- Department of Cardiovasology, Shanghai Changhai Hospital, Naval Medical University, Shanghai, China
Aims: Device related thrombus (DRT) is a known complication of left atrial appendage closure (LAAC). However, the relation between DRT and elevated risk of ischemic events remains controversial. This study is sought to reassessed the incidence of DRT following LAAC and the relation between DRT and elevated risk of ischemic stroke and systemic embolism (SE) with latest clinical trials included.
Methods: The PubMed, Embase, and Cochrane Library databases were systematically searched from their inception until April 2022 for studies that reported the incidence of DRT and compared the incidence of both stroke and SE between DRT patients and non-DRT patients.
Results: In 59 eligible studies, the incidence of DRT was 366/12,845 (2.8%, ranging from 0 to 11%, I2 = 64%). The incidence of DRT was not statistically different between single-seal device (SS) and dual-seal device (DS) in subgroup analysis [171/6,190 (2.8%) vs. 78/3,023 (3.6%); p = 0.93]. The pooled incidence of stroke (26 studies, 7,827 patients) in patients with and without DRT was 11.5% in DRT patients and 2.9% among non-DRT patients (OR: 5.08; 95% CI = 3.47–7.44). In the sensitivity analysis, DRT was associated with higher rate of stroke (12.1 vs. 3.2%; OR: 4.14; 95% CI = 2.69–6.38) and SE (16.0 vs. 3.8%; OR: 4.48; 95% CI = 3.04–6.62).
Conclusion: The incidence of DRT was low and similar between SS and DS devices. DRT was associated with increased rates of ischemic events. The occurrence rate of ischemic events associated DRT was comparable between two occlusion mechanism devices.
Systematic review registration: [https://www.crd.york.ac.uk/], identifier [CRD42022326179].
Introduction
Atrial fibrillation (AF) is one of the most common arrhythmias in clinical practice among the elderly (1). Moreover, AF is related to increased risk of several serious adverse events such as ischemic stroke and systemic embolism (SE) (2). Research have shown that patients with AF are four to five times more likely to develop thrombotic and embolic events than the general population (3, 4). Therefore, stroke prevention with oral anticoagulation (OAC) and direct oral anticoagulants (DOACs) is an important part of the treatment regime in patients with AF (5). However, it might be difficult to use these drugs in the clinic, for drug interactions may increase the bleeding risk in patients. Due to these limitations, left atrial appendage closure (LAAC) is recommended for patients who are intolerant to OAC (6). Growing operator experience coupled with technical improvements and device modification have reduced procedural complications and accelerated continued growth in LAAC (7). However, device related thrombus (DRT) is still a known complication of LAAC. Until now, the prevalence and possible risk factors of DRT were discussed based on the randomized trials and large registries (8–11). Saw et al. (9) concluded that independent predictors of DRT were smoking and female sex, while Plicht et al. (10) thought CHADS2 and CHA2DS2-VASc scores, platelet count, and ejection fraction were risk factors of DRT formation. Nevertheless, whether DRT increases the risk of stroke and SE remains controversial. In Cochet’s study (12), a non-significant trend for a lower rate of stroke and SE was observed in DRT group rather than normal patients; while in a large multicenter RCT led by Dukkipati et al. (13) concluded that a non-significant trend for higher occurrence rate of stroke and SE was observed in DRT group. It has been less clear if and to what degree the discovery of this finding increases the stroke risk (14). Recently, major published studies focused on the incidence and consequence of DRT, hence we conducted an updated meta-analysis of both latest observational cohort and randomized controlled trials (RCT) to investigate the association of DRT following LAAC with stroke and SE. Furthermore, the subgroup analysis was performed to explore whether there was a causal relationship between DRT and device types and post-implant anti-thrombotic strategy.
Materials and methods
Search strategy
We conducted a systematic search for relevant articles in PubMed, Embase, and Cochrane Library up to April 2022. The following keywords were used: “Left Atrial Appendage Closure/Occlusion,” “device thrombosis/thrombus/embolization,” “device related thrombus/ thrombosis,” and “device associated thrombus/thrombosis.” Both mesh terms and free terms were used to search for relevant studies. In addition, we searched the references of identified studies to find other satisfactory studies. Our meta-analysis was conducted and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Protocols (PRISRMA-P) (15). This task was completed by two reviewers (SZ and S-HX) independently. The full text of relevant papers will be reviewed if the titles and abstracts of the articles cannot provide enough information for our inclusion. When disagreements arose, a third reviewer (X-XZ or Z-FG) was consulted. The initial study protocol was pre-registered at PROSPERO (CRD42022326179).
Inclusion and exclusion criteria
The inclusion criteria were as following: (1) Patients received LAAC with left atrial occlusion device (Watchman, ACP, or Amulet); (2) With clearly outcomes of our interest reported. The outcomes were DRT, stroke, and SE. The definition of these outcomes and the classification of devices of LAAC (16) was showed in Table 1. Specifically, the single-seal (SS) device includes Watchman, Watchman FLX, Occlutect device, and the dual-seal (DS) device includes ACP, Amulet, LAmbre device; (3) The patients were followed-up for at least 6 months; (4) At least 30 patients recruited in the study; (5) Studies were published in English.
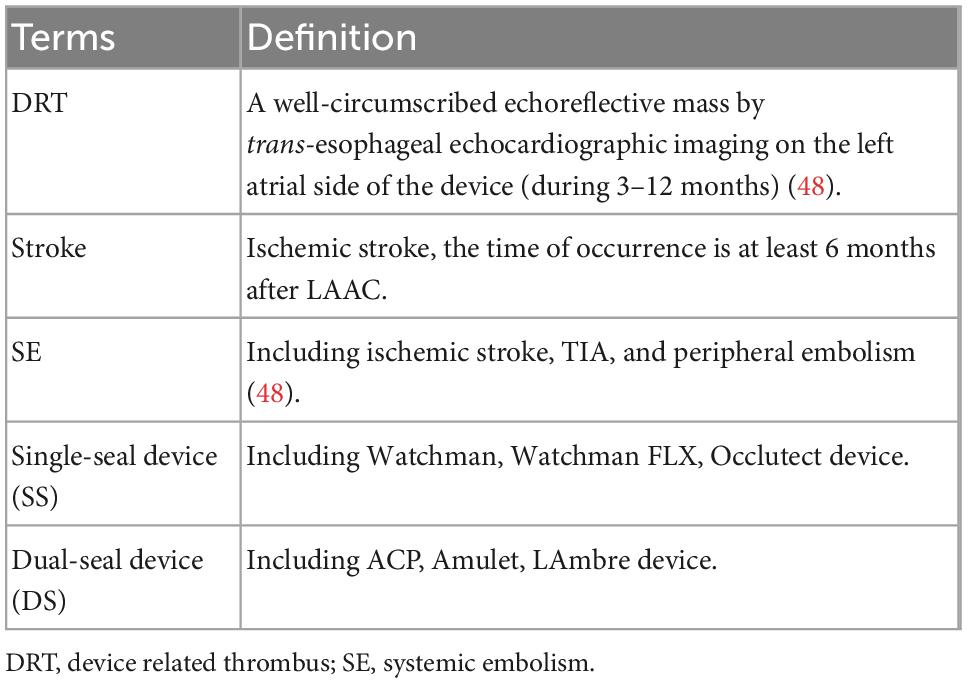
Table 1. Definition of device related thrombus (DRT), ischemic events, and classification of devices.
The exclusion criteria were as following: (1) Studies which were not belong to clinical trials; (2) Studies included other interventions excluded LAAC; (3) Data from non-human species; (4) Duplicate reports with identical data; (5) The imaging detection of DRT should be performed at least 3 months after LAAC.
Quality assessment
Cochrane Collaboration risk of bias (Cochrane ROB) (17) instrument was used to perform quality assessment for the RCTs. All the observational studies were assessed using the adapted methodological items for non-randomized studies (MINORS) (18). Strictly according to the scoring criteria, two reviewers (SZ and S-HX) assessed the quality of two types of studies independently. A third reviewer (X-XZ or Z-FG) was consulted when disagreements arose.
