
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 04 November 2022
Sec. Cardiovascular Epidemiology and Prevention
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.1026597
This article is part of the Research Topic Epidemiology and Clinical Researches in Atherosclerosis and Cardiovascular Disease View all 13 articles
 Jeonggeun Moon1
Jeonggeun Moon1 Pyung Chun Oh1
Pyung Chun Oh1 Kyounghoon Lee1
Kyounghoon Lee1 Ho-Jun Jang2
Ho-Jun Jang2 Tae-Hoon Kim3
Tae-Hoon Kim3 Sang-Don Park4
Sang-Don Park4 Sung Woo Kwon4
Sung Woo Kwon4 Min Gyu Kong5
Min Gyu Kong5 Jon Suh5
Jon Suh5 Woong Chol Kang1*
Woong Chol Kang1*Background: Height declines with age, and its degree differs among individuals. Despite epidemiologic evidence for the inverse relationship between adult height and cardiovascular disease (CVD) incidence, the clinical significance of height loss in CVD remains to be elucidated. Therefore, this study investigated the association between height loss and CVD incidence.
Methods: In total, 127,573 Korean participants were enrolled; their heights were monitored from 2002 to 2011. The annual height loss (cm/year) was the difference between the first and last height measurements within the observation period divided by the number of years. The participants were classified as Group 1 (height loss: <0.3 cm/year; n = 102,554), Group 2 (height loss: 0.3– < 0.6 cm/year; n = 17,324), or Group 3 (height loss: ≥0.6 cm/year; n = 7,695).
Results: The cumulative major adverse cardiac and cerebral event (MACCE: cardiac death, non-fatal myocardial infarction, and unplanned hospitalization for heart failure or stroke) incidence rate was 3.6% for Group 1, 4.5% for Group 2, and 5.2% for Group 3. Group 2 (hazard ratio [HR] = 1.27, 95% confidence interval [CI] = 1.17–1.37) and Group 3 (HR = 1.46, 95% CI = 1.32–1.62) had a significantly higher incidence of MACCE than Group 1. In the model adjusted for age, sex, comorbidities, income level, body mass index, smoking, and drinking status, the MACCE risk was higher in Group 2 (HR = 1.11, 95% CI = 1.07–1.20) and Group 3 (HR = 1.25, 95% CI = 1.13–1.39) than in Group 1.
Conclusion: The degree of height loss was independently associated with CVD occurrences in the Korean population.
Growing evidence suggests that short height is associated with cardiovascular disease (CVD) occurrence (1–4), although a rationale for this epidemiological finding remains unclear. Thus, genetically determined height may be a non-modifiable indicator for increased CVD risk. The maximal stature of a given individual is determined in the late teen years (5). However, height declines with aging due to senile changes in the musculoskeletal system (6). The degree of height loss varies among individuals based on the severity of osteoporosis or sarcopenia, which are significant contributors to stature decrease and have a known association with mortality (7–9). Hence, it can be hypothesized that the degree of height loss is correlated with future CVD occurrence. This topic was investigated once in elderly British men two decades ago, and the authors observed that marked height loss (≥3 cm over the preceding two decades) was an independent CVD risk factor (10). However, data from different countries, eras, and ethnicity are needed to confirm this hypothesis because anthropometric parameters, such as height, are influenced by demographic characteristics and the socioeconomic status of the society. The Republic of Korea is suitable for the research for that purpose because it is homogenous as regards ethnicity, and there is a government-driven annual medical check-up/treatment data for all eligible Koreans. This study used a large Korean cohort to investigate the relationship between height loss and CVD prevalence.
The National Health Insurance Service (NHIS) of the Republic of Korea operates a mandatory public insurance program for all citizens and supports public health policy and research activities by developing and maintaining the National Health Information Database (11). This study was performed using NHIS data.
Eligible participants were ethnically Korean, ≥40 years, underwent medical examinations in the Republic of Korea between January 1, 2002, and December 31, 2015; 332,579 individuals were eligible. The exclusion was based on the following: 89,194 individuals had only one height measurement, 57,164 had unreliable height data (such as increasing height, a decrease of ≥20 cm, or an annual decrease rate of ≥10 cm/year), 689 had missing demographic data during the observation period (2002–2011), 27,599 had major adverse cardiac and cerebral events (MACCE) during the observation period, and 30,360 died before 2012, which was the beginning of the outcome monitoring period. As a result, 127,573 individuals were included in the analysis (Figure 1).
Height was measured to the nearest 0.1 cm using a stadiometer with the participants standing upright. The observation period for measuring the decrease in height (measured in cm) was ten years (2002–2011). Each participant was included in the study at the time of the first height measurement recording. Heights were measured annually, and the interval between each height measurement was a year or more. The annual height decrease rate (cm/year) was calculated as the difference between the first and the last height measurement within the observation period divided by the number of years. According to annual height decrease rate, participants were classified as Group 1 (reference; height loss: <0.3 cm/year; n = 102,554), Group 2 (height loss: 0.3– < 0.6 cm/year; n = 17,324), or Group 3 (height loss: ≥0.6 cm/year; n = 7,695; Figure 1).
Using the Korean Standard Classification of Diseases (KCD-7), which is based on the International Statistical Classification of Diseases and Related Health Problems, MACCE included cardiac death (acute myocardial infarction [MI] [I21], heart failure [I50, I130, I132, I110], cardiac arrest [I46], all with death), non-fatal MI, unplanned hospitalization for heart failure (HF), and stroke (10). Stroke included ischemic and hemorrhagic pathologies. The demographic variables of sex, age, income deciles (1st to 10th deciles), diabetes mellitus, dyslipidemia, hypertension, smoking (individuals who answered “I still smoke” to their smoking status were considered smokers), drinking (individuals who drank > two times per week were considered drinkers), and body mass index (BMI, kg/m2) were collected. The BMI was calculated using body weight measured to the nearest 0.1 kg at enrolment. For stratified analysis, those ≥ 60 years were considered elderly (prone to height loss). Women ≥ 50 years were arbitrarily defined as “menopausal” (after which height prominently decreases) based on the previously reported mean menopausal age of Korean women (12).
