- 1College of Medicine, University of Kentucky, Lexington, KY, United States
- 2Tower Health, West Reading, PA, United States
As the burden of cardiovascular and cerebrovascular events continues to increase, emerging evidence supports the concept of plaque vulnerability as a strong marker of plaque rupture, and embolization. Qualitative assessment of the plaque can identify the degree of plaque instability. Ultrasound and computed tomography (CT) have emerged as safe and accurate techniques for the assessment of plaque vulnerability. Plaque features including but not limited to surface ulceration, large lipid core, thin fibrous cap (FC), intraplaque neovascularization and hemorrhage can be assessed and are linked to plaque instability.
Introduction
The most frequent cause of coronary and carotid artery disease is atherosclerosis. Plaque with high-risk features are characterized as “vulnerable” and are associated with a greater probability of neurologic and cardiovascular events (1). There is evidence that not only luminal narrowing but plaque morphology plays a vital role in characterizing such vulnerable plaques (2). Carotid plaques may rupture and lead to transient ischemic attacks or ischemic strokes (3). There are currently no therapies for vulnerable plaque beyond treatment with statins, as stenting and endarterectomy are recommended for the treatment of symptomatic patients with high stenosis. However, studies have shown that carotid plaques with high risk features albeit <50% stenosis may be linked to cryptogenic ischemic strokes (4). The dynamic nature of atherosclerotic plaque and its potential consequences has led researchers to focus on non-invasive methods for their early detection and identification. Duplex ultrasound is simple, inexpensive, and can be used to assess the morphology and degree of carotid stenosis. Determination of carotid plaque morphology including but not limited to ulceration, plaque area, intraplaque hemorrhage (IPH), and plaque echogenicity may be useful in identifying patients with asymptomatic carotid disease who are at higher risk of adverse events (2). CT including both dual source CT (DSCT) and multidetector CT angiography (MDCTA) has emerged as a reliable tool in the assessment of vulnerable plaque as well. This review summarizes the utility of ultrasound and CT in the evaluation of the vulnerable carotid plaque. Early detection via these modalities can prevent neurologic and cardiovascular events.
Ultrasound and the carotid vulnerable plaque
Carotid ultrasound has been utilized to predict the risk of cerebrovascular disease (CVD). Traditionally, measuring the carotid intima media thickness (CIMT) has been verified as an important estimator of CVD (5). However, potential pitfalls in the assessment of CIMT has made it fall out of favor. In contrast, recent studies have demonstrated the assessment of carotid plaque itself as a more accurate means of assessing the risk of CVD (6–8). Inflammatory changes in an unstable plaque have shown to contribute more to CVD events than direct extension of atherosclerosis (9). Ultrasound is a safe and non-invasive method to assess plaque vulnerability. Carotid plaque evaluation via ultrasound should include a detailed assessment of the number of plaques, plaque thickness/height, plaque area, surface features, neovascularization, and when possible, a 3D assessment of the entire vessel involved (7).
Various ultrasonographic methods can be used to assess atherosclerotic plaques including real time ultrasound, doppler ultrasound, non-doppler flow evaluation methods, optimal ultrasound, Contrast enhanced ultrasound (CEUS), and shear wave elastography (10).
Plaque surface irregularities
Plaque ulceration has long been shown to correlate with neurological symptoms and the occurrence of stroke (11). The detection of plaque ulceration is superior via CEUS as opposed to B-mode or color flow doppler sonography due superior sensitivity (88% compared to 29%) (12). In individual studies, B-mode and color flow doppler have shown sensitivities and specificities of 35.7–85.7% and 75%–81.3% respectively. The latter lacks sensitivity in high-grade stenosis (13).
Echo-intensity serves as a marker of surface morphology. Uniform echo intensity corresponds to a smooth and regular surface whereas a non-uniform pattern and mixed echo-intensities indicate surface heterogeneity (14). Criterion used to classify ulceration vary, largely the projection of a column of microbubbles within an atherosclerotic plaque of 1 × 1 mm or more has shown a high sensitivity (15, 12)
Plaque surface has broadly been classified over the spectrum of smooth, irregular, and ulcerated (Figure 1) and correlates with the risk of embolic strokes (16, 17). Smooth plaques lack surface irregularities, irregular plaques have surface irregularities ranging from 0.3 to 0.9 mm without ulceration (18), while ulcerated plaques have at least one focal cavity measuring from 1 to 2 mm in depth which leads to exposure of the underlying necrotic core (17).
