
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cardiovasc. Med. , 10 November 2022
Sec. Thrombosis and Haemostasis
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.1033416
This article is part of the Research Topic Advances in Thrombin Generation View all 12 articles
 Lars L. F. G. Valke1,2
Lars L. F. G. Valke1,2 Sanna Rijpma3
Sanna Rijpma3 Danielle Meijer3
Danielle Meijer3 Saskia E. M. Schols1,2
Saskia E. M. Schols1,2 Waander L. van Heerde1,2,4*
Waander L. van Heerde1,2,4*Treatment of bleeding and thrombotic disorders is highly standardized and based on evidence-based medicine guidelines. These evidence-based treatment schemes are well accepted but may lead to either insufficient treatment or over-dosing, because the individuals’ hemostatic properties are not taken into account. This can potentially introduce bleeding or thrombotic complications in individual patients. With the incorporation of pharmacokinetic (PK) and pharmacodynamic (PK-PD) parameters, based on global assays such as thrombin generation assays (TGAs), a more personalized approach can be applied to treat either bleeding or thrombotic disorders. In this review, we will discuss the recent literature about the technical aspects of TGAs and the relation to diagnosis and management of bleeding and thrombotic disorders. In patients with bleeding disorders, such as hemophilia A or factor VII deficiency, TGAs can be used to identify patients with a more severe bleeding phenotype and also in the management with non-replacement therapy and/or bypassing therapy. These assays have also a role in patients with venous thrombo-embolism, but the usage of TGAs in patients with arterial thrombosis is less clear. However, there is a potential role for TGAs in the monitoring of (long-term) antithrombotic therapy, for example with the use of direct oral anticoagulants. Finally this review will discuss controversies, limitations and knowledge gaps in relation to the introduction of TGAs to personalize medicine in daily medical practice.
Hemostasis consists of a number of highly balanced processes to ensure blood flow and prevent unnecessary thrombosis and bleeding. A shift in this balance can lead to either of these conditions with associated morbidity and mortality, and an impairment in quality of life (1). In patients with thrombosis this balance has shifted to a state with increased activation of prothrombogenic factors while in patients with bleeding disorders it is associated with an inability to ensure sufficient stable platelet plug formation. Treatment of both disorders is highly standardized and is shaped according to evidence based medicine guidelines.
The most well-known coagulation related bleeding disorder, hemophilia A (HA), is associated with a high bleeding risk due to a deficiency of coagulation factor (F) VIII (2). Severe HA patients, who have a FVIII activity level of <1 IU/dL, are treated with prophylactic coagulation factor replacement therapy to prevent bleeding and subsequent joint damage (3). The schemes for prophylactic therapy are standardized and adjusted according to FVIII activity trough levels (4). However, some patients with adequate FVIII activity levels still experience bleeding symptoms (5). On the other hand, patients with thrombosis are treated with anticoagulant therapy, for example direct oral anticoagulants (DOACs) in venous thrombo-embolism (VTE) (6). The dosage of this therapy is based on large scale randomized controlled trials (RTCs) and it is effective to prevent recurrent thrombosis in most patients, without the introduction of bleeding complications. Nonetheless, some patients experience recurrent thrombosis despite adequate therapy compliance, while others experience life-threatening bleeding with the same therapeutic dosage (6). Therefore, despite current state-of-the-art evidence-based medicine, diagnosis and treatment of patients with hemostatic disorders is possibly suboptimal due to either insufficient treatment in one patient, while over-dosing in the other. Both introduces a risk for bleeding and thrombotic complications in the individual patient.
The assays to analyze patients with thrombotic and bleeding disorders consists of screening assays like the prothrombin time (PT) and activated partial thromboplastic time (APTT) and on confirmation assays of specific coagulation factors, like FVIII and protein C activity level determinations. Both kind of assays investigate a certain part of the coagulation cascade and do not take the intertwining processes into account. A global hemostasis assay can measure these multiple processes (7). Several global hemostasis assays exist (8), all with the idea to provide a more detailed impression of the individual patients hemostatic balance. The physician can use these parameters together with personal characteristics of the patient, like concomitant use of medication and comorbidities that interfere with coagulation, to provide a clinical applicable picture to eventually adapt therapy upon (9).
In this review we will discuss the recent literature about thrombin generation assays in relation to the management of bleeding and thrombotic disorders. Assays using whole blood or investigating fibrinolysis are beyond the scope of this review. For bleeding disorders, the main focus will lay on HA, as this is the most prevalent coagulation related bleeding disorder. For thrombotic disorders, the focus will lie on venous thrombo-embolic disorders and the treatment with DOACs, heparinoids and vitamin K-antagonists (VKA). Furthermore, we will discuss controversies, limitations and knowledge gaps in relation to the introduction of plasma-based global assays to personalize medicine in daily medical practice.
The first reports of manual thrombin generation assays (TGAs) were published in 1953 (10, 11). Generation of thrombin is the result of effective activation of procoagulant factors of coagulation. Thrombin is a pivotal enzyme in hemostasis, as its generation represents a rate limiting step in fibrin formation, amongst its other key functions in hemostatic processes (12). It also functions as initiator of anticoagulant processes through potentiation of protein C upon binding of thrombin to thrombomodulin. In vitro measurement of thrombin generation uses substrates specific for thrombin cleaving activity that release a chromogenic or fluorogenic signal, to represent the net balance between these processes. Upon activation of the coagulation pathways through addition of tissue factor, phospholipids and calcium, the production of thrombin is initiated and accelerates exponentially, slows down, until it reaches a plateau phase (13). This can be measured in time, which gives insight in the net result of the hemostatic capacity.
The process of thrombin generation is visually represented by the first derivative of the thrombin generation signal as the signal accumulates in time, when measured with a chromogenic or fluorescent substrate (Figure 1). Important parameters describing this curve include lag time (1), which describes the time between application of the trigger and the initiation of thrombin generation, time to thrombin peak (2) when the generation of thrombin has reached its maximum, thrombin peak height (3) evaluating maximum generated quantity of thrombin, area under the curve (AUC, also called endogenous thrombin potential (ETP), (4) where the total amount of thrombin generation is evaluated, as well as the velocity index (5) which describes the slope of the curve during the amplification phase. These parameters vary in close conjunction; an increased thrombin peak height is mostly accompanied by an increased velocity index and ETP, while time to thrombin peak and also lag time are often shortened.
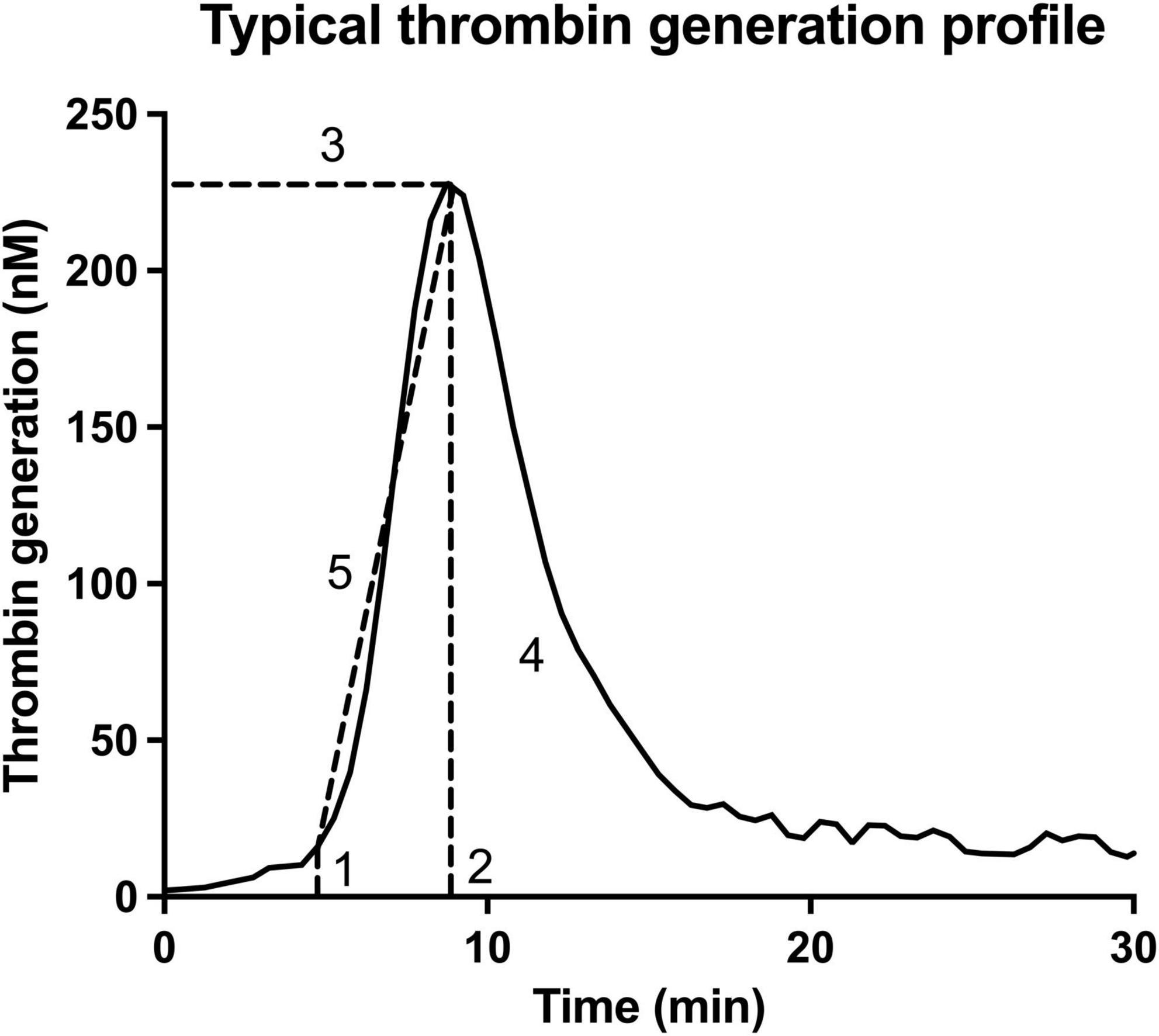
Figure 1. Essential parameters obtained with the thrombin generation assay. This thrombin generation assay is visually represented by the first derivative of the thrombin generation signal. The important parameters describing this curve include lag time (1), which describes the time between application of the trigger and the initiation of thrombin generation; time to thrombin peak (2) when the generation of thrombin is maximal; thrombin peak height (3) evaluating maximum generated quantity of thrombin; the area under the curve (AUC, also called endogenous thrombin potential (ETP), (4) where the total amount of thrombin generation is evaluated; as well as the velocity index (5) which describes the slope of the curve during the amplification phase.
The lack of standardization of test parameters and composition of reagents has hampered comparability and harmonization of thrombin generation results to a great extent. First, the type of thrombin generation matrix varies from platelet poor plasma (PPP), platelet rich plasma (PRP) to whole blood. In most cases, PPP is used. For the TGA, standardization of preanalytical procedures to prepare PPP is essential. Comparison of different pre-analytical protocols where different blood collection systems, blood collection tubes, centrifugation method and time between collection and testing were evaluated, showed significant effects on thrombin generation results (14). Exemplifying centrifugation; a two-step centrifugation significantly decreased ETP potential compared to a one-step centrifugation. This is probably due to phospholipid contamination through microparticles or vesicles in samples that are centrifuged only once at a low centrifugation speed. Another factor that introduces pre-analytical variation is the venipuncture system and type of collection tube used. Addition of corn trypsin inhibitor (CTI) interferes with contact-activation, and thereby may reduce thrombin potential if contact activation occurs. However, this is also dependent on other factors like tissue factor concentration. The time and temperature at which a sample is preserved is another factor that can have a significant effect on thrombin generation results, as instability of coagulation factors does not allow prolonged storage times at room temperature. In conclusion, the establishment of a standardized pre-analytical protocol will aid significantly in harmonizing TGAs. The International Society on Thrombosis and Haemostasis (ISTH) Scientific and Standardization Subcommittee (SSC) suggested specific preanalytical conditions for the measurement of thrombin generation for the indication of hemophilia, which are described in ref (15).
Composition of the method that is applied to produce a fluorescent signal, is another important factor that hampers harmonization of thrombin generation results. Two main specific thrombin substrates have been applied to measure its generation, either attached to a chromogenic or fluorogenic signal, para-nitroaniline (pNA) and 7-amino-4-methylcoumar (AMC), respectively (16, 17). In both methods, the caged signal molecule is released by thrombin mediated proteolysis. The peptide is specific for thrombin and has a relative high Km and low Vmax to avoid substrate depletion. The chromogenic pNA signal is quantified through extinction measurement at 405 nM. Defibrination or inhibition of fibrin polymerization is required for this assay due to interference of the chromogenic signal during clot formation. Fluorescent AMC signal is not sensitive toward interference through clot formation and is excitated at a wavelength of 390 nm resulting in a peak at an emission wavelength of 460 nm. The rate at which these signal molecules are released varies between chromogenic and fluorescent substrates, and also the amino acid composition of the tri-peptide, which hampers comparison of results for these two methods (8).
Calibration of the assay can be performed by measurement of a known range of thrombin concentrations, or thrombin bound to alpha-2-macroglobulin (α2M). Fluorescent substrates in the TGA are also cleaved by thrombin complexed to α2M, whereas thrombin complexed to α2M is not biologically active in fibrinogen activation (18). Moreover, in specific patients, such as the pediatric population and neonates, the α2M concentrations can be significantly altered compared to the adult population (19). The biological active free thrombin component can therefore differ from the measured total thrombin activity. In some assays, the thrombin generation curve is corrected for the amount of thrombin that is bound to α2M, while in other assays no correction for α2M is applied/necessary (20).
Furthermore, correction for quenching of the fluorescent signal differs between methods. Correcting for quenching, or the inner filter effect, of the fluorescent signal in patient samples with varying composition is a non-linear phenomenon and may lead to an underestimation of thrombin generation and increased variation in test results (21). Some methods correct for quenching by the application of mathematical correction using an intra-assay control sample, in other methods no correction for quenching is applied (22).
