
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med., 07 November 2022
Sec. Cardiac Rhythmology
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.1036574
This article is part of the Research TopicInsights in Cardiac Rhythmology: 2022View all 17 articles
 Massimiliano Marini1,2*†
Massimiliano Marini1,2*† Luigi Pannone2†
Luigi Pannone2† Stefano Branzoli3,4
Stefano Branzoli3,4 Francesca Tedoldi1
Francesca Tedoldi1 Giovanni D’Onghia1
Giovanni D’Onghia1 Diego Fanti1
Diego Fanti1 Emanuele Sarao1
Emanuele Sarao1 Fabrizio Guarracini1
Fabrizio Guarracini1 Silvia Quintarelli1
Silvia Quintarelli1 Cinzia Monaco2
Cinzia Monaco2 Angelo Graffigna3
Angelo Graffigna3 Roberto Bonmassari1
Roberto Bonmassari1 Mark La Meir4
Mark La Meir4 Gian Battista Chierchia2
Gian Battista Chierchia2 Carlo de Asmundis2
Carlo de Asmundis2Background: Left atrial appendage (LAA) is a common source of thrombi in patients with atrial fibrillation (AF). The effect on left atrial (LA) function of the Totally Thoracoscopic (TT)-LAA exclusion with epicardial clip is currently unknown. This study aims at evaluating the effect of TT-LAA exclusion on LA function.
Methods: Standalone TT-LAA exclusion with the clip device was performed in 26 patients with AF and contraindication to oral anticoagulation (OAC). A 3D CT scan, trans-esophageal echocardiography, spirometry and cerebrovascular doppler ultrasound were performed preoperatively. Clip positioning and LAA exclusion were guided and confirmed by intraoperative trans-esophageal echo. To evaluate LA function, standard transthoracic echocardiography and 2D strain of LA were performed before surgery, at discharge and at 3-month follow-up.
Results: The mean CHA2DS2-VASc and HASBLED scores were 4.6 and 2.4 respectively. There were no major complications during the procedure. At median follow-up of 10.3 months, 1 (3.8%) non-cardiovascular death, 1 (3.8%) stroke and 4 (15.4%) cardiovascular hospitalizations occurred. At 2D strain of LA, the reservoir function decreased significantly at discharge, compared to baseline and recovered at 3-months follow-up. Furthermore, NT-proBNP increased significantly after the procedure with a return to baseline after 3 months. Changes in E/A were persistent at 3 months.
Conclusion: Our data in a small cohort suggest that TT-LAA exclusion with epicardial clip can be a safe procedure with regards to the atrial function. The LAA amputation impairs the reservoir LA function on the short term, that recovers over time.
Atrial fibrillation (AF) is the most common sustained arrhythmia in humans and oral anticoagulation (OAC) to prevent ischemic stroke in AF patients with high CHA2DS2-VASc risk score is a guideline-recommended therapy (1, 2).
Despite the recent advances in pharmacological stroke prevention the perceived risk of OAC-associated bleeding may result in significant under prescription or under dosage of this therapy (3). The surgical exclusion of the left atrial appendage (LAA) is a therapeutic strategy for stroke prevention in AF patients with an absolute contraindication to OAC or a high risk of life-threatening bleeding on OAC or antiplatelet therapy (APT) (3) and unsuitable for percutaneous LAA occlusion. This intervention effect on left atrial (LA) function has not been studied. The LAA produces vasoactive neuroendocrine hormones activated by stretch-sensitive receptors (4) and this suggests a role in cardiovascular homeostasis as a “decompression chamber.” LAA closure results in an increase LA size and mean pressure from animal models and human studies (5). Recent techniques have been introduced to assess the LA function such as two-dimensional speckle tracking echocardiography (2D STE), and specifically the strain and strain rate parameters. Through these parameters, the three LA function stages (reservoir, conduit and contractile) can be assessed.
This study aims at evaluating the effect of totally thoracoscopic (TT)-LAA exclusion on the LA function, evaluated with 2D STE.
This observational and retrospective study enrolled patients with AF at high risk for ischemic stroke and at high risk of life-threatening bleeding on OAC or APT or with a contraindication to long-term OAC. All patients underwent TT-LAA exclusion in the period between March 2020 and June 2021 at S. Chiara Hospital, Trento, Italy.
