
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 09 November 2022
Sec. Coronary Artery Disease
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.1051078
This article is part of the Research Topic Debates in Coronary Artery Disease: 2022 View all 6 articles
 Lifei Pan†
Lifei Pan† Zhitong Li†
Zhitong Li† Chenglin Li
Chenglin Li Xiaopeng Dong
Xiaopeng Dong Tesfaldet H. Hidru
Tesfaldet H. Hidru Fei Liu
Fei Liu Yunlong Xia
Yunlong Xia Xiaolei Yang
Xiaolei Yang Lei Zhong*
Lei Zhong* Ying Liu*
Ying Liu*Background: The occurrence of new-onset atrial fibrillation (NOAF) post-acute myocardial infarction (AMI) is associated with worse outcomes. In this study, we sought to assess the predictive effect of stress hyperglycemia ratio (SHR) and neutrophil to lymphocyte ratio (NLR) to predict NOAF in patients with AMI.
Materials and methods: We recruited 3,194 individuals with AMI but free of atrial fibrillation (AF). AMI cases were stratified into groups according to SHR and NLR quartiles and were further categorized based on diabetes status. High SHR and high NLR were defined as the highest quartile of SHR and NLR. A nomogram incorporating risk factors for NOAF was constructed using multivariate logistic regression analyses. The performance of the novel nomogram was tested for predictive performance, agreement between the actual and predicted probability, and clinical utility using area under the curve (AUC), bootstrapped calibration curves, and decision curve analysis, respectively.
Result: A total of 245 (7.67%) patients developed NOAF post-AMI. The NOAF cases had higher values of SHR and NLR than non-NOAF patients after AMI regardless of diabetes status. After adjusting for potential confounders, high SHR and NLR were independently associated with NOAF post-AMI. Moreover, the novel nomogram incorporating high NLR and high SHR for NOAF risk estimation in patients with AMI showed satisfactory performance assessed by the AUC, calibration curves, decision curve analysis.
Conclusion: SHR and NLR were independently associated with NOAF in AMI patients. The constructed novel nomogram that incorporates SHR and NLR might assist in NOAF risk stratification post-AMI.
Among many complications of acute myocardial infarction (AMI), new-onset atrial fibrillation (NOAF) is popular. The incidence of NOAF ranges from 6 to 21% and its presence predicts an increased risk of death (1, 2). However, the development of NOAF is complex and the mechanisms are still unclear (2, 3). Therefore, identifying high-NOAF-risk patients earlier could be helpful for better prognosis prediction, and tailoring the individual therapy strategy.
Stress hyperglycemia is regarded as the transient increase in blood glucose concentrations. It is suggested that stress hyperglycemia link with worse outcomes in cases of AMI regardless of diabetic status (4). In the past, many studies have utilized admission blood glucose (ABG) to mark stress hyperglycemia levels. However, ABG can be influenced by acute physiological stress or the presence of a chronic rise in baseline glucose levels (5). Consequently, Roberts et al. (6) proposed stress hyperglycemia ratio (SHR) to control bias related to background glycemia or acute hyperglycemia. In fact, SHR has been suggested as an accurate index to identify stress hyperglycemia than ABG and exhibited better predictive value in the prognosis of AMI (7). However, the predictive value of SHR on the risk of NOAF after AMI has been rarely reported.
Neutrophil to lymphocyte ratio (NLR), a surrogate marker of the state of systematic inflammation, has been widely studied. Established evidence indicates that NLR was closely linked to slow progress of cardiovascular events (CVE) or increased severity of CVD conditions (8, 9) and this association was more significant than neutrophil or lymphocyte alone and various leukocyte parameters (10). However, the predictive value of NLR for NOAF in patients with AMI has been less investigated.
As such, the current study is investigated to evaluate the independent predictive value of the SHR and NLR for NOAF in patients with AMI. Furthermore, in this study, we established a quantitative method for clinicians to predict the incidence of NOAF in patients with AMI by nomogram analysis.
The present study was a sub analysis of a previous study (11). The study was conducted based on clinical examinations carried out among AMI patients at the First Affiliated Hospital of Dalian Medical University (FAHDMU) between January 2016 and June 2021. We initially considered 5,763 AMI patients for coronary arteriography (CAG). We excluded participants with (i) a history of atrial fibrillation (AF) and/or atrial flutter (AFL) (n = 101), (ii) a history of cardiac valvular disease (n = 52), (iii) fasting blood glucose (FBG) < 3.9 mmol/L (n = 64), Hb < 100 g/L (n = 27), (iv) estimate glomerular filtration rate (eGFR) < 30 ml/min/1.73 m2 (n = 79), (v) active infection, chronic inflammatory, or immunologic disease or a history of glucocorticoid therapy within the past 3 months (n = 107), and (vi) lack of crucial laboratory data (glucose or HbA1c results) or other clinical data (n = 2,139). Finally, 3,194 patients were included in the analysis (Figure 1). The study was approved by the Ethics Committee of FAHDMU and complied with the principles of the Declaration of Helsinki. The electronic medical record of FAHDMU was utilized to retrieve data, therefore our study waived the obligation for informed consent.