Extraction
The data were extracted from the selected studies by two researchers (SZ and S-HX) independently. When disagreements arose, a third researcher (YB or Y-WQ) was consulted. The extracted data were as follows: first author’s name, basic characteristics, LAAC device, the type of anti-thrombotic therapy, the follow-up date and protocol and the rate and diagnosis time of DRT, stroke and SE. In addition, in case of multiple studies from the same pool of patients, we will include the most recent study only (19).
Statistical analysis
We combined of each article using standard meta-analytic methods to estimate overall incidence of DRT and compared the incidence of both stroke and SE in DRT patients with non-DRT patients (defined as patients received LAAC without the occurrence of DRT). Revman (Version 5.4, The Cochrane Collaboration, London, UK) and Statistical Product and Service Solutions (SPSS) were used to analyze available data. For the incidence of DRT, stroke and SE each eligible studies, pooled estimates of odds ratio with 95% confidence interval (CI) were calculated (20, 21). The mean difference (MD) and 95% CI were used to estimate the basic characteristics of patients. Chi-square and I-square tests were used for statistical heterogeneity measurement between eligible studies. A p-value < 0.05 was considered to be statistically significant. A fixed-effects model was used if significant heterogeneity (I2 < 50%) was found among those studies. Otherwise, a random-effect model was used (22). Sensitivity analysis was performed to investigate potential source of inconsistency by including multicenter registries (MCR) and randomized controlled studies (RCT) only. Funnel plot and Begg’s test were used to access publication bias (Supplementary Figure 1). Furthermore, to find out the potential predictor of DRT, eligible studies with DRT predictors [effect estimate with risk ratio (RR)] were included in secondary analysis. Separate meta-analyses were conducted for predictor variables that were reported in at least three different studies (23). When two or more RR were present per predictor, the pooled RRs with 95% CI were computed using random model (24).
Results
Search results and quality assessment
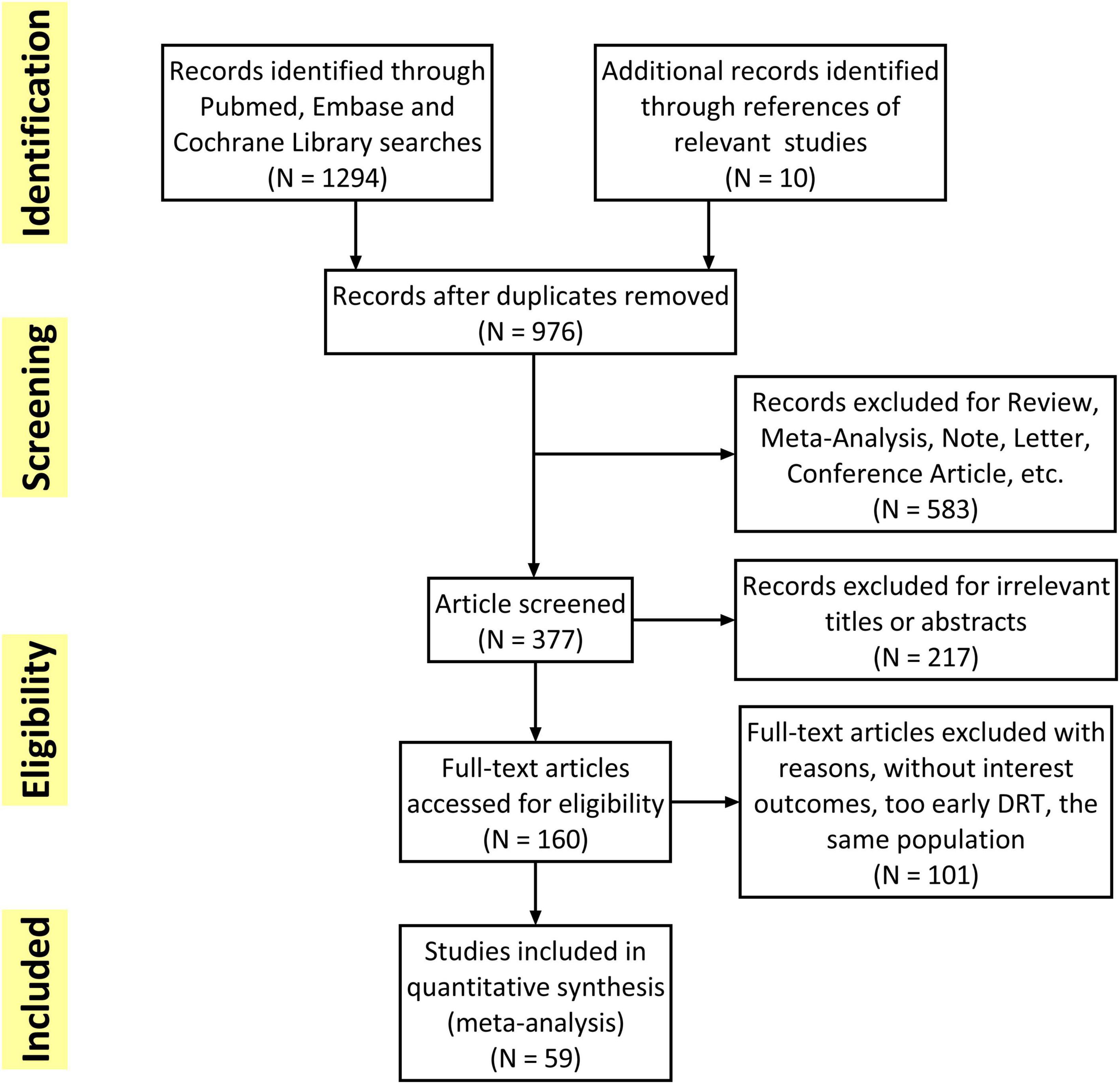
The flowchart of the study selection was presented in Figure 1. Overall, 1,304 studies were initially retrieved and a total of 59 studies met the inclusion criteria and were finally included. Subsequently, 101 studies were excluded because of without interest outcomes (DRT, stroke or SE), too early DRT (detection in less than 3 months) and unacceptable time for imaging follow-up. The quality of all included studies was shown in Supplementary Table 4. The mean MINORS score for the observational studies was 10.7 ± 1.50. The only one RCT (25) assessed by Cochrane ROB showed low risk in all eight items.