Inclusion criteria were: (1) AF defined following current guidelines (1); (2) Patients deemed at high risk for ischemic stroke, defined as CHADSVASC > 1 or ≥ 2 if female sex; and (3) contraindication to long term OAC/APT, defined as at least one of the following: HASBLED > 3, previous severe bleeding on OAC/APT or refractory anemia; or (4) refractory LAA thrombosis or recurrent stroke despite different OAC therapies (1). Previous severe bleeding was defined as at least one of the following:, diffuse gastrointestinal hemorrhage requiring transfusions or prior cerebral hemorrhage or other bleeding scenario with BARC > 1 (6).
Final decision on inclusion in the study was taken by the “AF Heart Team,” including a cardiac surgeon, a cardiologist, neurologist/neurosurgeon and referring physician following current guidelines on LAA exclusion (6).
All patients underwent preoperative computed tomography (CT) with 3-dimensional reconstruction, transthoracic and transesophageal echocardiography (TEE) to rule out thrombi in the LAA and to exclude other cardiac surgery indications for structural or functional heart diseases. Spirometry and bilateral carotid ultrasound doppler were also performed during the preoperative work out. Clinical history and laboratory data were collected and analyzed. Patient provided written informed consent to the procedure. The study complied with the Declaration of Helsinki as revised in 2013; the ethic committee approved the study.
All patients were treated using the video assisted thoracoscopic LAA exclusion approach with Atriclip PRO2 device (AtriCure Inc., Mason, OH). The procedure has been previously described in details (7). Briefly, patients were placed in a supine position, selective right lung ventilation was chosen with double lumen ventilation and intraoperative TEE monitoring to evaluate and guide the correct device positioning. Three 12 Fr thoracoscopic access ports were used, including the following: (1) a camera port placed along the mid axillary line at mid sternal level and (2) working ports, placed along the anterior axillary line in the third intercostal space and in the intercostal space at the intersection between the line in the middle of anterior and midaxillary line and a sagittal line crossing the xiphoid process. After insufflation with CO2, visualization of the intrathoracic anatomy, and freeing of adhesions, the opening of the pericardium was performed. The LAA was mobilized and the base measured with a dedicated sizer to select the device size. The AtriClip PRO2 (Atri-Cure Inc., West Chester, OH) device was positioned using the dedicated deployment device and deployed under TEE guidance and camera visualization.
Echocardiographic analysis was performed in all patients by experienced cardiologists, a specific protocol was compiled, and the echocardiographic measurements were obtained following current guidelines (8). Standard 2D measurements were performed using GE Vivid E9 or GE Vivid E80, (GE-Healthcare, Chicago, Illinois) and LA deformation was evaluated with a 2D STE software. All images were acquired in a DICOM format and digitally stored for offline analysis. Two different experienced cardiologists performed offline analysis. Echocardiographic parameters analyzed included the following: LV end diastolic volume (EDV), left ventricular (LV) ejection fraction (LVEF), LA volume indexed to body surface area (BSA), mitral peak velocity in early diastole (E) and in late diastole (A), average (mean of septal and lateral) early diastolic mitral annulus velocity (e’) estimated by tissue Doppler. Simpson’s biplane method of discs was used to perform volumetric calculation of both LV and LA. All measurements were performed following ASE guidelines (9).
Two-dimensional speckle tracking echocardiography was performed with a standard protocol following current guidelines (10). The apical four-chamber view was utilized for the strain measurements of LA and LV. Briefly, first the LA endocardium edge was traced manually and then the tracings based on the 2D STE were generated by the software, Figure 1. The mean deformation (strain) expressed in percentage was then calculated by the software. The reservoir function of the LA (LA reservoir strain) was calculated as the maximal wall deformation of LA during LV systole as compared to the end diastole, that was considered as preset reference point (11). 2D STE LA strain refers to reservoir strain if not otherwise specified. In patients with permanent AF, atrial strain was performed during ongoing AF. In patients with non-permanent AF, atrial strain was performed during sinus rhythm. Global longitudinal strain (GLS) of LV was measured as the longitudinal shortening of the myocardium (change in length compared to the baseline length).