Thrombin generation is initiated by the addition of tissue factor and phospholipids, which will activate the extrinsic pathway and stimulate coagulation, respectively. The concentration of these trigger components can differ and will alter sensitivity of the assay toward different types of coagulation disorders. Reagent compositions applying low concentration of tissue factor and phospholipids are most sensitive toward bleeding disorders (23), whereas a higher tissue factor concentration can be predictive for a prothrombotic phenotype (24). In the anticoagulated patient, higher concentrations of tissue factor and phospholipids are suggested to be more appropriate (25). Tissue factor can be derived from either tissue (human plasma, human placenta, and rabbit), or through recombinant expression. Apart from the concentrations in trigger reagent, also the composition of the applied phospholipids (e.g., percentage of phosphatidylserine, phosphatidylcholine, phosphatidylethanolamine) and the size distribution of the vesicles containing these phospholipids may vary. Imprecision in thrombin generation results is increased using different sources of tissue factor and phospholipids, especially at low concentrations (26). For most commercial available reagents, exact composition for the different reagents is not disclosed, and variation between batches could be expected (25).
Normalization of the obtained thrombin generation results, as suggested by the ISTH-SSC, is often advocated to reduce inter-assay and inter-laboratory variation, and to aid in the interpretation of the results of the different thrombin generation parameters (15, 26). However, determination and application of a normal sample is complex because no reference material exists, different pool samples, or commercially available control samples have varying composition and therefore may increase variation between assays as well as hamper comparability and interpretation of results of different assays.
Finally, thrombin generation can be determined with a manual, semi-automated or automated assay. The automated assays that are currently available for the evaluation of thrombin generation apply fluorescent substrates, but reagent composition, and calibration and mathematical procedures differ (8). The (semi-)automated assays that are often applied are the Calibrated Automated Thrombography (CAT, Stago), ST Genesia (Stago), and Ceveron TGA (Technoclone). A comparison of thrombin generation performance of these assays for patient populations with specific bleeding or thrombotic phenotypes, as well as patients treated with anticoagulants, has not been reported. Therefore, the effect of differences in reagent composition, calibration, correction methods and normalization on thrombin generation results cannot be interpreted for these patient population when different assays are used.
TGAs are used in the analysis of bleeding disorders, to give an impression of the clinical phenotype of patients with bleeding disorders and to monitor treatment (for summary of most important findings, see Table 1). These different applications will be discussed here.
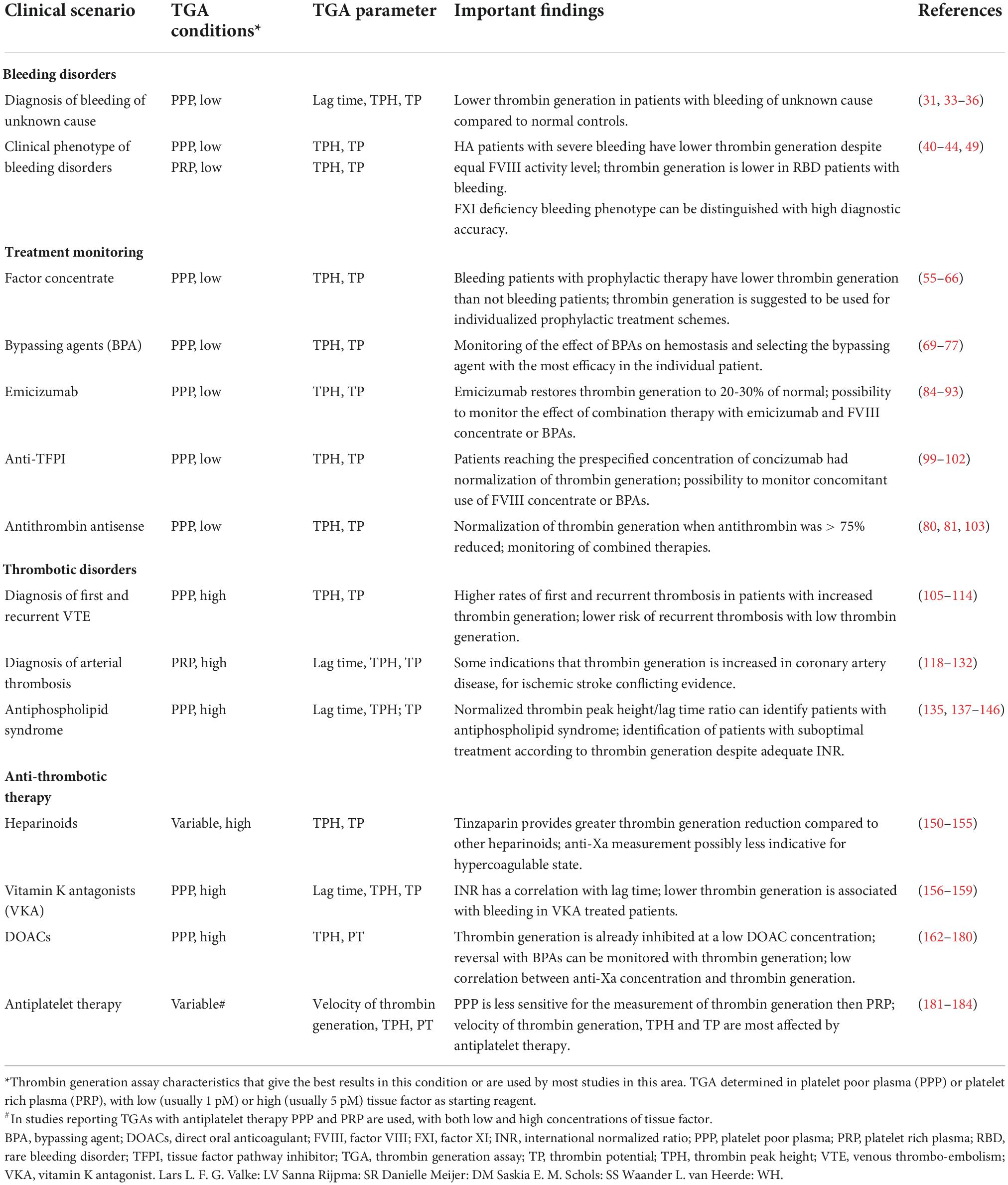
Table 1. Summary of thrombin generation assay characteristics and findings in bleeding and thrombotic diseases.
Patients with a mild to moderate bleeding tendency are subjected to multiple diagnostic assays to detect the hemostatic abnormality (27). However, in one in three patients a definitive diagnosis cannot be established (28), a condition called “bleeding of unknown cause” (BUC) (29). Several research groups have investigated the additional value of thrombin generation measured in PPP in patients with BUC (30–36). These studies provided conflicting results, with two, older studies showing no association (30, 32), while other studies found abnormalities in thrombin generation parameters (31, 33–36). The studies that found decreased thrombin generation were generally larger than the studies that did not, and used lower TF concentrations to start the TGA. As a hypocoagulable state can best be detected with a low TF concentration, it is possible that the amount of TF can explain this difference. The most prevalent described abnormalities were a prolonged lag time (31, 33–36), decreased thrombin peak height (31, 33), and decreased ETP (31, 33, 34, 36). However, the main limitation of TGAs in BUC patients is the overlap in thrombin generation results between patients with BUC and healthy controls. Therefore, reference ranges for diagnosis of bleeding based on decreased thrombin generation or increased lag time need to be determined. A specific disease entity (for example, bleeding based on impaired thrombin generation, as data from previous mentioned studies imply) could improve scientific research and treatment for these patients.
The value of TGAs is best researched in patients with HA, where it shows an association with clinical bleeding phenotype. Only a few papers report about TGA and bleeding tendency in patients with rare bleeding disorders (RBDs) (37–39).
Hemophilia patients with the same genotypic variant and factor coagulation activity level often show differences in clinical bleeding phenotype, possibly due to alterations in other coagulation factors than FVIII (in HA) and FIX (in hemophilia B; HB). This hypothesis was confirmed by multiple small studies in which the bleeding phenotype correlated with one or multiple TGA parameters in PPP (40–44). One study was unable to establish this association (45), possible due to the use of the Tosetto bleeding score, that is developed for Von Willebrand disease (VWD), instead of a hemophilia specific bleeding score (46). Another study investigated the relationship between FVIII activity level, genetic variations and inhibitor status. Patients with an inhibitor, a neutralizing antibody against FVIII making replacement therapy ineffective, showed decreased thrombin generation capacity compared to patients without inhibitors, despite equal FVIII activity levels (47). Thereby it can be concluded that TGAs can play a role in unraveling the clinical bleeding phenotype of hemophilia patients and even may play a role in how to treat these patients.
It is known that in patients with FXI deficiency (also known as hemophilia C) FXI activity level and bleeding phenotype do not correlate with each other (48). However, thrombin generation could possibly distinguish patients with different FXI activity levels and patients with and without bleeding. It appeared that certain sampling and testing conditions influenced the results of thrombin generation. In PPP with CTI, thrombin generation did not identify FXI deficient patients from normal controls. But when PRP with CTI and low TF was used, it could differentiate between patients with and without FXI deficiency. Furthermore, differences in thrombin peak height and thrombin potential had a high diagnostic accuracy for identifying bleeding from non-bleeding patients (49).
RBDs are a heterogenous group of diseases with different coagulation factor defects. All these diseases have a variable bleeding tendency, that only partially can be explained by the activity level of the missing or depleted coagulation factor. A retrospective study of RBD patients showed that thrombin peak height and ETP measured in PPP were significant lower in patients with major bleeding, compared to patients with minor bleeding (38). Major bleeding patients had ETP values <20% of normal (38). This was comparable to the results of another study that found that all patients with major bleeding had an ETP of <20% of normal, while RBD patients with an ETP >30% of normal had no clinically relevant bleeding symptoms (37). The added value of TGAs in patients with RBDs was confirmed in a third study which showed that it could better predict significant bleeding compared to factor activity level (39).
Lastly, a study involving patients with von Willebrand disease (VWD) showed that patients with a mild bleeding phenotype had higher thrombin peak height compared to patients with more severe bleeding. The thrombin peak height and velocity of thrombin generation both correlated with VWF activity level and FVIII activity levels. This was observed in both PRP as well as PPP. Plasma FVIII activity level was the main driver of thrombin generation in this study (50).
The use of TGAs is mainly investigated in the treatment of HA patients. Spiking studies were first reported, in which plasma of HA patients was spiked with multiple concentrations of FVIII and thrombin generation in PPP was measured. Multiple in vitro spiking studies showed that thrombin generation reaches a plateau phase when FVIII activity level is between 20 and 50 IU/dL (40, 51–53), with only one study failed to show this plateau phase and suggested a linear correlation between FVIII activity level and thrombin generation (54). All studies reported major variation in the FVIII activity level at which thrombin generation was normalized in individual patients, indicating a need to individualize FVIII replacement therapy dosage to obtain normal thrombin generation in the TGA.
The individual response to FVIII replacement therapy was also reported in multiple in vivo studies with HA patients. A strong correlation between FVIII activity level and thrombin generation parameters (except lag time) in PPP was found (55–61), but the inter-patient variation of thrombin generation was large after a standard infusion of FVIII. For example, some patients generate normal total amounts of thrombin with subtherapeutic FVIII activity levels while others don’t show normalization of thrombin generation despite adequate FVIII activity levels (56). On the other hand, the intra-patient variation was small, thereby suggesting that the thrombin generation in an individual patients is predictable (57, 60).
Furthermore, multiple studies showed that the thrombin generation response had a prolonged duration after a single bolus of factor VIII concentrate compared to FVIII activity level. FVIII activity level declined over time after administration of FVIII replacement therapy, while thrombin generation remained elevated (55, 57, 60, 62). This effect was, again, variable between patients, implicating that other factors, like very low-titer inhibitors or level of other coagulation factors could play a role. This was further investigated in a pharmacokinetic (PK)/pharmacodynamic (PD) modeling study which showed that on average a 50% ETP level (EC50) was reached with only 11.6 IU/dL FVIII activity level increase. However, the inter-individual differences were large, underscoring the existence of an individual unique thrombin generation profile. For example, three patients with similar PK-profiles exhibited EC50 values that varied from 7.9 to 29.8 IU/dL FVIII (63).
A second PK/PD-modeling study also incorporated bleeding in their analysis. This study was based on the data of the GENA-21 study, which already had shown that FVIII activity level did not correlate with bleeding symptoms during prophylactic FVIII replacement therapy (64). However, patients with bleeding had significant lower thrombin generation compared to patients who had no bleeding symptoms (65). In this PK/PD-modeling study the authors found that patients with the highest ETP at baseline, had the lowest bleeding rate even with the lowest FVIII replacement therapy dosage compared to patients with the lowest ETP at baseline and highest FVIII replacement therapy dosage (66). This study showed that individualized dosing of FVIII replacement therapy based on ETP is superior in bleeding outcomes with this specific FVIII product.
In patients with hemophilia B (HB) a PK-PD model study was performed with a recombinant FIX-Fc fusion protein (eftrenonacog-alfa) and showed that velocity of thrombin generation showed the best correlation with FIX activity level. Thrombin peak height and ETP were the following parameters that decreased over time after replacement therapy. However, bleeding was not assessed in this study (67).
Lastly, a cross-over study assessed the PK-PD relationship between supplementation of plasma derived (pd) FVII and recombinant activated FVII (rFVIIa) in patients with FVII deficiency. This study identified lag time as the best effect-response parameter. In the PD-analysis, it was shown that the EC50 was only 2 IU/dL FVII activity for both pdFVII and rFVIIa. Furthermore, they showed that a plasma FVII activity level of 3-4 IU/dL was sufficient to reach lag time values comparable with the upper limit of healthy controls (68). These data underscore the discriminating value of thrombin generation in RBDs, especially if supplementation therapy is difficult to monitor because of long turn-around times for certain coagulation factor activity level determinations or because of replacement with rFVIIa, activated prothrombin complex concentrate (aPCC) or plasma (see section “Bypassing agents”).
Thrombin generation assays have an additional value in the monitoring of bypassing agents (BPAs), like rFVIIa and aPCC, in hemophilia patients. These products are mainly used in patients with inhibitors because FVIII replacement therapy is ineffective. Since BPAs cannot be monitored with a single factor assay, especially if it is given in combination with other treatment modalities, performing thrombin generation is an attractive alternative (9).
Activated prothrombin complex concentrates are shown to restore thrombin generation by spiking plasma samples of HA patients with 1–2 IU/mL aPCC (which corresponds with the therapeutic dose of 50–100 IU/kg) (69–71). In a PK/PD-study with aPCC in three HA patients, thrombin generation was restored after administration of 65–100 IU/kg aPCC and it diminished to 50% between 4 and 7 h (71). In pediatric HA patients with inhibitors, thrombin generation was restored to 80% of normal at peak aPCC levels after administration of 60–100 IU/kg. Thrombin potential remained enhanced 2.6 fold at trough aPCC level compared to control inhibitor plasma, indicating longer lasting effects on thrombin generation (72).
