- 1Second Clinical School of Medicine, Beijing University of Chinese Medicine, Beijing, China
- 2Department of Cardiology, Dongfang Hospital, Beijing University of Chinese Medicine, Beijing, China
- 3Department of Cardiology, Dongzhimen Hospital, Beijing University of Chinese Medicine, Beijing, China
Objectives: In China, Danhong injection (DHI) is recommended by expert consensus and is widely used in the perioperative management of patients with acute coronary syndrome (ACS). This study investigates the effect of perioperative DHI administration and the timing of DHI administration on patients with ACS undergoing percutaneous coronary intervention (PCI) by analyzing the prognosis and anti-inflammatory effects. This article summarizes the most up-to-date clinical evidence on DHI, and in this study, we assesses treatment efficacy of DHI in patients with ACS.
Methods: A total of seven databases (PubMed, Embase, Cochrane Library, SINOMED, CNKI, Wanfang, and VIP) were searched from the time of their inception to 1 July 2022. Clinical randomized controlled trials (RCTs) of DHI combined with PCI for the treatment of ACS were included. RCT quality was assessed using the Cochrane Handbook risk-of-bias tool, and STATA 17.0 was used for meta-analysis.
Results: In total, 33 studies including 3,458 patients with ACS undergoing PCI were included in the meta-analysis. Compared with conventional therapy alone, the combination of DHI and conventional therapy significantly decreased the incidence of major adverse cardiovascular events (MACEs; P<0.001) and improved the reperfusion rate (P < 0.001). Serum high-sensitivity C-reactive protein (hs-CRP) and interleukin (IL)-6 levels were substantially reduced in the test group (P<0.001). In addition, the plasma levels of myocardial injury markers and cardiac troponin T (cTnT) declined significantly (P < 0.01). Compared with the control group, DHI improved the left ventricular ejection fraction (LVEF; P < 0.001) and reduced B-type natriuretic peptide (BNP; P < 0.001) levels. Subgroups were established based on different timings of DHI administration: preoperative, intraoperative, and postoperative groups. The results showed that the incidence of MACEs and the reperfusion rate did not differ between the groups. Among the subgroups, the postoperative group exhibited significantly lower levels of BNP, hs-CRP, and IL-6 serum and a significantly higher level of LVEF (P < 0.05).
Conclusion: The combination of DHI and conventional therapy results in a better therapeutic effect than that observed with conventional therapy alone in patients with ACS. To improve treatment efficacy, postoperative initiation of DHI is recommended as a standard treatment. Further research is needed to confirm these results.
Systematic review registration: Identifier: CRD42022344830.
Introduction
Acute coronary syndrome (ACS) is caused by the rupture of atherosclerotic plaque and subsequent thrombosis, resulting in unstable angina, non-ST segment elevation myocardial infarction, and ST segment elevation myocardial infarction (1, 2). In China, the incidence of coronary heart disease (CHD) and associated mortality rates is increasing annually (3). ACS is the most extreme type of CHD. Percutaneous coronary intervention (PCI) has an immediate effect on the revascularization of the infarct-related artery, and it may be more effective in restoring myocardial perfusion, reducing the incidence of myocardial ischemia or infarction, and improving clinical outcomes. PCI is widely used for the treatment of ACS (4). However, PCI may be complicated by no reflow, slow coronary flow, diverse arrhythmias, myocardial ischemia–reperfusion injuries (MIRIs), and in-stent restenosis (ISR). MIRI seriously affects patients' heart function and prognosis. Therefore, these complications of PCI cannot be ignored.
As a complementary or adjuvant therapy, DHI is a standardized traditional Chinese medicine (TCM) product. The main active ingredients are protocatechuic aldehyde, tanshinone, salvianolic acid, and catechin (5). Based on the TCM theory, the pathogenesis of CHD is closely related to stagnant blood, while DHI promotes blood flow and resolves the blood stasis. Modern pharmacological studies have reported that DHI promotes multiple pharmacological activities that have anti-thrombotic, anti-platelet aggregate, anti-inflammatory, hypolipidemic, anti-oxidative damage, and pro-human microcirculation effects (6). In clinical practice, DHI has been used to treat cardiovascular diseases and to reduce the incidence of major adverse cardiovascular events (MACEs), myocardial necrosis marker levels, and inflammatory factor levels (7–10).
A previous meta-analysis reported that DHI combined with conventional therapy for the treatment of patients with ACS improved the total efficacy rate and decreased the incidence of MACEs after PCI (11). However, it did not measure indicators such as myocardial injury or analyze the effect of the timing of DHI.
Therefore, this systemic review and meta-analysis summarizes the results of more recent RCTs regarding DHI. The efficacy of DHI in patients with ACS undergoing PCI and the effect of the timing of DHI on the incidence of MACEs and myocardial injury and inflammatory biomarker levels are assessed to provide clinical evidence regarding DHI.
Materials and methods
This analysis followed the PRISMA guidelines (12), and the review protocol was registered with PROSPERO (CRD42022344830).
Search strategy
For this study, seven databases (PubMed, Embase, Cochrane Library, SINOMED, CNKI, Wanfang, and VIP) were searched from their inception to 1 July 2022, using the following subject terms: “percutaneous coronary intervention,” “Danhong injection,” “acute coronary syndrome,” “myocardial infarction,” “unstable anginas,” “percutaneous coronary intervention,” and “randomized controlled trial.” The search terms were changed according to databases and languages. Language restrictions were not applied for included studies. The different databases used a corresponding combination of subject words, free words, and keywords. In total, two researchers (YXL and YL) independently evaluated the eligibility of the retrieved studies. A third researcher (DL) was consulted in case of disagreement. The bibliography of each article was manually searched for additional studies.
Inclusion and exclusion criteria
The prespecified eligibility criteria were as follows: (a) RCTs including patients with ST elevation myocardial infarction or unstable angina/non-ST elevation myocardial infarction, as defined by the European Society of Cardiology guidelines, and undergoing PCI were included in the meta-analysis; (b) all studies including a control group undergoing conventional therapy and a test group undergoing DHI combined with conventional treatment; and (c) studies reporting at least one of the following findings or outcomes: MACEs, thrombolysis in myocardial infarction (TIMI) flow grade, ST segment resolution (STR), high-sensitivity C-reactive protein (hs-CRP), interleukin (IL)-6, creatine kinase (CK), CK–myocardial band (MB), cardiac troponin T (cTnT), left ventricular ejection fraction (LVEF), or brain natriuretic peptide (BNP).
The exclusion criteria were as follows: (a) duplicate studies, (b) studies in which other TCMs were used in control or experimental groups, and (c) case reports, narrative reviews, meta-analyses, systematic literature reviews, observational studies, animal studies, or in vitro studies.
Data extraction
In this study, two researchers (YXL and YL) independently extracted data from each study, including the first author, year of publication, participant characteristics, sample size, intervention, duration of intervention, and outcome assessment, and any differences were resolved via discussion.
Quality assessment
The quality of the included studies was assessed following the Cochrane Handbook of Systematic Review. Random sequence generation, assignment confounding, blinding of participants and hospital staff, blind outcome assessment, incomplete outcome data, selective reporting, and other sources of bias were considered in the quality assessment. The results were cross-checked by the same two researchers (YXL and YL), and any disagreements were resolved via discussion.
Data synthesis and statistical analysis
STATA 17.0 was used for the meta-analysis (13). Data are presented as risk ratios (RRs) and standardized mean differences (SMDs) with 95% confidence intervals (CIs). Potential heterogeneity was assessed using Cochran Q and I2 statistical tests. A fixed-effects model was used to compare data from studies with low heterogeneity (14), whereas a random-effects model was used to compare data from studies with high heterogeneity (P < 0.05, I2 > 50%). Subgroup, sensitivity, and meta-regression analyses were used to examine heterogeneity between the outcomes. The potential of a publication bias was assessed using Egger's and Begg's tests.
Results
Literature search and screening
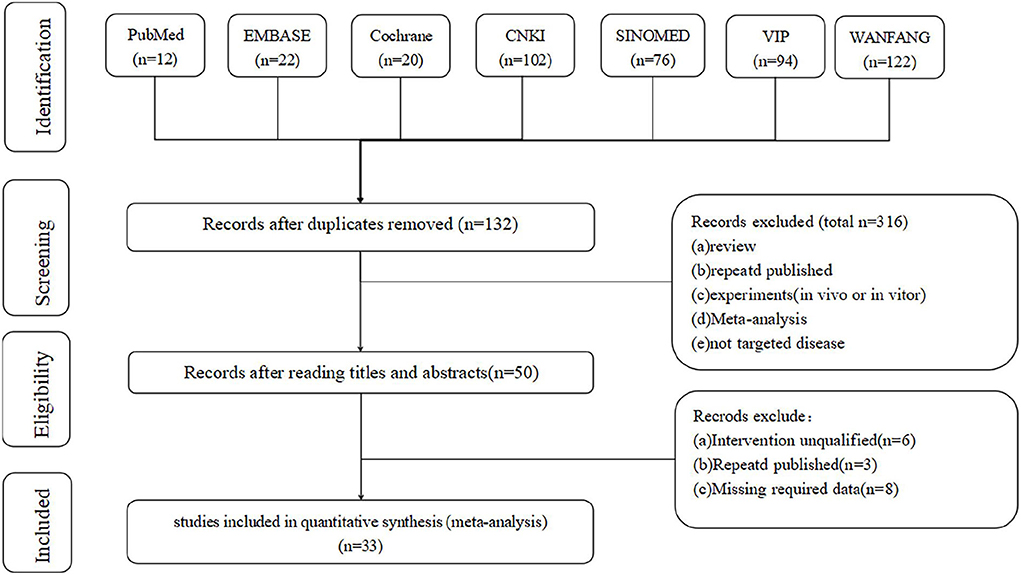
First, the database search identified 448 articles for evaluation (Wanfang: 120 articles; CNKI: 102 articles; VIP: 93 articles; SINOMED: 76 articles; Embase: 22 articles; Cochrane Library: 20 articles; and PubMed: 12 articles). Subsequently, 316 duplicate records were identified and removed. After screening titles and abstracts, 82 articles met the exclusion criteria. Finally, 33 articles (7–10, 15–43) were included in the meta-analysis (Figure 1).