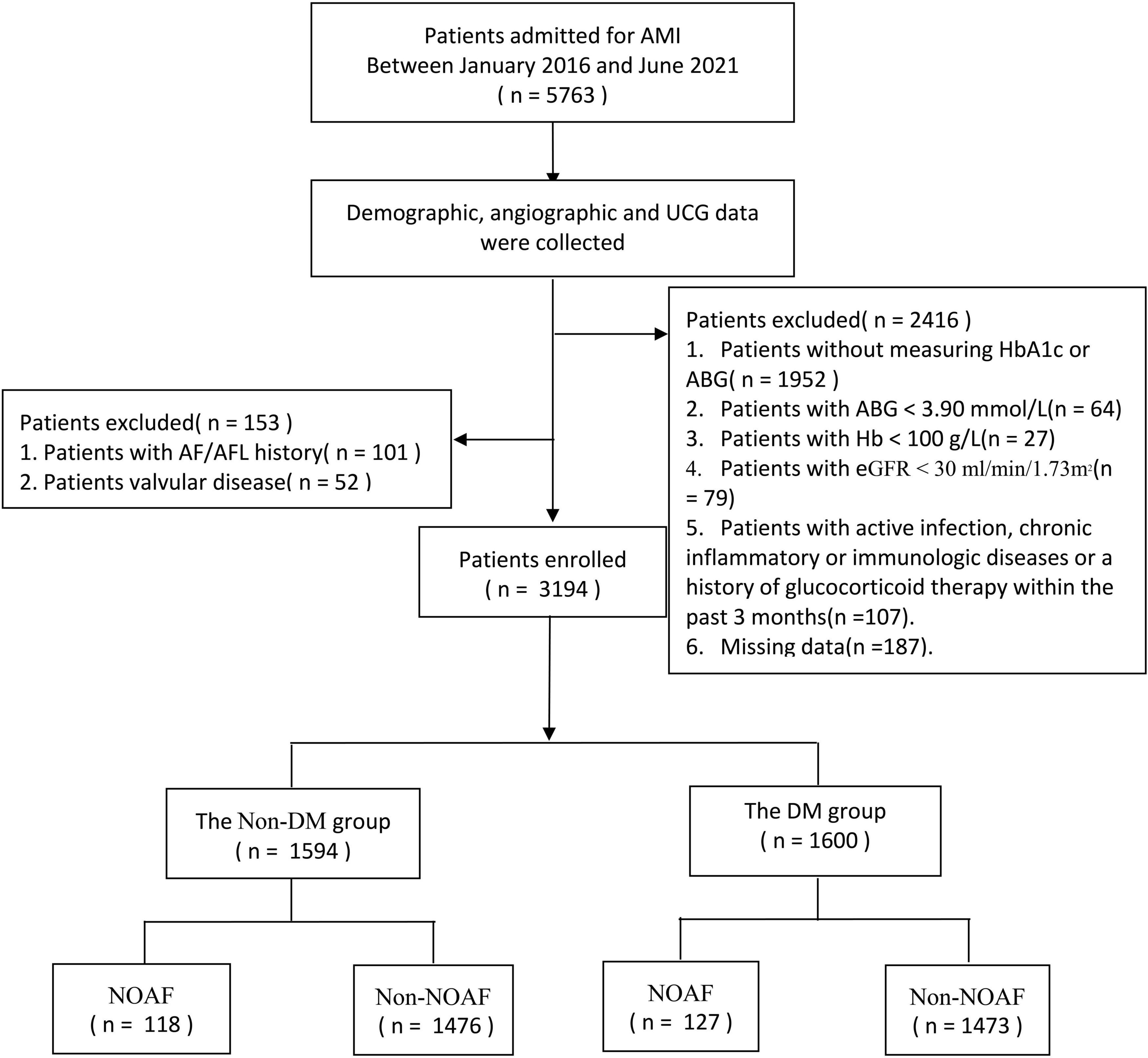
Figure 1. Flow chart. AMI, acute myocardial infarction; UCG, ultrasound cardiogram; AF, atrial fibrillation; AFL, atrial flutter; HbA1c, hemoglobin A1c; ABG, admission fasting blood gulcose; eGFR, estimated glomerular filtration rate; DM, diabetes mellitus; NOAF, new-onset atrial fibrillation.
In our study, those AMI cases with history of diabetes or HbA1c value of >6.5% were considered to have diabetes mellitus (DM) (12). Hypertension (HTN) was defined based on the rise of blood pressure (SBP ≥ 140 mmHg or DBP ≥ 90 mmHg) and/or positive history of antihypertensive drug use (13). Congestive heart failure (CHF) was defined as per previous studies (14). Elevated cardiac troponin values accompanied by clinical evidence for myocardial ischemia, presence of pathological Q wave on electrocardiogram (ECG), or evidence of new regional wall motion abnormality in echocardiography was defined as acute myocardial infarction (15). The SHR was calculated as the admission FBG divided by the average glucose: SHR = admission FBG/[1.59 × HbA1c (%) - 2.59] (16). The NLR output was computed as plasma neutrophil count (*109/L)/plasma lymphocyte count (*109/L) (17). AF was diagnosed as the absence of P waves, coarse or fine fibrillatory waves, and irregular RR intervals (18). If there was any proof of AF from the electrocardiography readings during the hospitalization period in AMI cases with no previous history of AF/AFL, we referred to them as new-onset AF cases.
The data for this study were analyzed using SPSS software (V.24.0, IBM, New York, NY, USA) and R software (V.4.0, R Foundation for Statistical Computing, Vienna, Austria). The population studied was stratified by diabetes status and the presence of NOAF. The distribution of continuous variables was evaluated by using the Kolmogorov–Smirnov test. The output of the normally distributed variables was presented as mean ± standard deviation (SD) whereas the output of the variables with skewed distribution was expressed as median (25th–75th interquartile). Categorical variables were expressed using frequency and percentage. Based on the results from Kolmogorov–Smirnov test, continuous data were compared using the Mann–Whitney U test or independent-samples T-test. For categorical variables, the chi-square test or Fisher exact test was used for comparison analysis and presented as a proportion. To compare the incidence of NOAF after AMI at different SHR and NLR levels, patients were further divided into four groups according to quartiles (Q) of SHR and NLR levels at baseline. High SHR and high NLR were defined as the highest quartile of SHR and NLR. We run binary logistic regression analysis to estimate the risk of NOAF for Q2, Q3, and Q4 of NLR and SHR, with Q1 of NLR/SHR serving as the reference for comparison. Only these variables with a P-value < 0.05 in the univariate analysis were included in the multivariate logistic model.
In this study, rms package of R, version 4.01 was used to construct a nomogram. The area under the receiver operating characteristic curve AUC was used to assess the predictive capacity of the novel nomogram. We estimated the bootstrapped calibration curves (1,000 resampling) to reflect the consistency between the actual and predicted probability. The decision curve analysis was checked to understand whether the net advantage of the nomogram remains appropriate for clinical use. A two-tailed P-value < 0.05 was considered statistically significant.
As shown in Table 1, 3,194 participants with AMI were enrolled in this research and stratified by diabetes status and the presence of NOAF. NOAF patients accounted for 7.67% (245/3,194) of the overall cohort, 7.94% (127/1,600) of DM patients, and 7.40% (118/1,594) of non-DM patients, respectively. Among these patients, 72 patients had AF/AFL rhythm at admission, the remaining patients were in sinus rhythm (SR). The baseline characteristics were comparable between participants with SR and AF/AFL rhythm at admission except for a higher heart rate, larger left atrium diameter, and longer hospitalization duration. At discharge, participants with AF/AFL rhythm at admission received more oral anticoagulants prescription and less antiplatelet drug prescription than those with NOAF. However, we found no significant difference in SHR and NLR in the two groups (Supplementary Table 1).
Overall, NOAF patients had increased values of SHR and NLR than those with SR after AMI regardless of diabetes status. Compared to patients without NOAF, patients with NOAF were older, more frequently females, or had more comorbidities. Moreover, patients with NOAF had higher CHA2DS2-VASc Score, GRACE score, and Killip classification compared to those with SR. Likewise, AMI cases who developed NOAF had elevated brain natriuretic peptide (BNP) levels, and left atrial dimension but lower eGFR and left ventricular ejection fraction (LVEF). Noteworthy, patients with NOAF experienced prolonged hospital stay compared with the patients in the SR group. At discharge, the use of OAC and diuretics were higher in patients with NOAF than in those without NOAF.
