
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 11 January 2023
Sec. Hypertension
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.1026606
This article is part of the Research Topic Blood Pressure in Children and Adolescents: Moving forward View all 10 articles
 Norrarath Nimkarn
Norrarath Nimkarn Anyamanee Sewarit
Anyamanee Sewarit Kwanchai Pirojsakul*
Kwanchai Pirojsakul* Witchuri Paksi
Witchuri Paksi Songkiat Chantarogh
Songkiat Chantarogh Pawaree Saisawat
Pawaree Saisawat Kanchana Tangnararatchakit
Kanchana TangnararatchakitBackground: Waist-to-height-ratio (WHtR) has been proposed as another indicator for cardiometabolic risk factors including hypertension. Normally, hypertension can be diagnosed in the office setting by detecting high blood pressure for three occasions. However, patients with high office blood pressure may not exhibit high blood pressure outside the office. Ambulatory blood pressure monitoring (ABPM) is a procedure to measure blood pressure over 24-h. Sustained hypertension is characterized as hypertension detected by both office measurement and ABPM. This study aimed to evaluate the performance of WHtR in the diagnosis of sustained hypertension in patients with high office blood pressure.
Materials and methods: Demographic data, height, body weight, body mass index (BMI), and waist circumference were retrospectively reviewed in children and adolescents who underwent ABPM due to persistently high office blood pressure. Patients were separated into two groups: a sustained hypertension group and a normal ABPM group. BMI was adjusted to z-score using the WHO Anthroplus software. WHtR was calculated by the formula: waist circumference (cm)/height (m). The performances of different parameters were analyzed using the receiver operating characteristic (ROC) curve and multivariate logistic regression.
Results: Sixty patients (63% male) with a mean age of 12.9 ± 3.7 years had persistently high office blood pressure. Twenty-nine (48.3%) had high ambulatory blood pressure parameters so-called “sustained hypertension.” The sustained hypertension group had a higher mean BMI z-score (2.32 vs. 1.31, p = 0.01) and a higher mean WHtR (57.7 vs. 49.2 cm/m, p < 0.001) than those of the normal ABPM group. For the diagnosis of sustained hypertension, the ROC analysis revealed that WHtR had a greater area under the ROC curve (AUC) than that of BMI z-score (0.772 vs. 0.723). WHtR remained associated with sustained hypertension (OR 1.2, 95% CI 1.022–1.408, p = 0.026) after adjusting for age, gender, and BMI z-score.
Conclusions: Apart from being a more user-friendly metric, WHtR tended to outperform BMI z-score in predicting sustained hypertension in children and adolescents with persistently high office blood pressure.
The increased prevalence of hypertension in children and adolescents has become a major public health issue (1, 2). Several studies have found that high blood pressure in childhood increased the likelihood of adult hypertension, which is a major contributor to cardiovascular disease later in life (3, 4). A report from the Thai National Health Examination Survey (5) showed that 9.4% of adolescents aged 10–19 years had high blood pressure and this prevalence was higher than those of the recent national surveys from South Korea and Canada (6, 7). Obesity was found to be an important determinant to high blood pressure seen in Thai school-aged children. It was revealed that obesity was significantly associated with high blood pressure and it increased the risk of pre-hypertension and hypertension by 9 and 10.6-fold, respectively (8, 9).
Systematic reviews showed an association between hypertension and body mass index (BMI) together with various measures of abdominal adiposity and the hypertension rates increased in a graded manner as adiposity increased (10–14). Generally, adiposity indicator such as BMI is correlated with hypertension (15–18). Meanwhile, abdominal obesity has also been recognized as a risk factor for hypertension in children and adolescents by using different measuring methods and various indices such as waist circumference (WC) and waist-to-height-ratio (WHtR) (18–24). WHtR can be calculated by dividing WC by height to represent an individual's size. WHtR varies only slightly across age and gender, therefore it does not need to be expressed as a z-score as does BMI (25). Some studies in children even suggested that WHtR was more strongly linked to high blood pressure than was BMI (26–28), while the others indicated that WHtR had a weaker relation to blood pressure compared to that of BMI (29, 30). As a result, the performances of these parameters for predicting hypertension in children and adolescents remain unclear.