The primary endpoint was MACCE occurrences between 2012 and 2015. Participants whose last height measurement was at least five years after the first measurement between 2002 and 2011 were included in this study. The incidence of MACCE was collectively followed-up between 2012 and 2015. The start date of MACCE incidences from the last height measurement differed for each participant; therefore, those who developed MACCE before 2012 were excluded from the data set.
Differences in demographic characteristics based on the annual height decrease rate were evaluated using the Chi-square test and the Kruskal–Wallis test. MACCE hazard ratios (HR) and 95% confidence intervals (CI) were calculated based on the annual height decrease rate using a Cox proportional-hazard model adjusted for sex, age, income deciles, diabetes, dyslipidemia, hypertension, smoking, drinking, and BMI. The proportional assumption of the Cox analysis was conducted for Cox proportional-hazard modeling. In univariate analysis, a Log-rank test was conducted to select significant variables. Subsequently, multiple analyses were conducted using Cox’s proportional hazard model. Factors affecting height decreases were analyzed using multivariate linear regression and Kaplan–Meier survival curves and presented to estimate the cumulative MACCE incidence rates with time. Data presents the stratified analyses for the elderly and menopausal populations. All the analyses were performed using the SAS 9.4 software (SAS Institute, Cary, NC, USA) at a statistical significance level of α = 0.05.
The overall number of height measurements was 4.1 ± 2.0 (4.3 ± 2.0 times for Group 1, 3.5 ± 1.6 times for Group 2, and 2.8 ± 1.3 times for Group 3). The overall time gap between the first and the last height measurements was 69 ± 28 months (median: 72, interquartile range [IQR, 47–94]) and 73 ± 27 months (median: 78, IQR [51–96]) for Group 1, 58 ± 27 months (median: 57, IQR [32–77]) for Group 2, and 39 ± 27 months (median: 30, IQR [19–55]) for Group 3. Table 1 presents the demographic and clinical characteristic distributions based on the height decrease rate. There were 57,623 males (45.2%) and 69,950 females (54.8%). Group 1 had the highest proportion of males, and Group 3 had the highest proportion of females with a significant inter-group difference (p < 0.0001). The highest number of participants were in their 50 s, while the lowest were in their 90 s, with a significant inter-group difference (p < 0.0001). The highest number of participants was in the 10th decile of income, and the lowest number was in the 5th decile; this inter-group difference was also significant (p < 0.0001). Overall, 48, 488 participants (38.0%) had hypertension, and Group 2 had the highest proportion of patients with hypertension; the inter-group difference was significant (p < 0.0001). Further, 44,619 participants (35.0%) had dyslipidemia, and Group 1 had the largest number; the inter-group difference was also significant (p < 0.0001). In total, 20,004 participants (15.7%) were smokers, and Group 1 had the highest proportion; the inter-group difference was significant (p = 0.0485). Finally, Group 1 had the highest proportion of drinkers, and the inter-group difference was significant (p < 0.0001).
Table 2 presents the cumulative MACCE incidence based on the ranges of the 4-year height decrease. The cumulative MACCE incidence rates were 3.6% for Group 1, 4.5% for Group 2, and 5.2% for Group 3. The rates were 4.3% in Group 1, 5.0% in Group 2, and 6.1% in Group 3 among men, and 3.0% in Group 1, 4.2% in Group 2, and 4.7% in Group 3 among women.
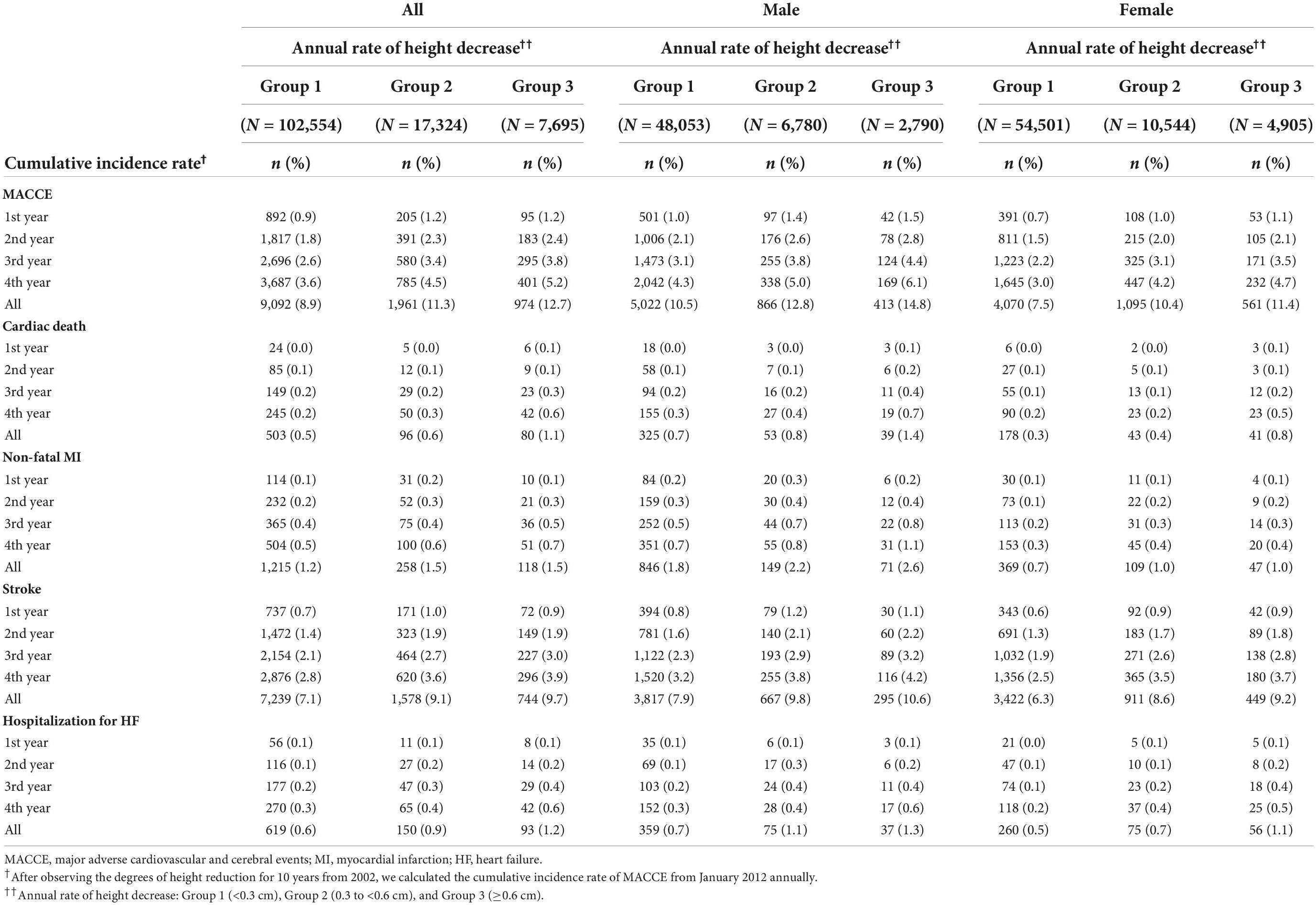
Table 2. The cumulative adverse cardiovascular and cerebral event incidence rates per the annual height decrease rate.