In our study, a total of 245 AMI patients were newly diagnosed with AF. Figure 2 shows the incidence of NOAF between diabetes and non-diabetes patients with AMI based on the quartiles of SHR/NLR. Regardless of the presence of diabetes, the incidence of NOAF was higher in the Q4 of SHR compared with AMI patients in Q1, Q2, and Q3 of SHR (Figure 2A). Similarly, as shown in Figure 2B, the incidence of NOAF was highest in Q4 of NLR compared with Q1, Q2, and Q3 of NLR regardless of diabetes status.
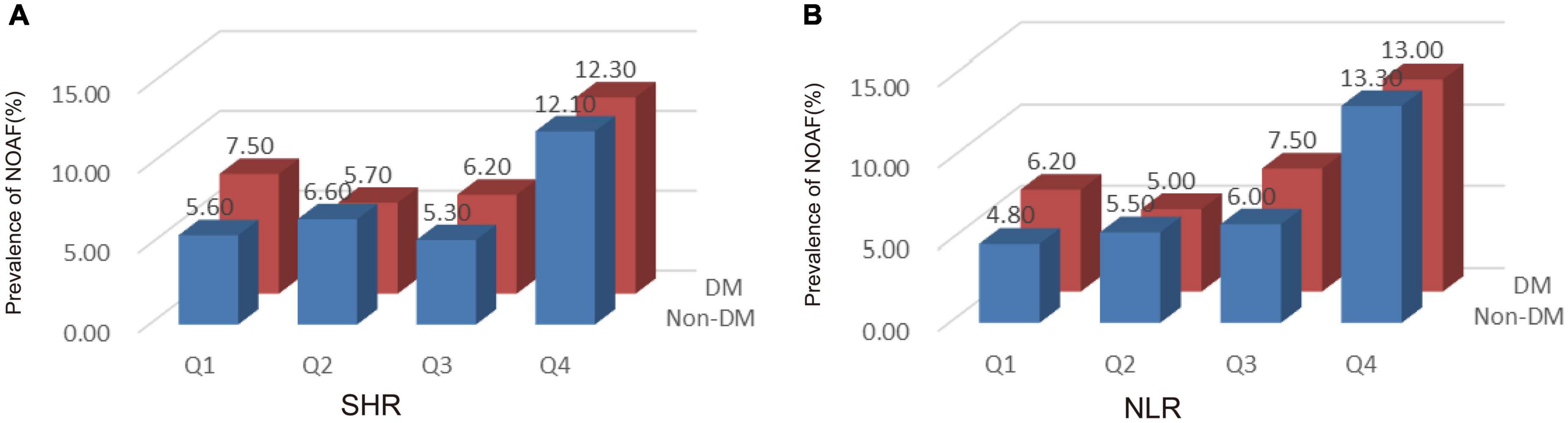
Figure 2. The incidence of new-onset atrial fibrillation (NOAF) in patients with acute myocardial infarction (AMI) according to tertiles of stress hyperglycemia ratio (SHR) (A) and neutrophil to lymphocyte ratio (NLR) (B). SHR, stress hyperglycemia ratio; DM, diabetes mellitus; NOAF, new-onset atrial fibrillation.
The corresponding increase in SHR and NLR was parallel with an increase in NOAF prevalence, suggesting the interaction between SHR and NLR may predict NOAF. As shown in Figure 3, patients in the highest quartile of SHR and NLR had the highest prevalence of NOAF.
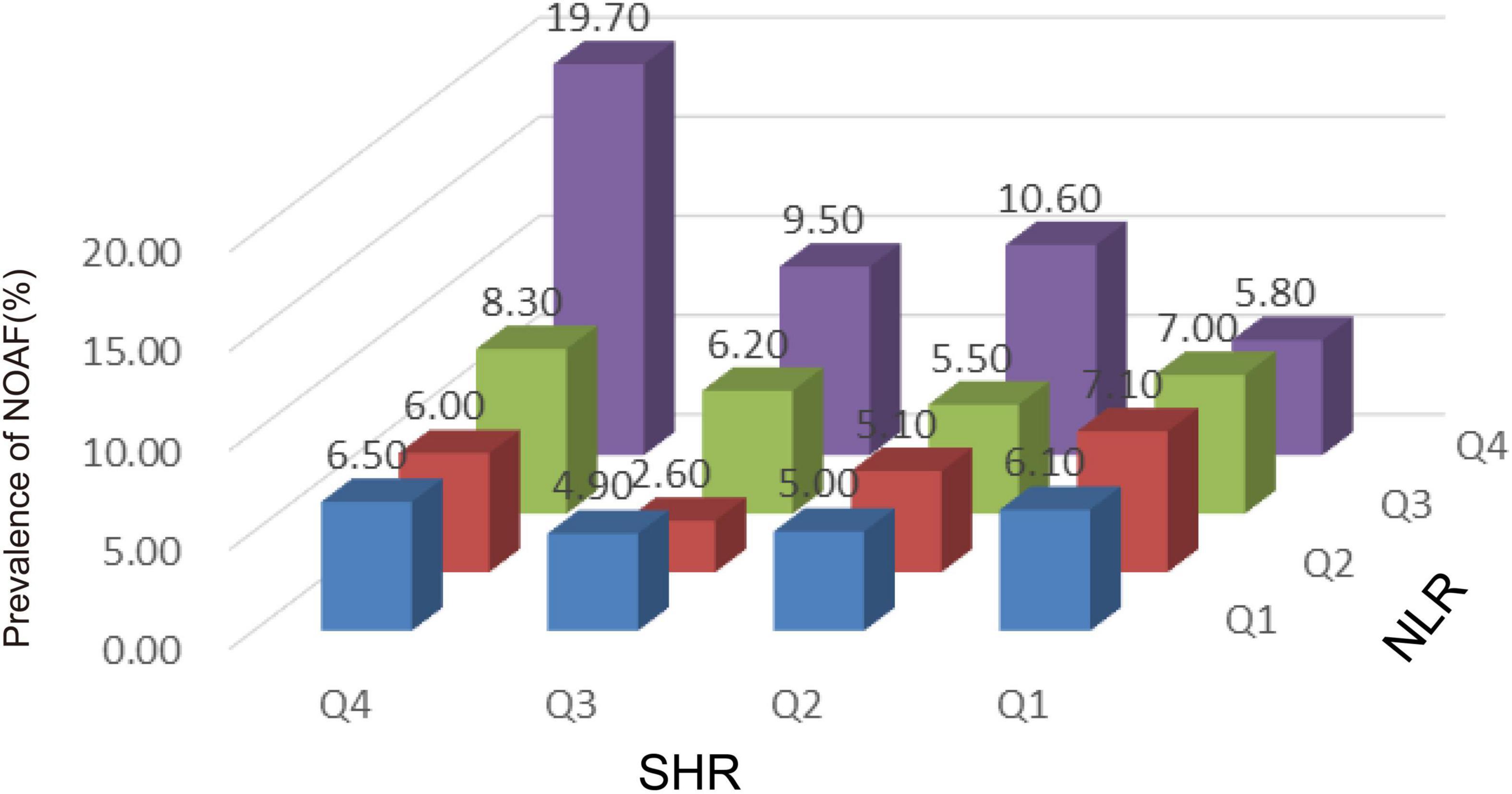
Figure 3. The incidence of new-onset atrial fibrillation (NOAF) in patients with acute myocardial infarction (AMI) based on stress hyperglycemia ratio (SHR) tertiles in patients grouped by neutrophil to lymphocyte ratio (NLR) tertiles. NLR, neutrophil to lymphocyte ratio; SHR, stress hyperglycemia ratio; NOAF, new-onset atrial fibrillation.
