
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med., 03 November 2022
Sec. General Cardiovascular Medicine
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.1021692
This article is part of the Research TopicPeripheral Artery Disease: Identification, management and prognosis in diverse populationsView all 4 articles
Background: Socioeconomic factors have been shown to be associated with amputation in peripheral artery disease (PAD); however, analyses have normally focused on insurance status, race, or median income. We sought to determine whether community-level socioeconomic distress was associated with major amputation and if that association differed by race.
Materials and methods: Community-level socioeconomic distress was measured using the distressed communities index (DCI). The DCI is a zip code level compositive socioeconomic score (0–100) that accounts for unemployment, education level, poverty rate, median income, business growth, and housing vacancies. A distressed community was defined as a zip code with DCI of 40 or greater. We calculated one-year risk of major amputation by DCI score for individuals with peripheral artery disease in South Carolina, 2012–2017. Treating death as competing event, we reported Fine and Gray subdistribution hazards ratios (sdHR), adjusted for patient demographic and clinical comorbidities associated with amputation. Further analyses were completed to identify potential differences in outcomes within strata of race and DCI.
Results: Among 82,848 individuals with peripheral artery disease, the one-year incidence of amputation was 3.5% (95% CI: 3.3%, 3.6%) and was significantly greater in distressed communities than non-distressed communities (3.9%; 95% CI: 3.8%, 4.1% vs. 2.4%; 95% CI: 2.2%, 2.6%). After controlling for death and adjusting for covariates, we found an increased hazard of amputation among individuals in a distressed community (sdHR: 1.25; 95% CI: 1.14, 1.37), which persisted across racial strata. However, regardless of DCI score, Black individuals had the highest incidence of amputation.
Conclusion: Socioeconomic status is independently predictive of limb amputation after controlling for demographic characteristics and clinical comorbidities. Race continues to be an important risk factor, with Black individuals having higher incidence of amputation, even in non-distressed communities, than White individuals had in distressed communities.
Peripheral artery disease (PAD) is a progressive circulatory disorder that occurs when arteries carrying blood from the heart to the legs are obstructed (1). PAD currently affects between 8 million to 12 million adults in the United States, but that number is expected to increase as the population ages and the prevalence of PAD risk factors increases (2–4). Individuals with PAD have reduced quality of life (5, 6) and increased risk for functional limitation (7, 8), cardiovascular disease (9, 10), and mortality (11, 12). Major limb amputation is a serious complication of PAD that arises when significant tissue loss has occurred, when medical or surgical intervention is not possible, or when an intervention has been unsuccessful (13, 14). Some amputations are medically necessary, usually in individuals with PAD who have chronic limb threatening ischemia. However, amputation is generally considered a failure of treatment and should only be attempted after considering all other treatment options. The majority of amputations are preventable, especially among individuals with PAD who have claudication, with timely diagnosis and optimal medical treatment (15, 16).
Socioeconomic and demographic characteristics are important factors that contribute to an individual’s access to medical care and health outcomes (17). Amongst individuals with PAD, low socioeconomic status is associated with poor access to care (18, 19), increased risk of hospitalization (20), and high prevalence of comorbid conditions (20, 21). Similarly, people from racial minority groups also have higher incidence of PAD (22), are disproportionately affected by poor access to care (1, 19), and have worse outcomes (23, 24). As more granular measures of economic status are often unavailable, race is commonly used as a proxy for SES (2, 25). We argue that race and socioeconomic status (SES) have a distinct process through which they impact health and there may be important differences that are ignored when SES and race are conflated (26).
To date, most socioeconomic disparities in amputation risk are based on individual-level (27) measures of SES or a specific socioeconomic component, such as median household income (28, 29). Comprehensive SES measures may improve predictions but are rarely included in models (30). In this study we utilize a composite ranking that incorporates social, economic, and financial metrics at the zip code level. The objectives of this study were to (1) investigate the association between community-level socioeconomic distress and major amputation among individuals with peripheral artery disease; and (2) determine if the association differed by race.
The present study used data from the South Carolina Patient Encounter database (SCPED; 2010–2018) obtained from the South Carolina Revenue and Fiscal Affairs Office. Detailed information about the SCPED and the data collection process has been described previously (31). Briefly, the SCPED serves as the central repository of all health and human services data in South Carolina, including claims and administrative data from all hospital inpatient and outpatient facilities within the state. Every individual is assigned a unique identifier that is used to link across multiple providers and data sources and allows for unduplicated cases across the data systems care. This study was approved by the Clemson University Institutional Review Board (IRB2020-035).
