- 1Department of Paediatric and Congenital Cardiology, M3C Regional Reference Centre, University Hospital, Montpellier, France
- 2PhyMedExp, Univ Montpellier, INSERM, French National Centre for Scientific Research (CNRS), Montpellier, France
- 3Department of Paediatric and Adult Congenital Cardiology, M3C National Reference Centre, Bordeaux University Hospital, Pessac, France
- 4Department of Physiology, University Hospital of Montpellier, Montpellier, France
- 5Paediatric Cardiology and Rehabilitation Unit, Saint-Pierre Institute, Palavas-Les-Flots, France
- 6Inserm, U1045, Institut Hospitalo-Universitaire (IHU) Liryc, Bordeaux Cardio-Thoracic Research Centre, Electrophysiology and Heart Modelling Institute, University of Bordeaux, Pessac, France
Background: In children with congenital long QT syndrome (LQTS), the risk of arrhythmic events during exercise commonly makes it difficult to balance exercise restrictions versus promotion of physical activity. Nevertheless, in children with LQTS, cardiorespiratory fitness, muscle fitness, and physical activity, have been scarcely explored.
Materials and methods: In this prospective, controlled, cross-sectional study, 20 children with LQTS (12.7 ± 3.7 years old) and 20 healthy controls (11.9 ± 2.4 years old) were enrolled. All participants underwent a cardiopulmonary exercise test, a muscular architecture ultrasound assessment, (cross-sectional area on right rectus femoris and pennation angle), a handgrip muscular strength evaluation, and a standing long broad jump test. The level of physical activity was determined using with a waist-worn tri-axial accelerometer (Actigraph GT3X).
Results: Peak oxygen uptake (VO2peak) and ventilatory anaerobic threshold (VAT) were lower in children with LQTS than in healthy controls (33.9 ± 6.2 mL/Kg/min vs. 40.1 ± 6.6 mL/Kg/min, P = 0.010; 23.8 ± 5.1 mL/Kg/min vs. 28.8 ± 5.5 mL/Kg/min, P = 0.007, respectively). Children with LQTS had lower standing long broad jump distance (119.5 ± 33.2 cm vs. 147.3 ± 36.1 cm, P = 0.02) and pennation angle (12.2 ± 2.4° vs. 14.3 ± 2.8°, P = 0.02). No differences in terms of moderate-to-vigorous physical activity were observed (36.9 ± 12.9 min/day vs. 41.5 ± 18.7 min/day, P = 0.66), but nearly all children were below the WHO guidelines.
Conclusion: Despite similar physical activity level, cardiorespiratory fitness and muscle fitness in children with LQTS were lower than in healthy controls. The origin of this limitation seemed to be multifactorial, involving beta-blocker induced chronotropic limitation, physical and muscle deconditioning. Cardiovascular rehabilitation could be of interest in children with LQTS with significant physical limitation.
1. Introduction
Habits and beliefs about physical activity in the general population are frequently not in line with the guidelines and recommendations (1). In pediatrics, according to the recent guidelines on physical activity from the World Health Organization (WHO), children aged 5 to 17 years old should perform at least 60 min of moderate-to-vigorous physical activity, daily (2, 3). Despite the physiological and psychological health benefits related to physical activity across the lifespan (4, 5), nearly 80% of children does not meet these recommendations (6). This is particularly true for children with chronic diseases, for which exercise intolerance and physical inactivity (7) contribute to increase cardiovascular risk during adulthood (8, 9). Cardiorespiratory fitness reflects children’s global health and stands as an independent predictor of all-cause mortality (10).
In the wide spectrum of pediatric chronic diseases, children with cardiac diseases are particularly affected by physical inactivity, as a consequence of their underlying cardiac condition, but also of parental overprotection and social barriers to sports practice, which negatively affect health-related quality of life (11, 12). Similarly, in children with inherited cardiac arrhythmia, such as long QT syndrome (LQTS) (13, 14), the risk of arrhythmic events during exercise commonly makes it difficult to balance exercise restrictions versus promotion of physical activity (15–19). However, the association between exercise and sudden cardiac death in LQTS remains unclear (20, 21).
In the past two decades, the guidelines on sports participation in inherited cardiac disorders have become progressively less restrictive (22–25) and the concept of shared-decision making, involving patients, families, and physicians, has recently emerged to promote physical activity, even in patients with LQTS (22). These guidelines are not primarily dedicated to pediatric patients, and physical fitness in children with LQTS has been scarcely explored.
In this study, we aimed to evaluate cardiorespiratory fitness, muscle fitness (strength and architecture), and physical activity, in children with LQTS, in comparison with healthy controls.
2. Materials and methods
2.1. Study design and population
This prospective controlled cross-sectional study was carried out from February to June 2021 in two pediatric cardiology tertiary care hospitals: the Pediatric and Congenital Cardiology Department of Montpellier University Hospital, France; and the Pediatric Cardiology and Rehabilitation Unit of the St-Pierre Institute in Palavas-Les-Flots, France.
Children aged between 6 and 18 years old were screened consecutively during a regular pediatric cardiology outpatient visit. Two groups were identified:
1. The LQTS group consisted of children diagnosed with congenital long QT syndrome, which is characterized by QT prolongation in repeated 12-lead electrocardiogram (ECG), and/or genetic mutation in children screened for known causal familial LQTS (26).
2. The control group consisted of children referred for a non-severe functional symptom (cardiac murmur, chest pain). These children were classified in the control group only after a comprehensive cardiac evaluation which revealed no cardiopulmonary abnormalities, including physical examination, ECG, and echocardiography. Children with any chronic disease, medical condition (cardiac, neurological, respiratory, muscular, or renal), or medical treatment and those requiring any further specialized medical consultation were not eligible.
Children with absolute contraindications for cardiopulmonary exercise test (CPET) were not eligible: fever, uncontrolled asthma, respiratory failure, acute myocarditis or pericarditis, uncontrolled arrhythmias causing symptoms or hemodynamic compromise, uncontrolled heart failure, acute pulmonary embolus or pulmonary infarction, and children with intellectual or developmental disability which impaired their ability to complete the exercise protocol.
2.2. Physical fitness
Two main components of physical fitness were evaluated: (1) cardiorespiratory fitness, and (2) muscle fitness (27).