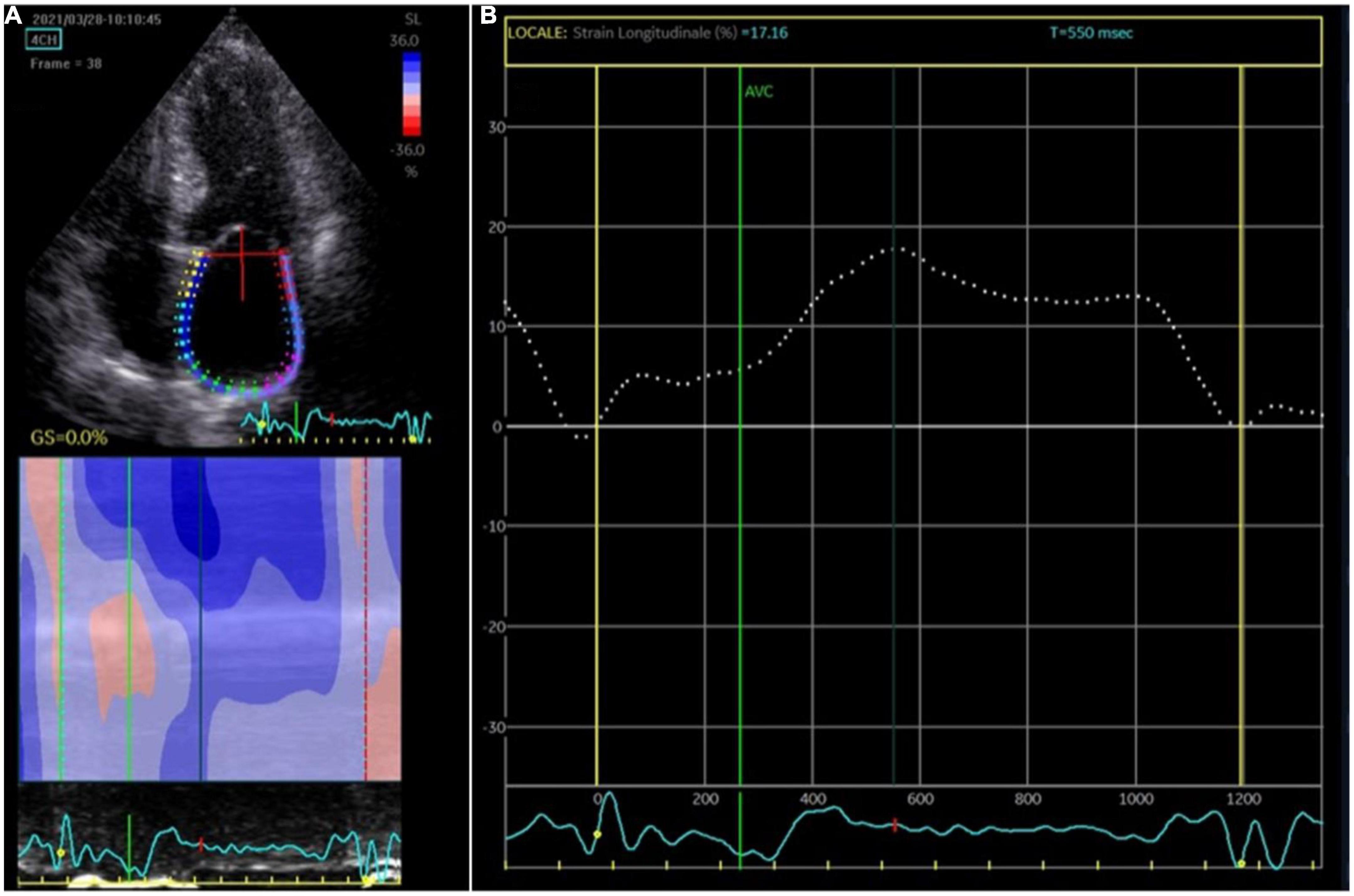
Figure 1. Atrial strain measurement. (A) The apical four-chamber view was utilized for the strain measurements of the left atrium (LA); the edge of the LA endocardium was manually traced. (B) The software generated tracings based on the 2D strain of LA. The mean deformation (strain) is expressed in percentage and calculated by the software.