Study characteristics and quality assessment
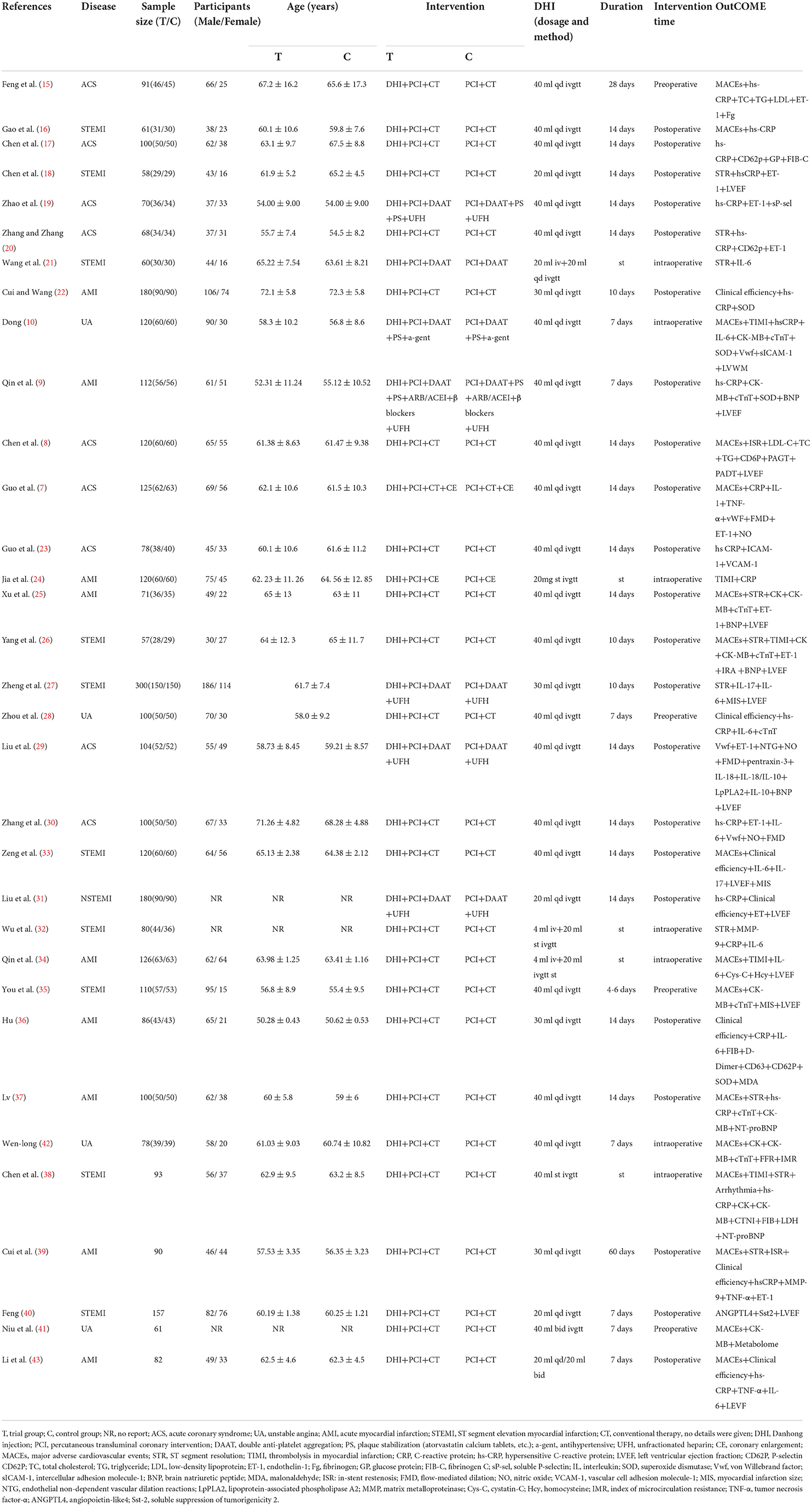
The 33 studies included in this meta-analysis were published between 2007 and 2022 (Table 1) and were conducted in China. Among them, two studies were published in English (35, 42) and 31 studies were in Chinese. A total of 3,458 patients with ACS who underwent PCI were enrolled, among whom 1,722—control group—patients received a PCI-based conventional treatment, including medicines for anti-platelet aggregation, anti-coagulation, lipid lowering and plaque stabilization, and inhibition of ventricular remodeling, and the remaining 1,736—test group—patients received DHI and the conventional treatment. A DHI dose of 20–40 mL was used, administered by intravenous drip or injection. DHI was administered before (preoperatively), during (intraoperatively), or after (postoperatively) PCI. The treatment duration was 7–14 days. The outcome indicators of response prognosis observed in the included studies were MACEs, STR, and ISR, and the inflammatory factors were hs-CRP, tumor necrosis factor (TNF)-α, IL-6, IL-10, and matrix metalloproteinase (MMP)-9. Markers of myocardial injury (CK, CK-MB, and cTnT) and cardiac function (LVEF and BNP) were also reported.
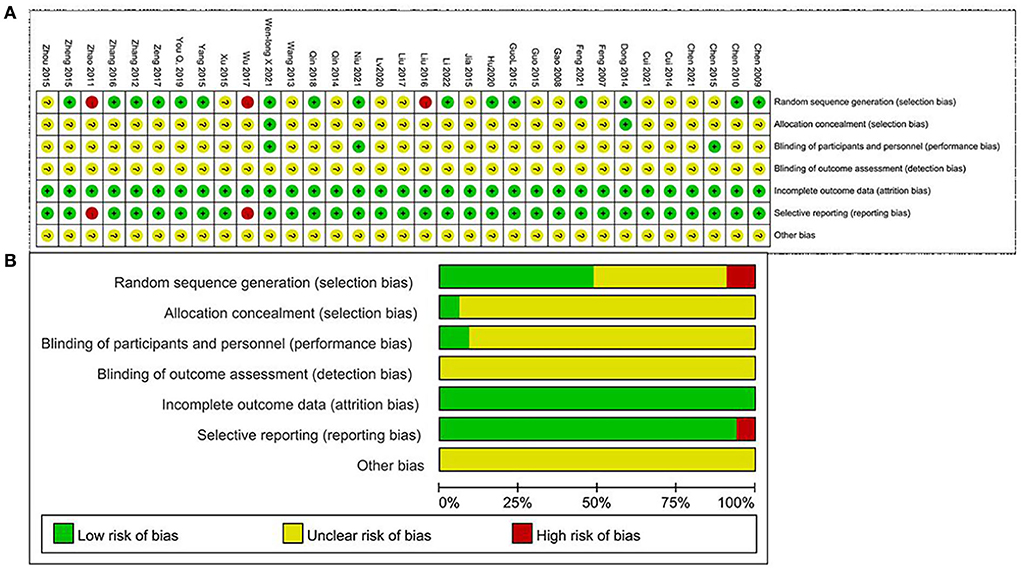
The Cochrane Handbook tool was used for assessing the risks of bias in this study. In all the studies, subjects were randomly assigned. A total of 15 studies that provided the detailed information about that random assignment method had a low risk of bias; two studies that randomized subjects according to the time of admission and treatment protocol had a high risk of bias; eight studies that did not report information about the randomization method had an unclear risk of bias. Regarding allocation concealment, two studies that provided descriptions of allocation methods had a low risk of bias. The remaining 29 studies that did not describe the allocation process had an unclear risk of bias. Regarding blinding methods, two studies that blinded the patients but not the investigators had a low risk of bias. The remaining studies that did not report patient or investigator blinding had an unclear risk of bias. In addition, two studies that did not report the results of the pre-specified indicators had a high risk of bias due to selective outcome reporting. The remaining 31 studies that included complete data results had a low risk of bias. All included studies stated that the baseline characteristics of the two groups were not significantly different (Figure 2).