The effect of rFVIIa on thrombin generation was found to be less than aPCC in a cross-over study (73). The thrombin generation response after a bolus rFVIIa was highest after 30–60 min and decreased over a period of 4 h, anticipating the half-life of rFVIIa (74). All studies investigating the effect of rFVIIa in HA patients have shown that thrombin peak height and ETP are increased in PPP, but do not reach normal values (75, 76). Furthermore, individual patients show a difference in thrombin generation response to rFVIIa, with some patients having a poor response (76).
Since it is impossible to predict the hemostatic response of an individual patient to BPA therapy, Dargaud et al. proposed a three step model to individually tailor therapy. They investigated the performance of this model in six HA patients during ten invasive procedures. No bleeding occurred in the patients in whom ETP was normalized with the selected therapy (77).
During recent years, non-factor replacement therapies have been introduced in the treatment landscape of HA. Emicizumab, a bispecific monoclonal antibody that forms a pseudo-tenase complex (78), is the first non-factor replacement drug receiving market authorization, with other treatments like anti-tissue factor pathway inhibitor (anti-TFPI, e.g., concizumab) (79) and a mRNA against antithrombin (fitusiran) (80, 81) following pursuit. Because the hemostatic effects of these non-factor replacement therapies cannot be monitored with conventional assays, or lead to falsely normalized FVIII activity level (82), it is better to assess the end-product of hemostasis with TGAs, especially if used in combination with other treatment modalities (83).
The first study of emicizumab in FVIII depleted plasma showed thrombin generation parameters increasing to half of normal (82). One study found a linear correlation between emicizumab concentration and thrombin peak height measured in PPP (84). Moreover, results showed that thrombin potential in emicizumab treated HA patients reached a plateau at 20–30% of normal (84), which was replicated in other studies (85, 86). Moreover, it was shown that thrombin generation was lower in infants younger than 1 year, compared to older children and adults, possibly due to a faster clearance of emicizumab (85, 87, 88). Finally, thrombin peak height and ETP were significantly lower in patients who presented with major bleedings (85, 89).
TGAs can mainly be used to monitor combined treatment modalities, in which measurement of individual components of therapy is impossible. Because emicizumab was first investigated in HA patients with inhibitors, most data exist about the combination of emicizumab with BPAs. However, one in vitro spiking study showed that combination therapy of emicizumab with plasma derived FVIII/VWF (pdFVIII/VWF) did not increase thrombin generation above levels observed in PPP with monotherapy pdFVIII/VWF. This is expected because FVIIIa has a greater affinity for the tenase complex than emicizumab (90). Moreover, in multiple spiking studies (90–92), it was observed that combination therapy of emicizumab with aPCC in low dosage (5 IU/kg) already normalized thrombin generation. APCC in higher dosage (>30 IU/kg) increase thrombin generation above normal values, to even more than eight-fold normal values with a dosage of 100 IU/kg (90–92). On the other hand, when HA plasma with emicizumab was spiked with rFVIIa in the highest dosage of 270 μg/kg, thrombin generation did not exceed normal values (90–92). Most importantly, it was discovered that activated FIX, in aPCC was responsible for the synergistic effect of emicizumab and aPCC in vitro (93). Whether this also occurs in vivo is still in debate. It can be concluded, however, that HA patients treated with emicizumab who need additional therapies should be treated with care and thrombin generation should be closely monitored. Patients without inhibitors can safely be treated with FVIII replacement therapy, because activated FVIII has a greater affinity for FIXa and FX than emicizumab and a synergistic effect is not expected (83, 94). However, patients with inhibitors can be treated with rFVIIa in normal dosage, and if not available or not effective, with very low dose aPCC with careful thrombin generation monitoring as multiple patients have developed thrombosis after administration of aPCC (dosed >200 IU/kg/day) in combination with emicizumab (78).
Anti-TFPI treatment enhances the initiation phase of coagulation by inhibiting the shutdown effect of TFPI resulting in a prolonged TF-FVIIa activity, leading to an increased activation of FX and eventually thrombin (95). The now discontinued agent BAX-499 already showed improved thrombin generation in hemophilic plasma (96). Additionally, spiking studies with two different anti-TFPI antibodies (marstacimab and befovacimab) both increased thrombin generation to a level that was approximately equal to a FVIII activity level of 40% (97, 98). Most pharmacodynamic studies are performed with concizumab which showed a dose-dependent increase in thrombin generation, even in plasma of healthy volunteers (79, 99). Afterward, pharmacodynamic monitoring was used to determine the eventually investigated dose of concizumab (100). Patients who reached the prespecified concentration of concizumab (>100 ng/ml) showed normalization of thrombin generation (101). Lastly, concomitant therapy of concizumab with aPCC, rFVIIa, and FVIII showed additive effects, instead of exponentially effects such as between aPCC and emicizumab (102). Therefore, concizumab can be combined with other treatment modalities, but dosages should be adjusted and monitored with TGAs to provide safe and effective therapy.
Antithrombin lowering can be established with fitusiran, an anti-sense oligonucleotide directed against antithrombin mRNA, leading to decreased inhibition of coagulation. Studies showed increasing amounts of thrombin generation with further reducing antithrombin with reaching near normal levels of thrombin generation when antithrombin was >75% reduced (80, 81). Comparable with concizumab, fitusiran combined with aPCC or rFVIIa had additive effects on thrombin generation (103).
The TGA can be used in the analysis of patients with thrombosis, for example to analyze the prothrombogenic phenotype in patients with (recurrent) VTE (104), and to monitor treatment with anticoagulant therapy (for most important findings, see Table 1).
Venous thrombo-embolism consists of pulmonary embolism and deep vein thrombosis and is common in the general population. Patients are treated with anticoagulants to prevent further progression of the thrombus and preventing recurrent thrombosis. However, the ideal duration of anticoagulation in the individual patient is unknown and decisions about stopping/continuing anticoagulation were made on clinical characteristics and patient preferences. Possibly, the TGA could help indicate which patient has a hypercoagulable phenotype and has a high risk for first or recurrent VTE. This is investigated in multiple studies (105–114). The first indication that thrombin generation could influence VTE recurrence was with a RCT in which D-dimer level measured one month after discontinuation was used to indicate prolonged anticoagulation. Patients with an elevated D-dimer level who restarted anticoagulation had a significant lower chance of recurrent VTE than patients without anticoagulation (115). Furthermore, it was shown that patients with VTE have significantly higher ETP values than controls without VTE (105, 106, 109). One study found that thrombin generation was higher in individuals with an additional risk factor for the development of VTE than patients with an idiopathic VTE (105). Another study, however, reported higher thrombin generation in patients with idiopathic VTE compared to those with provoked VTE, even after correction for FVIII and D-dimer levels (112). Furthermore, multiple studies showed that addition of thrombomodulin to the TGA was able to magnify the differences found between patients and controls (105–107).
The risk of recurrent VTE can be estimated with TGA with increased hazard ratios (HR) ranging from 1.6 to 4.0 for increased ETP and subsequent recurrent VTE (107, 108, 113). The HR for recurrent VTE based on thrombin peak height was even 4.6 in one study (107). One study could not establish an increased risk for recurrent VTE (HR 1.1) when elevated ETP was used to distinguish patients and controls (109). This different conclusion can be explained by the study design, as the last study was a case-control study, while all others were prospective cohort studies. Interestingly, a cohort study also showed that patients with low thrombin generation had a lower risk of recurrent VTE (HR 0.40) (111). Thereby confirming the risk association between thrombin generation and the risk of recurrent VTE.
Lastly, patients with cancer have a high risk of VTE development. Ay et al. performed TGA in 1033 patients with various types of solid tumors and found a HR of 2.1 for the development of a VTE event in patients with the highest quartile of thrombin generation. Incidence of VTE in the first 6 months was 11% in this quartile, compared to 4% in patients with lower thrombin peak height (116). Therefore, it can be concluded that thrombin generation might be a useful tool to predict first and recurrent VTE incidence, in patients with idiopathic and provoked VTE, and in patients with a malignancy. However, it should be noted that absolute cut-off values of thrombin generations parameters are not possible, because a large overlap in thrombin generation profiles exists between VTE patients (114).
In contrast to VTE, the role of thrombin generation measurement is less clear in patients with arterial thrombosis. Arterial thrombosis is a leading cause of death worldwide and consists of coronary artery disease (CAD) and ischemic stroke. It is a complex interaction between the long lasting process of atherosclerosis of the main arteries, in combination with acute rupture of an atherosclerotic plaque that provokes thrombus formation at the site of injury. Only if the thrombus limits blood flow to the affected organ, symptoms can be reported by the patient. Since atherosclerosis and inflammation are strongly linked to each other and inflammation has a role in thrombin generation, the exact relationship between arterial thrombosis and thrombin generation is hard to establish and conflicting evidence is reported (117).
It is shown that patients with CAD have higher thrombin generation during an acute myocardial infarction and during the chronic phase, compared to patients with stable disease (118). This suggests that these patients are in a more hypercoagulable state and are more prone to arterial thrombosis (119). Increased thrombin generation parameters (thrombin potential and thrombin peak height) are often described in patients with acute MI or CAD (118, 120–122), but also a prolonged lag time is described (123). However, other studies describe a more U-shaped association between thrombin potential and CAD (124, 125). The association between enhanced thrombin generation and arterial thrombosis was further investigated in a case-control study with patients with an in-stent thrombosis after myocardial infarction. Here again, it showed that patients had higher thrombin generation compared to controls who did not have in-stent thrombosis (126). Furthermore, patients with residual detectable thrombin generation after percutaneous coronary intervention (PCI) despite optimal antiplatelet and periprocedural anticoagulant therapy had a higher risk of cardiovascular death (127).
For ischemic stroke, evidence is less clear. In one study, young stroke patients had an increased thrombin potential in PRP, while the association was not found in PPP (128). Multiple, smaller studies did not show an association between thrombin generation and adverse events (129, 130). These studies could be hampered by their sample size, since in one cohort study of more than 9,000 persons, a significant association was found between thrombin generation and the development of ischemic stroke. This study suggests that ischemic stroke could be prevented by diminishing the hypercoagulable state in these patients (122). On the other hand, another prospective cohort study found a significant inverse relationship between thrombin generation and the development of stroke (121).
In summary, the relationship between thrombin generation and arterial thrombosis is not readily defined. Thrombin generation in patients with CAD is increased in most studies, but the effect is only substantial. In patients with ischemic stroke, the evidence is even less clear-cut. These differences can be explained by study design or study population (117). Furthermore, the influence of traditional risk factors for cardiovascular disease on thrombin generation cannot be excluded. For example, obesity has been shown to increase thrombin potential (131). Also, another study showed that the concentration of apolipoprotein C-III was an independent risk factor for CAD, but also that it was associated with thrombin peak height and thrombin potential (132). Still, it is established that higher thrombin potential is associated with increased total mortality (131). Further research in this field should elaborate on thrombin generation in both PPP and PRP, because thrombin generation in arterial thrombosis is an interplay between vessel wall, platelets and coagulation factors.
The antiphospholipid syndrome (APS) is characterized by obstetric complications and/or arterial/venous thrombosis in combination with typical antiphospholipid antibodies (aPL antibodies: lupus anticoagulant (LAC) and/or anti-β2 glycoprotein I (aβ2GPI) and/or anti-cardiolipin (AC)) measured twice with at least 12 weeks in between (133). Major assay heterogeneity and lack of standardization cause problems with the diagnosis of APS (134). Also, non-pathogenic aPL antibodies can be encountered, for example in the presence of certain infections or medication. Furthermore, not all carriers of aPL antibodies develop thrombo-embolic complications. In this regard, the TGA may play an important distinguishable role (134).
In the diagnostic process, a chromogenic TGA was able to detecting all three aPL antibodies and could even distinguish between APS antibodies and antibodies arisen from transient causes, such as infections. However, this assay used purified antibodies and NPP, making it not available for use in routine clinical practice (135). Multiple groups have shown that aPL antibodies cause a lag time prolongation in the TGA, potentially due to shielding of the exogenous added phospholipids (136, 137). However, in these patients, thrombin peak height was increased as well, which led to the proposition of the use of the normalized peak height/lag time ratio (PH/LT-ratio) (138). This ratio was able to detect LAC antibodies with high sensitivity, even in anticoagulated patients (139). But additional research is necessary to establish that increased thrombin generation is due to APS instead of other causes, since increased thrombin generation is also seen in patients with VTE (as described in section “First and recurrent venous thrombo-embolism”).
Multiple studies have shown that the increased thrombin generation observed in patients with APS is mainly due to increased activated protein C (APC) resistance (137, 140, 141). This APC resistance was associated with thrombotic events (139–143), and was even incorporated in a ratio that could predict thrombosis over time (144). Lastly, TGAs can also be used to determine the degree of anticoagulation in patients with APS (145, 146). It even showed that a subgroup of patients had increased thrombin generation despite adequate international normalized ratio (INR) values. Thereby it is a possible tool to identity APS patients with an ongoing prothrombotic state despite therapy with vitamin K-antagonists (VKAs) (145). In the next paragraph, anticoagulation monitoring with TGAs will be further described. Thereby, it can be concluded that TGAs can be used in combination with classic APS assays to provide a more detailed impression of the hypercoagulable state of patients with thrombosis due to APS. Furthermore, monitoring of anticoagulation in these patients can be helpful, as recurrent thrombosis is common in APS patients (147).
Arterial and venous thrombo-embolic disorders are treated with different kinds of anticoagulant therapies, depending on the indication and patient characteristics. For most of these treatments, some kind of test to monitor the effect exists in the laboratory. However, mostly this encompasses a part of the coagulation cascade, like anti-Xa monitoring for heparinoids or LMWH, and it does not take hyper- or hypocoagulability of the patient into account (148). This part of the review will focus on anticoagulant therapy with heparinoids, VKA, DOACs and lastly antiplatelet therapy.
Heparin treatment can be divided in unfractionated heparin (UFH) and low molecular weight heparin (LMWH) therapy. UFH treatment needs to be monitored by measurement of APTT and/or anti-Xa, while treatment with LMWH is often fixed-dosed or weight-based dosed (149). However, LMWH is sometimes monitored with anti-Xa determination at the extremes of the weight spectrum (e.g., cachexia and morbid obesity) and in patients suffering from renal insufficiency. With anti-Xa monitoring, it appears that some patients show widely different anti-Xa activity levels with the same dosage, therefore, thrombin generation monitoring could be of interest in patients treated with UFH or LMWH (149).