Study characteristics
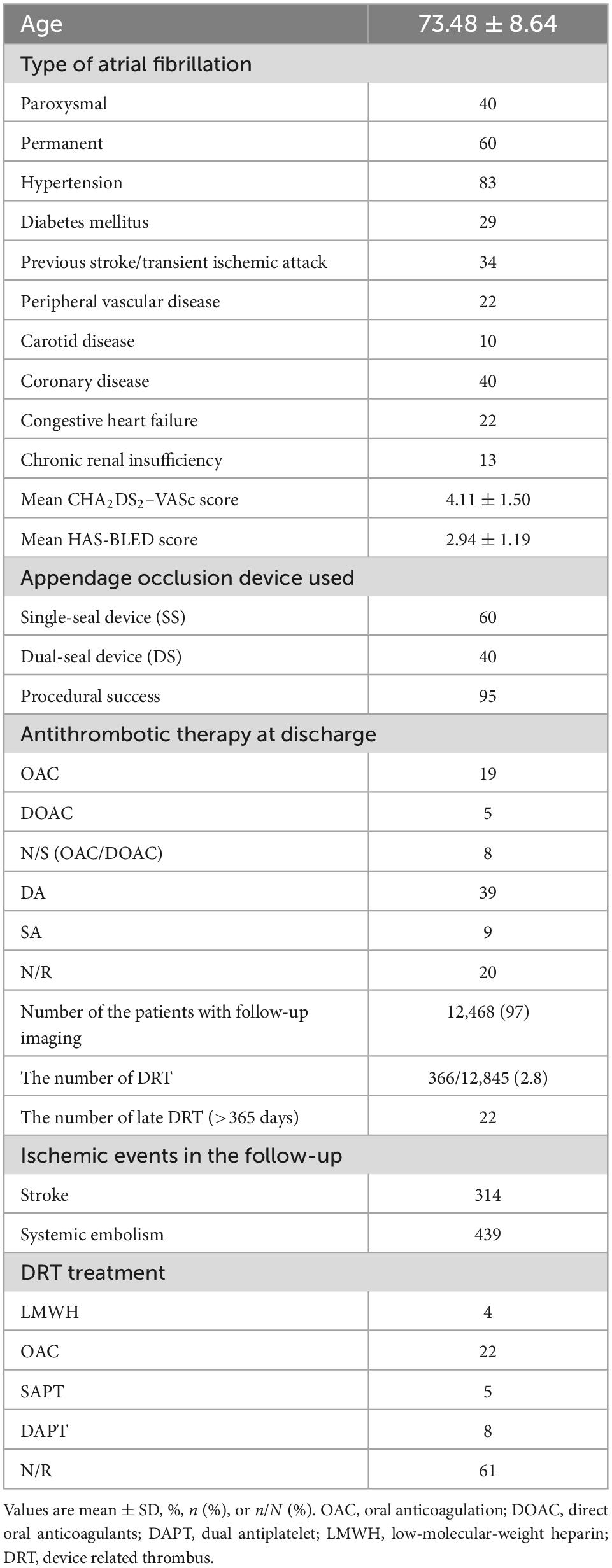
All the included studies were clinical trials with 6 months to several years follow-up. The eligible studies enrolled 12,845 patients with sample size ranging from 30 to 1,739. Most of the patients were elderly people (73.48 ± 8.64 years) and 60% of them had permanent AF. The mean CHA2DS2–VASc score, and HAS-BLED score was 4.11 ± 1.50 and 2.94 ± 1.19, respectively. About the device used in appendage occlusion, 60% patients used the SS device while the remaining 40% used DS device. The total procedural success rate among all the patients was 95%. Antithrombotic therapy at discharge was OAC in 19%, DOAC in 5%, single/dual antiplatelet (SAPT/DAPT) in 48%, and other/not reported (NR) in 28%. The pooled baseline data of patients in the included studies was summarized in Table 2. The detailed data of each eligible studies was summarized in Supplementary Table 1.
DRT and ischemic events
A total of 12,468 (97%) patients had at least once imaging follow-up for LACC device. The number of DRT, and late DRT was 366 and 22, respectively. Among all patients included in our study, the pooled incidence of DRT was 2.8 (366/12,845), with significant variation in the reported incidence (ranging from 0 to 11.3%; I2 = 64%) (Figure 2). In the follow-up period, 314 ischemic stroke events and 439 SE were detected. Studies which reported the specific number of ischemic events between DRT patients and non-DRT patients were included for additional analysis. In the studies that reported the incidence of stroke between DRT and non-DRT (26 studies, 7,827 patients), the pooled incidence of stroke was 11.5% in DRT patients and 2.9% among non-DRT patients (OR: 5.08; 95% CI = 3.47–7.44; p < 0.001; I2 = 21%) (Figure 3A). And in the studies that reported the incidence of SE (29 studies, 7,977 patients), the pooled incidence of SE was 15.0% and 3.4% among patients without DRT (OR: 5.40; 95% CI = 3.82–7.63; p < 0.001; I2 = 35%) (Figure 3B).
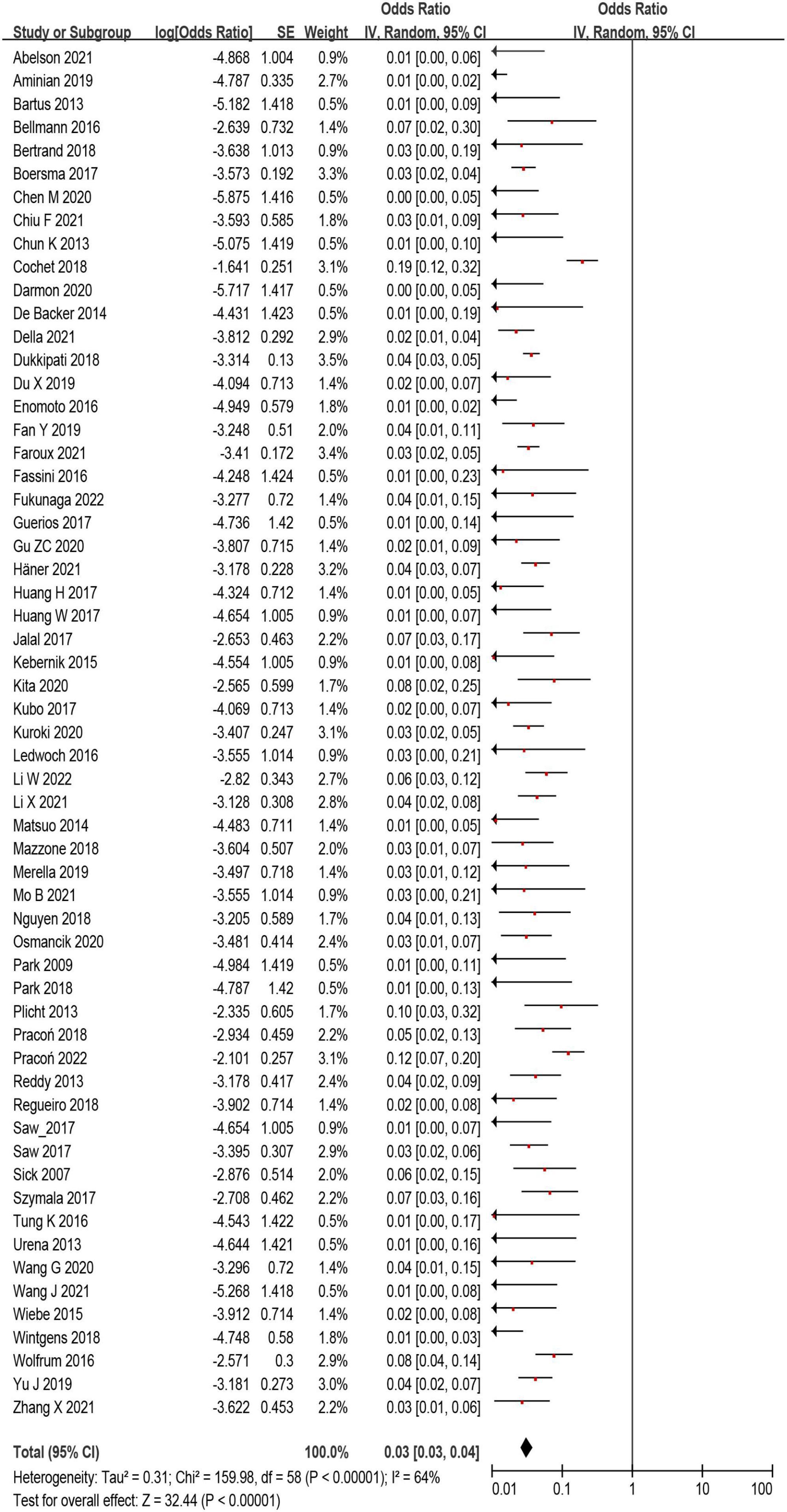
Figure 2. The forest plot of the device related thrombus (DRT) incidence after left atrial appendage closure (LAAC) of all eligible studies. The odds ratio represents the incidence of DRT.