Outcome measures and subgroup analyses
MACE
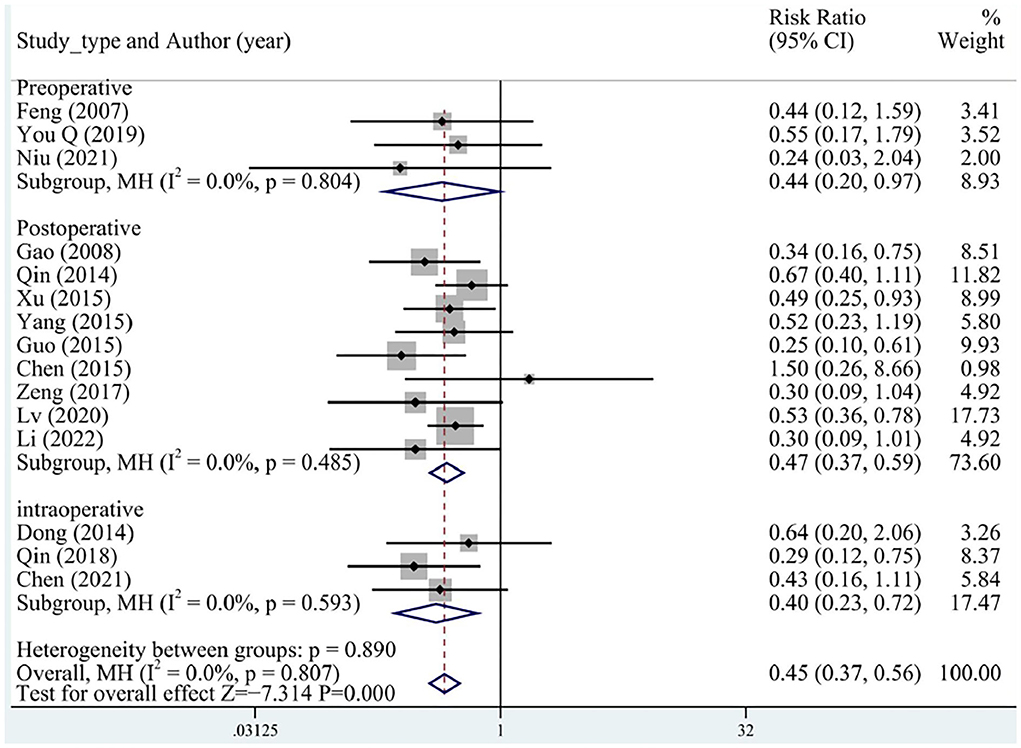
A total of 15 (three preoperative, three intraoperative, and nine postoperative) studies reported MACEs, including malign arrhythmias, angina pectoris, recurrent myocardial infarction, recurrent hemodialysis, heart failure, and cardiac death that occurred during follow-up. No significant heterogeneity was determined between the studies. Overall, the incidence of MACEs was significantly lower in the test group than in the control group (RR = 0.45, 95% CI [0.37, 0.56], P < 0.05, I2 = 0%), and no differences between preoperative, intraoperative, and postoperative groups were identified (Figure 3).
Reperfusion rate
The reperfusion rate was assessed using the postprocedural TIMI flow grade and STR. A TIMI ≥ grade 3 and an STR rate ≥ 50% indicated successful reperfusion.
A total of three studies reported TIMI flow grades, and all studies applied DHI intraoperatively. Low heterogeneity was detected between the studies. The TIMI flow grade was significantly better in the test group than in the control group (RR = 0.22, 95% CI [0.10, 0.50], P < 0.05, I2 = 0%), suggesting that DHI could improve the TIMI flow grade of patients (Figure 4A).
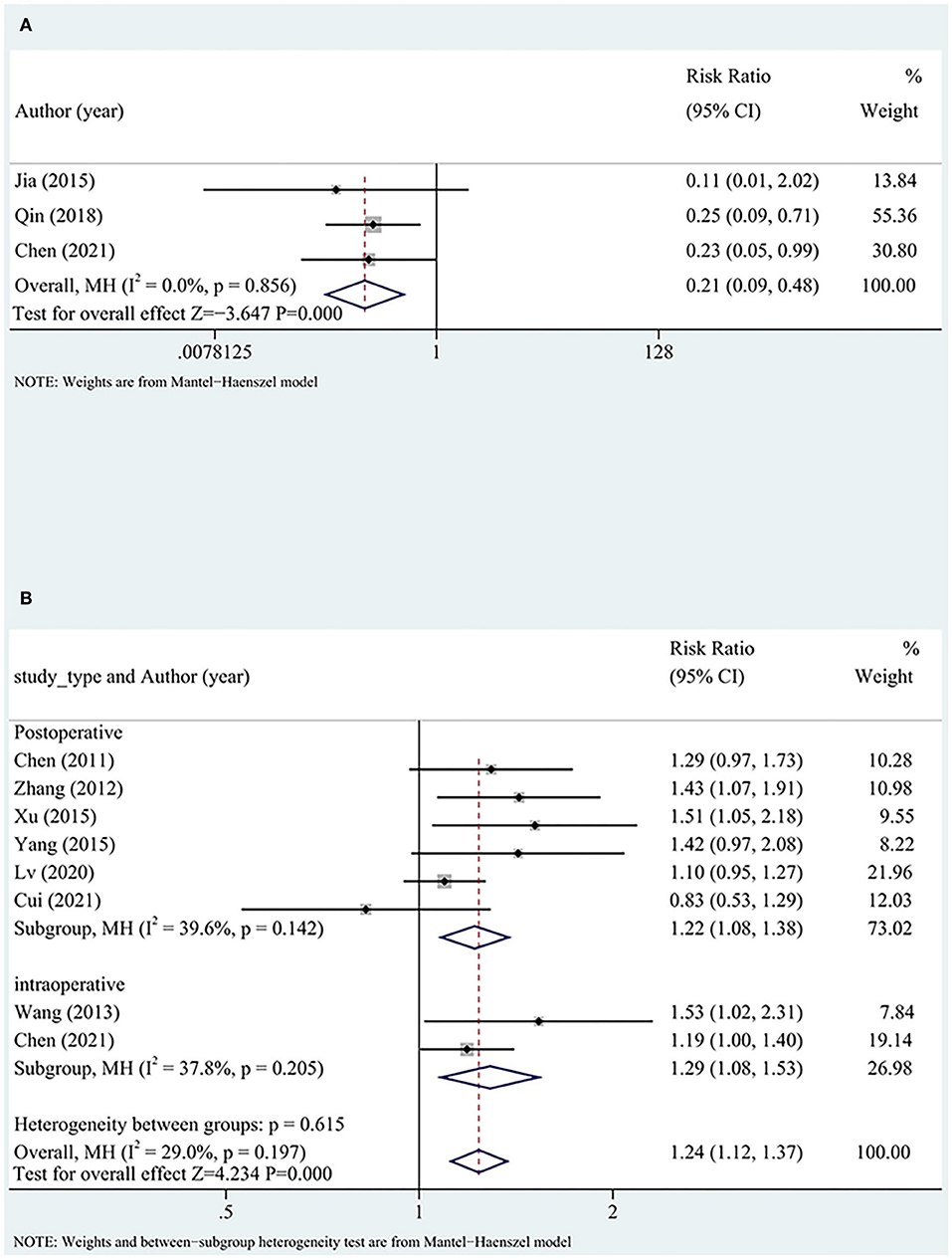
Figure 4. Reperfusion subgroup analysis including (A) TIMI flow grade and (B) STR. TIMI, thrombolysis in myocardial infarction; STR, ST segment resolution.
Overall nine (three intraoperative and six postoperative) studies with high heterogeneity reported STR. STR was significantly better in the test group than in the control group (RR = 1.33, 95% CI [1.13, 1.58], P < 0.05, I2 = 66%). After excluding one study (32), the heterogeneity significantly reduced, although the findings did not significantly change (RR = 1.24, 95% CI [1.12, 1.37], P < 0.05, I2 = 29%).
The comparison of intraoperative and postoperative indicators between the two groups showed no differences. The reperfusion rate was more favorable in the test group than in the control group. The timing of DHI (intraoperatively or postoperatively) did not significantly affect the reperfusion rate (Figure 4B).
Inflammatory factors
A total of 18 studies reported serum hs-CRP levels. Random-effects models were applied owing to the high heterogenicity. The serum hs-CRP levels more significantly decreased in the test group than in the control group (SMD = −1.14, 95% CI [−1.58, −0.7], P < 0.05, I2 = 94.2%). The hs-CRP level more significantly decreased when DHI was administered postoperatively than pre- or intraoperatively (Figure 5A). A meta-regression was conducted to identify the possible sources of the high heterogeneity, and the hs-CRP test method was identified as a source of heterogeneity (P < 0.05; Figure 5B). According to the test method, a subgroup analysis was undertaken based on the explicit test method. We excluded one study (22) to reduce the heterogeneity of the explicit test method groups. Subsequently, there was no heterogeneity in the explicit test method groups. The use of the unspecified assay method to measure the hs-CRP level was identified as a specific potential source of heterogeneity (Figure 5C).

Figure 5. (A) hs-CRP subgroup analysis. (B) Results of meta-regression. (C) Subgroup analysis of test methods. Hs-CRP; high sensitivity C-reactive protein.