The anticoagulant effect of UFH is comparable with different kinds of LMWH in spiked PRP (150). This study showed that tinzaparin had greater thrombin generation inhibitory effects compared to UFH and other LMWHs at the same anti-Xa activity level (150), which was confirmed in a second study that compared enoxaparin with tinzaparin (151). Moreover, it was shown that fondaparinux, a synthetic pentasaccharide which inhibits Xa formation via antithrombin, had less inhibitory effect on thrombin generation if compared to LMWH (150, 152).
Thrombin generation in the presence of LMWH was also measured in some specific populations. It is known that thrombin generation increases during pregnancy. In one study, healthy pregnant women, pregnant women with mild (e.g., heterozygous factor V Leiden) and severe thrombophilia (e.g., homozygous factor V Leiden) were followed each trimester with thrombin generation measurement. In women with severe thrombophilia, thrombin generation increased more than in women without thrombophilia (153). Prophylactic LMWH dosage inhibited thrombin generation. However, in the third trimester, thrombin generation was significantly elevated despite stable anti-Xa activity levels over time (153). Suggesting that pregnant women are in a hypercoagulable state despite fixed prophylactic LMWH therapy. This effect was also shown in morbidly obese pregnant women, which showed higher thrombin generation parameters compared to normal weight pregnant women. Interestingly, the authors showed that a weight-based prophylactic LMWH dosage led to significant lower ETP values compared to standard-dosed LMWH (154). Lastly, the TGA was able to detect a hypercoagulable state in patients with cancer and showed normalization of thrombin generation whilst patients were on LMWH therapy (155).
Before the introduction of DOACs, VKA were the main oral anticoagulants used. Dosing of VKA was personalized by measurement of the INR with subsequent dosage adjustments because multiple factors, like diet and genetic variants, influence the effect of VKA. Bleeding is the main risk of anticoagulation, therefore the goal is to keep the INR in a prespecified range. However, the INR only gives an impression of procoagulant factors, while anticoagulant factors are also influenced by VKAs. Therefore it would be interesting to know if VKAs could also be monitored with TGA.
Thrombin generation in the VKA treated patient showed a significant correlation with INR values, especially for lag time (156). In another study, however, some patients showed persisting thrombin generation despite adequate INR values (145), possibly indicating that they were still prone to recurrent VTE. When warfarin was compared with rivaroxaban, a DOAC, it appeared that overall thrombin generation parameters were comparable. Rivaroxaban exhibited slightly longer lag time, time to thrombin peak and lower thrombin peak height, while warfarin showed a lower ETP (157). However, in a study investigating an APS patient, rivaroxaban showed higher thrombin generation compared to warfarin and enoxaparin (146).
Interestingly, in a prospective study investigating bleeding episodes in patients using VKAs, it appeared that patients with bleeding had significant lower thrombin peak height and ETP values measured with whole blood TGA, compared to patients who did not bleed. The patients with bleeding also had higher HAS-BLED scores, indicating that both whole blood TGA and HAS-BLED score showed an association with bleeding (158). In another prospective cross-sectional study ETP was lower in warfarin treated patients who presented at the emergency department with bleeding, compared to warfarin treated patients who presented with another medical emergency, while INR was within target range in both groups (159).
Direct oral anticoagulants can inhibit thrombin (dabigatran) or FXa (apixaban, edoxaban, and rivaroxaban) and are given in a fixed dosing regimen, either once daily (edoxaban and rivaroxaban) or twice daily (apixaban and dabigatran). The main advantage of DOACs over VKAs is that monitoring of anticoagulation is not required (160). However, in some instances, for example in case of bleeding, recurrent thrombosis, or renal insufficiency, monitoring the effect of anticoagulation with DOACs can be of interest. In this regard, the anti-IIa or anti-Xa can be useful, but only gives an impression of the effect of the drug and not of the overall hemostatic capacity of the patient (161). The effect of DOACs on thrombin generation has been studied quite extensively, with in vitro studies as well as with plasma from patients using DOACs.
The in vitro studies showed that thrombin generation is hampered by DOACs (162–170). However, the parameters that are affected differ with the kind of DOAC. For example, dabigatran resulted in an increased lag time, while thrombin peak height and ETP remained relatively normal (162–164). On the other hand, presence of FXa inhibitors (apixaban, edoxaban and rivaroxaban) was shown by an increased lag time, but also an increased time to thrombin peak, with additional decreased thrombin peak height and ETP (163–167). Most studies that compare different DOACs, have shown that rivaroxaban has a stronger inhibitory effect on thrombin peak height and ETP compared to apixaban and edoxaban (163, 164). Furthermore, in vitro spiking plasma of pediatric and adult patients with edoxaban showed an equal inhibitory effect on thrombin generation among different age groups, except children <2 years of age, who had a stronger inhibition of thrombin generation at the same concentration of edoxaban (169).
Concerning thrombin generation and the use of DOACs, in healthy volunteers taking dabigatran, rivaroxaban and apixaban on different occasions, the same parameters were affected as with in vitro measurements (171). Dabigatran only increased lag time, while apixaban and rivaroxaban both inhibited thrombin peak height and ETP (171–174). Further studies showed that apixaban and rivaroxaban have a non-linear inhibitory function for thrombin generation. This indicates that most thrombin generation inhibition occurs at low anti-Xa concentrations (i.e., with a low concentration of DOAC, thrombin generation is still inhibited) (175, 176). Therefore, the authors further investigated how much thrombin was generated 12 h after intake of a DOAC. Thrombin generation was still inhibited at this time point, suggesting that an urgent surgery was not possible when thrombin generation is used as surrogate marker for hemostatic normalization (177). Furthermore, plasma levels of DOACs did not correlate with the extend of thrombin generation inhibition (178, 179). Therefore, thrombin generation measurement to provide an individual thrombin generation profile could be of more importance in a patient presenting with an acute bleeding or in need of urgent surgery than measuring DOAC anti-Xa activity level.
Lastly, several studies have investigated in vitro the effects of DOAC reversal therapy. These studies show that a TGA can help to establish correction of thrombin generation after addition of reversal agents (162, 166, 180). This is of major importance because other laboratory assays that do not measure hemostasis as a whole and cannot be used (i.e., anti-Xa assays or factor level activity assays) (161).
Even though antiplatelet therapy, like aspirin and clopidogrel, do not affect coagulation factors, the effect of these therapies on thrombin generation were studied in PRP as well as PPP. A case-control study showed that patients with CAD followed by PCI who were treated with standard dosage of dual antiplatelet therapy with aspirin and clopidogrel had significant longer time to thrombin peak, decreased thrombin peak height and ETP than controls in PRP (181). Velocity of thrombin generation was most impaired in patients. These differences in thrombin generation parameters were not found in PPP but only in PRP suggesting the importance of platelets (181). In a longitudinal study investigating thrombin generation after ischemic stroke, it was shown that aspirin in combination with dipyridamole significantly decreased thrombin peak height and ETP, while aspirin monotherapy and clopidogrel did not significantly change thrombin generation compared to baseline measured in PPP and not in PRP (182). Another study showed that platelet reactivity, measured with different platelet-activity assays, did not correlate with thrombin generation, measured in PPP (183). These studies show the importance of measurement thrombin generation in PRP, because in PPP it is less sensitive to assess the effects of these drugs.
In a study by de Breet et al., thrombin generation was measured in PPP one and six month after PCI for CAD. Patients were followed for one year to assess bleeding. It appeared that patients with bleeding had a significant lower thrombin peak height, ETP and velocity of thrombin generation at 1 and 6 months after PCI compared to patients without bleeding. Suggesting that performing TGA is possibly to identify patients with clinical relevant risk for bleeding episodes whilst using dual antiplatelet therapy (184).
Personalized medicine is becoming increasingly important in research and clinical practice and aims that “medial decisions, practices, interventions, and/or therapeutic agents are being tailored to the individual patient, based on their predicted response to treatment or risk of disease” (185). In other words, it aims to adjust treatment to each patient individual needs and preferences.
The processes of thrombosis and hemostasis are often depicted as a balance or two crossing lines (Figure 2). The lines represent the chance of bleeding or thrombosis, the pathological outcomes, which is probably more represented by thrombin generation in the individual patient. Even within the small target area, patients are still at risk for bleeding and thrombotic events, even though this risk is smaller than at the extremes of the curve. This can for example be seen in a patient with thrombosis (the black dot in Figure 2) due to a hypercoagulable state. With the use of anticoagulation, for example VKA with a target INR of 2–3, the risk of VTE recurrence is lowered, but some patients can still be hypercoagulable while having an INR within the target range (145). Therefore this patient can experience a recurrent VTE and only after intensification of the INR target range to 3–4, the patient’s hemostatic balance is within the referred range. In this example, VKA therapy and monitoring can be seen as a form of personalized medicine, because the number of tablets is dependent of the measured INR value. However, PT-based INR monitoring is highly artificial (due to a high TF concentration) and anticoagulant factors, like APC resistance, are not part of the INR test. Therefore INR is not really monitoring the hemostatic balance, while TGAs are more physiological and are expected to better reflect the patients hemostatic potential.
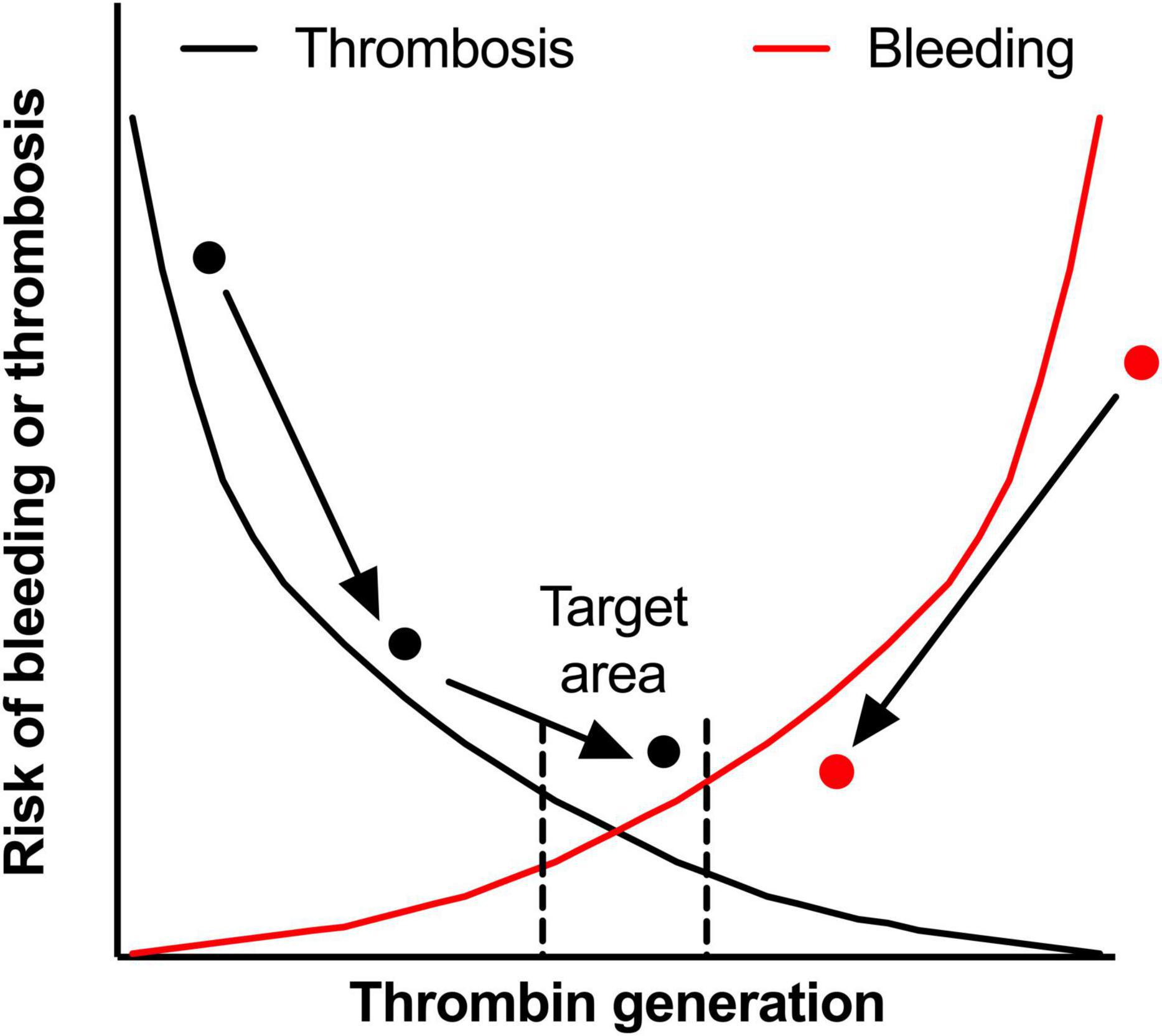
Figure 2. The hemostatic balance. The hemostatic balance is depicted as the risk of thrombosis (black line) and the risk of bleeding (red line), which is dependent on thrombin generation (on the x-axis). In the target area, both the risk of thrombosis and the risk of bleeding are acceptably low, but can still occur in an individual patient in certain circumstances. Two patients are shown in the figure, the black dot represents a patient with a venous thrombo-embolism during an hypercoagulable state. With treatment the hypercoagulable state is diminished, but the patient experiences a second thrombotic event while on adequate anticoagulant therapy. By intensifying anticoagulant treatment, this patient reaches the target area. On the other side of the curve, the red dot represents an hemophilia A patient with a high bleeding rate. After starting prophylactic therapy with factor VIII concentrate, the bleeding phenotype improves, but doesn’t reach the target area. However, for this patient the reduction in bleeding is acceptable, while intensifying treatment could lead to overshoot to a risk of thrombosis. In both patients, monitoring with TGAs could identify the target area better. This could have prevented the second thrombosis in the patient represented by the black dot as the hypercoagulable state was recognized earlier.
Because the INR can be sub- or supratherapeutic, with an accessory risk of thrombosis or bleeding, respectively, DOACs were developed. Since DOAC therapy has a standard dosage regimen based on evidence-based medicine, the manufactures advocate that monitoring is not required. However, patients can also be over- or underdosed. This is illustrated by the percentage of bleeding and recurrent thrombotic events in the DOAC trials (6), thereby indicating that for the main population, the dosage of the DOAC is correct, but for a number of patients it still results in either bleeding or thrombosis. TGAs, however, could give an impression of the hemostatic balance of the individual patient. This was illustrated in a case report in which the dosage of rivaroxaban was adapted based on thrombin generation results (186).