Figure 2 presents the Kaplan–Meier survival curves for the MACCE incidence rates based on the extent of height decrease. The cumulative MACCE incidence rate was highest in Group 3 (HR = 1.46, 95% CI = 1.32–1.62), followed by Group 2 (HR = 1.27, 95% CI = 1.17–1.37) and Group 1 (Reference); the inter-group difference was significant (p < 0.0001). All the MACCE components, including cardiac death (HR = 2.29, 95% CI = 1.65–3.17), non-fatal MI (HR = 1.35, 95% CI = 1.01–1.80), and stroke (HR = 1.38, 95% CI = 1.22–1.55) occurred more frequently in Group 3 than in Group 1.
Table 3 presents the Cox proportional-hazard model results for the annual height decrease rate and MACCE incidences. In the crude model, the MACCE and cardiac death risks were 1.46 (95% CI = 1.32–1.62) and 2.29 (95% CI = 1.65–3.17) times higher in Group 3 than in Group 1, respectively, and the inter-group difference was significant, considering the 95% CI. In the adjusted model, the risks of MACCE (HR = 1.25, 95% CI = 1.13–1.39), cardiac death (HR = 1.67, 95% CI = 1.20–2.33), stroke (HR = 1.18, 95% CI = 1.04–1.33), and hospitalization for HF (HR = 1.56, 95% CI = 1.12–2.17) were higher in Group 3 than in Group 1; the inter-group difference was significant. The subgroup analysis of the elderly population (≥60 years) demonstrated similar results (Tables 4, 5). In females, height loss increased dramatically after menopause. The analysis of premenopausal versus menopausal groups demonstrated that MACCE rarely occurred in premenopausal women (Tables 6, 7), and height loss was significantly associated with MACCE in postmenopausal women (≥50 years).
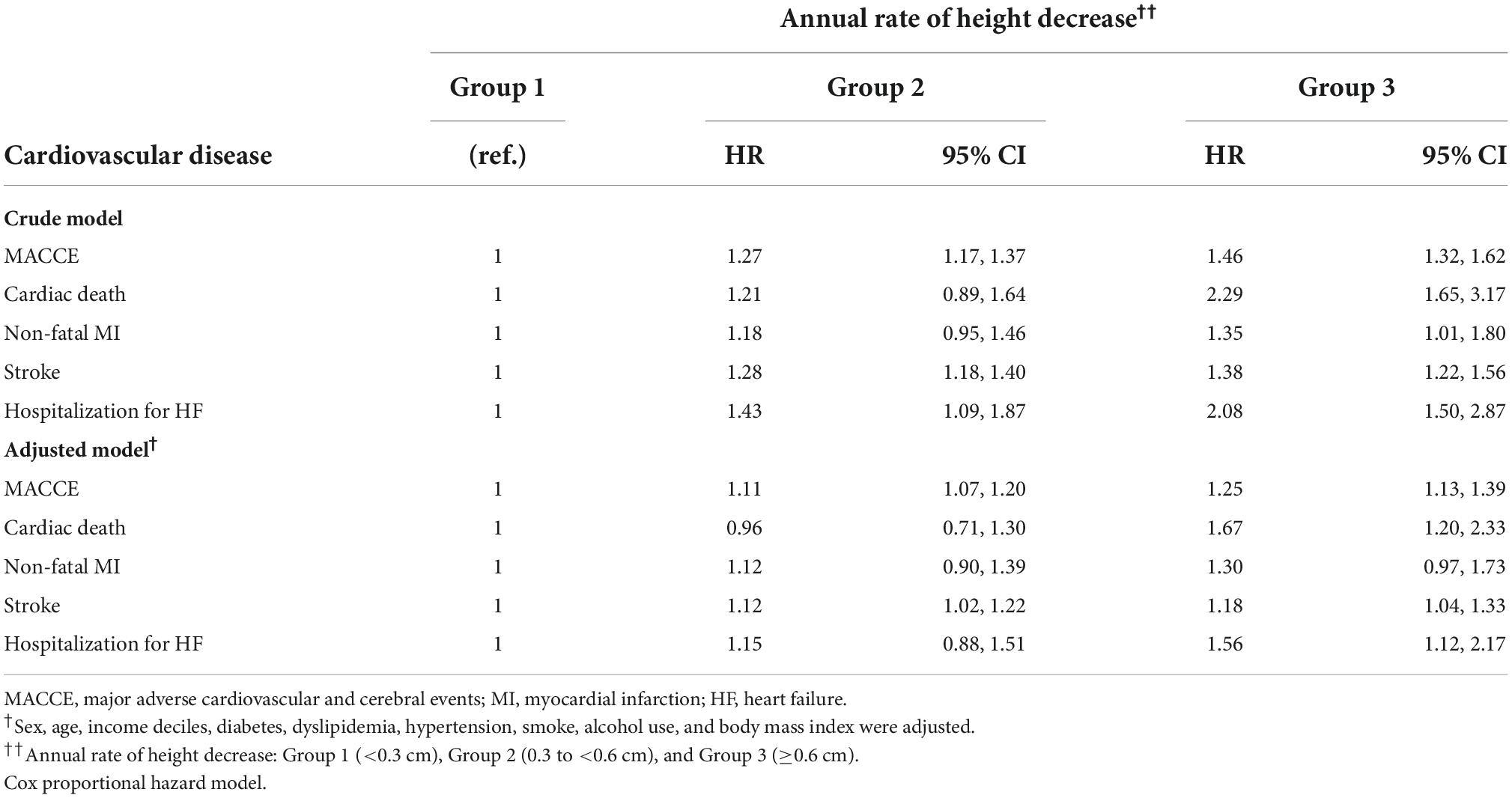
Table 3. Hazard ratios of adverse cardiovascular and cerebral events per the annual height decrease rate.