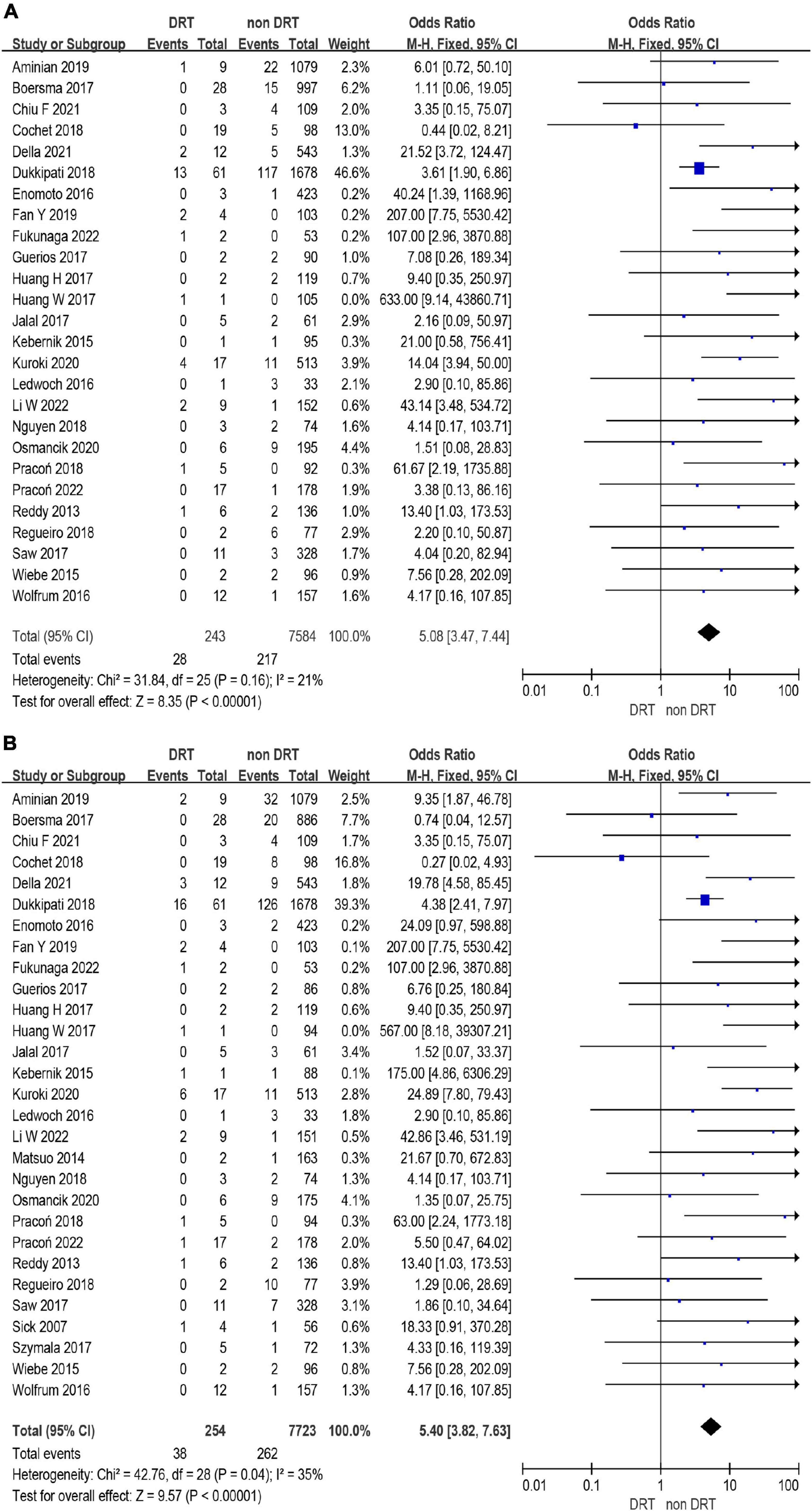
Figure 3. Forest plots of stroke (A) and systemic embolism (SE) (B) in patients with device related thrombus (DRT) and non-DRT.
Sensitivity analysis
In a sensitivity analysis, we conducted a meta-analysis to compare the incidence of ischemic events between DRT patients and non-DRT patients in MCR and RCT only. In these studies (25 studies, 9,320 patients), the pooled incidence of DRT was 2.8% (259 of 9,320) (Supplementary Figure 2). In the comparison of ischemic events in these studies, the DRT was associated with higher rate of stroke (12.1 vs. 3.2%; OR: 4.14; 95% CI = 2.69–6.38; p < 0.001; I2 = 24%) and SE (16.0 vs. 3.8%; OR: 4.48; 95% CI = 3.04–6.62; p < 0.001; I2 = 44%) (Supplementary Figures 3, 4).
Subgroup analysis
The incidence of DRT was not statistically different in patients who underwent LAAC using SS vs. DS device [171/6,190 (2.8%) vs. 78/3,023 (3.6%); p = 0.93] (Supplementary Tables 2, 3 and Figure 4). In the subgroup analysis, the incidence of stroke (p = 0.99) and SE (p = 0.98) associated DRT was not statistically different between SS and DS devices (Figure 5). And in the subgroup analysis based on anti-thrombotic therapy, the difference of the incidence of DRT was similar in patients who received OAC vs. APT (p = 0.21) (Supplementary Figure 5).
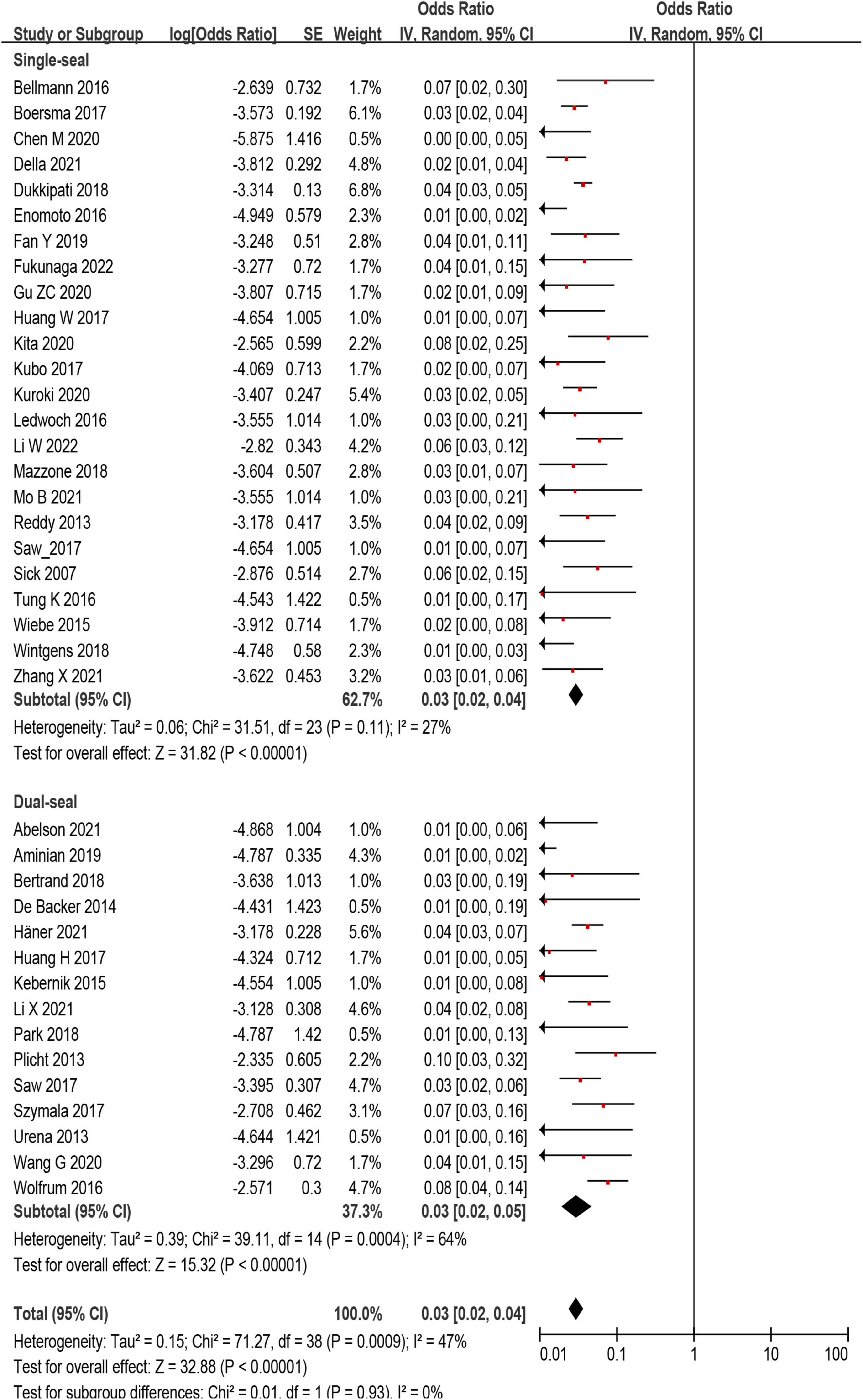
Figure 4. Forest plot comparing the incidence of device related thrombus (DRT) with subgroup analysis based on occlusion mechanism.
