- 1Klinik für Herz- und Kreislauferkrankungen, Deutsches Herzzentrum München, Technical University Munich, Munich, Germany
- 2Institut für Anästhesiologie, Deutsches Herzzentrum München, Technical University Munich, Munich, Germany
- 3Deutsches Zentrum für Herz- und Kreislauf-Forschung (DZHK) e.V. (German Center for Cardiovascular Research), Partner Site Munich Heart Alliance, Munich, Germany
Aims: To quantify extra-valvular cardiac damage associated with severe aortic valve stenosis (AS), a novel staging model was proposed. This study aimed to validate this model in patients undergoing transcatheter aortic valve replacement (TAVR) as well as to assess its prognostic impact.
Methods and results: Based on echocardiographic findings, the following stages were applied: isolated AS (stage 0), left ventricular (LV) damage (stage 1), left atrial or mitral valve damage (stage 2), pulmonary hypertension or tricuspid regurgitation (stage 3), or right ventricular dysfunction (stage 4). The primary endpoint was 2-year all-cause mortality. The distribution across stages was 0.8% at stage 0, 7.5% at stage 1, 63.3% at stage 2, 18.3% at stage 3, and 10.1% at stage 4. All-cause mortality increased at all stages 1–4 (12.1%, 18.2%, 26.6%, and 28.2%; p = 0.023). In the multivariate model, the stage of cardiac damage, age, New York Heart Association (NYHA) class III/IV, peripheral artery disease, and previous pacemaker were independent predictors of the primary endpoint.
Conclusions: Patients treated for severe AS show a high prevalence of extra-valvular cardiac damage. An increase in stage is associated with higher 2-year all-cause mortality. The application of this staging model may add value to current treatment algorithms.
Introduction
Aortic valve stenosis (AS) is the most commonly acquired heart valve defect in industrial nations, and its incidence rate is 4.4%/year in patients above 65 years of age (1). Surgical or transcatheter aortic valve replacement (TAVR) remains the standard therapy for severe AS. Currently, an indication for the treatment of AS is driven by the severity of stenosis and the clinical manifestation of symptoms. The decision to recommend conventional surgical vs. catheter-based aortic valve replacement depends on patients‘ age and comorbidities (2). With the exception of a reduced left ventricular (LV) ejection fraction (< 50%), no additional structural cardiac changes are currently included in the decision algorithm, although extra-valvular cardiac damage is believed to occur over time. After an initial adaptive response with concentric hypertrophy to maintain cardiac output in severe AS, this initially beneficial mechanism leads to both systolic and diastolic LV dysfunction, the time point at which most patients will develop symptoms (3). The damage then extends to the left atrium due to elevated filling pressures and leads to left atrial dilatation and remodeling with an increased risk of atrial fibrillation and mitral regurgitation (4). A secondary increase in pulmonary artery pressure may eventually lead to tricuspid regurgitation and right ventricular dysfunction (5, 6). Although concomitant cardiac damage has been described in numerous studies in patients with severe AS (7–10) and is expected to affect long-term outcomes, it is currently under debate whether it can be used as a prognostic parameter and thus be relevant for decision-making regarding the optimal timing of therapy. Recently, in the PARTNER II trial, Généreux et al. proposed the quantification of extra-valvular cardiac damage in patients with AS and found that this classification was significantly correlated with a worse outcome when applied to patients undergoing TAVR for severe AS (11). Therefore, we aimed to validate extra-valvular cardiac damage staging initially proposed by Généreux et al. in patients undergoing TAVR for severe AS stenosis in a real world, single-center population and to assess its impact on mid-term outcome. To increase statistical power, we dichotomized the proposed cardiac damage stages corresponding to isolated left heart dysfunction (0–2) vs. stages 3–4, i.e., the damage extending to the pulmonary circulation and right heart.
Methods
Patient population
Between 2011 and 2016, 1,118 patients underwent TAVR for symptomatic severe AS at the Deutsches Herzzentrum München, Munich, Germany. For the scope of this analysis, only patients treated by transfemoral access and without previous valve surgery (aortic, mitral, or tricuspid) were considered (n = 1,063). Of these, for 841 (79%) patients a complete echocardiographic data set was obtained at baseline to allow staging. For a detailed study flow chart, see Supplementary Figure S1. All cases were discussed in the multidisciplinary heart team, and a consensus on the therapeutic strategy was achieved. All patients gave their written informed consent for the procedure. TAVR was performed in a hybrid operating suite under general anesthesia or conscious sedation. Data were prospectively collected and classified according to the Valve Academic Research Consortium criteria (12). Data collection was approved by the ethics committee of the Faculty of Medicine at Technische Universität München.
Echocardiography and staging
Echocardiography was performed prior to the procedure in the hospital's echocardiography department. Two-dimensional, color-wave, pulsed-wave, and continuous Doppler technique images were obtained in parasternal and apical views according to current recommendations (13). The following parameters were assessed: LV ejection fraction, LV mass index, E/e', left atrial volume, aortic valve area, mean and maximum transaortic valve gradient, the grade of aortic, mitral, and tricuspid regurgitation, pulmonary artery pressure, and right ventricular function.
The extent of extra-aortic cardiac damage was retrospectively categorized into five stages according to the model previously described by Généreux et al. (11): stage 0: showing no extra-valvular cardiac damage; stage 1 with isolated LV damage defined by increased LV mass, LV ejection fraction < 50% or E/e'>14; stage 2 with left atrial dilatation >34 ml/m2, moderate-to-severe mitral regurgitation or the presence of atrial fibrillation; stage 3 with pulmonary hypertension ≥ 60 mmHg or moderate-to-severe tricuspid regurgitation; and stage 4 with moderate-to-severe right ventricular dysfunction.