Periprocedural adverse events were registered. Thoracoscopic access was evaluated after 10 days from the procedure. Clinical evaluations included laboratory work-out at pre-discharge and after 3 months and physical examination. A protocol echocardiogram was performed at baseline (pre-surgery), at pre-discharge (after surgery) and at 3 months. At 3 months follow-up, a cardiac synchronized CT scan or TEE were also performed to measure the size of the residual stump, if any and to assess the efficacy of LAA exclusion. A satisfactory outcome was considered as a residual stump < 1 cm (12). The primary endpoint was LA function, defined with 2D STE at pre-discharge and at 3 months. Secondary endpoints were the following: all-cause mortality, cardiovascular hospitalizations and stroke at long-term follow-up.
Descriptive statistics are reported as medians and interquartile range (IQR) for non-normally distributed continuous variables or mean ± standard deviation for normally distributed continuous variables. T-test was used to compare numerical normal variables, and Wilcoxon test for non-parametric variables. The categorical variables were compared by Chi-squared test or Fisher’s exact test and described as frequencies and percentages. A p-value < 0.05 was considered significant for all tests. The analysis was performed using R software version 3.6.2 (R Foundation for Statistical Computing, Vienna, Austria).
The study enrolled 26 consecutive patients (77.3 ± 6 years, 76.9% males). The mean HAS-BLED and CHA2DS2-VASc scores were 2.4 ± 0.6 and 4.6 ± 1.1 respectively. Permanent AF was present in 8 patients (30.8%). The indication for LAA exclusion was the following: history of cerebral hemorrhage (n = 10), diffuse gastrointestinal hemorrhage requiring transfusions (n = 4), clinical scenario of high bleeding risk (n = 4), refractory anemia (n = 3), other bleeding scenarios (n = 2), refractory LAA thrombosis (n = 1) and recurrent stroke despite different OAC therapies (n = 2). Baseline characteristics are summarized in Table 1.
A total of 26 patients underwent thoracoscopic LAA exclusion. Mean operation time (skin-to-skin) was 69.2 ± 18.5 min. No deaths procedure-related or pulmonary morbidity were observed. No patient required conversion to mini thoracotomy. Intraoperative TEE showed complete LAA exclusion with minimal residual stump (<1 cm) in all cases. Following the procedure, no patients were prescribed OAC, APT or heparin. There were no major complications during and after the procedure.
Compared with baseline, NT-proBNP was significantly higher at pre-discharge evaluation (1000.3 ± 950.1 pg/ml vs. 3170.2 ± 2011.6 pg/ml, p < 0.001); after 3 months there was no differences in NT-proBNP value compared with baseline (p = 0.17) (Table 2). There were no significant differences in creatinine and hemoglobin at pre-discharge and at 3-months follow-up (Table 2).
The 2D STE of LA measured at pre-discharge decreased significantly compared with the baseline values (11.8 ± 8% vs. 16.9 ± 7.7%, p = 0.028) with a recovery at 3-months (18.6 ± 10.5% vs. 16.9 ± 7.7%, p = 0.55) (Table 2). When compared with baseline, E/A increased significantly after 3 months (1.3 ± 0.4 vs. 0.9 ± 0.3, p = 0.004) (Table 2). Of note, there was a non-significant trend toward higher E/A values at pre-discharge evaluation compared to baseline (1.1 ± 0.3 vs. 0.9 ± 0.3, p = 0.25). E/e’ decreased throughout serial evaluation with no significant change (13.1 ± 6.8 vs. 12.7 ± 6.8 vs. 10.6 ± 3.0, p = NS for all comparisons). At pre-discharge and at 3-months follow-up echocardiography, the LA volume indexed to BSA was unchanged compared with baseline measurements (56.6 ± 26.9 ml/mq vs. 59.2 ± 28.3 ml/mq vs. 59.3 ± 33.0 ml/mq, p = NS for all comparisons). There was no significant difference in LV EDV, LVEF and GLS of LV (Table 2). The results of 2D STE of LA for permanent AF patients compared with non-permanent AF patients are summarized in Table 3.
Follow up was completed and available for all 26 patients. At a median follow-up of 10.3 ± 4.7 months, no patients were on OAC, APT or heparin therapy. One (3.8%) non-cardiovascular death, 1 (3.8%) stroke and 4 (15.4%) cardiovascular hospitalizations occurred at long-term follow up. Evaluation by TEE or CT after 3 months showed stable and appropriate device position with LAA stump < 1 cm in all patients.
