
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 22 December 2022
Sec. Pediatric Cardiology
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.1077339
This article is part of the Research Topic Pulmonary Hypertension Associated with Congenital Heart Disease View all 7 articles
Background: Persistent pulmonary hypertension of the newborn (PPHN) is a major lethal disorder in neonates that leads to an extremely high mortality rate. Thus, the early identification of adverse outcomes in PPHN is critical for clinical practice. This research attempted to develop a nomogram prediction system for assessing the mortality of newborns with PPHN.
Methods: Two hundred and three newborns with PPHN diagnosed from January 2015 to March 2022 were involved in the study. The clinical features of these newborns and pregnancy details were compared between newborns in the survival and lethal groups. Univariable and multivariate analyses were established in sequence to demonstrate the essential risk factors. The nomogram prediction model was built.
Results: A total of 203 newborns were included in the analysis. 136 (67.0%) newborns represented the hospital survival group. Plasma pH value (OR = 0.606, p = 0.000, 95% CI 0.45715–0.80315), septicemia (OR = 3.544, p = 0.000, 95% CI 1.85160–6.78300), and abnormal pregnancy history (OR = 3.331, p = 0.008, 95% CI 1.37550–8.06680) were identified as independent risk factors for neonatal death in newborns associated with PPHN. Finally, the nomogram predictive model was established based on multivariate analysis results, indicating the efficacies of prediction and calibration.
Conclusion: This study generated an applicable risk score formula using the plasma pH value, septicemia, and abnormal pregnancy history to recognize neonatal death in newborns with PPHN, presenting a sufficient predictive value and calibration.
Persistent pulmonary hypertension of the newborn (PPHN) is a disease that occurs during the prenatal to postnatal circulatory transition, leading to a phenotype with elevated pulmonary vascular resistance and hypoxemia. According to previous research, the mortality rate of PPHN is significantly high (approximately 33%) in the perinatal phase. In the past, studies have been conducted to reduce the lethal rate associated with PPHN. Such studies predicted that the mortality rate could decrease by 7–15% in developed high-income countries. However, an extensive mortality rate distribution (12–40%) exists in lower-income regions (1–4). Given the high mortality rate, identifying efficient risk factors for mortality is essential to manage the clinical practice of PPHN.
In clinical practice, it is urgently necessary to establish a helpful tool for predicting adverse outcomes or early mortality of newborns with PPHN, leading to personalized management based on risk stratification. Several studies have identified risk factors in predicting early death in neonates with PPHN, including an exclusive right-to-left ductal shunt, non-response to inhaled nitric oxide (iNO), 1-min Apgar score ≤3 points, infants with congenital anomalies of the respiratory tract, gestational age <34 weeks, neonates with pneumothorax and acute kidney injury, lung hypoplasia (LH), cesarean section mode of delivery, congenital diaphragmatic hernia (CDH), treatment with high-frequency oscillation ventilation (HFOV), and a decreased pH value (1–3, 5–7). However, the risk factors derived from different studies are controversial, and a more applicable and efficient predictive model needs to be established. Encouragingly, the nomogram prediction score, which incorporates the disease characteristics of an individual instead of a single indicator, has been widely identified as a helpful tool in predicting the prognosis of different diseases in clinical practice (8, 9). The nomogram system has demonstrated higher efficiency in assessing several conditions’ prognoses than simple models (10, 11). This study aimed to determine the risk factors for early death in newborns with PPHN. Based on the risk factors, we attempted to set up a nomogram prediction model.
This was a retrospective, single-center, observational study that enrolled neonates with established PPHN who presented with a history of singleton delivery between January 2015 and March 2022 in West China Second University Hospital, Sichuan University. The present study conformed to the principles of the Declaration of Helsinki. The Ethics Committee approved this study of the West China Second Hospital of Sichuan University (2014-034). Two trained individual physicians collected the data. Electronic medical records confirmed all clinical data.