2.2.1. Cardiorespiratory fitness
Children from both groups underwent a CPET, using a pediatric cycle ergometer protocol adapted to children to obtain a homogeneous incremental overall duration between 8 and 12 min, as previously described by our group (28). CPET procedures in both centers were harmonized before the study started. We used the Quark CPET calibrated gas analyzer (Cosmed Srl., Pavonna di Albano, Italy). The following CPET parameters were measured: peak oxygen uptake (VO2peak), peak heart rate (HRpeak) ventilatory anaerobic threshold (VAT), ventilatory efficiency (VE/VCO2 slope), oxygen pulse (VO2/HR), maximal power, respiratory exchange ratio (RER), respiratory reserve (RR). Spirometry was systematically performed before the exercise test, to measure forced expiratory volume in 1 s (FEV1), forced vital capacity (FVC), and the FEV1/FVC ratio. The CPET was considered as maximal when the following four criteria were reached: respiratory exchange ratio (RER = VCO2/VO2) ≧ 1.1, and limit of the child’s tolerance despite verbal encouragement. VO2peak values were normalized as percentage of the predicted VO2peak. A single investigator manually calculated the VO2peak and the VAT using Beaver’s method (29). VO2peak and VAT values were normalized in a percentage of the predicted maximum oxygen uptake using Cooper’s pediatric reference values (30). A VO2peak value below 80% and/or a VAT value below 55% of predicted VO2peak were indicative of physical deconditioning, in reference to reported values in adults and children (31).
2.2.2. Muscle fitness
Muscle fitness was determined by evaluating muscle architecture and muscle strength (32).
2.2.2.1. Muscle architecture
Muscle architecture reflects the contractile properties of muscle and is a determinant of strength capacity (33). It was evaluated by muscular ultrasound technique (34), the patient being in dorsal decubitus, with legs and arms relaxed, feet in neutral position, and arm in supination. Analyses of cross-sectional area on rectus femoris showed good feasibility and reliability (35). We measured two parameters: (1) the anatomical cross-sectional area on right rectus femoris, defined as the area of the cross-section of the muscle perpendicular to its longitudinal axis; (2) the pennation angle of vastus lateralis (Figure 1). Pennation angle was defined as the angle formed between muscular fascicles and intramuscular tendon insertion. A greater pennation angle enables more myofibrillar packing and promotes fascicle rotation during dynamic contraction strength, increasing muscle cross-sectional area and strength (36, 37). Thus, a lesser pennation angle may suggest reduced strength capacity (38). Five measures were performed for each one of the two parameters by a single operator. Minimal and maximal values were excluded, and the mean of the three remaining values was calculated. Image J software was used for image analysis. Muscular ultrasound examinations were performed using the EPIQ CVx (Philips®, Andover, MA, USA), and the Vivid E95 (General Electric®, New York, NY, USA).
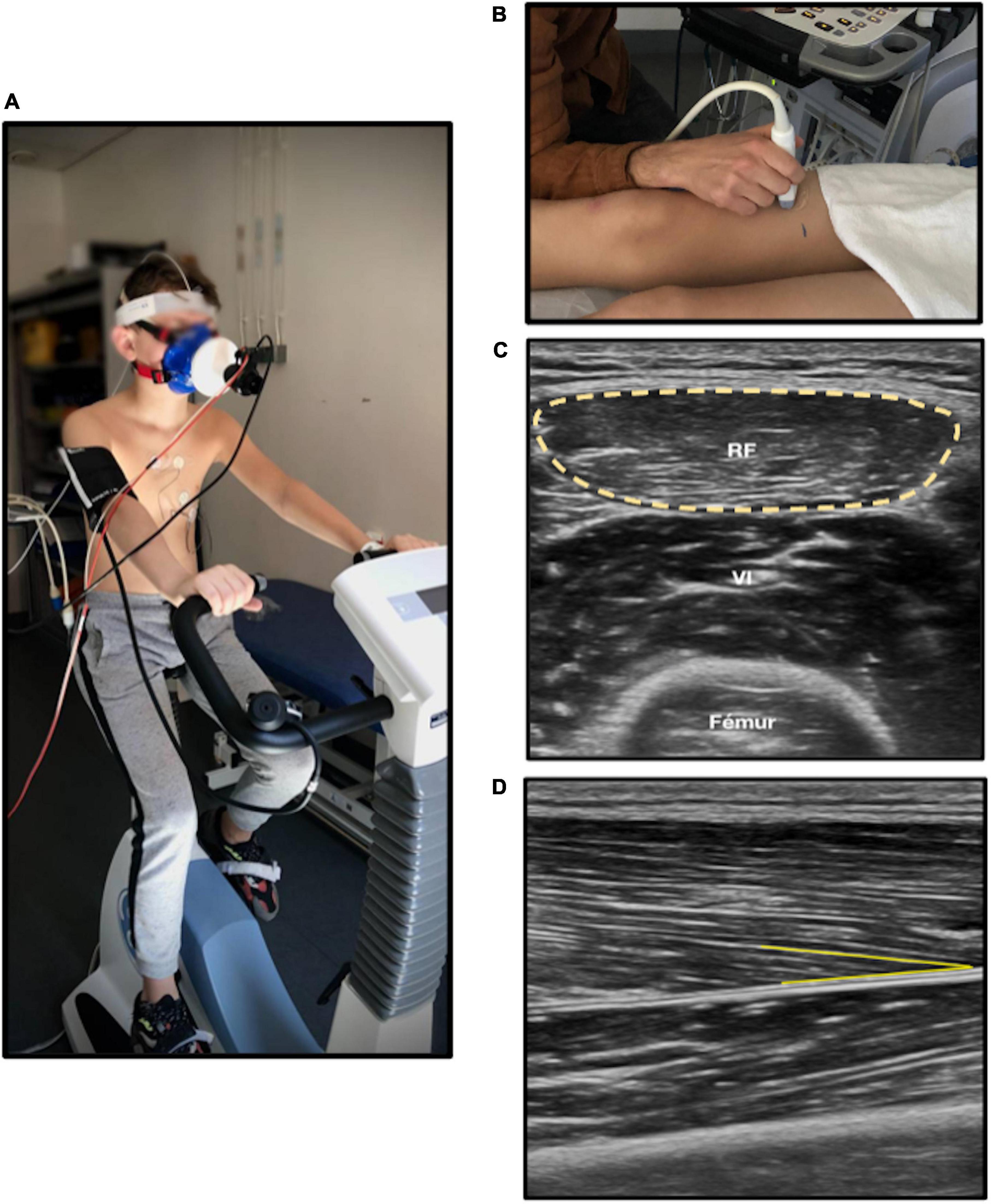
Figure 1. Cardiorespiratory and muscle fitness analysis. (A) Cardiopulmonary exercise test on ergocycle. (B) Muscular ultrasound on right leg. (C) Anatomical cross-sectional area (yellow circle) of rectus femoris measured with ultrasound. (D) Pennation angle (yellow line) of right vastus lateralis; RF, Rectus femoris. The chosen anatomic site was at two third of the length from iliac spine anterior superior to upper edge of the patella on right leg. The probe was put in transversal plane and longitudinal plane for cross-sectional area and pennation angle, respectively.