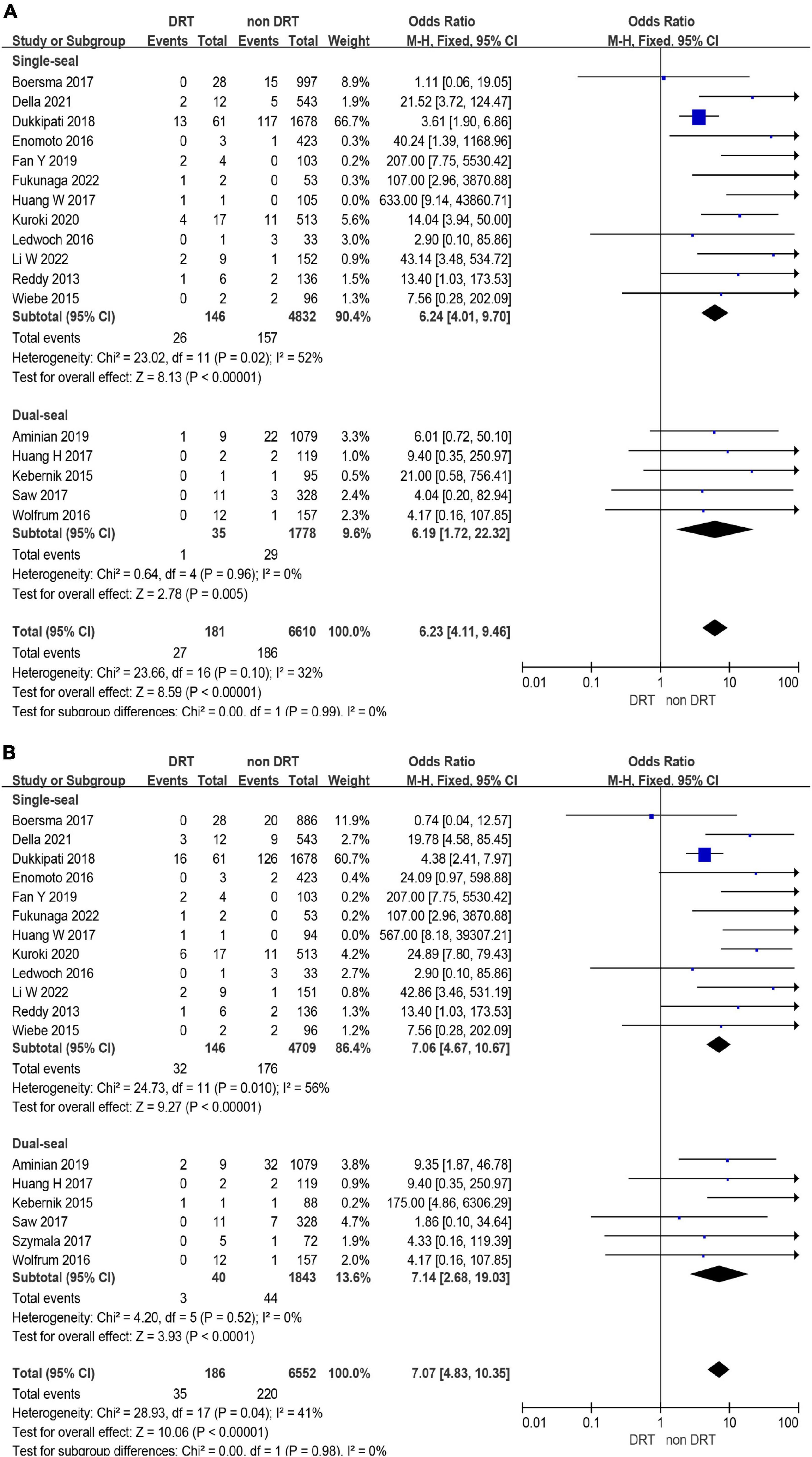
Figure 5. Forest plots of stroke (A) and systemic embolism (SE) (B) in device related thrombus (DRT) and non-DRT patients with subgroup analysis based on occlusion mechanism.
Resolution of DRT
Among all the patients diagnosed with DRT, 144 patients (39%) were reported using specific regimen to treat DRT. In these patients, 16 patients (4%) received low-molecular-weight heparin (LMWH), 81 patients (22%) received OAC, and 47 patients (13%) were treated as SAPT or DAPT. In the follow-up period, most of the DRT was resolved by drugs, while only few patients (<5%) needed surgical intervention.
Predictive and non-predictive factors of DRT
Among those eligible studies, five studies (9, 13, 26–28) investigating potential predictors of DRT were included. Five predictors were used in the meta-analysis, including age, smoking, history of stroke/TIA, hypertension and CHA2DS2–VASc score (Figure 6). Of the five predictors included in the current meta-analysis, history of stroke/TIA and CHA2DS2–VASc score remained statistically significant (Figures 6B, E), while the other three predictors (age, smoking, and hypertension) were non-predictive after effect size aggregation (Figures 6A, C, D). Only the meta-analysis of smoking predictor showed high heterogeneity.
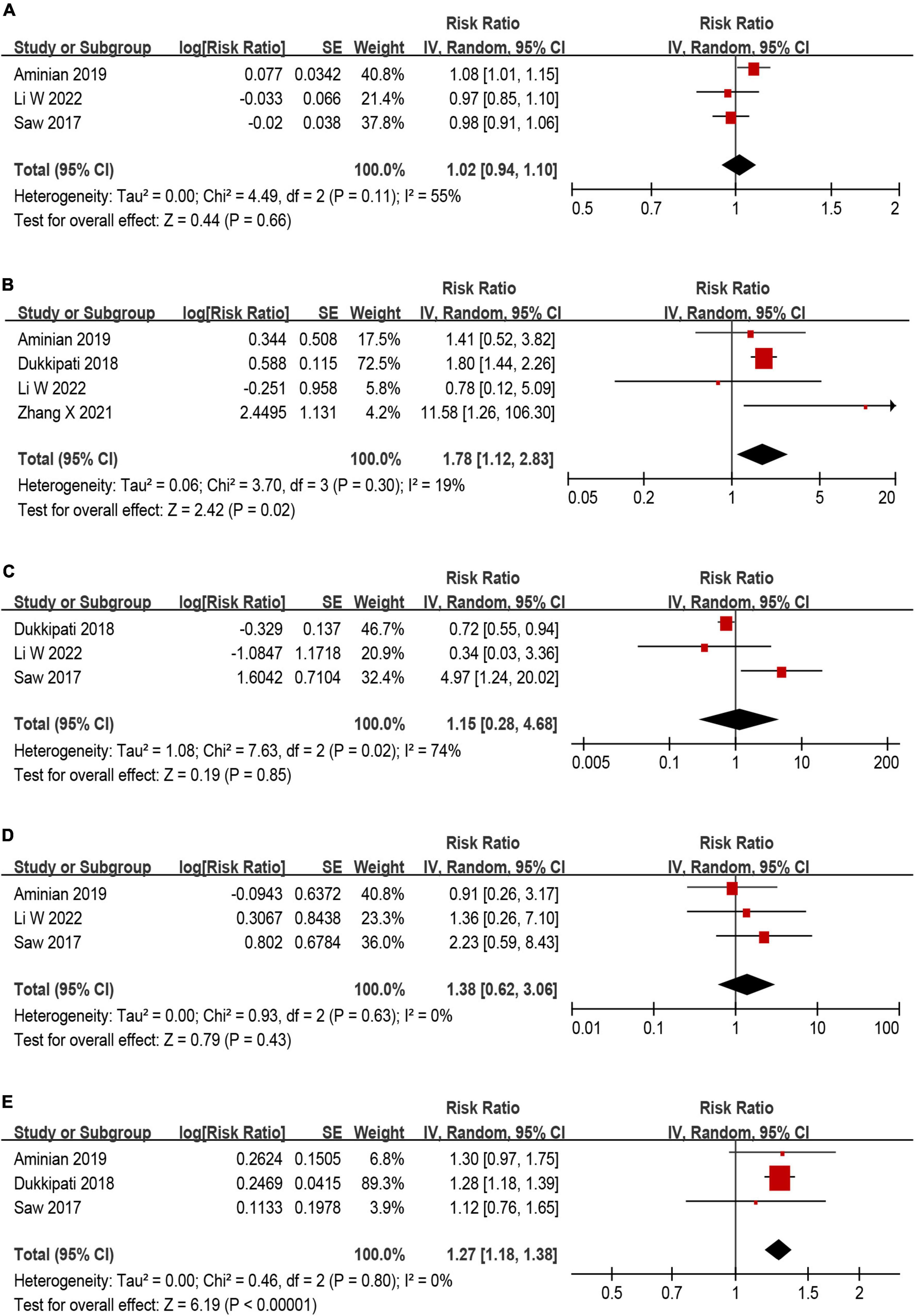
Figure 6. Forest plot of risk ratios (RR) for (A) age, (B) history of stroke/TIA, (C) smoking, (D) hypertension, and (E) CHA2DS2–VASc score as predictors for device related thrombus (DRT).
