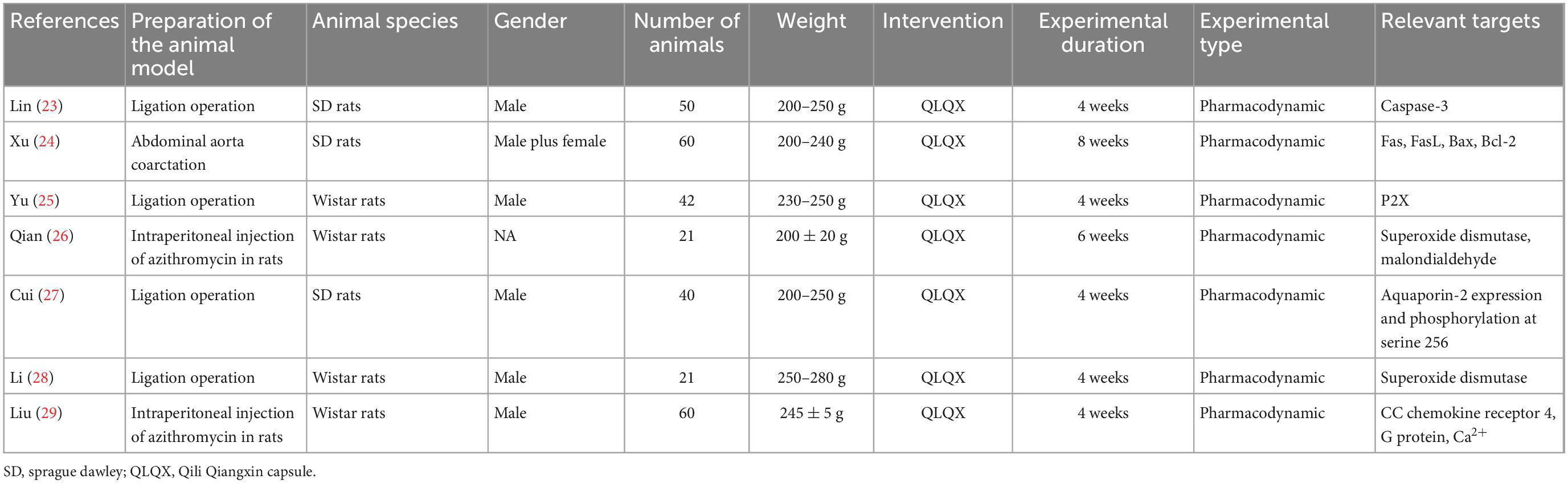
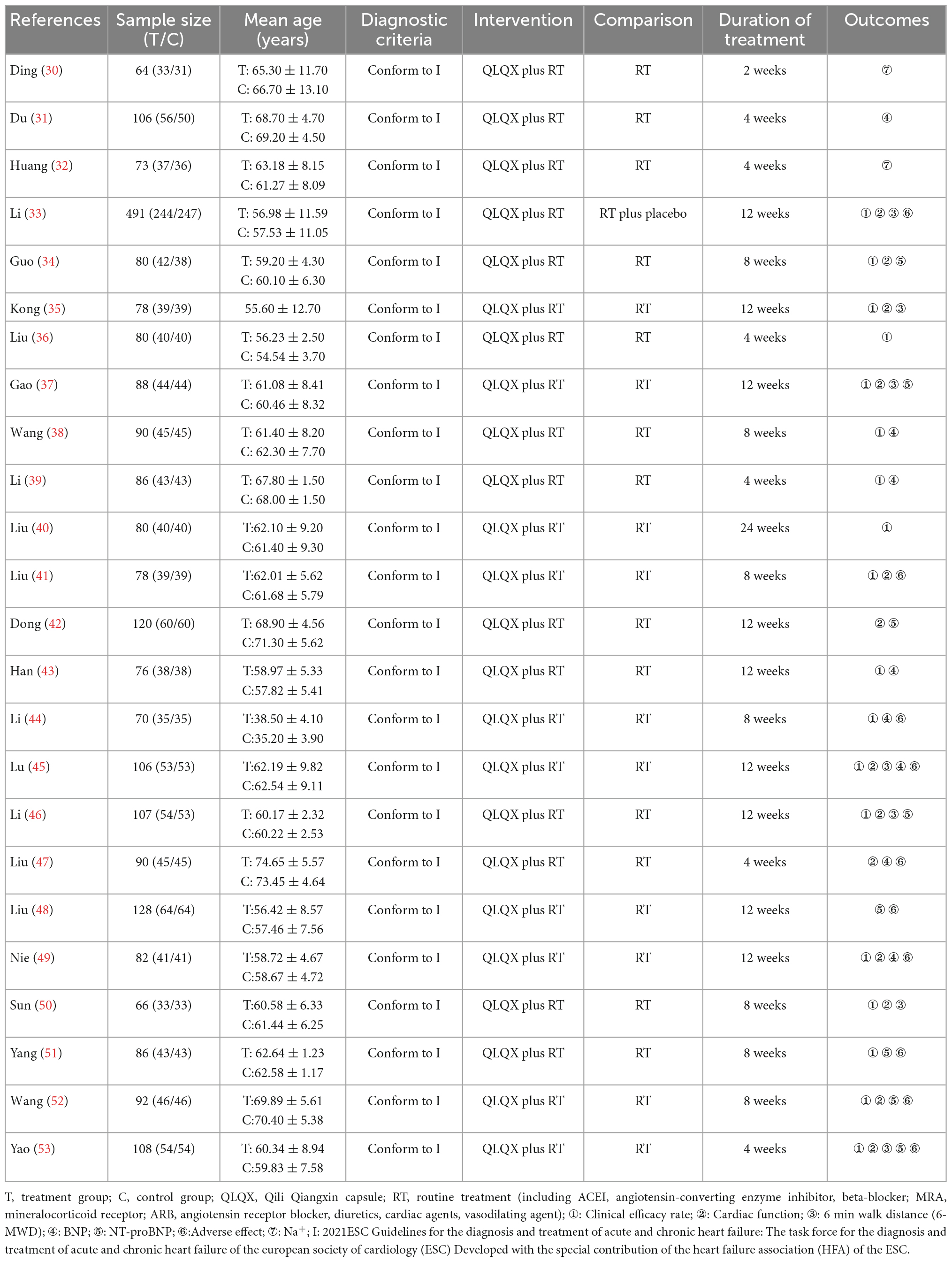
Seven PEs (23–29) and 24 RCTs (30–53) included 294 rats and 2,731 patients respectively. PE studies range from 4 to 8 weeks including 21–60 animals in each study. The clinical trial duration ranges from 2 to 24 weeks with patients from 64 to 491 each. In the PEs, all experiments were pharmacodynamic studies investing the biochemical effects of QLQX in animal CHF models. In the included RCTs, 1 trial (33) used the placebo in the control group. The characteristics of included studies are shown in Tables 1, 2.
