- Department of Cardiology, Xiangyang No.1 People’s Hospital, Hubei University of Medicine, Xiangyang, Hubei, China
Background: Perforation of the interventricular septum and left ventricular (LV) free wall by a right ventricular (RV) lead is an extremely rare and potentially life-threatening complication. In this case report, we discussed the diagnosis and management of a very unusual complication of pacemaker (PM) implantation, i.e., LV perforation brought on by an RV pacing lead.
Case summary: A 92-year-old man was admitted to Xiangyang No.1 People’s Hospital due to a complete atrioventricular block. We performed a dual-chamber PM implantation; however, on the second postoperative day (POD), pacemaker failure occurred. Thoracic computed tomography (CT) scan showed that RV lead had pierced the interventricular septum and LV free wall. A transvenous lead extraction of the penetrating lead was performed uneventfully, and RV lead was refixed at the lower RV septum on the 5th POD.
Discussion: Identification of high-risk patients is mandatory to prevent this serious complication, and transvenous lead extraction with cardiac surgery backup may be an option.
1. Introduction
Iatrogenic ventricle perforation by pacing/defibrillation leads is a rare but potentially life-threatening complication which occurs in only 0.3–0.8% of pacemaker (PM) or implantable cardioverter defibrillator (ICD) implantation procedures (1). Although the right ventricular (RV) apex is the site of lead perforation that occurs most frequently, few cases have been reported of lead penetration through the interventricular septum and the left ventricular (LV) free wall to the left hemithorax (2). Appropriate management of lead migration in the left hemithorax is still uncertain. Among these reported cases, most patients underwent surgical extraction of the penetrating lead (3).
2. Case presentation
A 92-year-old man was referred to our center for syncope due to a complete atrioventricular block (Figure 1A). His medical history includes coronary atherosclerotic heart disease and chronic obstructive pulmonary disease. Coronary angiography was performed on account of effort angina 3 years ago (Figures 1E, F). Prior coronary angiography revealed a diffuse lesion (percent diameter stenosis 70–80%) in the middle segment of the left anterior descending (LAD) branch (Figure 1E). However, he refused to receive revascularization or medication treatment. During hospitalization, he had no finding of heart failure, and the transthoracic echocardiography (TTE) revealed minor mitral insufficiency and normal LV contraction (ejection fraction: 55%).