The inclusion criteria were as follows: (1) newborns diagnosed with PPHN according to the ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension (2022) and AHA guidelines of pediatric pulmonary hypertension (2015); (2) clinical manifestations of refractory hypoxemia identified according to one of the following three criteria: (i) pulmonary artery systolic pressure (PASP) >40 mmHg (12); (ii) a difference of preductal and postductal arterial oxygen saturation (SaO2) >5 to 10%; (iii) a difference of preductal and postductal partial pressure of oxygen (PaO2) >10–20 mmHg; (3) PPHN was diagnosed within the first 28 days after birth; (4) PPHN was confirmed by echocardiography; (5) newborns completed a follow-up minimum of 6 months; (6) enrolled newborns were singleton pregnancies; and (7) the parents or guardians of the newborns signed the agreement form to be involved in the research. The exclusion criteria included the following: (1) newborn had a suspected cardiomyopathy; (2) newborns had severe structural malformations of the pulmonary veins; (3) newborns suffered from neonatal or fetal neoplasm; (4) suspected or confirmed chromosomal abnormality; (5) lethal coagulopathy or severe bleeding; (6) positive family history of pulmonary hypertension or suspected inherited pulmonary hypertension; and (7) medical records were incomplete.
The newborns’ demographic data, maternal pregnancy history, and delivery conditions were documented. Echocardiography and blood tests were conducted and analyzed. Four categories of data were collected that contained the variables considered in our modeling: demographics [sex, birth weight, pregnancy complications, maternal age, abnormal pregnancy-labor history, in vitro fertilization and embryo transfer (IVF-ET), gestational age, Apgar score, cesarean or natural birth]; echocardiography [right atrium, right ventricle, left atrium, left ventricle, systolic pulmonary arteria pressure, fractional shortening (FS), ejection fraction (EF), size of patent ductus arteriosus (PDA)]; associated diseases [CDH, pneumonia, neonatal respiratory distress syndrome (NRDS), bronchopulmonary dysplasia (BPD), pneumothorax, asphyxia, hypoxic ischemic encephalopathy (HIE), neonatal meconium aspiration syndrome (MAS), acute kidney injury (AKI), septicemia, intracranial hemorrhage]; and blood test results [complete blood count, liver function, blood electrolyte, renal function, arterial blood gas analysis, coagulation function test, cardiac troponin I (I), creatine kinase-MB (CK-MB), brain natriuretic peptide (BNP)].
This study summarized the demographic and clinical characteristics of the patients as continuous and categorical variables. Early death in PPHN was identified as any lethality within the first 6 months postnatally. Associations of the risk of early death in newborns with PPHN with risk factors were evaluated by univariable analysis. Multivariable analysis was then performed using logistic regression to identify independent factors among the significant results found in the univariable analysis. Nomogram prediction scores were formulated based on the results of independent risk factors according to the multivariable analyses. Stepwise regression was used to identify the indicators for inclusion in the nomogram. The receiver operating characteristic curve (ROC) with the area under the ROC curve (AUC) values was used to evaluate the effects of the prediction. Calibration of the prediction model was established by a visual calibration plot comparing the predicted and actual probability of survival in newborns with PPHN to identify those at risk of early death.
Univariable analysis was conducted using IBM SPSS 26.0 (IBM SPSS Inc. Chicago, IL, USA). Quantitative data are presented as the means and standard deviations (SDs), while qualitative data are expressed as numbers of individuals. Differences between the two groups were assessed using independent t-tests or Mann–Whitney U-tests for continuous variables and the χ2 test or Fisher’s exact test for categorical variables. Multivariable logistic regression analyses, nomograms, and model evaluation were formulated and conducted using R (version 4.1.2) within the RStudio platform. P-values < 0.05 were considered statistically significant.
A total of 220 newborns matched the inclusion criteria. Seven newborns with suspected or confirmed chromosomal abnormalities or inherited pulmonary hypertension were excluded. Thus, 203 newborns were included to develop the nomogram prediction model. The baseline information of the participants is summarized in Table 1. Briefly, 67 out of 203 newborns (37%) suffered early death associated with PPHN (Figure 1). Furthermore, all the first-degree family members of the involved patients were absent from the history of PPHN.