3.3. Risk of bias
Seven PEs (23–29) were assessed by the SYRCLE risk of bias tool (Supplementary Table 4). All PEs were assessed as the “some concerns” in the item of “random housing” in all PEs due to lack of reporting. Three PEs (27–29) existed a “some concerns” risk in the “sequence generation” section due to lack of group method reporting, and other two PEs (25, 26) without baseline information were assessed as the “some concerns.” A total of 24 RCTs (30–53) were assessed by RoB 2.0 risk of bias tool (Supplementary Table 5). All RCTs were assessed as the “low” risks in the item of “bias due to deviations from intended intervention” and “bias arising from the randomization process.” From the item of “bias in measurement of the outcome” or “bias in selection of the reported result,” three RCTs (38, 42, 49) were assessed as “high” risks because of incomplete data and results.
4. Preclinical experiments
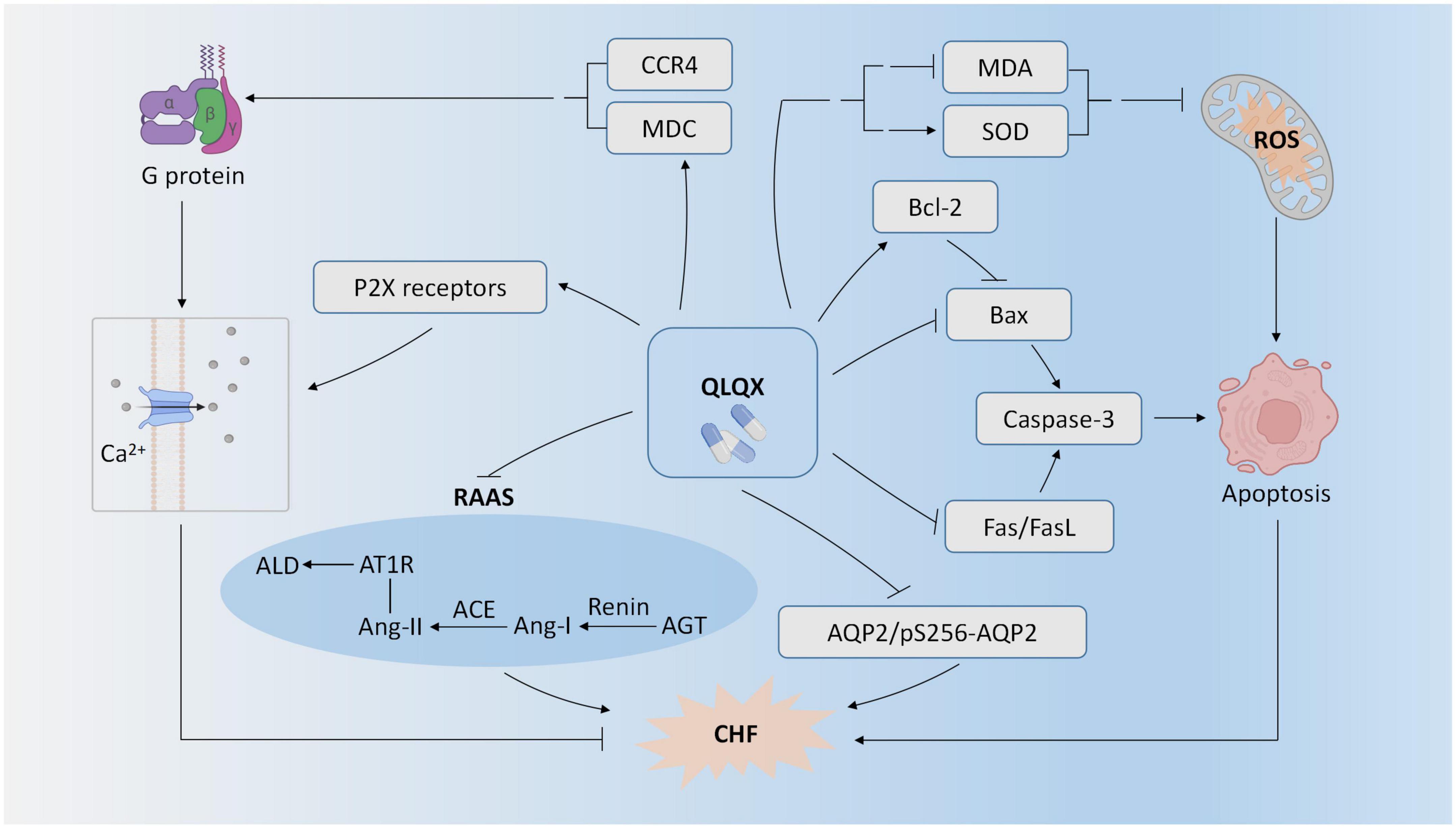
Seven PEs (23–29) studied the effect of QLQX for CHF in the animal models. As shown in Figure 2, one experimental study showed that QLQX improved cardiac function in the CHF rat model of the left anterior descending coronary artery ligation by suppressing caspase-3 mediated myocardiocyte apoptosis (23). Consistently, QLQX attenuated apoptosis in myocardiocytes of CHF rats by inhibiting Fas, FasL, and Bax and upregulating Bcl-2 (24). Two experimental studies indicated that QLQX reduced levels of oxidative stress in CHF rats as demonstrated by the downregulation of malondialdehyde (MDA) and upregulation of superoxide dismutase (SOD) in serum (26, 28). QLQX also improved cardiac function by inhibiting activation of cardiac RAAS, e.g., upregulation of renin, angiotensin II, and aldosterone, angiotensin converting enzyme (ACE), and angiotensin type 1 receptor (AT1R) (28). QLQX elevated MDC protein content and expression, and the binding of MDC to CC chemokine receptor 4 (CCR4) could activate G protein and promote Ca2+ influx, finally enhancing myocardial contractile function (29). QLQX improved cardiac output by increasing P2X-mediated ventricular contractility in CHF rats (25). QLQX also reduced water reabsorption by suppressing aquaporin-2 expression and phosphorylation at serine 256 (pS256-AQP2) (27).