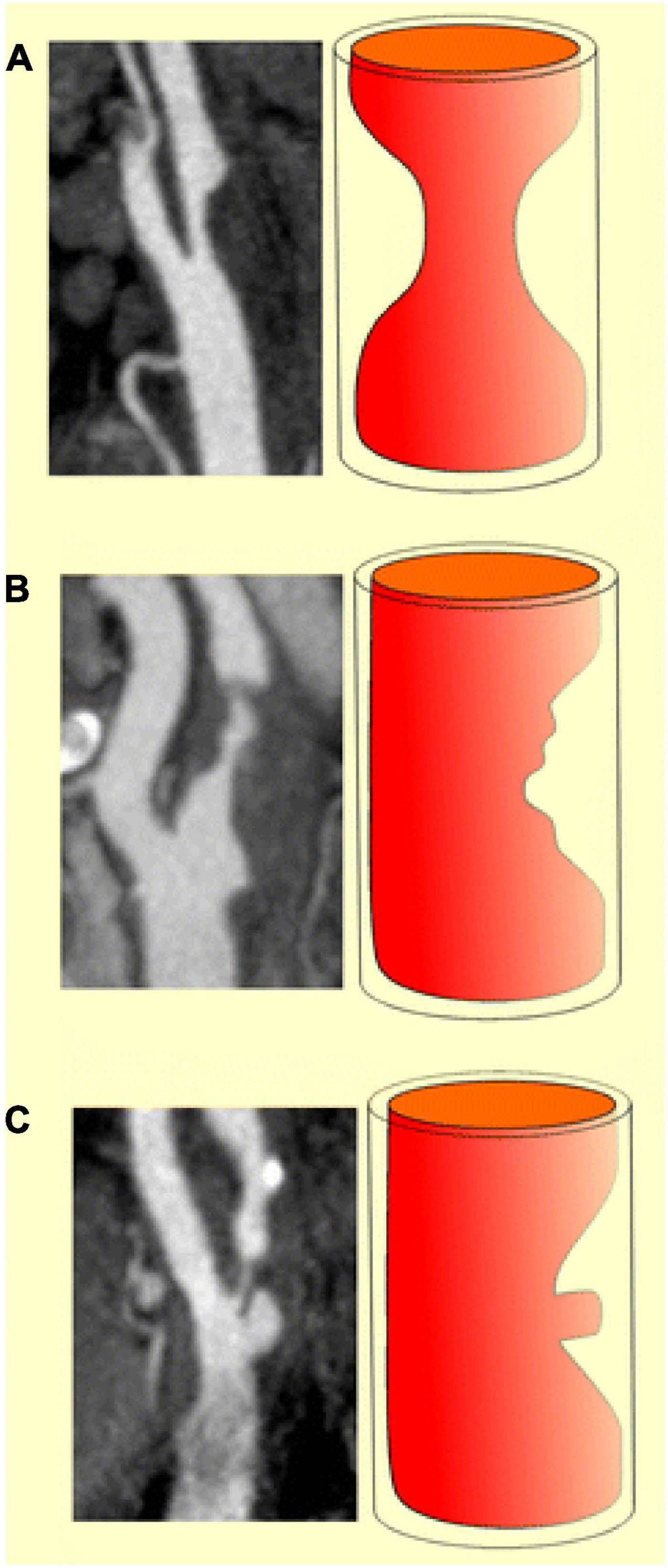
Figure 1. Diagrammatic representations and MDCTA images illustrating the classification of carotid plaques based on their surface morphology as smooth (a), irregular (b) and ulcerated (c) (Content copied in its original form from reference (19) under Creative Commons Attribution 4.0 International License).
Plaque echogenicity
Echogenicity of the plaque is directly related to the amount of calcification and fibrous tissue and inversely related to the lipid content of the plaque. IPH is directly related to lipid content and inversely related to the amount of fibrous tissue in the plaque. Therefore, the association between IPH and a high lipid content may support the theory of the lipid-rich plaque being more prone to rupture (20–24).
Echogenicity of a plaque is graded across type 1 to 5 based on the Gray-Weale-Nicolaides (GWN) classification (25, 26):
Type 1: Homogeneously translucent plaque which is difficult to distinguish from fluid inside the vessel. Plaque primarily composed of lipids and necrotic material.
Type 2: Echo lucent plaque with the presence of single calcifications not exceeding 25% of plaque volume or 20−25% of plaque size.
Type 3: Predominantly echogenic plaque−calcifications constitute up to 50% of plaque structure.
Type 4: Uniformly echogenic with greater than 50% uniform calcification.
Type 5: Heavily calcified plaque.