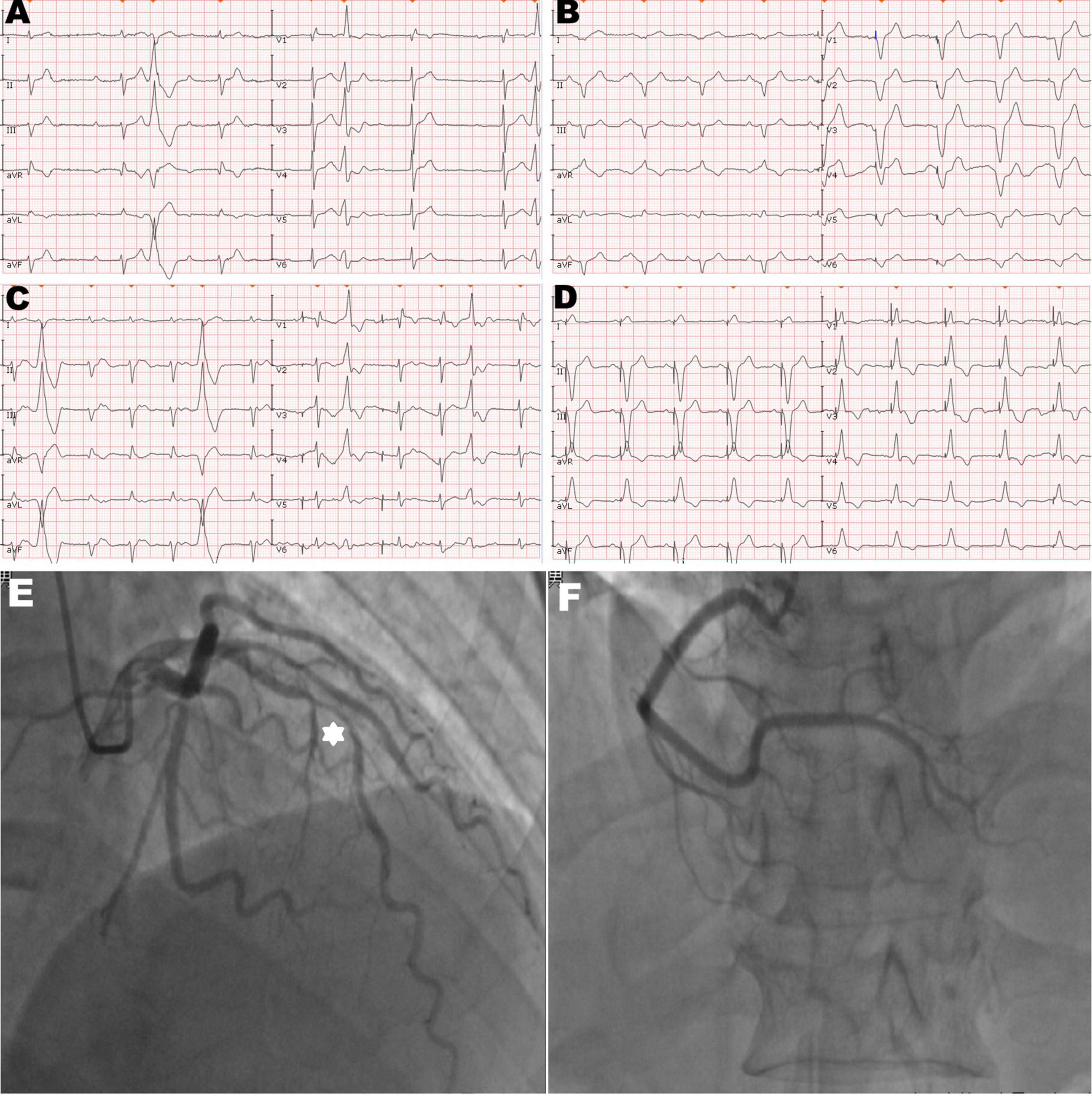
Figure 1. Twelve-lead electrocardiogram and coronary angiogram of the patient. (A) The initial electrocardiogram on the admission day showed complete atrioventricular block with frequent premature ventricular contractions. (B) The electrocardiogram immediately after pacemaker (PM) implantation showed the left bundle branch block pattern and the pacing model was VAT. (C) The electrocardiogram on the second post-operative day (POD) showed ventricular pacing failure. (D) The electrocardiogram after transvenous lead extraction and re-fixation showed the right bundle branch block and left axis deviation, and the right ventricular (RV) lead was refixed at the lower RV septum. (E) Hexagonal star symbol shows a diffuse atherosclerotic stenosis was observed in the middle segment of the left anterior descending branch, thus leading to insufficient coronary blood supply on the mid-portion of the interventricular septum. (F) Right coronary artery was relatively normal.
We performed dual-chamber PM (HeartTone LD300D, LifeTech Scientific Corporation, Shenzhen, China) implanted via the left subclavian vein on the next day after admission. The implantation procedure can be summarized as follows: under local anesthesia, after a successful puncture of the left subclavian vein, the patient was implanted with a common sheath. Although a superior vena cava stenosis might exist, we did not replace the common sheath with a long sheath. Thus, the manipulation of active fixation leads was very difficult; we attempted to fix RV lead at the RV septum, but it was not as successful. Finally, two active fixation leads were, respectively, placed at the RV apex (SureScan 5076; 58 cm, Medtronic, Minneapolis, MN, USA) and the right atrial appendage (SureScan 5076; 52 cm, Medtronic, Minneapolis, MN, USA) with excellent pacing parameters (intraoperative RV lead impedance and threshold were 1,300 Ω and 1.4 V, respectively). There were no abnormalities or complications during the implantation procedure. X-ray fluoroscopy image immediately after implantation showed that the RV lead was fixed properly (Figure 2A). Electrocardiogram immediately after implantation showed the left bundle branch block pattern (Figure 1B).