5. Clinical trials
5.1. Clinical efficacy rate
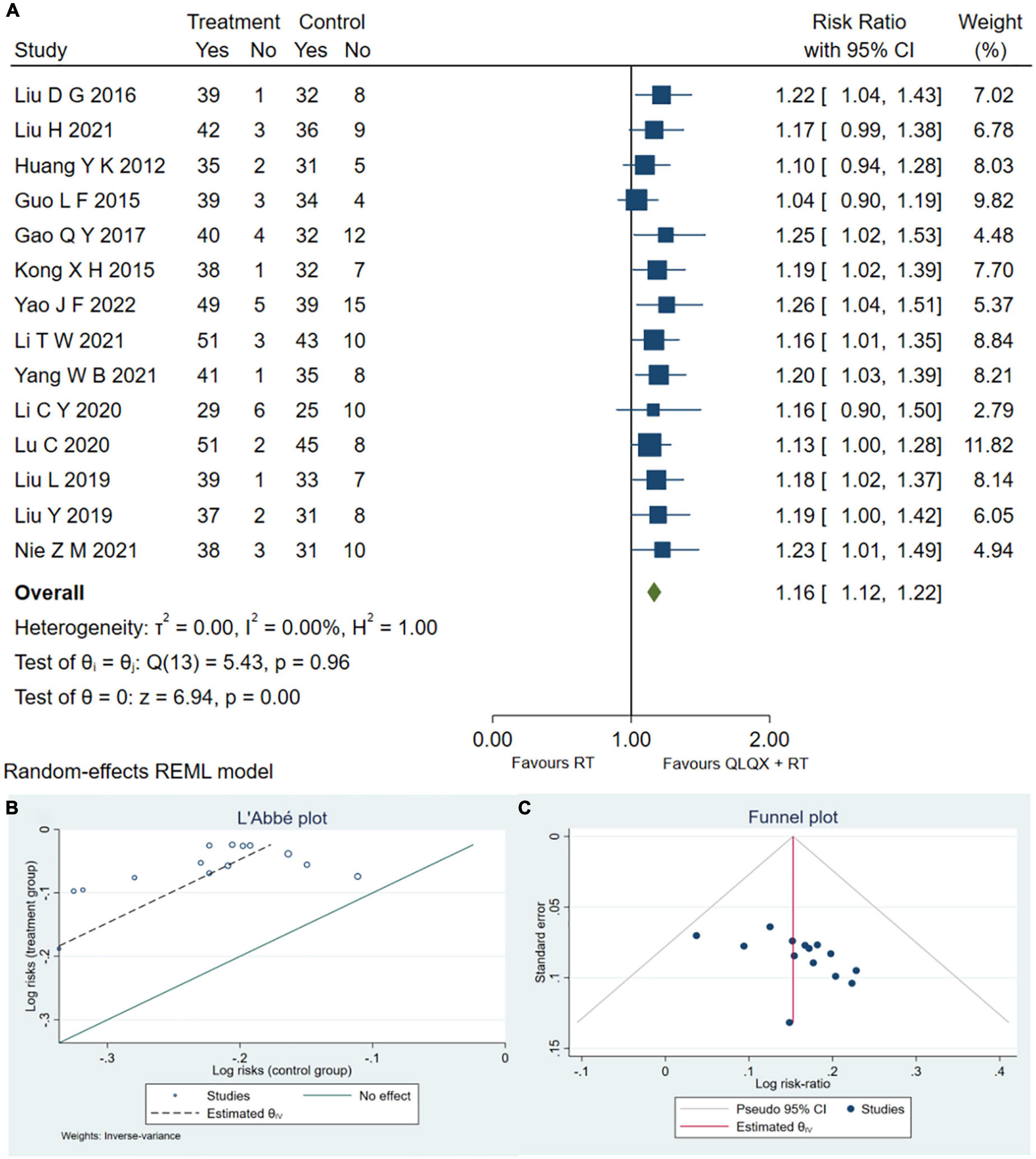
Fourteen RCTs (32, 34–37, 40, 41, 44–47, 49, 51, 53) reported the clinical efficacy rate. As shown in Figure 3A, QLQX plus RT significantly increased clinical efficacy rate [RR = 1.16, 95% CI (1.12, 1.22), p < 0.05, GRADE: moderate, as shown in Table 3], with no heterogeneity [Q (13) = 5.43, P = 0.00, I2 = 0]. Egger test results showed that there was no published bias (β1 = 2.02, SE of β1 = 1.636, z = 1.23, P = 0.2180 > 0.05). The L’Abbe and funnel plots are respectively presented in Figures 3B, C.
5.2. Cardiac function
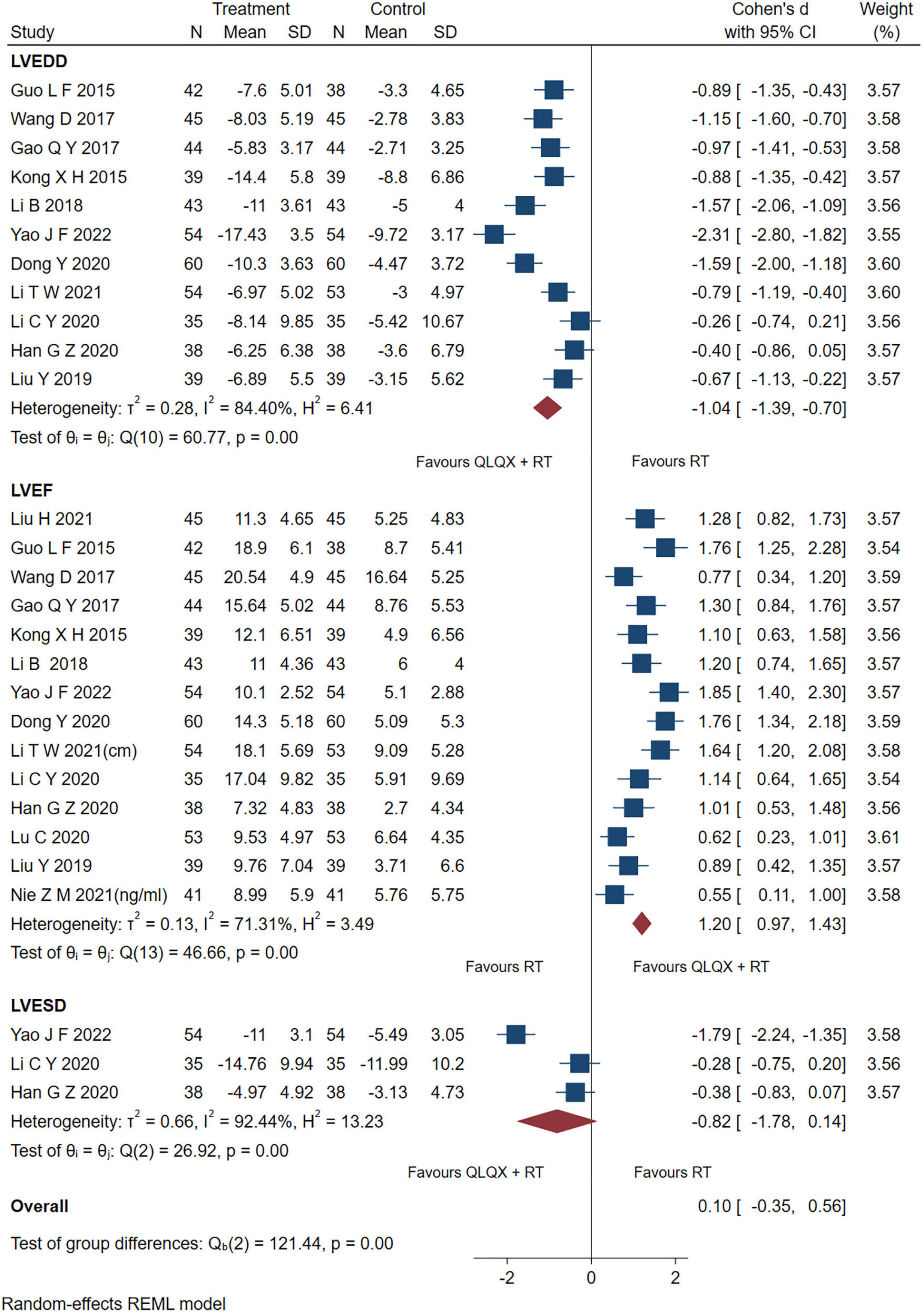
Fourteen studies reporting the results of LVEF, 7 studies reporting LVEDD and three studies (43, 44, 53) reporting LVESD were pooled and analyzed. As shown in Figure 4, the intervention group significantly improved the indicators of LVEF [SMD = 1.20, 95% CI (0.97, 1.43), p < 0.05; Q (13) = 46.66, p = 0.00, I2 = 71.31%, GRADE: moderate], and LVEDD [SMD = −1.04, 95% CI (−1.39, −0.70), p < 0.05; Q (10) = 60.77, p = 0.00, I2 = 84.40%, GRADE: low] compared to the control group. However, there was no significant difference between QLQX plus RT and RT on decreasing LVESD in patients with CHF [SMD = −0.82, 95% CI (−1.78, 0.14), p > 0.05; Q (2) = 26.92, p = 0.00, I2 = 92.44%, GRADE: low]. Heterogeneity analysis indicated that differences in drug dosage and sample size might cause high heterogeneity.