Follow-up and definition of endpoints
Follow-up data were prospectively collected during routine ambulatory visits at the outpatient clinic, by referring to the treating physician, hospital documentation, or through telephone interview. Events were censored after 2 years or when the last event-free contact was made.
The primary endpoint of this analysis was to assess the impact of extra-valvular cardiac damage on all-cause mortality, while the secondary endpoint comprised a composite of all-cause mortality and rehospitalization for worsening congestive heart failure (CHF).
Statistical analysis
Categorical and ordinal variables are expressed as frequencies and proportions and were compared using the chi-squared or Fisher exact test. Continuous variables are expressed as mean with standard deviation (SD) or median with interquartile range and compared using the Student‘s t-test or the Mann–Whitney U-test, as appropriate. Survival and event rates during follow-up were calculated as Kaplan–Meier estimates, and a comparison of cumulative event rates between these groups was performed by a log-rank test. Two-sided p < 0.05 was considered statistically significant for all analyses. To increase statistical power, cardiac damage stages were dichotomized in stages 0–2 (isolated left heart dysfunction) vs. stages 3–4 (left and right heart dysfunction). To identify significant predictors of the primary and secondary endpoints, univariate Cox regression analysis was performed for all variables; clinically relevant variables yielding p < 0.05 in the univariate analysis were used for the multivariate Cox regression analysis.
IBM SPSS Statistics (Version 28, SPSS, Inc. Chicago, IL, USA) and R (Version 1.4.1103, The R Foundation, Vienna, Austria) were used for analyses.
Results
Patient population and cardiac damage staging
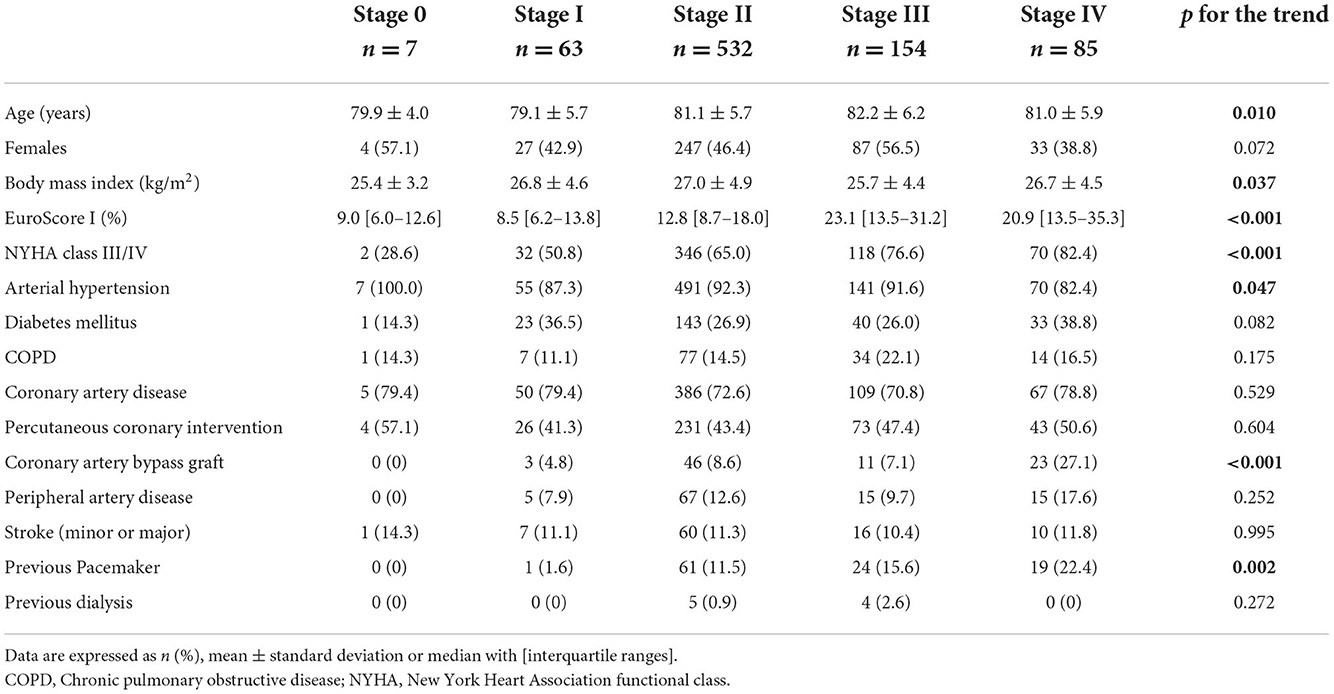
Overall, of the 841 patients enrolled in this study, the mean age was 81.1 ± 5.8 years and 47.3% (398/841) were women with a median logistic EuroScore of 14.2% (8.97–22.06). Pre-procedural echocardiographic staging classified 0.8% (7/841) of patients having no cardiac damage (stage 0), and 7.5% (63/841) and 63.3% (532/841) were graded in stages 1 and 2, respectively. 18.3% (154/841) of patients were in stage 3, while 10.1% (85/841) were in stage 4. The single component of each stage is depicted in Supplementary Table S1. Baseline characteristics according to the stage of cardiac damage are shown in Table 1. The number of patients suffering from New York Heart Association (NYHA) functional class III/IV increased stepwise with each stage of cardiac damage (p for the trend < 0.001). Furthermore, the logistic EuroScore showed a non-linear increase with each stage of cardiac damage, and patients in stages 2–4 had more frequently undergone coronary artery bypass grafting and previous pacemaker implantation.
