Effect of Alirocumab on Coronary Calcification in Patients With Coronary Artery Disease
- 1School of Medicine, Case Western Reserve University, Cleveland, OH, United States
- 2University Hospitals Cleveland Medical Center, Cleveland, OH, United States
- 3Center for Cardiovascular Prevention, Harrington Heart and Vascular Institute, Cleveland, OH, United States
Genetic and epidemiologic studies have demonstrated that elevated serum levels of Lp(a) increase risk for atherosclerotic vascular disease (ASCVD), with guidelines suggesting that levels ≥50 mg/dL or ≥125 nmol/L be used as a risk-enhancing factor when adjudicating ASCVD risk (1). However, several obstacles have limited routine Lp(a) assessment and result application in clinical practice. These include the lack of a standardized quantification method, variable expression among different races and ethnicities, and the lack of clear guidance when to treat along with limited therapeutic options when an elevated test result is received. There is a need for a more targeted approach that goes beyond contemporary risk stratification paradigms with the goal to be more aggressive at implementing known risk reducing therapies, especially among younger individuals with elevated risk [e.g., high Lp(a) and family history of ASCVD] who might not otherwise meet standard indications for statin and other therapies. One strategy would be to link Lp(a) testing with an established tool for assessing ASCVD risk, known to improve risk discrimination. Coronary artery calcium scoring (CAC) is an ideal candidate given its increasingly prevalent use, previously shown association with Lp(a), and the potential benefits of a dual risk stratification system (2–5).
To better understand the potential utility of CAC and Lp(a) for ASCVD risk, it is important to characterize the individual and joint associations that CAC and Lp(a) have with ASCVD incidence in diverse populations (Table 1). Given the varying pathophysiology between CAC and Lp(a), each test may contribute independent, and potentially synergistic, information for risk assessment. Lp(a) promotes atherogenesis and thrombosis with levels genetically determined and unaffected by diet, exercise, and use of statins. Conversely, CAC is a measure of atheroma calcium content that correlates with plaque burden and is directly related to traditional atherosclerotic risk factors such as blood pressure, weight, and serum cholesterols. In a study of the MESA and DHS cohorts, Mehta et al. (6) recently showed that elevated Lp(a) level and CAC score were each independently associated with ASCVD incidence. Moreover, among participants with elevated CAC (i.e., ≥100 AU), the top Lp(a) quintile (as stratified by race) was associated with an ASCVD event rate of >20% while the same elevated Lp(a) levels had ASCVD event rate of < 10% when coupled with CAC < 100. This was following adjustments for traditional risk factors and family history of heart disease (6). This suggests one of two possibilities. First is that CAC scoring can help inform care among patients with elevated Lp(a). Alternatively, elevated Lp(a) further potentiates elevated risk due to a high CAC score. This reinforces the current standard of practice where Lp(a) levels are adjudicated in individuals with strong predisposition for atherosclerotic disease. This includes those with strong family history of cardiovascular disease, repeat events despite appropriate therapy, and following a premature atherosclerotic event. Importantly, the association of Lp(a) with CAC is corroborated by additional studies illustrating a positive correlation (7). In an analysis of German participants without known coronary artery disease, Lp(a) levels and CAC were significantly associated despite adjusting for risk factors such as age, sex, smoking, BMI, lipids, diabetes, antihypertensive medication and lipid-lowering medication. However, it is noteworthy in this study that genetic variants responsible for elevated Lp(a) levels were not associated with CAC scores. This finding supports an independent pathophysiology for Lp(a) such that the burden of calcific disease is not necessarily indicative of long-term exposure to the oxidative and atherothrombotic properties of Lp(a) (8). Further evidence of heterogeneity of effects between CAC and Lp(a) come from a study by Qasim et al. that demonstrated Lp(a) to be a strong predictor of CAC in women with type 2 diabetes but not in men or those without diabetes (9). Explanations for this finding could include a cardioprotective effect of decreased estrogen levels in the postmenopausal female cohort studied; alternatively, Lp(a) function and atherogenicity may be modified by glycation in the milieu of diabetes. Nevertheless, the aforementioned studies did not explore rates of ASCVD incidence and therefore make it challenging to infer meaningful information to help guide clinical management. As such, outcomes driven data are required to fully understand the impact of joint Lp(a) and CAC testing on ASCVD events.