As shown in Table 1, the univariable analysis revealed several parameters associated with earth death in newborns with PPHN, including abnormal pregnancy history (P = 0.03), IVF-ET (P = 0.048), birth weight (P = 0.008), history of asphyxia (P = 0.026), septicemia (P = 0.000), intracranial hemorrhage (P = 0.002), acute kidney injury (P = 0.027), application of iNO (P = 0.046), high-dose nitric oxide administration (P = 0.012), dopamine application (P = 0.013), alprostadil administration (P = 0.048), treatment with noradrenaline (P = 0.011), red blood cell count (P = 0.013), mean platelet volume (P = 0.016), platelet-larger cell ratio (P = 0.020), serum calcium (P = 0.038), platelets (P = 0.025), globulin (P = 0.035), plasma pH (P = 0.005), and red cell distribution width (P = 0.007).
After univariable analysis, the variables of abnormal pregnancy history, IVF-E, birth weight, history of asphyxia, septicemia, intracranial hemorrhage, AKI, application of iNO, high-dose nitric oxide administration, dopamine application, alprostadil administration, treatment with noradrenaline, red blood cell count, mean platelet volume, platelet-larger cell ratio, serum calcium, platelet, globulin, plasma pH and red cell distribution width were entered into the multivariate logistic regression analysis. Multivariable logistic regression analysis identified pH (OR = 0.606, P = 0.000, 95% CI 0.457–0.803), septicemia (OR = 3.544, P = 0.000, 95% CI 1.851–6.783), and abnormal pregnancy history (OR = 3.331, P = 0.008, 95% CI 1.376–8.067) as independent predictors of survival in newborns with PPHN (Table 2).
Based on the multivariable logistic regression analysis, a nomogram prediction model was constructed, which was formulated with three independent risk factors to identify early death in newborns with PPHN (Figure 2). A total score was calculated using the plasma pH, septicemia incidence, and abnormal pregnancy history. Among these variables, plasma pH represented a continuous variable; the other two parameters were binary. The value of each of the involved variables was converted into a score on the point scale axis. The total score could then be calculated by adding every single score together and, by projecting the total score to the estimated risk, allowed us to determine the probability of early death among newborns with PPHN.

Figure 2. The nomogram prediction scores of multivariable analysis. The value of each variable was given a score on the point scale axis. A total score could be easily calculated by adding each single score, and by projecting the total score to the lower total point scale, we were able to estimate the probability of early death among newborns with PPHN.
The value of the established nomogram prediction was measured by predictive ability and calibration. AUC assessed the predictive ability of the nomogram model. The calibration of the established model was evaluated by a visual calibration plot comparing the predicted and actual survival rates of newborns with PPHN. Based on the ROC analysis, the nomogram prediction model presented an efficient prediction, with an AUC of 0.737 (95% CI 0.663–0.812) (Figure 3A). The calibration curve of the nomogram prediction is presented in Figure 3B and reveals that the early death prediction by the nomogram agrees well with the actual mortality rate. The Hosmer–Lemeshow test yielded no statistically significant difference (P = 0.636).