Normally, hypertension in children and adolescents can be diagnosed in the office setting by detecting blood pressure greater than the 95th percentile for gender, age, and height for three occasions. However, some children and adolescents may have high blood pressure in the office but do not show high blood pressure outside the office. In 2017, the American Academy of Pediatrics clinical practice guidelines recommended that ambulatory blood pressure monitoring (ABPM) be used to confirm hypertension in children and adolescents who have persistently high office blood pressure for three occasions (3). ABPM is a procedure in which blood pressure is measured every 20–30 min over 24-h using a portable device. Those with high blood pressure for both office measurement and ABPM are called having “sustained hypertension.” Previous studies on the relationship between BMI, WHtR, and the diagnosis of hypertension in children and adolescents typically defined hypertension mainly by using office blood pressure measurement with only a few studies employing ABPM in children and adolescents with obesity (31–33). The present study aimed to evaluate the performances of BMI z-score and WHtR in the diagnosis of sustained hypertension detected by ABPM in children and adolescents with high office blood pressure.
Patients aged ≥6 years referred to the pediatric hypertension clinic at Ramathibodi Hospital Mahidol University due to high office blood pressure on three occasions and subsequently underwent 24-h ABPM were enrolled in the present study. Exclusion criteria included patients who did not have sufficient ABPM data, were previously diagnosed with hypertension, and had any underlying diseases or received any medications that may affect blood pressure.
Demographic data, height, body weight, BMI, and waist circumference were collected. Waist circumference was measured while standing straight using a measurement tape with a precision of 1 mm. The tape was placed at the midline between the bottom of the lowest rib and the iliac crest. WHtR was calculated by the following formula: waist circumference (cm)/height (m) and BMI was adjusted to z-score for age and gender using the World Health Organization (WHO) Anthroplus software (34). Obesity was defined as a BMI z-score > 2.
Office blood pressure was measured with an oscillometric device two times in the right arm while seated, using standard blood pressure measurement practice and appropriate cuff size. An average of the two blood pressure values was considered to be a blood pressure value for each occasion. High office blood pressure is defined as a systolic blood pressure (SBP) or a diastolic blood pressure (DBP) ≥95th percentile for gender, age, and height in children aged < 13 years; or ≥ 130/80 mmHg in children aged ≥ 13 years for three occasions according to the current pediatric guidelines (3). For office blood pressure, mean blood pressure was an average of the blood pressure values from three occasions while maximum blood pressure was the highest blood pressure value among three occasions.
ABPM was performed using a TM-2430 (A&D, Japan) device, which has been validated for use in pediatric patients (35). An appropriate cuff for each patient was applied on the non-dominant arm by the trained healthcare provider. The device was set to record blood pressure every 20 min during awake and every 30 min during sleep for a period of 24-h. Patients were instructed to continue their normal daily activities, avoid strenuous activities, and record their activities including the actual sleep and awake periods. In each patient, ABPM data were considered sufficient if there were ≥40 valid blood pressure readings for the entire 24-h period. Hypertension by ABPM is defined as a mean SBP or a mean DBP ≥ 95th percentile for gender and height, and SBP or DBP load ≥25% for either awake or asleep or both periods while prehypertension is defined as a mean SBP or a mean DBP < 95th percentile for gender and height, but SBP or DBP load ≥25% for either awake or asleep or both periods according to the guidelines by the American Heart Association (36). Based on the results of ABPM, patients were separated into two groups: a sustained hypertension group and a normal ABPM group. The normal ABPM group included patients with prehypertension and white coat hypertension.
To compare blood pressure parameters between patients of different ages, genders, and heights, blood pressure parameters were converted to blood pressure indices with the following formula: blood pressure value/cut-off value for high blood pressure for each patient.