5.3. 6-min walk distance
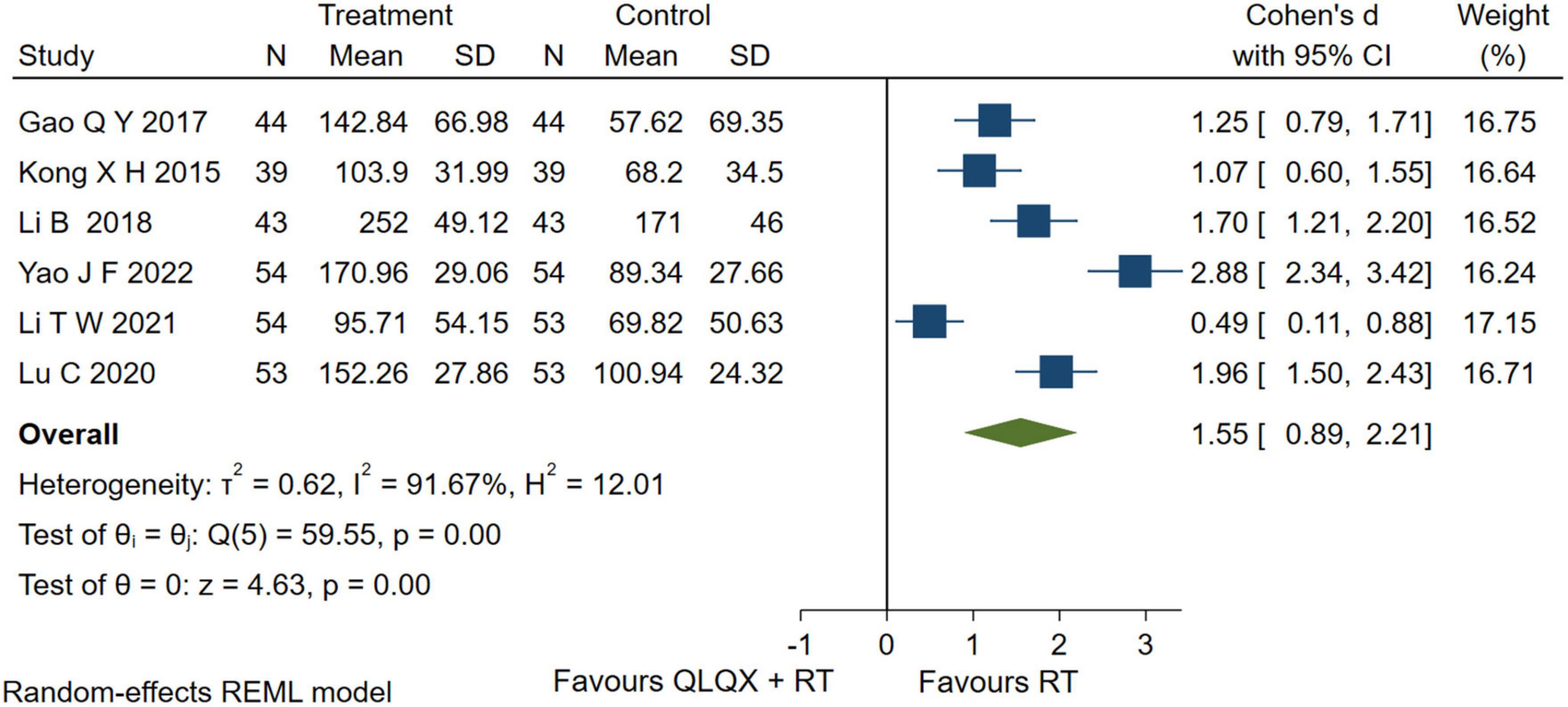
Six studies (35, 37, 39, 45, 46, 53) reported the 6-MWD. The forest plot in Figure 5 showed that intervention group improved the 6-MWD significantly [SMD = 1.55, 95% CI (0.89, 2.21), p < 0.05, GRADE: low]; but there was a higher heterogeneity [Q (5) = 59.55, p = 0.00, I2 = 91.67%]. The heterogeneity analysis indicated that different durations led to high heterogeneity.
5.4. BNP and NT-proBNP
Eight studies (31, 38, 39, 43–45, 47, 49) reported serum BNP levels. However, sensitivity analysis found that two studies (44, 49) caused a higher heterogeneity [Q (7) = 152.55, p = 0.01, I2 = 98.16%] and were excluded from the pooled analysis. The forest plot in Figure 6A reported that QLQX plus RT significantly reduced the level of serum BNP in patients with CHF [SMD = −0.78, 95% CI (−1.06, −0.51), p < 0.05, Q (5) = 12.29, p = 0.00, I2 = 59.27%, GRADE: low]. Six studies (34, 37, 42, 46, 51, 53) QLQX significantly decreased serum NT- proBNP levels [SMD = −2.15, 95% CI (−3.60, −0.71), p < 0.05, Q (5) = 168.27, p = 0.00, I2 = 98.06%, GRADE: low] as shown in Figure 6B.
