- 1Department of Clinical and Molecular Medicine, Sapienza University of Rome, Rome, Italy
- 2Department of Cardiovascular and Pneumological Sciences, Catholic University of Sacred Heart, Rome, Italy
- 3Fondazione Policlinico Universitario Agostino Gemelli Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS), Rome, Italy
- 4Department of Translational and Precision Medicine, Sapienza University of Rome, Rome, Italy
- 5Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Neuromed, Pozzilli, Italy
- 6San Raffaele Pisana Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS), Rome, Italy
- 7Division of Cardiology, Sant’Andrea Hospital, Rome, Italy
Background: The tricuspid annular plane systolic excursion/systolic pulmonary arterial pressure (TAPSE/sPAP) ratio is an echocardiographic estimation of the right ventricle to pulmonary artery (RV/PA) coupling, with a validated prognostic role in different clinical settings. Systemic sclerosis (SSc) patients without evident cardiovascular involvement frequently display subtle RV impairment. The amino-terminal atrial natriuretic peptide (NT-proANP) plasma level relates to SSc disease progression and mortality. We aimed to assess the prognostic value of the TAPSE/sPAP ratio and its relationship with NT-proANP plasma level in SSc patients without overt cardiovascular involvement.
Methods: We retrospectively analysed 70 SSc consecutive patients, with no clinical evidence of cardiovascular involvement or pulmonary hypertension (PH), and 30 healthy controls (HC) in a retrospective, single-centre study. All SSc patients underwent recurrent clinical and echocardiographic assessments and NT-proANP plasma level was assessed at baseline. SSc-related cardiovascular events and deaths were extracted during a 6-year follow-up. The complete work-up for the diagnosis, treatment and management of PH performed along the 6 years of follow-up referred to the 2015 European Society of Cardiology guidelines.
Results: Systemic sclerosis patients showed lower TAPSE/sPAP ratio at baseline compared to HC [SSc median value = 0.71 mm/mmHg, (IQR 0.62–0.88) vs. HC median value = 1.00 mm/mmHg, (IQR 0.96–1.05); p < 0.001]. Multivariable Cox analysis revealed TAPSE/sPAP ratio as an independent predictor for SSc-related cardiovascular events [HR = 3.436 (95% CI 1.577–7.448); p = 0.002] and mortality [HR = 3.653 (95% CI 1.712–8.892); p = 0.014]. The value of TAPSE/sPAP ratio < 0.7 mm/mmHg was identified as an optimal cut-off for predicting adverse outcomes (p < 0.001) by receiver operating characteristic (ROC) analyses. NT-proANP level significantly related to TAPSE/sPAP ratio (r = 0.52, p < 0.001). TAPSE/sPAP ratio combined with NT-proANP showed an overall significant prognostic role in this SSc population, confirmed by Kaplan–Meier analysis (Log rank p < 0.001).
Conclusion: The TAPSE/sPAP ratio, as an index of RV/PA coupling, is an affordable predictor of cardiovascular events and mortality in SSc and, combined with NT-proANP level, may improve the clinical phenotyping and prognostic stratification of SSc patients.
1. Introduction
Systemic sclerosis (SSc) is an autoimmune disease with a multisystem involvement characterised by widespread inflammation and microvascular alterations leading to ischemia and fibrosis (1, 2), particularly affecting the heart and lungs. Interstitial lung disease (ILD) and cardiovascular injuries are the most frequent complications, with a consequent severe impact on prognosis (3–5). Clinical evidence of cardiovascular disease may be found in 20–35% of patients with SSc, whereas the heart is affected in up to 80% of patients at post-mortem examination (6, 7).
Primary cardiac manifestations of SSc, due to myocardial fibrosis, impaired microcirculation and vascular wall remodelling, can range from myocardial disease, right and left heart failure (HF), conduction system abnormalities and arrhythmias, to coronary and pericardial disease and aggressive atherosclerosis. The involvement of the pulmonary circulation can lead to pulmonary arterial hypertension (PAH), with the consequent poorest prognosis (8). Despite a normal echocardiographic assessment, SSc patients often go through a right ventricle (RV) occult dysfunction and latent pulmonary vascular disease and hypertension, eventually revealed by stress echocardiography and haemodynamic evaluation (9, 10). Therefore, the active screening for an early diagnosis of sub-clinical cardiovascular and cardio-pulmonary impairment might be essential for timely treatment and adequate long-term management of SSc patients.
The mechanical and energetic efficiency of the cardiovascular system is determined by the dynamic interaction between the heart and vessels. The anatomical and functional link between the RV and pulmonary arterial (PA) vascular district underscores the importance to consider RV and PA as one combined unit. The ventricular-arterial coupling, defined by the end-systolic ventricular elastance (Ees) and arterial elastance (Ea) ratio is a reliable index of cardiovascular performance, derived from the invasive measurement of the pressure-volume relationship (11). In spite of the wide range of parameters assessing cardiac and pulmonary properties and functions, only the RV/PA coupling is a sensitive index of the cardio-pulmonary unit function (11, 12).
Among simpler surrogates that are therefore being validated, the tricuspid annular plane systolic excursion/systolic pulmonary arterial pressure (TAPSE/sPAP) ratio is an echocardiographic estimation of the RV/PA coupling, well approximating the invasive gold standard measurement (12, 13).
Previous studies demonstrated that the perturbation of the RV/PA coupling is a sensitive and prognostic parameter in different clinical settings. Likewise, TAPSE/sPAP ratio has been shown to have prognostic relevance in PAH (14, 15), HF with and without pulmonary hypertension (PH) (16, 17), pulmonary embolism (18) and PH in chronic lung disease (19). Based on its clinical relevance, the TAPSE/sPAP ratio was recently added to routine assessment for the diagnosis and management of PH according to the 2022 ESC/ERS guidelines (20). Instead, the role of the TAPSE/sPAP ratio in SSc patients without overt cardiovascular involvement has been so far poorly investigated.
Atrial natriuretic peptide (ANP), which participates in the cardiovascular system homeostasis, is synthesised in the heart as a pre-pro-hormone, subsequently cleaved into the biologically carboxy-terminal active peptide and the amino-terminal peptide (NT-proANP), which is a more stable circulating form (21). ANP is mainly released by atrial cardiomyocytes in response to hemodynamic stressors, such as pressure and volume overload. ANP release kinetic is highly triggered by the haemodynamic load applied to the right atrium and pulmonary circulation, mainly under exercise (22, 23). Simultaneously, the same hemodynamic triggers for ANP secretion contribute to impair the ventricular performance and arterial stiffness, promoting the RV/PA uncoupling.
Atrial natriuretic peptide is also secreted from the endothelium in response to vessel injuries and it exerts protective functions in an autocrine/paracrine manner (24, 25). NT-proANP plasma level predicted disease progression and mortality in SSc patients (26, 27). Since ANP arises in response to both the hemodynamic changes promoting RV/PA uncoupling and to the endothelial injury, a typical feature of SSc pathogenesis and progression, the increase of NT-proANP plasma level might be an early marker of the cardio-pulmonary unit involvement in asymptomatic SSc patients.
The present study aimed to investigate the prognostic value of the TAPSE/sPAP ratio as an index of RV/PA coupling and the relevance of TAPSE/sPAP ratio alone and combined with NT-proANP plasma level for the prediction of adverse outcomes in SSc patients.