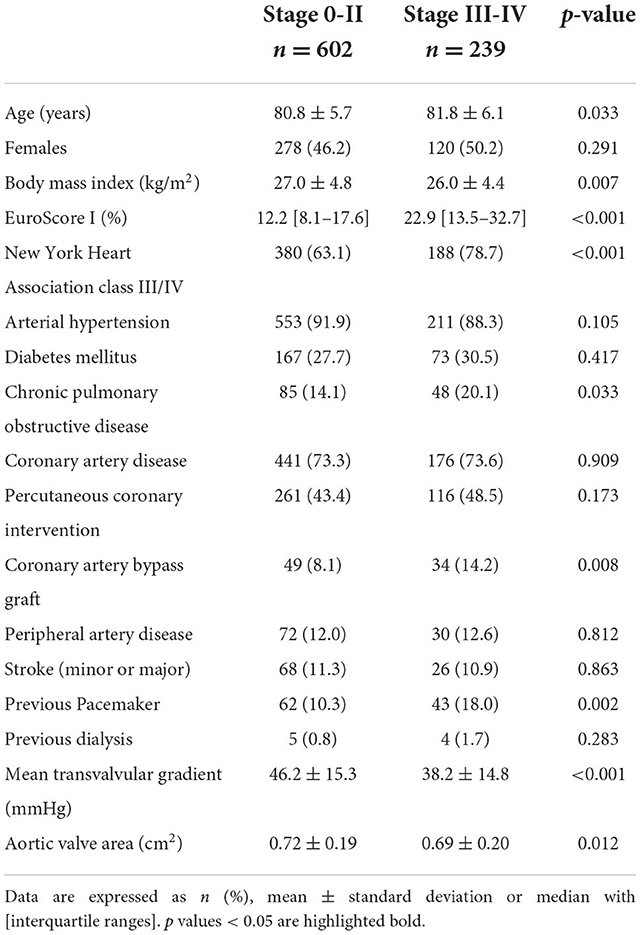
Table 2 shows baseline characteristics according to the dichotomized stages of cardiac damage. Patients in stages 3–4 were ~1 year older than patients in stages 0–2 (81.8 ± 6.1 vs. 80.8 ± 5.7 years; p = 0.033), and presented with significantly higher rates of comorbidities including higher rates of chronic obstructive pulmonary disease, previous coronary artery bypass grafting, and previous pacemaker implantation. Overall, patients in stages 3–4 had a higher preoperative logistic EuroScore I [22.9% (13.5–32.7) vs. 12.2% (8.1–17.6); p < 0.001] and higher rates of NYHA classes III–IV (78.7 vs. 63.1%; p < 0.001).
Two-year outcome according to cardiac damage
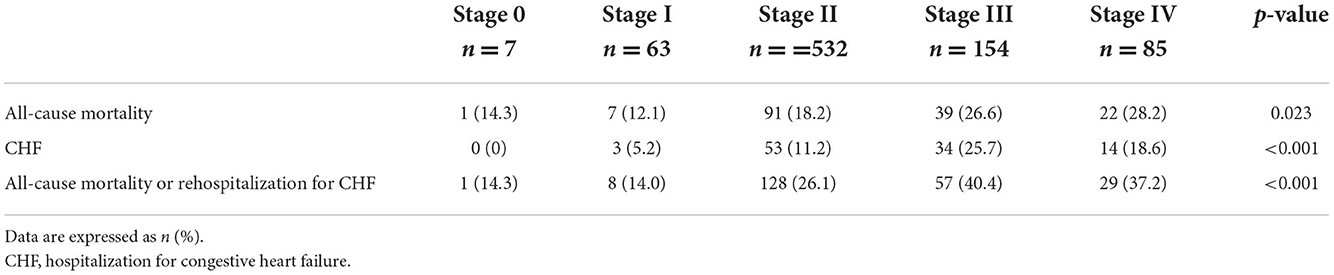
The median follow-up was 851 days (438–1,700), and a 2-year follow-up was completed for 722/841 (85.9) patients. At 2 years, all-cause mortality in the overall population was 20.3%, while the composite of all-cause mortality and CHF occurred in 26.5%. Clinical outcomes at 2 years differed between the individual stages of cardiac damage, as depicted in Table 3. The Kaplan–Meier survival curves showed a significant stepwise increase in all-cause mortality and the composite endpoint of all-cause mortality or rehospitalization for CHF across all stages of cardiac damage, except for stage 0 due to small numbers (Figures 1A,B).