Our study population included individuals aged 18 years or older who were admitted to an inpatient hospital or outpatient surgery facility in South Carolina for an index PAD encounter. PAD diagnosis was detected using ICD-9/10 codes (Supplementary material) and included individuals with claudication and chronic limb threatening ischemia A two-year lookback period was chosen to minimize misclassification of PAD diagnosis (32, 33). Individuals were followed for one year after index encounter. Patients who resided outside the state, had missing zip code, or for which socioeconomic distress could not be measured were excluded (7.3%; n = 6,559).
Socioeconomic distress was measured using the distressed communities index (DCI). Developed by the Economic Innovation Group, the DCI incorporates seven metrics to estimate socioeconomic distress of a community at the zip code level (34). A DCI score is available for every zip code with at least 500 individuals, which captures 99% of the US population. The metrics include no high school degree, housing vacancy rate, adults not working, poverty rate, mean income ratio, change in employment, and change in business establishments (Supplementary material). Each variable is equally weighted, compared to its nearest neighbors, and normalized to obtain a raw score (35). A score of 0 indicates that a community has no distress and a score of 100 indicates severe distress. Based on the distribution of the population by DCI score, a distressed community in this study was defined as a zip code with DCI score ≥40.
The primary outcome for this study was major limb amputation after index PAD encounter. Major limb amputation was defined as above or below the knee amputation, with toe and forefoot amputations excluded, and identified using ICD-9/10 procedural codes (Supplementary material).
Relevant covariates were identified a priori based on prior knowledge, published literature, and data availability. Demographics include age at index encounter, gender, and race (black/white). Payor was categorized as Medicare, Medicaid, Self-Pay, Private, and Other (HMO, Charitable/Indigent Organization, Worker’s Compensation). Clinical variables were identified during the two-year look period and at the index encounter using ICD 9/10 codes (Supplementary material). These variables included diabetes, renal failure, chronic obstructive pulmonary disease (COPD), congestive heart failure (CHF), coronary artery disease (CAD), chronic limb threatening ischemia (CLTI), and the Charlson comorbidity index (CCI).
We compared the distribution of demographic characteristics, comorbid conditions, and study outcomes across DCI groups. Normally distributed continuous variables were reported as mean ± standard deviation and statistical differences were identified using independent t-test. Non-normally distributed continuous variables were reported as median and interquartile range and statistical differences were identified using the Mann-Whitney test. Categorical variables were presented as proportions and the chi-square test was performed to identify differences across DCI groups.
Individuals were followed from index PAD encounter until the first occurrence of major amputation or death (a competing event, which precludes the occurrence of major amputation). Data was administratively censored at one year. We presented crude cumulative incidence curves of amputation free survival, stratified by DCI group, to describe the differences in amputation outcomes after index PAD encounter (36). Differences between DCI groups were assessed using Gray’s test (37).
We used Fine and Gray subdistribution proportional hazards models to calculate subdistribution hazard ratios (sdHR) for the association between DCI score and major amputation, accounting for death as a competing event and adjusting for the independent variables listed above (38). An interaction term for race and DCI score was added to the model, but found to be non-significant; therefore, we report only the results of the model without the interaction term. In sensitivity analysis, the DCI score was scaled by 25 so that every 1-point change represents a 25-point (quartile) increase in the DCI (28).
All analyses were performed using SAS version 9.4 software (SAS Institute, Cary, NC), with a P < 0.05 set as statistically significant.
We identified 82,848 individuals who had an index PAD encounter at an inpatient hospital or outpatient surgery facility in South Carolina from 2012 to 2017. There were 57,196 (69%) individuals who resided in distressed communities and 25,652 (31%) who resided in non-distressed communities. The majority of the cohort were white (70%) and male (53%) with a mean age of 67 ± 14.1 years (Table 1). Individuals from non-distressed communities were more likely to be older, white, and have Medicare or Private insurance. Individuals from distressed communities were more likely to have diagnosed comorbidities, including diabetes, rental failure, COPD, and CHF.