The association between SHR and NLR values and the risk of NOAF are given in Table 2 and Supplementary Table 2. We initially treated SHR and NLR as numerical variables. The estimated OR and 95% CI of the SHR and NLR for NOAF were 3.298 (95% CI: 1.415–7.690, P = 0.006) and 1.047 (95% CI: 1.013–1.082, P = 0.006), respectively. Again, when the values of SHR and NLR were categorized into quartiles, the adjusted OR and 95% CI for NOAF in Q4 of SHR/NLR in relation to their corresponding Q1 was 1.733 (95% CI: 1.130–2.657, P = 0.012) and 2.037 (95% CI: 1.341–3.095, P = 0.001), respectively.
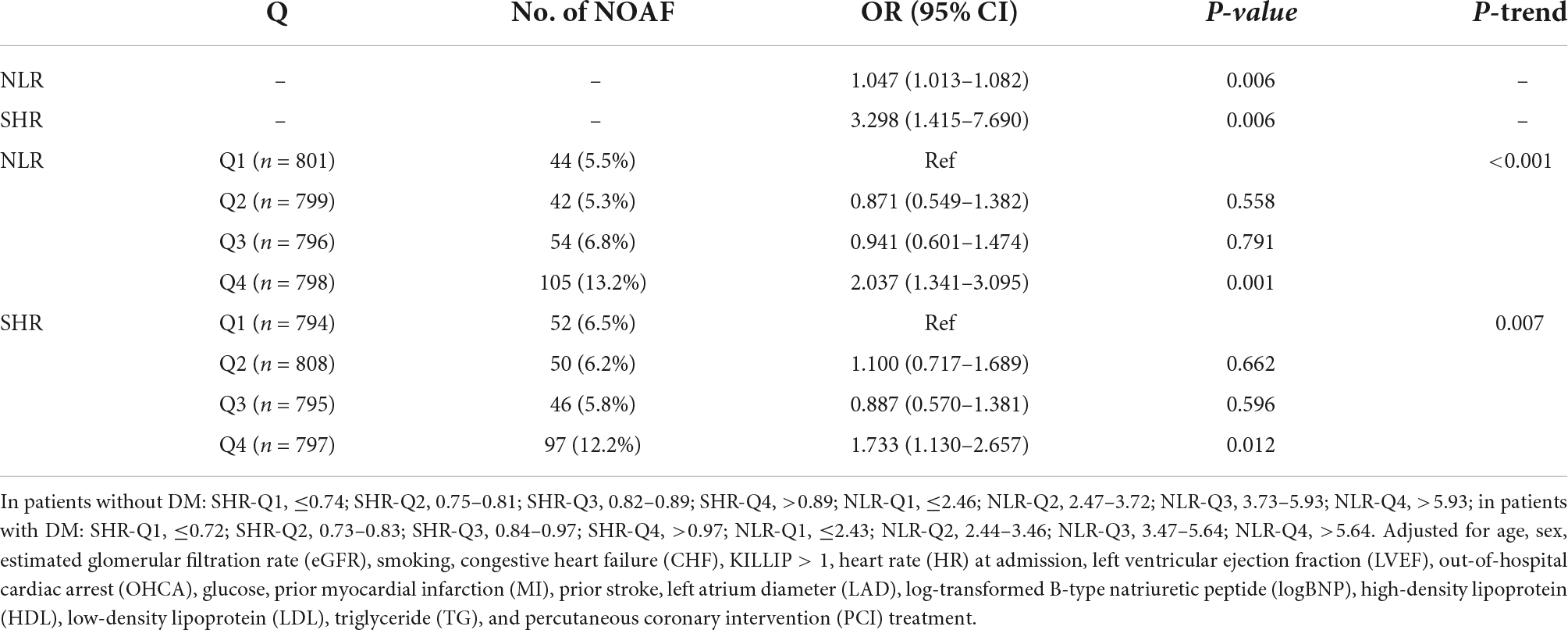
Table 2. The prevalence of new-onset atrial fibrillation (NOAF) and the risk estimate for NOAF based on the Q of stress hyperglycemia ratio (SHR).
In this study, age, prior MI, HR, log-transformed B-type natriuretic peptide (log BNP), HDL-C, LAD, LVEF, PCI treatment, high NLR, and high SHR were significant factors and were combined to establish a nomogram (Figure 4). As shown in Figure 5, the AUC of the nomogram was 0.795 (95% CI: 0.764–0.825). The calibration plots of the constructed nomogram confirmed that there was an adequate fit between the predicted risk and the observed risk of NOAF with a mean absolute error of 0.005 (Figure 6A). For a threshold probability between 10 and 90%, the nomogram had a higher net benefit for the prediction of NOAF, suggesting the clinical usefulness of the novel nomogram (Figure 6B).