Statistical analysis was performed using IBM SPSS® Software, Version 26. The distribution of each parameter was tested with the Kolmogorov-Smirnov test. Descriptive data were presented as number (percentage), mean ± standard deviation (SD), or median (interquartile range, IQR) as appropriate. Demographic data were compared between the sustained hypertension group and the normal ABPM group. For comparative analysis, the chi-square test or Fisher's exact test was used for categorical data; and the Student t-test or Mann–Whitney U-test was used for continuous data, as appropriate. The receiver operating characteristic (ROC) curve was used to analyze the performances of the BMI z-score and WHtR for the diagnosis of sustained hypertension. Univariate logistic regression analysis was used to test the parameters associated with sustained hypertension. The parameters that were significantly associated with sustained hypertension from the univariate analysis, were further added to the multivariate logistic regression model. A p ≤ 0.05 was defined as statistical significance.
A total of 72 patients with persistently high office blood pressure were enrolled in the present study. Twelve patients with congenital anomalies of the kidney and urinary tract, attention deficit hyperactivity disorder, systemic lupus erythematosus, vesicoureteral reflux, obstructive sleep apnea, and coarctation of aorta were excluded as their underlying diseases or medication uses might affect blood pressure. Among sixty patients (38 males) with a mean age of 12.9 years, 29 patients (48.3%) had sustained hypertension. The demographic and clinical data between the two groups are shown in Table 1. A higher mean BMI z-score (2.32 ± 1.51 vs. 1.31 ± 1.49, p = 0.01) and a more proportion of obesity [20 (69%) vs 10 (32%), p = 0.04] were detected in the sustained hypertension group compared with the normal ABPM group. The sustained hypertension group also had a substantially higher mean waist circumference (86.7 ± 17.8 vs. 77.7 ± 16.7, p = 0.048) and a higher mean WHtR (57.7 ± 8.5 vs. 49.2 ± 9.2, p < 0.001) than those of the normal ABPM group.
The ABPM parameters between the sustained hypertension and normal ABPM groups are shown in Table 2 and the ABPM phenotypes between the obesity and non-obesity groups are shown in Table 3. Of 31 patients in the normal ABPM group, 12 patients had white coat hypertension and 19 patients had prehypertension, accounting for 20 and 31.7% of all high office blood pressure patients, respectively. In the sustained hypertension group (N = 29), seven patients had isolated daytime hypertension, 11 patients had isolated nocturnal hypertension and the remaining 11 patients had both daytime and nocturnal hypertension, accounting for 11.7, 18.3, and 18.3% of all high office blood pressure patients, respectively.
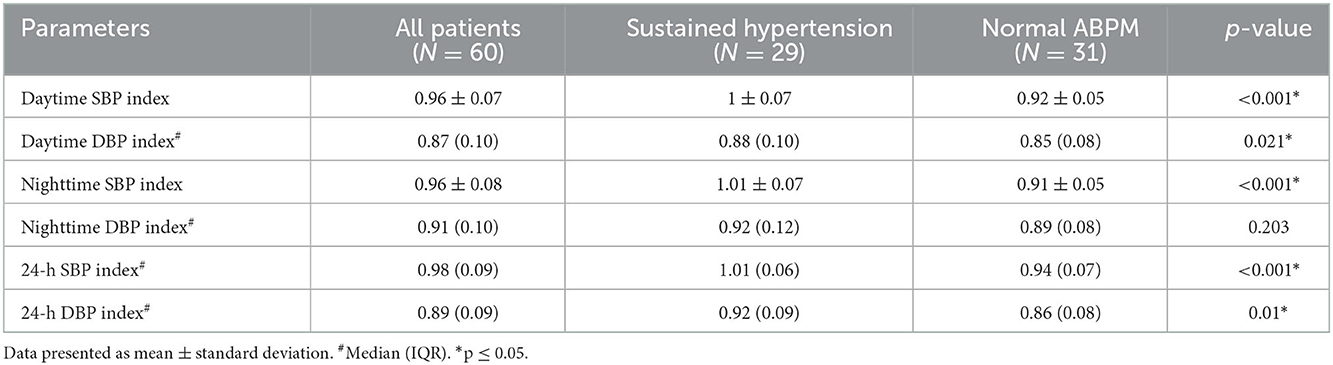
Table 2. Ambulatory blood pressure parameters between the sustained hypertension and normal ABPM groups.