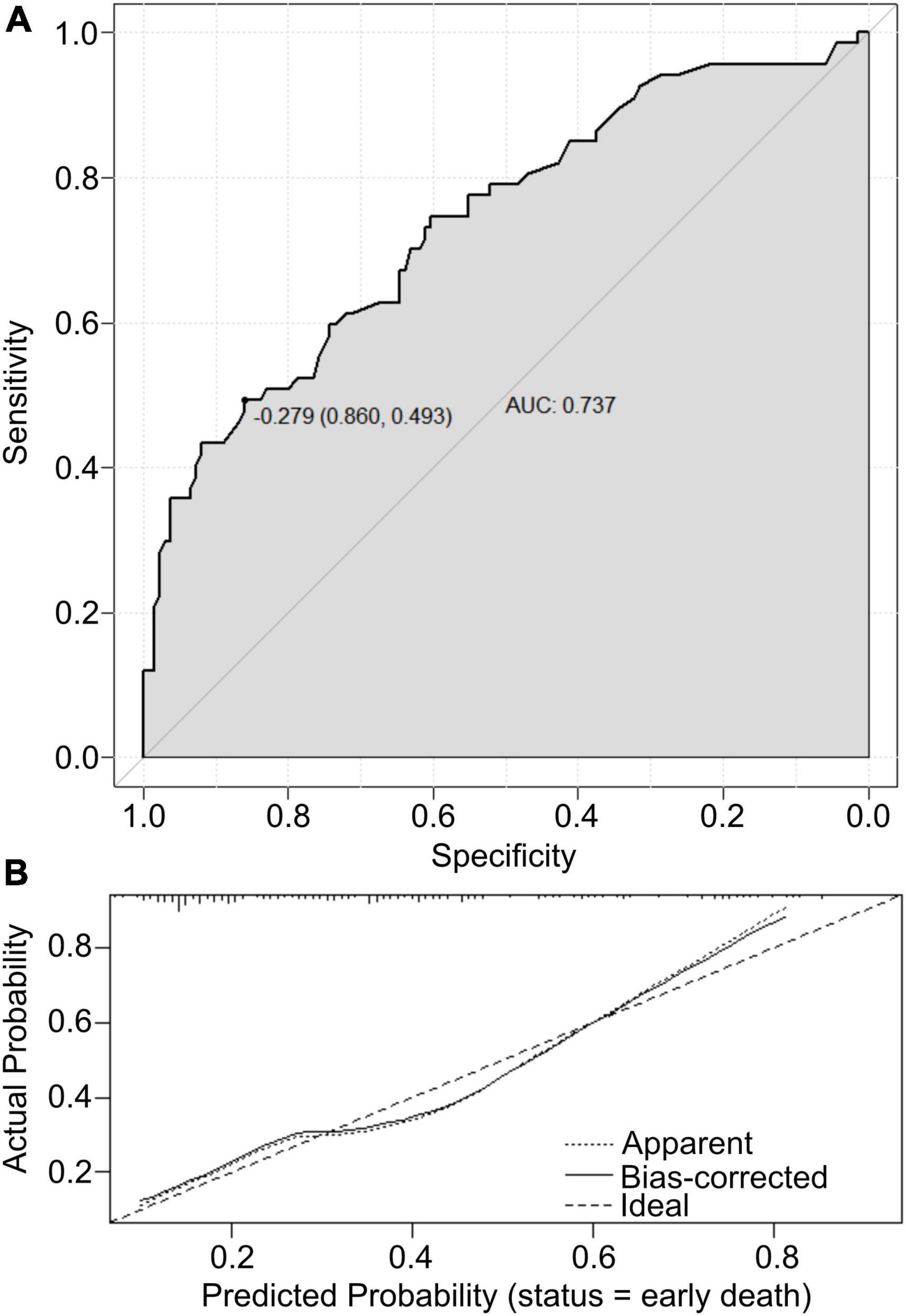
Figure 3. (A) The ROC curves of the nomogram scores. (B) Calibration plot analyses for nomogram scores.
The sustained elevation of PVR and pulmonary vessel remodeling characterize PPHN. PPHN represents a significant challenge in managing neonates due to the high rate of adverse outcomes. Lung recruitment, iNO, intratracheal exogenous pulmonary surfactant (PS) therapy, and gentle ventilation strategies would improve the outcomes of PPHN, which would also help to reduce the reliance on extracorporeal membrane oxygenation (ECMO). However, it has been identified that the early or prompt administration of ECMO might increase the survival rate of PPHN. In the present study, a prediction model for early death was developed and evaluated in newborns with PPHN. This nomogram model incorporated the plasma pH value, identification of septicemia, and abnormal pregnancy-labor history and showed an efficient predictive value (AUC = 0.737, 95% CI: 0.663–0.812) and calibration performance. Thus, this nomogram prediction score system was able to facilitate individualized prediction of early death in newborns with PPHN. As such, this nomogram may have great potential in clinical application.