Among all PAD individuals, one-year cumulative incidence of major amputation was 3.5% (95% CI: 3.3%, 3.6%). The cumulative incidence of major amputation was significantly greater in individuals from distressed communities (3.9%; 95% CI: 3.8%, 4.1%) compared to non-distressed communities (2.4%; 95% CI: 2.2%, 2.6%; Figure 1). Among White individuals, incidence of major amputation ranged from 1.8% (95% CI: 1.7%, 2.0%) in non-distressed communities to 2.2% (95% CI: 2.0%, 2.3%) in distressed communities (Figure 2). Among Black individuals, incidence of major amputation was 5.3% (95% CI: 4.6%, 6.0%) in non-distressed communities and 7.1% (95% CI: 6.8%, 7.5%) in distressed communities.
After adjusting for demographic and clinical covariates, individuals who lived in distressed communities had a hazard of major amputation 1.25 (95% CI: 1.14, 1.37) times the hazard among individuals in a non-distressed community (Figure 3). Among White individuals, the sdHR comparing individuals in distressed communities to non-distressed communities was 1.14 (95% CI: 1.01, 1.29). Black individuals who lived in distress communities had an adjusted hazard of major amputation 1.36 (95% CI: 1.18, 1.57) times the hazard of Black individuals living in non-distressed communities. Similar results were seen in the sensitivity analysis when the DCI score was scaled by 25. The sdHR comparing individuals in distressed communities to non-distressed communities was 1.09 (95% CI: 1.05, 1.13) in the overall population, 1.07 (95% CI: 1.02, 1.13) in White individuals, and 1.09 (95% CI: 1.04, 1.14) in Black individuals.
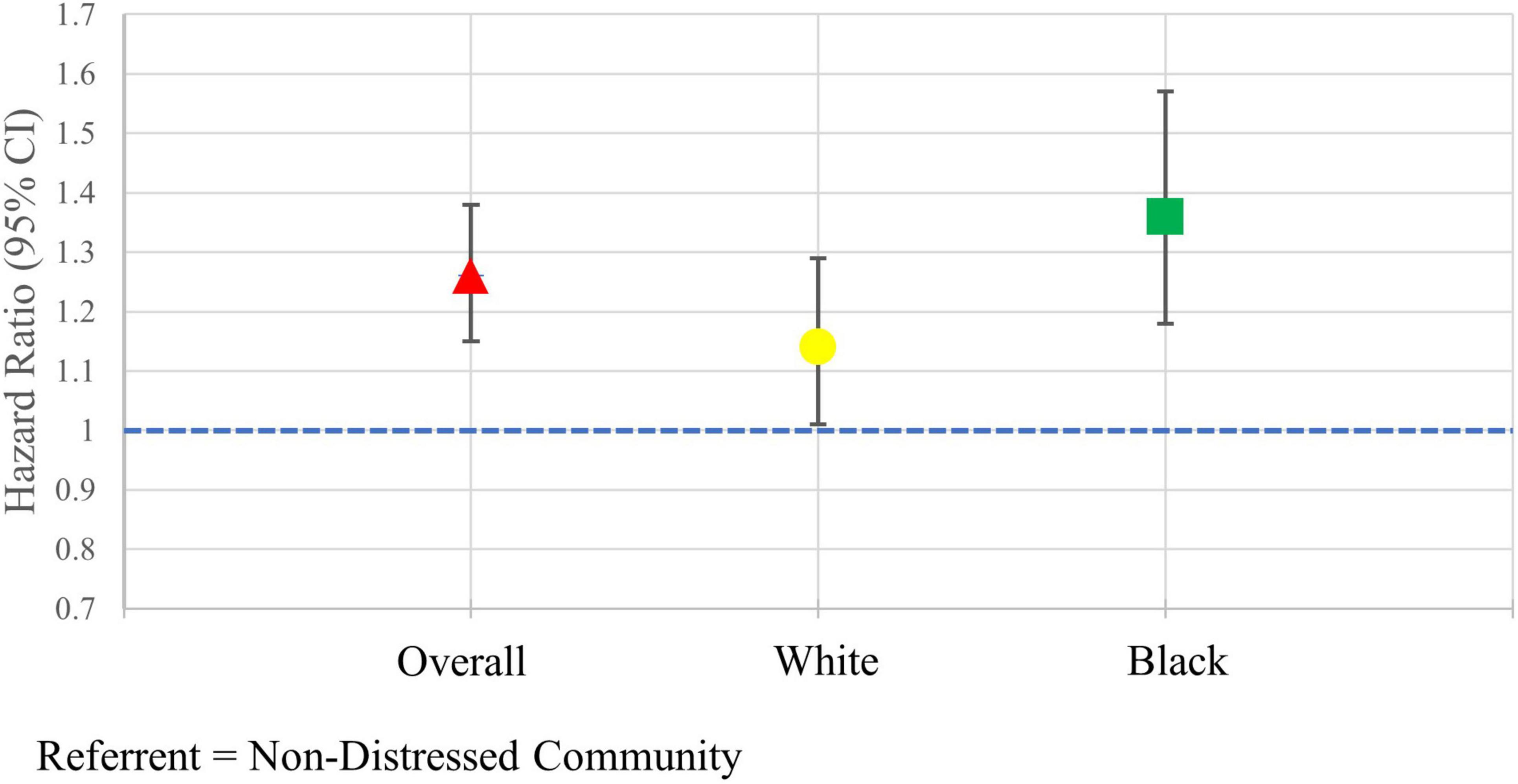
Figure 3. Adjusted hazard ratios of major amputation in the overall population and stratified by race.