Discussion
We performed a systematic review to assess the incidence of DRT and whether detected DRT is associated with a significantly elevated risk of ischemic events. The clinical trials in our meta-analysis included more than 12,800 patients (largest-to-date) and have established a solid evidence base supports that DRT is associated with a fivefold increase in ischemic events. The incidence of DRT after LAAC in included studies was 2.8%, which is lower than previous meta-analysis describing the occurrence rate of DRT (9). In the subgroup analysis of anti-thrombotic therapy, the incidence of DRT was similar between patients received post-implant APT or OAC. While the subgroup analyses based on occlusion mechanism showed the incidence of DRT was not statistically different in patients who underwent LAAC using DS or SS device. And the elevated risk of ischemic events associated DRT was not related to occlusion mechanisms of the device.
On the basis of the early successes of PROTECT-AF and PREVAIL trial, LAAC became rapidly adopted as stroke preventive strategy (20). Several major complications have been reported including pericardial tamponade and device embolization (29). However, DRT remains a major concern, with an estimated incidence of 4% (range 0–16% in eligible studies) (12, 30). In previous studies, plenty studies set 45 days after LAAC for DRT detection (31–35). However, DRT requires a certain development time after device implantation. Therefore, in combination with the definition of DRT in multiple literatures, the occurrence time of DRT was defined as at least 75 days after LAAC in our study. Studies which DRT reported within 3 months after LAAC were not included. Additionally, our meta-analysis included 59 eligible studies enrolled 12,845 patients with sample size ranging from 30 to 1,739. Compared with the previous meta-analysis, our study has included more literature published in the past 3 years. Also, more strictive definition of occurrence time was used in our study to calculate the pooled incidence of DRT. And this increased the credibility of our DRT incidence results. Therefore, the major procedure-related complication incidence has improved over time and with increasing physician. Furtherly, improvements can be made to the current device to further reduce the incidence of DRT (WATCHMAN FLX vs. WATCHMAN 2.5; Amulet vs. ACP) (36).
Current published reports suggests that DRT is associated with a significantly elevated risk of ischemic events (9, 13, 26). A significant positive association between the incidence of DRT and subsequent ischemic events was also observed in our meta-analysis, documented approximately fivefold greater rates of ischemic events in patients with DRT compared with those without DRT. Among patients who developed DRT and stroke, majority of them were diagnosed with DRT prior to the occurrence of stroke. Although different clinicians did not have a uniform opinion on the relationship between DRT and stroke, the persistent signal of increased ischemic events warrants attention. However, current result together indicate that DRT after LAAC represents a significant danger in patients with AF who are already at high risk for stroke or TIA. Ding et al. (37) suggested that the high-risk AF patients have a high rate of mortality and an ongoing significant risk of bleeding and thrombotic events during follow-up. While Teiger et al. (38) concluded that LAAC in high-risk patients seems reasonable to decrease the rate of stroke. The limitations of the uncertain definition of DRT occurrence time reduce the credibility of their results. It is noteworthy that the thrombus development after LAAC is not clear since DRT is mostly silent and its follow-up is highly depended on imaging tools. As a result, it is difficult for clinicians to diagnose the DRT occurrence time precisely. It is important to detect them in a timely manner during follow up through appropriate diagnostic imaging techniques such as coronary angiography and trans-esophageal echocardiography. Our findings suggest that DRT is associated with more severe complications (stroke and SE), so early diagnosis and treatment of DRT may lead to better prognosis of patients. However, some studies have shown that anticoagulant therapy has an effect on the endothelialization process (39–41), and the occurrence of DRT is related to the incomplete endothelialization of device, so the early use of anticoagulant to treat DRT may hinder the completion of endothelialization. Recent studies indicated a reduced risk of DRT in patients receiving post-LAAC anticoagulation (42).
Within the last decade, several LAAC devices have been developed and introduced into clinical practice. The included clinical trials in our study also covered 3–4 types of devices. In our study, the devices are divided into two categories, one is SS including Watchman, FLX, Occlutect, and the other is DS including ACP, Amulet, LAmbre (16). The subgroup analyses showed that the incidence of DRT was not statistically different between SS and DS group, and the occurrence rate of stroke and SE associated DRT was similar between two groups. Based on these results, it seemed that the safety for LAAC was comparable between devices under different occlusion mechanisms. As more clinical trials comparing the clinical outcomes between SS and DS devices are underway, it is important to consider not only the baseline characteristics of the patient but also the differences in device mechanisms when formulating post-operative therapy.
Identifying predictors or risk factors of DRT is important for DRT prevention. As mentioned in the preceding text, the independent predictors of DRT occurrence remained unknown. In our current meta-analysis, the predictors of DRT were history of stroke/TIA and CHA2DS2–VASc score, while the conclusion among studies were inconsistent. Saw et al. (9) concluded that smoking and female sex were independent predictors of DRT, while Plicht et al. (10) suggested that predictors might be CHADS2 and CHA2DS2–VASc scores, platelet count, etc. In our previous real-world study, peri-device leak was considered as a risk factor of DRT (43). In recent two studies deep implantation depth was found to be a risk factor for DRT (7, 42). Though other predictors such as pericardial effusion and renal insufficiency were found, these predictors should be confirmed in larger studies (7). Until more data become available, researchers should raise more concern on the treatment of DRT, though the selection of treatment is still in debate. Simard et al. (44) concluded that 8–12 weeks Vitamin K antagonist (VKA), NOAC, and 2–4 weeks LMWH were recommended for treatment of DRT. Sedaghat et al. (45) revealed that patients underwent the three regimens mentioned above showed no statistical difference in clinical outcomes. Patients diagnosed with DRT may have persistent DRT or DRT recurrence after receiving anti-thrombotic therapies. Recent studies reported that only approximate a quarter of cases demonstrating persistent DRT presence in clinical follow-up (7, 45). And patients with persistent DRT showed higher stroke rates and increased mortality rates (45). And Asmarats et al. (46) firstly evaluated the recurrence of DRT, suggesting that thrombus recurrence was common, thus long-term OAC was encouraged after a first DRT. More specified anti-thrombotic regimens and more RCT are needed in prevention of DRT. Except for DRT, late DRT is thought to be less frequent because of device sealing, which remains variable on an individual basis. Furthermore, the collection of late DRT in this study is not comprehensive enough to carry out effective analysis. Consistent with our results, Sedaghat et al. (45) suggested that no clinical or echocardiographic predictors for late DRT formation could be identified in their study.
This meta-analysis has several limitations. First, because of limited randomized data, this meta-analysis included both randomized and observational studies. The observational studies are subjected to unmeasured confounding and selection bias. Therefore, consistent with previous meta-analysis of DRT (47), we conducted sensitivity analysis by including MCR and RCT only to test the inconsistency, which could verify the feasibility of the inclusion of multiple studies. Second, the definition of some clinical events such as major procedure-related complications was not unanimous across studies; however, it was less likely to have a huge impact on our final conclusion. Third, the follow-up duration in each study varied, which may have negative influence on outcomes. Last but not least, due to the paucity of individual data in each eligible study, no subgroup analysis was done according to different generations of SS and DS.