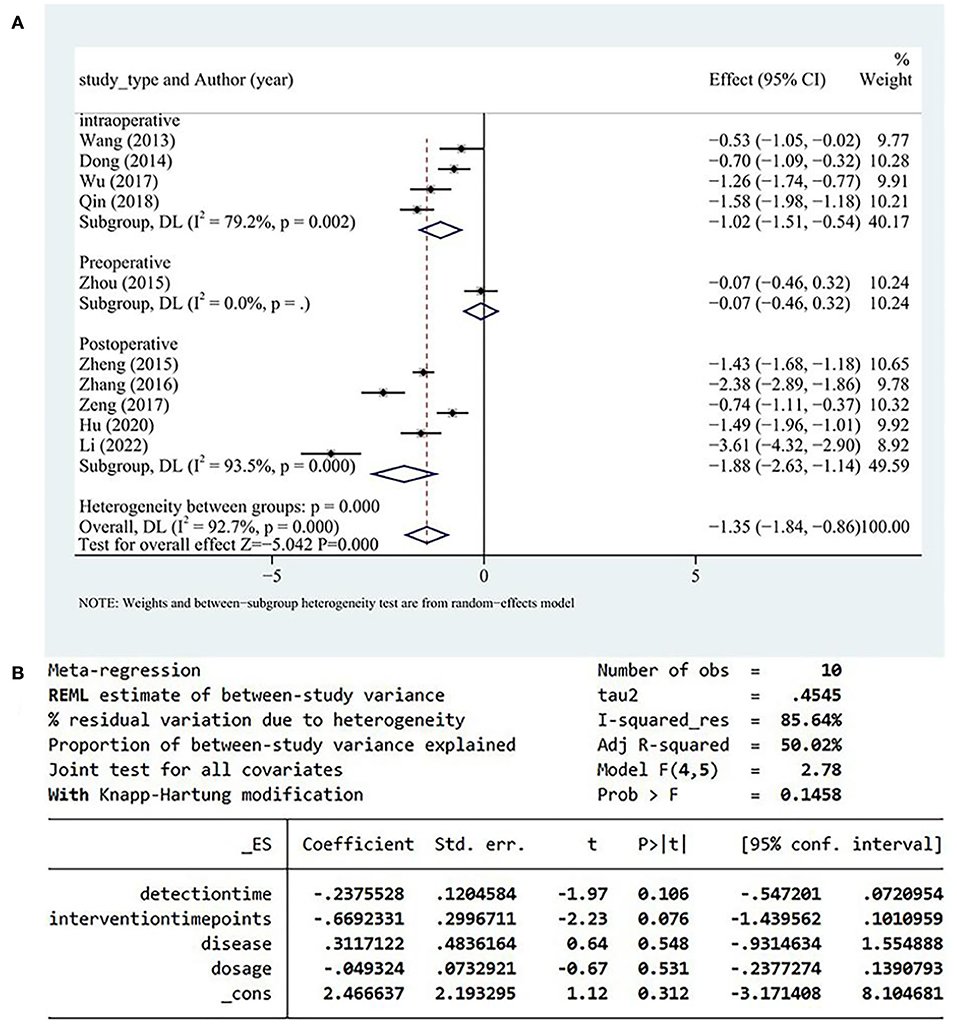
A total of 10 studies reported serum IL-6 levels, and high heterogeneity was detected among these studies. Serum IL-6 levels more significantly decreased in the test group than in the control group (SMD = −1.35, 95% CI [−1.84, −086], P < 0.05, I2 = 92.7%; Figure 6A). A meta-regression analysis determined that the heterogeneity was independent of disease type, detection time, and drug dose and was correlated with the timing of DHI (Figure 6B).
Heterogeneity within the data of the intraoperative group originated from one study (34). After excluding that study (34), heterogeneity of the intraoperative group decreased from I2 = 79.2% to I2 = 54%. But the source of heterogeneity in the postoperative group was not identified after the separate exclusion of each study; therefore, the postoperative group was identified as the main source of heterogeneity.
Myocardial injury index
In all, seven studies reported serum cTnT levels, five of which included patients with acute myocardial infarction in whom DHI was administered postoperatively, and the remaining two studies involved patients with unstable angina for whom DHI was administered preoperatively or intraoperatively. Random-effects models were applied because of high heterogeneity. The results indicated that the peak cTnT level more significantly decreased in the test group than in the control group (SMD = −1.59, 95% CI [−2.48, −0.69], P < 0.05, I2 = 96%). We removed one study (9) after sensitivity analysis with no significant heterogeneity within the subgroups. The results did not change significantly (SMD = −1.56, 95% CI [−2.60, −0.52], P<0.05, I2 = 96.5%; Figure 7A). Among the three subgroups, the intraoperative group had a significantly higher cTnT peak level.
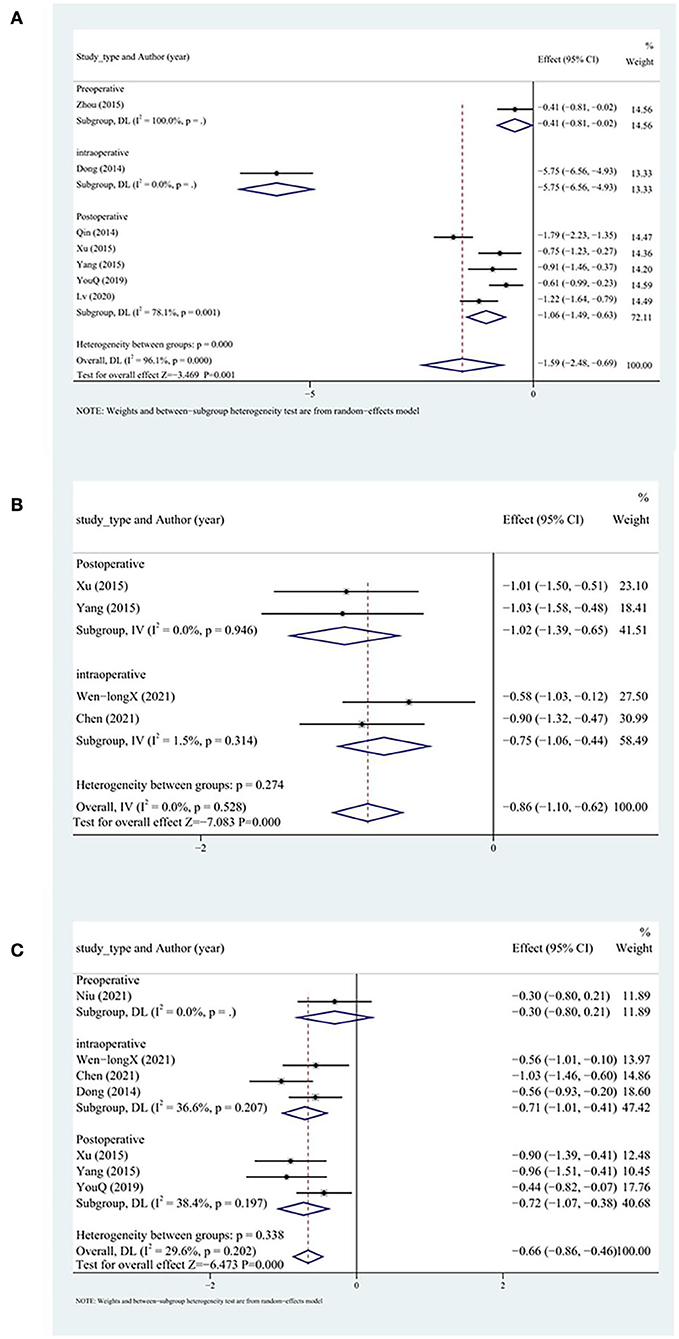
Figure 7. Myocardial injury index subgroup analysis including (A) cTnT, (B) CK, and (C) CK-MB. cTnT, cardiac troponin T; CK, creatine kinase.
A total of four studies reported CK levels. Fixed-effects models were applied because of low heterogeneity. The peak CK level more significantly decreased in the test group than in the control group (SMD = −0.86, 95% CI [−1.10, −0.62], P < 0.05, I2 = 0%; Figure 7B).
A total of nine studies reported CK-MB levels. Random-effects models were applied because of high heterogeneity. The peak CK-MB level more significantly decreased in the test group than in the control group (SMD = −1.05, 95% CI [−1.55, −0.55], P < 0.05, I2 = 90.6%). We searched for the source of heterogeneity by conducting a sensitivity analysis. After excluding two studies (9, 37), the heterogeneity decreased significantly. The peak CK-MB level more significantly decreased in the test group than in the control group (SMD = −0.66, 95% CI [−0.86, −0.46], P < 0.05, I2 = 29.6%). No differences between the intraoperative and postoperative groups were observed (Figure 7C).
Cardiac function
LVEF and BNP levels were analyzed to assess cardiac function. A total of 12 studies reported LVEF levels. LVEF levels more significantly increased in the test group than in the control group (SMD = 0.96, 95% CI [0.68, 1.25], P < 0.05, I2 = 85.1%). The source of heterogeneity was determined by conducting a sensitivity analysis, and one study was identified as the main source of heterogeneity (27). After excluding that study (27), each subgroup had significantly lower heterogeneity. LVEF levels more significantly increased in the test group than in the control group (SMD = 0.84, 95% CI [0.73, 0.96], P < 0.05, I2 = 44.5%). The postoperative group had the most significantly increased LVEF levels (P < 0.05; Figure 8A).