Thickness of the fibrous cap, size of the lipid necrotic core
Thinning and rupture of the fibrous cap (FC) is sentinel in plaque instability. Rupture is common in plaques with FC thickness less than 0.065 mm (27–30). Neovascularization found in the FC especially in the medial and lateral corners can become leakage sites of blood vessels through the accumulation of inflammatory cells. This adds to the vulnerability of the plaque (30). Thin or ruptured FC has also been linked to plaque ulceration which is a known marker of plaque instability. In addition to thickness, echogenicity of the cap is also important. A thin FC defines the plaque as thin cap atheromatic plaque (TCAP) which makes the plaque vulnerable to rupture (26).
Lipid core accounting for 40% of the plaque volume makes it prone to rupture (13). Echo lucent plaques are lipid rich while echogenic plaques are fibrin rich with calcification. Plaque echogenicity can be graded from one to four as described previously. Echo lucent plaques have a higher association with CVAs (31, 32).
Carotid neovascularization
Atherosclerosis within the plaque leads to local hypoxia promoting neovascularization and vessel wall injury. Vessel wall injury in turn leads to inward growth of the vasa vasorum leading to further neovascularization. This immature neovascularization leads to increased vessel wall density. As the vascularity grows, the size of the core grows which stretches the FC thin. This is referred to as thin fibrous cap atheroma/thin cap atheroma plaque (TFCA/TCAP). The microvessels lack wall integrity, bleed and lead to IPH which compromises stability of the plaque (10, 33–35). IPH has been correlated with increased incidence of CVD (36).
Studies have shown consistency of CEUS in the detection of neovascularization (37, 38). CEUS uses ultrasonographic contrast agent (UCA) consisting of micro bubbles which reflect ultrasound waves as harmonic frequencies back toward the transducer. The contrast agents are composed of small microbubbles that remain intra-arterial and can pick up microvasculature in the adventitia and the core of the plaque. The microbubbles give off signals back to the transducer which are reflective of the microvasculature. This intraplaque enhancement can represent IPH, immature leaky vasa vasorum and neovessels in vulnerable plaques. Signal intensity may correlate with the density of the microvasculature and be directly related to the vulnerability of the plaque (39, 40). Intraplaque enhancement has been graded for qualitative assessment. Grade 1 (mild) no intra plaque enhancement, Grade 2 (moderate) enhancement of the plaque shoulder and adventitia, or Grade 3 (severe) intraplaque enhancement (40, 41). Grade 4 has also been used which involves more extensive infiltration into the plaque body (Figure 2).
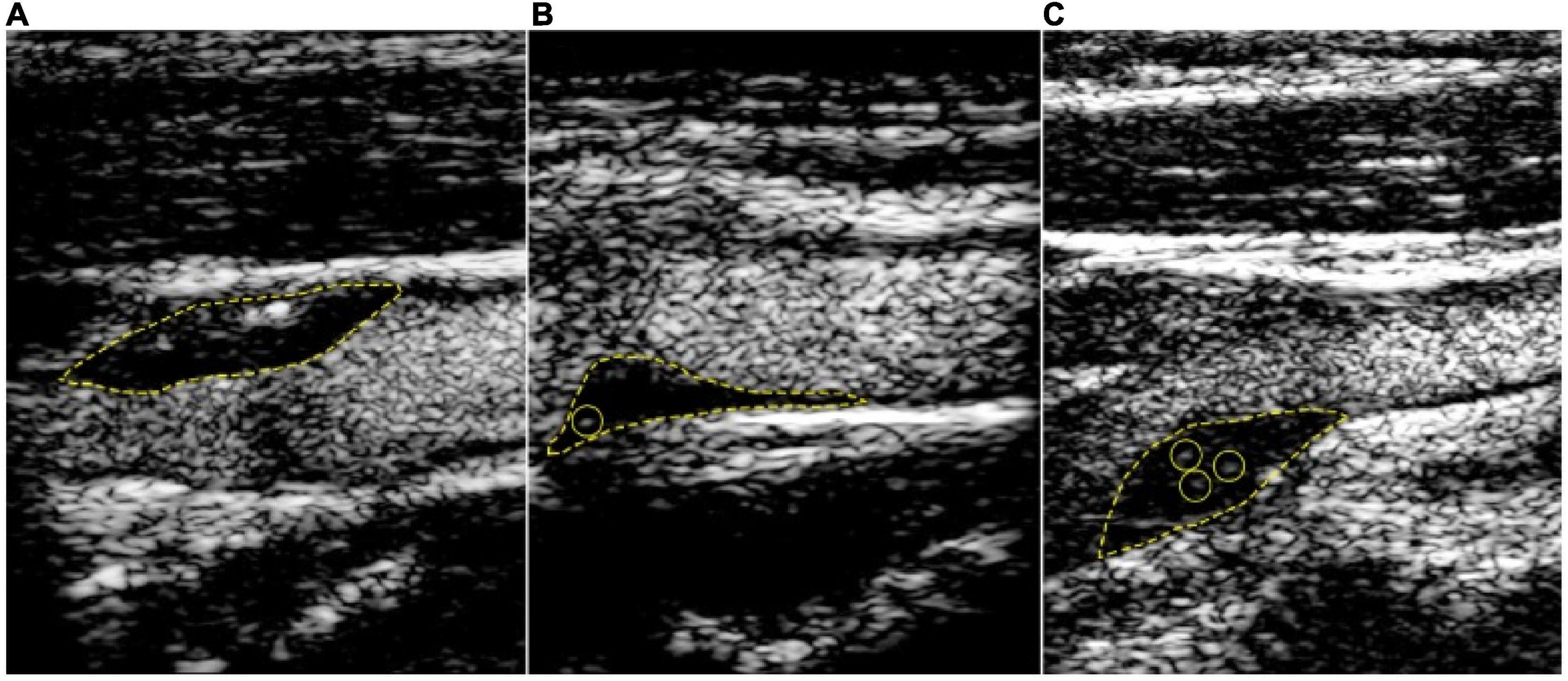
Figure 2. Contrast-enhanced carotid ultrasound for the detection of plaque neovascularization. Content copied in its original form from Mantella et al. with permission (42). Carotid intraplaque neovascularization scoring method. Representative contrast-enhanced ultrasound images of carotid plaques. (A) demonstrates a plaque score of 0, no visible microbubbles within the plaque; (B) demonstrates a plaque score of 1, minimal microbubbles confined to peri-adventitial area; (C) demonstrates a plaque score of 2, microbubbles present throughout the plaque core. The yellow dotted line outlines the plaque lesion. Yellow circles depict intraplaque contrast microbubbles.