Conclusion
Device related thrombus is an infrequent complication of LAAC, associated with increased rates of ischemic events. The incidence of DRT was comparable between SS and DS devices. The occurrence rate of stroke and SE associated DRT was similar in devices with different occlusion mechanisms. Further large multicenter prospective studies are needed to confirm the true prevalence of DRT and to evaluate the risk factors, associated complications and treatment regimens.
Data availability statement
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
SZ: data curation (lead), formal analysis (equal), investigation (equal), methodology (equal), software (lead), validation (equal), visualization (equal), writing – original draft (equal), and writing – review and editing (equal). S-HX: data curation (equal), formal analysis (equal), investigation (equal), methodology (equal), validation (equal), visualization (equal), writing – original draft (equal), and writing – review and editing (equal). Y-GG: methodology (equal), software (lead), and writing – review and editing (equal). X-XZ and Z-FG: supervision (equal), investigation (equal), methodology (equal), and writing – review and editing (equal). Y-WQ: supervision (equal), investigation (equal), and writing – review and editing (equal). YB: conceptualization (lead), resources (lead), writing – original draft (equal), writing – review and editing (equal), supervision (lead), and taken responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the 234 Discipline Promotion Foundation (YB, grant number 2020YXK010) and NSFC cultivation project (YB, grant number 2021JCMS20) of Changhai Hospital, Shanghai, China.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.1088782/full#supplementary-material
References
1. Go A, Hylek E, Phillips K, Chang Y, Henault L, Selby J, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the anticoagulation and risk factors in atrial fibrillation (atria) study. JAMA. (2001) 285:2370–5. doi: 10.1001/jama.285.18.2370
2. Bencivenga L, Komici K, Nocella P, Grieco F, Spezzano A, Puzone B, et al. Atrial fibrillation in the elderly: a risk factor beyond stroke. Ageing Res Rev. (2020) 61:101092. doi: 10.1016/j.arr.2020.101092
3. Wolf P, Abbott R, Kannel W. Atrial fibrillation as an independent risk factor for stroke: the framingham study. Stroke. (1991) 22:983–8. doi: 10.1161/01.str.22.8.983
4. Wang T, Massaro J, Levy D, Vasan R, Wolf P, D’Agostino R, et al. A risk score for predicting stroke or death in individuals with new-onset atrial fibrillation in the community: the framingham heart study. JAMA. (2003) 290:1049–56. doi: 10.1001/jama.290.8.1049
5. Alkhouli M, Noseworthy P, Rihal C, Holmes DJr. Stroke prevention in non-valvular atrial fibrillation: a stakeholder perspective. J Am Coll Cardiol. (2018) 71:2790–801. doi: 10.1016/j.jacc.2018.04.013
6. Yu J, Bai Y, Jiang L. Device related thrombus after left atrial appendage closure: state of the art. Pacing Clin Electrophysiol. (2021) 44:1253–8. doi: 10.1111/pace.14122
7. Simard T, Jung R, Lehenbauer K, Piayda K, Pracoń R, Jackson G, et al. Predictors of device-related thrombus following percutaneous left atrial appendage occlusion. J Am Coll Cardiol. (2021) 78:297–313. doi: 10.1016/j.jacc.2021.04.098
8. Viles-Gonzalez J, Kar S, Douglas P, Dukkipati S, Feldman T, Horton R, et al. The clinical impact of incomplete left atrial appendage closure with the watchman device in patients with atrial fibrillation: a protect af (percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation) substudy. J Am Coll Cardiol. (2012) 59:923–9. doi: 10.1016/j.jacc.2011.11.028
9. Saw J, Tzikas A, Shakir S, Gafoor S, Omran H, Nielsen-Kudsk J, et al. Incidence and clinical impact of device-associated thrombus and peri-device leak following left atrial appendage closure with the amplatzer cardiac plug. JACC Cardiovasc Interv. (2017) 10:391–9. doi: 10.1016/j.jcin.2016.11.029
10. Plicht B, Konorza T, Kahlert P, Al-Rashid F, Kaelsch H, Jánosi R, et al. Risk factors for thrombus formation on the amplatzer cardiac plug after left atrial appendage occlusion. JACC Cardiovasc Interv. (2013) 6:606–13. doi: 10.1016/j.jcin.2013.02.014
11. Main M, Fan D, Reddy V, Holmes D, Gordon N, Coggins T, et al. Assessment of device-related thrombus and associated clinical outcomes with the watchman left atrial appendage closure device for embolic protection in patients with atrial fibrillation (from the protect-af trial). Am J Cardiol. (2016) 117:1127–34. doi: 10.1016/j.amjcard.2016.01.039
12. Cochet H, Iriart X, Sridi S, Camaioni C, Corneloup O, Montaudon M, et al. Left atrial appendage patency and device-related thrombus after percutaneous left atrial appendage occlusion: a computed tomography study. Eur Heart J Cardiovasc Imag. (2018) 19:1351–61. doi: 10.1093/ehjci/jey010
13. Dukkipati S, Kar S, Holmes D, Doshi S, Swarup V, Gibson D, et al. Device-related thrombus after left atrial appendage closure: incidence, predictors, and outcomes. Circulation. (2018) 138:874–85. doi: 10.1161/CIRCULATIONAHA.118.035090
14. Bertog S, Sievert H. Left atrial appendage closure: prevalence and risk of device-associated thrombus formation. Cardiovasc Diagn Ther. (2019) 9:104–9. doi: 10.21037/cdt.2018.10.05
15. Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (prisma-p) 2015 statement. Systematic Rev. (2015) 4:1. doi: 10.1186/2046-4053-4-1
16. Sievert K, Asmarats L, Arzamendi D. Left atrial appendage occlusion strengths and weaknesses of the lobe-only occluder concept in theory and in practice. Int Cardiol Clin. (2022) 11:195–203. doi: 10.1016/j.iccl.2021.11.004
17. Higgins J, Altman D, Gøtzsche P, Jüni P, Moher D, Oxman A, et al. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. (2011) 343:d5928. doi: 10.1136/bmj.d5928
18. Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surgery. (2003) 73:712–6. doi: 10.1046/j.1445-2197.2003.02748.x
19. Osman M, Busu T, Osman K, Khan S, Daniels M, Holmes D, et al. Short-term antiplatelet versus anticoagulant therapy after left atrial appendage occlusion: a systematic review and meta-analysis. JACC Clin Electrophysiol. (2020) 6:494–506. doi: 10.1016/j.jacep.2019.11.009
20. Reddy V, Doshi S, Kar S, Gibson D, Price M, Huber K, et al. 5-year outcomes after left atrial appendage closure: from the prevail and protect AF trials. J Am Coll Cardiol. (2017) 70:2964–75. doi: 10.1016/j.jacc.2017.10.021
21. Fauchier L, Cinaud A, Brigadeau F, Lepillier A, Pierre B, Abbey S, et al. Device-related thrombosis after percutaneous left atrial appendage occlusion for atrial fibrillation. J Am Coll Cardiol. (2018) 71:1528–36. doi: 10.1016/j.jacc.2018.01.076
22. Higgins J, Thompson S, Deeks J, Altman D. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
23. Tunvirachaisakul C, Gould R, Coulson M, Ward E, Reynolds G, Gathercole R, et al. Predictors of treatment outcome in depression in later life: a systematic review and meta-analysis. J Affect Disord. (2018) 227:164–82. doi: 10.1016/j.jad.2017.10.008
24. Kauw F, Takx R, de Jong H, Velthuis B, Kappelle L, Dankbaar J. Clinical and imaging predictors of recurrent ischemic stroke: a systematic review and meta-analysis. Cerebrovasc Dis. (2018) 45:279–87. doi: 10.1159/000490422