In hemophilia treatment, personalized medicine is becoming the standard of care. Prophylactic therapy with factor replacement therapy decreases the bleeding phenotype from a regular and spontaneous bleeder to become a mild bleeder (red dot in Figure 2). By intensifying prophylactic treatment, either by increasing the dosage or shortening the interval, the treatment can be personalized to a situation in which the patient has even less bleeds. However, by intensifying treatment, the chance of thrombotic disease may increase and costs will rise temporarily. Global assays may overcome this as it is expected that these assays better reflect the hemostatic balance. Measurement of thrombin generation can be combined with FVIII activity level and this could be used as the basis for an individualized treatment scheme, with the help of a population based PK-PD model (63, 66). However, this strategy is not yet tested in daily clinical practice.
Despite the overwhelming amount of evidence in favor of implementing TGAs in thrombotic and bleeding disorders to personalize medicine, some controversies and limitations remain to be addressed. Four important points need to be addressed, of which two are related to the methodology of the TGA and two regarding the monitoring of the hemostatic balance.
First, different kinds of (commercial) platforms exist to determine thrombin generation. In general, all platforms use the same method, but with slightly different concentrations of reagents, which are often undisclosed. This leads to varying amounts of generated thrombin with associated variating normal values. This problem could be targeted by normalizing the TGA parameters with normal pooled plasma (NPP). Even though it is assumed that NPP should approximately contain a normal activity level of all coagulation factors, there is still difference in thrombin generation with different NPP. This was nicely illustrated by a study that investigated the coefficient of variation of three plasmas with different coagulation profiles. Despite using the same TGA, results differed more than the predefined 25% and normalizing the results with the laboratory’s own NPP did not improve variation (187). Therefore, before the TGA can be compared across studies and can be used in daily clinical practice, the methods and materials used should be harmonized.
Second, performance of the TGA is time consuming because of preparation of plasma and the duration of the assay itself. For research purposes this is not a problem, as the TGA is often performed in batches to minimize variation. However, in clinical practice, the need of TGA determination can be urgent, for example, as mentioned in case of bleeding in a HA patient treated with emicizumab, or a patient using a DOAC. The whole blood viscoelastic tests (e.g., ROTEM) can be determined directly and results are available within an hour. Therefore, the determination of the TGA should be faster and possibly applicable as a point-of-care test (POCT). Already progress is being made to develop a POCT TGA which can be utilized at home for monitoring hemophilia patients with a bleed or at the emergency services to screen for coagulation defects in multi-trauma patients.
Thirdly, the target range of TGAs on the hemostatic balance is not known. This means that normal values of a larger healthy population are known, but it is unknown if this range is also the target range to prevent bleeding and thrombosis for an individual patient. Furthermore, each individual has a certain amount of thrombin generation during stable situations. However, during a thrombotic event, thrombin generation can be higher compared to the normal situation due to an intercurrent event which may has caused the thrombosis. The question is to what extent the patient should be treated; to the amount of thrombin generation before the event (if this is known), or to a predefined target based on large evidence based studies. In both cases, the patients treatment is according the principals of personalized medicine. However, only in the first scenario the patient is treated according to its own hemostatic need, which probably prevents bleeding due to the right amount of anticoagulation.
Fourth, the TGA is just one parameter whereas the hemostatic balance is orchestrated by the vessel wall, platelets, coagulation factor and fibrinolysis. The ultimate goal would be a global assay that measures these parameters simultaneously.
Thrombin generation assays can play an important role in the assessment of bleeding and thrombotic diseases, like diagnosis and prognosis of coagulation disorders, both inherited and acquired, as well as monitoring of the treatment of these diseases. However, a number of important items need further attention. The most important is the comparison and standardization of different TGA platforms with uniform reporting of the (local) thrombin generation reference values and normalization to NPP. Furthermore, most of the studies described in this review contain only small numbers of patients and are often monocentric. Larger studies with the use of standardized TGA systems could help for better adaptation of the assay in clinical practice. Also, serial determination of thrombin generation during health and disease (bleeding, thrombosis, and/or hypercoagulable states like cancer) in an individual patient will help understanding the variation in thrombin generation over time. This will give insight in the determination of the target range for treatment of bleeding and thrombotic disorders. Lastly, rapidly available, POCT TGA testing are promising to determine the hemostatic balance of an individual patient with acute hemorrhage due to acquired or congenital bleeding disorders.
In conclusion, TGAs are a versatile tool to measure the coagulation cascade as a whole in bleeding and thrombotic diseases. Since it measures the individual patients hemostatic balance, it can be used to personalize medicine of patients with bleeding disorders, thrombosis and monitoring (anti)hemostatic therapy (see Table 1). Results of recent research show that personalized medicine based on TGAs is most promising for patients with HA. Especially with the emerging non-factor replacement therapies and concomitant usage of therapies, hemostatic monitoring on an individual patient level is essential. The personalization of therapies could ultimately lead to tailoring the treatment of these disorders to needs of the patients without exposing them to unnecessary bleeding or thrombotic risks. However, before utilization in clinical practice, some important hurdles should be taken.
LV and SR wrote the first draft of the manuscript. DM, SS, and WH critically revised the manuscript. All authors contributed to the article and approved the submitted version.
WH received unrestricted grants from Bayer, Shire, Novo Nordisk, and CSL Behring. WH is the founder and CSO of Enzyre BV, a Radboudumc spinoff company. SS received a research grant from Bayer.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Oladapo AO, Epstein JD, Williams E, Ito D, Gringeri A, Valentino LA. Health-related quality of life assessment in haemophilia patients on prophylaxis therapy: a systematic review of results from prospective clinical trials. Haemophilia. (2015) 21:e344–58. doi: 10.1111/hae.12759
2. Mannucci PM, Tuddenham EG. The hemophilias–from royal genes to gene therapy. N Engl J Med. (2001) 344:1773–9. doi: 10.1056/NEJM200106073442307
4. Srivastava A, Santagostino E, Dougall A, Kitchen S, Sutherland M, Pipe SW, et al. WFH guidelines for the management of hemophilia, 3rd edition. Haemophilia. (2020) 26(Suppl. 6):1–158.
5. Jimenez-Yuste V, Auerswald G, Benson G, Lambert T, Morfini M, Remor E, et al. Achieving and maintaining an optimal trough level for prophylaxis in haemophilia: the past, the present and the future. Blood Transfus. (2014) 12:314–9. doi: 10.2450/2014.0298-13
6. Elsebaie MAT, van Es N, Langston A, Büller HR, Gaddh M. Direct oral anticoagulants in patients with venous thromboembolism and thrombophilia: a systematic review and meta-analysis. J Thromb Haemost. (2019) 17:645–56.
7. van Geffen M, van Heerde WL. Global haemostasis assays, from bench to bedside. Thromb Res. (2012) 129:681–7. doi: 10.1016/j.thromres.2011.12.006
8. Kintigh J, Monagle P, Ignjatovic V. A review of commercially available thrombin generation assays. Res Pract Thromb Haemost. (2018) 2:42–8.
9. Verhagen MJA, Valke L, Schols SEM. Thrombin generation for monitoring hemostatic therapy in hemophilia A: a narrative review. J Thromb Haemost. (2022) 20:794–805. doi: 10.1111/jth.15640
10. Macfarlane RG, Biggs R. A thrombin generation test; the application in haemophilia and thrombocytopenia. J Clin Pathol. (1953) 6:3–8. doi: 10.1136/jcp.6.1.3
11. Pitney WR, Dacie JV. A simple method of studying the generation of thrombin in recalcified plasma; application in the investigation of haemophilia. J Clin Pathol. (1953) 6:9–14. doi: 10.1136/jcp.6.1.9
12. Davie EW, Kulman JD. An overview of the structure and function of thrombin. Semin Thromb Hemost. (2006) 32(Suppl. 1):3–15.
13. Duarte RCF, Ferreira CN, Rios DRA, Reis HJD, Carvalho MDG. Thrombin generation assays for global evaluation of the hemostatic system: perspectives and limitations. Rev Bras Hematol Hemoter. (2017) 39:259–65. doi: 10.1016/j.bjhh.2017.03.009
14. Loeffen R, Kleinegris MC, Loubele ST, Pluijmen PH, Fens D, van Oerle R, et al. Preanalytic variables of thrombin generation: towards a standard procedure and validation of the method. J Thromb Haemost. (2012) 10:2544–54.
15. Dargaud Y, Wolberg AS, Gray E, Negrier C, Hemker HC, Subcommittee on Factor VIII, et al. Proposal for standardized preanalytical and analytical conditions for measuring thrombin generation in hemophilia: communication from the SSC of the ISTH. J Thromb Haemost. (2017) 15:1704–7. doi: 10.1111/jth.13743
16. Hemker HC, Giesen P, AlDieri R, Regnault V, de Smed E, Wagenvoord R, et al. The calibrated automated thrombogram (CAT): a universal routine test for hyper- and hypocoagulability. Pathophysiol Haemost Thromb. (2002) 32:249–53. doi: 10.1159/000073575
17. Hemker HC, Wielders S, Kessels H, Béguin S. Continuous registration of thrombin generation in plasma, its use for the determination of the thrombin potential. Thromb Haemost. (1993) 70:617–24.
18. Chandler WL, Roshal M. Optimization of plasma fluorogenic thrombin-generation assays. Am J Clin Pathol. (2009) 132:169–79.
19. Ignjatovic V, Greenway A, Summerhayes R, Monagle P. Thrombin generation: the functional role of alpha-2-macroglobulin and influence of developmental haemostasis. Br J Haematol. (2007) 138:366–8. doi: 10.1111/j.1365-2141.2007.06663.x
20. Hemker HC, de Smedt E, Dieri R. Al. The contribution of alpha(2)-macroglobulin thrombin to the endogenous thrombin potential. Br J Haematol. (2007) 139:513. doi: 10.1111/j.1365-2141.2007.06834.x
21. De Smedt E, Al Dieri R, Spronk HM, Hamulyak K, ten Cate H, Hemker HC. The technique of measuring thrombin generation with fluorogenic substrates: 1. Necessity of adequate calibration. Thromb Haemost. (2008) 100:343–9.
23. Shima M, Matsumoto T, Ogiwara K. New assays for monitoring haemophilia treatment. Haemophilia. (2008) 14(Suppl. 3):83–92.
24. Baglin T. Using the laboratory to predict recurrent venous thrombosis. Int J Lab Hematol. (2011) 33:333–42.
25. Depasse F, Binder NB, Mueller J, Wissel T, Schwers S, Germer M, et al. Thrombin generation assays are versatile tools in blood coagulation analysis: a review of technical features, and applications from research to laboratory routine. J Thromb Haemost. (2021) 19:2907–17. doi: 10.1111/jth.15529
26. Dargaud Y, Luddington R, Gray E, Negrier C, Lecompte T, Petros S, et al. Effect of standardization and normalization on imprecision of calibrated automated thrombography: an international multicentre study. Br J Haematol. (2007) 139:303–9. doi: 10.1111/j.1365-2141.2007.06785.x
27. Boender J, Kruip MJ, Leebeek FW. A diagnostic approach to mild bleeding disorders. J Thromb Haemost. (2016) 14:1507–16.
28. Zegers SAM, Smit Y, Saes JL, van Duren C, Schuijt TJ, van Heerde WL, et al. Diagnostic work up of patients with increased bleeding tendency. Haemophilia. (2020) 26:269–77.
29. Moenen F, Nelemans PJ, Schols SEM, Schouten HC, Henskens YMC, Beckers EAM, et al. The diagnostic accuracy of bleeding assessment tools for the identification of patients with mild bleeding disorders: a systematic review. Haemophilia. (2018) 24:525–35. doi: 10.1111/hae.13486
30. Ay C, Haselböck J, Laczkovics C, Koder S, Pabinger I. Thrombin generation in patients with a bleeding tendency of unknown origin. Ann Hematol. (2011) 90:1099–104.
31. Holm E, Zetterberg E, Lövdahl S, Berntorp E. Patients referred for bleeding symptoms of unknown cause: does evaluation of thrombin generation contribute to diagnosis? Mediterr J Hematol Infect Dis. (2016) 8:e2016014.
32. Alves GS, Orsi FA, Santiago-Bassora FD, Quaino SK, Montalvão SA, Paula EV, et al. Laboratory evaluation of patients with undiagnosed bleeding disorders. Blood Coagul Fibrinolysis. (2016) 27:500–5.
33. Hofer S, Ay C, Rejtö J, Wolberg AS, Haslacher H, Koder S, et al. Thrombin-generating potential, plasma clot formation, and clot lysis are impaired in patients with bleeding of unknown cause. J Thromb Haemost. (2019) 17:1478–88. doi: 10.1111/jth.14529
34. MacDonald S, Wright A, Beuche F, Downes K, Besser M, Symington E, et al. Characterization of a large cohort of patients with unclassified bleeding disorder; clinical features, management of haemostatic challenges and use of global haemostatic assessment with proposed recommendations for diagnosis and treatment. Int J Lab Hematol. (2020) 42:116–25. doi: 10.1111/ijlh.13124
35. Veen CSB, Huisman EJ, Cnossen MH, Kom-Gortat R, Rijken DC, Leebeek FWG, et al. Evaluation of thromboelastometry, thrombin generation and plasma clot lysis time in patients with bleeding of unknown cause: a prospective cohort study. Haemophilia. (2020) 26:e106–15. doi: 10.1111/hae.13991
36. Cornette M, Monteyne T, De Kesel PM, Devreese KMJ. Thrombin generation measured by two platforms in patients with a bleeding tendency. J Thromb Haemost. (2021) 19:1460–71.
37. Al Dieri R, Peyvandi F, Santagostino E, Giansily M, Mannucci PM, Schved JF, et al. The thrombogram in rare inherited coagulation disorders: its relation to clinical bleeding. Thromb Haemost. (2002) 88:576–82.
38. Van Geffen M, Menegatti M, Loof A, Lap P, Karimi M, Laros-van Gorkom BA, et al. Retrospective evaluation of bleeding tendency and simultaneous thrombin and plasmin generation in patients with rare bleeding disorders. Haemophilia. (2012) 18:630–8. doi: 10.1111/j.1365-2516.2012.02759.x
39. Zekavat OR, Haghpanah S, Dehghani J, Afrasiabi A, Peyvandi F, Karimi M. Comparison of thrombin generation assay with conventional coagulation tests in evaluation of bleeding risk in patients with rare bleeding disorders. Clin Appl Thromb Hemost. (2014) 20:637–44.