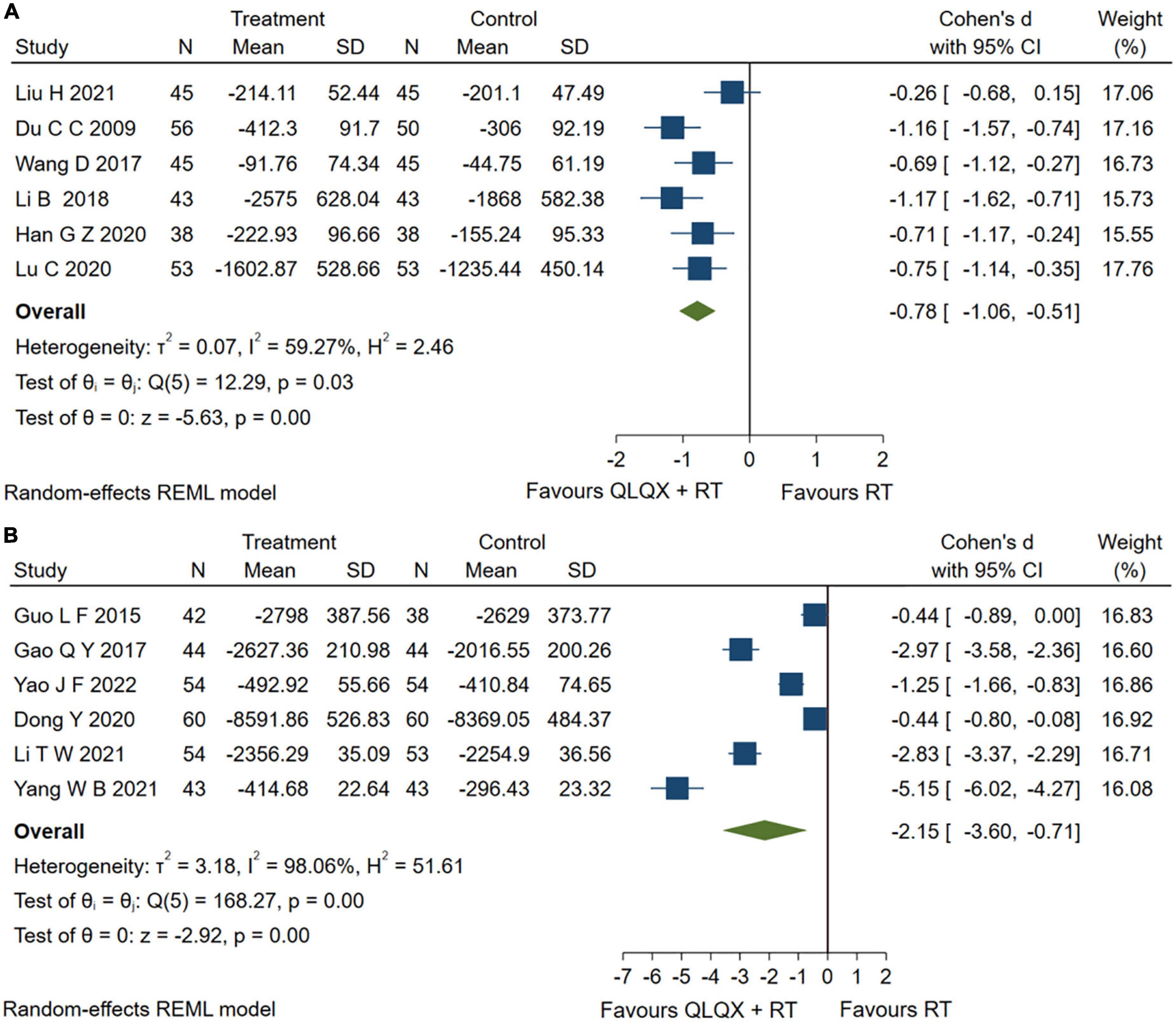
Figure 6. (A) Forest plots of brain natriuretic peptide; (B) N-terminal pro-brain natriuretic peptide.
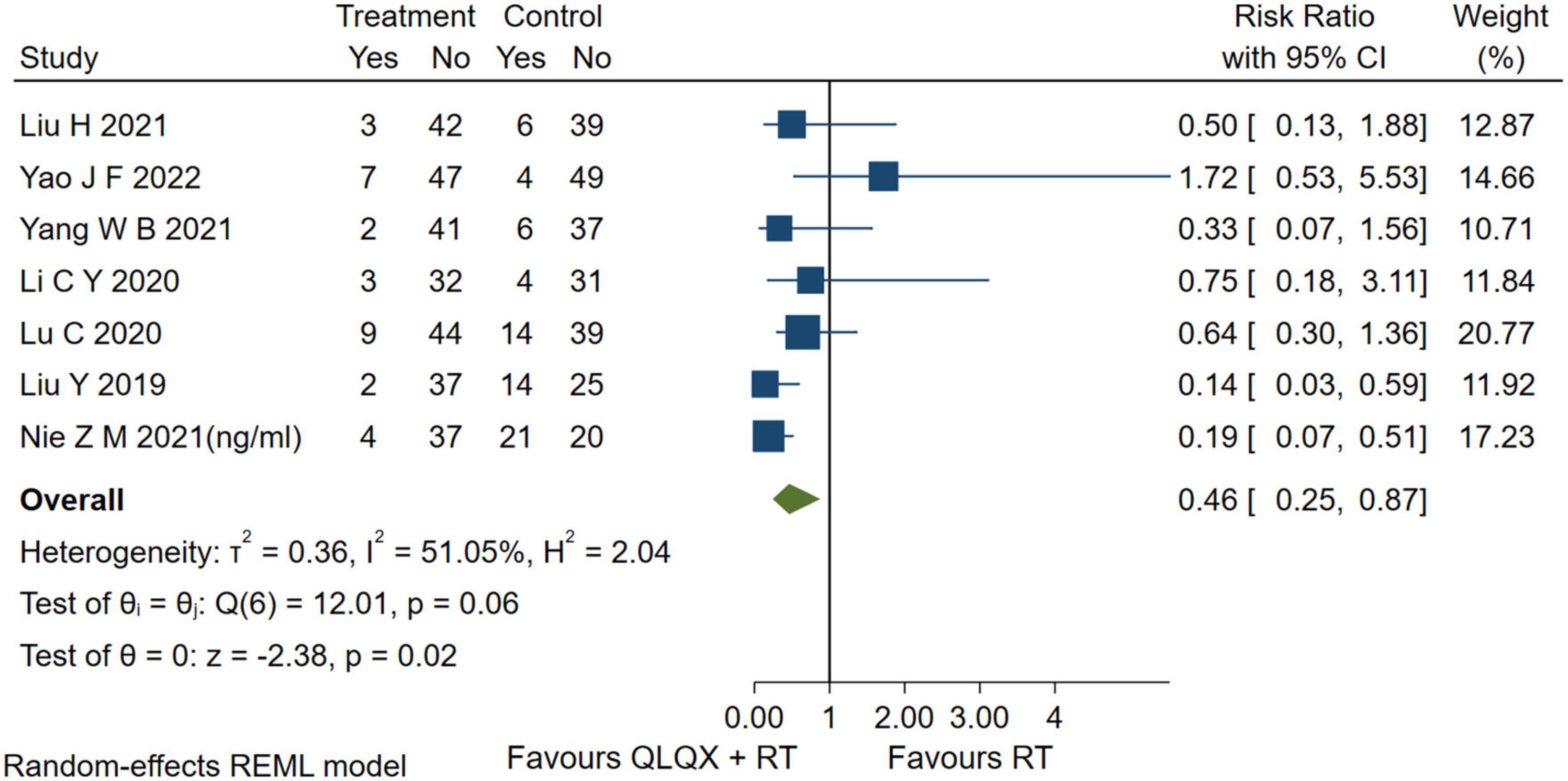
5.5. Adverse events
Seven studies (41, 44, 45, 47, 49, 51, 53) reported adverse events. The forest plot Figure 7 indicated that compared with the RT, QLQX treatment showed a decrease in adverse events [RR = 0.46, 95% CI (0.25, 0.87), p < 0.05, Q (6) = 12.01, p = 0.02, I2 = 51.05%, GRADE: low].