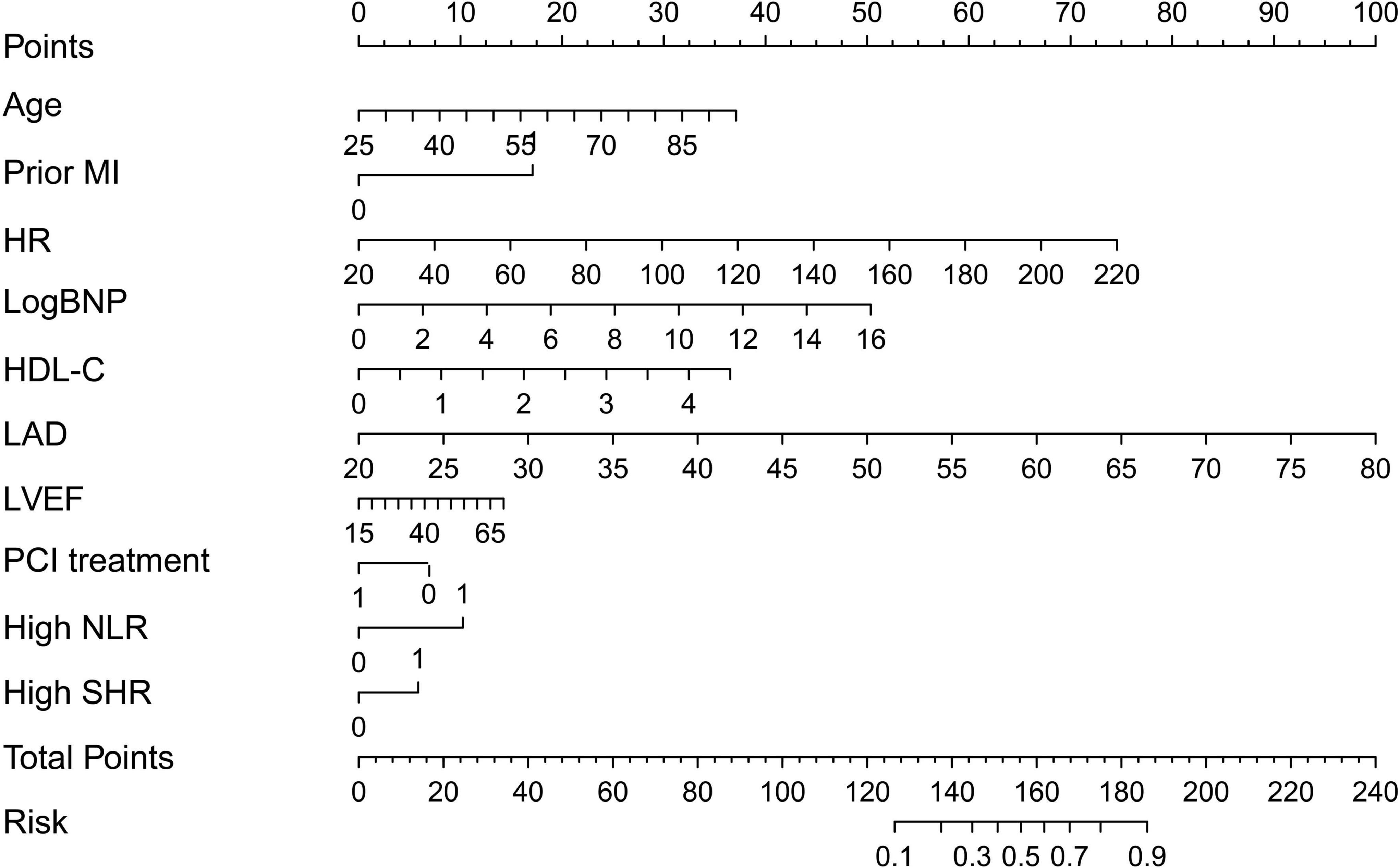
Figure 4. Nomogram to calculate risk score and predict the incidence of new-onset atrial fibrillation (NOAF) in patients with acute myocardial infarction (AMI). MI, myocardial infarction; HR, heart rate; LogBNP, log-transformed B-type natriuretic peptide; HDL, high-density lipoprotein; LAD, left atrium diameter; LVEF, left ventricular ejection fraction; PCI, percutaneous coronary intervention; NLR, neutrophil to lymphocyte ratio; SHR, stress hyperglycemia ratio.
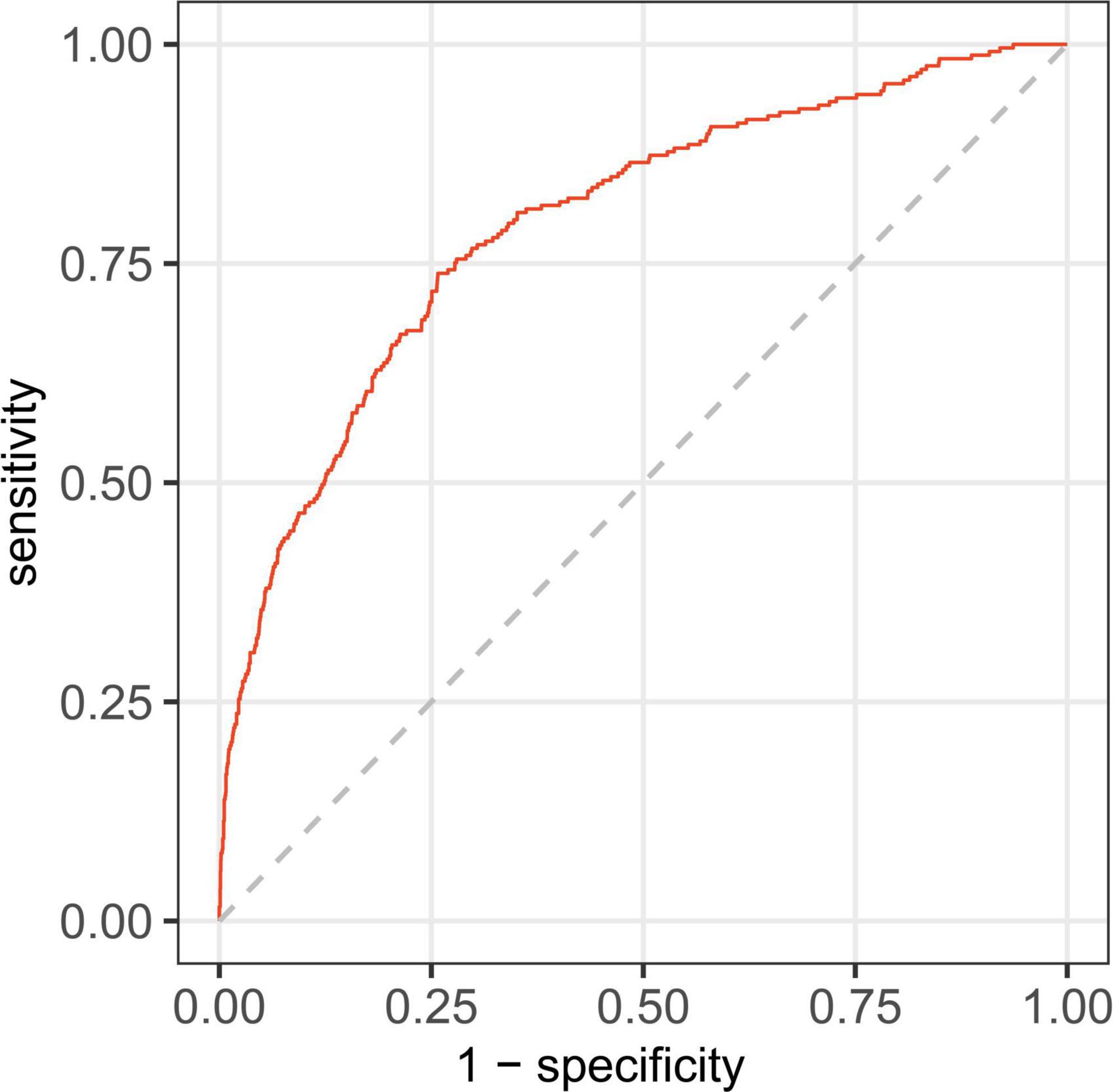
Figure 5. Receiver operating characteristic curve for nomogram in predicting the incidence of new-onset atrial fibrillation (NOAF) in patients with acute myocardial infarction (AMI).