2.2.2.2. Muscle strength
Muscle strength was evaluated using a handgrip test to assess upper limb strength, and a standing long broad jump to assess lower limb strength. Those tests are time-efficient, costless, validated as a general index of muscular fitness, and easily administered in clinical pediatric setting (39, 40). Measurements of upper limb strength from the handgrip test were based on protocols used in pediatrics (41). Grip notch was adjusted based on participant’s comfort. The participant squeezed the handgrip, with their right hand, for 3 to 4 s, as hard as they could. The procedure was repeated three times, with 30 s of rest between each trial. The maximum value of these three trials was reported. During standing long broad jump, the child stood behind jumping line, with feet together, and pushed off vigorously forward as far as possible (39). After the jump, participants were asked to stay still, with their two feet on the ground. If they lost their balance or touched any object, they were asked to repeat the jump. The distance between the jumping line and heel landing was measured. Among the two trials, the longest jump distance was reported.
2.3. Level of physical activity
The level of physical activity was evaluated using a waist-worn tri-axial accelerometer (ActiGraph GT3X, Pensacola, FL, USA) (42). The time spent at moderate and vigorous physical intensity was measured by the accelerometer. The device was assigned during the inclusion visit and the participants were instructed to always wear it at the waist for 14 days, except during sleep and water-based activities such as swimming or bathing. We chose an e-poch of 15 s (filtered acceleration signal over a user-defined time sampling interval) and non-wearing period was calculated by Choi’s algorithm (43). We fixed intensity thresholds (counts/min) using Romanzini’s equations for young adolescents (11–17 years old) (44), and Evenson’s equations, for children aged from 6 to 9 years old (45). Counts per minute thresholds were used to determine the level of moderate-to-vigorous physical activity (MVPA), the level of moderate physical activity and the level of vigorous physical activity. The three mean values were compared between LQTS and controls groups. A wearing period of 10 h per day for at least 4 days was necessary for the analysis (46).
2.4. Statistical analysis
Participant’s characteristics were presented using mean and standard deviation (SD) for continuous variables, and frequencies and proportions for categorical variables. After checking Q-Q plot, normality was violated. Therefore, we used Mann-Whitney test comparisons between groups. For categorical variable, we used Chi-square test. The effect size was estimated with Cohen’s d measure. The statistical significance was set at 0.05 and analyses were performed with R Studio software.
3. Results
3.1. Population
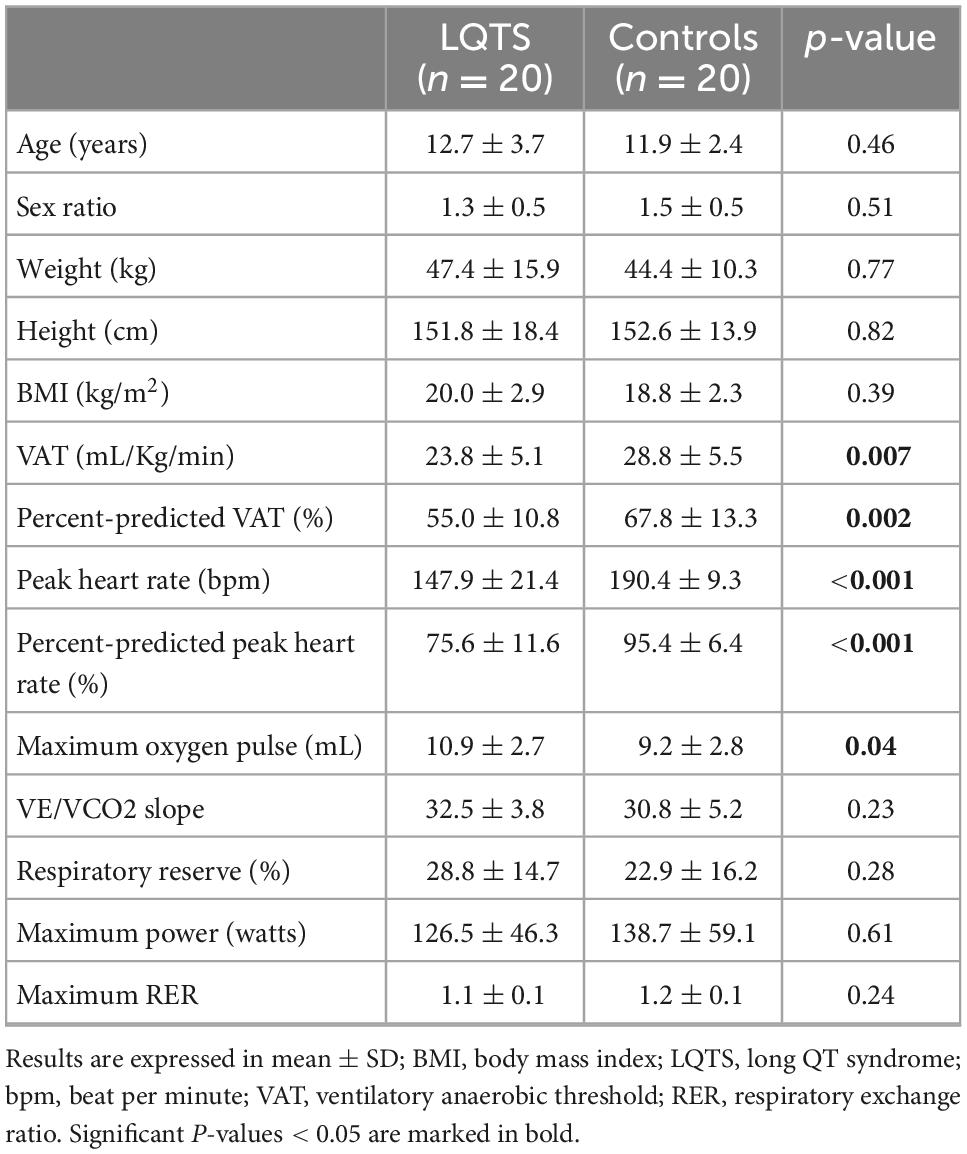
Twenty children with LQTS (12.7 ± 3.7 years old) and 20 healthy children (11.9 ± 2.4 years old) were included in the study. The two groups were similar in terms of age, gender, weight, height, and body mass index (Table 1). Children with LQTS were affected by the following genetic mutations, by descending order: KCNQ1 (n = 11, 55%), KCNH2 (n = 7, 35%), and KCNJ2 (n = 1, 5%). No mutation was found for one participant. Diagnosis modes were represented as follows: genetic diagnosis after known causal familial mutation (n = 12, 60% of which one prenatal diagnosis), incidental finding (n = 3, 15%), cardiac symptoms [n = 5, 25%, e.g., syncope (n = 2), ventricular extrasystole (n = 2), and bradycardia (n = 1)]. Most children with LQTS were prescribed beta-blockers (n = 18, 90%), of which nadolol (mean dose of 40 mg/m2/day) for 17 children and atenolol (0.5 mg/Kg/day) for one child. The mean QTc value was 458.3 ± 33.4 msec. None of LQTS had implantable cardioverter defibrillator.