Univariate analysis revealed that BMI z-score and WHtR were significantly associated with sustained hypertension as shown in Table 4. In addition, having obesity was 4.7 times (95% CI 1.571–13.866, p < 0.01) higher risk of sustained hypertension compared with those who did not have obesity while having WHtR ≥ 50 cm/m was 6.7 times (95% CI 2.004–22.041, p < 0.01) higher risk of sustained hypertension compared with those who had a WHtR < 50 cm/m. However, multivariate analysis revealed that WHtR was the only factor associated with sustained hypertension (OR 1.2; 95% CI 1.022–1.408, p = 0.026).
For the diagnosis of sustained hypertension, the ROC analysis revealed that WHtR had a greater area under the curve (AUC) than that of BMI z-score (0.772 vs. 0.723, respectively). WHtR ≥ 50 cm/m had a sensitivity of 82.8% and a specificity of 58.1% whereas BMI z-score > 2 had a sensitivity of 69% and a specificity of 67.7% to detect sustained hypertension (Figure 1, Table 5).
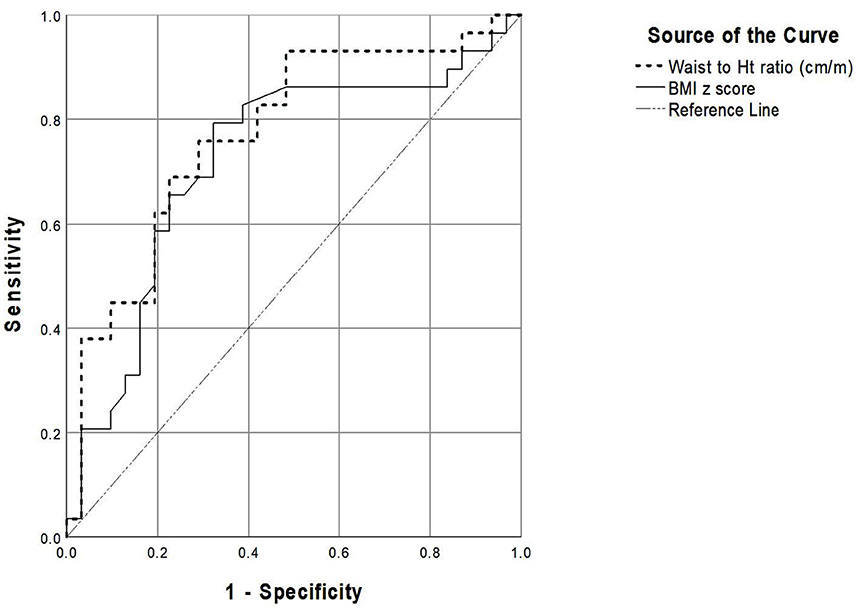
Figure 1. The receiver-operating characteristic curve representing performance of the parameters for detecting sustained hypertension.
Among children and adolescents suspected to have hypertension due to the detection of persistently high office blood pressure, the present study revealed that 48.3% had sustained hypertension. This was consistent with the previous studies reporting the prevalences of sustained hypertension ranging from 20 to 54% (37–39). The present study also showed that WHtR tended to outperform BMI z-score for the prediction of sustained hypertension.
The associations between ABPM parameters and obesity have been well-described. The previous studies reported that white coat hypertension was seen in 10–30% of pediatric patients with obesity (31, 40, 41). These results were consistent with the present study that white coat hypertension was seen in 10% of patients. For nocturnal hypertension, the prevalences ranged from 17 to 23% in pediatric patients with obesity (41, 42) compared to the prevalence of 46.6% in the present study. It was postulated that patients with obesity had high nighttime blood pressure than daytime blood pressure due to poor sleep quality caused by snoring, altered function of the autonomic nervous system, or an impaired ability to excrete sodium (40).
In the present study, not only BMI z-score but also was WHtR significantly higher in the sustained hypertension group than the normal ABPM group. After adjusting with age, gender, and BMI z-score, WHtR was found to be the only independent parameter associated with sustained hypertension. In addition, WHtR at the cut-off point > 0.5 showed a good sensitivity of 82.8% while BMI z-score at the cut-off point of >2 (WHO criteria for obesity) showed a sensitivity of 69% to detect sustained hypertension. Therefore, WHtR tended to be a slightly better parameter for predicting sustained hypertension than BMI z-score.