Computed tomography imaging of carotid artery vulnerable plaque
Traditionally, MRI has been considered the imaging of choice in evaluating high risk plaques. However, recent advances in CT have made it a reliable resource for detecting plaque vulnerability. MDCTA and DSCT are the two most widely available techniques utilized for plaque assessment. Compared to conventional CT which utilizes individual slices, MDCTA acquires volume data, has much higher data acquisition speed and therefore allows for a much higher spatial resolution. This allows for better visualization of the tissue components of a vulnerable plaque compared to MRI (43, 44).
Although MDCTA’s wide availability makes it a convenient resource, it does have noticeable difficulty in differentiating between plaque calcium and luminal contrast (38). These restrictions are overcome with the DSCT technology that visualizes distinct radiodensities in a carotid plaque. Clear distinction between luminal contrast and the plaque body is achieved with DSCT. Unlike MDCT, DSCT is not as widely available but provides greater temporal resolution compared to MRI (13, 45).
Multidetector computed tomography and dual source computed tomography also help assess details of a soft plaque. Soft plaque is a combination of IPH, lipid-rich necrotic core (LRNC) and fibrous elements. On CT, soft plaque is generally defined as a low attenuation plaque with roughly <60 Hounsfield units (HU), whereas fibrous tissue is considered between 60−130 HU and >130 HU is considered calcified plaque. Soft plaque is associated with a threefold increase in cerebrovascular events (46), hence it is increasingly important for imaging techniques to efficiently discover soft plaque of the carotid arteries.
Computed tomography evaluation of intraplaque-hemorrhage
Intra-plaque hemorrhage is a critical event preceding an acute ischemic events. Neo-vasculature that has invaded into the plaque can rupture and cause IPH. Factors contributing to micro hemorrhaging include diabetes, metabolic derangements, etc. (47).
Recent research has suggested that CT can identify these high risk features in a plaque, despite MRI having been considered the foremost imaging modality for IPH in the past. One study by Saba et al. in 2018 suggested that Hounsfield units <25 on CT consistently identified the presence of IPH. Utilizing this information, a retrospective study published in 2019 by Saba et al. evaluated components and subcomponents of plaque volume and IPH in 200 carotid arteries that underwent CTA. Their research suggested that Hounsfield units <25, which represented IPH, showed a statistically significant association with the presence of cerebrovascular events in patients (48).
Computed tomography evaluation of the lipid rich necrotic core
Differentiating between LRNC and IPH on CTA can be challenging as both have low attenuation (<60 HU). However, IPH is considered to have lower Hounsfield unit values on average than LRNC, 18 HU compared to 63 HU, respectively. In general, low attenuation still represents the presence of high risk soft plaque and differentiating between IPH and LRNC on CTA may not have a clinical importance (45).