3.2. Cardiorespiratory fitness
The mean VO2peak in the LQTS group was lower than in the control group, in raw values (33.9 ± 6.2 mL/Kg/min vs. 40.1 ± 6.6 mL/Kg/min, respectively, P = 0.010, d = −0.96), as well as in percent-predicts (78.6% ± 13.1% vs. 94.5% ± 15.2%, respectively, P = 0.002, d = –1,12) (Figure 2). The proportion of children with an impaired cardiorespiratory fitness (e.g., percent-predicted VO2peak < 80%) was 2.2 times higher in the LQTS than in the control group (45 vs. 20%). The VAT was lower in the LQTS group than in the control group, in raw values (23.8 ± 5.1 mL/Kg/min vs. 28.8 ± 5.5 mL/Kg/min, respectively, P = 0.007, d = –0.95), and in percent-predicts (55% ± 10.8% vs. 67.8% ± 13.3%, respectively, P = 0.002, d = –1,05). The proportion of children with an impaired VAT (e.g., percent-predicted VAT < 55%) was 2.5 times higher in the LQTS than in the control group (50 vs. 20%). Peak heart rate was lower in the LQTS group, and no significant difference was observed for maximal power and the main ventilatory parameters (VE/VCO2 slope, maximal RER). The mean VO2 in the LQTS group was not significantly different from the mean VO2 at the same heart rate (e.g., 140 bpm) in the control group (26.2 ± 4.35 mL/Kg/min vs. 27.4 ± 6.0 mL/Kg/min, respectively, P = 0.44, d = –0.23).
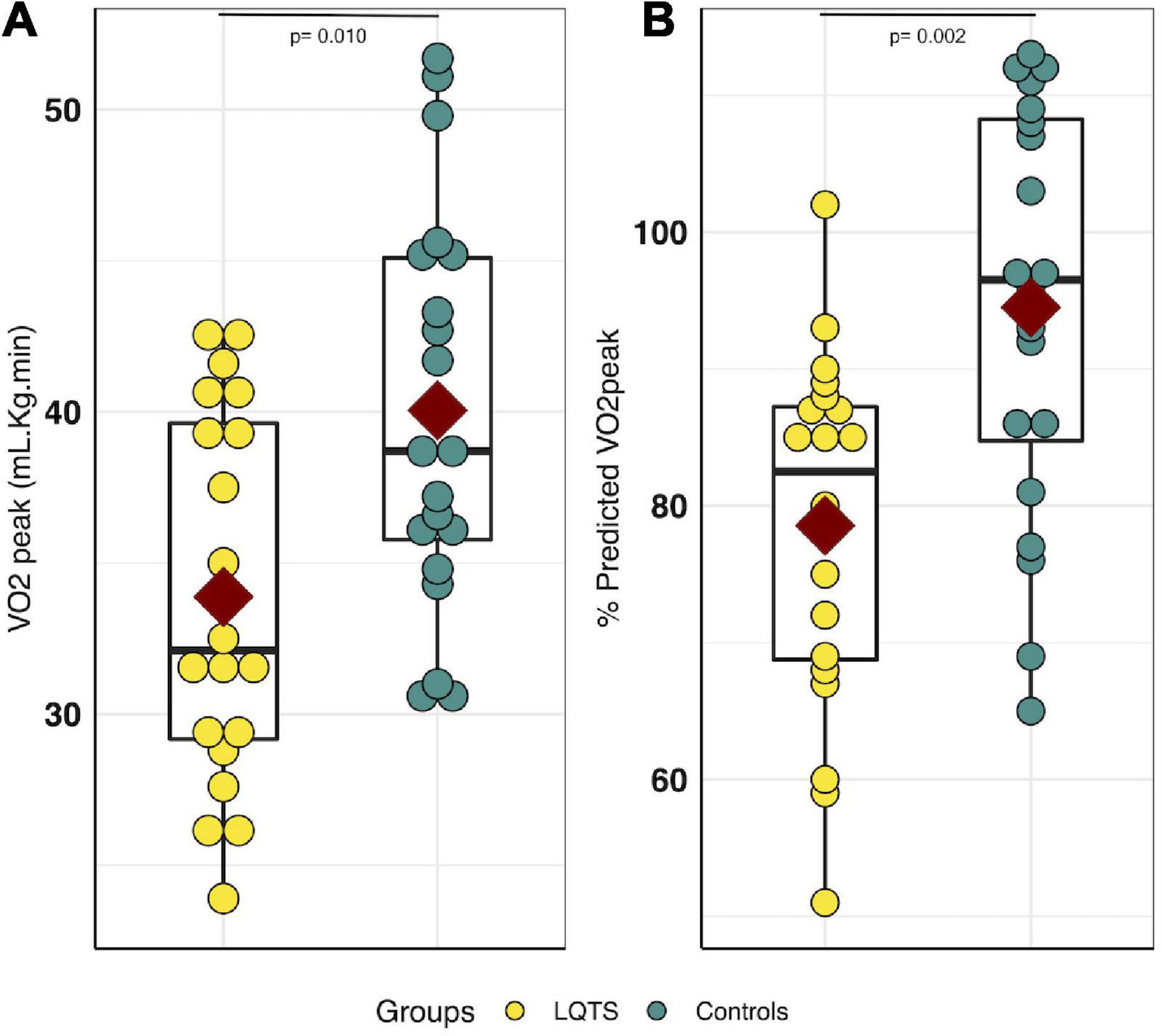
Figure 2. VO2peak comparison between LQTS and control groups. (A) VO2peak comparison. (B) Percent-predicted VO2peak comparison. For each group, the dark red square represents the mean value for each group, the black boxplot represents the first, second and third quartiles [e.g., Q1, median, Q3], respectively. LQTS, long QT syndrome.
3.3. Muscular fitness
The pennation angle was significantly lower in the LQTS group than in controls (12.2° ± 2.4° vs. 14.3° ± 2.8°, respectively, P = 0.02, d = –0.8) (Figure 3A). The anatomical cross-sectional area was not significantly different between LQTS and control groups (2.7 ± 1.0 cm2 vs. 3.5 ± 1.6 cm2, respectively, P = 0.10, d = –0.6) (Figure 3B). The upper limb strength assessed by the handgrip test was not significantly different between the two groups (20.8 ± 8.2 Kg in LQTS and 23.6 ± 8.3 Kg in controls, P = 0.29) (Figure 3C). The lower limb strength assessed by the standing long broad jump distance was lower in the LQTS group than in controls (119.5 ± 33.2 cm vs. 147.3 ± 36.1 cm, respectively, P = 0.02, d = –0.8) (Figure 3D).

Figure 3. Muscle fitness comparison between LQTS and control groups. (A) Pennation angle (°). (B) Anatomical cross-sectional area (cm2). (C) Handgrip strength (kg). (D) Standing long broad jump distance (cm). For each group, the dark red square represents the mean value for each group, the black boxplot represents the first, second, and third quartiles [e.g., Q1, median, Q3], respectively. LQTS, long QT syndrome.