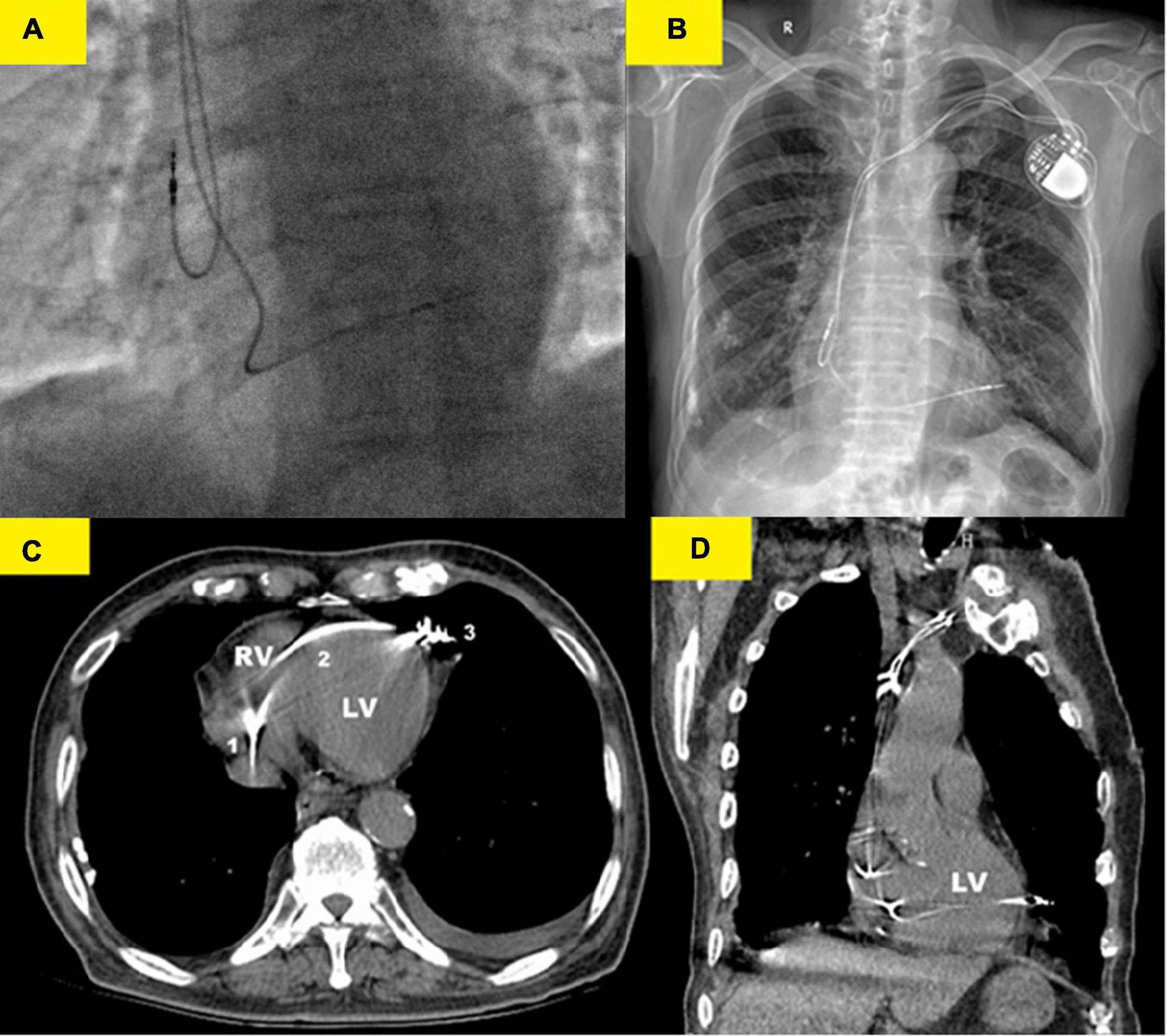
Figure 2. Various imaging modalities showed the lead perforation. (A) X-ray fluoroscopy image (right anterior oblique 30°) immediately after implantation showed that the RV lead was placed at the right ventricular (RV) apex. (B) Chest x-ray showed that the RV lead was beyond the left cardiac margin and clearly migrated in the left hemithorax. (C) Thoracic computed tomography (CT) scan showed the RV lead course from the right ventricle (1) to the left cardiac margin (3) in the patient and perforation of the interventricular septum (2) was well-evident. (D) Coronal CT images confirmed the RV lead migration in the left hemithorax. Thoracic CT scan should, therefore, be regarded as the gold standard for the strategical management of this complication.