2. Materials and methods
2.1. Study population
We analysed seventy consecutive patients (sixty-four women and six men) already diagnosed with SSc according to the criteria of the American College of Rheumatology/European League against Rheumatism (28, 29) in a single-centre, retrospective study, from 2014 to 2020, at Sant’Andrea Hospital, Rome, Italy. Exclusion criteria were cardiovascular, pulmonary and renal diseases, malignancies, coagulopathy, diabetes, dyslipidaemia, and smoking habit. Pregnant or breastfeeding women were excluded. Patients with severe SSc-related ILD were excluded (30, 31) (Supplementary Figure 1). Thirty healthy volunteers [healthy controls (HC)] were enrolled as the control group, with homologous characteristics compared to SSc patients.
All SSc patients underwent complete evaluation, including clinical examination, electrocardiogram, echocardiography, 6-min walking test, nailfold video capillaroscopy, high-resolution chest tomography (HRTC), pulmonary function test, diffusion lung carbon oxide (DLCO), both at baseline and yearly (or more when needed) for the 6-year follow-up (30, 31). DLCO and DLCO/VA assessment were performed using carbon monoxide and helium as tracer gas (26, 27).
According to the international recommendation (32–34) all SSc patients underwent comprehensive echocardiography yearly. All patients presented at least a mild tricuspid regurgitation, committed for sPAP estimation. Right heart catheterization was performed for the diagnosis of PH, in accordance with the 2015 ESC/ERS guidelines (35), when indicated.
Plasma NT-proANP level measurement was performed at baseline in all patients with SSc. The study protocol was approved by the locally appointed ethical committee and written informed consent was obtained from all participants, complied with the Declaration of Helsinki.
2.2. Outcomes
The primary composite outcome was the occurrence of all cardiovascular events referring to the cardiovascular manifestation of SSc, during the 6-year follow-up. The cardiovascular manifestations of SSc were defined as new diagnosis of PH (35), PAH (35), HF (36) requiring or not hospitalisation, arrhythmias and conduction disturbances, new onset of ischaemic heart disease and pericardial effusion. The secondary outcome was the SSc-related death occurring during the 6-year follow-up. Mortality for SSc was defined as death due to complications of ILD, PAH, PH, and HF.
2.3. Assessment of NT-proANP plasma level
Venous blood samples (3 ml) were collected from all SSc patients in EDTA tubes and centrifuged at 3000 rpm for 15 minutes. Measurements of the plasma level of NT-proANP were performed using an ELISA kit (Biomedica, Wien, Austria). The cut-off of normal value for NT-proANP was considered 2200 fmol/ml.
2.4. Echocardiography
Baseline echocardiographic data were acquired using commercially available equipment and standard views, in accordance with the international guidelines (35–38). For each patient, simple parameters and derived measures were obtained and recorded at baseline and during the 6-year follow-up. All echocardiographic data were performed by a board-certified cardiologist echocardiographer and independently reviewed by a board-certified echocardiographer blinded to the outcomes. Right ventricular outflow tract (RVOT) diameter was obtained in 2D long-axis parasternal view as a linear dimension measured from the anterior RV wall to the interventricular septal-aortic junction. RVOT Doppler acceleration time was measured from the beginning of the flow to the peak flow velocity. Proximal diameter of the main pulmonary artery was measured in 2D short axis view to evaluate dilatation. TAPSE was measured on M-mode images as the difference in RV basal motion from peak systole to end-diastole. RV and left ventricle (LV) diameters were measured at end-diastole in the four-chamber view to calculate RV/LV ratio. The tricuspid regurgitation (TR) severity was classified as mild, moderate, or severe. The maximal tricuspid regurgitation velocity (TRV) by continuous-wave Doppler was used to derive the RV–right atrial (RA) pressure gradient by the simplified Bernoulli equation. RA pressure (RAP) was estimated based on the inferior vena cava (IVC) end-expiratory diameter and collapsibility at the end-inspiratory phase, as recommended (38). The peak sPAP was assessed by adding the estimated RAP to the systolic trans-tricuspid pressure gradient. The TAPSE/sPAP ratio was then calculated for all patients and healthy subjects.
2.5. Statistical analysis
Continuous distributed variables were represented as median and interquartile range (IQR) and categorical data were expressed as frequencies. Data were analysed for normality by the coefficient of kurtosis and by the Shapiro–Wilk test. Appropriate statistical parametric and non-parametric (Student’s t, Mann–Whitney U, X2) tests were used in the different analyses. Receiver operating characteristic (ROC) analyses and the Youden Index were performed to test the TAPSE/sPAP ratio in the prediction of outcomes and to determine the optimal cut-off value. Univariable and multivariable Cox regression models were performed to assess the prognostic relevance of different variables to outcomes.
To avoid collinearity and overfitting, the multivariable Cox model included the following continuous variables: age, DLCO, TAPSE/sPAP ratio, and NT-proANP for both primary and secondary outcomes.
A reduced model by backward stepwise multivariable regression analysis was used to detect independent predictors. Linear correlation was assessed by Spearman’s coefficient.
Kaplan–Meier analysis was used to evaluate overall survival and events recurrence, with the log rank test for comparison. The subjects were censored after any of the composite outcomes were reached.
To evaluate the incremental value of TAPSE/sPAP and NT-proANP plasma level, two models were compared: model 1 as TAPSE/sPAP alone and model 2 as TAPSE/sPAP ratio combined with NT-proANP. Global X2 and incremental significance of these models were calculated. The strength of independent predictors was compared using the concordance index (C-index). Akaike information criterion (AIC) method was added for the comparison of model fit (Supplementary Table 1).
A two-sided p-value < 0.05 was considered statistically significant in all analyses. Coefficients obtained from regression models were expressed in terms of hazard ratio (HR) with 95% confidence intervals (CI). All statistical analyses were performed using SPSS software (version 27.0, IBM) and Prism GraphPad (version 8.1).
3. Results
3.1. Baseline characteristics, outcomes, and TAPSE/sPAP ratio distribution
Seventy SSc patients (sixty-four women and six men) without overt cardiovascular involvement were enrolled in this study. The majority of the study population (67%) presented with the limited cutaneous subtype of SSc while the remaining 33% manifested the diffuse cutaneous systemic scleroderma subtype. Demographic, clinical, and echocardiographic characteristics of the SSc population and controls are reported in Table 1.
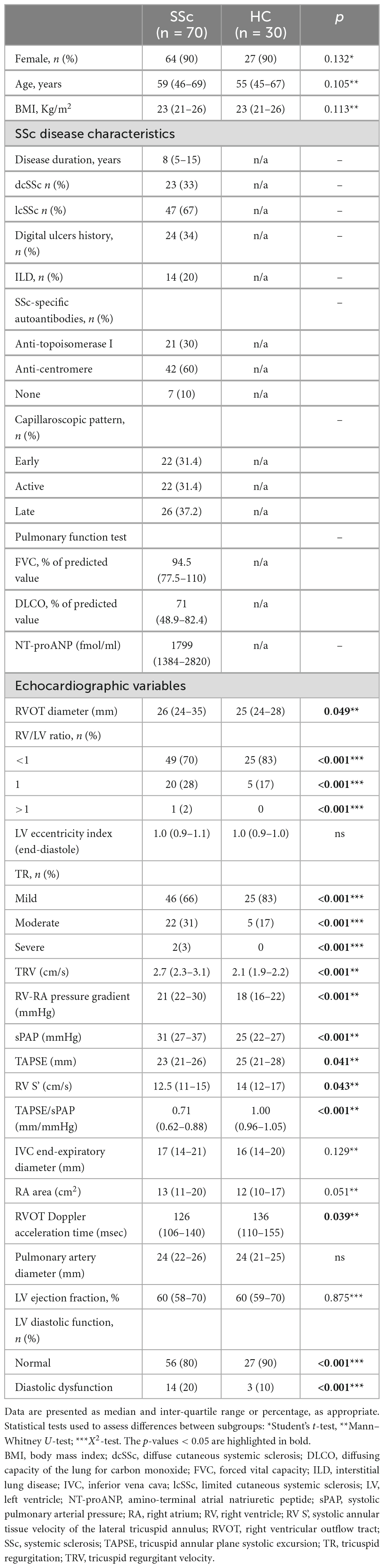
Table 1. Baseline demographic, clinical, and echocardiographic characteristics of the SSc population and healthy individuals (HC).