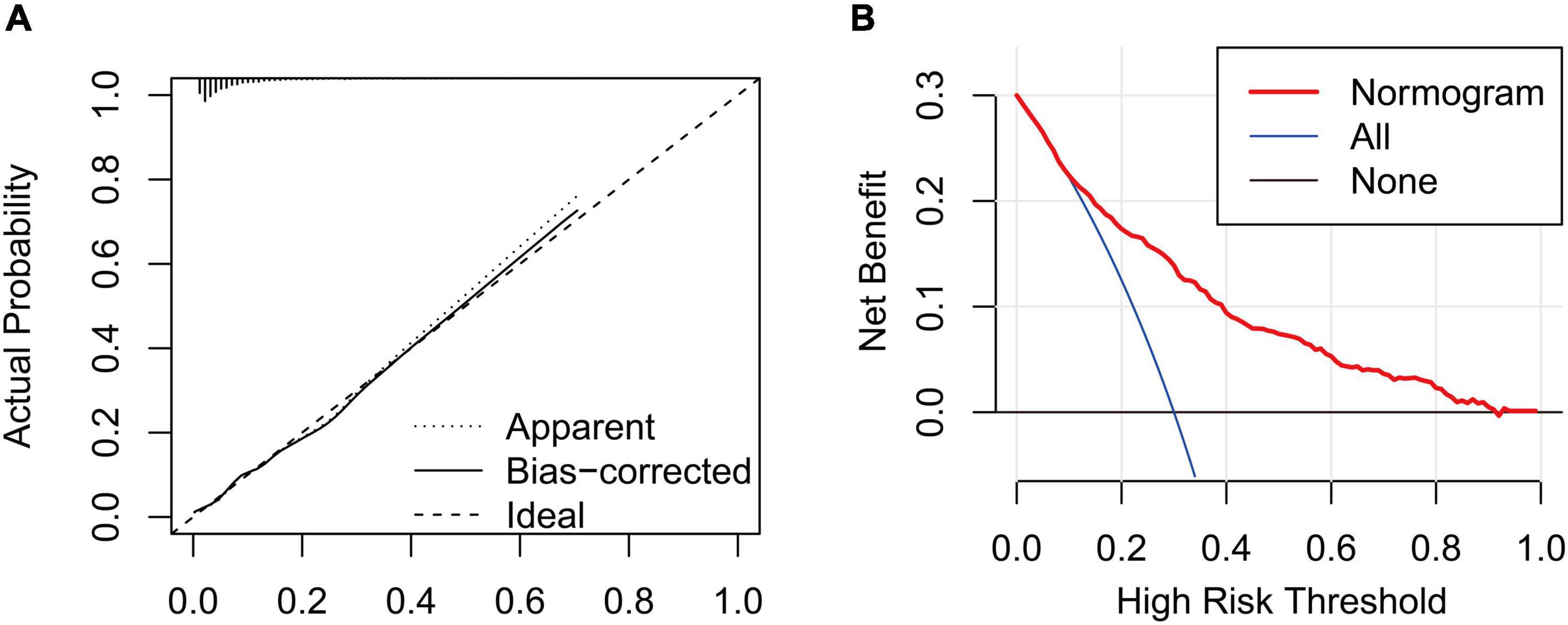
Figure 6. (A) Calibration curve for the nomogram to predict the incidence of new-onset atrial fibrillation (NOAF) in patients with acute myocardial infarction (AMI). The x-axis represents the predicted incidence of NOAF and the y-axis denotes the actual incidence of NOAF. The solid line shows the bias-corrected performance. 1,000 bootstrap repetitions; mean absolute error = 0.005; n = 3,194. (B) Decision curve analysis for the incidence of NOAF in patients with AMI, implicating the net benefit with respect to the use of the nomogram.
In this study, SHR and NLR were higher in patients with NOAF than in those with non-NOAF after AMI regardless of diabetes status. Also, SHR and NLR were independently associated with NOAF in AMI patients even after adjusting for other traditional risk factors. Importantly, a novel nomogram incorporating high NLR and high SHR was established and showed good prediction performance for NOAF in AMI patients. To the best of our knowledge, we are the first to construct a nomogram incorporating NLR and SHR to predict NOAF in AMI patients.
New-onset atrial fibrillation in patients with AMI is common in clinical practice and is highly associated with the risk of death and prolonged hospital stay (2, 19). The prevalence of NOAF in patients with AMI differs from one study to another (11, 20). In the present study, the prevalence of NOAF was 7.67%, which was consistent with previous studies (20).
The negative impact of SHR and its role in deteriorating the progress of AMI has been described before (12, 21). However, whether the effect of SHR was similar for NOAF in AMI patients with or without DM was not reported precisely. Here, we observed higher SHR values in AMI patients with NOAF than those without NOAF regardless of the diabetic condition. Interestingly, an elevated SHR index was significantly linked with NOAF than ABG or chronic hyperglycemia (surrogated by HbA1c). The mechanisms underlying the association between SHR and NOAF in patients with AMI have not been clearly elucidated, and several reasons may explain their relationship. First, the activation of adrenergic reaction and the flow of catecholamine’s after AMI during stress hyperglycemia may exacerbate inflammation and oxidative stress, which is associated with left atrial dilatation and increased incidence of AF (22, 23). Second, hyperglycemia leads to impaired mitochondrial function which associates with inflammation and oxidative stress (23). Third, the glycation end products formed from hyperglycemia (24) could lead to fibrosis and increased atrial stiffness. An increase in atrial stiffness and left atrial remodeling may be the major factors contributing to the occurrence of AF.
Inflammation plays a role in the repair and remodeling of infarcted heart tissue after the occurrence of AMI (25, 26). Moreover, inflammation participates in the initiation and perpetuation of AF (27). The inflammatory process during AMI involves neutrophils and lymphocytes. Neutrophils represent inflammation whereas lymphocytes represent the inflammatory reaction and the stress phase that exists in the body. In the past, these types of white blood cells (neutrophil and lymphocyte) were found to link with adverse outcomes in AMI cases (28, 29), where elevated neutrophils and depleted lymphocytes were associated with an increased risk of death. Recently, some studies demonstrated that NLR, as the ratio of neutrophil and lymphocyte, which may systematically and accurately reflect the degree of inflammation and stress, was turned out to be superior compared with neutrophil or lymphocyte alone and various leukocyte parameters (10). However, limited studies reported the relationship between NLR and NOAF in patients with AMI. Herein, we found a positive relationship between NLR and NOAF in patients with AMI, even after adjusting for confounding risk factors. Our findings are consistent with previous reports (30–32) highlighting the relationship between inflammation and AF development. Colchicine is an anti-inflammatory drug widely used to treat gout. Recent literature suggested that low-dose colchicine had cardiovascular benefits in patients with acute coronary syndrome through reducing inflammation (33). The effect of anti-inflammatory therapy on NOAF after AMI should also be studied in the future.