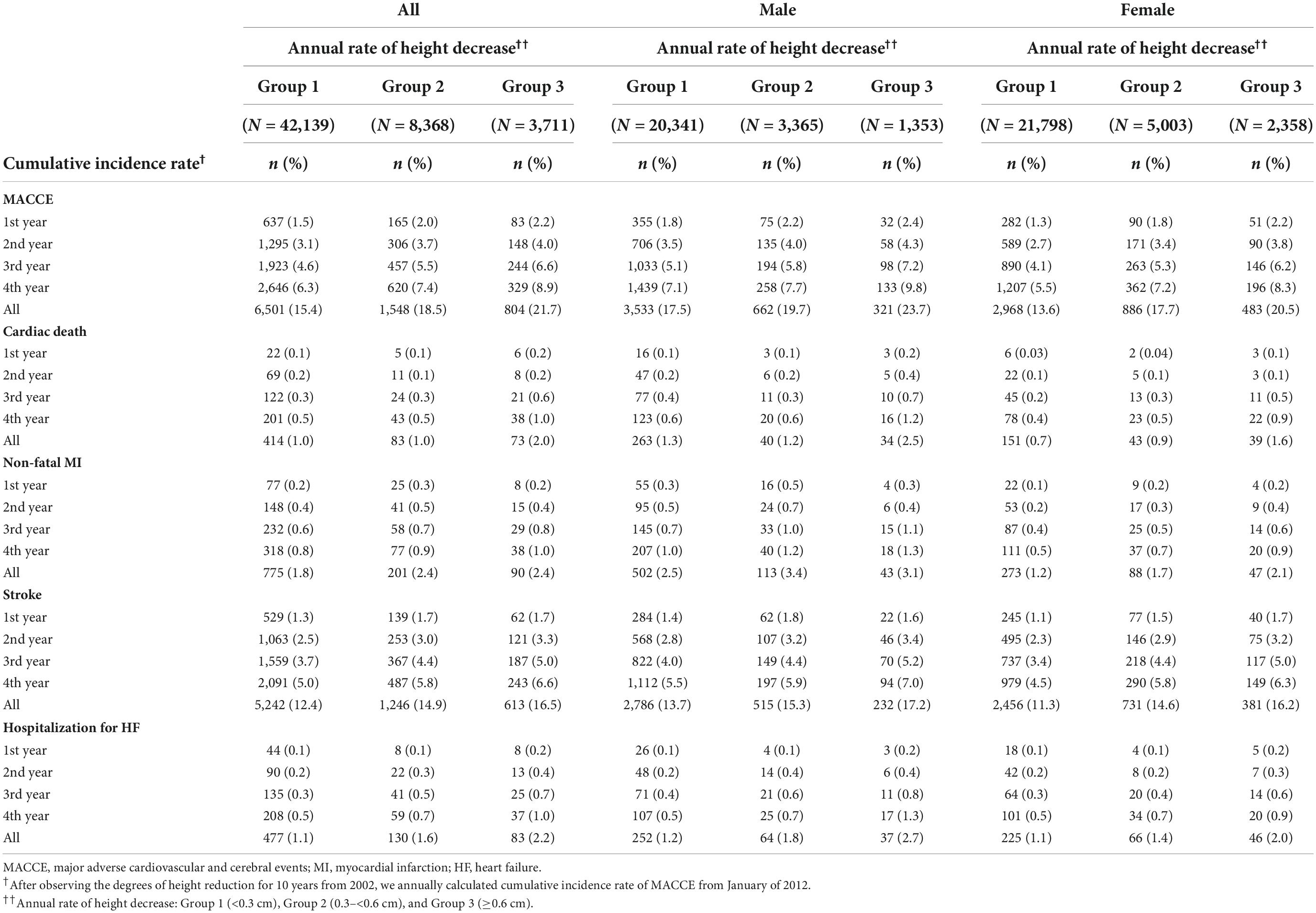
Table 4. The cumulative incidence rates of major adverse cardiovascular and cerebral events per the annual height decrease rate in patients aged ≥ 60 years.
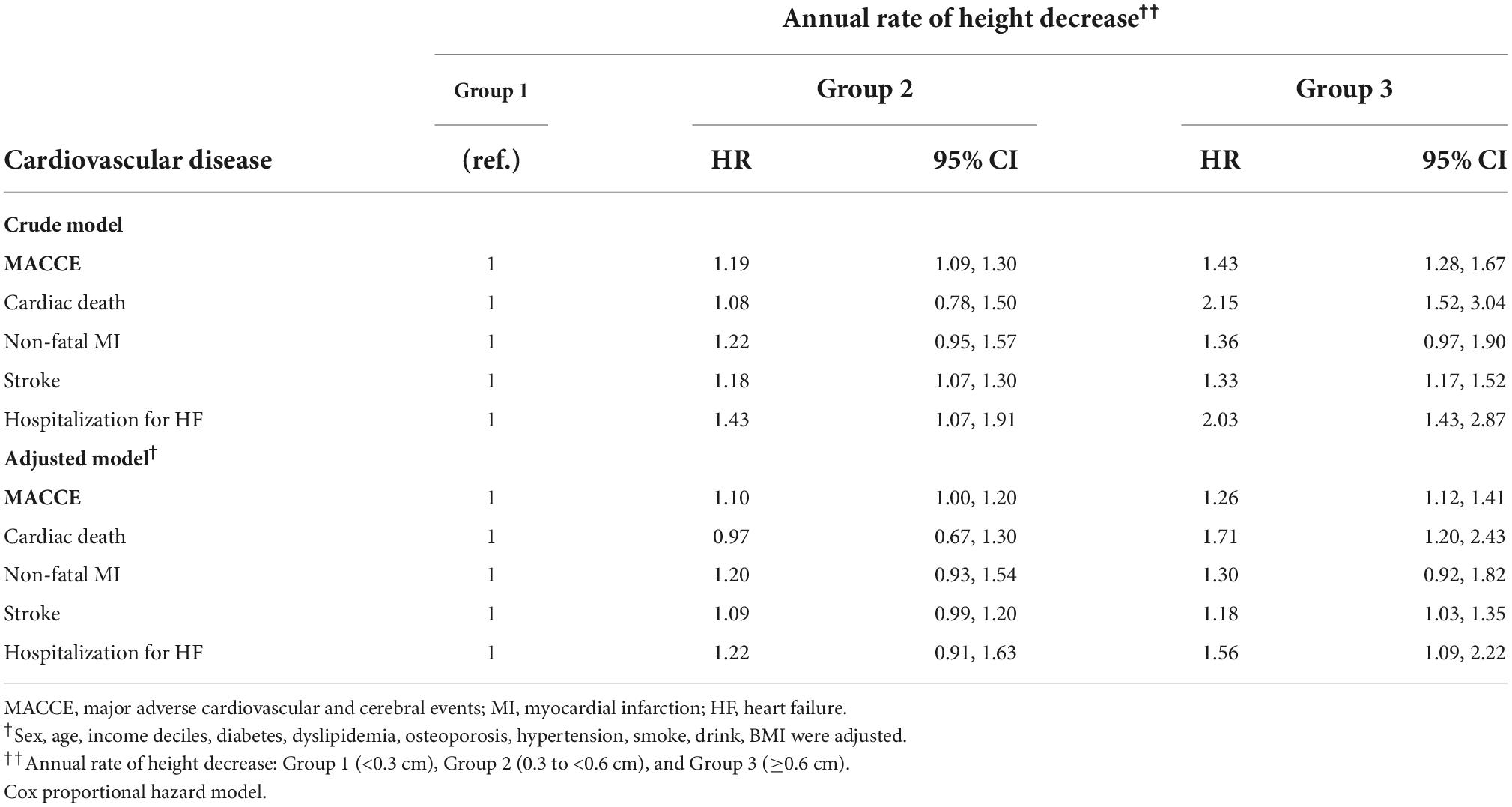
Table 5. The hazard ratios of major adverse cardiovascular