While WHtR is a parameter representing abdominal obesity, BMI is a parameter representing total body mass. As BMI cannot distinguish between fat and fat-free mass, an elevated BMI may not entirely reflect adiposity accumulation (43). On the other hand, WHtR is related to the amount of intra-abdominal visceral fat, which is more closely related to cardiovascular risk compared with total body mass represented by BMI (21, 44). For implementing across various age groups, BMI varies significantly according to child growth and pubertal development, so it must be stated as a z-score to age and gender. On the contrary, WHtR varies slightly by age and gender and does not need to be stated as a z-score because waist circumference and children's height increase continuously as they age in the same boundary value (25, 44). As a result, WHtR becomes a simpler index to calculate. Not only for sustained hypertension, WHtR also was reported to perform well to predict metabolic syndrome in a national survey of Thai adolescents (45).
Although many studies (10, 21, 26–29) had shown a strong association between WHtR and hypertension, some other studies showed that BMI z-score is slightly superior to or equal to WHtR (14, 18, 30, 46). The discordant results of those studies from the present study could be due to variation in the study designs. Those studies used different definitions of hypertension and most used one- to two-visits of office blood pressure measurements, or used persistently elevated office blood pressure. However, the present study categorized hypertension according to the results of ABPM as recommended by the current pediatric guidelines (3).
The available data regarding the performance of WHtR and the results of ABPM in children and adolescents with persistently high office blood pressure are limited. To our knowledge, this is the largest study that analyzed the performance of WHtR in predicting sustained hypertension by using ABPM. Nonetheless, the present study had some limitations. Firstly, auscultatory blood pressure was not performed for office blood pressure measurement. The other metabolic abnormalities associated with cardiovascular risks such as dyslipidemia and insulin resistance had not been collected. Data regarding the end-organ damages such as left ventricular mass index which is well-known and associated with sustained hypertension were unavailable. Lastly, the number of patients included in the present study was small. A larger study needs to be conducted to explore the performance of WHtR in predicting the risk of sustained hypertension among children with persistently high office blood pressure.
In conclusion, sustained hypertension was detected in 48.3% of the patients with persistently high office blood pressure. Apart from being a more user-friendly metric, WHtR tended to outperform BMI z-score in predicting sustained hypertension confirmed by ABPM.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Ethics Committee for Human Research of Ramathibodi Hospital (MURA 2022/327). Written informed consent from the participants' legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
NN, AS, KP, WP, SC, PS, and KT designed the study. NN, AS, KP, and WP performed the study. NN, AS, and KP drafted and revised the manuscript. All authors approved the final version of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Song P, Zhang Y, Yu J, Zha M, Zhu Y, Rahimi K, et al. Global prevalence of hypertension in children: a systematic review and meta-analysis. JAMA Pediatr. (2019) 173:1154–63. doi: 10.1001/jamapediatrics.2019.3310
2. Lurbe E, Agabiti-Rosei E, Cruickshank JK, Dominiczak A, Erdine S, Hirth A, et al. 2016 European society of hypertension guidelines for the management of high blood pressure in children and adolescents. J Hypertens. (2016) 34:1887–920. doi: 10.1097/HJH.0000000000001039