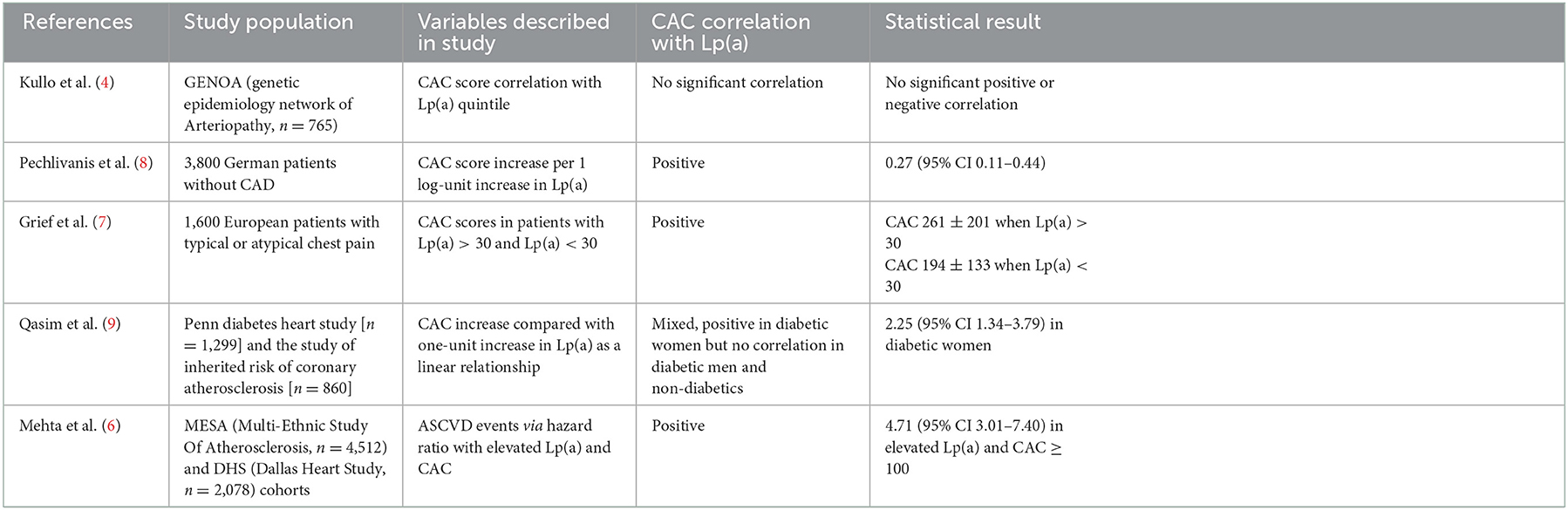
Table 1. Summary of contemporary evidence for the relationship between lipoprotein(a) and coronary artery calcium.
Further research is needed to define a potential role for joint testing of CAC and Lp(a) and how these tests could be selectively utilized in clinical practice to maximize the risk/benefit ratio and define a high-risk individual for aggressive preventive therapies. Based on the current evidence, it could be argued that there is less utility in pursuing Lp(a) levels without prior evidence of atherosclerotic burden. Alternatively, it raises questions as to whether CAC scoring should precede Lp(a) levels, acting as a gateway to Lp(a) testing, given the high likelihood that an elevated Lp(a) result in that context would prompt more aggressive preventive therapy. To address this gap in knowledge and practice, a prospective study is required to potentially expand our current indications for primary prevention. In particular, we must assess the ASCVD event rate for subjects with Lp(a) cutoff >50 mg/dL and CAC >100 who are without other common risk factors for atherosclerotic disease (i.e., hypertension, tobacco use, etc.). Additionally, with the advent of novel Lp(a) lowering therapies poised to hit the market soon, should there be consideration to add a Lp(a) lowering therapy in addition to statins in individuals with elevated CAC and Lp(a) given the high-risk phenotype and the lack of response of Lp(a) levels to conventional statin therapy? At this time, we lack medications that are proven to lower Lp(a) and provide more favorable cardiovascular outcomes. Niacin, for instance, reduces lipoprotein(a) serum levels by approximately 20% but does not reduce death or ischemic cardiovascular events (10, 11). Additionally, in a post-hoc analysis of the FOURIER study evaluating PCSK9 inhibition (PCSK9i), PCSK9i-treated groups incurred an estimated 20 mg/dL absolute reduction in Lp(a) associated with a 10% relative risk reduction in MACE (12). Nevertheless, we have not completed prospective clinical studies to corroborate these findings. For now, the cardiovascular community eagerly awaits the results of ongoing trials (i.e., HORIZON) investigating the impact of dedicated Lp(a) lowering compounds on Lp(a) and ASCVD events, and the potential role CAC scoring may have in defining the ideal patient for these novel therapies.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/ APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. (2019) 73:e285–e350. doi: 10.1016/j.jacc.2018.11.003
2. Garg PK, Guan W, Karger AB, Steffen BT, Budoff M, Tsai MY. Lipoprotein (a) and risk for calcification of the coronary arteries, mitral valve, and thoracic aorta: the Multi-Ethnic Study of Atherosclerosis. J Cardiovasc Comput Tomogr. (2021) 15:154–60. doi: 10.1016/j.jcct.2020.06.002