The main findings of this study can be summarized as follows: (1) The amputation of LAA significantly impairs LA reservoir function after the procedure, although this function recovers after 3 months. (2) TT-LAA exclusion results in a change in E/A that is persistent at 3 months follow-up.
The LA function consists of three components, namely: conduit, reservoir and pump. It is the result of a complex interplay between LV systolic and diastolic function, circulating blood volume and LAA function (13).
In the current study a transient impairment of LA reservoir function was observed after LAA exclusion; different mechanisms might contribute to this finding.
The sudden volume reduction of the LA after the procedure may affect LA distension, whereas its recovery might be explained with volume recovery or LA remodeling over time.
Changes in LA function could also be secondary to an altered neuro-humoral homeostasis expressed by changes in both atrial natriuretic peptide (ANP) and NT-proBNP. LAA endovascular occlusion is associated with an increase in ANP levels (14); ANP is produced by LAA and it plays an important role in LA physiology (15); its mutation is associated with a familial atrial dilated cardiomyopathy with standstill evolution (15). In a previous study on endovascular LAA closure, NT-proBNP was higher at 6 h and 24 h after procedure with no difference at 48 h (16). This is consistent with our results of a sudden increase in NT-proBNP followed by a return to baseline values.
Previous studies with percutaneous LAA closure, were characterized by heterogeneous results; different groups showed no changes in LA function after the procedure (17–19). A limitation of previous studies was the lack of routine 2D STE. Indeed, a subtle difference in LA function was evident only at 2D STE in our study. Furthermore, other groups demonstrated an improvement in LA function (20). The technical difference between the percutaneous and the surgical approach (with the latter causing a clean anatomical exclusion of the LAA) could explain the different behavior of the atrial function during the follow-up (21). In patients undergoing TT pulmonary veins isolation and LAA exclusion: De Maat et al. (21) concluded that the LAA exclusion does not impair the LA contractile function or the ejection fraction of LA, but there is only a reduction in LA reservoir function, in contrast Gelsomino et al. (22) described a gain of LA function and a reverse LA remodeling after the surgical ablation and LAA exclusion.
The recovery of reservoir strain of LA after 3 months is consistent with previous studies with 2D STE (20, 23); it might be explained by the recovery of both LA preload and neuro-humoral homeostasis. LAA exclusion might improve mechanical function of the LA and result in reverse LA remodeling (24).
Pulsed wave measures, in particular E/A remained increased at 3 months follow-up in the current study. In the pulsed wave analysis of transmitral flow, E wave represents the early fast diastolic filling and it is a measure of LA reservoir function. Its increase, with a consequent increase of E/A has been reported in previous studies (25); it could be a consequence of LA volume reduction following LAA exclusion, that represents the most distensible portion of LA (26).
The main limitation of the study is that it is retrospective. The included number of patients was relatively small, due to strict inclusion criteria. Limitations also included referral bias, being the center specialized in TT treatment of AF and TT-LAA exclusion. The reported changes in reservoir function might depend also on the appendage volume, which may differ among individuals. Data on LAA volume are lacking in all the published studies, and in the present one. In patients with permanent AF, A wave was not measured.
Our data in a small cohort suggest that TT-LAA exclusion with epicardial clip can be a safe procedure with regards to the atrial function. The LAA amputation impairs the reservoir LA function on the short term, that recovers over time.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Santa Chiara Hospital Ethics Committee. The patients/participants provided their written informed consent to participate in this study.
MMa, LP, and CA: conception and design of the work. MMa, LP, SB, FT, GD’O, DF, and ES: substantial contributions to the acquisition of data for the work. MMa and LP: substantial contributions to the analysis of data for the work and drafting the work. AG, RB, MMe, GC, and CA: substantial contributions to the interpretation of data for the work. FG, SQ, CM, AG, RB, MMe, GC, and CA: revising the draft of the work critically for important intellectual content. MMa, LP, SB, FT, GD’O, DF, ES, FG, SQ, CM, AG, RB, MMe, GC, and CA: final approval of the version to be published and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors contributed to the article and approved the submitted version.