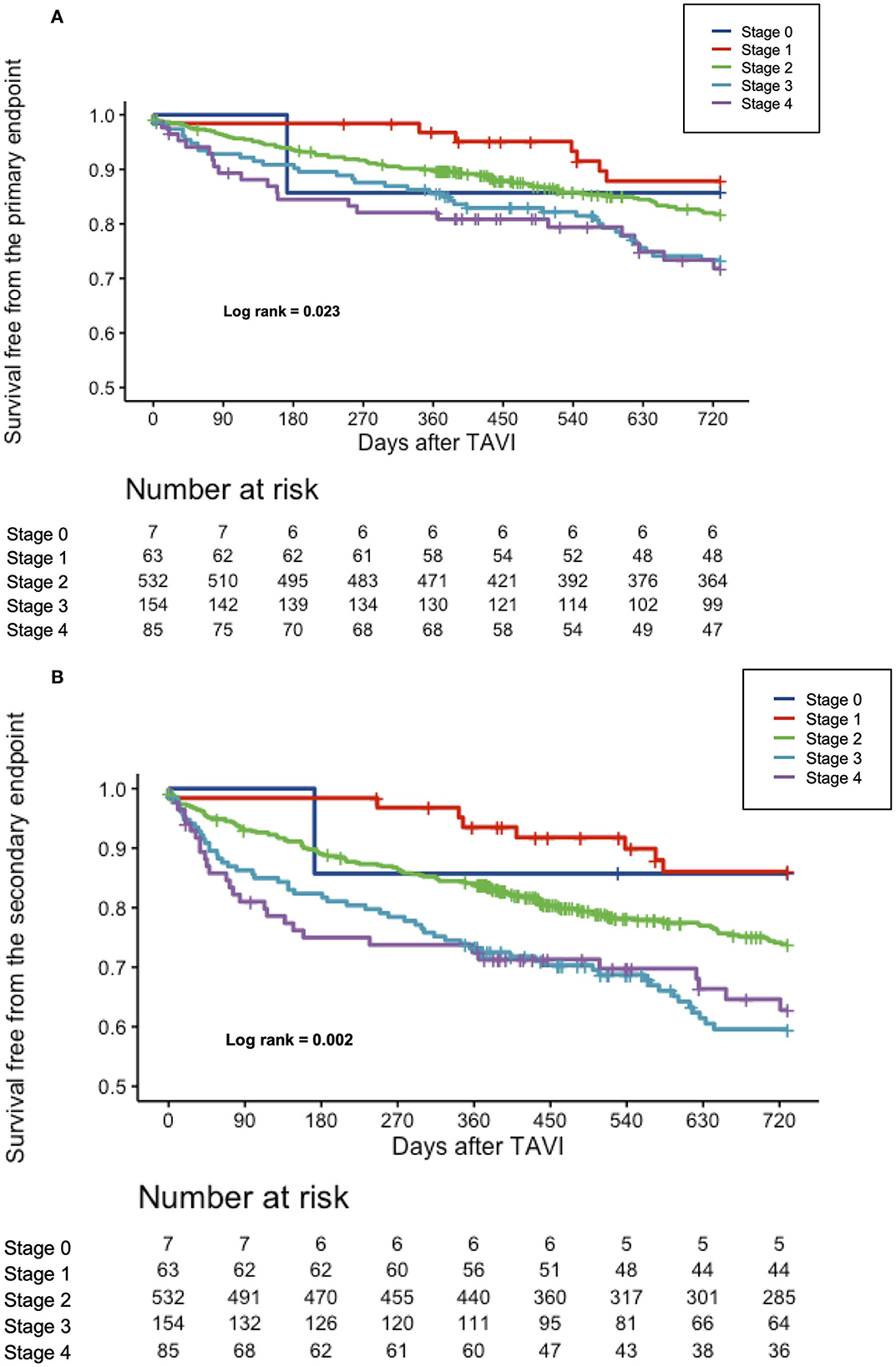
Figure 1. (A) Cumulative incidence of all-cause mortality according to the stages of cardiac damage. Kaplan–Meier curves showing cumulative incidence of all-cause mortality according to the stages of cardiac damage. (B) Cumulative incidence of the composite all-cause mortality or rehospitalization for worsening CHF according to the stages of cardiac damage. Kaplan–Meier curves showing cumulative incidence of the composite all-cause mortality according to the stages of cardiac damage.
The results were even more pronounced using the dichotomized stages of cardiac damage (stages 0–2 vs. 3–4), where the Kaplan–Meier survival analysis paralleled prior findings with almost doubled event rates for the primary and secondary endpoints for stages 3–4 of cardiac damage (Figures 2A,B). Supplementary Table S2 displays a 2-year clinical outcome according to the dichotomized stages of cardiac damage.
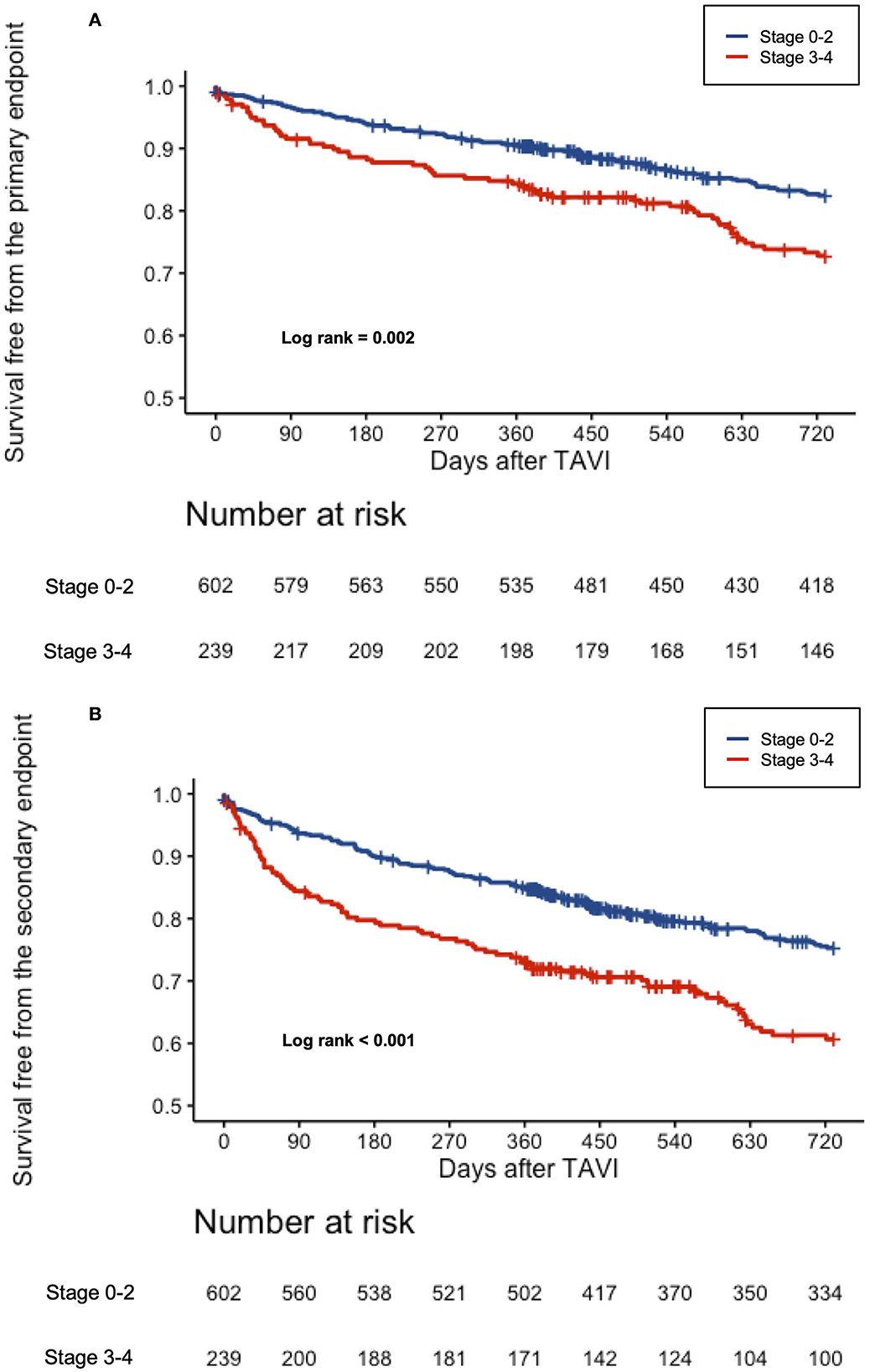
Figure 2. (A) Cumulative incidence of all-cause mortality according to the dichotomized stages of cardiac damage. Kaplan–Meier curves showing cumulative incidence of all-cause mortality according to the dichotomized