6. Discussion
From the clinical aspect of traditional Chinese medicine, QLQX could be used for the type of heart-Yang deficiency caused by Qi inadequacy and blood stasis in CHF (33). This study explored the possible molecular mechanism and clinical efficacy of QLQX in the treatment of CHF. In the clinical section, our pooled analysis showed that QLQX presented significant efficacy in clinical efficacy rate, LVEF, LVEDD, 6-MWD, BNP, NT-proBNP, and adverse events. Previous evidence (54) has shown that tailor-made medications by NT-proBNP guided approach reduce all-cause mortality of CHF by 20% compared with conventional treatments. Our findings show that QLQX has a larger reductive effect on NT-proBNP, suggesting a good clinical effect on CHF. The GRADE-assessed evidence added to this study demonstrates that most of the pooled analyses remain low-level evidence recommendations but show a moderate level of evidence in clinical efficacy rate and improved LVEF indicator due to the sufficient number of studies and patients.
Based on our preclinical findings and previous studies, PI3K/AKT may be a noteworthy signaling pathway regulated by QLQX during the treatment of CHF. QLQX could activate this signaling pathway, up-regulating GSK3β, increasing Bcl-2 and decreasing Bax (55, 56). The pathway could also activate enzymatic antioxidant systems, including SOD, catalase, and heme oxidase-1, which prevents the excessive production of ROS, and reduce the level of MDA. And SODs could catalytic O2– to H2O2, then catalase or glutathione peroxidase in the cytoplasm and mitochondria converts H2O2 to H2O and O2 (56, 57). Besides, it has been proved that the accumulation of ROS could induce apoptosis and excessive autophagy, while QLQX could inhibit it by down-regulating AMP-activated protein kinase phosphorylation, and up-regulating mechanistic target of rapamycin (mTOR) phosphorylation (10). Another study (58) indicates that the cardioprotective effect of QLQX is partially mediated through PI3K/AKT-dependent vascular endothelial-derived growth factor (VEGF) expression, which could prevent apoptosis and regulate cell survival. According to previous studies (59, 60), although the role of p53 gene in the heart is well established, it is not clear how p53 is regulated in CHF. In normal cells, p53 expression is kept at low levels by the E3 ubiquitin ligase mouse double minute 2 homolog (Mdm2), which targets p53 for proteasomal degradation. In response to acute stress, Mdm2 is inactivated and increased p53 levels block cell division and induce apoptosis (61). Therefore, we hypothesize that QLQX could downregulate p53 to curb the negative feedback loop of p53 activity, but further validation is needed.
There are some limitations in this study. First, even though one high-quality RCT (33) is included, the overall quality of the included RCTs is still not enough to provide a high certainty of evidence. Second, mechanisms of QLQX protecting against CHF are not fully explored. In the study, low to moderate levels of evidence indicated that QLQX attenuates CHF. In the future, more well-designed RCTs should be conducted to improve the reliability of evidence. Preclinical studies should delve into the signaling pathways associated with ventricular remodeling to explore the mechanisms by which QLQX exerts its effectiveness as well as determine the active compounds in QLQX.
7. Conclusion
QLQX has adjuvant efficacy and safety in patients with CHF and improves clinical efficacy rates, LVEF at moderate levels of evidence. QLQX could attenuate CHF by inhibiting apoptosis, suppressing RAAS activation, improving water retention, and enhancing cardiocyte remodeling.
Data availability statement
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
QH and HC conceived and designed the study. HC revised the manuscript. JM and HDL supervised the study and provided some comments. XX and JG performed the data analysis and wrote the manuscript. BS, YW, WY, and YJ assessed the literature. HSL, JW, CL, and HZ extracted the data. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the Beijing-Tianjin-Hebei Basic Research Cooperation Project 573 (No. J200020), Shenzhen Science & Innovation Fund (JCYJ20180306173745092 and JCYJ20210324114604013), HKSAR General Research Fund (17109019 and 17113416), and Seed Fund for Basic Research (202011159210 and 202111159235).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.1090616/full#supplementary-material
References
1. McDonagh T, Metra M, Adamo M, Gardner R, Baumbach A, Böhm M, et al. 2021 Esc guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the task force for the diagnosis and treatment of acute and chronic heart failure of the european society of cardiology (ESC). With the special contribution of the heart failure association (HFA) of the Esc. Eur J Heart Fail. (2022) 24:4–131. doi: 10.1002/ejhf.2333
2. Cleland J, Tendera M, Adamus J, Freemantle N, Polonski L, Taylor J. The perindopril in elderly people with chronic heart failure (Pep-Chf) study. Eur Heart J. (2006) 27:2338–45. doi: 10.1093/eurheartj/ehl250
3. Chioncel O, Lainscak M, Seferovic P, Anker S, Crespo-Leiro M, Harjola V, et al. Epidemiology and one-year outcomes in patients with chronic heart failure and preserved, mid-range and reduced ejection fraction: An analysis of the esc heart failure long-term registry. Eur J Heart Fail. (2017) 19:1574–85. doi: 10.1002/ejhf.813
4. Wang G, Hamid T, Keith R, Zhou G, Partridge C, Xiang X, et al. Cardioprotective and antiapoptotic effects of heme oxygenase-1 in the failing heart. Circulation. (2010) 121:1912–25. doi: 10.1161/circulationaha.109.905471
5. Costa S, Reina-Couto M, Albino-Teixeira A, Sousa T. Statins and oxidative stress in chronic heart failure. Rev Port Cardiol. (2016) 35:41–57. doi: 10.1016/j.repc.2015.09.006
6. Liu T, Cai J, Zhang L, Sun N, Cui J, Wang H, et al. The mechanism of rna oxidation involved in the development of heart failure. Free Radic Res. (2019) 53:910–21. doi: 10.1080/10715762.2019.1646424
7. Zhang Y. Clinical observation on treating chronic heart failure with the qili qiangxin capsule. Clin J Chinese Med. (2017) 9:83–4.