However, on the second postoperative day (POD), the patient complained of unrelenting chest pain and syncope. A subsequent immediate chest X-ray identified that the RV lead tip migrated to the region outside the left cardiac margin (Figure 2B). A thoracic computed tomography (CT) scan revealed that the RV lead passed through the interventricular septum and the left LV free wall, and finally reached the left of the pericardial cavity. Furthermore, as the thoracic CT scan also showed a small amount of left pleural effusion (Figure 2B) and there were no obvious symptoms of pericardial tamponade (Figures 2C, D), we deduced that the lead had already penetrated the pericardium. Electrocardiogram and interrogation showed loss of ventricular capture (Figure 1C).
The subsequent patient’s management was thoroughly debated. Patients may not tolerate surgical lead extraction due to the variety of severe chronic diseases and advanced age. Therefore, on the 5th POD, we performed transvenous lead extraction under transesophageal echocardiography monitored and cardiac surgery backup. Figures 3A–D depict and describe the entire procedure in detail. Transvenous lead extraction was uncomplicated and did not have any hemodynamic instability, and intraoperative ultrasonography monitoring showed no increase in pleural effusion and pericardial effusion. The original RV lead was refixed at the lower RV septum to reduce the risk of perforation and consequent tamponade. Subsequent electrocardiogram and PM interrogation revealed the PM functioning normally with excellent pacing parameters (intraoperative RV lead impedance and threshold were 1,020 Ω and 1.0 V, respectively) (Figure 1D). On the 10th POD, the patient was discharged, and the subsequent 6 months of follow-up were uneventful.