and cerebral events per the annual height decrease rate in patients aged ≥ 60 years.
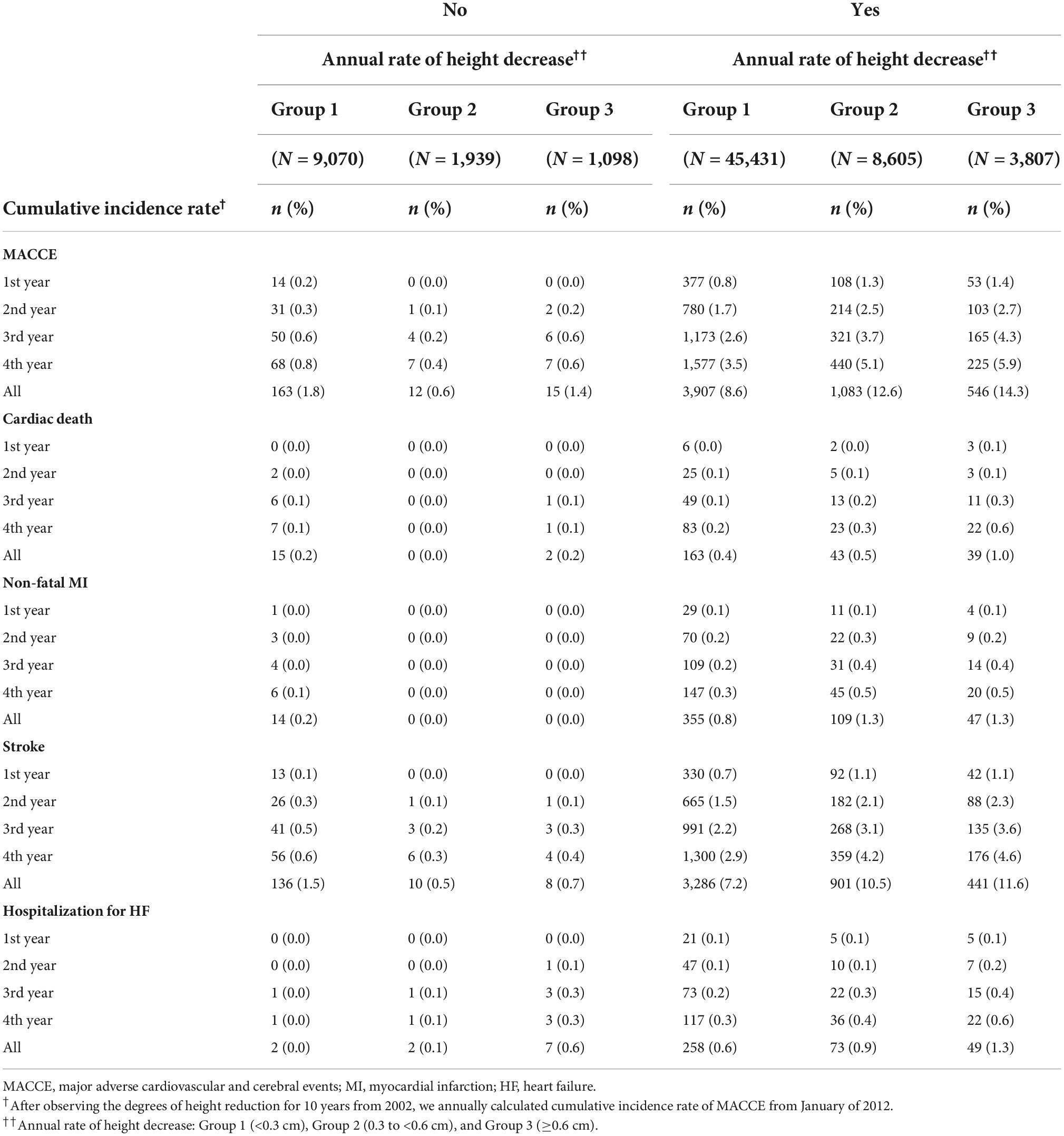
Table 6. The cumulative incidence rates of major adverse cardiovascular and cerebral events per the annual height decrease rate in female patients based on menopausal status.