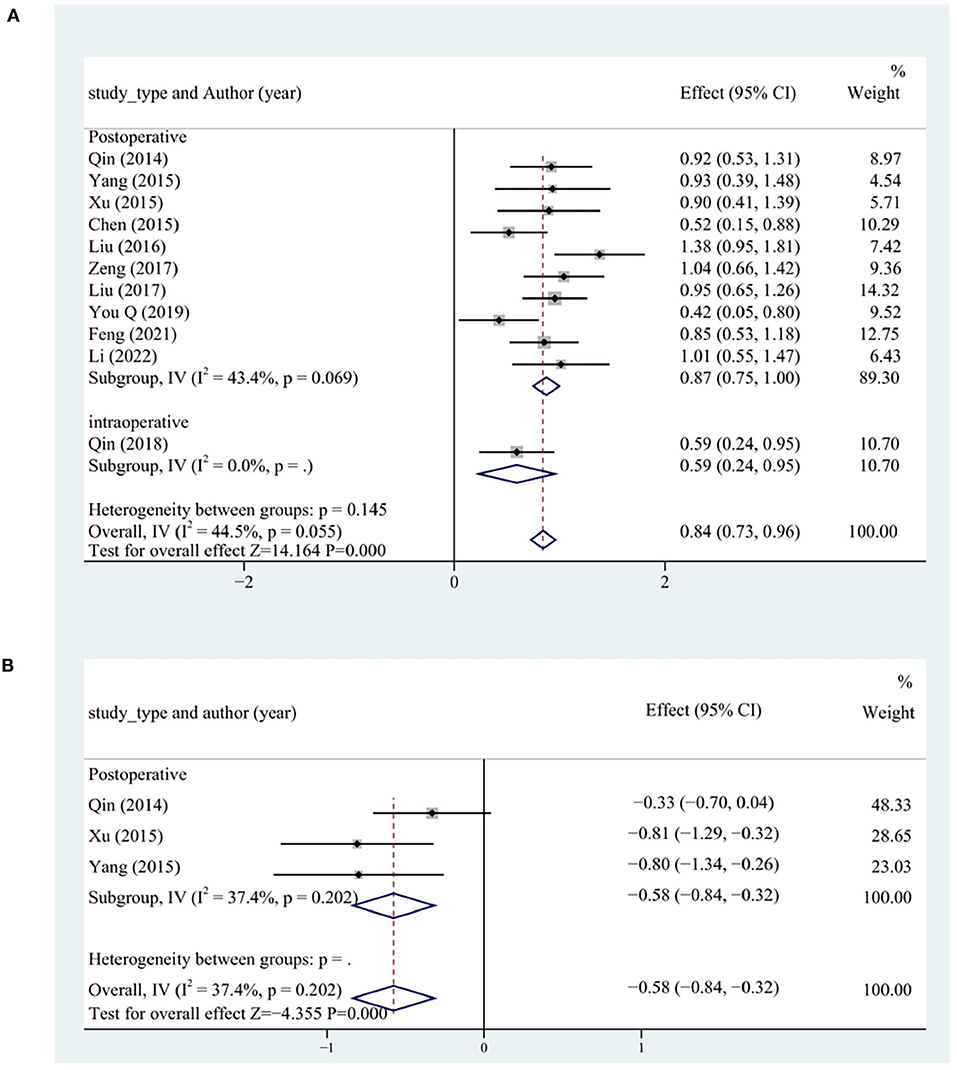
Figure 8. Cardiac function subgroup analysis including (A) LVEF and (B) BNP. LVEF, left ventricular ejection fraction; BNP, B-type natriuretic peptide.
In all, four studies reported BNP levels. The BNP levels more significantly decreased in the test group than in the control group (SMD = −1.96, 95% CI [−3.70, −0.21], P < 0.05, I2 = 97.7%). We searched for the source of heterogeneity by conducting a sensitivity analysis. After the exclusion of the study (29), the heterogeneity was significantly lower in each subgroup. The BNP levels more significantly decreased in the test group than in the control group (SMD = −0.58, 95% CI [−0.84, −0.32], P < 0.05, I2 = 37.4%). The postoperative group had the most significantly decreased BNP levels (Figure 8B).
Publication bias
Since more than 10 studies reported MACEs, hs-CRP, IL-6, and LVEF, we performed Egger's and Begg's tests to identify publication bias for these studies. The results showed that there was no possibility of publication bias (Figure 9).
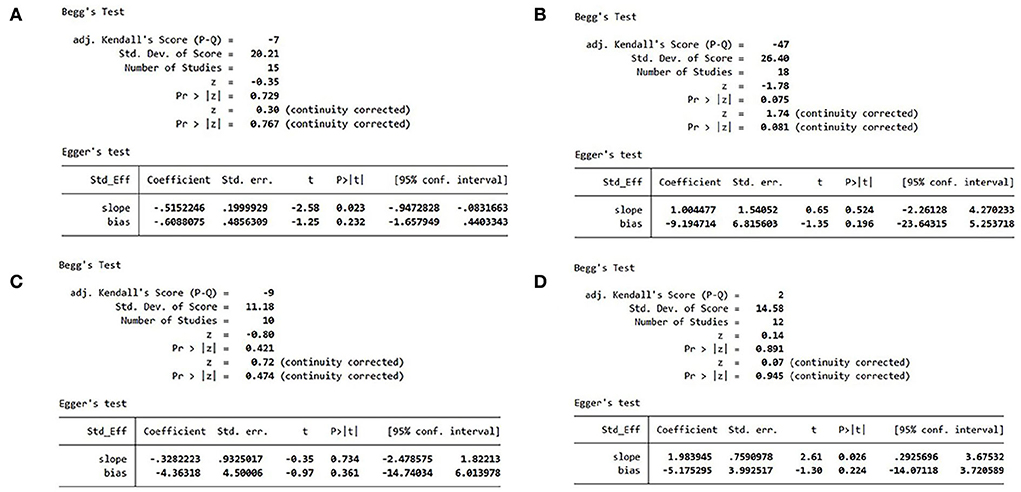
Figure 9. (A) MACE publication bias analysis. (B) hs-CRP publication bias analysis. (C) IL-6 publication bias analysis. (D) LVEF publication bias analysis. MACE, major adverse cardiovascular event; hs-CRP, high-sensitivity C-reactive protein; IL, interleukin; LVEF, left ventricular ejection fraction.
Discussion
Overview of evidence
A total of 33 studies, including four in which DHI was administered preoperatively, seven in which DHI was administered intraoperatively, and 22 in which DHI was administered postoperatively. In our study, 3,458 patients were included in meta-analysis. Data regarding the use of DHI in patients with ACS during the perioperative period of PCI were summarized. The combination of DHI and conventional treatment effectively decreased the number of inflammatory factors, the incidence of no reflow, myocardial injury, and the incidence of MACEs, and increased cardiac function. Postoperative DHI may result in more favorable suppression of the inflammatory response and improvement in cardiac function and patients' quality of life.
Clinical application of DHI to ACS
The annual CHD incidence in China is increasing. ACS is the most serious type of CHD, and PCI is an effective treatment for ACS (3). PCI may be complicated by no reflow, arrhythmias, and MIRI. MIRI impairs cardiac function and negatively affects prognosis in patients undergoing cardiac surgery (44); there is a high risk of MIRI even when patients are administered conventional drug therapy, and MIRI contributes to up to 50% of the final infarct size (45). Therefore, the development of a method for attenuating myocardial injury to increase PCI efficacy is necessary.
In China, DHI is recommended for patients with ACS and patients undergoing PCI (46, 47). DHI is widely used in clinical practice and includes phenolic acid, C-glycosyl quinone chalcones, flavonoid glycosides, cyclic enol ether terpene glycosides, organic acids, amino acids, and nucleosides (46).
Several clinical studies had reported that the combination of DHI and conventional therapy may result in more favorable clinical outcomes than standard therapy alone. This meta-analysis also clarifies the clinical efficacy of DHI. Moreover, subgroup analyses are conducted to determine the optimal timing of DHI. Angina pectoris, heart failure, severe arrhythmia, recurrent myocardial infarction, re-bleeding, and cardiogenic death during follow-up are some of the MACEs. MACE is a reflection of the prognosis of patients; therefore, MACE is a primary endpoint in this study.
DHI improves patients' prognosis. When combined with conventional therapy, DHI decreases the incidence of MACEs. MIRI leads to adverse ventricular remodeling, resulting in progressive heart failure and poor outcomes. The results of the current meta-analysis indicate that DHI ameliorates cardiac function. After treatment with DHI, the LVEF level significantly improved (P < 0.05), and the BNP level significantly reduced (P < 0.05).
As a TCM standardized product, DHI has several targets and multiple effects. Hence, the mechanism of action of DHI remains unclear. DHI improves the reperfusion rate (P < 0.05) and decreases the CK, CK-MB, and cTnT levels in patients with ACS during the perioperative period of PCI (P < 0.05), indicating that DHI decreases the incidence of myocardial injury by improving the reperfusion rate. This may be the mechanism of action of DHI to increase cardiac function and improve patients' prognosis.