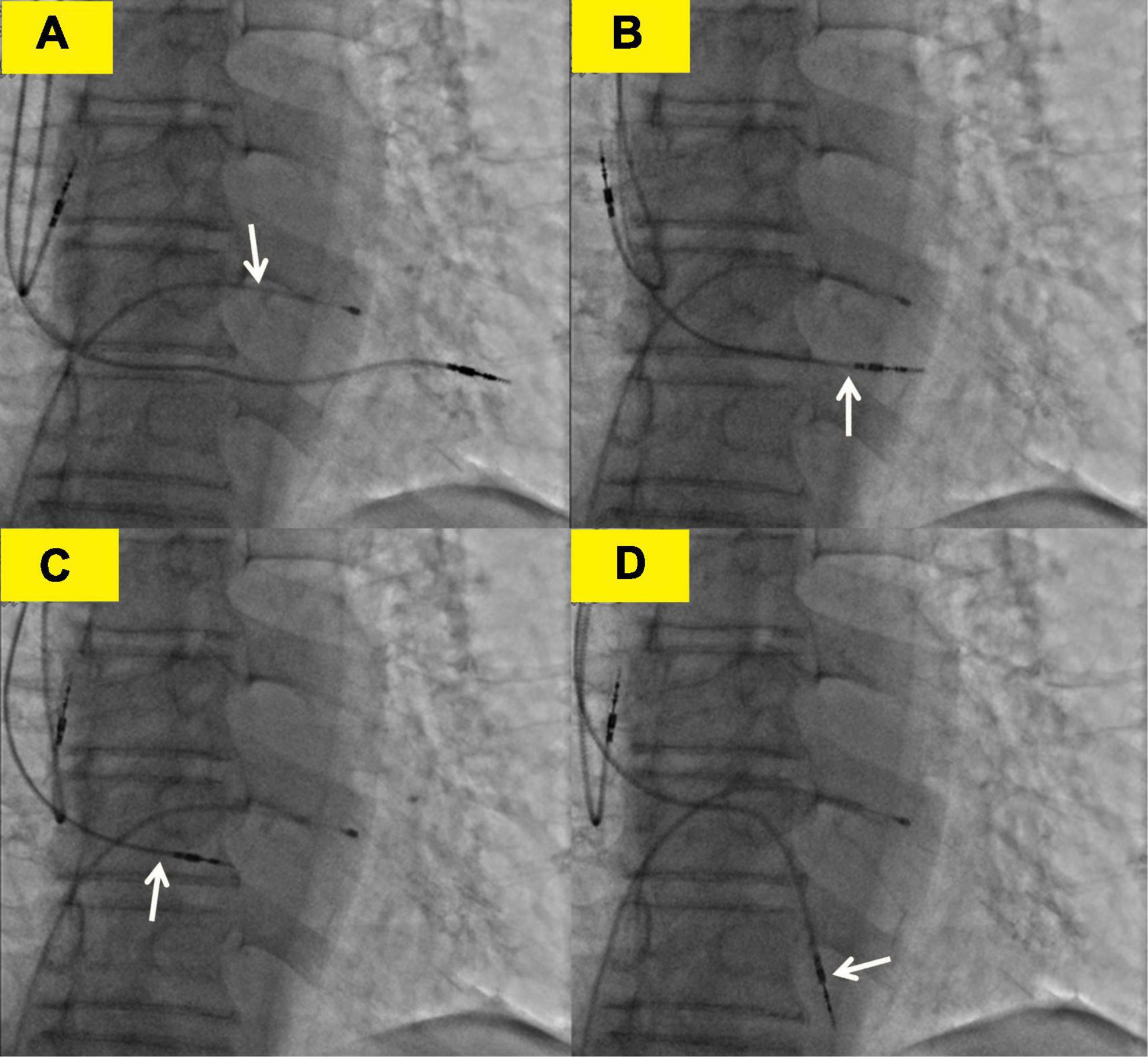
Figure 3. Transvenous lead extraction process. (A) The white arrow shows the lead of the temporary transvenous pacemaker. (B) The white arrow shows the right ventricular (RV) lead was screwed back to the left ventricle. (C) The white arrow shows the RV lead was screwed back to the right ventricle. (D) The white arrow shows the right ventricular lead was refixed at the lower right ventricular septum.
3. Discussion
Left ventricular perforation caused by an RV pacing lead is a rare and potentially life-threatening complication, and the lead can migrate to the outside of the pericardium with a potential risk of vascular or pulmonary damage. Although late-onset (> 1 month) ventricular perforations have also been observed, they often started within < 24 h after device placement (4). To the best of our knowledge, no specific recommendations are known to exist for this uncommon complication. In addition, it has not yet been extensively investigated what pathophysiological mechanisms are involved (5). All of the clinical cases with this complication documented in the literature are shown in Table 1, together with their lead type, model, location, migration course, clinical characteristics, treatment, and follow-up (2, 3, 5–12).
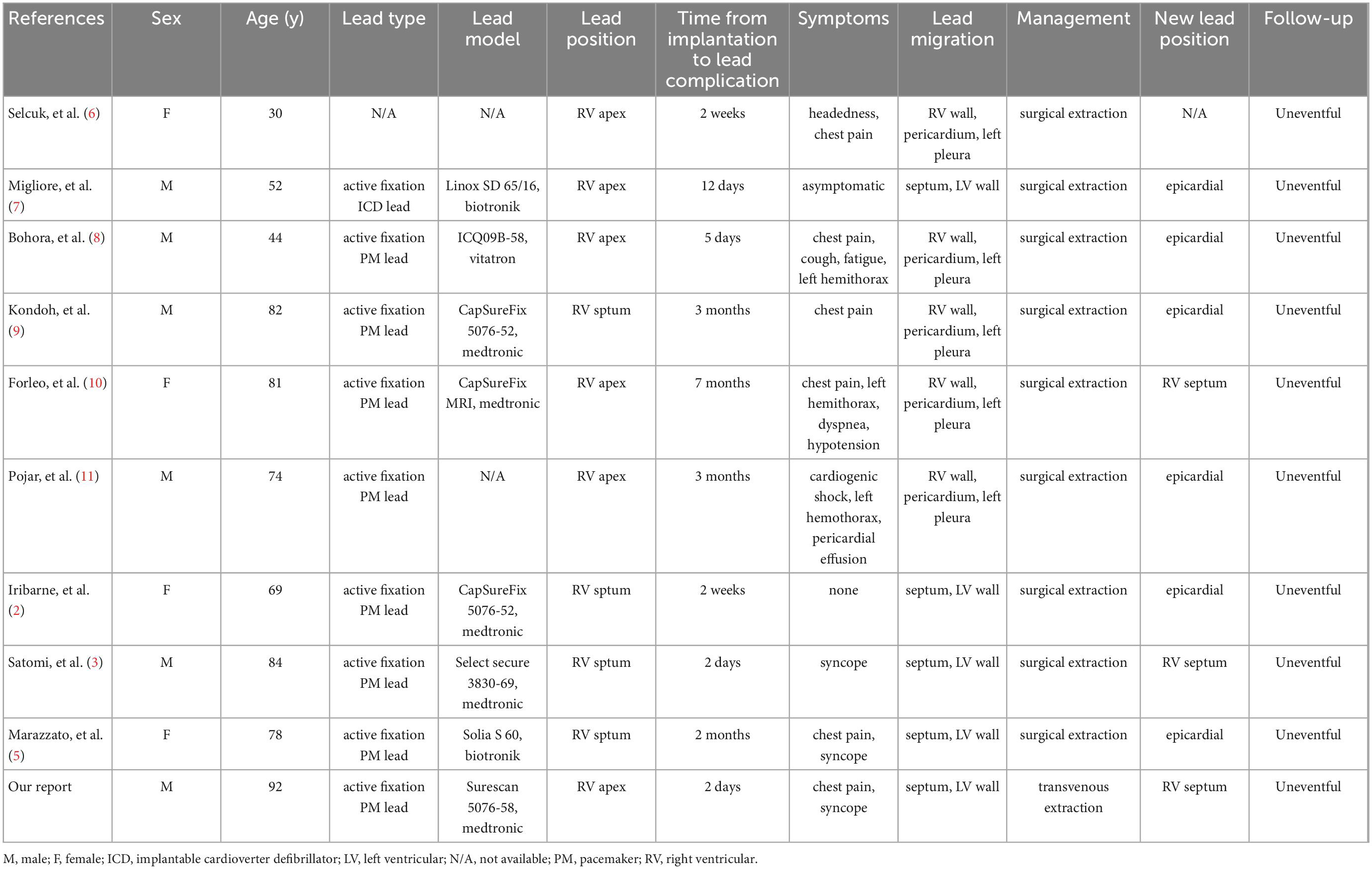
Table 1. Summary of clinical cases of ventricular pacemaker/implantable cardioverter defibrillator (PM/ICD) leads migration in the left hemithorax including our case.
Although the mechanisms of interventricular septal perforation are still unknown, the reported cases suggest that inadequate blood supply and the twisting motion of the septal musculature from beat to beat may be associated with this complication (5). Besides, active fixation leads positioned on the thin-walled RV apex also seem to be related to an increased risk of left-sided lead migration, according to earlier observations on patients with lead perforation. Indeed, in this case, the perforation portion of the septal was the coronary angiogram that had previously shown insufficient coronary blood supply. Although active fixation leads positioned in the RV apex result in greater stability, the straight shape of the distal RV lead may bring about significant pressure perpendicularly transferred to the ventricular wall (13). Moreover, features of the lead (SureScan 5076; 58 cm, Medtronic) also seem to play a pivotal role in our case. This lead’s non-retractable bare screw and substantial tipping load may have created excessive stress on the ventricular wall (12). Finally, excessive loop of the RV lead may generate more tension in the ventricular wall, which is also a potential cause of ventricular perforation. In this regard, to prevent the recurrence of this lead implantation complication, a leadless PM should be used in conjunction with a functioning AAI system as an alternate treatment.