Computed tomography evaluation of the fibrous cap
Assessment of FC integrity plays a critical role in differentiating between low and high risk plaques. Low risk plaques have an intact FC, thinned FC is related with mildly increased risk of rupture, while a fissured FC overlying a large LRNC carries a very high risk of plaque rupture. Once rupture occurs, the thrombogenic subendothelial plaque and its matrix are exposed to intraluminal blood flow and can lead to thromboembolism.
Saba et al. in 2013 showed a correlation between fissured FC and contrast enhancement on MDCTA. Forty-seven symptomatic patients underwent MDCTA scans and contrast enhancement of the plaques were analyzed. Patients then underwent carotid endarterectomy followed by histologic analysis of the plaque to evaluate for fissured FC’s. Of the forty-seven patients, twelve were found to have fissured FC’s, and 92% (11/12 patients) of fissured FC’s had contrast enhancement on CTA. Of the non-fissured FC’s, 69% (24/35 patients) also had contrast enhancement, however, to a much lesser degree (22.6 HU as opposed to 12.9 HU) (49). This suggests that MDCTA can evaluate for vulnerable plaque using contrast enhancement on MDCTA.
Surface morphology
Ulcerated plaque surfaces, defined as a cavity of >1 mm, are most concerning on VT imaging. MDCTA has a higher sensitivity and specificity than digital subtraction angiography and ultrasound at detecting these ulcerations (44).
Discussion
The emerging concept of plaque vulnerability has been well documented in the recent years. Assessment of plaques for their vulnerability as opposed to traditional vascular stenosis can better quantify the risk of embolic events. The current review summarizes the sentinel features of the vulnerable plaque, and outlines the role of carotid ultrasound and CT in the identification of such vulnerable features (6, 7). Figure 3 shows a graphical summary.
The connection between vulnerable plaque and vulnerable patient was described in the SHAPE taskforce report. Iliofemoral and carotid atherosclerosis are CHD risk equivalents. These predict atherosclerosis in other vascular beds and should be treated aggressively (50) In asymptomatic patients with carotid atherosclerosis, the utility of revascularization remains to be proven. However, individual risk factor assessment including features of plaque vulnerability may identify a high risk patient for a near term event. Therapeutic strategies focusing these patients can decrease the burden of palliative care for CVD.
Ultrasound serves as the first line modality for many vascular studies. In comparison to CT and MRI, CEUS can be used for the assessment of thrombus vascularity via real-time and continuous scanning (51). Compared to standard carotid intima-media thickness (cIMT), the presence of plaque on carotid ultrasound is a superior predictor of future vascular events.
Ultrasound has a lower overall cost, and is relatively safe compared to CT modalities due to the lack of iodinated contrast media. One of the major limitation of ultrasound and CEUS is subjective interpretation by the investigator which is constant along the spectrum of ultrasound based studies. Secondly, due to the specialized nature of CEUS and its contrast agents, wider availability is yet to be attained. In addition, CEUS requires superior software modalities to better process impulses received by the transducer. Contrast medium consists of microbubbles filled with high molecular weight gas which can rarely cause headaches, injection site bruising, pain, and paresthesia’s. (40)
Limitations of CT imaging include beam hardening artifacts which can be common in MDCTA secondary to calcification in the arteries and plaques. In comparison to ultrasound, contrast agents used in CT carry the risk of hepatotoxicity, renal toxicity, and allergic reactions. In comparison, contrast agents used for CEUS contain microbubbles which are mainly metabolized by respiration and are safer (44, 51). In addition to being more expensive, CT also carries the risk of ionizing radiation.
Conclusion
Plaque vulnerability is a superior marker in predicting future risk of cerebrovascular events. CEUS and CT assessment are emerging as easy non-invasive tools for quantitative and qualitative assessment of plaque vulnerability. These modalities can identify surface and intraplaque irregularities which are markers for plaque instability. The use of these modalities should be increased in routine carotid plaque assessment.
Author contributions
UN took responsibility for all aspects of the reliability and freedom from bias of the data presented and the discussed interpretation. UN, MA, JS, HH, and TW contributed equally toward manuscript preparation, literature search, getting references, and revising the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
Authors UN, JS, TW, MA, and HH were employed by Tower Health.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Naghavi M, Falk E, Hecht HS, Jamieson MJ, Kaul S, Berman D, et al. From vulnerable plaque to vulnerable patient–Part III: executive summary of the screening for heart attack prevention and education (SHAPE) task force report. Am J Cardiol. (2006) 98:2H–15. doi: 10.1016/j.amjcard.2006.03.002
2. Staub D, Schinkel AF, Coll B, Coli S, van der Steen AF, Reed JD, et al. Contrast-enhanced ultrasound imaging of the vasa vasorum: from early atherosclerosis to the identification of unstable plaques. JACC Cardiovasc Imaging. (2010) 3:761–71. doi: 10.1016/j.jcmg.2010.02.007
3. Verhoeven B, Hellings WE, Moll FL, de Vries JP, de Kleijn DP, de Bruin P, et al. Carotid atherosclerotic plaques in patients with transient ischemic attacks and stroke have unstable characteristics compared with plaques in asymptomatic and amaurosis fugax patients. J Vasc Surg. (2005) 42:1075–81.