3.4. Level of physical activity
Valid wear time was appropriate in the LQTS group (mean 11 ± 3.6 days and 11.9 ± 1.8 h per day), as well as in the control group (mean 12.3 ± 2.2 days and 11.81 ± 1.5 h per day). Three participants were excluded from this analysis (two in LQTS group, one in control group) because the valid wear time period was not respected. MVPA was not significantly different between LQTS and control groups (36.9 ± 12.9 min/day vs. 41.5 ± 18.7 min/day, respectively, P = 0.66). The levels of physical activity were not significantly different between LQTS and control groups in terms of MVPA (36.9 ± 12.9 min/day vs. 41.5 ± 18.7 min/day, respectively, P = 0.66, d = –0.2), moderate physical activity (22.13 ± 12 min/day vs. 22.57 ± 11.16 min/day, respectively, P = 0.88, d = 0.03), and vigorous physical activity (14.31 ± 7.65 min/day vs. 19.97 ± 16.58 min/day, respectively, P = 0.46, d = 0.4). Two participants from the control group, and none in the LQTS group, complied with the WHO guidelines (e.g., ≥ 60 min per day of moderate-to-vigorous physical activity).
4. Discussion
In this prospective controlled study, two major components of physical fitness, e.g., cardiorespiratory and muscular fitness, were significantly impaired in children with LQTS, in comparison with healthy participants, despite similar levels of physical activity in both groups.
In terms of cardiorespiratory fitness, the lower peak VO2 values in the LQTS group may reflect chronotropic limitation induced by beta-blockers, which represents the standard treatment in children with LQTS. Indeed, the VO2 at the same heart rate was similar between LQTS and controls. During exercise, heart rate has a greater contribution to peak VO2 than stroke volume (47, 48). Nevertheless, the origin of the cardiorespiratory fitness limitation seemed multifactorial. Whereas peak VO2 remains the most common method of assessing aerobic fitness, our results also found that the VAT was impaired in half of children with LQTS, suggesting the existence of peripherical limiting factors to physical capacity in this population. Classically, cardiac adaptation at submaximal exercise, up to 50% of peak VO2, mostly relies on stroke volume increase, rather than on heart rate (49). Similarly, Bratt et al. found that beta-blockers did not reduce exercise capacity in adolescents with hypertrophic cardiomyopathy despite lower heart rate (50). Indeed, the VAT is more related to lactate metabolism than chronotropic adaptation (51). Physiologically, the lactate shuttle mechanism combines the production of lactate by active muscle fibers during exercise and the simultaneous consumption of lactate by adjacent fibers or distant sites as source of energy (52, 53). Previous studies have shown that lactate accumulation was mainly driven by oxidative capacity mechanism, e.g., oxidative enzyme, mitochondrial reticulum volume density (54), and capillary density. Therefore, the impaired VAT in children with LQTS may reflect some degree of altered oxidative mechanism and dysfunction of blood lactate shuttle mechanism.
In terms of muscular fitness, the lower strength muscle in lower limb may suggest the existence of neuro-muscular and/or glycolytic metabolism alteration in LQTS. Indeed, previous studies showed subclinical electromyographic alterations in adult with LQTS, but whether these abnormalities are neurogenic or myogenic remains unclear (55, 56). Pennation angle was significantly lower in children with LQTS, while anatomical cross-sectional area, although diminished, did not reach statistical significance. Considering that muscular adaptation to increase muscle strength after undergoing resistance training in children is mainly due to neuromuscular adaptation and coordination rather than hypertrophy (57), the same process may occur during muscle loss, reversely. Therefore, lower pennation angle and lower strength could result more from decreased muscle tension or poorer neuromuscular adaptation than from decreased muscle mass. The direct implication of beta-blockers on altered muscular fitness seems irrelevant, as they commonly have no effect on muscle excitability and fatiguability (58, 59). Muscular fitness assessment in clinical follow-up of children with chronic diseases may be of interest, however further studies are necessary to evaluate the underlying causal mechanism.
In this study, despite lower aerobic fitness, the level of physical activity assessed by accelerometer in children with LQTS was similar to that of healthy controls, as in previous studies using physical activity questionnaires (60). The weak association between objectively measured daily physical activity and aerobic fitness has been previously highlighted (61–64). Overall, no child with LQTS and only two healthy participants respected the WHO guidelines on physical activity (e.g., ≥ 60 min per day of MVPA). These results reflect the major public health issue of physical inactivity in children, whether they have a chronic disease or not (6). Previous studies observed that children with LQTS were commonly withdrawn from competitive sports, and as a precautionary principle, from many recreational sports activities (18, 23, 25). Considering that a cardiovascular training effect requires moderate-to-vigorous exercise intensity, the decrease in aerobic fitness observed in children with LQTS may reflect imposed activity restrictions. Yet, exercise-related events are exceptional in appropriately managed children with LQTS (65). Fortunately, the recent 2020 ESC guidelines on competitive sports participation in athletes with cardiovascular disease have taken an important step forward in promoting physical activity in patients with cardiac diseases, especially by introducing the concept of shared-decision making between patients and physicians (22). Nevertheless, the appropriate level of intensity and the type of physical activity for patients with LQTS need to be clarified, especially for pediatric patients. Therefore, it could be relevant to enroll patients with LQTS into cardiovascular rehabilitation programs, from pediatric age, as in congenital heart disease (31). Indeed, cardiovascular rehabilitation involves established core components, including exercise, patient education, psychosocial counseling, risk factor reduction and behavior modification, with a goal of optimizing patient’s quality of life (12, 31).
5. Study limitations
Some factors of cardiorespiratory fitness, such as nutrition, psychological factors, environment, or patients compliance to medical guidelines (19) have not been investigated in this study. Control participants were enrolled during a consultation at the hospital, and therefore may not be considered as healthy as if they were recruited from the general population. Some participants were enrolled during the COVID-19 pandemic lock-down, however, during this period (April 2021), subjects from both groups were evenly recruited and outdoor physical activities remained allowed within a 10 kilometer-perimeter. Because of the sample size study, no multivariate analysis could be performed, however, this pilot study led to the larger ongoing QUALIMYORYTHM trial, which will further explore the main determinants of physical components in children with inherited cardiac diseases (66).
6. Conclusion
In children with LQTS, cardiorespiratory fitness determined by peak oxygen uptake and ventilatory anaerobic threshold, and muscle fitness were lower than in healthy controls, despite similar levels of physical activity. The origin of this limitation seemed to be multifactorial, involving beta-blocker induced chronotropic limitation, physical and muscle deconditioning. Cardiopulmonary exercise testing and muscular evaluation may be of interest in pediatric LQTS follow-up and participate in promoting safe physical activity in this population.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
This study was conducted in compliance with the Good Clinical Practices protocol and Declaration of Helsinki principles. It was approved by a drawn national Ethics Committee (CPP Sud-Est VI, 2020-A00411-38). Informed consent was obtained from all parents or legal guardians, and oral assent was obtained from all children. Written informed consent was obtained from the minor’s legal guardian for the publication of the potentially identifiable image (Figure 1). The study was registered on Clinicaltrials.gov (NCT04712136).