In our study of more than 80,000 individuals with PAD, we found that DCI score is independently predictive of limb amputation after controlling for demographic characteristics and clinical comorbidities. The increased amputation risk was found across racial strata indicating the importance of an individual’s community for all races. The increased risk was greater in Black individuals than White individuals. Additionally, we also showed that Black individuals had higher incidence of amputation, even in non-distressed communities, than White individuals had in distressed communities. Race continues to be an important predictor of amputation risk among individuals with PAD, even after controlling for SES. Future studies should incorporate both race and SES to avoid missing important differences in outcomes when race is used as a proxy for SES.
Socioeconomic disparities among individuals with PAD have been well documented (39). Individuals with lower SES are disproportionately affected by PAD risk factors (40), have a higher risk of being hospitalized for PAD (20), and have worse outcomes (18). However, previous studies often focus more on individual level SES and may miss the important effects of where patients live. Our study contributes to a growing body of evidence that suggests that community-level socioeconomic characteristics also play a critical role in individuals with PAD (21, 28). Our findings confirm results from a recent study among veterans with PAD, extending these findings to highlight the importance of community-level socioeconomic disparities in amputation risks among different PAD populations (21). Furthermore, our study found that these community-level disparities persisted across racial groups, even after controlling for insurance status, which is an important determinant in access to health care. Our findings confirm that both individual-level and community-level SES are important factors in health disparities among individuals with PAD.
There are several possible mechanisms behind the association between community-level SES and amputation. Living in a distressed community exerts psychosocial stress on individuals due to high poverty, high unemployment, high crime, limited green space, and lack of access to nutritional food, amongst other contributing factors (39, 41, 42). Cumulative stress in these individuals correlates with the development and progression of atherosclerosis in the lower extremities, which is the main cause of PAD (43, 44). Furthermore, compared to non-distressed communities, distressed communities often have limited access to care and less availability of health care resources, such as vascular specialty care, that make it difficult to receive timely diagnosis and up-to-date management of PAD (28). Taken together, individuals that live in distressed communities are more likely to develop PAD due to cumulative stress, but less likely to have the necessary resources to manage it.
We identified a racial disparity in amputation risk among individuals with PAD, a finding that has been documented for decades (29, 45, 46). Racial disparities are believed to be due to differences in SES, insurance coverage, disease severity at presentation, and comorbid conditions (24). However, even after adjusting for these potential factors, we still observed that race was a major independent predictor of amputation. Further, we found that Black individuals in non-distressed communities had higher rates of amputation compared to White individuals in distressed communities. These findings indicate that regardless of the community they reside in, Black individuals are at higher risk of worse limb outcomes than Whites. Provider bias in clinical decision making and medical management of PAD has been explored in recent studies as a potential contributing factor in racial disparities (1, 21, 24). Although provider bias may partially explain the persistent differences in amputations rates among races, we are not able to properly explore this potential factor due to data limitations.
Structural racism has been identified as an important contributor to health disparities in the United States (47). Historically, racial minorities, particularly Black individuals, have been segregated to less-desirable neighborhoods that have higher concentrations of poverty. In these areas, Black individuals are exposed to more air pollution, have fewer job and educational opportunities, and have lower access to green space (48). As a result, it is more difficult for these individuals to practice healthy behaviors, such as adequate physical activity and proper nutrition, that can help prevent PAD. Living in these distressed neighborhoods also decreases their access to primary care, specialty care, and pharmacy services, which are necessary to manage chronic conditions that are comorbidities of PAD (16). Structural racism is particularly relevant in our study, which is based on data from South Carolina. We found that the proportion of Black individuals that reside in distressed communities is almost double that of non-distressed communities. Future analysis will explore the impact of structural racism through the inclusion of index of concentrations (ICE) at the zip code level (49).