A previous study found that pericardial effusion and tamponade only occurred in 38 and 19% of patients (14). However, detecting cardiac perforation brought on by ventricular leads with clinical symptoms was not difficult. Therefore, in instances with unclear results and/or a lack of symptoms, thoracic CT scans are essential to determine if the pleural or LV free wall is implicated and to establish an appropriate diagnosis.
According to a consensus endorsed by the American Heart Association, transvenous lead extraction is not the preferred strategy for patients with cardiac perforation due to pacing/defibrillation lead (15). As indicated in Table 1, surgical lead extraction was carried out without incident in all instances of lead migration in the left hemithorax. Still, for patients who may not tolerate surgical extraction or refuse surgical extraction, transvenous lead extraction could be a potential alternative, especially in recent implantations. It is a less invasive method than open surgery that avoids the potential complications of sternotomy as well as long-term hospital stays (16).
In this report, to the best of our knowledge, we first demonstrated the transvenous lead extraction to treat the lead migration in the left hemithorax. From our experience, spontaneous closure of the perforated site with myocardial contraction is likely to occur immediately after lead withdrawal in LV or RV perforation. Therefore, transvenous lead extraction is feasible, but considering its high-risk characteristics, the whole procedure should be performed by an experienced intervention cardiologist specializing in transvenous lead extraction, preferably in a hybrid operating room, under careful hemodynamic and transesophageal echocardiographic monitoring with a cardiac surgical backup. In addition, transvenous lead extraction should be followed by a new lead implantation or re-fixing of the perforating lead in a different location.
4. Conclusion
The presence of unrelenting chest pain should consider the possibility of a lead migration in the left hemithorax in cases of suspected cardiac perforation. To assess whether the pleural or LV free wall is involved, thoracic CT scans are essential. With respect to lead extraction, transvenous lead extraction with cardiac surgery backup can be feasible. In addition, less traumatic passive-fixation leads or leadless PM may be employed in these high-risk individuals with evidence of non-revascularizable coronary atherosclerotic heart disease and unequivocal need for PM/ICD implantation.
Data availability statement
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article. The patient consent form was read and signed by the patient.
Author contributions
XH: methodology, investigation, formal analysis, and writing—original draft. HZ: conceptualization and visualization. X-ML: investigation and writing—review. X-LL: project administration and supervision. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.1089694/full#supplementary-material
Supplementary Figure 1 | Transvenous lead extraction process.
References
1. Carlson M, Freedman R, Levine P. Lead perforation: incidence in registries. Pacing Clin Electrophysiol. (2008) 31:13–5. doi: 10.1111/j.1540-8159.2007.00943.x
2. Iribarne A, Sangha R, Bostock I, Rothstein E, McCullough J. Right ventricular lead perforation through the septum, left ventricle, and pleura, managed by an open surgical approach. Heart Rhythm Case Rep. (2018) 4:397–400. doi: 10.1016/j.hrcr.2018.05.004
3. Satomi N, Enta K, Otsuka M, Ishii Y, Asano R, Sawa S. Left ventricular free wall perforation by a right ventricular pacemaker lead: a case report. Eur Heart J Case Rep. (2021) 5:ytab125. doi: 10.1093/ehjcr/ytab125
4. Khan M, Joseph G, Khaykin Y, Ziada K, Wilkoff B. Delayed lead perforation: a disturbing trend. Pacing Clin Electrophysiol. (2005) 28:251–3. doi: 10.1111/j.1540-8159.2005.40003.x