stages of cardiac damage. (B) Cumulative incidence of the composite all-cause mortality or rehospitalization for worsening CHF according to the dichotomized stages of cardiac damage. Kaplan–Meier curves showing cumulative incidence of the composite all-cause mortality according to the dichotomized stages of cardiac damage.
Prognostic relevance of cardiac damage
To assess the impact of cardiac damage on the outcome, univariate and multivariate Cox regression analyses for the primary and secondary endpoints were performed; significant results are shown in Table 4. The stage of cardiac damage, age, NYHA class III/IV, peripheral artery disease, and previous pacemaker implantation were significant predictors of all-cause mortality. In detail, each step increase in the stage of cardiac damage resulted in a 30% higher risk for 2-year all-cause mortality (odds ratio (OR) 1.306, 95% confidence interval (CI): (1.051–2.622); p = 0.016).
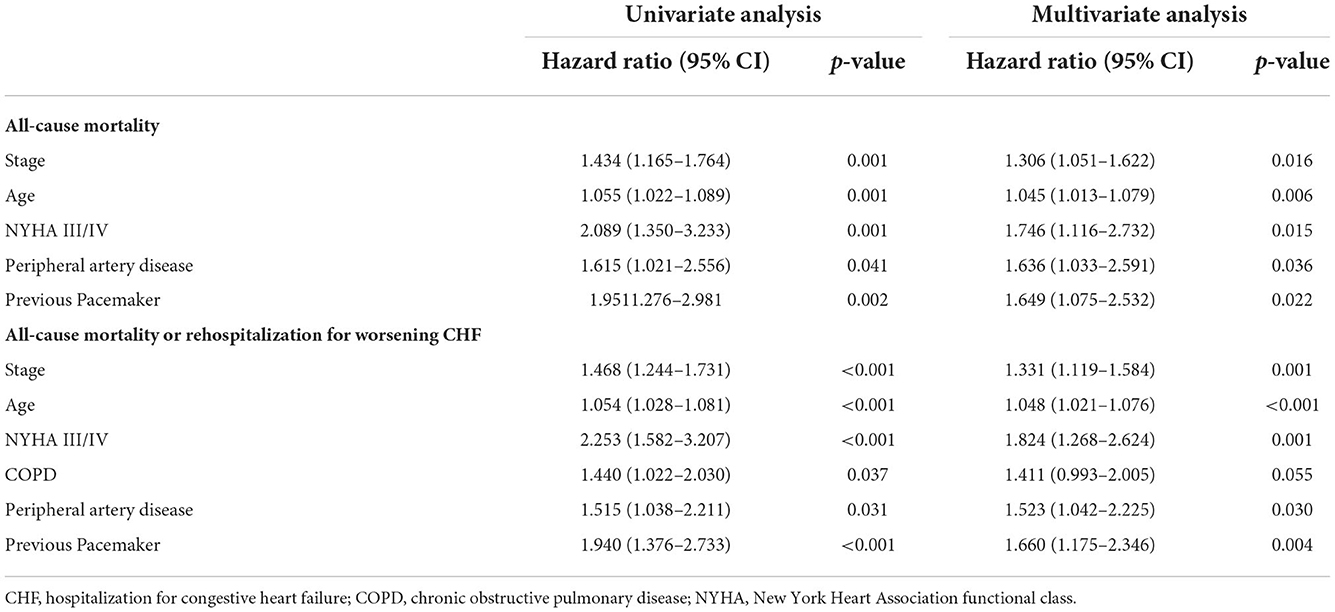
Table 4. Univariate and multivariate Cox proportional hazard analysis for variables yielding p < 0.05 for all-cause mortality and the composite of all-cause mortality or rehospitalization for worsening CHF at 2 years.