Addressing the racial and socioeconomic disparities in amputation risk among individuals with PAD will need to involve coordinated efforts across multiple stakeholders (50). Our findings suggest that interventions need to be targeted on distressed communities, particularly those with a large Black population. Policy makers should invest in the development and implementation of community-based interventions that increase awareness of PAD and promote healthy behaviors. Community screening programs and exercise programs have proven to be effective in identifying individuals with PAD and improving functionality among these individuals (51, 52). Additionally, community and patient engagement interventions have proven to be effective in decreasing racial disparities in amputations among individuals with diabetes and may be adapted to the address such disparities in PAD (53).
There are several limitations in the study that should be noted. Our study is based on administrative claims data, which is subject to errors, inaccuracies, and discrepancies in coding practices (54). Residual confounding due to undocumented and undiagnosed relevant medical conditions can bias results. It is also possible that some of socioeconomic disparity found in this study is due to variations in hospital related practices and not the community level SES. Furthermore, this study assessed community-level SES using DCI scores at the zip code level. The DCI scores are only calculated for zip codes with > 500 people and were not available for every individual. Finally, although the DCI score is an accurate assessment of community-level SES, not every individual within a given zip code will be impacted the same way. Individuals with a high individual-level SES may be able to overcome the impacts of lower community-level SES.
Individuals who reside in distressed communities had increased risk for amputation, even after adjusting for race and clinical conditions. Race continues to be an important predictor of amputation, with Black individuals having higher rates of amputation than White individuals, regardless of the SES of their communities.
The data analyzed in this study is subject to the following licenses/restrictions: The datasets analyzed for this study were obtained from the South Carolina Revenue and Fiscal Affairs Office and are available from the authors with the permission of the South Carolina Revenue and Fiscal Affairs Office. Requests to access these datasets should be directed to https://rfa.sc.gov/boards-committees/dataoversight.
The studies involving human participants were reviewed and approved by Clemson University Institutional Review Board (IRB2020-035). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
BW, BH, CAK, LS, and RM: conceptualization. BW and CAK: methodology and formal analysis. BW: software and writing—original draft preparation. CAK, BH, LS, and RM: supervision and writing—review and editing. All authors have read and agreed to the published version of the manuscript.
CAK was funded by Career Development Awards from the American Heart Association (19CDA34760135) and National Institute of Health/National Heart Lung and Blood Institute (K01HL146900). BW and BH effort was supported by NIH NIGMS 2U54GM104942-07 and NIH NIMHD RADx-Up 1U01MD017419-01.
We thank the staff of the South Carolina Revenue and Fiscal Affairs Office for their important contributions.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.1021692/full#supplementary-material
1. Hackler IIIEL, Hamburg NM, White Solaru KT. Racial and ethnic disparities in peripheral artery disease. Circ Res. (2021) 128:1913–26. doi: 10.1161/CIRCRESAHA.121.318243