Myocardial ischemic injury and MIRI are associated with inflammation. Previous clinical studies have examined whether DHI exerts a protective effect by suppressing the inflammatory response. In the current meta-analysis, IL-6 and hs-CRP are used as indicators of pro-inflammatory responses. IL-6 induces inflammatory cell adhesion and injures vascular endothelium, and hs-CRP is a predictor of cardiovascular events. DHI more significantly decreases hs-CRP and IL-6 levels than standard therapy (P < 0.05). The mechanism of action of DHI in inhibiting inflammation may be multi-faceted. It is reported that DHI reduces inflammatory cytokines, such as IL-1, IL-18, MMP-9, and TNF-α, resulting in a broad anti-inflammatory effect (7, 27, 30, 32, 39, 43). Inhibition of inflammation by DHI is an important mechanism for its cardioprotective effect.
Although DHI is recommended for patients with ACS or patients undergoing PCI, the timing of DHI has not optimized yet. Patients included in this meta-analysis were divided into preoperative, intraoperative, and postoperative subgroups. The incidence of MACEs, TIMI flow grade, and STR was not significantly different between the subgroups. However, hs-CRP and IL-6 levels reduced more significantly in patients in whom DHI was administered postoperatively. The peak cTnT level was significantly decreased in the intraoperative group than in the other subgroups. In the postoperative group, the LVEF level was significantly improved (P < 0.05), and the BNP level was significantly reduced.
The results of this study suggest that DHI is effective in patients with ACS. While the incidence of MACEs is not affected by the timing of DHI, postoperative DHI may suppress the inflammatory response and improve cardiac function more significantly. Therefore, postoperative DHI is recommended to optimizing the efficacy of conventional treatment. However, the potential adverse effects of DHI must be considered. The incidence of adverse reactions of DHI is 3.50 per 1,000, and common adverse reactions include pruritus, rash, sweating, dizziness, and headache. Severe adverse effects such as anaphylactic shock are very rare (48). Current clinical studies do not report adequate data regarding the adverse effects of DHI. We suggest all clinical studies in progress to report adverse effects.
Anti-inflammatory effects of DHI
DHI enhances cardiac function by inhibiting the inflammatory response, increasing the reperfusion rate, alleviating myocardial injuries, and ultimately reducing the incidence of MACEs. Inflammatory response plays an important role in MACEs and is closely related to ischemia–reperfusion injury. DHI alleviates myocardial injuries via anti-inflammatory effects exerted by multi-target pathways.
Shortly after myocardial ischemic injury, necrotic cardiomyocytes release alarmins to activate the immune system and trigger neutrophil infiltration in the ischemic necrosis area (49). Neutrophils generate pro-inflammatory responses that trigger the infiltration of monocytes (50). When reperfusion is performed at this time, fibroblasts release granulocyte–macrophage colony-stimulating factor to promote neutrophil and monocyte infiltration in the ischemic necrotic area (51). Activation or degranulation of mast cells results in the release of pro-inflammatory mediators, and the derived angiotensin II (AngII) induces reperfusion arrhythmias by activating the renin–angiotensin system (52, 53). Few hours to days after myocardial ischemic injury, the composition of immune cells changes, and the spleen becomes a major source of monocytes. Monocyte migration to sites of myocardial injury is regulated by IL-1β, AngII, and the binding of chemokine ligand 2 and chemokine receptor 2. The first monocytes to migrate to the site of myocardial injury are pro-inflammatory Ly6Chigh monocytes, which differentiate into activated pro-inflammatory macrophages; express IL-1β, IL-6, TNF-α, and protein hydrolases; and secrete MMPs, which degrade the extracellular matrix (54–57). At a later stage, both Ly-6Clow monocytes and the M2 phenotype are involved in angiogenesis and collagen deposition, forming scar tissue to replace lost cardiomyocytes in areas of ischemic necrosis and promoting the healing response of the ischemic myocardium (Figure 10).
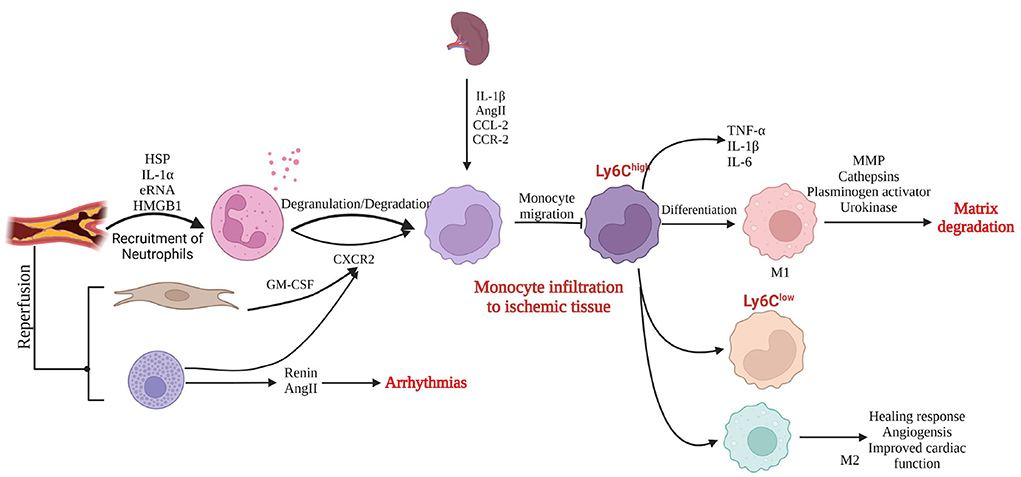
Figure 10. Inflammatory response to myocardial injury (created with BioRender.com). HSP, heat shock protein; IL, interleukin, RNA, ribonucleic acid; HMGB1, high mobility group box 1; GM-CSF, granulocyte–macrophage colony-stimulating factor; AngII, angiotensin II; CXCR2, CXC chemokine receptor 2; CCL-2, C-C chemokine ligand 2; CCR-2, C-C chemokine receptor type 2; TNF, tumor necrosis factor; MMP, matrix metalloproteinase.
The sustained and severe pro-inflammatory response during this process leads to adverse ventricular remodeling. Inflammation is an important novel target to ameliorate the prognosis of patients after PCI. Postoperative DHI better inhibits the inflammatory response and increases cardiac function, which may be related to adverse ventricular remodeling caused by the inhibition of the inflammatory response by DHI.
The results of this study indicate that DHI significantly reduces the hs-CRP and IL-6 levels (P < 0.05). IL-6 induces inflammatory cell adhesion and injures vascular endothelium, and hs-CRP is a predictor of cardiovascular events. In addition, DHI reduces the TNF level in patients with ACS (39, 43), leading to reduced expression of chemoattractant protein-1 in monocytes to inhibit inflammatory responses (58). Indicators of myocardial injury are further reduced after decreased inflammation in patients with ACS, and cardiac function is significantly improved. Cardiac function is closely related to the prognosis of patients, and the improvement of cardiac function reduces the incidence of MACEs.
Strengths and limitations
A previous meta-analysis reported that DHI improved the total efficacy rate, reduced the inflammatory response, and inhibited oxidative stress in patients with ACS (59). Liao et al. reported that DHI reduced mortality and the incidence of MACEs, which were considered to be associated with improved cardiac function and reperfusion, in patients with acute myocardial infarction (60). Zou et al. conducted a meta-analysis of the effects of DHI in patients with ACS undergoing interventional procedures and showed that DHI improved the overall response rate of treatment and reduced the incidence of MACEs (11). However, no previous study evaluated the effects of the timing of DHI.
This study is a state-of-the-art study involving 3,458 patients and evaluates the clinical effects of the combination of DHI and conventional therapy in patients with ACS. Also, this study clarifies the effectiveness of DHI during the perioperatively period of PCI in patients with ACS, proposes a possible mechanism of action, and assesses the timing of DHI.
This study also has several limitations. First, the included studies were all conducted in China. To generalize these results to other populations, multi-national investigations should be conducted in future. Second, some included studies achieved low scores on quality assessment. Thus, future trials should be designed to meet the CONSORT criteria. Third, the number of studies regarding preoperative/intraoperative DHI was low, limiting the strength of the conclusions, which should be interpreted carefully.
Conclusion
The combination of DHI and conventional therapy results showed a better therapeutic effect than conventional therapy alone in patients with ACS undergoing PCI. DHI decreases the incidence of MACEs and improves the reperfusion rate. DHI has multiple effects, including reducing inflammation, reducing myocardial injury, and adjusting cardiac function to play a cardioprotective role. DHI could be a useful supplement to perioperative PCI for patients with ACS. Therefore, postoperative DHI is recommended as a standard treatment for patients with ACS.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
YuL and DL carried out the protocol and drafted the article. DL and WW assisted in data collection, quality control, and project administration. WW and XL assisted with analysis methods. XL, PL, and YZ were involved in data management and analysis. YaL and QL designed and managed this protocol. All authors contributed to the final version of the article.