8. Jiao C, Pei X. Study on the therapeutic effect of qiliqiangxin capsule on diastolic heart failure. Cardiovasc Dis J Intergra Traditional Chinese Western Med. (2017) 5:165–6. doi: 10.16282/j.cnki.cn11-9336/r.2017.30.134
9. Popova T, Muzyko E, Kustova M, Bychenkova M, Perfilova V, Prokofiev I, et al. Influence of the dense extract from herb of Primula veris L. On the oxidative stress development and the functional state of the cardiomyocytes mitochondria of rats with experimental chronic heart failure. Biomed Khim. (2018) 64:334–43. doi: 10.18097/pbmc20186404334
10. Fan C, Cai W, Ye M, Chen M, Dai Y. Qili qiangxin, a compound herbal medicine formula, alleviates hypoxia-reoxygenation-induced apoptotic and autophagic cell death via suppression of Ros/Ampk/Mtor Pathway in Vitro. J Integr Med. (2022) 20:365–75. doi: 10.1016/j.joim.2022.04.005
11. Xu X, Yang Y, Zhou G, Du Z, Zhang X, Mao W, et al. Clinical efficacy of qili qiangxin capsule combined with western medicine in the treatment of chronic heart failure: A systematic review and meta-analysis. Evid Based Complement Alternat Med. (2021) 2021:9761159. doi: 10.1155/2021/9761159
12. Xiang Q, Wang M, Ding Y, Fan M, Tong H, Chen J, et al. Qili qiangxin capsule combined with Sacubitril/Valsartan for Hfref: A systematic review and meta-analysis. Front Pharmacol. (2022) 13:832782. doi: 10.3389/fphar.2022.832782
13. Sun J, Zhang K, Xiong W, Yang G, Zhang Y, Wang C, et al. Clinical effects of a standardized chinese herbal remedy, qili qiangxin, as an adjuvant treatment in heart failure: Systematic Review and meta-analysis. BMC Complement Altern Med. (2016) 16:201. doi: 10.1186/s12906-016-1174-1
14. Liberati A, Altman D, Tetzlaff J, Mulrow C, Gøtzsche P, Ioannidis J, et al. The prisma statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ. (2009) 339:b2700. doi: 10.1136/bmj.b2700
15. Hooijmans C, Rovers M, de Vries R, Leenaars M, Ritskes-Hoitinga M, Langendam M. Syrcle’s risk of bias tool for animal studies. BMC Med Res Methodol. (2014) 14:43. doi: 10.1186/1471-2288-14-43
16. Sterne J, Savović J, Page M, Elbers R, Blencowe N, Boutron I, et al. Rob 2: A revised tool for assessing risk of bias in randomised trials. BMJ. (2019) 366:l4898. doi: 10.1136/bmj.l4898
17. Guyatt G, Oxman A, Akl E, Kunz R, Vist G, Brozek J, et al. Grade Guidelines: 1. introduction-grade evidence profiles and summary of findings tables. J Clin Epidemiol. (2011) 64:383–94. doi: 10.1016/j.jclinepi.2010.04.026
18. Guyatt G, Oxman A, Vist G, Kunz R, Brozek J, Alonso-Coello P, et al. Grade Guidelines: 4. Rating the Quality of Evidence–Study Limitations (Risk of Bias). J Clin Epidemiol. (2011) 64:407–15. doi: 10.1016/j.jclinepi.2010.07.017
19. Guyatt G, Oxman A, Kunz R, Woodcock J, Brozek J, Helfand M, et al. Grade Guidelines: 7. rating the quality of evidence–inconsistency. J Clin Epidemiol. (2011) 64:1294–302. doi: 10.1016/j.jclinepi.2011.03.017
20. Guyatt G, Oxman A, Kunz R, Woodcock J, Brozek J, Helfand M, et al. Grade Guidelines: 8. rating the quality of evidence–indirectness. J Clin Epidemiol. (2011) 64:1303–10. doi: 10.1016/j.jclinepi.2011.04.014
21. Guyatt G, Oxman A, Kunz R, Brozek J, Alonso-Coello P, Rind D, et al. Grade Guidelines 6. rating the quality of evidence–imprecision. J Clin Epidemiol. (2011) 64:1283–93. doi: 10.1016/j.jclinepi.2011.01.012
22. Guyatt G, Oxman A, Montori V, Vist G, Kunz R, Brozek J, et al. Grade Guidelines: 5. rating the quality of evidence–publication bias. J Clin Epidemiol. (2011) 64:1277–82. doi: 10.1016/j.jclinepi.2011.01.011
23. Lin R, Zhu J, Wang W, Fu Y. The effect of qiliqiangxin on chronic heart failure rat model. J Med Res. (2010) 39:62–6.
24. Xu T, Guo L, Chen L, Li F, Li Y. Effects of Qili Qiangxin Capsules on Apoptosis of Cardiac Muscle Cells of Rats with Chronic Heart Failure. Tradit Chinese Drug Res Clin Pharmacol. (2010) 21:366–9. doi: 10.19378/j.issn.1003-9783.2010.04.011
25. Yu C, Li X, Xiao Y, Xin Y, Wang Y. Effect of qili qiangxin capsule on p2x receptor mrna expression in rat myocardium with chronic heart failure. J Tradit Chinese Med. (2012) 53:416–9. doi: 10.13288/j.11-2166/r.2012.05.024
26. Qian Y, Li Z, Wang M, Fan Z, Zhang L, Shi J. Experimental study of qiliqiangxin capsule against adriamycin induced oxidative damage in rats with heart failure. Chinese J Difficult Complicated Cases. (2014) 13:625–33.
27. Cui X, Zhang J, Li Y, Sun Y, Cao J, Zhao M, et al. Effects of Qili Qiangxin Capsule on Aqp2, V2r, and At1r in Rats with Chronic Heart Failure. Evid Based Complement Alternat Med. (2015) 2015:639450. doi: 10.1155/2015/639450
28. Li Y, Jin X. Effect of qiliqiangxin capsules on the raas activity in rat with heart failure. Chinese J Clin (Electronic Edition). (2016) 10:238–42. doi: 10.1016/j.phymed.2018.03.003
29. Liu C, Jiang D, Chen F, Xie Y, Wang J, Wang W, et al. Effect of qili qiangxin capsule on expression of myocardial macrophage derived chemokines in chronic heart failure rats. J Guangzhou Univ Chinese Med. (2020) 37:1321–6. doi: 10.13359/j.cnki.gzxbtcm.2020.07.022
30. Ding M, Yang P, He M, Feng Z. Efficacy evaluation of qili qiangxin capsule in the treatment of chronic congestive heart failure. J Jilin Univ (Medicine Edition). (2009) 35:1147–50. doi: 10.13481/j.1671-587x.2009.06.012
31. Du C, Hao X, Zhao Y. The effect of qiliqiangxin capsule about plasma concentration of BNP and D – dimer on the patient with chronic Heart failure. J Pract Tradit Chin Intern Med. (2009) 23:312.
32. Huang Y, Pan Q. Effect of qili qiangxin capsules on serum sodium in patients with chronic heart failure. J New Chinese Med. (2012) 44:16–8. doi: 10.13457/j.cnki.jncm.2012.12.031
33. Li X, Zhang J, Huang J, Ma A, Yang J, Li W, et al. A multicenter, randomized, double-blind, parallel-group, placebo-controlled study of the effects of qili qiangxin capsules in patients with chronic heart failure. J Am Coll Cardiol. (2013) 62:1065–72. doi: 10.1016/j.jacc.2013.05.035
34. Guo L, Yan K. Effects of adjuvant therapy of qili qiangxin capsule on angiotensin II level and cardiac function in patients with chronic heart failure. Med Herald. (2015) 34:208–10.
35. Kong X, Wang Z. Observation of the therapeutic effect of qiliqiangxin capsule on the treatment of chronic heart failure. J Taishan Med Coll. (2015) 36:295–6.
36. Liu D, Li Y. Therapeutic effect and safety evaluation of qili qiangxin capsule in patients with chronic systolic heart failure. Electron J Cardiovasc Dis Integr Tradit Chinese West Med. (2016) 4:19–22. doi: 10.16282/j.cnki.cn11-9336/r.2016.11.013
37. Gao Q, Wang M. Observation on qiliqiangxin capsules in treatment of chronic heart failure. Eval Anal Drug-Use Hosp China. (2017) 17:1186. doi: 10.14009/j.issn.1672-2124.2017.09.012
38. Wang D, Xiao L. Clinical study of qili qiangxin capsule combined with western medicine in the treatment of chronic heart failure. Xinjiang Tradit Chinese Med. (2017) 35:7–9.