Persistent pulmonary hypertension of the newborn may be primary or secondary to conditions such as pulmonary hypoplasia, NRDS, MAS, CDH, pneumonia, asphyxia, sepsis, or cardiac disorders (5, 13). In animal and human studies, sepsis has been reported as an independent risk factor for PPHN (5, 14). Sepsis complicated by PPHN can lead to severe hypoxemia and worsen the outcomes and course of affected newborns. Several cases of neonatal sepsis-induced PPHN have demonstrated high morbidity and mortality in newborns (15–17). Early detection of PPHN with sepsis may lead to specific interventions associated with more favorable outcomes (18). Severe infection may cause pulmonary vasoconstriction, leading to hypoxia and PPHN (18). Mean PASP was significantly higher in neonates with sepsis than those without infection or other known risk factors for PPHN (19). Previous studies have found that group B Streptococcus (GBS) infection contributes to early onset sepsis in neonates. At the same time, phosphatidylglycerol and cardiolipin, which GBS produces, are considered to be associated with PPHN (20, 21) due to their ability to induce pulmonary hypertensive and pulmonary endothelial injuries. Thus, early administration of penicillin after birth may help to reduce the severity of PPHN associated with GBS infection. However, penicillin treatment of Streptococci also induces an immediate secretion of phospholipids, which may contribute to developing PPHN (22). Furthermore, long-term usage of penicillin increases lipid synthesis. Thus, the timing and duration of antibiotic application are critical in managing PPHN in newborns (14).
This study identified a history of abnormal pregnancy as an independent risk factor for early death associated with PPHN. An abnormal pregnancy-labor history was related to genetic abnormalities and harmful environmental exposures. Although the present study excluded cases of chromosomal diseases and inherited cardiovascular diseases, some newborns may have suffered from genetic malformations that contributed to adverse outcomes, as the subjects of this study were not required to undergo genetic testing. As such, the present study failed to determine whether PPHN was associated with genetic mutations. Alterations in lung development and genetic conditions are essential in developing PPHN. Moreover, several maternal and fetal issues encode the origins of PPHN prenatally. Previous studies have demonstrated a link between genetic polymorphisms of the urea cycle enzyme and the onset of PPHN (23). In addition, transforming growth factor-β (TGF-β) and the endothelin system have been implicated in PPHN (24–26). Impaired expression of vasoactive substances also contributes to progressive changes in pulmonary vasoreactivity, which are tightly related to the variant expression of the NOS3, VEGFA, and EDN1 genes (27–30). EDN1 has been involved in several cardiovascular disorders, including high blood pressure and pulmonary hypertension (31–33). The rs2070699 SNP of EDN1 has been suspected to be associated with neonatal respiratory distress and PPHN. In addition, the ABCA3 gene has also been identified to contribute to neonatal recurrent pulmonary hypertension and respiratory failure (34). Accordingly, prenatal genetic screens are critical for newborns with highly suspected PPHN. These screens may also be beneficial for the subsequent pregnancy. However, genetic variance contributes to identifying the risk factor of PPHN. Nevertheless, the application of genetic tests was expensive and led to an extended analysis duration. So, we only enrolled genetic tests in the highly suspected syndrome patients. As all the PPHN patients with syndromes were excluded, the general patients did not receive any genetic tests in this study.
Responses to iNO are also correlated with the severity of PPHN and facilitate optimal lung aeration by intratracheal exogenous PS. However, evidence has demonstrated that a large number of infants fail to respond to the administration of iNO. The plasma pH value is critical to predict the responses of iNO treatment, and the normal range of pH values between 7.40 and 7.45 is more likely to correspond to iNO treatment. Previous studies have shown that a PaO2/FiO2 < 50 and a pH value < 7.2 indicate more severe PPHN and a higher probability of early death. The degree of acidosis forms a negative feedback loop with pulmonary hypertension, resulting in poor iNO treatment. Morel et al. showed that low PaO2 and pH values cause a negative response to iNO treatment (35). Heidersbach et al. revealed that the administration of iNO, alkalosis and oxygen presented a dose-dependent effect on pulmonary vasodilation in a lamb model. They revealed that iNO-induced pulmonary vasodilation is efficient when the systemic arterial pH > 7.40 (36, 37). Thus, the arterial pH value was crucial to assess the outcomes of PPHN.