Author MMe was consultant for AtriCure. Author GC received compensation for teaching purposes and proctoring from Medtronic, Abbott, Biotronik, Boston Scientific, and Acutus Medical. Author CA received research grants on behalf of the center from Biotronik, Medtronic, Abbott, LivaNova, Boston Scientific, AtriCure, Philips, and Acutus Medical. Author CA received compensation for teaching purposes and proctoring from Medtronic, Abbott, Biotronik, Livanova, Boston Scientific, AtriCure, Acutus Medical, and Daiichi Sankyo.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European association for cardio-thoracic surgery (EACTS). Eur Heart J. (2021). 42:373–498.
2. January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation. J Am Coll Cardiol. (2019) 74:104–32.
3. Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. (2014) 383:955–62.
4. Regazzoli D, Ancona F, Trevisi N, Guarracini F, Radinovic A, Oppizzi M, et al. Left atrial appendage: physiology, pathology, and role as a therapeutic target. Biomed Res Int. (2015) 2015:205013.
5. Melduni RM, Schaff HV, Lee HC, Gersh BJ, Noseworthy PA, Bailey KR, et al. Impact of left atrial appendage closure during cardiac surgery on the occurrence of early postoperative atrial fibrillation, stroke, and mortality. Circulation. (2017) 135:366–78.
6. Glikson M, Wolff R, Hindricks G, Mandrola J, Camm AJ, Lip GYH, et al. EHRA/EAPCI expert consensus statement on catheter-based left atrial appendage occlusion – an update. Europace. (2020) 22:184.
7. Branzoli S, Marini M, Guarracini F, Pederzolli C, D’Onghia G, Centonze M, et al. Standalone totally thoracoscopic left appendage clipping: safe, simple, Standardized. Ann Thorac Surg. (2021) 111:e61–3. doi: 10.1016/j.athoracsur.2020.04.130
8. Mitchell C, Rahko PS, Blauwet LA, Canaday B, Finstuen JA, Foster MC, et al. Guidelines for performing a comprehensive transthoracic echocardiographic examination in adults: recommendations from the American society of echocardiography. J Am Soc Echocardiogr. (2019) 32:1–64.
9. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American society of echocardiography and the European association of cardiovascular imaging. Eur Heart J Cardiovasc Imaging. (2015) 16:1–39.e14.
10. Badano LP, Kolias TJ, Muraru D, Abraham TP, Aurigemma G, Edvardsen T, et al. Standardization of left atrial, right ventricular, and right atrial deformation imaging using two-dimensional speckle tracking echocardiography: a consensus document of the EACVI/ASE/industry task force to standardize deformation imaging. Eur Heart J Cardiovasc Imaging. (2018) 19:591–600. doi: 10.1093/ehjci/jey042
11. Mor-Avi V, Lang RM, Badano LP, Belohlavek M, Cardim NM, Derumeaux G, et al. Current and evolving echocardiographic techniques for the quantitative evaluation of cardiac mechanics: ASE/EAE consensus statement on methodology and indications endorsed by the Japanese society of echocardiography. Eur J Echocardiogr. (2011) 12:167–205.
12. Osmancik P, Budera P, Zdarska J, Herman D, Petr R, Fojt R, et al. Residual echocardiographic and computed tomography findings after thoracoscopic occlusion of the left atrial appendage using the AtriClip PRO device. Interact Cardiovasc Thorac Surg. (2018) 26:919–25. doi: 10.1093/icvts/ivx427
13. Leischik R, Littwitz H, Dworrak B, Garg P, Zhu M, Sahn DJ, et al. Echocardiographic evaluation of left atrial mechanics: function, history, novel techniques, advantages, and pitfalls. Biomed Res Int. (2015) 2015:765921. doi: 10.1155/2015/765921
14. Behnes M, Sartorius B, Wenke A, Lang S, Hoffmann U, Fastner C, et al. Percutaneous closure of left atrial appendage affects mid-term release of MR-proANP. Sci Rep. (2017) 7:9028. doi: 10.1038/s41598-017-08999-4
15. Disertori M, Quintarelli S, Grasso M, Pilotto A, Narula N, Favalli V, et al. Autosomal recessive atrial dilated cardiomyopathy with standstill evolution associated with mutation of natriuretic peptide precursor A. Circ Cardiovasc Genet. (2013) 6:27–36. doi: 10.1161/CIRCGENETICS.112.963520
16. Huakang L, Qing Y, Bing S, Zhihui Z, Zhiyuan S. The influence of left atrial appendage closure on the structure and function of the left atrium. Int J Clin Exp Med. (2018) 11:3845–51. doi: 10.1378/chest.128.3.1853
17. Hanna IR, Kolm P, Martin R, Reisman M, Gray W, Block PC. Left atrial structure and function after percutaneous left atrial appendage transcatheter occlusion (PLAATO): six-month echocardiographic follow-up. J Am Coll Cardiol. (2004) 43:1868–72. doi: 10.1016/j.jacc.2003.12.050
18. Madeira M, Teixeira R, Reis L, Dinis P, Paiva L, Botelho A, et al. Does percutaneous left atrial appendage closure affect left atrial performance? Int J Cardiovasc Sci. (2018) 31:569–77.