3. Flynn JT, Kaelber DC, Baker-Smith CM, Blowey D, Carroll AE, Daniels SR, et al. Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics. (2017) 140:e20171904. doi: 10.1542/peds.2017-1904
4. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Executive summary: heart disease and stroke statistics–2016 update: a report from the American heart association. Circulation. (2016) 133:447–54. doi: 10.1161/CIR.0000000000000366
5. Pirojsakul K, Aekplakorn W, Siwarom S, Paksi W, Kessomboon P, Neelapaichit N, et al. Sleep duration and risk of high blood pressure in Thai adolescents: the Thai national health examination survey V, 2014 (NHES-V). BMC Public Health. (2022) 22:1983. doi: 10.1186/s12889-022-14430-z
6. Cho H, Kim JH. Secular trends in hypertension and elevated blood pressure among Korean children and adolescents in the Korea national health and nutrition examination survey 2007–2015. J Clin Hypertens. (2020) 22:590–7. doi: 10.1111/jch.13842
7. Robinson SK, Rodd CJ, Metzger DL, Sharma AK. Prevalence of high blood pressure among Canadian Children: 2017 American academy of pediatrics guidelines with the canadian health measures survey. Paediatr Child Health. (2021) 26:e158–65. doi: 10.1093/pch/pxaa026
8. Sukhonthachit P, Aekplakorn W, Hudthagosol C, Sirikulchayanonta C. The association between obesity and blood pressure in Thai public school children. BMC Public Health. (2014) 14:729. doi: 10.1186/1471-2458-14-729
9. Rerksuppaphol L, Rerksuppaphol S. Prevalence and risk factors of hypertension in school children from central Thailand: a cross-sectional study. Int J Prev Med. (2021) 12:28. doi: 10.4103/ijpvm.IJPVM_110_20
10. Kelishadi R, Mirmoghtadaee P, Najafi H, Keikha M. Systematic review on the association of abdominal obesity in children and adolescents with cardio-metabolic risk factors. J Res Med Sci. (2015) 20:294–307.
11. Friedemann C, Heneghan C, Mahtani K, Thompson M, Perera R, Ward AM. Cardiovascular disease risk in healthy children and its association with body mass index: systematic review and meta-analysis. BMJ. (2012) 345:e4759. doi: 10.1136/bmj.e4759
12. Skinner AC, Perrin EM, Moss LA, Skelton JA. Cardiometabolic risks and severity of obesity in children and young adults. N Engl J Med. (2015) 373:1307–17. doi: 10.1056/NEJMoa1502821
13. Falkner B, Gidding SS, Ramirez-Garnica G, Wiltrout SA, West D, Rappaport EB. The relationship of body mass index and blood pressure in primary care pediatric patients. J Pediatr. (2006) 148:195–200. doi: 10.1016/j.jpeds.2005.10.030
14. Tao JM, Wei W, Ma XY, Huo YX, Hu MD, Li XF, et al. Diagnostic accuracy of anthropometric indices for discriminating elevated blood pressure in pediatric population: a systematic review and a meta-analysis. BMC Pediatr. (2022) 22:19. doi: 10.1186/s12887-021-03062-8
15. Ribeiro RC, Lamounier JA, Oliveira RG, Bensenor IM, Lotufo PA. Measurements of adiposity and high blood pressure among children and adolescents living in belo horizonte. Cardiol Young. (2009) 19:436–40. doi: 10.1017/S1047951109990606
16. Moser DC, Giuliano Ide C, Titski AC, Gaya AR, Coelho-e-Silva MJ, Leite N. Anthropometric measures and blood pressure in school children. J Pediatr. (2013) 89:243–9. doi: 10.1016/j.jped.2012.11.006
17. Gröber-Grätz D, Widhalm K, de Zwaan M, Reinehr T, Blüher S, Schwab KO, et al. Body mass index or waist circumference: which is the better predictor for hypertension and dyslipidemia in overweight/obese children and adolescents? Association of cardiovascular risk related to body mass index or waist circumference. Horm Res Paediatr. (2013) 80:170–8. doi: 10.1159/000354224
18. Fowokan AO, Punthakee Z, Waddell C, Rosin M, Morrison KM, Gupta M, et al. Adiposity measures and their validity in estimating risk of hypertension in South Asian children: a cross-sectional study. BMJ Open. (2019) 9:e024087. doi: 10.1136/bmjopen-2018-024087