Author contributions
LS, MA, AB, OW, and PA contributed to the study design and participants enrollment. AR, SM, MV, GD, VP, J-LP, and SG contributed to participants enrollment. LS, MA, and PA contributed to writing the manuscript. All authors approved the final version of the manuscript.
Funding
This study belongs to the QUALIMYORYTHM national research program, dedicated to evaluating and improving the quality of life of children with inherited cardiac disorders, sponsored by Montpellier University Hospital, France. The study was funded by the French Federation of Cardiology (2019 FFC research team grant, www.fedecardio.org), the French National Department of Health (DGOS, GIRCI-SOHO, APITHEM 2019, www.girci-soho.fr), and the French National Rare Cardiac Disease Network (2018 CARDIOGEN grant, www.filiere-cardiogen.fr). Additional research funding was obtained from young researcher award (Luc Souilla, 2021 doctoral research grant, University of Montpellier; Oscar Werner, AOI 2019, Montpellier University Hospital; Martina Avesani, EACVI training grant, App000088435).
Acknowledgments
We are grateful to Stanislava Nenova, Anne Cadene, Yousra Mermat, Christelle Sarran, and Annie Auer, for their organizational support. We thank the children and their families for their participation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Palermi S, Sacco AM, Belviso I, Romano V, Montesano P, Corrado B, et al. Guidelines for physical activity-a cross-sectional study to assess their application in the general population. Have we achieved our goal? Int J Environ Res Public Health. (2020) 17:E3980. doi: 10.3390/ijerph17113980
2. Ding D, Mutrie N, Bauman A, Pratt M, Hallal P, Powell K. Physical activity guidelines 2020: comprehensive and inclusive recommendations to activate populations. Lancet Lond Engl. (2020) 396:1780–2. doi: 10.1016/S0140-6736(20)32229-7
4. Dimitri P, Joshi K, Jones N, Moving Medicine for Children Working Group. Moving more: physical activity and its positive effects on long term conditions in children and young people. Arch Dis Child. (2020) 105:1035–40. doi: 10.1136/archdischild-2019-318017
5. Eime R, Young J, Harvey J, Charity M, Payne WR. A systematic review of the psychological and social benefits of participation in sport for children and adolescents: informing development of a conceptual model of health through sport. Int J Behav Nutr Phys Act. (2013) 10:98. doi: 10.1186/1479-5868-10-98
6. Guthold R, Stevens G, Riley L, Bull F. Global trends in insufficient physical activity among adolescents: a pooled analysis of 298 population-based surveys with 1.6 million participants. Lancet Child Adolesc Health. (2020) 4:23–35. doi: 10.1016/S2352-4642(19)30323-2
7. West SL, Banks L, Schneiderman JE, Caterini JE, Stephens S, White G, et al. Physical activity for children with chronic disease; a narrative review and practical applications. BMC Pediatr. (2019) 19:12. doi: 10.1186/s12887-018-1377-3
8. Ruiz J, Cavero-Redondo I, Ortega F, Welk G, Andersen L, Martinez-Vizcaino V. Cardiorespiratory fitness cut points to avoid cardiovascular disease risk in children and adolescents; what level of fitness should raise a red flag? A systematic review and meta-analysis. Br J Sports Med. (2016) 50:1451–8. doi: 10.1136/bjsports-2015-095903
9. Lee I, Shiroma E, Lobelo F, Puska P, Blair S, Katzmarzyk P. Impact of physical inactivity on the world’s major non-communicable diseases. Lancet. (2012) 380:219–29. doi: 10.1016/S0140-6736(12)61031-9
10. Raghuveer G, Hartz J, Lubans DR, Takken T, Wiltz JL, Mietus-Snyder M, et al. Cardiorespiratory fitness in youth: an important marker of health: a scientific statement from the American heart association. Circulation. (2020) 142:e101–18. doi: 10.1161/CIR.0000000000000866
11. Moola F, Fusco C, Kirsh J. The Perceptions of caregivers toward physical activity and health in youth with congenital heart disease. Qual Health Res. (2011) 21:278–91. doi: 10.1177/1049732310384119
12. Amedro P, Dorka R, Moniotte S, Guillaumont S, Fraisse A, Kreitmann B, et al. Quality of life of children with congenital heart diseases: a multicenter controlled cross-sectional study. Pediatr Cardiol. (2015) 36:1588–601. doi: 10.1007/s00246-015-1201-x
13. Schwartz PJ, Stramba-Badiale M, Crotti L, Pedrazzini M, Besana A, Bosi G, et al. Prevalence of the congenital long-QT syndrome. Circulation. (2009) 120:1761–7. doi: 10.1161/CIRCULATIONAHA.109.863209
14. Schwartz P, Periti M, Malliani A. The long Q-T syndrome. Am Heart J. (1975) 89:378–90. doi: 10.1016/0002-8703(75)90089-7
15. Corrado D, Basso C, Rizzoli G, Schiavon M, Thiene G. Does sports activity enhance the risk of sudden death in adolescents and young adults? J Am Coll Cardiol. (2003) 42:1959–63. doi: 10.1016/j.jacc.2003.03.002
16. Emery M, Kovacs R. Sudden cardiac death in athletes. JACC Heart Fail. (2018) 6:30–40. doi: 10.1016/j.jchf.2017.07.014
17. Harmon KG, Asif IM, Maleszewski JJ, Owens DS, Prutkin JM, Salerno JC, et al. Incidence, cause, and comparative frequency of sudden cardiac death in national collegiate athletic association athletes: a decade in review. Circulation. (2015) 132:10–9. doi: 10.1161/CIRCULATIONAHA.115.015431
18. Maron BJ, Chaitman BR, Ackerman MJ, Luna AB, Corrado D, Crosson JE, et al. Recommendations for physical activity and recreational sports participation for young patients with genetic cardiovascular diseases. Circulation. (2004) 109:2807–16. doi: 10.1161/01.CIR.0000128363.85581.E1
19. Boisson A, Villeon GD, Huguet H, Abassi H, Pasquie J, Lavastre K, et al. Physical activity and aerobic fitness in children with inherited cardiac diseases. Arch Cardiovasc Dis. (2021) 114:727–36. doi: 10.1016/j.acvd.2021.07.004
20. Ritt L, Milani M, Stein R. Long QT syndrome: to exercise safely or not to exercise, that’s the question!!! Eur J Prev Cardiol. (2022) 29:1630–2. doi: 10.1093/eurjpc/zwac109
21. Johnson J, Ackerman M. Return to play? Athletes with congenital long QT syndrome. Br J Sports Med. (2013) 47:28–33. doi: 10.1136/bjsports-2012-091751
22. Pelliccia A, Sharma S, Gati S, Bäck M, Börjesson M, Caselli S, et al. 2020 ESC guidelines on sports cardiology and exercise in patients with cardiovascular disease. Eur Heart J. (2021) 42:17–96. doi: 10.1093/eurheartj/ehaa605
23. Pelliccia A, Fagard R, Bjørnstad H, Anastassakis A, Arbustini E, Assanelli D, et al. Recommendations for competitive sports participation in athletes with cardiovascular disease: a consensus document from the study group of sports cardiology of the working group of cardiac rehabilitation and exercise physiology and the working group of myocardial and pericardial diseases of the European society of cardiology. Eur Heart J. (2005) 26:1422–45. doi: 10.1093/eurheartj/ehi325
24. Ackerman M, Zipes D, Kovacs R, Maron B, American Heart Association Electrocardiography and Arrhythmias Committee of Council on Clinical Cardiology, Council on Cardiovascular Disease in Young, Council on Cardiovascular and Stroke Nursing, et al. Eligibility and disqualification recommendations for competitive athletes with cardiovascular abnormalities: task force 10: the cardiac channelopathies: a scientific statement from the American heart association and American college of cardiology. Circulation. (2015) 132:e326–9. doi: 10.1161/CIR.0000000000000246
25. Maron B, Zipes D. Introduction: eligibility recommendations for competitive athletes with cardiovascular abnormalities-general considerations. J Am Coll Cardiol. (2005) 45:1318–21. doi: 10.1016/j.jacc.2005.02.006
26. Morita H, Wu J, Zipes D. The QT syndromes: long and short. Lancet Lond Engl. (2008) 372:750–63. doi: 10.1016/S0140-6736(08)61307-0