During the 6 years of follow-up, thirty-six patients (51% of the study population) experienced an SSc-related cardiovascular event. We reported six new PAH diagnoses (17%) and seven new diagnoses of right HF (19%), four of them requiring hospitalisations. Ten patients (28%) experienced arrhythmias (seven cases of documented supraventricular tachycardia, two cases of documented ventricular tachycardia, and one case of atrioventricular block requiring a pacemaker implantation). Seven patients (19%) had angina without evidence of coronary artery disease and six patients (17%) experienced more than mild pericardial effusion, one of them requiring pericardiocentesis (Supplementary Figure 2A).
The diagnosis of PH and PAH was performed according to the current 2015 ESC/ERS guidelines (35), during the 6-year follow-up. However, by applying the last 2022 ESC/ERS guidelines and the new cut-off for the diagnosis of PH (20), our patients fulfil the inclusion criteria and there would be no difference on the primary outcome.
Out of the seventy SSc patients included in our study, we reported sixteen SSc-related deaths (23%): nine (56%) were attributed to ILD complications, four (25%) to PAH progression and three (19%) to HF-related fatalities (Supplementary Figure 2B). These outcomes are concordant with those reported in the international SSc registries (3–5).
Systemic sclerosis patients showed lower values of the TAPSE/sPAP ratio compared to controls at baseline [SSc median value = 0.71 mm/mmHg, (IQR 0.62–0.88) vs. HC median value = 1.00 mm/mmHg, (IQR 0.96–1.05); p < 0.001] (Table 1).
The distribution of the TAPSE/sPAP ratio in the SSc population at baseline is reported in Figure 1.
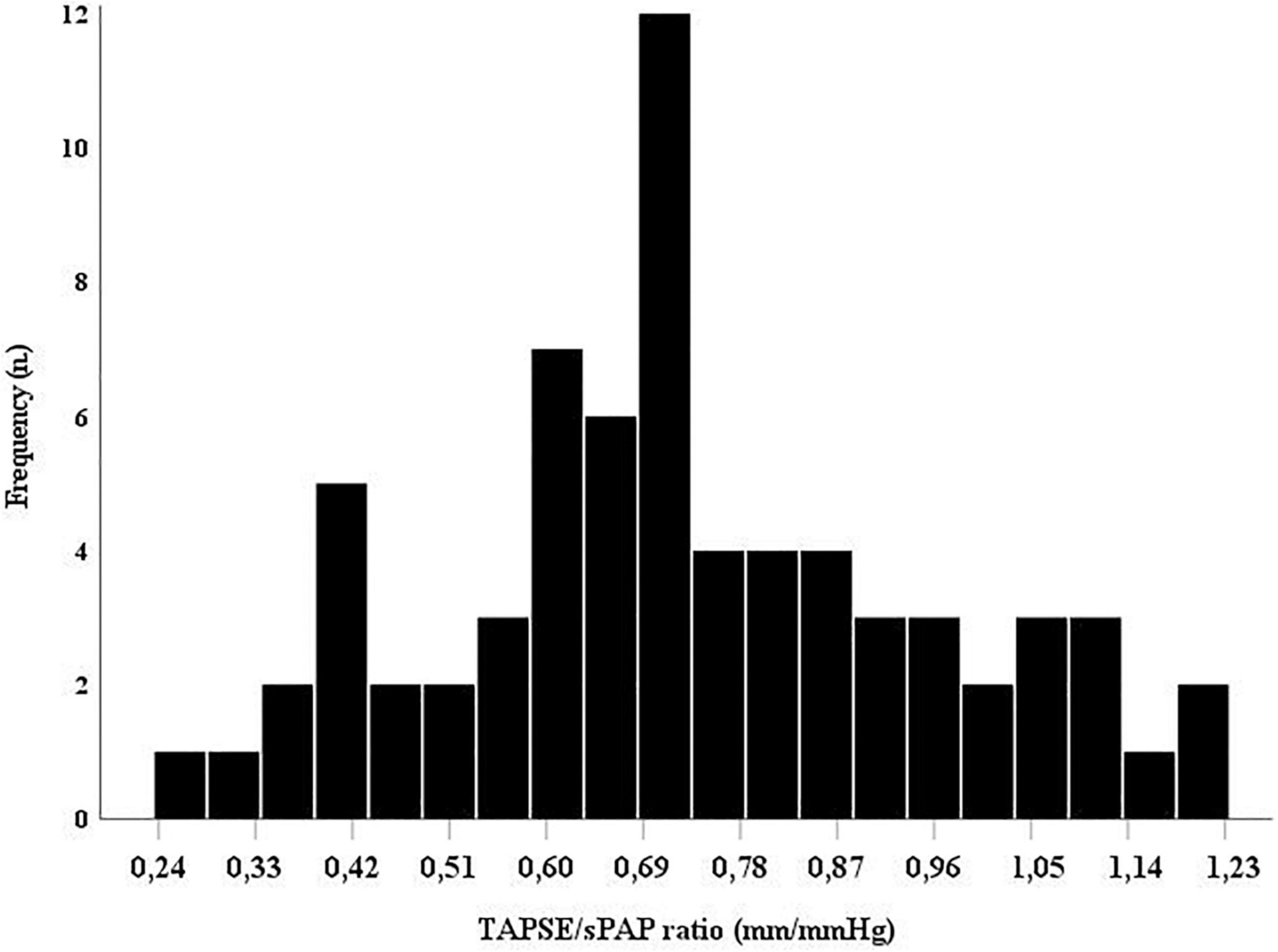
Figure 1. The TAPSE/sPAP ratio distribution in this population of systemic sclerosis (SSc) patients. Histogram showing the overall TAPSE/sPAP ratio distribution in the SSc population at baseline. sPAP, systolic pulmonary arterial pressure; TAPSE, tricuspid annular plane systolic excursion.
The median value of the TAPSE/sPAP ratio at baseline was significantly lower in SSc patients facing primary outcome vs. SSc patients without cardiovascular events [0.62 (0.47–0.74) vs. 0.77 (0.71–0.95) mm/mmHg; p < 0.001] on 6-year follow-up.
According to the secondary outcome, the baseline TAPSE/sPAP ratio was significantly lower in patients who died for SSc-related causes in comparison with survived patients [0.51 (0.41–0.62) vs. 0.76 (0.68–0.95) mm/mmHg; p < 0.001].
3.2. Receiver operating characteristic (ROC) analysis for the TAPSE/sPAP ratio and outcomes
In accordance with the ROC analysis, the value of TAPSE/sPAP ratio < 0.7 mm/mmHg resulted as a significant predictor of both primary [sensitivity = 82%, specificity = 70%, AUC 0.748 (95% CI 0.628–0.868); p < 0.001] and secondary [sensitivity = 70%, specificity = 94%, AUC 0.874 (95% CI 0.788–0.959); p < 0.001] outcomes (Figures 2A, B, respectively).