Funding
This work was supported by the Capital's Funds for Health Improvement and Research (2020-2-4201, 2020-4-4204) and the National Natural Science Foundation of China (81973622).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Collet JP, Thiele H, Barbato E, Barthélémy O, Bauersachs J, Bhatt DL, et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. (2021) 42:1289–367. doi: 10.1093/eurheartj/ehaa909
2. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. (2018) 39:119–77. doi: 10.1093/eurheartj/ehx393
3. TWCotRoCHaDiC. Key points of report on cardiovascular health and diseases in China 2021. Chin J Cardiovasc Res. (2022) 20:577–96. doi: 10.3969/j.issn.1007-5410.2021.03.001
4. Lawton JS, Tamis-Holland JE, Bangalore S, Bates ER, Beckie TM, Bischoff JM, et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: A report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. J Am Coll Cardiol. (2022) 79:e21–129. doi: 10.1016/j.jacc.2021.09.006
5. Feng X, Li Y, Wang Y, Li L, Little PJ, Xu SW, et al. Danhong injection in cardiovascular and cerebrovascular diseases: Pharmacological actions, molecular mechanisms, and therapeutic potential. Pharmacol Res. (2019) 139:62–75. doi: 10.1016/j.phrs.2018.11.006
6. Nijat D, Xu L, Kuang Y, Yu R, Zhang Y, Hasan A, et al. A pharmacokinetic-pharmacodynamic study to elucidate the cardiovascular protective constituents in Danhong Injection. J Pharm Biomed Anal. (2022) 219:114953. doi: 10.1016/j.jpba.2022.114953
7. Guo J, Wu S, Yang JP, Sun GD, Wang YF. Treatment of Dnhong injection with atorvastatin on endothelial function and inflammation factors in patients with acute coronary syndrome after percutaneous coronary intervention. Chin J Exp Tradit Med. (2015) 21:154–7. doi: 10.13422/j.cnki.syfjx.2015120154
8. Chen J, Xu H, Zhou DP, Dong M, Du HL. The curative effect of Danhong injection in patients with acute coronary syndrome undergoing percutaneous coronary intervention. Liaoning J Tradit Chin Med. (2015) 42:341–4. doi: 10.13192/j.issn.1000-1719.2015.02.048
9. Qin L, Zuo H, Wang A, Sun X. Effect of Danhong injection on interventional treatment of ST segment elevation acute myocardial infarction. Shaanxi J Tradit Chin Med. (2014) 35:648–50. doi: 10.3969/j.issn.1000-7369.2014.06.004
10. Dong J. Evaluation of myocardial protective effect of Danhong injection during perioperative period of PCI in patients with unstable angina pectoris using velocity vector imaging (Master's thesis). Chinese PLA School of Medicine, Beijing, China (2014).
11. Zou JB, Zhang XF, Wang J, Wang F, Cheng JX, Yang FY, et al. The therapeutic efficacy of Danhong injection combined with percutaneous coronary intervention in acute coronary syndrome: a systematic review and meta-analysis. Front Pharmacol. (2018) 9:550. doi: 10.3389/fphar.2018.00550
12. Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. (2015) 4:1. doi: 10.1186/2046-4053-4-1
13. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. (2011) 343:d5928. doi: 10.1136/bmj.d5928
14. Lau J, Ioannidis JP, Schmid CH. Quantitative synthesis in systematic reviews. Ann Intern Med. (1997) 127:820–6. doi: 10.7326/0003-4819-127-9-199711010-00008
15. Feng K, Ji XB, Qiu WW, Jiang MY, Shen W, Jiang W. The effect of Danhong injection on cardiovascular event in earlier period and inflammatory re-action of the patients of ACS with PCI. J Chin Microcirc. (2007) 6:390–2. Available online at: https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CJFD&dbname=CJFD2007&filename=ZWXH200706014&uniplatform=NZKPT&v=yinMans226DbfRYiL-mG0Kvxl7mx6aK4lEtk9DAYZHm-pASucCi9z3XscTomg4vk
16. Gao X, Zhou PS, Kang WW. Clinical effect of emergency interventional therapy combined with Danhong injection on acute myocardial infarction. Pract J Card Cereb Pneumal Vasc Dis. (2008) 6:28–9. Available online at: https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CJFD&dbname=CJFD2008&filename=SYXL200806016&uniplatform=NZKPT&v=XRS-W4_NZGNtESe2K6Y2Z-kc2RFZ0jcmpuYQ_sawIN3NlxIqto65U7WLQHrXbaPV
17. Chen Z, Hong L, Wang H, Yin QL, Lai HL, Lu LX. Effect of Danhong injection on platelet activation and inflammatory factors in patients of acute coronary syndrome after intervention therapy. Chin J Integr Tradit Western Med. (2009) 29:692–4. Available online at: https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CJFD&dbname=CJFD2009&filename=ZZXJ200908008&uniplatform=NZKPT&v=rAfuIZj74_vNz6_znvkmlXKx6IXDltMb4wMPcMdmaiyBsxf1Q1XyvGRg9U0slcUW
18. Chen H, Zhao LX, Ma XN, Chu RP, Lv XH, Wang SC. Myocardial protective effect of Danhong injection in patients with acute myocardial infarction after interventional procedure. Clin Focus. (2010) 25:563–6. Available online at: https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CJFD&dbname=CJFD2010&filename=LCFC201007006&uniplatform=NZKPT&v=RRnPP4DpeGhTnIhKEbBG92ByL3_BsWRNtIxyDDGcCVnE4ssjigeN2YcPdK9vDNrl
19. Zhao P, Jiang S. Effect of Danhong injection on ET-1, sP-sel and hsCRP in patients with acute coronary syndrome undergoing percutaneous coronary intervention. Chin J Integr TraditWest Med. (2011) 31:11–4. Available online at: https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CJFD&dbname=CJFD2011&filename=ZZXJ201101005&uniplatform=NZKPT&v=xUmwDKcnfVqQ3vHOv_CdKTrN59PR1xrqbkmsy3rFpOpIRdIu3OOgUkCBLl5MOrzW
20. Zhang Y, Zhang, Q. Effects of Danhong injection on protect myocardium of acute coronary syndrome patients after selective percutaneous coronary artery intervention. Chin J Exp Tradit Med. (2012) 18:308–11. doi: 10.13422/j.cnki.syfjx.2012.23.097
21. Wang B, Zhou C, Guo M, Li P, Zhang X. Effect of Danhong on myocardial no reflow after PCI operation in ST segment elevation acute myocardial infarction and its mechanism. Med J Qilu. (2013) 28:250–2. doi: 10.11712/qlyx201303021
22. Cui Y, Wang L. Effect of Danhong injection on oxidative stress and inflammation reaction in patients with acute myocardial infarction undergoing percutaneous coronary intervention. Chin J Hosp Pharm. (2014) 34:215–8. doi: 10.13286/j.cnki.chinhosppharmacyj.2014.03.14
23. Guo L, An Y, Sun B, Chu XM, Li TD. Influence of Danhong injection combining external counterpulsation on inflammatory factors in patients with acute coronary syndrome after percutaneous coronary intervention. Chin J Evid Based Cardiovasc Med. (2015) 7:631–4. doi: 10.3969/j.issn.1674-4055.2015.05.14
24. Jia M, Liu J, Gai F, Wang Y, Zhou F, Zhang Y. Effect of Danhong injection combined with nitroglycerin on no-reflow and CRPin PCI treatment for AMI PATIENTS. Hebei Tradit Chin Med Pharmacol. (2015) 30:5–7. doi: 10.16370/j.cnki.13-1214/r.2015.01.002
25. Xu X, Cui H, Yang J, Gui Y, Wang J, Ren YS. Protective effect of Danhong injection on the ischemic myocardium after percutaneous coronary intervention for acute myocardial infarction. Mil Med J Southeast China. (2015) 17:451–4. doi: 10.3969/j.issn.1672-271X.2015.05.001
26. Yang J, Cui HM, Ren YS, Wu ZG. Danhong injection in patients with no-reflow/slow-flow following primary PCI of ST-segment elevation myocardial infarction. World J Integr Tradit Western Med. (2015) 10:210–213+225. doi: 10.13935/j.cnki.sjzx.150220
27. Zheng W, Wu X, Zhang A. Influence of Danhong Injection on IL-17 Expression after PCI in STEMI Patients. Cardiovasc Rehabil Med. (2015) 24:84–7. doi: 10.3969/j.issn.1008-0074.2015.01.25
28. Zhou W, Zhou Y, Zhang H. Perioperative effect of Danhong Injection in patients with unstable angina pectoris during percutaneous; coronary intervention. Chin J Evid-Based Cardiol Med. (2015) 7:336–8. doi: 10.3969/j.1674-4055.2015.03.15
29. Liu Y, Xu JP, Di WY, Li J, Xu ZW, Zhao XZ. Effect of Danhong injection on endothelial injury, degree of inflammation and cardiac function of patients with acute coronary syndrome after intervention therapy. J Hainan Med Univ. (2016) 22:1619–22. doi: 10.13210/j.cnki.jhmu.20160427.002
30. Zhang W, Dong HW, Zhang WQ. Influence of Danhong Injection on endothelial function and inflammatory factor in treatment of elderly patients with acute coronary syndrome after PCI intervention. Chin J Gerontol. (2016) 36:5591–3. doi: 10.3969/j.issn.1005-9202.2016.22.042