27. Caspersen C, Powell K, Christenson G. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep. (1985) 100:126–31.
28. Amedro P, Gavotto A, Guillaumont S, Bertet H, Vincenti M, Villeon GD, et al. Cardiopulmonary fitness in children with congenital heart diseases versus healthy children. Heart. (2018) 104:1026–36. doi: 10.1136/heartjnl-2017-312339
29. Beaver W, Wasserman K, Whipp BJ. A new method for detecting anaerobic threshold by gas exchange. J Appl Physiol. (1986) 60:2020–7. doi: 10.1152/jappl.1986.60.6.2020
30. Cooper D, Weiler-Ravell D, Whipp B, Wasserman K. Aerobic parameters of exercise as a function of body size during growth in children. J Appl Physiol. (1984) 56:628–34. doi: 10.1152/jappl.1984.56.3.628
31. Amedro P, Gavotto A, Legendre A, Lavastre K, Bredy C, Villeon GD, et al. Impact of a centre and home-based cardiac rehabilitation program on the quality of life of teenagers and young adults with congenital heart disease: the QUALI-REHAB study rationale, design and methods. Int J Cardiol. (2019) 283:112–8. doi: 10.1016/j.ijcard.2018.12.050
32. Smith J, Eather N, Morgan P, Plotnikoff R, Faigenbaum A, Lubans D. The health benefits of muscular fitness for children and adolescents: a systematic review and meta-analysis. Sports Med. (2014) 44:1209–23. doi: 10.1007/s40279-014-0196-4
33. Orsso CE, Tibaes JR, Oliveira CL, Rubin DA, Field CJ, Heymsfield SB, et al. Low muscle mass and strength in pediatrics patients: why should we care? Clin Nutr. (2019) 38:2002–15. doi: 10.1016/j.clnu.2019.04.012
34. Jacobs J, Jansen M, Janssen H, Raijmann W, Van Alfen N, Pillen S. Quantitative muscle ultrasound and muscle force in healthy children: a 4-year follow-up study. Muscle Nerve. (2013) 47:856–63. doi: 10.1002/mus.23690
35. Ponti F, De Cinque A, Fazio N, Napoli A, Guglielmi G, Bazzocchi A. Ultrasound imaging, a stethoscope for body composition assessment. Quant Imaging Med Surg. (2020) 10:1699–722. doi: 10.21037/qims-19-1048
36. Bourdier P, Birat A, Rochette E, Dor É, Courteix D, Dutheil F, et al. Muscle function and architecture in children with juvenile idiopathic arthritis. Acta Paediatr. (2021) 110:280–7. doi: 10.1111/apa.15335
37. Selva Raj I, Bird S, Shield A. Ultrasound measurements of skeletal muscle architecture are associated with strength and functional capacity in older adults. Ultrasound Med Biol. (2017) 43:586–94. doi: 10.1016/j.ultrasmedbio.2016.11.013
38. Kawakami Y, Abe T, Fukunaga T. Muscle-fiber pennation angles are greater in hypertrophied than in normal muscles. J Appl Physiol. (1993) 74:2740–4. doi: 10.1152/jappl.1993.74.6.2740
39. Castro-Piñero J, Ortega FB, Artero EG, Girela-Rejón MJ, Mora J, Sjöström M, et al. Assessing muscular strength in youth: usefulness of standing long jump as a general index of muscular fitness. J Strength Cond Res. (2010) 24:1810–7. doi: 10.1519/JSC.0b013e3181ddb03d
40. Wind A, Takken T, Helders P, Engelbert R. Is grip strength a predictor for total muscle strength in healthy children, adolescents, and young adults? Eur J Pediatr. (2010) 169:281–7. doi: 10.1007/s00431-009-1010-4
41. Gąsior JS, Pawłowski M, Jele PJ, Rameckers EA, Williams CA, Makuch R, et al. Test-retest reliability of handgrip strength measurement in children and preadolescents. Int J Environ Res Public Health. (2020) 17:E8026. doi: 10.3390/ijerph17218026
42. Hänggi J, Phillips L, Rowlands A. Validation of the GT3X ActiGraph in children and comparison with the GT1M ActiGraph. J Sci Med Sport. (2013) 16:40–4. doi: 10.1016/j.jsams.2012.05.012
43. Choi L, Liu Z, Matthews C, Buchowski M. Validation of accelerometer wear and nonwear time classification algorithm. Med Sci Sports Exerc. (2011) 43:357–64. doi: 10.1249/MSS.0b013e3181ed61a3
44. Romanzini M, Petroski E, Ohara D, Dourado A, Reichert F. Calibration of ActiGraph GT3X, Actical and RT3 accelerometers in adolescents. Eur J Sport Sci. (2014) 14:91–9. doi: 10.1080/17461391.2012.732614
45. Evenson K, Catellier D, Gill K, Ondrak K, McMurray R. Calibration of two objective measures of physical activity for children. J Sports Sci. (2008) 26:1557–65. doi: 10.1080/02640410802334196
46. Voss C, Duncombe S, Dean P, de Souza A, Harris K. Physical activity and sedentary behavior in children with congenital heart disease. J Am Heart Assoc Cardiovasc Cerebrovasc Dis. (2017) 6:e004665. doi: 10.1161/JAHA.116.004665
47. Sullivan M, Cobb F, Higginbotham M. Stroke volume increases by similar mechanisms during upright exercise in normal men and women. Am J Cardiol. (1991) 67:1405–12. doi: 10.1016/0002-9149(91)90472-w
48. Higginbotham M, Morris K, Williams R, McHale P, Coleman R, Cobb F. Regulation of stroke volume during submaximal and maximal upright exercise in normal man. Circ Res. (1986) 58:281–91. doi: 10.1161/01.res.58.2.281
49. Nottin S, Vinet A, Stecken F, Nguyen L, Ounissi F, Lecoq A, et al. Central and peripheral cardiovascular adaptations during a maximal cycle exercise in boys and men. Med Sci Sports Exerc. (2002) 34:456–63. doi: 10.1097/00005768-200203000-00012
50. Bratt E, Östman-Smith I. Effects of lifestyle changes and high-dose β-blocker therapy on exercise capacity in children, adolescents, and young adults with hypertrophic cardiomyopathy. Cardiol Young. (2015) 25:501–10. doi: 10.1017/S1047951114000237
51. Armstrong N, McManus A editors. Aerobic fitness. In: Children’s Sport and Exercise Medicine. Oxford: Oxford University Press (2017). p. 161–80.