There are some limitations in this study. Selective bias may have occurred, as this study was performed in a single institution. Some of the data in this study are not comprehensive and cannot be included in the multivariate analysis. The ratio of ECMO application was not high enough, which may have affected the survival rate of newborns. This study discusses the genetic variance associated with PPHN, but the subjects of this study did not undergo genetic testing. Genetic data combining clinical characteristics may improve the prediction model performance.
Persistent pulmonary hypertension of the newborn is a life-threatening condition that reveals significant challenges in neonatal management, leading to a high morbidity and mortality rate. An advanced predictive system would help to improve the prognosis among such patients and maintain the therapeutic strategy. This study generated a novel applicable risk score formula using the plasma pH value, septicemia, and abnormal pregnancy history to recognize neonatal death in newborns with PPHN by nomogram prediction method. This score formula presented an efficient predictive value and calibration in PPHN.
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
This study was approved by the Ethics Committee of West China Second Hospital of Sichuan University (2014-034). Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
CL, JM, YZ, SD, and JW collected the clinical data. CL and JM reviewed the literature, contributed to the manuscript drafting, and performed the nomogram analysis. JW and YL conceptualized and designed the study, coordinated and supervised data collection, critically reviewed the manuscript for important intellectual content, and responsible for the revision of the manuscript for important intellectual content. All authors issued final approval for the version to be submitted.
This work was supported by grants from the Technology Project of Sichuan Province of China (2021YFQ0061) and the National Natural Science Foundation of China (82270249). The funding did not participate in the design of the study and collection, analysis, and interpretation of data or in writing the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Steurer MA, Baer RJ, Oltman S, Ryckman KK, Feuer SK, Rogers E, et al. Morbidity of persistent pulmonary hypertension of the newborn in the first year of life. J Pediatr. (2019) 213:58–65.e4. doi: 10.1016/j.jpeds.2019.06.053
2. Nakwan N, Jain S, Kumar K, Hosono S, Hammoud M, Elsayed YY, et al. An Asian multicenter retrospective study on persistent pulmonary hypertension of the newborn: incidence, etiology, diagnosis, treatment and outcome. J Matern Fetal Neonatal Med. (2020) 33:2032–7. doi: 10.1080/14767058.2018.1536740
3. Nakwan N, Pithaklimnuwong S. Acute kidney injury and pneumothorax are risk factors for mortality in persistent pulmonary hypertension of the newborn in Thai neonates. J Matern Fetal Neonatal Med. (2016) 29:1741–6. doi: 10.3109/14767058.2015.1060213
4. Nakwan N, Nakwan N, Wannaro J. Predicting mortality in infants with persistent pulmonary hypertension of the newborn with the score for neonatal acute physiology-version II (SNAP-II) in Thai neonates. J Perinat Med. (2011) 39:311–5. doi: 10.1515/jpm.2011.011
5. Steurer MA, Jelliffe-Pawlowski LL, Baer RJ, Partridge JC, Rogers EE, Keller RL. Persistent pulmonary hypertension of the newborn in late preterm and term infants in California. Pediatrics. (2017) 139:e20161165. doi: 10.1542/peds.2016-1165