39. Li B, Kang A, Shen J. Effect of qiliqiangxin capsule on Bnp level and prognosis in elderly patients with chronic heart failure. Shanxi Med J. (2018) 47:2642–4.
40. Liu L. Efficacy observation of metoprolol combined with qili qiangxin capsules in the treatment of chronic heart failure. Chinese Pract Med. (2019) 14:95–6. doi: 10.14163/j.cnki.11-5547/r.2019.33.052
41. Liu Y. Observation on the effect of metoprolol combined with qili qiangxin capsules in the treatment of chronic heart failure. Clin Med. (2019) 39:116–7. doi: 10.19528/j.issn.1003-3548.2019.12.048
42. Dong Y, Du Q. Effect of qili qiangxin capsule combined with sacubitril-valsartan sodium in the treatment of chronic heart failure and its effect on the level of nt-probnp. Appli Mod Med China. (2020) 14:153–5. doi: 10.14164/j.cnki.cn11-5581/r.2020.24.070
43. Han G. Effects of metoprolol combined with qili qiangxin capsules on Bnp level and cardiac function in patients with chronic heart failure. Pract Clin Pract Integr Tradit Chinese West Med. (2020) 20:60–1. doi: 10.13638/j.issn.1671-4040.2020.11.028
44. Li C, Zhang M, Zhao H, Yuan J, Sang J, He Z, et al. Clinical observation on the curative effect of sakubatrovalsartan combined with qiliqiangxin capsule in the treatment of patient with chronic failure. Chinese J Difficult Complicated Cases. (2020) 19:667–71.
45. Lu C, Li F, Zhang J, Mo Y. Effect of metoprolol combined with qiliqiangxin capsule on Gal-3, Hcy in patients with chronic heart failure. Chinese J Rational Drug Use. (2020) 17:69–73.
46. Li T, Wang J, Li J, Jiang W. Efficacy of qiliqiangxin capsules combined with shakuba trivalsartan sodium on chronic heart failure. Northwest J Pharm. (2021) 36:824–8.
47. Liu H, Li W. Effect of qili qiangxin capsule on the level of cardiac function and plasma Bnp, Pra, Il-6 in patients with chronic heart failure. J Hubei Univ Chinese Med. (2021) 23:59–61.
48. Liu Y. Application of qili qiangxin capsule combined with metoprolol succinate sustained-release tablet in patients with chronic heart failure. Med Equipment. (2021) 34:113–4.
49. Nie Z, Liu Z, Chen Z, Liu H, Huang C. Effect of qili qiangxin capsules combined with metoprolol on plasma Bnp, Hs-Ctnt Levels and cardiac function in patients with chronic heart failure. Chinese Med Mod Dis Educ China. (2021) 19:134–6.
50. Sun C. Efficacy evaluation of qili qiangxin capsule combined with valsartan in the treatment of chronic heart failure. Cardiovasc Dis Prev Knowl. (2021) 11:11–2.
51. Yang W. Effect of qili qiangxin capsule combined with sacubitril-valsartan sodium on chronic heart failure. Pract Clin Pract Integr Tradit Chinese West Med. (2021) 21:66–7. doi: 10.13638/j.issn.1671-4040.2021.14.029
52. Wang Y, Fu C. Clinical effect of qili qiangxin capsule combined with candesartan medoxomil in the treatment of chronic heart failure. J Clin Rational Drug Use. (2022) 15:8–10. doi: 10.15887/j.cnki.13-1389/r.2022.08.003
53. Yao J, Liu D. Clinical effect of qili qiangxin capsule combined with sacubitril-valsartan in patients with chronic heart failure. J Healthc Eng. (2022) 2022:8598806. doi: 10.1155/2022/8598806
54. Bajaj N, Patel N, Prabhu S, Arora G, Wang T, Arora P. Effect of Nt-Probnp-Guided therapy on all-cause mortality in chronic heart failure with reduced ejection fraction. J Am Coll Cardiol. (2018) 71:951–2. doi: 10.1016/j.jacc.2017.11.070
55. Han X, Li C, Yang P, Jiang T. Potential mechanisms of qili qiangxin capsule to prevent pulmonary arterial hypertension based on network pharmacology analysis in a rat model. Ann Transl Med. (2022) 10:453. doi: 10.21037/atm-22-901
56. Zhao Q, Li H, Chang L, Wei C, Yin Y, Bei H, et al. Qiliqiangxin attenuates oxidative stress-induced mitochondrion-dependent apoptosis in cardiomyocytes via Pi3k/Akt/Gsk3β signaling pathway. Biol Pharm Bull. (2019) 42:1310–21. doi: 10.1248/bpb.b19-00050
57. Xiao J, Deng S, She Q, Li J, Kao G, Wang J, et al. Traditional chinese medicine qili qiangxin inhibits cardiomyocyte apoptosis in rats following myocardial infarction. Exp Ther Med. (2015) 10:1817–23. doi: 10.3892/etm.2015.2759
58. Liang T, Zhang Y, Yin S, Gan T, An T, Zhang R, et al. Cardio-Protecteffect of qiliqiangxin capsule on left ventricular remodeling, dysfunction and apoptosis in heart failure rats after chronic myocardial infarction. Am J Transl Res. (2016) 8:2047–58.
59. Wang J, Zhou J, Ding X, Zhu L, Jiang K, Fu M, et al. Qiliqiangxin improves cardiac function and attenuates cardiac remodeling in rats with experimental myocardial infarction. Int J Clin Exp Pathol. (2015) 8:6596–606.
60. Chunhacha P, Pinkaew D, Sinthujaroen P, Bowles D, Fujise K. Fortilin Inhibits P53, halts cardiomyocyte apoptosis, and protects the heart against heart failure. Cell Death Discov. (2021) 7:310. doi: 10.1038/s41420-021-00692-w
Keywords: chronic heart failure, Qili Qiangxin, systematic review, myocardial apoptosis, adjuvant efficacy
Citation: Xing X, Guo J, Mo J, Li H, Zhang H, Shao B, Wang Y, Li H, Wang J, Leung CL, Jiang Y, Yin W, Chen H and He Q (2023) Qili Qiangxin capsules for chronic heart failure: A GRADE-assessed clinical evidence and preclinical mechanism. Front. Cardiovasc. Med. 9:1090616. doi: 10.3389/fcvm.2022.1090616
Received: 05 November 2022; Accepted: 23 December 2022;
Published: 11 January 2023.
Edited by:
Hongcai Shang, Dongzhimen Hospital, Beijing University of Chinese Medicine, ChinaReviewed by:
Iokfai Cheang, Nanjing Medical University, ChinaShichao Lv, First Teaching Hospital of Tianjin University of Traditional Chinese Medicine, China
Yonghong Gao, Dongzhimen Hospital, Beijing University of Chinese Medicine, China
Copyright © 2023 Xing, Guo, Mo, Li, Zhang, Shao, Wang, Li, Wang, Leung, Jiang, Yin, Chen and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qingyong He,  heqingyongg@163.com; Haiyong Chen,
heqingyongg@163.com; Haiyong Chen,  haiyong@hku-szh.org
haiyong@hku-szh.org
†These authors have contributed equally to this work
 Xiaoxiao Xing
Xiaoxiao Xing