19. Maffeis C, Banzato C, Brambilla P, Cerutti F, Corciulo N, Cuccarolo G, et al. Insulin resistance is a risk factor for high blood pressure regardless of body size and fat distribution in obese children. Nutr Metab Cardiovasc Dis. (2010) 20:266–73. doi: 10.1016/j.numecd.2009.04.005
20. Adegboye AR, Andersen LB, Froberg K, Sardinha LB, Heitmann BL. Linking definition of childhood and adolescent obesity to current health outcomes. Int J Pediatr Obes. (2010) 5:130–42. doi: 10.3109/17477160903111730
21. Graves L, Garnett SP, Cowell CT, Baur LA, Ness A, Sattar N, et al. Waist-to-height ratio and cardiometabolic risk factors in adolescence: findings from a prospective birth cohort. Pediatr Obes. (2014) 9:327–38. doi: 10.1111/j.2047-6310.2013.00192.x
22. Kovacs VA, Gabor A, Fajcsak Z, Martos E. Role of waist circumference in predicting the risk of high blood pressure in children. Int J Pediatr Obes. (2010) 5:143–50. doi: 10.3109/17477160903111771
23. Khoury M, Manlhiot C, Dobbin S, Gibson D, Chahal N, Wong H, et al. Role of waist measures in characterizing the lipid and blood pressure assessment of adolescents classified by body mass index. Arch Pediatr Adolesc Med. (2012) 166:719–24. doi: 10.1001/archpediatrics.2012.126
24. Hu YH, Reilly KH, Liang YJ, Xi B, Liu JT, Xu DJ, et al. Increase in body mass index, waist circumference and waist-to-height ratio is associated with high blood pressure in children and adolescents in China. J Int Med Res. (2011) 39:23–32. doi: 10.1177/147323001103900103
25. Ashwell M, Hsieh SD. Six reasons why the waist-to-height ratio is a rapid and effective global indicator for health risks of obesity and how its use could simplify the international public health message on obesity. Int J Food Sci Nutr. (2005) 56:303–7. doi: 10.1080/09637480500195066
26. Madruga JG, Moraes Silva F, Scherer Adami F. Positive association between waist-to-height ratio and hypertension in adolescents. Rev Port Cardiol. (2016) 35:479–84. doi: 10.1016/j.repce.2016.03.006
27. Campagnolo PD, Hoffman DJ, Vitolo MR. Waist-to-height ratio as a screening tool for children with risk factors for cardiovascular disease. Ann Hum Biol. (2011) 38:265–70. doi: 10.3109/03014460.2010.526147
28. Hara M, Saitou E, Iwata F, Okada T, Harada K. Waist-to-height ratio is the best predictor of cardiovascular disease risk factors in Japanese schoolchildren. J Atheroscler Thromb. (2002) 9:127–32. doi: 10.5551/jat.9.127
29. Dong B, Wang Z, Wang HJ, Ma J. Associations between adiposity indicators and elevated blood pressure among Chinese children and adolescents. J Hum Hypertens. (2015) 29:236–40. doi: 10.1038/jhh.2014.95
30. Kim NY, Hong YM, Jung JW, Kim NS, Noh CI, Song YH. The relationships of body mass index, waist-to-height ratio, and body fat percentage with blood pressure and its hemodynamic determinants in Korean adolescents: a school-based study. Korean J Pediatr. (2013) 56:526–33. doi: 10.3345/kjp.2013.56.12.526
31. Hvidt KN, Olsen MH, Ibsen H, Holm JC. Effect of changes in BMI and waist circumference on ambulatory blood pressure in obese children and adolescents. J Hypertens. (2014) 32:1470–7; discussion 7. doi: 10.1097/HJH.0000000000000188
32. Tepe D, Yilmaz S. Is office blood pressure measurement reliable in obese adolescents? Diabetes Metab Syndr Obes. (2021) 14:3809–17. doi: 10.2147/DMSO.S329273
33. Rujirakan P, Siwarom S, Paksi W, Wecharak A, Phoonlapdacha P, Pirojsakul K. Masked hypertension and correlation between body composition and nighttime blood pressure parameters in children and adolescents with obesity. Blood Press Monit. (2021) 26:419–25. doi: 10.1097/MBP.0000000000000555
34. World Health Organization. Growth Reference Data for 5–19 Years. World Health Organization (2007). Available online at: https://www.who.int/tools/growth-reference-data-for-5to19-years/application-tools (accessed July 01, 2022).