Nomograms are of great utility in predicting an individual’s probability of a clinical event using individual factors, and they are common prognostic tools in clinical settings (34, 35). In the present study, we constructed a novel nomogram composed of different risk factors for NOAF. The established nomogram may improve individualized risk stratification and tailor the individual therapy strategy for the primary prevention of NOAF in AMI patients. The satisfactory performance of this model was reflected by an AUC of more than 0.79. In addition, the calibration curve shows the prediction of NOAF in patients with AMI was similar to the actual NOAF in patients with AMI. Furthermore, decision curve analysis indicated that the nomogram would gain more net benefits with threshold probabilities of 0.1–0.9.
First of all, we could not exclude the possibility of selection bias given that part of the subjects were excluded due to missing data. Also, the number of patients who developed NOAF in this study is limited, further prospective, multicenter, large-sample studies are highly desirable. Second, we recorded the existence of NOAF only by ECG, which may underestimate the incidence of AF in our study. It is also possible that some patients with pre-existing asymptomatic AF have been erroneously regarded to have NOAF. Thirdly, very few patients had intensive ECG Holter monitoring after discharge, as a result, we have only data regarding NOAF during hospitalization period in patients with AMI. Future studies with long-term follow up are required. Finally, the nomogram has not been verified in the external validation queue, thus, external validations are needed to verify our findings in the future.
SHR and NLR are positively linked with the incidence of NOAF in patients with AMI. Furthermore, the novel nomogram might be helpful for risk stratification and tailoring the individual therapy strategy for the primary prevention of NOAF in patients with AMI.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Ethics Committee of the First Affiliated Hospital of Dalian Medical University. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
YL and LZ designed the study. LP and ZL were in charge of the data analysis. LP drafted the manuscript. LP, ZL, CL, XD, and FL conducted the data collection. TH, YX, and XY did the critical revision of the manuscript. All authors have read and approved the final manuscript.
This research was funded by the National Natural Science Foundation of China (grant number 81970286), the Chang Jiang Scholars Program (grant number T2017124), the Dalian Talents Innovation Supporting Project (grant number 2018RD09), the Program of Liaoning Distinguished Professor, and the Liaoning Revitalization Talents Program (grant number XLYC2002096).
We would like to acknowledge Yidu Cloud (Beijing) Technology Ltd., for their worthy cooperation in data searching, extraction, and processing.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.1051078/full#supplementary-material
1. Zarich SW, Nesto RW. Implications and treatment of acute hyperglycemia in the setting of acute myocardial infarction. Circulation. (2007) 115:e436–9. doi: 10.1161/circulationaha.105.535732
2. Petersen JK, Butt JH, Yafasova A, Torp-Pedersen C, Sørensen R, Kruuse C, et al. Incidence of ischaemic stroke and mortality in patients with acute coronary syndrome and first-time detected atrial fibrillation: a nationwide study. Eur Heart J. (2021) 42:4553–61. doi: 10.1093/eurheartj/ehab575
3. Boriani G, Vitolo M, Diemberger I, Proietti M, Valenti AC, Malavasi VL, et al. Optimizing indices of atrial fibrillation susceptibility and burden to evaluate atrial fibrillation severity, risk and outcomes. Cardiovasc Res. (2021) 117:1–21. doi: 10.1093/cvr/cvab147
4. Sönnerqvist C, Brus O, Olivecrona M. Validation of the scandinavian guidelines for initial management of minor and moderate head trauma in children. Eur J Trauma Emerg Surg. (2021) 47:1163–73. doi: 10.1007/s00068-019-01288-x
5. Wexler DJ, Nathan DM, Grant RW, Regan S, Van Leuvan AL, Cagliero E. Prevalence of elevated hemoglobin A1c among patients admitted to the hospital without a diagnosis of diabetes. J Clin Endocrinol Metab. (2008) 93:4238–44. doi: 10.1210/jc.2008-1090
6. Roberts GW, Quinn SJ, Valentine N, Alhawassi T, O’Dea H, Stranks SN, et al. Relative hyperglycemia, a marker of critical illness: introducing the stress hyperglycemia ratio. J Clin Endocrinol Metab. (2015) 100:4490–7. doi: 10.1210/jc.2015-2660
7. Xu W, Yang YM, Zhu J, Wu S, Wang J, Zhang H, et al. Predictive value of the stress hyperglycemia ratio in patients with acute ST-segment elevation myocardial infarction: insights from a multi-center observational study. Cardiovasc Diabetol. (2022) 21:48. doi: 10.1186/s12933-022-01479-8
8. Zahorec R. Ratio of neutrophil to lymphocyte counts–rapid and simple parameter of systemic inflammation and stress in critically ill. Bratislavske Lek Listy. (2001) 102:5–14.