3. Guerra R, Yu Z, Marcovina S, Peshock R, Cohen JC, Hobbs HH. Lipoprotein (a) and apolipo-protein (a) isoforms: no association with coronary artery calcification in the Dallas Heart Study. Circulation. (2005) 111:1471–9. doi: 10.1161/01.CIR.0000159263.50305.BD
4. Kullo IJ, Bailey KR, Bielak LF, Sheedy PF, Klee GG, Kardia SL, et al. Lack of association between lipoprotein(a) and coronary artery calcification in the Genetic Epidemiology Network of Arteriopathy (GENOA) study. Mayo Clin Proc. (2004) 79:1258–63. doi: 10.4065/79.10.1258
5. Cainzos-Achirica M, Bittencourt MS, Osei AD, Haque W, Bhatt DL, Blumenthal RS, et al. Coronary artery calcium to improve the efficiency of randomized controlled trials in primary cardiovascular prevention. J Am Coll Cardiol Img. (2021) 14:1005–16. doi: 10.1016/j.jcmg.2020.10.016
6. Mehta A, Vasquez N, Ayers CR, Patel J, Hooda A, Khera A, et al. Independent association of lipoprotein(a) and coronary artery calcificationp with atherosclerotic cardiovascular risk. J Am Coll Cardiol. (2022) 79:757–68. doi: 10.1016/j.jacc.2021.11.058
7. Greif M, Arnoldt T, von Ziegler F, Ruemmler J, Becker C, Wakili R, et al. Lipoprotein (a) is independently correlated with coronary artery calcification. Eur J Intern Med. (2013) 24:75–9. doi: 10.1016/j.ejim.2012.08.014
8. Pechlivanis S, Mahabadi AA, Hoffmann P, Nöthen MM, Broecker-Preuss M, Erbel R, et al. Association between lipoprotein(a) (Lp(a)) levels and Lp(a) genetic variants with coronary artery calcification. BMC Med Genet. (2020) 21:62. doi: 10.1186/s12881-020-01003-3
9. Qasim AN, Martin SS, Mehta NN, Wolfe ML, Park J, Schwartz S, et al. Lipoprotein(a) is strongly associated with coronary artery calcification in type-2 diabetic women. Int J Cardiol. (2011) 150:17–21. doi: 10.1016/j.ijcard.2010.02.021
10. Landray MJ, Haynes R, Hopewell JC, Parish S, Aung T, Tomson J, et al. Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med. (2014) 371:203–12. doi: 10.1056/NEJMoa1300955
11. Boden WE, Probstfield JL, Anderson T, Chaitman BR, Desvignes-Nickens P, Koprowicz K, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. (2011) 365:2255–67. doi: 10.1056/NEJMoa1107579
Keywords: coronary artery calcification (CAC), lipoprotein(a), atherosclerotic vascular disease, risk stratification, prevention
Citation: Naami R, Miller DM, Al-Kindi S and Neeland IJ (2022) Coronary artery calcium scoring as a tool for risk stratification in patients with an elevated lipoprotein(a) level. Front. Cardiovasc. Med. 9:1084814. doi: 10.3389/fcvm.2022.1084814
Received: 31 October 2022; Accepted: 02 December 2022;
Published: 22 December 2022.
Edited by:
Helena B. Thomaides-Brears, Perspectum Diagnostics, United KingdomReviewed by:
Preethi Mani, University of Texas Southwestern, United StatesCopyright © 2022 Naami, Miller, Al-Kindi and Neeland. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ian J. Neeland,  SWFuLk5lZWxhbmQmI3gwMDA0MDtVSGhvc3BpdGFscy5vcmc=
SWFuLk5lZWxhbmQmI3gwMDA0MDtVSGhvc3BpdGFscy5vcmc=
 Robert Naami
Robert Naami Drew M. Miller2
Drew M. Miller2