4. Kleindorfer DO, Towfighi A, Chaturvedi S, Cockroft KM, Gutierrez J, Lombardi-Hill D, et al. 2021 Guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the american heart association/american stroke association. Stroke. (2021) 52:e364–467. doi: 10.1161/STR.0000000000000375
5. Gepner AD, Keevil JG, Wyman RA, Korcarz CE, Aeschlimann SE, Busse KL, et al. Use of carotid intima-media thickness and vascular age to modify cardiovascular risk prediction. J Am Soc Echocardiogr. (2006) 19:1170–4.
6. Naqvi TZ, Lee MS. Carotid intima-media thickness and plaque in cardiovascular risk assessment. JACC Cardiovasc Imaging. (2014) 7:1025–38.
7. Johri AM, Nambi V, Naqvi TZ, Feinstein SB, Kim ESH, Park MM, et al. Recommendations for the assessment of carotid arterial plaque by ultrasound for the characterization of atherosclerosis and evaluation of cardiovascular risk: from the American society of echocardiography. J Am Soc Echocardiogr. (2020) 33:917–33.
8. Sillesen H, Sartori S, Sandholt B, Baber U, Mehran R, Fuster V. Carotid plaque thickness and carotid plaque burden predict future cardiovascular events in asymptomatic adult Americans. Eur Heart J Cardiovasc Imaging. (2018) 19:1042–50. doi: 10.1093/ehjci/jex239
9. Marnane M, Prendeville S, McDonnell C, Noone I, Barry M, Crowe M, et al. Plaque inflammation and unstable morphology are associated with early stroke recurrence in symptomatic carotid stenosis. Stroke. (2014) 45:801–6. doi: 10.1161/STROKEAHA.113.003657
11. Barnett HJ, Taylor DW, Eliasziw M, Fox AJ, Ferguson GG, Haynes RB, et al. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis: north american symptomatic carotid endarterectomy trial collaborators. N Engl J Med. (1998) 339:1415–25. doi: 10.1056/NEJM199811123392002
12. ten Kate GL, van Dijk AC, van den Oord SC, Hussain B, Verhagen HJ, Sijbrands EJ, et al. Usefulness of contrast-enhanced ultrasound for detection of carotid plaque ulceration in patients with symptomatic carotid atherosclerosis. Am J Cardiol. (2013) 112:292–8. doi: 10.1016/j.amjcard.2013.03.028
13. Muraki M, Mikami T, Yoshimoto T, Fujimoto S, Tokuda K, Kaneko S, et al. New criteria for the sonographic diagnosis of a plaque ulcer in the extracranial carotid artery. AJR Am J Roentgenol. (2012) 198:1161–6. doi: 10.2214/AJR.11.7018
14. Reilly LM, Lusby RJ, Hughes L, Ferrell LD, Stoney RJ, Ehrenfeld WK. Carotid plaque histology using real-time ultrasonography: clinical and therapeutic implications. Am J Surg. (1983) 146:188–93. doi: 10.1016/0002-9610(83)90370-7
15. Rafailidis V, Chryssogonidis I, Xerras C, Nikolaou I, Tegos T, Kouskouras K, et al. A comparative study of color Doppler imaging and contrast-enhanced ultrasound for the detection of ulceration in patients with carotid atherosclerotic disease. EurRadiol. (2019) 29:2137–45. doi: 10.1007/s00330-018-5773-8
16. Saba L, Anzidei M, Sanfilippo R, Montisci R, Lucatelli P, Catalano C, et al. Imaging of the carotid artery. Atherosclerosis. (2012) 220:294–309.
17. Saba L, Caddeo G, Sanfilippo R, Montisci R, Mallarini G. CT and ultrasound in the study of ulcerated carotid plaque compared with surgical results: potentialities and advantages of multidetector row CT angiography. AJNR Am J Neuroradiol. (2007) 28:1061–6. doi: 10.3174/ajnr.A0486
18. Saba L, Anzidei M, Marincola BC, Piga M, Raz E, Bassareo PP, et al. Imaging of the Carotid Artery Vulnerable Plaque. Cardiovasc Intervent Radiol. (2014) 37:572–85.