40. Beltran-Miranda CP, Khan A, Jaloma-Cruz AR, Laffan MA. Thrombin generation and phenotypic correlation in haemophilia A. Haemophilia. (2005) 11:326–34.
41. Gilmore R, Harmon S, Gannon C, Byrne M, O’Donnell JS, Jenkins PV. Thrombin generation in haemophilia A patients with mutations causing factor VIII assay discrepancy. Haemophilia. (2010) 16:671–4.
42. Brummel-Ziedins KE, Whelihan MF, Gissel M, Mann KG, Rivard GE. Thrombin generation and bleeding in haemophilia A. Haemophilia. (2009) 15:1118–25.
43. Tarandovskiy ID, Balandina AN, Kopylov KG, Konyashina NI, Kumskova MA, Panteleev MA, et al. Investigation of the phenotype heterogeneity in severe hemophilia A using thromboelastography, thrombin generation, and thrombodynamics. Thromb Res. (2013) 131:e274–80. doi: 10.1016/j.thromres.2013.04.004
44. Santagostino E, Mancuso ME, Tripodi A, Chantarangkul V, Clerici M, Garagiola I, et al. Severe hemophilia with mild bleeding phenotype: molecular characterization and global coagulation profile. J Thromb Haemost. (2010) 8:737–43. doi: 10.1111/j.1538-7836.2010.03767.x
45. Haghpanah S, Bazrafshan A, Silavizadeh S, Dehghani J, Afrasiabi A, Karimi M. Evaluation of Thrombin Generation Assay in Patients With Hemophilia. Clin Appl Thromb Hemost. (2016) 22:322–6.
46. Tosetto A, Rodeghiero F, Castaman G, Goodeve A, Federici AB, Batlle J, et al. A quantitative analysis of bleeding symptoms in type 1 von Willebrand disease: results from a multicenter European study (MCMDM-1 VWD). J Thromb Haemost. (2006) 4:766–73. doi: 10.1111/j.1538-7836.2006.01847.x
47. Mancuso ME, Chantarangkul V, Clerici M, Fasulo MR, Padovan L, Scalambrino E, et al. The thrombin generation assay distinguishes inhibitor from non-inhibitor patients with severe haemophilia A. Haemophilia. (2016) 22:e286–91.
48. Saes JL, Verhagen MJA, Meijer K, Cnossen MH, Schutgens REG, Peters M, et al. Bleeding severity in patients with rare bleeding disorders: real-life data from the RBiN study. Blood Adv. (2020) 4:5025–34.
49. Pike GN, Cumming AM, Hay CR, Bolton-Maggs PH, Burthem J. Sample conditions determine the ability of thrombin generation parameters to identify bleeding phenotype in FXI deficiency. Blood. (2015) 126:397–405.
50. Rugeri L, Beguin S, Hemker C, Bordet JC, Fleury R, Chatard B, et al. Thrombin-generating capacity in patients with von Willebrand’s disease. Haematologica. (2007) 92:1639–46.
51. Bassus S, Wegert W, Krause M, Escuriola-Ettinghausen C, Siegemund A, Petros S, et al. Platelet-dependent coagulation assays for factor VIII efficacy measurement after substitution therapy in patients with haemophilia A. Platelets. (2006) 17:378–84. doi: 10.1080/09537100600757448
52. Brophy DF, Martin EJ, Ninivaggi M, Mohammed BM, Barrett JC, Kuhn J, et al. Evaluating the thrombin generation profiles of four different rFVIII products in FVIII-deficient plasma using FIXa and FXIa activation. Haemophilia. (2018) 24:815–22. doi: 10.1111/hae.13597
53. Salvagno GL, Astermark J, Lippi G, Ekman M, Franchini M, Guidi GC, et al. Thrombin generation assay: a useful routine check-up tool in the management of patients with haemophilia? Haemophilia. (2009) 15:290–6. doi: 10.1111/j.1365-2516.2008.01877.x
54. Chelle P, Montmartin A, Piot M, Ardillon L, Wibaut B, Frotscher B, et al. Prediction of individual factor VIII or IX level for the correction of thrombin generation in haemophilic patients. Haemophilia. (2018) 24:995–1001.
55. Dargaud Y, Béguin S, Lienhart A, Al Dieri R, Trzeciak C, Bordet JC, et al. Evaluation of thrombin generating capacity in plasma from patients with haemophilia A and B. Thromb Haemost. (2005) 93:475–80. doi: 10.1160/TH04-10-0706
56. van Veen JJ, Gatt A, Bowyer AE, Cooper PC, Kitchen S, Makris M. Calibrated automated thrombin generation and modified thromboelastometry in haemophilia A. Thromb Res. (2009) 123:895–901. doi: 10.1016/j.thromres.2008.09.011
57. Lewis SJ, Stephens E, Florou G, Macartney NJ, Hathaway LS, Knipping J, et al. Measurement of global haemostasis in severe haemophilia A following factor VIII infusion. Br J Haematol. (2007) 138:775–82.
58. Ay Y, Balkan C, Karapinar DY, Akin M, Bilenoðlu B, Kavakli K, et al. Feasibility of using thrombin generation assay (TGA) for monitoring of haemostasis during supplementation therapy in haemophilic patients without inhibitors. Haemophilia. (2012) 18:911–6. doi: 10.1111/j.1365-2516.2012.02849.x
59. Al Hawaj MA, Martin EJ, Venitz J, Barrett JC, Kuhn JG, Nolte ME, et al. Monitoring rFVIII prophylaxis dosing using global haemostasis assays. Haemophilia. (2013) 19:409–14. doi: 10.1111/hae.12110
60. Valke L, Meijer D, Nieuwenhuizen L, Laros-van Gorkom BAP, Blijlevens NMA, van Heerde WL, et al. Pharmacodynamic monitoring of factor VIII replacement therapy in hemophilia A: combining thrombin and plasmin generation. J Thromb Haemost. (2020) 18:3222–31. doi: 10.1111/jth.15106
61. Augustsson C, Norström E, Lind V, Martin M, Astermark J, Strandberg K. Validation of factor VIII activity for monitoring standard and extended half-life products and correlation to thrombin generation assays. Haemophilia. (2021) 27:494–500. doi: 10.1111/hae.14317
62. Hugenholtz GC, Luddington R, Baglin T. Haemostatic response to factor VIII administration in patients with haemophilia A measured by thrombin generation and correlation with factor concentrate use. Haemophilia. (2016) 22:e42–5. doi: 10.1111/hae.12798
63. Bukkems LH, Valke LLFG, Barteling W, Laros-van Gorkom BAP, Blijlevens NMA, Cnossen MH, et al. Combining factor VIII levels and thrombin/plasmin generation: a population pharmacokinetic-pharmacodynamic model for patients with haemophilia A. Br J Clin Pharmacol. (2022) 88:2757–68. doi: 10.1111/bcp.15185
64. Dargaud Y, Negrier C, Rusen L, Windyga J, Georgiev P, Bichler J, et al. Individual thrombin generation and spontaneous bleeding rate during personalized prophylaxis with Nuwiq((R)) (human-cl rhFVIII) in previously treated patients with severe haemophilia A. Haemophilia. (2018) 24:619–27. doi: 10.1111/hae.13493
65. Dargaud Y, Lienhart A, Janbain M, Le Quellec S, Enjolras N, Negrier C. Use of thrombin generation assay to personalize treatment of breakthrough bleeds in a patient with hemophilia and inhibitors receiving prophylaxis with emicizumab. Haematologica. (2018) 103:e181–3. doi: 10.3324/haematol.2017.185330
66. Delavenne X, Ollier E, Lienhart A, Dargaud Y. A new paradigm for personalized prophylaxis for patients with severe haemophilia A. Haemophilia. (2020) 26:228–35.
67. Atsou S, Furlan F, Duchemin J, Ellouze S, Sourdeau É, Launois A, et al. Pharmacodynamics of eftrenonacog-alfa (rFIX-Fc) in severe hemophilia B patients: a real-life study. Eur J Pharmacol. (2021) 891:173764. doi: 10.1016/j.ejphar.2020.173764
68. van Geffen M, Mathijssen NC, Holme PA, Laros-van Gorkom BA, van Kraaij MG, Masereeuw R, et al. Pharmacodynamics of recombinant activated factor VII and plasma-derived factor VII in a cohort of severe FVII deficient patients. Thromb Res. (2013) 132:116–22. doi: 10.1016/j.thromres.2013.04.021
69. Luna-Zaizar H, Beltrán-Miranda CP, Esparza-Flores MA, Soto-Padilla J, Bergés-García A, Rodríguez-Zepeda MD, et al. Thrombin generation as objective parameter of treatment response in patients with severe haemophilia A and high-titre inhibitors. Haemophilia. (2014) 20:e7–14. doi: 10.1111/hae.12309
70. Turecek PL, Váradi K, Keil B, Negrier C, Berntorp E, Astermark J, et al. Factor VIII inhibitor-bypassing agents act by inducing thrombin generation and can be monitored by a thrombin generation assay. Pathophysiol Haemost Thromb. (2003) 33:16–22. doi: 10.1159/000071637
71. Varadi K, Negrier C, Berntorp E, Astermark J, Bordet JC, Morfini M, et al. Monitoring the bioavailability of FEIBA with a thrombin generation assay. J Thromb Haemost. (2003) 1:2374–80. doi: 10.1046/j.1538-7836.2003.00450.x
72. Ettingshausen CE, Kreuz W. Early long-term FEIBA prophylaxis in haemophilia A patients with inhibitor after failing immune tolerance induction: a prospective clinical case series. Haemophilia. (2010) 16:90–100. doi: 10.1111/j.1365-2516.2009.02116.x
73. Tran HTT, Peterburs P, Seibel J, Abramov-Sommariva D, Lamy E. Monitoring bypassing agent therapy – a prospective crossover study comparing thromboelastometry and thrombin generation assay. Haemophilia. (2015) 21:275–83. doi: 10.1111/hae.12570
74. Eichinger S, Lubsczyk B, Kollars M, Traby L, Zwiauer K, Gleiss A, et al. Thrombin generation in haemophilia A patients with factor VIII inhibitors after infusion of recombinant factor VIIa. Eur J Clin Invest. (2009) 39:707–13.
75. Fernandez-Bello I, Stenmo C, Butta N, Lind V, Ezban M, Jiménez-Yuste V. The pharmacokinetics and pharmacodynamics of single-dose and multiple-dose recombinant activated factor VII in patients with haemophilia A or B. Haemophilia. (2017) 23:868–76.
76. Qi X, Zhao Y, Li K, Fan L, Hua B. Evaluating and monitoring the efficacy of recombinant activated factor VIIa in patients with haemophilia and inhibitors. Blood Coagul Fibrinolysis. (2014) 25:754–60.
77. Dargaud Y, Lienhart A, Negrier C. Prospective assessment of thrombin generation test for dose monitoring of bypassing therapy in hemophilia patients with inhibitors undergoing elective surgery. Blood. (2010) 116:5734–7. doi: 10.1182/blood-2010-06-291906
78. Oldenburg J, Mahlangu JN, Kim B, Schmitt C, Callaghan MU, Young G, et al. Emicizumab Prophylaxis in hemophilia A with inhibitors. N Engl J Med. (2017) 377:809–18.
79. Eichler H, Angchaisuksiri P, Kavakli K, Knoebl P, Windyga J, Jiménez-Yuste V, et al. Concizumab restores thrombin generation potential in patients with haemophilia: pharmacokinetic/pharmacodynamic modelling results of concizumab phase 1/1b data. Haemophilia. (2019) 25:60–6. doi: 10.1111/hae.13627
80. Pasi KJ, Lissitchkov T, Mamonov V, Mant T, Timofeeva M, Bagot C, et al. Targeting of antithrombin in hemophilia A or B with investigational siRNA therapeutic fitusiran-results of the phase 1 inhibitor cohort. J Thromb Haemost. (2021) 19:1436–46.
81. Pasi KJ, Rangarajan S, Georgiev P, Mant T, Creagh MD, Lissitchkov T, et al. Targeting of antithrombin in hemophilia A or B with RNAi therapy. N Engl J Med. (2017) 377:819–28.
82. Uchida N, Sambe T, Yoneyama K, Fukazawa N, Kawanishi T, Kobayashi S, et al. A first-in-human phase 1 study of ACE910, a novel factor VIII-mimetic bispecific antibody, in healthy subjects. Blood. (2016) 127:1633–41. doi: 10.1182/blood-2015-06-650226
83. Muller J, Pekrul I, Pötzsch B, Berning B, Oldenburg J, Spannagl M. Laboratory monitoring in emicizumab-treated persons with hemophilia A. Thromb Haemost. (2019) 119:1384–93. doi: 10.1055/s-0039-1692427
84. Schmitt C, Adamkewicz JI, Xu J, Petry C, Catalani O, Young G, et al. Pharmacokinetics and pharmacodynamics of emicizumab in persons with hemophilia A with factor VIII inhibitors: HAVEN 1 study. Thromb Haemost. (2021) 121:351–60.
85. Barg AA, Budnik I, Avishai E, Brutman-Barazani T, Bashari D, Misgav M, et al. Emicizumab prophylaxis: prospective longitudinal real-world follow-up and monitoring. Haemophilia. (2021) 27:383–91.
86. Brophy DF, Martin EJ, Kuhn J. Use of global assays to monitor emicizumab prophylactic therapy in patients with haemophilia A with inhibitors. Haemophilia. (2019) 25:e121–3.