35. Yip GW, So HK, Li AM, Tomlinson B, Wong SN, Sung RY. Validation of A&D TM-2430 upper-arm blood pressure monitor for ambulatory blood pressure monitoring in children and adolescents, according to the British hypertension society protocol. Blood Press Monit. (2012) 17:76–9. doi: 10.1097/MBP.0b013e328351d4a4
36. Flynn JT, Daniels SR, Hayman LL, Maahs DM, McCrindle BW, Mitsnefes M, et al. Update: ambulatory blood pressure monitoring in children and adolescents: a scientific statement from the american heart association. Hypertension. (2014) 63:1116–35. doi: 10.1161/HYP.0000000000000007
37. Swartz SJ, Srivaths PR, Croix B, Feig DI. Cost-effectiveness of ambulatory blood pressure monitoring in the initial evaluation of hypertension in children. Pediatrics. (2008) 122:1177–81. doi: 10.1542/peds.2007-3432
38. Kavey RE, Kveselis DA, Atallah N, Smith FC. White coat hypertension in childhood: evidence for end-organ effect. J Pediatr. (2007) 150:491–7. doi: 10.1016/j.jpeds.2007.01.033
39. Stergiou GS, Nasothimiou E, Giovas P, Kapoyiannis A, Vazeou A. Diagnosis of hypertension in children and adolescents based on home versus ambulatory blood pressure monitoring. J Hypertens. (2008) 26:1556–62. doi: 10.1097/HJH.0b013e328301c411
40. Hvidt KN, Olsen MH, Holm JC, Ibsen H. Obese children and adolescents have elevated nighttime blood pressure independent of insulin resistance and arterial stiffness. Am J Hypertens. (2014) 27:1408–15. doi: 10.1093/ajh/hpu055
41. Bhatt GC, Pakhare AP, Gogia P, Jain S, Gupta N, Goel SK, et al. Predictive model for ambulatory hypertension based on office blood pressure in obese children. Front Pediatr. (2020) 8:232. doi: 10.3389/fped.2020.00232
42. Macumber IR, Weiss NS, Halbach SM, Hanevold CD, Flynn JT. The association of pediatric obesity with nocturnal non-dipping on 24-hour ambulatory blood pressure monitoring. Am J Hypertens. (2016) 29:647–52. doi: 10.1093/ajh/hpv147
43. Freedman DS, Wang J, Maynard LM, Thornton JC, Mei Z, Pierson RN, et al. Relation of BMI to fat and fat-free mass among children and adolescents. Int J Obes. (2005) 29:1–8. doi: 10.1038/sj.ijo.0802735
44. Garnett SP, Baur LA, Cowell CT. Waist-to-height ratio: a simple option for determining excess central adiposity in young people. Int J Obes. (2008) 32:1028–30. doi: 10.1038/ijo.2008.51
45. Siwarom S, Pirojsakul K, Aekplakorn W, Paksi W, Kessomboon P, Neelapaichit N, et al. Waist-to-Height ratio is a good predictor of metabolic syndrome in adolescents: a report from the thai national health examination survey V, 2014. Asia Pac J Public Health. (2022) 34:36–43. doi: 10.1177/10105395211046474
Keywords: waist-to-height-ratio, body mass index, ambulatory blood pressure monitoring, sustained hypertension, children, adolescents
Citation: Nimkarn N, Sewarit A, Pirojsakul K, Paksi W, Chantarogh S, Saisawat P and Tangnararatchakit K (2023) Waist-to-height-ratio is associated with sustained hypertension in children and adolescents with high office blood pressure. Front. Cardiovasc. Med. 9:1026606. doi: 10.3389/fcvm.2022.1026606
Received: 24 August 2022; Accepted: 27 December 2022;
Published: 11 January 2023.
Edited by:
Maria Lorenza Muiesan, University of Brescia, ItalyReviewed by:
Priya Pais, St. John's Medical College, IndiaCopyright © 2023 Nimkarn, Sewarit, Pirojsakul, Paksi, Chantarogh, Saisawat and Tangnararatchakit. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kwanchai Pirojsakul,  cHVnZ3l0aEBnbWFpbC5jb20=
cHVnZ3l0aEBnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.