2. Allison MA, Ho E, Denenberg JO, Langer RD, Newman AB, Fabsitz RR, et al. Ethnic-specific prevalence of peripheral arterial disease in the United States. Am J Prev Med. (2007) 32:328–33. doi: 10.1016/j.amepre.2006.12.010
3. Fowkes FGR, Rudan D, Rudan I, Aboyans V, Denenberg JO, McDermott MM, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. (2013) 382:1329–40. doi: 10.1016/S0140-6736(13)61249-0
4. Rudofker EW, Hogan SE, Armstrong EJ. Preventing major amputations in patients with critical limb ischemia. Curr Cardiol Rep. (2018) 20:1–7. doi: 10.1007/s11886-018-1019-2
5. Kalbaugh CA, Taylor SM, Blackhurst DW, Dellinger MB, Trent EA, Youkey JR. One-year prospective quality-of-life outcomes in patients treated with angioplasty for symptomatic peripheral arterial disease. J Vasc Surg. (2006) 44:296–303. doi: 10.1016/j.jvs.2006.04.045
6. Breek JC, De Vries J, Van Heck GL, van Berge Henegouwen DP, Hamming JF. Assessment of disease impact in patients with intermittent claudication: discrepancy between health status and quality of life. J Vasc Surg. (2005) 41:443–50. doi: 10.1016/j.jvs.2004.12.042
7. McDermott MM. Epidemiology and clinical significance. Cleve Clin J Med. (2006) 73:S3. doi: 10.3949/ccjm.73.Suppl_4.S2
8. Pell JP. Impact of intermittent claudication on quality of life. Eur J Vasc Endovasc Surg. (1995) 9:469–72. doi: 10.1016/S1078-5884(05)80018-8
9. Stoyioglou A, Jaff MR. Medical treatment of peripheral arterial disease: a comprehensive review. J Vasc Interv Radiol. (2004) 15:1197–207. doi: 10.1097/01.RVI.0000137978.15352.C6
10. Bradbury AW, Ruckley CV, Fowkes F, Forbes JF, Gillespie I, Adam DJ. Bypass versus angioplasty in severe ischaemia of the leg (BASIL): multicentre, randomised controlled trial. Lancet. (2005) 366:1925–34. doi: 10.1016/S0140-6736(05)67704-5
11. Jones WS, Patel MR, Dai D, Vemulapalli S, Subherwal S, Stafford J, et al. High mortality risks after major lower extremity amputation in Medicare patients with peripheral artery disease. Am Heart J. (2013) 165:809–15. doi: 10.1016/j.ahj.2012.12.002
12. Diehm C, Allenberg JR, Pittrow D, Mahn M, Tepohl G, Haberl RL, et al. Mortality and vascular morbidity in older adults with asymptomatic versus symptomatic peripheral artery disease. Circulation. (2009) 120:2053–61. doi: 10.1161/CIRCULATIONAHA.109.865600
13. Swaminathan A, Vemulapalli S, Patel MR, Jones WS. Lower extremity amputation in peripheral artery disease: improving patient outcomes. Vasc Health Risk Manag. (2014) 10:417. doi: 10.2147/VHRM.S50588
14. Torbjörnsson E, Ottosson C, Blomgren L, Boström L, Fagerdahl A. The patient’s experience of amputation due to peripheral arterial disease. J Vasc Nurs. (2017) 35:57–63. doi: 10.1016/j.jvn.2016.11.002
15. Creager MA, Matsushita K, Arya S, Beckman JA, Duval S, Goodney PP, et al. Reducing nontraumatic lower-extremity amputations by 20% by 2030: time to get to our feet: a policy statement from the American Heart Association. Circulation. (2021) 143:e875–91. doi: 10.1161/CIR.0000000000000967
16. Fanaroff AC, Yang L, Nathan AS, Khatana SAM, Julien H, Wang TY, et al. Geographic and socioeconomic disparities in major lower extremity amputation rates in metropolitan areas. J Am Heart Assoc. (2021) 10:e021456. doi: 10.1161/JAHA.121.021456
17. Arpey NC, Gaglioti AH, Rosenbaum ME. How socioeconomic status affects patient perceptions of health care: a qualitative study. J Prim Care Commun Health. (2017) 8:169–75. doi: 10.1177/2150131917697439
18. Henry AJ, Hevelone ND, Belkin M, Nguyen LL. Socioeconomic and hospital-related predictors of amputation for critical limb ischemia. J Vasc Surg. (2011) 53:330–9. doi: 10.1016/j.jvs.2010.08.077
19. Regenbogen SE, Gawande AA, Lipsitz SR, Greenberg CC, Jha AK. Do differences in hospital and surgeon quality explain racial disparities in lower-extremity vascular amputations? Ann Surg. (2009) 250:424–31. doi: 10.1097/SLA.0b013e3181b41d53