87. Barg AA, Livnat T, Budnik I, Avishai E, Brutman-Barazani T, Tamarin I, et al. Emicizumab treatment and monitoring in a paediatric cohort: real-world data. Br J Haematol. (2020) 191:282–90. doi: 10.1111/bjh.16964
88. Barg AA, Avishai E, Budnik I, Levy-Mendelovich S, Barazani TB, Kenet G, et al. Emicizumab prophylaxis among infants and toddlers with severe hemophilia A and inhibitors-a single-center cohort. Pediatr Blood Cancer. (2019) 66:e27886. doi: 10.1002/pbc.27886
89. Misgav M, Brutman-Barazani T, Budnik I, Avishai E, Schapiro J, Bashari D, et al. Emicizumab prophylaxis in haemophilia patients older than 50 years with cardiovascular risk factors: real-world data. Haemophilia. (2021) 27:253–60. doi: 10.1111/hae.14261
90. Bravo MI, Raventós A, Pérez A, Costa M, Willis T. Non-additive effect on thrombin generation when a plasma-derived factor VIII/von Willebrand factor (FVIII/VWF) is combined with emicizumab in vitro. J Thromb Haemost. (2020) 18:1934–9. doi: 10.1111/jth.14887
91. Kizilocak H, Marquez-Casas E, Phei Wee C, Malvar J, Carmona R, Young G. Comparison of bypassing agents in patients on emicizumab using global hemostasis assays. Haemophilia. (2021) 27:164–72. doi: 10.1111/hae.14213
92. Schultz NH, Glosli H, Bjørnsen S, Holme PA. The effect of emicizumab and bypassing agents in patients with hemophilia – an in vitro study. Res Pract Thromb Haemost. (2021) 5:e12561. doi: 10.1002/rth2.12561
93. Hartmann R, Feenstra T, Valentino L, Dockal M, Scheiflinger F. In vitro studies show synergistic effects of a procoagulant bispecific antibody and bypassing agents. J Thromb Haemost. (2018) 16:1580–91. doi: 10.1111/jth.14203
94. Lenting PJ, Denis CV, Christophe OD. Emicizumab, a bispecific antibody recognizing coagulation factors IX and X: how does it actually compare to factor VIII? Blood. (2017) 130:2463–8. doi: 10.1182/blood-2017-08-801662
95. Nordfang O, Valentin S, Beck TC, Hedner U. Inhibition of extrinsic pathway inhibitor shortens the coagulation time of normal plasma and of hemophilia plasma. Thromb Haemost. (1991) 66:464–7.
96. Gorczyca ME, Nair SC, Jilma B, Priya S, Male C, Reitter S, et al. Inhibition of tissue factor pathway inhibitor by the aptamer BAX499 improves clotting of hemophilic blood and plasma. J Thromb Haemost. (2012) 10:1581–90. doi: 10.1111/j.1538-7836.2012.04790.x
97. Patel-Hett S, Martin EJ, Mohammed BM, Rakhe S, Sun P, Barrett JC, et al. Marstacimab, a tissue factor pathway inhibitor neutralizing antibody, improves coagulation parameters of ex vivo dosed haemophilic blood and plasmas. Haemophilia. (2019) 25:797–806. doi: 10.1111/hae.13820
98. Martin EJ, Nolte ME, Kuhn J, Schmidt N, Pfaff N, Brophy DF. An in vitro pharmacodynamic spiking study of befovacimab, a tissue factor pathway inhibitor monoclonal antibody, in blood samples from patients with severe FVIII deficiency. Haemophilia. (2021) 27:690–8. doi: 10.1111/hae.14314
99. Waters EK, Sigh J, Friedrich U, Hilden I, Sørensen BB. Concizumab, an anti-tissue factor pathway inhibitor antibody, induces increased thrombin generation in plasma from haemophilia patients and healthy subjects measured by the thrombin generation assay. Haemophilia. (2017) 23:769–76. doi: 10.1111/hae.13260
100. Eichler H, Angchaisuksiri P, Kavakli K, Knoebl P, Windyga J, Jiménez-Yuste V, et al. A randomized trial of safety, pharmacokinetics and pharmacodynamics of concizumab in people with hemophilia A. J Thromb Haemost. (2018) 16:2184–95.
101. Shapiro AD, Angchaisuksiri P, Astermark J, Benson G, Castaman G, Chowdary P, et al. Subcutaneous concizumab prophylaxis in hemophilia A and hemophilia A/B with inhibitors: phase 2 trial results. Blood. (2019) 134:1973–82. doi: 10.1182/blood.2019001542
102. Kjalke M, Kjelgaard-Hansen M, Andersen S, Hilden I. Thrombin generation potential in the presence of concizumab and rFVIIa, APCC, rFVIII, or rFIX: in vitro and ex vivo analyses. J Thromb Haemost. (2021) 19:1687–96. doi: 10.1111/jth.15323
103. Livnat T, Sehgal A, Qian K, Van Nguyen H, Madigan K, Sorensen B, et al. Thrombin generation in plasma of patients with haemophilia A and B with inhibitors: effects of bypassing agents and antithrombin reduction. Blood Cells Mol Dis. (2020) 82:102416. doi: 10.1016/j.bcmd.2020.102416
104. Lim HY, Han J, Yoon H, Jang KL. A review of global coagulation assays – is there a role in thrombosis risk prediction? Thromb Res. (2019) 179:45–55. doi: 10.1016/j.thromres.2019.04.033
105. Dargaud Y, Trzeciak MC, Bordet JC, Ninet J, Negrier C. Use of calibrated automated thrombinography +/- thrombomodulin to recognise the prothrombotic phenotype. Thromb Haemost. (2006) 96:562–7.
106. Tripodi A, Martinelli I, Chantarangkul V, Battaglioli T, Clerici M, Mannucci PM. The endogenous thrombin potential and the risk of venous thromboembolism. Thromb Res. (2007) 121:353–9.
107. Tripodi A, Legnani C, Chantarangkul V, Cosmi B, Palareti G, Mannucci PM. High thrombin generation measured in the presence of thrombomodulin is associated with an increased risk of recurrent venous thromboembolism. J Thromb Haemost. (2008) 6:1327–33. doi: 10.1111/j.1538-7836.2008.03018.x
108. Besser M, Baglin C, Luddington R, van Hylckama Vlieg A, Baglin T. High rate of unprovoked recurrent venous thrombosis is associated with high thrombin-generating potential in a prospective cohort study. J Thromb Haemost. (2008) 6:1720–5. doi: 10.1111/j.1538-7836.2008.03117.x
109. van Hylckama Vlieg A, Christiansen SC, Luddington R, Cannegieter SC, Rosendaal FR, Baglin TP. Elevated endogenous thrombin potential is associated with an increased risk of a first deep venous thrombosis but not with the risk of recurrence. Br J Haematol. (2007) 138:769–74.
110. van Hylckama Vlieg A, Baglin TP. The risk of a first and a recurrent venous thrombosis associated with an elevated D-dimer level and an elevated thrombin potential: results of the THE-VTE study: reply. J Thromb Haemost. (2015) 13:2286–7. doi: 10.1111/jth.13170
111. Hron G, Kollars M, Binder BR, Eichinger S, Kyrle PA. Identification of patients at low risk for recurrent venous thromboembolism by measuring thrombin generation. JAMA. (2006) 296:397–402.
112. Lutsey PL, Folsom AR, Heckbert SR, Cushman M. Peak thrombin generation and subsequent venous thromboembolism: the longitudinal investigation of thromboembolism etiology (LITE) study. J Thromb Haemost. (2009) 7:1639–48. doi: 10.1111/j.1538-7836.2009.03561.x
113. Eichinger S, Hron G, Kollars M, Kyrle PA. Prediction of recurrent venous thromboembolism by endogenous thrombin potential and D-dimer. Clin Chem. (2008) 54:2042–8. doi: 10.1373/clinchem.2008.112243
114. Chaireti R, Jennersjo C, Lindahl TL. Is thrombin generation at the time of an acute thromboembolic episode a predictor of recurrence? The linkoping study on thrombosis (LIST)–a 7-year follow-up. Thromb Res. (2013) 131:135–9. doi: 10.1016/j.thromres.2012.11.015
115. Palareti G, Cosmi B, Legnani C, Tosetto A, Brusi C, Iorio A, et al. D-dimer testing to determine the duration of anticoagulation therapy. N Engl J Med. (2006) 355:1780–9.
116. Ay C, Dunkler D, Simanek R, Thaler J, Koder S, Marosi C, et al. Prediction of venous thromboembolism in patients with cancer by measuring thrombin generation: results from the Vienna cancer and thrombosis study. J Clin Oncol. (2011) 29:2099–103.
117. Ten Cate H, Hemker HC. Thrombin generation and atherothrombosis: what does the evidence indicate? J Am Heart Assoc. (2016) 5:e003553.
118. Orbe J, Zudaire M, Serrano R, Coma-Canella I, Martínez de Sizarrondo S, Rodríguez JA, et al. Increased thrombin generation after acute versus chronic coronary disease as assessed by the thrombin generation test. Thromb Haemost. (2008) 99:382–7.
119. Brummel-Ziedins K, Undas A, Orfeo T, Gissel M, Butenas S, Zmudka K, et al. Thrombin generation in acute coronary syndrome and stable coronary artery disease: dependence on plasma factor composition. J Thromb Haemost. (2008) 6:104–10. doi: 10.1111/j.1538-7836.2007.02799.x
120. Smid M, Dielis AW, Winkens M, Spronk HM, van Oerle R, Hamulyák K, et al. Thrombin generation in patients with a first acute myocardial infarction. J Thromb Haemost. (2011) 9:450–6.
121. Loeffen R, Winckers K, Ford I, Jukema JW, Robertson M, Stott DJ, et al. Associations between thrombin generation and the risk of cardiovascular disease in elderly patients: results from the PROSPER study. J Gerontol A Biol Sci Med Sci. (2015) 70:982–8.
122. Carcaillon L, Alhenc-Gelas M, Bejot Y, Spaft C, Ducimetière P, Ritchie K, et al. Increased thrombin generation is associated with acute ischemic stroke but not with coronary heart disease in the elderly: the three-city cohort study. Arterioscler Thromb Vasc Biol. (2011) 31:1445–51.
123. Kuliczkowski W, Szewczyk M, Kaczmarski J, Sztohryn E, Greif M, Pres D, et al. Thrombin generation and platelet reactivity at hospital discharge and 6-month outcome after the acute coronary syndrome in diabetic and nondiabetic patients. Cardiology. (2014) 128:25–33. doi: 10.1159/000356492
124. Borissoff JI, Joosen IA, Versteylen MO, Spronk HM, ten Cate H, Hofstra L, et al. Accelerated in vivo thrombin formation independently predicts the presence and severity of CT angiographic coronary atherosclerosis. JACC Cardiovasc Imaging. (2012) 5:1201–10. doi: 10.1016/j.jcmg.2012.01.023
125. Schneider JG, Isermann B, Kleber ME, Wang H, Boehm BO, Grammer TB, et al. Inverse association of the endogenous thrombin potential (ETP) with cardiovascular death: the Ludwigshafen risk and cardiovascular health (LURIC) study. Int J Cardiol. (2014) 176:139–44. doi: 10.1016/j.ijcard.2014.07.026
126. Loeffen R, Godschalk TC, van Oerle R, Spronk HM, Hackeng CM, ten Berg JM, et al. The hypercoagulable profile of patients with stent thrombosis. Heart. (2015) 101:1126–32.
127. Attanasio M, Marcucci R, Gori AM, Paniccia R, Valente S, Balzi D, et al. Residual thrombin potential predicts cardiovascular death in acute coronary syndrome patients undergoing percutaneous coronary intervention. Thromb Res. (2016) 147:52–7. doi: 10.1016/j.thromres.2016.09.020
128. Faber CG, Lodder J, Kessels F, Troost J. Thrombin generation in platelet-rich plasma as a tool for the detection of hypercoagulability in young stroke patients. Pathophysiol Haemost Thromb. (2003) 33:52–8. doi: 10.1159/000071642
129. Rooth E, Sobocinski-Doliwa P, Antovic J, Frykman Kull V, Von Arbin M, Rosenqvist M, et al. Thrombin generation in acute cardioembolic and non-cardioembolic ischemic stroke. Scand J Clin Lab Invest. (2013) 73:576–84.
130. Balogun IO, Roberts LN, Patel R, Pathansali R, Kalra L, Arya R. Thrombin generation in acute ischaemic stroke. Stroke Res Treat. (2016) 2016:7940680.
131. van Paridon PCS, Panova-Noeva M, van Oerle R, Schulz A, Prochaska JH, Arnold N, et al. Thrombin generation in cardiovascular disease and mortality – results from the Gutenberg health study. Haematologica. (2020) 105:2327–34.
132. Olivieri O, Martinelli N, Girelli D, Pizzolo F, Friso S, Beltrame F, et al. Apolipoprotein C-III predicts cardiovascular mortality in severe coronary artery disease and is associated with an enhanced plasma thrombin generation. J Thromb Haemost. (2010) 8:463–71. doi: 10.1111/j.1538-7836.2009.03720.x
133. Miyakis S, Lockshin MD, Atsumi T, Branch DW, Brey RL, Cervera R, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost. (2006) 4:295–306. doi: 10.1111/j.1538-7836.2006.01753.x
134. Sciascia S, Baldovino S, Schreiber K, Solfietti L, Radin M, Cuadrado MJ, et al. Thrombotic risk assessment in antiphospholipid syndrome: the role of new antibody specificities and thrombin generation assay. Clin Mol Allergy. (2016) 14:6. doi: 10.1186/s12948-016-0043-2
135. Sheng Y, Hanly JG, Reddel SW, Kouts S, Guerin J, Koike T, et al. Detection of ‘antiphospholipid’ antibodies: a single chromogenic assay of thrombin generation sensitively detects lupus anticoagulants, anticardiolipin antibodies, plus antibodies binding beta(2)-glycoprotein I and prothrombin. Clin Exp Immunol. (2001) 124:502–8. doi: 10.1046/j.1365-2249.2001.01555.x
136. Dienava-Verdoold I, Boon-Spijker MG, de Groot PG, Brinkman HJ, Voorberg J, Mertens K, et al. Patient-derived monoclonal antibodies directed towards beta2 glycoprotein-1 display lupus anticoagulant activity. J Thromb Haemost. (2011) 9:738–47. doi: 10.1111/j.1538-7836.2011.04212.x
137. Regnault V, Béguin S, Wahl D, de Maistre E, Coenraad Hemker H, Lecompte T. Thrombinography shows acquired resistance to activated protein C in patients with lupus anticoagulants. Thromb Haemost. (2003) 89:208–12.
138. Devreese K, Peerlinck K, Arnout J, Hoylaerts MF. Laboratory detection of the antiphospholipid syndrome via calibrated automated thrombography. Thromb Haemost. (2009) 101:185–96.
139. Devreese K, Peerlinck K, Hoylaerts MF. Thrombotic risk assessment in the antiphospholipid syndrome requires more than the quantification of lupus anticoagulants. Blood. (2010) 115:870–8. doi: 10.1182/blood-2009-09-244426
140. Liestol S, Sandset PM, Mowinckel MC, Wisløff F. Activated protein C resistance determined with a thrombin generation-based test is associated with thrombotic events in patients with lupus anticoagulants. J Thromb Haemost. (2007) 5:2204–10.