19. Rafailidis V, Chryssogonidis I, Tegos T, Kouskouras K, Charitanti-Kouridou A. Imaging of the ulcerated carotid atherosclerotic plaque: a review of the literature. Insights Imaging. (2017) 8:213–25. doi: 10.1007/s13244-017-0543-8
20. Grønholdt ML, Wiebe BM, Laursen H, Nielsen TG, Schroeder TV, Sillesen H. Lipid-rich carotid artery plaques appear echolucent on ultrasound B-mode images and may be associated with intraplaque haemorrhage. Eur J Vasc Endovasc Surg. (1997) 14:439–45. doi: 10.1016/s1078-5884(97)80121-9
21. Di Stefano R, Felice F, Balbarini A. Angiogenesis as risk factor for plaque vulnerability. Curr Pharm Des. (2009) 15:1095–106.
22. Kume S, Hama S, Yamane K, Wada S, Nishida T, Kurisu K. Vulnerable carotid arterial plaque causing repeated ischemic stroke can be detected with B-mode ultrasonography as a mobile component: jellyfish sign. Neurosurg Rev. (2010) 33:419–30. doi: 10.1007/s10143-010-0270-9
23. Hellings WE, Peeters W, Moll FL, Piers SR, van Setten J, Van der Spek PJ, et al. Composition of carotid atherosclerotic plaque is associated with cardiovascular outcome: a prognostic study. Circulation. (2010) 121:1941–50.
24. Ota H, Yu W, Underhill HR, Oikawa M, Dong L, Zhao X, et al. Hemorrhage and large lipid-rich necrotic cores are independently associated with thin or ruptured fibrous caps: an in vivo 3T MRI study. Arterioscler Thromb Vasc Biol. (2009) 29:1696–701. doi: 10.1161/ATVBAHA.109.192179
25. Brinjikji W, Huston J III, Rabinstein AA, Kim GM, Lerman A, Lanzino G. Contemporary carotid imaging: from degree of stenosis to plaque vulnerability. J Neurosurg. (2016) 124:27–42.
26. Fedak A, Ciuk K, Urbanik A. Ultrasonography of vulnerable atherosclerotic plaque in the carotid arteries: B-mode imaging. J Ultrason. (2020) 20:e135–45. doi: 10.15557/JoU.2020.0022
27. Finn AV, Nakano M, Narula J, Kolodgie FD, Virmani R. Concept of vulnerable/unstable plaque. Arterioscler Thromb Vasc Biol. (2010) 30:1282–92. doi: 10.1161/ATVBAHA.108.179739
29. Kolodgie FD, Burke AP, Farb A, Gold HK, Yuan J, Narula J, et al. The thin-cap fibroatheroma: a type of vulnerable plaque: the major precursor lesion to acute coronary syndromes. Curr Opin Cardiol. (2001) 16:285–92. doi: 10.1097/00001573-200109000-00006
30. McCarthy MJ, Loftus IM, Thompson MM, Jones L, London NJ, Bell PR, et al. Angiogenesis and the atherosclerotic carotid plaque: an association between symptomatology and plaque morphology. J Vasc Surg. (1999) 30:261–8.
31. Mathiesen EB, Bønaa KH, Joakimsen O. Echolucent plaques are associated with high risk of ischemic cerebrovascular events in carotid stenosis: the tromsø study. Circulation. (2001) 103:2171–5. doi: 10.1161/01.cir.103.17.2171
32. Kitta Y, Obata JE, Takano H, Nakamura T, Kodama Y, Fujioka D, et al. Echolucent carotid plaques predict in-stent restenosis after bare metal stenting in native coronary arteries. Atherosclerosis. (2008) 197:177–82. doi: 10.1016/j.atherosclerosis.2007.03.021
33. Feinstein SB. Contrast ultrasound imaging of the carotid artery vasa vasorum and atherosclerotic plaque neovascularization. J Am Coll Cardiol. (2006) 48:236–43.
34. Naghavi M, Libby P, Falk E, Casscells SW, Litovsky S, Rumberger J, et al. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: part II. Circulation. (2003) 108:1772–8. doi: 10.1161/01.CIR.0000087481.55887.C9
35. Badimon L, Vilahur G. Thrombosis formation on atherosclerotic lesions and plaque rupture. J Intern Med. (2014) 276:618–32.
36. Altaf N, MacSweeney ST, Gladman J, Auer DP. Carotid intraplaque hemorrhage predicts recurrent symptoms in patients with high-grade carotid stenosis. Stroke. (2007) 38:1633–5. doi: 10.1161/STROKEAHA.106.473066
37. Oura K, Kato T, Ohba H, Terayama Y. Evaluation of intraplaque neovascularization using superb microvascular imaging and contrast-enhanced ultrasonography. J Stroke Cerebrovasc Dis. (2018) 27:2348–53.