9. Dentali F, Nigro O, Squizzato A, Gianni M, Zuretti F, Grandi AM, et al. Impact of neutrophils to lymphocytes ratio on major clinical outcomes in patients with acute coronary syndromes: a systematic review and meta-analysis of the literature. Int J Cardiol. (2018) 266:31–7. doi: 10.1016/j.ijcard.2018.02.116
10. Horne BD, Anderson JL, John JM, Weaver A, Bair TL, Jensen KR, et al. Which white blood cell subtypes predict increased cardiovascular risk? J Am Coll Cardiol. (2005) 45:1638–43. doi: 10.1016/j.jacc.2005.02.054
11. Zhitong Chenglin Zhitong Chenglin Li Z, Fei Liu Q, Liu F, Hidru TH, Yang Y, Wang S, et al. Atrial cardiomyopathy markers and new-onset atrial fibrillation risk in patients with acute myocardial infarction. Eur J Intern Med. (2022) 102:72–9. doi: 10.1016/j.ejim.2022.04.019
12. Sia CH, Chan MH, Zheng H, Ko J, Ho AF, Chong J, et al. Optimal glucose, HbA1c, glucose-HbA1c ratio and stress-hyperglycaemia ratio cut-off values for predicting 1-year mortality in diabetic and non-diabetic acute myocardial infarction patients. Cardiovasc Diabetol. (2021) 20:211. doi: 10.1186/s12933-021-01395-3
13. Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. (2018) 39:3021–104. doi: 10.1093/eurheartj/ehy339
14. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European society of cardiology (ESC)developed with the special contribution of the heart failure association (HFA) of the ESC. Eur Heart J. (2016) 37:2129–200. doi: 10.1093/eurheartj/ehw128
15. Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, et al. Fourth universal definition of myocardial infarction (2018). Circulation. (2018) 138:e618–51. doi: 10.1161/cir.0000000000000617
16. Chu H, Huang C, Tang Y, Dong Q, Guo Q. The stress hyperglycemia ratio predicts early hematoma expansion and poor outcomes in patients with spontaneous intracerebral hemorrhage. Ther Adv Neurol Disord. (2022) 15:17562864211070681. doi: 10.1177/17562864211070681
17. Wu S, Yang YM, Zhu J, Ren JM, Wang J, Zhang H, et al. Impact of baseline neutrophil-to-lymphocyte ratio on long-term prognosis in patients with atrial fibrillation. Angiology. (2021) 72:819–28. doi: 10.1177/00033197211000495
18. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. (2016) 37:2893–962. doi: 10.1093/eurheartj/ehw210
19. Xue Y, Zhou Q, Shen J, Liu G, Zhou W, Wen Y, et al. Lipid profile and new-onset atrial fibrillation in patients with acute ST-segment elevation myocardial infarction (an observational study in Southwest of China). Am J Cardiol. (2019) 124:1512–7. doi: 10.1016/j.amjcard.2019.07.070
20. Schmitt J, Duray G, Gersh BJ, Hohnloser STesfaldet H Hidrua Atrial fibrillation in acute myocardial infarction: a systematic review of the incidence, clinical features and prognostic implications. Eur Heart J. (2009) 30:1038–45. doi: 10.1093/eurheartj/ehn579
21. Yang Y, Kim TH, Yoon KH, Chung WS, Ahn Y, Jeong MH, et al. The stress hyperglycemia ratio, an index of relative hyperglycemia, as a predictor of clinical outcomes after percutaneous coronary intervention. Int J Cardiol. (2017) 241:57–63. doi: 10.1016/j.ijcard.2017.02.065
22. Wang A, Green JB, Halperin JL, Piccini JP Sr. Atrial fibrillation and diabetes mellitus: JACC review topic of the week. J Am Coll Cardiol. (2019) 74:1107–15. doi: 10.1016/j.jacc.2019.07.020
23. Karam BS, Chavez-Moreno A, Koh W, Akar JG, Akar FG. Oxidative stress and inflammation as central mediators of atrial fibrillation in obesity and diabetes. Cardiovasc Diabetol. (2017) 16:120. doi: 10.1186/s12933-017-0604-9
24. Zelniker TA, Jarolim P, Scirica BM, Braunwald E, Park JG, Das S, et al. Biomarker of collagen turnover (C-terminal telopeptide) and prognosis in patients with non- ST -elevation acute coronary syndromes. J Am Heart Assoc. (2019) 8:e011444. doi: 10.1161/jaha.118.011444
25. Frangogiannis NG. The inflammatory response in myocardial injury, repair, and remodelling. Nat Rev Cardiol. (2014) 11:255–65. doi: 10.1038/nrcardio.2014.28
26. Seropian IM, Toldo S, Van Tassell BW, Abbate A. Anti-inflammatory strategies for ventricular remodeling following ST-segment elevation acute myocardial infarction. J Am Coll Cardiol. (2014) 63:1593–603. doi: 10.1016/j.jacc.2014.01.014
27. Guo Y, Lip GY, Apostolakis S. Inflammation in atrial fibrillation. J Am Coll Cardiol. (2012) 60:2263–70. doi: 10.1016/j.jacc.2012.04.063
28. Meissner J, Irfan A, Twerenbold R, Mueller S, Reiter M, Haaf P, et al. Use of neutrophil count in early diagnosis and risk stratification of AMI. Am J Med. (2011) 124:534–42. doi: 10.1016/j.amjmed.2010.10.023
29. Núñez J, Núñez E, Bodí V, Sanchis J, Mainar L, Miñana G, et al. Low lymphocyte count in acute phase of ST-segment elevation myocardial infarction predicts long-term recurrent myocardial infarction. Coron Artery Dis. (2010) 21:1–7. doi: 10.1097/mca.0b013e328332ee15
30. Aronson D, Boulos M, Suleiman A, Bidoosi S, Agmon Y, Kapeliovich M, et al. Relation of C-reactive protein and new-onset atrial fibrillation in patients with acute myocardial infarction. Am J Cardiol. (2007) 100:753–7. doi: 10.1016/j.amjcard.2007.04.014
31. Hwang HJ, Ha JW, Joung B, Choi EH, Kim J, Ahn MS, et al. Relation of inflammation and left atrial remodeling in atrial fibrillation occurring in early phase of acute myocardial infarction. Int J Cardiol. (2011) 146:28–31. doi: 10.1016/j.ijcard.2009.05.065
32. Gedikli O, Orem C, Baykan M, Karahan C, Kucukosmanoglu M, Sahin S, et al. Association between serum C-reactive protein elevation and atrial fibrillation after first anterior myocardial infarction. Clin Cardiol. (2008) 31:482–7. doi: 10.1002/clc.2027653
33. Grajek S, Michalak M, Urbanowicz T, Olasinska-Wisniewska AA. Meta-analysis evaluating the colchicine therapy in patients with coronary artery disease. Front Cardiovasc Med. (2021) 8:740896. doi: 10.3389/fcvm.2021.740896
34. Yang M, Tao L, An H, Liu G, Tu Q, Zhang H, et al. A novel nomogram to predict all-cause readmission or death risk in Chinese elderly patients with heart failure. ESC Heart Fail. (2020) 7:1015–24. doi: 10.1002/ehf2.12703
Keywords: stress hyperglycemia ratio, neutrophil to lymphocyte ratio, acute myocardial infarction, atrial fibrillation, diabetes mellitus
Citation: Pan L, Li Z, Li C, Dong X, Hidru TH, Liu F, Xia Y, Yang X, Zhong L and Liu Y (2022) Stress hyperglycemia ratio and neutrophil to lymphocyte ratio are reliable predictors of new-onset atrial fibrillation in patients with acute myocardial infarction. Front. Cardiovasc. Med. 9:1051078. doi: 10.3389/fcvm.2022.1051078
Received: 22 September 2022; Accepted: 24 October 2022;
Published: 09 November 2022.
Edited by:
Tommaso Gori, Johannes Gutenberg University Mainz, GermanyReviewed by:
Ivan Zeljkovic, Sisters of Charity Hospital, CroatiaCopyright © 2022 Pan, Li, Li, Dong, Hidru, Liu, Xia, Yang, Zhong and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lei Zhong, ZG11emhvbmdsZWlAeWVhaC5uZXQ=; Ying Liu, eWluZ2xpdS5tZWRAZ21haWwuY29t
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.