6. Razzaq A, Iqbal Quddusi A, Nizami N. Risk factors and mortality among newborns with persistent pulmonary hypertension. Pak J Med Sci. (2013) 29:1099–104. doi: 10.12669/pjms.295.3728
7. Fraisse A, Geva T, Gaudart J, Wessel DL. Doppler echocardiographic predictors of outcome in newborns with persistent pulmonary hypertension. Cardiol Young. (2004) 14:277–83. doi: 10.1017/s1047951104003051
8. Balachandran VP, Gonen M, Smith JJ, DeMatteo RP. Nomograms in oncology: more than meets the eye. Lancet Oncol. (2015) 16:e173–80. doi: 10.1016/s1470-2045(14)71116-7
9. Hijazi Z, Oldgren J, Lindbäck J, Alexander JH, Connolly SJ, Eikelboom JW, et al. The novel biomarker-based ABC (age, biomarkers, clinical history)-bleeding risk score for patients with atrial fibrillation: a derivation and validation study. Lancet. (2016) 387:2302–11. doi: 10.1016/s0140-6736(16)00741-8
10. Shariat SF, Karakiewicz PI, Suardi N, Kattan MW. Comparison of nomograms with other methods for predicting outcomes in prostate cancer: a critical analysis of the literature. Clin Cancer Res. (2008) 14:4400–7. doi: 10.1158/1078-0432.Ccr-07-4713
11. Shariat SF, Capitanio U, Jeldres C, Karakiewicz PI. Can nomograms be superior to other prediction tools? BJU Int. (2009) 103:492–5. doi: 10.1111/j.1464-410X.2008.08073.x
12. Heys JJ, Holyoak N, Calleja AM, Belohlavek M, Chaliki HP. Revisiting the simplified bernoulli equation. Open Biomed Eng J. (2010) 4:123–8. doi: 10.2174/1874120701004010123
13. Rocha G, Baptista MJ, Guimarães H. Persistent pulmonary hypertension of non cardiac cause in a neonatal intensive care unit. Pulm Med. (2012) 2012:818971. doi: 10.1155/2012/818971
14. Curtis J, Kim G, Wehr NB, Levine RL. Group B streptococcal phospholipid causes pulmonary hypertension. Proc Natl Acad Sci USA. (2003) 100:5087–90. doi: 10.1073/pnas.0931493100
15. Shankaran S, Farooki ZQ, Desai R. beta-hemolytic streptococcal infection appearing as persistent fetal circulation. Am J Dis Child. (1982) 136:725–7. doi: 10.1001/archpedi.1982.03970440069020
16. Girardin E, Berner M, Grau GE, Dayer JM, Roux-Lombard P, Suter S. Tumour necrosis factor in neonatal listeriosis: a case report. Eur J Pediatr. (1989) 148:644–5. doi: 10.1007/bf00441521
17. Walter-Nicolet E, Leblanc M, Leruez-Ville M, Hubert P, Mitanchez D. Congenital cytomegalovirus infection manifesting as neonatal persistent pulmonary hypertension: report of two cases. Pulm Med. (2011) 2011:293285. doi: 10.1155/2011/293285
18. Verma B, Daga SR, Mahapankar A. Persistent pulmonary hypertension among neonates with sepsis. Indian J Pediatr. (2006) 73:250–1. doi: 10.1007/bf02825496
19. Waites KB, Crouse DT, Philips JB III, Canupp KC, Cassell GH. Ureaplasmal pneumonia and sepsis associated with persistent pulmonary hypertension of the newborn. Pediatrics. (1989) 83:79–85.
20. Fischer W. The polar lipids of group B Streptococci. I. Glucosylated diphosphatidylglycerol, a novel glycopholipid. Biochim Biophys Acta. (1977) 487:74–88. doi: 10.1016/0005-2760(77)90045-5
21. Filgueiras MH, Op den Kamp JA. Cardiolipin, a major phospholipid of Gram-positive bacteria that is not readily extractable. Biochim Biophys Acta. (1980) 620:332–7. doi: 10.1016/0005-2760(80)90215-5
22. Curtis J, Kim G, Wehr NB, Levine RL. Group B Streptococcus, phospholipids and pulmonary hypertension. J Perinatol. (2011) 31(Suppl. 1):S24–8. doi: 10.1038/jp.2010.168
23. El-Khazragy N, El Barbary M, Fouad H, Abdelgawad A, Rabie D. Association between genetic variations in carbamoyl-phosphate synthetase gene and persistent neonatal pulmonary hypertension. Eur J Pediatr. (2021) 180:2831–8. doi: 10.1007/s00431-021-04053-8