25. Osmancik P, Herman D, Neuzil P, Hala P, Taborsky M, Kala P, et al. Left atrial appendage closure versus direct oral anticoagulants in high-risk patients with atrial fibrillation. J Am Coll Cardiol. (2020) 75:3122–35. doi: 10.1016/j.jacc.2020.04.067
26. Aminian A, Schmidt B, Mazzone P, Berti S, Fischer S, Montorfano M, et al. Incidence, characterization, and clinical impact of device-related thrombus following left atrial appendage occlusion in the prospective global amplatzer amulet observational study. JACC. (2019) 12:1003–14. doi: 10.1016/j.jcin.2019.02.003
27. Li W, Gao R, Zhao J, Ren Y, Chen G, Zhu J, et al. Safety and efficacy of different anticoagulation regimens after left atrial appendage occlusion. Ann Palliat Med. (2022) 11:201–9. doi: 10.21037/apm-21-3654
28. Zhang X, Jin Q, Hu J, Kong D, Pan C, Chen D, et al. Impact of thrombocytopenia in patients with atrial fibrillation undergoing left atrial appendage occlusion: a propensity-matched comparison of 190 consecutive watchman implantations. Front Cardiovasc Med. (2021) 8:603501. doi: 10.3389/fcvm.2021.603501
29. Lempereur M, Aminian A, Freixa X, Gafoor S, Kefer J, Tzikas A, et al. Device-associated thrombus formation after left atrial appendage occlusion: a systematic review of events reported with the watchman, the amplatzer cardiac plug and the amulet. Catheter Cardiovasc Interv. (2017) 90:E111–21. doi: 10.1002/ccd.26903
30. Bartus K, Han F, Bednarek J, Myc J, Kapelak B, Sadowski J, et al. Percutaneous left atrial appendage suture ligation using the lariat device in patients with atrial fibrillation: initial clinical experience. J Am Coll Cardiol. (2013) 62:108–18. doi: 10.1016/j.jacc.2012.06.046
31. Meincke F, Schmidt-Salzmann M, Kreidel F, Kuck K, Bergmann M. New technical and anticoagulation aspects for left atrial appendage closure using the watchman® device in patients not taking warfarin. EuroIntervention. (2013) 9:463–8. doi: 10.4244/EIJV9I4A75
32. Galea R, De Marco F, Meneveau N, Aminian A, Anselme F, Gräni C, et al. Amulet or watchman device for percutaneous left atrial appendage closure: primary results of the swiss-apero randomized clinical trial. Circulation. (2022) 145:724–38. doi: 10.1161/CIRCULATIONAHA.121.057859
33. Chen Y, Zhang Y, Qu L, Huang W, Su X, Chen Y. Short-term non-vitamin k antagonist oral anticoagulants vs. warfarin in preventing device-related thrombosis after left atrial appendage closure. J Thromb Thrombolysis. (2021) 52:872–9. doi: 10.1007/s11239-021-02408-4
34. Alkhouli M, Du C, Killu A, Simard T, Noseworthy P, Friedman P, et al. Clinical impact of residual leaks following left atrial appendage occlusion: insights from the ncdr laao registry. JACC Clin Electrophysiol. (2022) 8:766–78. doi: 10.1016/j.jacep.2022.03.001
35. Søndergaard L, Wong Y, Reddy V, Boersma L, Bergmann M, Doshi S, et al. Propensity-matched comparison of oral anticoagulation versus antiplatelet therapy after left atrial appendage closure with watchman. JACC. (2019) 12:1055–63. doi: 10.1016/j.jcin.2019.04.004
36. Lakkireddy D, Thaler D, Ellis C, Swarup V, Sondergaard L, Carroll J, et al. Amplatzer amulet left atrial appendage occluder versus watchman device for stroke prophylaxis (amulet ide): a randomized. controlled trial. Circulation. (2021) 144:1543–52. doi: 10.1161/circulationaha.121.057063
37. Ding W, Lip G, Bartoletti S, Morrison L, Khalatbari A, Aggarwal S, et al. Long-term outcomes of left atrial appendage occlusion in high-risk atrial fibrillation patients: 4-year follow up data. J Thromb Thrombolysis. (2021) 51:1090–3. doi: 10.1007/s11239-020-02333-y
38. Teiger E, Thambo J, Defaye P, Hermida J, Abbey S, Klug D, et al. Percutaneous left atrial appendage closure is a reasonable option for patients with atrial fibrillation at high risk for cerebrovascular events. Circulation. (2018) 11:e005841. doi: 10.1161/CIRCINTERVENTIONS.117.005841
39. Achilles A, Mohring A, Dannenberg L, Grandoch M, Hohlfeld T, Fischer J, et al. Dabigatran enhances platelet reactivity and platelet thrombin receptor expression in patients with atrial fibrillation. J Thromb Haemost. (2017) 15:473–6. doi: 10.1111/jth.13595
40. Olivier C, Weik P, Meyer M, Weber S, Anto-Michel N, Diehl P, et al. Trap-induced platelet aggregation is enhanced in cardiovascular patients receiving dabigatran. Thromb Rese. (2016) 138:63–8. doi: 10.1016/j.thromres.2015.10.038
41. Vinholt P, Nielsen C, Söderström A, Brandes A, Nybo M. Dabigatran reduces thrombin-induced platelet aggregation and activation in a dose-dependent manner. J Thromb Thrombolysis. (2017) 44:216–22. doi: 10.1007/s11239-017-1512-2
42. Vij V, Piayda K, Nelles D, Gloekler S, Galea R, Fürholz M, et al. Clinical and echocardiographic risk factors for device-related thrombus after left atrial appendage closure: an analysis from the multicenter euroc-drt registry. Clin Res Cardiol. (2022) 111:1276–85. doi: 10.1007/s00392-022-02065-4
43. Bai Y, Xue X, Duenninger E, Muenzel M, Jiang L, Keil T, et al. Real-world survival data of device-related thrombus following left atrial appendage closure: 4-year experience from a single center. Heart Vessels. (2019) 34:1360–9. doi: 10.1007/s00380-019-01364-7
44. Simard T, Hibbert B, Alkhouli M, Abraham N, Holmes D Jr. Device-related thrombus following left atrial appendage occlusion. EuroIntervention. (2022) 18:224–32. doi: 10.4244/eij-d-21-01010
45. Sedaghat A, Vij V, Al-Kassou B, Gloekler S, Galea R, Fürholz M, et al. Device-related thrombus after left atrial appendage closure: data on thrombus characteristics, treatment strategies, and clinical outcomes from the euroc-drt-registry. Circulation. (2021) 14:e010195. doi: 10.1161/CIRCINTERVENTIONS.120.010195
46. Asmarats L, Cruz-González I, Nombela-Franco L, Arzamendi D, Peral V, Nietlispach F, et al. Recurrence of device-related thrombus after percutaneous left atrial appendage closure. Circulation. (2019) 140:1441–3. doi: 10.1161/circulationaha.119.040860
47. Alkhouli M, Busu T, Shah K, Osman M, Alqahtani F, Raybuck B. Incidence and clinical impact of device-related thrombus following percutaneous left atrial appendage occlusion: a meta-analysis. JACC Clin Electrophysiol. (2018) 4:1629–37. doi: 10.1016/j.jacep.2018.09.007
Keywords: atrial fibrillation, left atrial appendage closure, stroke, stroke prevention, thromboembolism
Citation: Zhang S, Xiong S-h, Guan Y-g, Zhao X-x, Qin Y-w, Guo Z-f and Bai Y (2022) An updated meta-analysis of device related thrombus following left atrial appendage closure in patients with atrial fibrillation. Front. Cardiovasc. Med. 9:1088782. doi: 10.3389/fcvm.2022.1088782
Received: 03 November 2022; Accepted: 06 December 2022;
Published: 23 December 2022.
Edited by:
Philippe P. M. Garot, Institut Cardiovasculaire Paris Sud (ICPS), FranceReviewed by:
Adel Aminian, Centre Hospitalier Universitaire de Charleroi, BelgiumXavier Freixa, Hospital Clinic of Barcelona, Spain
Copyright © 2022 Zhang, Xiong, Guan, Zhao, Qin, Guo and Bai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuan Bai, ✉ eXVhbmJhaUBzbW11LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
 Song Zhang
Song Zhang Si-huai Xiong
Si-huai Xiong Yu-gen Guan
Yu-gen Guan Yuan Bai
Yuan Bai