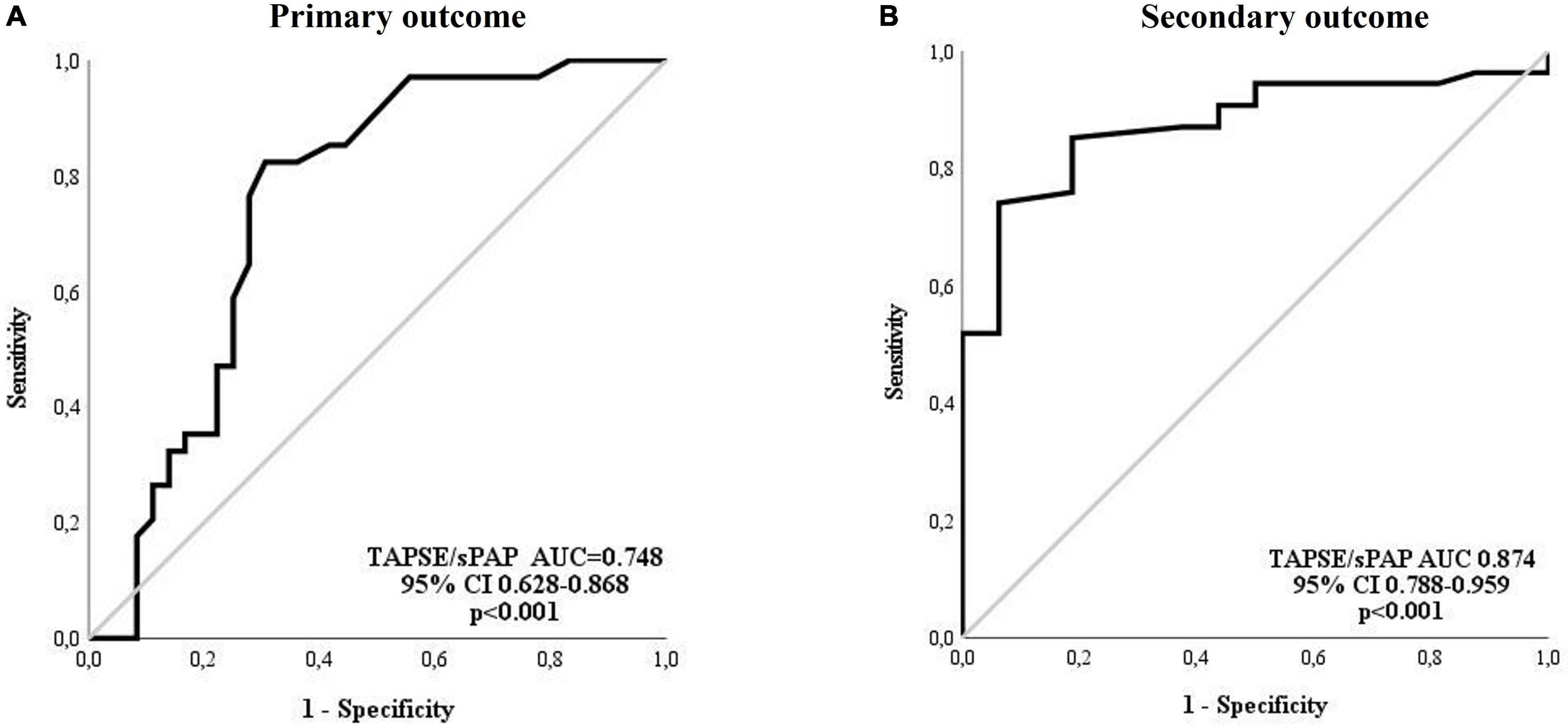
Figure 2. Receiver operating characteristic (ROC) analysis for the TAPSE/sPAP ratio and outcomes. (A) Primary outcome: The TAPSE/sPAP ratio had an AUC of 0.748 (95% CI 0.628–0.868; p < 0.001) for the prediction of cardiovascular events (sensitivity 82% and specificity 70%). (B) Secondary outcome: The TAPSE/sPAP ratio had an AUC of 0.874 (95% CI 0.788–0.959; p < 0.001) for the prediction of death (sensitivity 70% and specificity 94%). AUC, area under the curve; CI, confidence interval; sPAP, systolic pulmonary arterial pressure; TAPSE, tricuspid annular plane systolic excursion.
Patients were dichotomised into high or low TAPSE/sPAP ratio assuming the value of 0.7 mm/mmHg as the threshold. A significantly higher proportion of all reported cardiovascular events (75%) and deaths (100%) occurred in patients with a low TAPSE/sPAP ratio (<0.7 mm/mmHg) compared to patients with a high TASPE/sPAP ratio (≥0.7 mm/mmHg) during the 6 years of follow-up (Supplementary Figures 3A, B).
3.3. Univariable and multivariable Cox analyses and outcomes
In the univariable Cox regression analysis, the TAPSE/sPAP ratio, as a continuous variable, was significantly associated with both primary [HR 4.616 (95% CI 2.240–9.514); p < 0.001] and secondary outcomes [HR 4.048 (95% CI 3.177–8.262); p = 0.002] (Table 2).
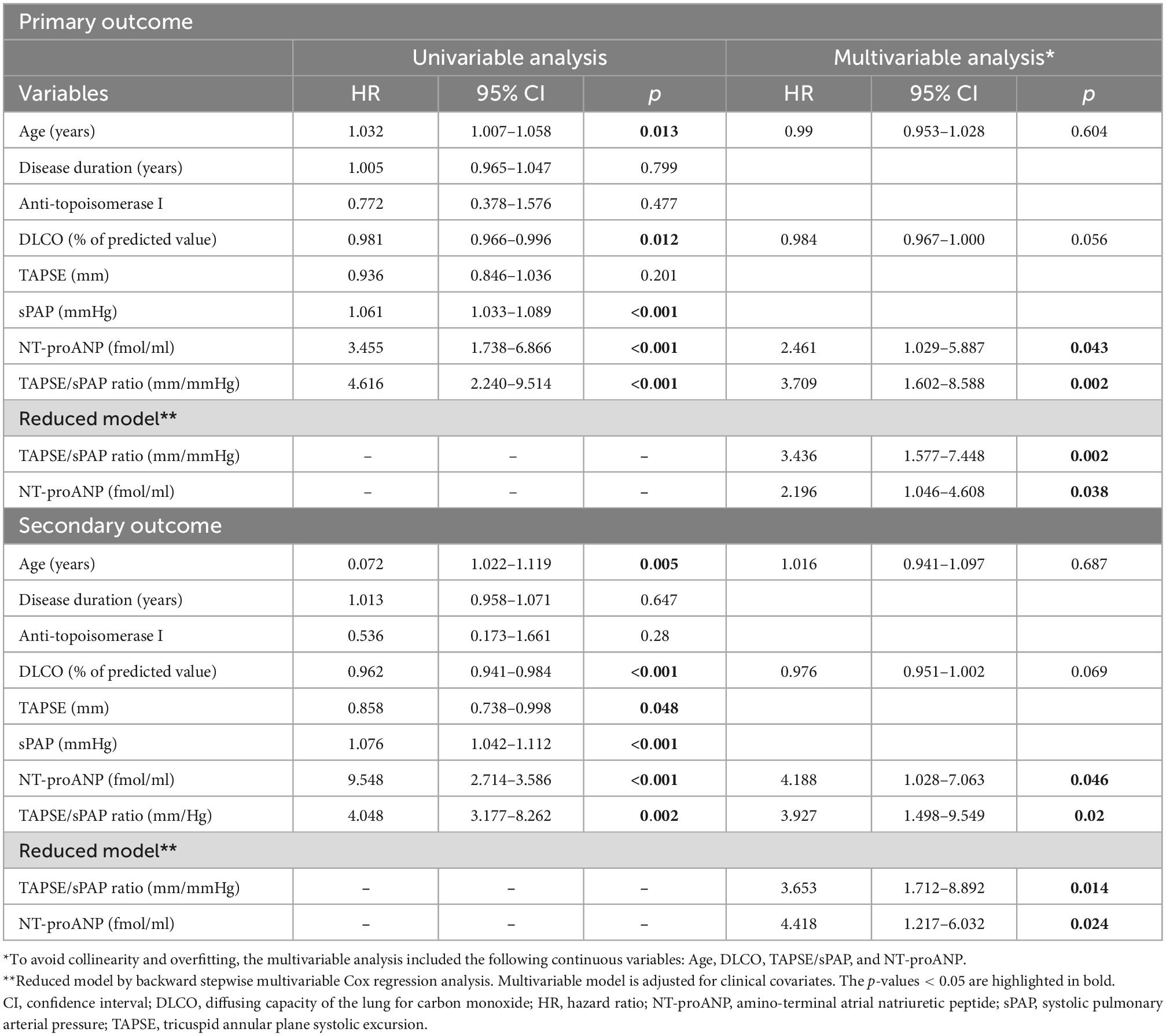
Table 2. Univariable and multivariable Cox regression analyses for independent predictors of outcomes.