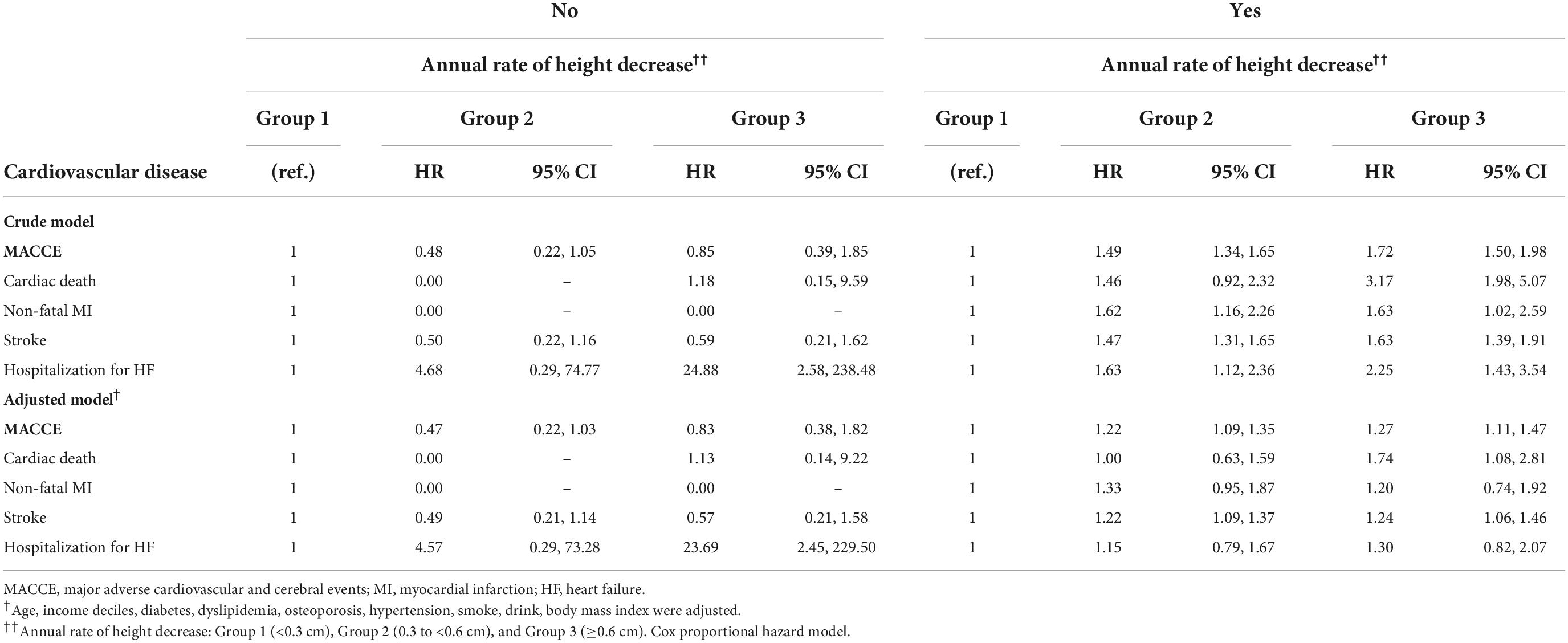
Table 7. The hazard ratios of major adverse cardiovascular and cerebral events per the annual height decrease rate in female patients based on menopausal status.
Table 8 presents the multivariate linear regression results on the demographic and clinical characteristics and the annual height decrease. Significant variables affecting the decrease in height included sex, age, low-income, hypertension, dyslipidemia, smoking, drinking, BMI, and baseline height (p < 0.0001). Diabetes did not affect the annual height decrease. Particularly, sex (female), age ≥ 60 years, low-income, hypertension, smoking and baseline height positively affect the decrease in height.
In this current research using Korean big data, we observed that the degree of height loss was independently associated with an increased risk of MACCE in a dose-dependent manner. Subgroup analyses of the elderly and postmenopausal women, who have a greater decline in height, also demonstrated this association. These results parallel the British males’ data (n = 4,213) published approximately two decades ago and supported the hypothesis that loss of height is more than a common aging process and is a marker for increased CVD risk (10). Compared to that study, the foremost strength of this study is the large sample size. In addition, our study included approximately 54.8% of female participants that were not included in the British data. Homogeneity in the study sample is also noteworthy, as all data were obtained from one country in a relatively short time frame, and the cohort was composed of one ethnicity: Korean. Adult height and its loss are influenced by many factors. As regards confounders affecting height and its loss, the study sample was relatively free from the effects of ethnicity and the chronological change in national socioeconomic status. Based on our study results, patients with significant height decreases should be monitored for CVD occurrence in clinical practice because it might represent an increased risk of CVD. Additionally, considering that determinants of height loss included older age, female sex, hypertension, smoking, baseline height, and low-income level, more attention should be paid to such individuals. The prevention and treatment of senile changes in the musculoskeletal system could also be helpful for cardiovascular health.
For decades, the inverse relationship between adult height and CVD has been repeatedly reported in epidemiological investigations. However, no single theory could explain the mechanism for the phenomenon. Although we and our other colleagues have claimed that dyslipidemia, arterial stiffening represented by a higher pulse wave velocity, or diastolic dysfunction of the heart serve as links between short height and CVD occurrences and outcomes (13–17), entirely satisfactory explanations are still lacking. In this study, we revealed that height loss is associated with increased CVD risk, and we reckon this is clinically meaningful because (1) An individual’s maximum height is genetically determined and is a non-modifiable factor; (2) Conversely, loss of height resulting from senile bone, muscle, and joint changes, is affected by environmental or personal factors, and thus, might be modifiable. The pathophysiology of osteoporosis and sarcopenia, which are responsible for senile height loss, is poorly understood and likely multifactorial; nonetheless, some factors can be targeted for intervention, such as physical inactivity, alcohol use, tobacco use, vitamin D deficiency, or an unhealthy diet. Osteoporosis, characterized by reduced bone density and increased fracture risk, is the primary contributor to height loss by reducing vertebral body strength and inducing the loss of mineral content and trabecular connectivity. Notably, a decrease in stature from osteoporosis and fracture is often remarkable and is likely to reach several centimeters (10, 18). CVD and osteoporosis, which often coexist, are public health challenges with multiple epidemiological links, pathophysiological mechanisms, and economic consequences (19–22). Studies have demonstrated that cardiovascular morbidity and mortality are associated with bone fractures and reduced bone mineral density which is related to vascular calcification, a well-known cardiovascular risk factor (19–22). Additionally, sarcopenia and poor muscle strength, as part of aging, are associated with bone loss and diseased bone structure, eventually resulting in height loss. Osteoporosis and sarcopenia are predictors of mortality and are, at least theoretically, preventable and treatable conditions through environmental modifications and medical interventions (9).