38. Hoshino M, Shimizu T, Ogura H, Hagiwara Y, Takao N, Soga K, et al. Intraplaque microvascular flow signal in superb microvascular imaging and magnetic resonance imaging carotid plaque imaging in patients with atheromatous carotid artery stenosis. J Stroke Cerebrovasc Dis. (2018) 27:3529–34. doi: 10.1016/j.jstrokecerebrovasdis.2018.08.017
39. Partovi S, Loebe M, Aschwanden M, Baldi T, Jäger KA, Feinstein SB, et al. Contrast-enhanced ultrasound for assessing carotid atherosclerotic plaque lesions. AJR Am J Roentgenol. (2012) 198:W13–9.
40. Staub D, Partovi S, Schinkel AF, Coll B, Uthoff H, Aschwanden M, et al. Correlation of carotid artery atherosclerotic lesion echogenicity and severity at standard US with intraplaque neovascularization detected at contrast-enhanced US. Radiology. (2011) 258:618–26. doi: 10.1148/radiol.10101008
41. Rajaram V, Pandhya S, Patel S, Meyer PM, Goldin M, Feinstein MJ, et al. Role of surrogate markers in assessing patients with diabetes mellitus and the metabolic syndrome and in evaluating lipid-lowering therapy. Am J Cardiol. (2004) 93:32C–48C. doi: 10.1016/j.amjcard.2004.02.004
42. Mantella LE, Colledanchise KN, Hétu MF, Feinstein SB, Abunassar J, Johri AM. Carotid intraplaque neovascularization predicts coronary artery disease and cardiovascular events. Eur Heart J Cardiovasc Imaging. (2019) 20:1239–47. doi: 10.1093/ehjci/jez070
43. Burrill J, Dabbagh Z, Gollub F, Hamady M. Multidetector computed tomographic angiography of the cardiovascular system. Postgrad Med J. (2007) 83:698–704.
44. Murgia A, Erta M, Suri JS, Gupta A, Wintermark M, Saba L. CT imaging features of carotid artery plaque vulnerability. Ann Transl Med. (2020) 8:1261.
45. Baradaran H, Gupta A. Carotid vessel wall imaging on CTA. AJNR Am J Neuroradiol. (2020) 41:380–6.
46. Baradaran H, Al-Dasuqi K, Knight-Greenfield A, Giambrone A, Delgado D, Ebani EJ, et al. Association between carotid plaque features on CTA and cerebrovascular ischemia: a systematic review and meta-analysis. AJNR Am J Neuroradiol. (2017) 38:2321–6. doi: 10.3174/ajnr.A5436
48. Saba L, Micheletti G, Brinjikji W, Garofalo P, Montisci R, Balestrieri A, et al. Carotid Intraplaque-Hemorrhage Volume and Its Association with Cerebrovascular Events. AJNR Am J Neuroradiol. (2019) 40:1731–7.
49. Saba L, Tamponi E, Raz E, Lai L, Montisci R, Piga M, et al. Correlation between fissured fibrous cap and contrast enhancement: preliminary results with the use of CTA and histologic validation. AJNR Am J Neuroradiol. (2014) 35:754–9. doi: 10.3174/ajnr.A3759
50. Tarantino L, Ambrosino P, Di Minno MN. Contrast-enhanced ultrasound in differentiating malignant from benign portal vein thrombosis in hepatocellular carcinoma. World J Gastroenterol. (2015) 21:9457–60. doi: 10.3748/wjg.v21.i32.9457
Keywords: plaque vulnerability, carotid ultrasound, CEUS, unstable plaque, CT
Citation: Singh A, Nasir U, Segal J, Waheed TA, Ameen M and Hafeez H (2022) The utility of ultrasound and computed tomography in the assessment of carotid artery plaque vulnerability–A mini review. Front. Cardiovasc. Med. 9:1023562. doi: 10.3389/fcvm.2022.1023562
Received: 19 August 2022; Accepted: 25 October 2022;
Published: 16 November 2022.
Edited by:
Giulia Elena Mandoli, University of Siena, ItalyCopyright © 2022 Singh, Nasir, Segal, Waheed, Ameen and Hafeez. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Usama Nasir, dXNhbWEubmFzaXJAdG93ZXJoZWFsdGgub3Jn
 Aniruddha Singh
Aniruddha Singh Usama Nasir
Usama Nasir Jared Segal
Jared Segal Tayyab Ali Waheed2
Tayyab Ali Waheed2 Muhammad Ameen
Muhammad Ameen