In the multivariable analysis, the TAPSE/sPAP ratio showed a significant independent association with both primary [HR 3.709 (95% CI 1.602–8.588); p = 0.002] and secondary [HR 3.927 (95% CI 1.498–9.549); p = 0.020] outcomes (Table 2).
Also, DLCO showed a significant correlation with mortality in the univariable analysis [HR 0.962 (95% CI 0.941–0.984); p < 0.001], but not in the multivariable (Table 2). Of note, the presence of anti-topoisomerase I antibodies, linked to a poor prognosis and SSc-related mortality, was not significant in our cohort of patients (p = 0.280) (Table 2).
The duration of the disease was not significant according to cardiovascular events and mortality in our population.
Additional stepwise multivariable regression analysis confirmed both the TAPSE/sPAP ratio and NT-proANP plasma level as independent prognostic factors for both primary [HR 3.436 (95% CI 1.577–7.448); p = 0.002 and HR 2.196 (95% CI 1.046–4.608); p = 0.038, respectively] and secondary outcomes [HR 3.653 (95% CI 1.712–8.892); p = 0.014 and HR 4.418 (95% CI 1.217–6.032); p = 0.024, respectively] (Table 2).
3.4. NT-proANP plasma level distribution and its relationship with the TAPSE/sPAP ratio
The overall median value of NT-proANP plasma level in this cohort was 1799 fmol/ml (1384–2820 fmol/ml). NT-proANP level was significantly higher in SSc patients experiencing cardiovascular complications vs. SSc patients free from events, and in patients who died for SSc-related causes vs. survived population, consistently with previous evidence (26, 27).
In the present work, we aimed at exploring the interaction between TAPSE/sPAP ratio and NT-proANP plasma level in SSc patients. A moderate but significant correlation was observed between the two parameters [r = 0.52, p < 0.001] (Figure 3A).
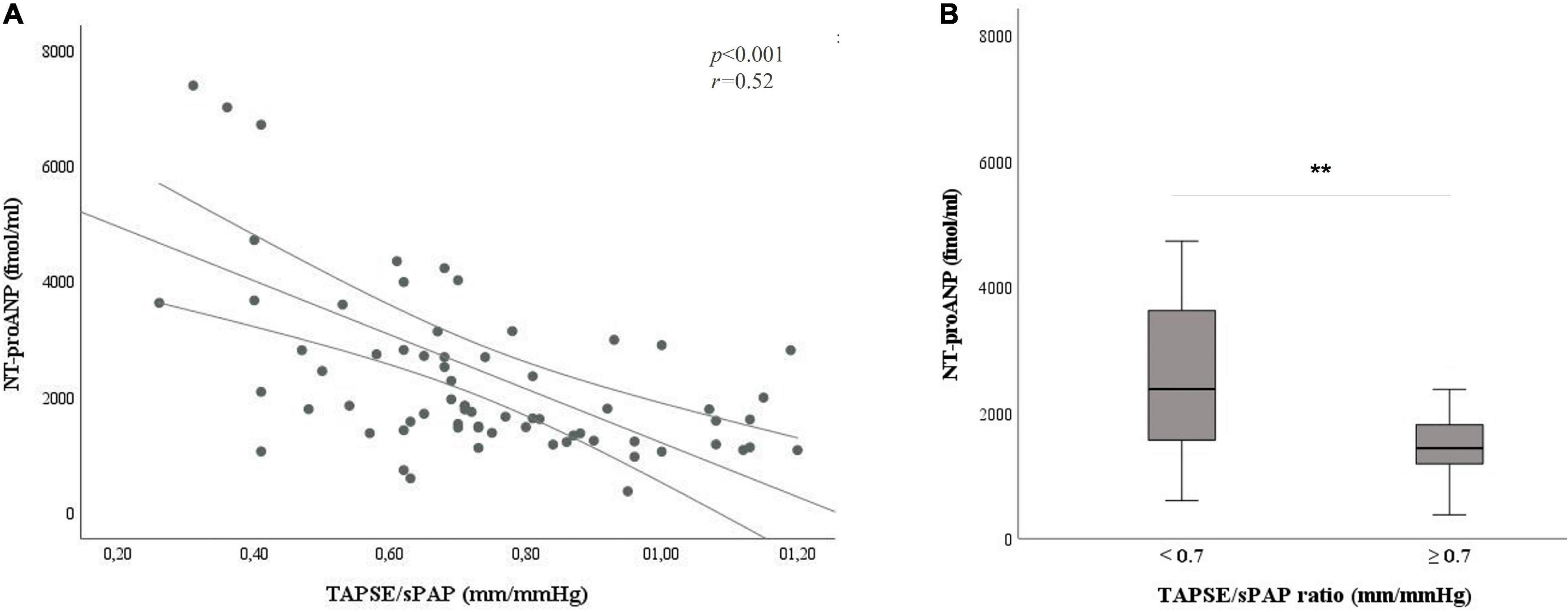
Figure 3. Relation between the TAPSE/sPAP ratio and NT-proANP plasma level. (A) Correlation between the TAPSE/sPAP ratio and NT-proANP plasma level (r = 0.52, *p < 0.001). (B) NT-proANP plasma level median value in patients with TAPSE/sPAP ratio < 0.7 vs. ≥ 0.7 mm/mmHg. [2735 (1815–4023) vs. 1511 (1221–1821) fmol/ml; **p < 0.001]. The cut-off value of TAPSE/sPAP ratio 0.7 mm/mmHg was derived from the ROC curves and the Youden Index. NT-proANP, amino-terminal atrial natriuretic peptide; sPAP, systolic pulmonary arterial pressure; ROC, receiver operating characteristic; TAPSE, tricuspid annular plane systolic excursion; *Spearman’s correlation coefficient; **Student’s t-test.
NT-proANP plasma level was significantly increased in SSc patients with the TAPSE/sPAP ratio < 0.7 mm/mmHg compared with patients with the TAPSE/sPAP ratio ≥ 0.7 mm/mmHg [2735 (1815–4023) vs. 1511 (1221–1821) fmol/ml; p < 0.001] (Figure 3B).
The distribution of the TAPSE/sPAP ratio and NT-proANP plasma levels, according to both primary and secondary outcomes, is shown in Figures 4A, B, respectively.