The mechanism relating to the loss of height and subsequent CVD remains unclear. However, the effect of height decrease, a predictor of vertebral fracture, on the respiratory and gastrointestinal systems is noteworthy. Vertebral fractures induce thoracic deformity or pain and disturb pulmonary function subsequently. Conversely, percutaneous vertebroplasty and kyphoplasty for vertebral fractures improve lung function. Further, lung capacity and gastrointestinal function decrease as height declines, potentially leading to less exercise, effort intolerance, early satiety, and poor nutritional status, resulting in sarcopenia and senile fragility (23–28). Weight loss and leanness, attributed to the loss of skeletal muscle mass in the elderly, are risk factors for CVD (the so-called “obesity paradox”) (29–32). Moreover, a musculoskeletal deformity significantly affects psychological conditions, such as depression and anxiety and the quality of life. From a different perspective, however, bone loss and poor bone quality (important determinants of height decline) share common pathophysiological mechanisms with CVD, such as oxidative stress, inflammation, abnormal homocysteine levels, and metabolic risk factors (such as hypertension, diabetes, and dyslipidemia) (33). CVD prevalence increases with age, and the same is true for bone loss and muscle mass decrease: these phenomena might result from shared mechanisms. Hence, from that viewpoint, loss of height might merely be a co-finding or a bystander with CVD rather than a primary cause. Further study is warranted to shed light on the pathophysiological link between loss of height and CVD.
The current investigation was performed based on KCD-7. Thus, biomarkers known as cardiovascular risk factors, such as laboratory findings, were unavailable for analysis. Further, each patient’s medication history was unavailable. The main contributor to height loss is osteoporosis; however, we could not objectively assess each patient’s bone mass density beyond the KCD-7 code. Hence, further study is warranted to clarify the potential pathophysiological association between senile changes in the musculoskeletal system and MACCE. In addition, unreliable height data were excluded from the analysis, and the real change in height cannot be fully distinguished from possible measurement errors. This limitation was due to the retrospective nature of this study. Annual height decrease rate is the main parameter in analysis; however, it could be too crude to represent senile changes in the musculoskeletal system meticulously. In addition, no consideration was given to the potential for non-linear height reduction in data analysis. This was another limitation of this study because it is plausible that serious adverse health events or a period of poor nutrition could lead to a significant loss of height.
The degree of height loss was independently associated with the occurrence of MACCE in a Korean population, although it has yet to be determined if there is a causal relationship between the two.
The data analyzed in this study is subject to the following licenses/restrictions: Restrictions apply to the availability of the raw data, which were used under a policy of the NHIS. The data and materials other than the raw data underlying the study are available from the corresponding author, WCK, on reasonable request. Requests to access these datasets should be directed to WCK, a2FuZ3djaEBnaWxob3NwaXRhbC5jb20=.
The studies involving human participants were reviewed and approved by Gachon University Gil Medical Center (IRB No. GFIRB2019-304). The patients/participants provided their written informed consent to participate in this study.
JGM and WCK designed the study and prepared the manuscript as submitted. JGM analyzed and interpreted the data. WCK supervised the project. All authors reviewed and critically revised the manuscript, read and approved the final manuscript.
We thank the Research Institute of The Way Health Care (Seoul, South Korea) for the statistical analyses and the Editage (www.editage.co.kr, Seoul, South Korea) for English language editing.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Silventoinen K, Zdravkovic S, Skytthe A, McCarron P, Herskind AM, Koskenvuo M, et al. Association between height and coronary heart disease mortality: a prospective study of 35,000 twin pairs. Am J Epidemiol. (2006) 163:615–21. doi: 10.1093/aje/kwj081
2. Nelson CP, Hamby SE, Saleheen D, Hopewell JC, Zeng L, Assimes TL, et al. Genetically determined height and coronary artery disease. N Engl J Med. (2015) 372:1608–18. doi: 10.1056/NEJMoa1404881
3. Park CS, Choi EK, Han KD, Lee HJ, Rhee TM, Lee SR, et al. Association between adult height, myocardial infarction, heart failure, stroke and death: a Korean nationwide population-based study. Int J Epidemiol. (2018) 47:289–98. doi: 10.1093/ije/dyx175
4. Park YM, Moon J, Hwang IC, Lim H, Cho B. Short stature is associated with incident sudden cardiac death in a large Asian cohort. Heart Rhythm. (2020) 17:931–6. doi: 10.1016/j.hrthm.2020.01.026
5. Solomons NW. Vision of research on human linear growth. Food Nutr Bull. (2019) 40:416–31. doi: 10.1177/0379572119885475
6. Sorkin JD, Muller DC, Andres R. Longitudinal change in height of men and women: implications for interpretation of the body mass index: the baltimore longitudinal study of aging. Am J Epidemiol. (1999) 150:969–77. doi: 10.1093/oxfordjournals.aje.a010106
7. Szulc P, Beck TJ, Marchand F, Delmas PD. Low skeletal muscle mass is associated with poor structural parameters of bone and impaired balance in elderly men–the MINOS study. J Bone Miner Res. (2005) 20:721–9. doi: 10.1359/JBMR.041230
9. Metter EJ, Talbot LA, Schrager M, Conwit R. Skeletal muscle strength as a predictor of all-cause mortality in healthy men. J Gerontol A Biol Sci Med Sci. (2002) 57:B359–65. doi: 10.1093/gerona/57.10.b359