141. Zuily S, Ait Aissa K, Membre A, Regnault V, Lecompte T, Wahl D. Thrombin generation in antiphospholipid syndrome. Lupus. (2012) 21:758–60.
142. Zuily S, Regnault V, Guillemin F, Kaminsky P, Rat AC, Lecompte T, et al. Superficial vein thrombosis, thrombin generation and activated protein C resistance as predictors of thromboembolic events in lupus and antiphospholipid patients. A prospective cohort study. Thromb Res. (2013) 132:e1–7. doi: 10.1016/j.thromres.2013.04.012
143. Arachchillage DR, Efthymiou M, Mackie IJ, Lawrie AS, Machin SJ, Cohen H. Anti-protein C antibodies are associated with resistance to endogenous protein C activation and a severe thrombotic phenotype in antiphospholipid syndrome. J Thromb Haemost. (2014) 12:1801–9. doi: 10.1111/jth.12722
144. Zuily S, de Laat B, Guillemin F, Kelchtermans H, Magy-Bertrand N, Desmurs-Clavel H, et al. Anti-domain I beta2-glycoprotein I antibodies and activated protein C resistance predict thrombosis in antiphospholipid syndrome: TAC(I)T study. J Appl Lab Med. (2020) 5:1242–52. doi: 10.1093/jalm/jfaa072
145. Efthymiou M, Lawrie AS, Mackie I, Arachchillage D, Lane PJ, Machin S, et al. Thrombin generation and factor X assays for the assessment of warfarin anticoagulation in thrombotic antiphospholipid syndrome. Thromb Res. (2015) 135:1191–7. doi: 10.1016/j.thromres.2015.03.030
146. Bergstrom CP, Zia A, Sarode R, Nagalla S. Thrombin generation in a patient with triple positive antiphospholipid syndrome treated with three different anticoagulants. Transfus Apher Sci. (2020) 59:102815. doi: 10.1016/j.transci.2020.102815
147. Cohen H, Hunt BJ, Efthymiou M, Arachchillage DR, Mackie IJ, Clawson S, et al. Rivaroxaban versus warfarin to treat patients with thrombotic antiphospholipid syndrome, with or without systemic lupus erythematosus (RAPS): a randomised, controlled, open-label, phase 2/3, non-inferiority trial. Lancet Haematol. (2016) 3:e426–36.
148. Binder NB, Depasse F, Mueller J, Wissel T, Schwers S, Germer M, et al. Clinical use of thrombin generation assays. J Thromb Haemost. (2021) 19:2918–29.
149. Spadarella G, Di Minno A, Donati MB, Mormile M, Ventre I, Di Minno G, et al. From unfractionated heparin to pentasaccharide: paradigm of rigorous science growing in the understanding of the in vivo thrombin generation. Blood Rev. (2020) 39:100613. doi: 10.1016/j.blre.2019.100613
150. Gerotziafas GT, Petropoulou AD, Verdy E, Samama MM, Elalamy I. Effect of the anti-factor Xa and anti-factor IIa activities of low-molecular-weight heparins upon the phases of thrombin generation. J Thromb Haemost. (2007) 5:955–62. doi: 10.1111/j.1538-7836.2007.02477.x
151. Thomas O, Lybeck E, Strandberg K, Tynngård N, Schött U. Monitoring low molecular weight heparins at therapeutic levels: dose-responses of, and correlations and differences between aPTT, anti-factor Xa and thrombin generation assays. PLoS One. (2015) 10:e0116835. doi: 10.1371/journal.pone.0116835
152. Gerotziafas GT, Depasse F, Chakroun T, Van Dreden P, Samama MM, Elalamy I. Comparison of the effect of fondaparinux and enoxaparin on thrombin generation during in-vitro clotting of whole blood and platelet-rich plasma. Blood Coagul Fibrinolysis. (2004) 15:149–56. doi: 10.1097/00001721-200403000-00006
153. Selmeczi A, Roach RE, Móré C, Batta Z, Hársfalvi J, van der Bom JG, et al. Thrombin generation and low-molecular-weight heparin prophylaxis in pregnant women with thrombophilia. Thromb Haemost. (2015) 113:283–9.
154. Ismail SK, Norris L, O’Shea S, Higgins JR. Weight-adjusted LMWH prophylaxis provides more effective thrombin inhibition in morbidly obese pregnant women. Thromb Res. (2014) 134:234–9. doi: 10.1016/j.thromres.2014.04.006
155. D’Alessio A, Marchetti M, Tartari CJ, Russo L, Cecchini S, Lambregts KWFM, et al. Long term low molecular weight heparin anticoagulant therapy modulates thrombin generation and D-dimer in patients with cancer and venous thromboembolism. Cancer Invest. (2017) 35:490–9. doi: 10.1080/07357907.2017.1340480
156. Schmidt DE, Chaireti R, Bruzelius M, Holmström M, Antovic J, Ågren A. Correlation of thromboelastography and thrombin generation assays in warfarin-treated patients. Thromb Res. (2019) 178:34–40. doi: 10.1016/j.thromres.2019.03.022
157. Arachchillage DR, Efthymiou M, Mackie IJ, Lawrie AS, Machin SJ, Cohen H. Rivaroxaban and warfarin achieve effective anticoagulation, as assessed by inhibition of TG and in-vivo markers of coagulation activation, in patients with venous thromboembolism. Thromb Res. (2015) 135:388–93. doi: 10.1016/j.thromres.2014.11.037
158. Bloemen S, Zwaveling S, Ten Cate H, Ten Cate-Hoek A, de Laat B. Prediction of bleeding risk in patients taking vitamin K antagonists using thrombin generation testing. PLoS One. (2017) 12:e0176967. doi: 10.1371/journal.pone.0176967
159. Dargaud Y, Hoffman M, Lefrapper L, Lin FC, Genty A, Chatard B, et al. Bleeding risk in warfarinized patients with a therapeutic international normalized ratio: the effect of low factor IX levels. J Thromb Haemost. (2013) 11:1043–52.
160. Kustos SA, Fasinu PS. Direct-acting oral anticoagulants and their reversal agents-an update. Medicines (Basel). (2019) 6:103. doi: 10.3390/medicines6040103
161. Wieland E, Shipkova M. Pharmacokinetic and pharmacodynamic drug monitoring of direct-acting oral anticoagulants: where do we stand? Ther Drug Monit. (2019) 41:180–91.
162. Bloemen S, Zwaveling S, Douxfils J, Roest M, Kremers R, Mullier F. The anticoagulant effect of dabigatran is reflected in the lag time and time-to-peak, but not in the endogenous thrombin potential or peak, of thrombin generation. Thromb Res. (2018) 171:160–6.
163. Wan H, Yang Y, Zhu J, Wu S, Zhou Z, Huang B, et al. An in-vitro evaluation of direct thrombin inhibitor and factor Xa inhibitor on tissue factor-induced thrombin generation and platelet aggregation: a comparison of dabigatran and rivaroxaban. Blood Coagul Fibrinolysis. (2016) 27:882–5.
164. Wong PC, White A, Luettgen J. Inhibitory effect of apixaban compared with rivaroxaban and dabigatran on thrombin generation assay. Hosp Pract (1995). (2013) 41:19–25. doi: 10.3810/hp.2013.02.1009
165. Tripodi A, Yang Y, Zhu J, Wu S, Zhou Z, Huang B, et al. How the direct oral anticoagulant apixaban affects thrombin generation parameters. Thromb Res. (2015) 135:1186–90.
166. Schenk B, Würtinger P, Streif W, Sturm W, Fries D, Bachler M. Ex vivo reversal of effects of rivaroxaban evaluated using thromboelastometry and thrombin generation assay. Br J Anaesth. (2016) 117:583–91.
167. Molenaar PJ, Dinkelaar J, Leyte A. Measuring Rivaroxaban in a clinical laboratory setting, using common coagulation assays, Xa inhibition and thrombin generation. Clin Chem Lab Med. (2012) 50:1799–807. doi: 10.1515/cclm-2012-0055
168. Samama MM, Mendell J, Guinet C, Le Flem L, Kunitada S. In vitro study of the anticoagulant effects of edoxaban and its effect on thrombin generation in comparison to fondaparinux. Thromb Res. (2012) 129:e77–82. doi: 10.1016/j.thromres.2011.07.026
169. Sinegre T, Zlobecki M, Doré E, Pereira B, Grèze V, Lebreton A. In vitro assessment of edoxaban anticoagulant effect in pediatric plasma. Thromb Res. (2019) 178:112–8. doi: 10.1016/j.thromres.2019.04.014
170. Morishima Y, Kamisato C. Laboratory measurements of the oral direct factor Xa inhibitor edoxaban: comparison of prothrombin time, activated partial thromboplastin time, and thrombin generation assay. Am J Clin Pathol. (2015) 143:241–7. doi: 10.1309/AJCPQ2NJD3PXFTUG
171. Artang R, Anderson M, Riley P, Nielsen JD. Assessment of the effect of direct oral anticoagulants dabigatran, rivaroxaban, and apixaban in healthy male volunteers using a thrombin generation assay. Res Pract Thromb Haemost. (2017) 1:194–201.
172. Kyriakou E, Katogiannis K, Ikonomidis I, Giallouros G, Nikolopoulos GK, Rapti E, et al. Laboratory assessment of the anticoagulant activity of apixaban in patients with nonvalvular atrial fibrillation. Clin Appl Thromb Hemost. (2018) 24(Suppl. 9):194S–201S.
173. Graff J, von Hentig N, Misselwitz F, Kubitza D, Becka M, Breddin HK, et al. Effects of the oral, direct factor xa inhibitor rivaroxaban on platelet-induced thrombin generation and prothrombinase activity. J Clin Pharmacol. (2007) 47:1398–407. doi: 10.1177/0091270007302952
174. Bertaggia-Calderara D, Kröll D, Gerschheimer C, Nicolas N, Nett P, Stirnimann G, et al. Effect of rivaroxaban on thrombin generation in vivo. A study in obese patients. Int J Lab Hematol. (2018) 40:e11–4. doi: 10.1111/ijlh.12767
175. Pfrepper C, Behrendt LC, Bönigk H, Siegemund T, Metze M, Franke D, et al. Influence of direct oral anticoagulants on thrombin generation on ceveron TGA. Int J Lab Hematol. (2022) 44:193–201. doi: 10.1111/ijlh.13721
176. Pfrepper C, Metze M, Siegemund A, Klöter T, Siegemund T, Petros S. Direct oral anticoagulant plasma levels and thrombin generation on ST genesia system. Res Pract Thromb Haemost. (2020) 4:619–27. doi: 10.1002/rth2.12340
177. Metze M, Pfrepper C, Klöter T, Stöbe S, Siegemund R, Siegemund T, et al. Inhibition of thrombin generation 12 hours after intake of direct oral anticoagulants. Res Pract Thromb Haemost. (2020) 4:610–8.
178. Metze M, Klöter T, Stöbe S, Rechenberger B, Siegemund R, Siegemund T, et al. Plasma levels do not predict thrombin generation in patients taking direct oral anticoagulants. Int J Lab Hematol. (2021) 43:1539–48.
179. Meihandoest T, Studt JD, Mendez A, Alberio L, Fontana P, Wuillemin WA, et al. Automated thrombin generation assay for rivaroxaban, apixaban, and edoxaban measurements. Front Cardiovasc Med. (2021) 8:717939. doi: 10.3389/fcvm.2022.817826
180. Khoo TL, Weatherburn C, Kershaw G, Reddel CJ, Curnow J, Dunkley S. The use of FEIBA(R) in the correction of coagulation abnormalities induced by dabigatran. Int J Lab Hematol. (2013) 35:222–4.
181. Berezovskaya G, Smirnova O, Malev E, Khromov-Borisov N, Klokova E, Karpenko M, et al. Thrombin generation test for evaluation of antiplatelet treatment in patients with coronary artery disease after percutaneous coronary intervention. Platelets. (2018) 29:185–91.
182. Tobin WO, Kinsella JA, Kavanagh GF, O’Donnell JS, McGrath RA, Collins DR, et al. Longitudinal assessment of thrombin generation potential in response to alteration of antiplatelet therapy after TIA or ischaemic stroke. J Neurol. (2013) 260:590–6.
183. Gremmel T, Panzer S, Steiner S, Seidinger D, Koppensteiner R, Pabinger I, et al. Response to antiplatelet therapy is independent of endogenous thrombin generation potential. Thromb Res. (2013) 132:e24–30. doi: 10.1016/j.thromres.2013.04.008
184. de Breet C, Zwaveling S, Vries MJA, van Oerle RG, Henskens YMC, Van’t Hof AWJ, et al. Thrombin generation as a method to identify the risk of bleeding in high clinical-risk patients using dual antiplatelet therapy. Front Cardiovasc Med. (2021) 8:679934. doi: 10.3389/fcvm.2021.679934
185. FORUM. Stratified, Personalised or P4 Medicine: A New Direction for Placing the Patient at the Centre of Healthcare and Health Education. Southampton: Academy of Medical Sciences (2015).
186. Menon N, Sarode R, Zia A. Rivaroxaban dose adjustment using thrombin generation in severe congenital protein C deficiency and warfarin-induced skin necrosis. Blood Adv. (2018) 2:142–5. doi: 10.1182/bloodadvances.2017012047
187. Perrin J, Depasse F, Lecompte T, French-speaking CAT, Group and Under the Aegis of GEHT, French-Speaking Cat Group. Large external quality assessment survey on thrombin generation with CAT: further evidence for the usefulness of normalisation with an external reference plasma. Thromb Res. (2015) 136:125–30. doi: 10.1016/j.thromres.2014.12.015
Keywords: bleeding, personalized medicine, thrombin generation, thrombosis, hemophilia
Citation: Valke LLFG, Rijpma S, Meijer D, Schols SEM and van Heerde WL (2022) Thrombin generation assays to personalize treatment in bleeding and thrombotic diseases. Front. Cardiovasc. Med. 9:1033416. doi: 10.3389/fcvm.2022.1033416
Received: 31 August 2022; Accepted: 26 October 2022;
Published: 10 November 2022.
Edited by:
Romy De Laat-Kremers, Synapse BV, NetherlandsReviewed by:
Hugo Ten Cate, Maastricht University Medical Centre, NetherlandsCopyright © 2022 Valke, Rijpma, Meijer, Schols and van Heerde. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Waander L. van Heerde, d2FhbmRlci52YW5oZWVyZGVAcmFkYm91ZHVtYy5ubA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.