20. Vart P, Coresh J, Kwak L, Ballew SH, Heiss G, Matsushita K. Socioeconomic status and incidence of hospitalization with lower-extremity peripheral artery disease: Atherosclerosis Risk in Communities study. J Am Heart Assoc. (2017) 6:e004995. doi: 10.1161/JAHA.116.004995
21. Arya S, Binney Z, Khakharia A, Brewster LP, Goodney P, Patzer R, et al. Race and socioeconomic status independently affect risk of major amputation in peripheral artery disease. J Am Heart Assoc. (2018) 7:e007425. doi: 10.1161/JAHA.117.007425
22. Kalbaugh CA, Kucharska-Newton A, Wruck L, Lund JL, Selvin E, Matsushita K, et al. Peripheral artery disease prevalence and incidence estimated from both outpatient and inpatient settings among medicare fee-for-service beneficiaries in the atherosclerosis risk in communities (ARIC) study. J Am Heart Assoc. (2017) 6:e003796. doi: 10.1161/JAHA.116.003796
23. Newhall K, Spangler E, Dzebisashvili N, Goodman DC, Goodney P. Amputation rates for patients with diabetes and peripheral arterial disease: the effects of race and region. Ann Vasc Surg. (2016) 30:292–8. doi: 10.1016/j.avsg.2015.07.040
24. Holman KH, Henke PK, Dimick JB, Birkmeyer JD. Racial disparities in the use of revascularization before leg amputation in Medicare patients. J Vasc Surg. (2011) 54:420–6. doi: 10.1016/j.jvs.2011.02.035
25. Durazzo TS, Frencher S, Gusberg R. Influence of race on the management of lower extremity ischemia: revascularization vs amputation. JAMA Surg. (2013) 148:617–23. doi: 10.1001/jamasurg.2013.1436
26. Williams DR, Priest N, Anderson NB. Understanding associations among race, socioeconomic status, and health: Patterns and prospects. Health Psychol. (2016) 35:407. doi: 10.1037/hea0000242
27. Hughes K, Mota L, Nunez M, Sehgal N, Ortega G. The effect of income and insurance on the likelihood of major leg amputation. J Vasc Surg. (2019) 70:580–7. doi: 10.1016/j.jvs.2018.11.028
28. Hawkins RB, Charles EJ, Mehaffey JH, Williams CA, Robinson WP, Upchurch GR, et al. Socioeconomic Distressed Communities Index associated with worse limb-related outcomes after infrainguinal bypass. J Vasc Surg. (2019) 70:786–94. doi: 10.1016/j.jvs.2018.10.123
29. Eslami MH, Zayaruzny M, Fitzgerald GA. The adverse effects of race, insurance status, and low income on the rate of amputation in patients presenting with lower extremity ischemia. J Vasc Surg. (2007) 45:55–9. doi: 10.1016/j.jvs.2006.09.044
30. Mehaffey JH, Hawkins RB, Charles EJ, Turrentine FE, Hallowell PT, Friel C, et al. Socioeconomic “Distressed Communities Index” improves surgical risk-adjustment. Ann Surg. (2020) 271:470–4. doi: 10.1097/SLA.0000000000002997
31. South Carolina Data Oversight Council,. Principles and protocol for the release of health care data. Columbia, SC: South Carolina Data Oversight Council (2014).
32. Kalbaugh CA, Loehr L, Wruck L, Lund JL, Matsushita K, Bengtson LG, et al. Frequency of care and mortality following an incident diagnosis of peripheral artery disease in the inpatient or outpatient setting: the ARIC (Atherosclerosis Risk in Communities) study. J Am Heart Assoc. (2018) 7:e007332. doi: 10.1161/JAHA.117.007332
33. Griffiths RI, O’Malley CD, Herbert RJ, Danese MD. Misclassification of incident conditions using claims data: impact of varying the period used to exclude pre-existing disease. BMC Med Res Methodol. (2013) 13:32. doi: 10.1186/1471-2288-13-32
34. Economic Innovation Group,. Distressed Communities Index. (2022). Available online at: https://eig.org/dci (accessed March 30, 2022).
35. Economic Innovation Group,. Methodology. (2022). Available online at: https://eig.org/dci/methodology (accessed March 30, 2022).