5. Marazzato J, Caravati F, Blasi F, Refugjati T, Vilotta M, Torchio F, et al. Ventricular pacemaker lead in the left hemithorax: mechanisms and evidence-based management of a late-onset hazardous complication. Clin Case Rep. (2021) 9:e04617. doi: 10.1002/ccr3.4617
6. Selcuk H, Selcuk M, Maden O, Ozeke O, Celenk M, Turkvatan A, et al. Uncomplicated heart and lung perforation by a displaced ventricular pacemaker lead: a case report. Pacing Clin Electrophysiol. (2006) 29:429–30. doi: 10.1111/j.1540-8159.2006.00366.x
7. Migliore F, Leoni L, Torregrossa G, Guglielmi C, Tarantini G, Buja G, et al. Asymptomatic right ventricular perforation by an implantable cardioverter defibrillator lead detected by home monitoring system. J Electrocardiol. (2010) 43:673–5. doi: 10.1016/j.jelectrocard.2010.07.022
8. Bohora S, Unnikrishnan M, Ajit Kumar V, Nayyar S, Tharakan J. Left hemothorax: a presentation of a late ventricular perforation caused by an active fixation pacing lead. Int J Cardiol. (2010) 141:e43–6. doi: 10.1016/j.ijcard.2008.11.152
9. Kondoh H, Funatsu T, Taniguchi K. Late left ventricular perforation by active fixation pacemaker lead implanted in the right ventricular septum. J Card Surg. (2012) 27:530–1. doi: 10.1111/j.1540-8191.2012.01479.x
10. Forleo G, Zeitani J, Perretta T, Della Rocca D, Santini L, Simonetti G, et al. Acute left hemothorax as a late complication of an active-fixation pacemaker lead. Ann Thorac Surg. (2013) 95:1081–4. doi: 10.1016/j.athoracsur.2012.07.064
11. Pojar M, Vobornik M, Novy J. Left hemothorax: an unusual complication of delayed right ventricular perforation by a permanent pacemaker lead. J Card Surg. (2013) 28:325–7. doi: 10.1111/jocs.12080
12. Gammage M, Lieberman R, Yee R, Manolis A, Compton S, Khazen C, et al. Multi-center clinical experience with a lumenless, catheter-delivered, bipolar, permanent pacemaker lead: implant safety and electrical performance. Pacing Clin Electrophysiol. (2006) 29:858–65. doi: 10.1111/j.1540-8159.2006.00452.x
13. Akbarzadeh M, Mollazadeh R, Sefidbakht S, Shahrzad S, Bahrololoumi Bafruee N. Identification and management of right ventricular perforation using pacemaker and cardioverter-defibrillator leads: a case series and mini review. J Arrhythm. (2017) 33:1–5. doi: 10.1016/j.joa.2016.05.005
14. Schwerg M, Stockburger M, Schulze C, Bondke H, Poller W, Lembcke A, et al. Clinical, anatomical, and technical risk factors for postoperative pacemaker or defibrillator lead perforation with particular focus on myocardial thickness. Pacing Clin Electrophysiol. (2014) 37:1291–6. doi: 10.1111/pace.12431
15. Wilkoff B, Love C, Byrd C, Bongiorni M, Carrillo R, Crossley G III, et al. Transvenous lead extraction: heart Rhythm Society expert consensus on facilities, training, indications, and patient management: this document was endorsed by the American heart association (AHA). Heart Rhythm. (2009) 6:1085–104. doi: 10.1016/j.hrthm.2009.05.020
16. Archontakis S, Sideris K, Aggeli K, Gatzoulis K, Demosthenous M, Tolios P, et al. Percutaneous lead extraction and repositioning: an effective and safe therapeutic strategy for early ventricular lead perforation with dislocation both inside and outside the pericardial sac following a cardiac device implantation. J Cardiovasc Electrophysiol. (2019) 30:299–307. doi: 10.1111/jce.13804
Keywords: pacemaker, lead migration, left ventricular perforation, transvenous lead extraction, right ventricular pacemaker lead
Citation: Huang X, Zhou H, Li X-M and Li X-L (2023) Case report: Left ventricular perforation caused by right ventricular pacemaker lead. Front. Cardiovasc. Med. 9:1089694. doi: 10.3389/fcvm.2022.1089694
Received: 04 November 2022; Accepted: 19 December 2022;
Published: 12 January 2023.
Edited by:
Masateru Takigawa, Tokyo Medical and Dental University, JapanReviewed by:
Stepan Havranek, Charles University, CzechiaSeigo Yamashita, Jikei University School of Medicine, Japan
Copyright © 2023 Huang, Zhou, Li and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiao-Lan Li,  d2FuZGVyczEzOTZAaG90bWFpbC5jb20=
d2FuZGVyczEzOTZAaG90bWFpbC5jb20=
 Xiang Huang
Xiang Huang Heng Zhou
Heng Zhou