10. Wannamethee SG, Shaper AG, Lennon L, Whincup PH. Height loss in older men: associations with total mortality and incidence of cardiovascular disease. Arch Intern Med. (2006) 166:2546–52. doi: 10.1001/archinte.166.22.2546
11. Lee YH, Han K, Ko SH, Ko KS, Lee KU. Data analytic process of a nationwide population-based study using national health information database established by national health insurance service. Diabetes Metab J. (2016) 40:79–82. doi: 10.4093/dmj.2016.40.1.79
12. Lim HS, Kim TH, Lee HH, Park YH, Kim JM, Lee BR. Hypertension and age at onset of natural menopause in Korean postmenopausal women: results from the korea national health and nutrition examination survey (2008-2013). Maturitas. (2016) 90:17–23. doi: 10.1016/j.maturitas.2016.04.019
13. Moon J, Lee HJ, Kim YJ, Kim JY, Pak HN, Ha JW, et al. Short stature and ischemic stroke in nonvalvular atrial fibrillation: new insight into the old observation. Int J Cardiol. (2014) 174:541–4. doi: 10.1016/j.ijcard.2014.04.154
14. Moon J, Suh J, Oh PC, Lee K, Park HW, Jang HJ, et al. Relation of stature to outcomes in Korean patients undergoing primary percutaneous coronary intervention for acute st-elevation myocardial infarction (from the INTERSTELLAR registry). Am J Cardiol. (2016) 118:177–82. doi: 10.1016/j.amjcard.2016.04.046
15. Moon J, Hwang IC, Han SH. Short stature is associated with higher pulse wave velocity in subjects without overt cardiovascular disease. Medicine. (2020) 99:e22219. doi: 10.1097/MD.0000000000022219
16. Hwang IC, Park YM, Kang WC, Moon J. Association between height and lipid profile among Korean men: results from the 10-year Korea national health and nutrition examination survey. Eur J Prev Cardiol. (2020) 27:2205–7. doi: 10.1177/2047487319877055
17. Hwang IC, Park YM, Kang WC, Moon J. Height is associated with dyslipidemia in korean premenopausal women: data from the korea national health and nutrition examination survey. Cardiology. (2020) 145:736–9. doi: 10.1159/000509631
18. Siminoski K, Warshawski RS, Jen H, Lee K. The accuracy of historical height loss for the detection of vertebral fractures in postmenopausal women. Osteoporos Int. (2006) 17:290–6. doi: 10.1007/s00198-005-2017-y
19. Farhat GN, Cauley JA. The link between osteoporosis and cardiovascular disease. Clin Cases Miner Bone Metab. (2008) 5:19–34.
20. Crepaldi G, Maggi S. Epidemiologic link between osteoporosis and cardiovascular disease. J Endocrinol Invest. (2009) 32:2–5.
21. McFarlane SI, Muniyappa R, Shin JJ, Bahtiyar G, Sowers JR. Osteoporosis and cardiovascular disease: brittle bones and boned arteries, is there a link. Endocrine. (2004) 23:1–10. doi: 10.1385/ENDO:23:1:01
22. Whitney C, Warburton DE, Frohlich J, Chan SY, McKay H, Khan K. Are cardiovascular disease and osteoporosis directly linked. Sports Med. (2004) 34:779–807. doi: 10.2165/00007256-200434120-00001
23. Lombardi I, Oliveira LM, Mayer AF, Jardim JR, Natour J. Evaluation of pulmonary function and quality of life in women with osteoporosis. Osteoporos Int. (2005) 16:1247–53. doi: 10.1007/s00198-005-1834-3
24. Kjensli A, Falch JA, Ryg M, Blenk T, Armbrecht G, Diep LM, et al. High prevalence of vertebral deformities in COPD patients: relationship to disease severity. Eur Respir J. (2009) 33:1018–24. doi: 10.1183/09031936.00073908
25. Leech JA, Dulberg C, Kellie S, Pattee L, Gay J. Relationship of lung function to severity of osteoporosis in women. Am Rev Respir Dis. (1990) 141:68–71. doi: 10.1164/ajrccm/141.1.68
26. Ross PD, Davis JW, Epstein RS, Wasnich RD. Pain and disability associated with new vertebral fractures and other spinal conditions. J Clin Epidemiol. (1994) 47:231–9. doi: 10.1016/0895-4356(94)90004-3
27. Ross PD. Clinical consequences of vertebral fractures. Am J Med. (1997) 103:30S–42S; discussion 42S–43S. doi: 10.1016/s0002-9343(97)90025-5.
28. Yuan HA, Brown CW, Phillips FM. Osteoporotic spinal deformity: a biomechanical rationale for the clinical consequences and treatment of vertebral body compression fractures. J Spinal Disord Tech. (2004) 17:236–42. doi: 10.1097/00024720-200406000-00012
29. Hughes VA, Frontera WR, Roubenoff R, Evans WJ, Singh MA. Longitudinal changes in body composition in older men and women: role of body weight change and physical activity. Am J Clin Nutr. (2002) 76:473–81. doi: 10.1093/ajcn/76.2.473
30. Carnethon MR, De Chavez PJ, Biggs ML, Lewis CE, Pankow JS, Bertoni AG, et al. Association of weight status with mortality in adults with incident diabetes. JAMA. (2012) 308:581–90. doi: 10.1001/jama.2012.9282
31. Logue J, Walker JJ, Leese G, Lindsay R, McKnight J, Morris A, et al. Association between BMI measured within a year after diagnosis of type 2 diabetes and mortality. Diabetes Care. (2013) 36:887–93. doi: 10.2337/dc12-0944
32. Pischon T, Boeing H, Hoffmann K, Bergmann M, Schulze MB, Overvad K, et al. General and abdominal adiposity and risk of death in Europe. N Engl J Med. (2008) 359:2105–20. doi: 10.1056/NEJMoa0801891
Keywords: height loss, CVD, MACCE, aging, cardiovascular disease
Citation: Moon J, Oh PC, Lee K, Jang H-J, Kim T-H, Park S-D, Kwon SW, Kong MG, Suh J and Kang WC (2022) Association of height loss and cardiovascular disease: Data from a large Korean cohort. Front. Cardiovasc. Med. 9:1026597. doi: 10.3389/fcvm.2022.1026597
Received: 24 August 2022; Accepted: 19 October 2022;
Published: 04 November 2022.
Edited by:
Wuxiang Xie, Peking University, ChinaReviewed by:
In Sook Kang, Ewha Womans University, South KoreaCopyright © 2022 Moon, Oh, Lee, Jang, Kim, Park, Kwon, Kong, Suh and Kang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Woong Chol Kang, a2FuZ3djaEBnaWxob3NwaXRhbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.