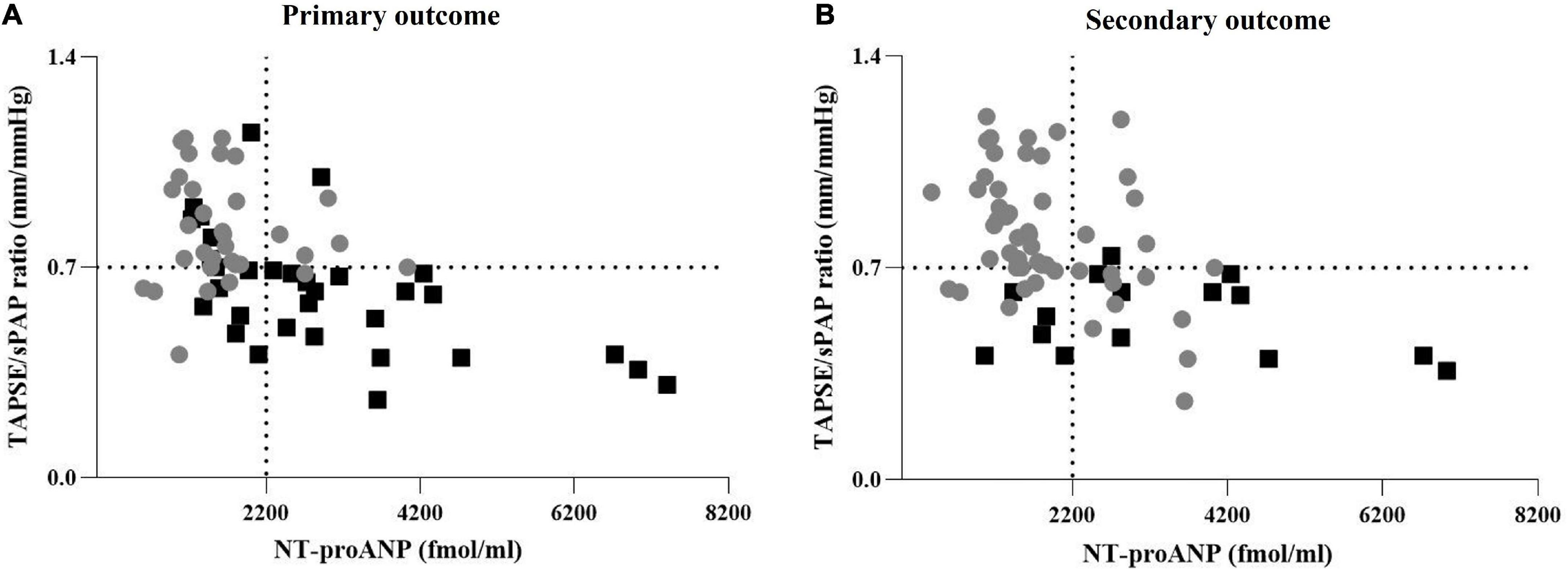
Figure 4. Distribution of the TAPSE/sPAP ratio and NT-proANP plasma level according to outcomes. (A) Distribution of TAPSE/sPAP ratio and NT-proANP plasma levels according to the primary outcome. Grey points: SSc patients free from cardiovascular events. Black squares: Patients with SSc-related cardiovascular events. (B) Distribution of TAPSE/sPAP ratio and NT-proANP plasma levels according to the secondary outcome. Grey points: Survived SSc patients. Black squares: SSc-related deaths. The TAPSE/sPAP ratio value of 0.7 mm/mmHg and NT-proANP value of 2,200 fmol/ml were set as thresholds. The cut-off value of TAPSE/sPAP ratio < 0.7 mm/mmHg was derived from ROC curves and the Youden Index. NT-proANP, amino-terminal atrial natriuretic peptide; sPAP, systolic pulmonary arterial pressure; ROC, receiver operating characteristic; TAPSE, tricuspid annular plane systolic excursion.
The prognostic significance of the TAPSE/sPAP ratio was confirmed by Kaplan–Meier analysis for both primary and secondary outcomes (Log rank p < 0.001). SSc patients with TAPSE/sPAP ratio ≥ 0.7 mm/mmHg showed a significantly lower rate of cardiovascular events recurrence (Figure 5A) and better survival (Figure 5B) compared with SSc patients with TAPSE/sPAP ratio < 0.7 mm/mmHg. Also, our data showed a significant prognostic value of the TAPSE/sPAP ratio combined with NT-proANP plasma level in this SSc population (Figures 5C, D).
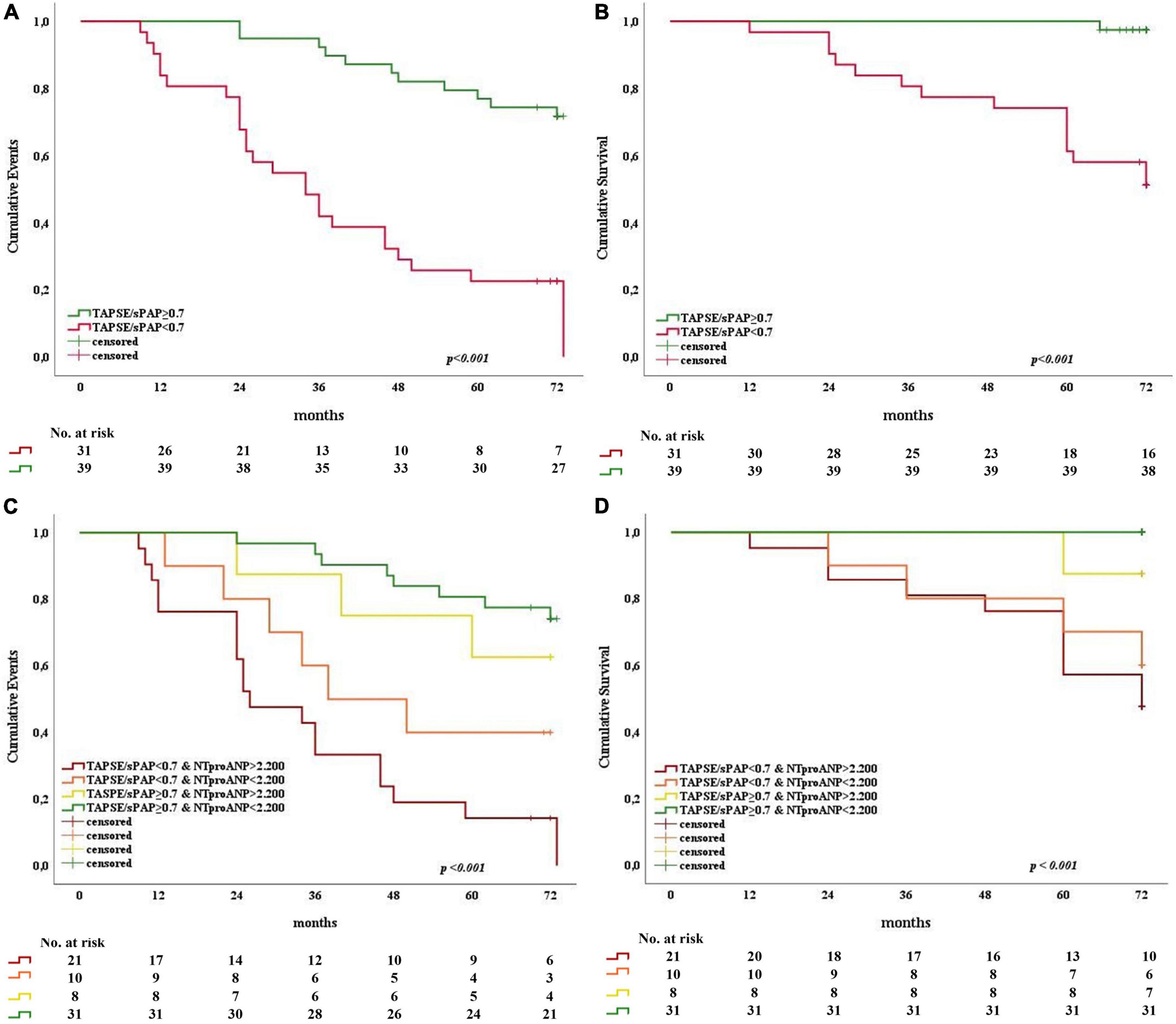
Figure 5. Kaplan–Meier analysis for primary and secondary outcomes as a function of the TAPSE/sPAP ratio alone and combined with NT-proANP plasma level in SSc patients. Patients were dichotomised at the TAPSE/sPAP ratio value of 0.7 mm/mmHg and NT-proANP value of 2200 fmol/ml. Stratifications by TAPSE/sPAP ratio alone (A,B) and combined with high and low NT-proANP plasma levels (C,D) showed significantly increased adverse outcomes (n = 70; Log-rank p < 0.001). (A) TAPSE/sPAP ratio and primary outcome (X2 25.182; Log rank p < 0.001). (B) TAPSE/sPAP ratio and secondary outcome (X2 20.783; Log rank p < 0.001). (C) TAPSE/sPAP ratio combined with NT-proANP and primary outcome (X2 30.933; Log rank p < 0.001). (D) TAPSE/sPAP ratio combined with NT-proANP and secondary outcome (X2 22.473; Log rank p < 0.001). NT-proANP, amino-terminal atrial natriuretic peptide; sPAP, systolic pulmonary arterial pressure; TAPSE, tricuspid annular plane systolic excursion.