36. Austin PC, Lee DS, Fine JP. Introduction to the analysis of survival data in the presence of competing risks. Circulation. (2016) 133:601–9. doi: 10.1161/CIRCULATIONAHA.115.017719
37. Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. (1988) 16:1141–54. doi: 10.1214/aos/1176350951
38. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Statist Assoc. (1999) 94:496–509. doi: 10.1080/01621459.1999.10474144
39. Pande RL, Creager MA. Socioeconomic inequality and peripheral artery disease prevalence in US adults. Circulation: Cardiovascular Quality and Outcomes. (2014) 7:532–9. doi: 10.1161/CIRCOUTCOMES.113.000618
40. Kaplan GA, Keil JE. Socioeconomic factors and cardiovascular disease: a review of the literature. Circulation. (1993) 88:1973–98. doi: 10.1161/01.CIR.88.4.1973
41. Albert MA, Durazo EM, Slopen N, Zaslavsky AM, Buring JE, Silva T, et al. Cumulative psychological stress and cardiovascular disease risk in middle aged and older women: Rationale, design, and baseline characteristics. Am Heart J. (2017) 192:1–12. doi: 10.1016/j.ahj.2017.06.012
42. Theall KP, Drury SS, Shirtcliff EA. Cumulative neighborhood risk of psychosocial stress and allostatic load in adolescents. Am J Epidemiol. (2012) 176(suppl_7):S164–74. doi: 10.1093/aje/kws185
43. Meng L, Zhang Y, Luo Y, Gong T, Liu D. Chronic stress a potential suspect zero of atherosclerosis: A systematic review. Front Cardiovasc Med. (2021) 20:738654. doi: 10.3389/fcvm.2021.738654
44. Olin JW, Sealove BA. Peripheral artery disease: current insight into the disease and its diagnosis and management. Mayo Clin Proc. (2010) 85:678–92. doi: 10.4065/mcp.2010.0133
45. Rowe VL, Weaver FA, Lane JS, Etzioni DA. Racial and ethnic differences in patterns of treatment for acute peripheral arterial disease in the United States, 1998-2006. J Vasc Surg. (2010) 51:S21–6. doi: 10.1016/j.jvs.2009.09.066
46. Dillingham TR, Pezzin LE, MacKenzie EJ. Racial differences in the incidence of limb loss secondary to peripheral vascular disease: a population-based study. Arch Phys Med Rehabil. (2002) 83:1252–7. doi: 10.1053/apmr.2002.34805
47. Churchwell K, Elkind MS, Benjamin RM, Carson AP, Chang EK, Lawrence W, et al. Call to action: structural racism as a fundamental driver of health disparities: a presidential advisory from the American Heart Association. Circulation. (2020) 142:e454–68. doi: 10.1161/CIR.0000000000000936
48. Williams DR, Lawrence JA, Davis BA. Racism and health: evidence and needed research. Annu Rev Public Health. (2019) 40:105–25. doi: 10.1146/annurev-publhealth-040218-043750
49. Chambers BD, Baer RJ, McLemore MR, Jelliffe-Pawlowski LL. Using index of concentration at the extremes as indicators of structural racism to evaluate the association with preterm birth and infant mortality—California, 2011–2012. J Urban Health. (2019) 96:159–70. doi: 10.1007/s11524-018-0272-4
50. Derose KP, Gresenz CR, Ringel JS. Understanding disparities in health care access—and reducing them—through a focus on public health. Health Aff. (2011) 30:1844–51. doi: 10.1377/hlthaff.2011.0644
51. Hirsch AT, Halverson SL, Treat-Jacobson D, Hotvedt PS, Lunzer MM, Krook S, et al. The Minnesota regional peripheral arterial disease screening program: toward a definition of community standards of care. Vasc Med. (2001) 6:87–96. doi: 10.1191/135886301701568756
52. Treat-Jacobson D, McDermott MM, Bronas UG, Campia U, Collins TC, Criqui MH, et al. Optimal exercise programs for patients with peripheral artery disease: a scientific statement from the American Heart Association. Circulation. (2019) 139:e10–33. doi: 10.1161/CIR.0000000000000623
53. Colleran K, Harding E, Kipp BJ, Zurawski A, MacMillan B, Jelinkova L, et al. Building capacity to reduce disparities in diabetes: training community health workers using an integrated distance learning model. Diabetes Educ. (2012) 38:386–96. doi: 10.1177/0145721712441523
Keywords: peripheral artery disease (PAD), amputation, distressed communities index (DCI), socioeconomic, health disparity
Citation: Witrick B, Shi L, Mayo R, Hendricks B and Kalbaugh CA (2022) The association between socioeconomic distress communities index and amputation among patients with peripheral artery disease. Front. Cardiovasc. Med. 9:1021692. doi: 10.3389/fcvm.2022.1021692
Received: 17 August 2022; Accepted: 17 October 2022;
Published: 03 November 2022.
Edited by:
Katharine L. McGinigle, University of North Carolina at Chapel Hill, United StatesReviewed by:
Yousef Shahin, The University of Sheffield, United KingdomCopyright © 2022 Witrick, Shi, Mayo, Hendricks and Kalbaugh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Brian Witrick, YnJpYW4ud2l0cmlja0Boc2Mud3Z1LmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.