24. Machado RD, Aldred MA, James V, Harrison RE, Patel B, Schwalbe EC, et al. Mutations of the TGF-beta type II receptor BMPR2 in pulmonary arterial hypertension. Hum Mutat. (2006) 27:121–32. doi: 10.1002/humu.20285
25. Pearson DL, Dawling S, Walsh WF, Haines JL, Christman BW, Bazyk A, et al. Neonatal pulmonary hypertension–urea-cycle intermediates, nitric oxide production, and carbamoyl-phosphate synthetase function. N Engl J Med. (2001) 344:1832–8. doi: 10.1056/nejm200106143442404
26. Germain M, Eyries M, Montani D, Poirier O, Girerd B, Dorfmüller P, et al. Genome-wide association analysis identifies a susceptibility locus for pulmonary arterial hypertension. Nat Genet. (2013) 45:518–21. doi: 10.1038/ng.2581
27. Pepke-Zaba J, Morrell NW. The endothelin system and its role in pulmonary arterial hypertension (PAH). Thorax. (2005) 60:443–4. doi: 10.1136/thx.2004.031724
28. Pepke-Zaba J, Higenbottam TW, Dinh-Xuan AT, Stone D, Wallwork J. Inhaled nitric oxide as a cause of selective pulmonary vasodilatation in pulmonary hypertension. Lancet. (1991) 338:1173–4. doi: 10.1016/0140-6736(91)92033-x
29. Abman SH. Impaired vascular endothelial growth factor signaling in the pathogenesis of neonatal pulmonary vascular disease. Adv Exp Med Biol. (2010) 661:323–35. doi: 10.1007/978-1-60761-500-2_21
30. Müller AM, Gruhn K, Lange S, Franke FE, Müller KM. [Angiotensin converting enzyme (ACE, CD143) in the regular pulmonary vasculature]. Pathologe. (2004) 25:141–6. doi: 10.1007/s00292-004-0681-x
31. Vadapalli S, Rani HS, Sastry B, Nallari P. Endothelin-1 and endothelial nitric oxide polymorphisms in idiopathic pulmonary arterial hypertension. Int J Mol Epidemiol Genet. (2010) 1:208–13.
32. Asai T, Ohkubo T, Katsuya T, Higaki J, Fu Y, Fukuda M, et al. Endothelin-1 gene variant associates with blood pressure in obese Japanese subjects: the Ohasama study. Hypertension. (2001) 38:1321–4. doi: 10.1161/hy1101.095333
33. Jin JJ, Nakura J, Wu Z, Yamamoto M, Abe M, Tabara Y, et al. Association of endothelin-1 gene variant with hypertension. Hypertension. (2003) 41:163–7. doi: 10.1161/01.hyp.0000043680.75107.cf
34. Kunig AM, Parker TA, Nogee LM, Abman SH, Kinsella JP. ABCA3 deficiency presenting as persistent pulmonary hypertension of the newborn. J Pediatr. (2007) 151:322–4. doi: 10.1016/j.jpeds.2007.05.054
35. Morel AA, Shreck E, Mally PV, Kim Y, Bailey SM, Wachtel EV. Clinical characteristics and factors associated with term and late preterm infants that do not respond to inhaled nitric oxide (iNO). J Perinat Med. (2016) 44:663–8. doi: 10.1515/jpm-2015-0210
36. Heidersbach RS, Johengen MJ, Bekker JM, Fineman JR. Inhaled nitric oxide, oxygen, and alkalosis: dose-response interactions in a lamb model of pulmonary hypertension. Pediatr Pulmonol. (1999) 28:3–11. doi: 10.1002/(sici)1099-0496(199907)28:1<3::aid-ppul2>3.0.co;2-s
Keywords: persistent pulmonary hypertension of the newborn, neonatal death, nomogram prediction, risk factors, clinical outcome
Citation: Lin C, Mi J, Zhang Y, Duan S, Wu J and Li Y (2022) A nomogram prediction model for early death in patients with persistent pulmonary hypertension of the newborn. Front. Cardiovasc. Med. 9:1077339. doi: 10.3389/fcvm.2022.1077339
Received: 22 October 2022; Accepted: 07 December 2022;
Published: 22 December 2022.
Edited by:
Siyi He, General Hospital of Western Theater Command, ChinaReviewed by:
Liqun Sun, University of Toronto, CanadaCopyright © 2022 Lin, Mi, Zhang, Duan, Wu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinlin Wu, ✉ MzczMDUzNzg1QHFxLmNvbQ==; Yifei Li, ✉ bGl5Zndjc2hAc2N1LmVkdS5jbg==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.