4. Discussion
In the present study, we provide evidence that SSc patients without evident cardiovascular involvement show a lower median value of the TAPSE/sPAP ratio, as an index of RV/PA uncoupling, compared to healthy subjects. The NT-proANP plasma level showed a correlation with the TAPSE/sPAP ratio. The TAPSE/sPAP ratio alone and in combination with NT-proANP plasma levels correlate with worse outcomes in our SSc population.
Cardiac involvement is common in SSc, even in the absence of clinical manifestations. Endomyocardial biopsies from SSc patients free from any cardiovascular complications (HF, PH and PAH, LV hypertrophy, arrhythmias, pericardial, and myocardial diseases) demonstrated an abnormal collagen deposition which is the distinguishing histological feature of SSc (39). Previous studies showed that patchy myocardial fibrosis and coronary microvascular dysfunction affect both RV and LV performance, impairing systolic (contractile) and diastolic (filling) functions (40, 41) in SSc. Echocardiographic studies showed that RV diastolic dysfunction can be detected even in the presence of normal sPAP, due to SSc per se and to the associated pulmonary vasculopathy (9). The immune abnormalities, the persistent interstitial inflammation, the increased extracellular matrix deposition together with the abnormal collagen cross-linking, the severe microangiopathy, characterised by arteriolar hyperplasia of the heart and lungs might explain, at least in part, the intrinsic myocardial impairment and the pulmonary vasculopathy contributing to RV/PA uncoupling in SSc patients.
The RV/PA uncoupling can be considered the driving cause of RV maladaptation and eventually RV failure. Previous echocardiographic and haemodynamic studies suggest that RV dysfunction is more severe in SSc patients (regardless of the presence of PH and ILD) compared to idiopathic PAH and it is also due to a greater afterload mismatch. This condition is related to the intrinsic myocardial dysfunction, decreased pulmonary arterial compliance, latent PH, leading to RV/PA uncoupling, RV negative remodelling and failure. This evidence underlines the poor survival of SSc patients with PAH (9).
According to the pathophysiology of SSc, characterised by endothelial injury, fibrosis and vascular remodelling of the heart and lungs, it appears mandatory to investigate the RV and the pulmonary vascular circuit as one combined physical unit. To this aim, the RV/PA coupling assessment better describes the functional state and efficiency of the cardio-pulmonary unit and can detect an impairment even when other indices are still preserved. The gold standard method for the quantification of RV/PA coupling relies on the invasive measurement of the Ees/Ea ratio calculated from a pressure-volume relationship, based on single-beat or multi-beat methods (11, 12, 42, 43). However, these measurements are invasive, technically, and costly demanding. A non-invasive gold standard approach for the evaluation of coupling has been developed based on RV volumes assessment by cardiac magnetic resonance (CMR) imaging (44–46).
The traditional echocardiographic evaluation of RV function and afterload may not fully detect the critical aspects of RV and PA interaction and dysfunction, especially in SSc population. Indeed, the echocardiographic TAPSE/sPAP ratio is a simpler, non-invasive, surrogate index of the RV/PA coupling and is more feasible in routine clinical practice (11).
The normal values of the Ees/Ea ratio range between 1.5 and 2.0 (11) whereas a normal TAPSE/sPAP ratio is about 1.0 in healthy subjects, ranging from 0.8 and 1.3 in healthy populations described in different studies (47–49).
The RV/PA coupling expresses a considerable reserve, since the Ees/Ea ratio must decrease from normal values to less than 0.8 before substantial RV maladaptation and clinically evident symptoms occur. Previous studies about the invasive measurements of RV/PA coupling demonstrated that a value of the Ees/Ea ratio < 0.7 in patients with PH was associated with clinical worsening (12). It is well known that patients with PAH receiving targeted therapies may remain stable for several years unless a severe uncoupling precipitates hemodynamic and clinical conditions. In this setting, RV/PA uncoupling is a sensitive marker of disease progression (46).
In this study, SSc patients showed a lower median value of TAPSE/sPAP ratio in comparison to controls (0.71 vs. 1.00 mm/mmHg) at baseline. A TAPSE/sPAP ratio < 0.7 mm/mmHg might reflect the intrinsic myocardial dysfunction, decreased pulmonary arterial compliance and increased vulnerability to ischemia, all typical features of the SSc disease.
Our original findings introduce the TAPSE/sPAP ratio as an independent prognostic echocardiographic parameter in patients with SSc. Although several indicators of RV function have been related to adverse events and survival (50), our data demonstrated that the TAPSE/sPAP ratio might be more specific and accurate, as it better reflects the RV performance and the load-dependent status of the pulmonary circulation.
Our previous studies demonstrated that NT-proANP plasma level correlated with vascular complications such as digital ulcers, worsening of PH and new onset of PAH in SSc patients (26) and it significantly predicted death due to the disease (27). In the present study, the NT-proANP moderately correlated with the TAPSE/sPAP ratio. Despite the lack of a strong statistical correlation coefficient (r = 0.52; p < 0.001), this evidence still carries a clinical and pathophysiological significance, linking plasma NT-proANP elevation to RV/PA uncoupling in SSc population.
The ANP release pattern is highly dependent on the hemodynamic stress triggers applied to the right atrium and pulmonary circulation, mainly under exertion (22, 23). For these reasons, the NT-proANP might represent a potential accurate biomarker related to the right atrial stress and impaired pulmonary hemodynamic.
Notably, the analysis of NT-proBNP level in this cohort did not reveal a statistically significant association with the TAPSE/sPAP ratio and outcomes.
Pulmonary involvement in SSc consists more often of interstitial fibrosis and pulmonary vascular disease, potentially leading to PH and PAH. Overall, chronic inflammation and consequent endothelial dysfunction are the main causes of SSc-related pulmonary injury, also contributing to ANP release (51). According to the pathophysiological link of this cardiac hormone with the endothelial homeostasis, pulmonary injury and hemodynamic stress contributing to ventricular-arterial uncoupling, NT-proANP is expected to behave as an appropriate biomarker for SSc-related cardiac, vascular, and pulmonary complications.