Similar findings were found for the secondary endpoint, where the stage of cardiac damage, age, NYHA III/IV, peripheral artery disease, and previous pacemaker were significant predictors in multivariate analysis. In particular, each increase in the stage of cardiac damage resulted in a 33% higher risk for all-cause mortality or rehospitalization for CHF [OR 1.331, 95% CI: (1.119–1.584); p = 0.001].
Discussion
The main findings of this study can be summarized as follows: in a large, single-center, real-world population, the application of the staging classification proposed by Généreux et al. showed a high prevalence of significant extra-valvular cardiac damage among patients referred for TAVR with symptomatic severe AS. Furthermore, clinical outcome worsened stepwise with increasing stage of cardiac damage. In multivariate analyses, increased cardiac damage stage independently predicted adverse outcome in terms of all-cause mortality and a composite of all-cause mortality or rehospitalization for worsening CHF. This finding was even more pronounced when considering a simplified dichotomized staging classification of stages 0–2 vs. 3–4.
In the original study of 1,661 patients with a mean age of 73 years undergoing aortic valve replacement for severe AS, Généreux et al. found the stage of cardiac damage to be the strongest predictor of 1-year all-cause mortality along with frailty and oxygen-dependent chronic obstructive pulmonary disease. For each increase in the stage of cardiac damage, the 1-year mortality risk increased by 45%. Similarly, in a strong population of 1,189 patients with severe AS with a mean age of 73 years, Vollema et al. confirmed the negative impact of cardiac damage staging on the outcome, in addition to age, previous myocardial infarction, renal function, and surgical or transcatheter AVR, which were all independently associated with all-cause mortality in the multivariable analysis (14). The current analysis differs from previous studies in that it only recruits patients undergoing TAVR, leading to obvious demographic differences, with a mean age of 81 years. Nevertheless, we found that cardiac damage staging was an independent predictor of all-cause mortality up to 2 years after TAVR, confirming its negative impact and reproducibility across different study populations. Similarly, Okuno et al. found that elderly patients with a high prevalence of right ventricular damage had a 5- to 7-fold increased risk of mortality at 1 year (15). It is not surprising that in this elderly population, age and clinical manifestation with regard to NYHA class III/IV were also independently associated with mortality. Recently, the cardiac damage staging model was applied to patients with asymptomatic moderate-to-severe AS, and 61% of patients were found to be in stage ≥2, with a markedly increased risk of mortality during a follow-up. The staging system may be applied to optimize the timing of TAVR procedures and identify asymptomatic patients with severe AS who may benefit from early valve replacement (16). It should also be noted that mortality and worsening of CHF in the current TAVI population, which is older and has many comorbidities, are multifactorial events that are not solely influenced by the extent of cardiac damage. Indeed, the presence of peripheral artery disease was associated with a poorer 2-year clinical outcome. Further, COPD, which was previously was associated with increased mortality after TAVR (17, 18), showed a trend toward a poorer clinical outcome, albeit without reaching statistical significance. To achieve the optimal clinical outcome, operators need to consider such comorbidities when planning treatment.
An indication for treatment is currently based on the extent of valve stenosis severity, symptoms, and LV ejection fraction, and guidelines further consider the presence of additional risk factors, such as STS-score or EuroScore, frailty, or other major organ dysfunction (19). The ongoing extension of an indication with TAVR will likely entail the need to tailor treatment and identify those who benefit the most. Finally, establishing the simplification of the staging classification using the dichotomized stages presented in this paper and differentiating patients with isolated LV damage from those with damage extending to the pulmonary circuit and right ventricle may help to increase the efficiency and accuracy of the clinical decision process in identifying patients who may best benefit from TAVR. Similarly, a recent retrospective analysis identified structural alterations in left and right heart morphology as sensitive indicators of poor prognosis after TAVR by applying unsupervised machine learning (20).
Furthermore, the question arises as to the best timing of treatment: symptomatic manifestation of AS is the principle underlying decision-making to proceed to TAVR; however, the onset of symptoms is often delayed and may manifest when irreversible damage has already occurred. In the analysis by Généreux et al., the stage of cardiac damage was a stronger predictor of mortality compared to any hemodynamic parameter, suggesting that the impact of extra-valvular damage secondary to AS often persists even after successful aortic valve replacement (11). In these cases, patients may possibly benefit from early intervention when irreparable extra-valvular damage has not yet occurred, especially since there is evidence that LV (21) and RV dysfunction can improve immediately after the relief of LV obstruction with TAVR (22). The treatment of this specific subset of patients is still controversial: while guidelines recommend treatment for asymptomatic very severe AS or rapid progression with low surgical risk, evidence is scarce (23, 24). Meanwhile, recent evidence suggests that early surgery in asymptomatic patients with aortic stenosis reduces mortality and death from cardiovascular causes (25), so further research on the optimal timing of transcatheter replacement is crucial. The ongoing randomized clinical Evaluation of TAVR Compared to SurveilLance for Patients With AsYmptomatic Severe Aortic Stenosis (Early TAVR) trial (ClinicalTrials.gov Identifier: NCT03042104) will soon shed light on this debate by enrolling asymptomatic patients with severe AS and comparing clinical surveillance to TAVR.