19. Jalal Z, Iriart X, Dinet ML, Corneloup O, Pillois X, Cochet H, et al. Evaluation of left atrial remodelling following percutaneous left atrial appendage closure. J Geriatr Cardiol. (2017) 14:496–500.
20. Ijuin S, Hamadanchi A, Haertel F, Baez L, Schulze PC, Franz M, et al. Improvement in left atrial strain among patients undergoing percutaneous left atrial appendage closure. J Cardiovasc Echogr. (2020) 30:15–21. doi: 10.4103/jcecho.jcecho_42_19
21. De Maat GE, Benussi S, Hummel YM, Krul S, Pozzoli A, Driessen AHG, et al. Surgical left atrial appendage exclusion does not impair left atrial contraction function: a pilot study. Biomed Res Int. (2015) 2015:318901.
22. Gelsomino S, Lucà F, Rao CM, Parise O, Pison L, Wellens F, et al. Improvement of left atrial function and left atrial reverse remodeling after surgical treatment of atrial fibrillation. Ann Cardiothorac Surg. (2014) 3:70–4.
23. Coisne A, Pilato R, Brigadeau F, Klug D, Marquie C, Souissi Z, et al. Percutaneous left atrial appendage closure improves left atrial mechanical function through frank–starling mechanism. Hear Rhythm. (2017) 14:710–6. doi: 10.1016/j.hrthm.2017.01.042
24. Dar T, Afzal MR, Yarlagadda B, Kutty S, Shang Q, Gunda S, et al. Mechanical function of the left atrium is improved with epicardial ligation of the left atrial appendage: insights from the LAFIT-LARIAT registry. Hear Rhythm. (2018) 15:955–9. doi: 10.1016/j.hrthm.2018.02.022
25. Kamohara K, Popović ZB, Daimon M, Martin M, Ootaki Y, Akiyama M, et al. Impact of left atrial appendage exclusion on left atrial function. J Thorac Cardiovasc Surg. (2007) 133:174–81.
Keywords: left atrial appendage exclusion, oral anticoagulation therapy, totally thoracoscopic surgery, atrial fibrillation, left atrial appendage
Citation: Marini M, Pannone L, Branzoli S, Tedoldi F, D’Onghia G, Fanti D, Sarao E, Guarracini F, Quintarelli S, Monaco C, Graffigna A, Bonmassari R, La Meir M, Chierchia GB and de Asmundis C (2022) Left atrial function after standalone totally thoracoscopic left atrial appendage exclusion in atrial fibrillation patients with absolute contraindication to oral anticoagulation therapy. Front. Cardiovasc. Med. 9:1036574. doi: 10.3389/fcvm.2022.1036574
Received: 04 September 2022; Accepted: 19 October 2022;
Published: 07 November 2022.
Edited by:
Roberto Rordorf, San Matteo Hospital Foundation (IRCCS), ItalyReviewed by:
Ida Iafelice, Istituto Clinico Città Studi (ICCS), ItalyCopyright © 2022 Marini, Pannone, Branzoli, Tedoldi, D’Onghia, Fanti, Sarao, Guarracini, Quintarelli, Monaco, Graffigna, Bonmassari, La Meir, Chierchia and de Asmundis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Massimiliano Marini, bWFzc2ltaWxpYW5vLm1hcmluaUBhcHNzLnRuLml0
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.