52. Poole D, Rossiter H, Brooks G, Gladden L. The anaerobic threshold: 50+ years of controversy. J Physiol. (2021) 599:737–67. doi: 10.1113/JP279963
53. Brooks G. Lactate production under fully aerobic conditions: the lactate shuttle during rest and exercise. Fed Proc. (1986) 45:2924–9.
54. Holloszy J. Biochemical adaptations in muscle. Effects of exercise on mitochondrial oxygen uptake and respiratory enzyme activity in skeletal muscle. J Biol Chem. (1967) 242:2278–82.
55. Finsterer J, Stöllberger C. Skeletal muscle involvement in congenital long QT syndrome. Neurol Sci. (2004) 25:238–40. doi: 10.1007/s10072-004-0329-x
56. Finsterer J, Stöllberger C. Subclinical skeletal muscle involvement in long-QT syndrome. J Electromyogr Kinesiol. (1999) 9:401–5. doi: 10.1016/s1050-6411(99)00013-9
57. Ramsay J, Blimkie C, Smith K, Garner S, MacDougall J, Sale D. Strength training effects in prepubescent boys. Med Sci Sports Exerc. (1990) 22:605–14. doi: 10.1249/00005768-199010000-00011
58. Cupido C, Hicks A, McKelvie R, Sale D, McComas A. Effect of selective and nonselective beta-blockade on skeletal muscle excitability and fatiguability. J Appl Physiol. (1994) 76:2461–6. doi: 10.1152/jappl.1994.76.6.2461
59. Kaiser P. Physical performance and muscle metabolism during beta-adrenergic blockade in man. Acta Physiol Scand Suppl. (1984) 536:1–53.
60. Chen C, De Souza A, Franciosi S, Harris K, Sanatani S. Physical activity in paediatric long QT syndrome patients. CJC Pediatr Congenit Heart Dis. (2022) 1:80–5. doi: 10.1016/j.cjcpc.2021.12.001
61. Banks L, Rosenthal S, Manlhiot C, Fan CS, McKillop A, Longmuir PE, et al. Exercise capacity and self-efficacy are associated with moderate-to-vigorous intensity physical activity in children with congenital heart disease. Pediatr Cardiol. (2017) 38:1206–14. doi: 10.1007/s00246-017-1645-2
62. Armstrong N, Tomkinson G, Ekelund U. Aerobic fitness and its relationship to sport, exercise training and habitual physical activity during youth. Br J Sports Med. (2011) 45:849–58. doi: 10.1136/bjsports-2011-090200
63. Müller J, Christov F, Schreiber C, Hess J, Hager A. Exercise capacity, quality of life, and daily activity in the long-term follow-up of patients with univentricular heart and total cavopulmonary connection. Eur Heart J. (2009) 30:2915–20. doi: 10.1093/eurheartj/ehp305
64. McCrindle BW, Williams RV, Mital S, Clark BJ, Russell JL, Klein G, et al. Physical activity levels in children and adolescents are reduced after the Fontan procedure, independent of exercise capacity, and are associated with lower perceived general health. Arch Dis Child. (2007) 92:509–14. doi: 10.1136/adc.2006.105239
65. Chambers KD, Ladouceur VB, Alexander ME, Hylind RJ, Bevilacqua L, Mah DY, et al. Cardiac events during competitive, recreational, and daily activities in children and adolescents with long QT syndrome. J Am Heart Assoc. (2017) 6:e005445. doi: 10.1161/JAHA.116.005445
66. Amedro P, Werner O, Abassi H, Boisson A, Souilla L, Guillaumont S, et al. Health-related quality of life and physical activity in children with inherited cardiac arrhythmia or inherited cardiomyopathy: the prospective multicentre controlled QUALIMYORYTHM study rationale, design and methods. Health Qual Life Outcomes. (2021) 19:187. doi: 10.1186/s12955-021-01825-6
Keywords: pediatrics, long QT syndrome, inherited cardiac arrythmia, cardiorespiratory fitness, muscle fitness, physical activity
Citation: Souilla L, Avesani M, Boisson A, Requirand A, Matecki S, Vincenti M, Werner O, De La Villeon G, Pommier V, Pasquie J-L, Guillaumont S and Amedro P (2023) Cardiorespiratory fitness, muscle fitness, and physical activity in children with long QT syndrome: A prospective controlled study. Front. Cardiovasc. Med. 9:1081106. doi: 10.3389/fcvm.2022.1081106
Received: 26 October 2022; Accepted: 28 December 2022;
Published: 11 January 2023.
Edited by:
Oswin Grollmuss, Université Paris-Sud, FranceReviewed by:
Stefan Gross, University Medicine Greifswald, GermanyStefano Palermi, University of Naples Federico II, Italy
David Alexander White, Children’s Mercy Hospital, United States
Patricia Longmuir, University of Ottawa, Canada
Copyright © 2023 Souilla, Avesani, Boisson, Requirand, Matecki, Vincenti, Werner, De La Villeon, Pommier, Pasquie, Guillaumont and Amedro. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pascal Amedro,  cGFzY2FsLmFtZWRyb0BjaHUtYm9yZGVhdXguZnI=
cGFzY2FsLmFtZWRyb0BjaHUtYm9yZGVhdXguZnI=
 Luc Souilla
Luc Souilla Martina Avesani
Martina Avesani Aymeric Boisson1
Aymeric Boisson1 Pascal Amedro
Pascal Amedro