Strengths and limitations
Despite the inclusion of 841 strong, real-world patients treated at a high-volume center and presenting complete data with a 2-year follow-up, this analysis has some limitations: first of all, it is an observational study with self-adjudication of events, lacking a central core laboratory. Furthermore, intra- and inter-observer variability inherent in echocardiography cannot be ruled out as a possible confounder in this analysis. There was no systematic assessment of symptomatic status improvement during the follow-up.
Conclusions
Patients undergoing TAVR for severe AS show a high prevalence of extra-valvular cardiac damage. An increase in the stage of cardiac damage is associated with higher 2-year all-cause mortality. The application of this staging model may add value to current treatment algorithms. Further prospective studies are warranted to confirm the additive value of the proposed staging system in patients with severe AS.
Impacts on daily practice
Severe AS may be associated with extra-valvular cardiac damage. Recently, Généreux et al. proposed a staging model with stages 0–4 according to the severity of the echocardiographic findings of extra-valvular cardiac damage. In a large population of patients undergoing TAVR, we found a high prevalence of extended cardiac damage. Further, the stage of cardiac damage was associated with higher 2-year all-cause mortality and was found to be an independent predictor of 2-year all-cause mortality in a multivariable model. The application of this staging model may add value to current treatment algorithms for patients with severe AS undergoing TAVR.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics Committee of the Faculty of Medicine at Technische Universität München. The patients/participants provided their written informed consent to participate in this study.
Author contributions
CP, CD, TR, AB, PN, FS, and HA-C contributed to the conception and implementation of the study, to acquire and process the data, perform the statistical analysis, and to write the manuscript. TT, NM, and HA-C verified the analytical methods. EX and MJ supervised the analysis and writing of the manuscript. All authors discussed the results and contributed to the final manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.1039208/full#supplementary-material
Supplementary Figure 1. Study flow chart.
Supplementary Table 1. Incidence of cardiac damage stages and their individual components.
Supplementary Table 2. 2-year outcome according to dichotomized stages of cardiac damage.
Abbreviations
AS, aortic valve stenosis; CHF, congestive heart failure; LV, left ventricular; TAVR, transcatheter aortic valve replacement.
References
1. Durko AP, Osnabrugge RL, Van Mieghem NM, Milojevic M, Mylotte D, Nkomo VT, et al. Annual number of candidates for transcatheter aortic valve implantation per country: current estimates and future projections. Eur Heart J. (2018) 39:2635–42. doi: 10.1093/eurheartj/ehy107
2. Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, et al. 2021 ESC/EACTS Guidelines for the management of valvular heart diseaseDeveloped by the Task Force for the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. (2022) 43:561–632. doi: 10.1093/eurheartj/ehab395
3. Carabello BA, Paulus WJ. Aortic stenosis. Lancet. (2009) 373:956–66. doi: 10.1016/S0140-6736(09)60211-7
4. Tarantini G, Mojoli M, Urena M, Vahanian A. Atrial fibrillation in patients undergoing transcatheter aortic valve implantation: epidemiology, timing, predictors, and outcome. Eur Heart J. (2017) 38:1285–93. doi: 10.1093/eurheartj/ehw456
5. Bohbot Y, Guignant P, Rusinaru D, Kubala M, Maréchaux S, Tribouilloy C. Impact of right ventricular systolic dysfunction on outcome in aortic stenosis. Circ Cardiovasc Imaging. (2020) 13:9802. doi: 10.1161/CIRCIMAGING.119.009802
6. Kammerlander AA, Marzluf BA, Graf A, Bachmann A, Kocher A, Bonderman D, et al. Right ventricular dysfunction, but not tricuspid regurgitation, is associated with outcome late after left heart valve procedure. J Am Coll Cardiol. (2014) 64:2633–42. doi: 10.1016/j.jacc.2014.09.062
7. Zilberszac R, Gleiss A, Binder T, Laufer G, Grimm M, Gabriel H, et al. Prognostic relevance of mitral and tricuspid regurgitation in patients with severe aortic stenosis. Eur Heart J Cardiovasc Imaging. (2018) 19:985–92. doi: 10.1093/ehjci/jey027
8. Schwartz LA, Rozenbaum Z, Ghantous E, Kramarz J, Biner S, Ghermezi M, et al. Impact of right ventricular dysfunction and tricuspid regurgitation on outcomes in patients undergoing transcatheter aortic valve replacement. J Am Soc Echocardiogr. (2017) 30:36–46. doi: 10.1016/j.echo.2016.08.016
9. Zlotnick DM, Ouellette ML, Malenka DJ, Desimone JP, Leavitt BJ, Helm RE, et al. Effect of preoperative pulmonary hypertension on outcomes in patients with severe aortic stenosis following surgical aortic valve replacement. Am J Cardiol. (2013) 112:1635–40. doi: 10.1016/j.amjcard.2013.07.025