31. Liu Z, Li YB, Zhao LP, Zhang FC. Efficacy of emergency PCI combined with Danhong injection in the treatment of non ST segment elevation acute myocardial infarction. Shaanxi J Traditional Chin Med. (2017) 38:869–70. doi: 10.3969/j.issn.1000-7369.2017.07.028
32. Wu J, Zheng QS, Yan CY, Wang XY, Hu JL. Effect of danhong injection on preventing no reflow after pci in ami patients and its mechanism. Med J Natl Defending Forces Southwest China. (2017) 27:986–8. doi: 10.3969/j.issn.1004-0188.2017.09.029
33. Zeng G, Wang CY, Xue YG, Qin CS, Kang XJ. Effect of Danhong injection on the serum IL-6 and IL-7 level in patients with acute myocardial infarction after percutaneous coronary intervention. Int J Pathol Clin Med. (2017) 37:1887–93. doi: 10.3978/j.issn.2095-6959.2017.09.020
34. Qin L, Zhao Y, Zhang B. Effects of Danhong injection combined with rosuvastatin on myocardial no-reflow and IL-6,Cys-C and Hcy in patients with acute myocardial infarction after PCI. Chin J Integr Med Cardio/Cerebrovasc Dis. (2018) 16:2453–6. doi: 10.12102/j.issn.1672-1349.2018.17.004
35. You Q, Wang J, Dong W, Tian F, Liu HX, Jing J, et al. Protective effect of Danhong injection in patients with acute myocardial infarction at a high risk of no-reflow during primary percutaneous coronary intervention. J Geriatr Cardiol. (2019) 16:406–3. doi: 10.11909/j.issn.1671-5411.2019.05.001
36. Hu Z. Effects of Danhong injection on coagulation and fibrinolysis function, platelet activation, inflammatory factors and oxidative stress after emergency PCI. J Clin Res. (2020) 37:350–3. doi: 10.3969/j.issn.1671-7171.2020.03.009
37. Lv T. Protective effect of Danhong injection on myocardial reperfusion injury in acute myocardial infarction. Liaoning J Traditional Chin Med. (2020) 47:118–20. doi: 10.13192/j.issn.1000-1719.2020.08.035
38. Chen Q, Lin ZS, Ma TY, Liu Z. Efficacy of loading ticagrelor combined with Danhong injection in preventing and treating reperfusion arrhythmia after PCI in patients with acute anterior wall STEMI and its influence on myocardial injury indexes. Mod J Integr Tradit Chin West Med. (2021) 30:2555–60. doi: 10.3969/j.issn.1008-8849.2021.23.010
39. Cui X, Yan W, He JH. Clinical effect of tigrillo combined with Danhong injection on postoperative PCI of acute myo-cardial infarction. J Guangxi Med Univ. (2021) 38:1609–14. doi: 10.16190/j.cnki.45-1211/r.2021.08.026
40. Feng H. Clinical effect exploration of Danhong injection combined with routine treatment on patients with ST segment elevation acute myocardial infarction with percutaneous coronary intervention (PCI). World Latest Med Inf . (2021) 66:72–4. doi: 10.3969/j.issn.1671-3141.2021.66.032
41. Niu Q, Xing WL, Liu HX, Wang YT, Zhu Y. Effects of Danhong injection on perioperative metabolomics in patients with unstable angina pectoris. World J Tradit Chin Med. (2021) 16:1900–8. doi: 10.3969/j.issn.1673-7202.2021.12.023
42. Xing WL, Wu YJ, Liu HX, Liu QR, Zhou Q, Li AY, et al. Effects of Danhong injection (丹红注射液) on peri-procedural myocardial injury and microcirculatory resistance in patients with unstable angina undergoing elective percutaneous coronary intervention: a pilot randomized study. Chin J Integr Med. (2021) 27:846–53. doi: 10.1007/s11655-021-2872-1
43. Li C, Cha L, Luo CM, He XC. Danhong injection in acute myocardial infarction after PCI with heart blood stasis syndrome. Guangming J Chin Med. (2022) 37:803–6. doi: 10.3969/j.issn.1003-8914.2022.05.025
44. Majidi M, Kosinski AS, Al-Khatib SM, Lemmert ME, Smolders L, van Weert A, et al. Reperfusion ventricular arrhythmia ‘bursts' predict larger infarct size despite TIMI 3 flow restoration with primary angioplasty for anterior ST-elevation myocardial infarction. Eur Heart J. (2009) 30:757–64. doi: 10.1093/eurheartj/ehp005
45. Hausenloy DJ, Botker HE, Engstrom T, Erlinge D, Heusch G, Ibanez B, et al. Targeting reperfusion injury in patients with ST-segment elevation myocardial infarction: trials and tribulations. Eur Heart J. (2017) 38:935–41. doi: 10.1093/eurheartj/ehw145
46. Fu C, Wnag Y, Li C. Danhong injection clinical application Chinese expert consensus. Chin J Integr Tradit West Med. (2018) 38:389–97. doi: 10.7661/j.cjim.20180312.064
47. Liu H, Wang X, Zhang M, Zhang J, Zhu M, Lin Q. Consensus of traditional Chinese medicine specialists on percutaneous coronary intervention (PCI) perioperative myocardial injury. Chin J Integr Tradit West Med. (2017) 37:389–93. doi: 10.7661/j.cjim.20170219.029
48. Li XL, Tang JF, Li WX, Li CX, Zhao T, Zhao BC, et al. Postmarketing safety surveillance and reevaluation of Danhong injection: clinical study of 30888 cases. Evid Based Complement Alternat Med. (2015) 2015:610846. doi: 10.1155/2015/610846
49. Huang S, Frangogiannis NG. Anti-inflammatory therapies in myocardial infarction: failures, hopes and challenges. Br J Pharmacol. (2018) 175:1377–400. doi: 10.1111/bph.14155
50. Soehnlein O, Zernecke A, Eriksson EE, Rothfuchs AG, Pham CT, Herwald H, et al. Neutrophil secretion products pave the way for inflammatory monocytes. Blood. (2008) 112:1461–71. doi: 10.1182/blood-2008-02-139634
51. Anzai A, Choi JL, He S, Fenn AM, Nairz M, Rattik S, et al. The infarcted myocardium solicits GM-CSF for the detrimental oversupply of inflammatory leukocytes. J Exp Med. (2017) 214:3293–310. doi: 10.1084/jem.20170689
52. Mackins CJ, Kano S, Seyedi N, Schäfer U, Reid AC, Machida T, et al. Cardiac mast cell-derived renin promotes local angiotensin formation, norepinephrine release, and arrhythmias in ischemia/reperfusion. J Clin Invest. (2006) 116:1063–70. doi: 10.1172/JCI25713
53. Morrey C, Brazin J, Seyedi N, Corti F, Silver RB, Levi R. Interaction between sensory C-fibers and cardiac mast cells in ischemia/reperfusion: activation of a local renin-angiotensin system culminating in severe arrhythmic dysfunction. J Pharmacol Exp Ther. (2010) 335:76–84. doi: 10.1124/jpet.110.172262
54. Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo JL, et al. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med. (2007) 204:3037–47. doi: 10.1084/jem.20070885
55. Nahrendorf M, Swirski FK. Monocyte and macrophage heterogeneity in the heart. Circ Res. (2013) 112:1624–33. doi: 10.1161/CIRCRESAHA.113.300890
56. Leuschner F, Panizzi P, Chico-Calero I, Lee WW, Ueno T, Cortez-Retamozo V, et al. Angiotensin-converting enzyme inhibition prevents the release of monocytes from their splenic reservoir in mice with myocardial infarction. Circ Res. (2010) 107:1364–73. doi: 10.1161/CIRCRESAHA.110.227454
57. Zouggari Y, Ait-Oufella H, Bonnin P, Simon T, Sage AP, Guérin C, et al. B lymphocytes trigger monocyte mobilization and impair heart function after acute myocardial infarction. Nat Med. (2013) 19:1273–80. doi: 10.1038/nm.3284
58. Ren ZH, Tong YH, Xu W, Ma J, Chen Y. Tanshinone II A attenuates inflammatory responses of rats with myocardial infarction by reducing MCP-1 expression. Phytomedicine. (2010) 17:212–8. doi: 10.1016/j.phymed.2009.08.010
59. Wu JR, Zhang XM, Zhang B. Danhong injection in the treatment of acute coronary syndrome: a systematic review and meta-analysis. Am J Chin Med. (2015) 43:199–214. doi: 10.1142/S0192415X15500135
Keywords: Danhong injection (DHI), perioperative period, acute coronary syndrome (ACS), prognosis, inflammatory factor expression, percutaneous coronary intervention (PCI)
Citation: Li Y, Li D, Wang W, Li X, Li P, Zhang Y, Lin Q and Li Y (2022) Effect of Danhong injection on prognosis and inflammatory factor expression in patients with acute coronary syndrome during the perioperative period of percutaneous coronary intervention: A systematic review and meta-analysis. Front. Cardiovasc. Med. 9:1029387. doi: 10.3389/fcvm.2022.1029387
Received: 27 August 2022; Accepted: 24 October 2022;
Published: 21 November 2022.
Edited by:
Wuqiang Zhu, Mayo Clinic Arizona, United StatesReviewed by:
Liu Wang, Mayo Clinic Arizona, United StatesShiyue Xu, The First Affiliated Hospital of Sun Yat-sen University, China
Copyright © 2022 Li, Li, Wang, Li, Li, Zhang, Lin and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dong Li, ZG9uZ2Rvbmc4MTA3QDE2My5jb20=; Yan Li, ZXJ6aXRhYmFAMTYzLmNvbQ==
 Yuxuan Li
Yuxuan Li Dong Li2*
Dong Li2* Wujiao Wang
Wujiao Wang Xingxing Li
Xingxing Li Yan Li
Yan Li