10. Barbanti M, Webb JG, Hahn RT, Feldman T, Boone RH, Smith CR, et al. Impact of preoperative moderate/severe mitral regurgitation on 2-year outcome after transcatheter and surgical aortic valve replacement: insight from the Placement of Aortic Transcatheter Valve (PARTNER) Trial Cohort A. Circulation. (2013) 128:2776–84. doi: 10.1161/CIRCULATIONAHA.113.003885
11. Généreux P, Pibarot P, Redfors B, Mack MJ, Makkar RR, Jaber WA, et al. Staging classification of aortic stenosis based on the extent of cardiac damage. Eur Heart J. (2017) 38:3351–8. doi: 10.1093/eurheartj/ehx381
12. Kappetein AP, Head SJ, Généreux P, Piazza N, Van Mieghem NM, Blackstone EH, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document (VARC-2). Eur J Cardiothorac Surg. (2012) 42:45–60. doi: 10.1093/ejcts/ezs533
13. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. (2015) 16:233–71. doi: 10.1093/ehjci/jev014
14. Vollema EM, Amanullah MR, Ng ACT, van der Bijl P, Prevedello F, Sin YK, et al. Staging cardiac damage in patients with symptomatic aortic valve stenosis. J Am Coll Cardiol. (2019) 74:538–49. doi: 10.1016/j.jacc.2019.05.048
15. Okuno T, Heg D, Lanz J, Praz F, Brugger N, Stortecky S, et al. Refined staging classification of cardiac damage associated with aortic stenosis and outcomes after transcatheter aortic valve implantation. Eur Hear J. (2021) 7:532–41. doi: 10.1093/ehjqcco/qcab041
16. Tastet L, Tribouilloy C, Maréchaux S, Vollema EM, Delgado V, Salaun E, et al. Staging cardiac damage in patients with asymptomatic aortic valve stenosis. J Am Coll Cardiol. (2019) 74:550–63. doi: 10.1016/j.jacc.2019.04.065
17. Mok M, Nombela-Franco L, Dumont E, Urena M, Delarochellière R, Doyle D, et al. Chronic obstructive pulmonary disease in patients undergoing transcatheter aortic valve implantation: insights on clinical outcomes, prognostic markers, and functional status changes. JACC Cardiovasc Interv. (2013) 6:1072–84. doi: 10.1016/j.jcin.2013.06.008
18. Ribeiro HB, Lerakis S, Gilard M, Cavalcante JL, Makkar R, Herrmann HC, et al. Transcatheter aortic valve replacement in patients with low-flow, low-gradient aortic stenosis: the TOPAS-TAVI Registry. J Am Coll Cardiol. (2018) 71:1297–308. doi: 10.1016/j.jacc.2018.01.054
19. Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. (2017) 38:2739–86. doi: 10.1093/eurheartj/ehx391
20. Lachmann M, Rippen E, Schuster T, Xhepa E, von Scheidt M, Pellegrini C, et al. Subphenotyping of patients with aortic stenosis by unsupervised agglomerative clustering of echocardiographic and hemodynamic data. Cardiovasc Interv. (2021) 14:2127–40. doi: 10.1016/j.jcin.2021.08.034
21. Graziani F, Cialdella P, Lillo R, Locorotondo G, Genuardi L, Ingrasciotta G, et al. Acute haemodynamic impact of transcatheter aortic valve implantation in patients with severe aortic stenosis. ESC Hear Fail. (2022) 9:1721. doi: 10.1002/ehf2.13846
22. Eleid MF, Padang R, Pislaru S V, Greason KL, Crestanello J, Nkomo VT, et al. Effect of transcatheter aortic valve replacement on right ventricular–pulmonary artery coupling. JACC Cardiovasc Interv. (2019) 12:2145–54. doi: 10.1016/j.jcin.2019.07.025
23. Lim WY, Ramasamy A, Lloyd G, Bhattacharyya S. Meta-analysis of the impact of intervention versus symptom-driven management in asymptomatic severe aortic stenosis. Heart. (2017) 103:268–72. doi: 10.1136/heartjnl-2016-309830
24. Généreux P, Stone GW, O'Gara PT, Marquis-Gravel G, Redfors B, Giustino G, et al. Natural history, diagnostic approaches, and therapeutic strategies for patients with asymptomatic severe aortic stenosis. J Am Coll Cardiol. (2016) 67:2263–88. doi: 10.1016/j.jacc.2016.02.057
Keywords: aortic stenosis, cardiac damage, femoral, transcatheter aortic valve implantation (TAVI), transthoracic echocardiogram, stages of cardiac damage
Citation: Pellegrini C, Duesmann C, Rheude T, Berg A, Alvarez-Covarrubias HA, Trenkwalder T, Mayr NP, Schürmann F, Nicol P, Xhepa E and Joner M (2022) The impact of extra-valvular cardiac damage on mid-term clinical outcome following transcatheter aortic valve replacement in patients with severe aortic stenosis. Front. Cardiovasc. Med. 9:1039208. doi: 10.3389/fcvm.2022.1039208
Received: 07 September 2022; Accepted: 01 November 2022;
Published: 01 December 2022.
Edited by:
Sebastian Ludwig, University Medical Center Hamburg-Eppendorf, GermanyReviewed by:
Hannah Billig, University Hospital Bonn, GermanyAtsushi Sugiura, University of Bonn, Germany
Copyright © 2022 Pellegrini, Duesmann, Rheude, Berg, Alvarez-Covarrubias, Trenkwalder, Mayr, Schürmann, Nicol, Xhepa and Joner. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michael Joner, am9uZXJAZGhtLm1obi5kZQ==
†These authors have contributed equally to this work
 Costanza Pellegrini
Costanza Pellegrini Charlotte Duesmann
Charlotte Duesmann Tobias Rheude
Tobias Rheude Amelie Berg
Amelie Berg Hector A. Alvarez-Covarrubias
Hector A. Alvarez-Covarrubias Teresa Trenkwalder1
Teresa Trenkwalder1 N. Patrick Mayr
N. Patrick Mayr Philipp Nicol
Philipp Nicol Erion Xhepa
Erion Xhepa