As the main original observation of our study, we report the first evidence that the TAPSE/sPAP ratio alone and in combination with NT-proANP plasma level are significant predictors of adverse outcomes due to SSc.
5. Conclusion
As far as our knowledge goes, the present study is the first one investigating the prognostic role of TAPSE/sPAP ratio, alone and in combination with NT-proANP plasma level, in an SSc population free from clinically evident cardiovascular manifestations. We provide evidence that the combined echocardiographic ratio of RV function (TAPSE) to afterload (sPAP) is an affordable predictor of adverse outcomes in SSc. This parameter can improve the clinical phenotyping and risk assessment of patients that may suffer from clinical deterioration and consequent worse prognosis on long-term follow-up. Importantly, the combination of TAPSE/sPAP ratio with NT-proANP plasma level, a known predictive biomarker in SSc, may open new perspectives in the clinical assessment and prognostic stratification of SSc patients.
5.1. Clinical implementations
The expression “the sooner, the better” is a paradigm in medicine, which suggests that an early diagnosis allows for a better outcome. SSc provides a perfect example of the need for useful tools to identify early subclinical cardiac impairment and improve survival through timely intervention.
Among the connective tissue diseases, SSc demonstrates a unique physiopathology and clinical phenotype with the poorest survival. Although screening and therapeutic strategies for SSc patients significantly evolved, important clinical needs remain unmet, particularly the availability of more appropriate tests and biomarkers for the screening and monitoring of early cardiovascular involvement. Echocardiographic measurements and biomarkers are already included in diagnostic algorithms and prognostic risk scores, but none of them strongly refers to the right ventricular function and the RV-PA unit. Also, a hormonal indicator of both pulmonary vascular and cardiac remodelling and impaired hemodynamic, like ANP, could better fit the need for an early diagnosis. In this scenario, the TAPSE/sPAP ratio and plasma NT-proANP level seem to be appropriate parameters to be used in the early clinical assessment of SSc patients, due to their greater sensitivity, specificity, and prognostic relevance. We provide the first evidence that the TAPSE/sPAP ratio, both when used alone and in combination with NT-proANP plasma level, appears the most suitable parameter for the clinical and prognostic assessment of SSc patients.
5.2. Limitations
First, this was a retrospective and single-centre study including a small moderately heterogeneous cohort of SSc patients. However, we believe it could represent a useful pilot study to validate the TAPSE/sPAP ratio and NT-proANP as early markers of cardiovascular involvement in a wider population of SSc patients. Further investigation is needed to strengthen and better explain the pathophysiological relationship between the TAPSE/sPAP ratio and NT-proANP plasma level and its clinical impact.
Moreover, our SSc population included a small number of male patients, in accordance with the typical epidemiological features of the disease. Patients involved in our study presented different clinical SSc subtypes and disease duration at the time of enrolment.
The echocardiographic evaluation of RV function has some limitations. Although TAPSE is a common estimate of RV function, it does not reflect the whole RV performance since longitudinal RV function may differ from radial or apical RV contractility. To this aim, the speckle tracking-derived measurement of myocardial deformation could be an accurate tool for the early detection of subclinical myocardial dysfunction, with the potential to provide additional insights into the course of SSc cardiovascular disease (52, 53). On the other hand, TAPSE/sPAP ratio is a simple, well-validated quantitative parameter easy to apply in clinical practice. Furthermore, a large validation of the TAPSE/sPAP ratio with an invasive assessment of the Ees/Ea ratio is lacking in this SSc population.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethical Committee of the Sant’Andrea Hospital. The patients/participants provided their written informed consent to participate in this study.
Author contributions
MCG performed the study conception, data management and interpretation, statistical analyses, first draft, revisions, and proofread of the manuscript. MCG, AR, and SR designed the research, contributed to suggestions and critical reading of the manuscript, and edited the final version. SR provided funding support. SR and AR collected biological materials. SM and SR processed biological materials. AR, MCG, ER, AD’A, and EC performed the selection and screening of the patients. All authors read and approved the submitted version.
Funding
This work was supported in part by a grant from the Italian Ministry of Health and by Progetto PRIN 2017 (from the Italian Ministry of Instruction, University and Research, no. 2017PZY5K7) to SR.
Acknowledgments
We thank all our patients, colleagues, technicians, and nurses for their clinical, technical, and scientific support. We strongly believe in translational research involving clinicians and biologists to promote personalised medicine.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.1021048/full#supplementary-material
References
1. Gabrielli A, Avvedimento E, Krieg T. Scleroderma. N Engl J Med. (2009) 360:1989–2003. doi: 10.1056/NEJMra0806188
2. Wollheim F. Classification of systemic sclerosis. Visions and reality. Rheumatology. (2005) 44:1212–6. doi: 10.1093/rheumatology/keh671
3. Tyndall A, Bannert B, Vonk M, Airò P, Cozzi F, Carreira P, et al. Causes and risk factors for death in systemic sclerosis: a study from the EULAR scleroderma trials and research (EUSTAR) database. Ann Rheum Dis. (2010) 69:1809–15. doi: 10.1136/ard.2009.114264
4. Walker UA, Tyndall A, Czirják L, Denton C, Farge-Bancel D, Kowal-Bielecka O, et al. Clinical risk assessment of organ manifestations in systemic sclerosis: a report from the EULAR Scleroderma Trials and Research group database. Ann Rheum Dis. (2007) 66:754–63. doi: 10.1136/ard.2006.062901
5. Meier FM, Frommer KW, Dinser R, Walker UA, Czirjak L, Denton CP, et al. Update on the profile of the EUSTAR cohort: an analysis of the EULAR Scleroderma trials and research group database. Ann Rheum Dis. (2012) 71:1355–60. doi: 10.1136/annrheumdis-2011-200742
6. Meune C, Vignaux O, Kahan A, Allanore Y. Heart involvement in systemic sclerosis: evolving concept and diagnostic methodologies. Arch Cardiovasc Dis. (2010) 103:46–52. doi: 10.1016/j.acvd.2009.06.009
7. Költő G, Faludi R, Aradi D, Bartos B, Kumánovics G, Minier T, et al. Impact of cardiac involvement on the risk of mortality among patients with systemic sclerosis: a 5-year follow-up of a single-center cohort. Clin Rheumatol. (2014) 33:197–205. doi: 10.1007/s10067-013-2358-4
8. Launay D, Sitbon O, Hachulla E, Mouthon L, Gressin V, Rottat L, et al. Survival in systemic sclerosis-associated pulmonary arterial hypertension in the modern management era. Ann Rheum Dis. (2013) 72:1940–6. doi: 10.1136/annrheumdis-2012-202489
9. Huez S, Roufosse F, Vachiéry J, Pavelescu A, Derumeaux G, Wautrecht J, et al. Isolated right ventricular dysfunction in systemic sclerosis: latent pulmonary hypertension? Eur Respir J. (2007) 30:928–36. doi: 10.1183/09031936.00025607
10. Douschan P, Tello K, Rieth A, Wiedenroth C, Sassmann T, Kovacs G, et al. Right ventricular-pulmonary arterial coupling and its relationship to exercise haemodynamics in a continuum of patients with pulmonary vascular disease due to chronic thromboembolism. Eur Respir J. (2022) 60:2200450. doi: 10.1183/13993003.00450-2022
11. Sanz J, Sánchez-Quintana D, Bossone E, Bogaard H, Naeije R. Anatomy, function, and dysfunction of the right ventricle: JACC state-of-the-art review. J Am Coll Cardiol. (2019) 73:1463–82. doi: 10.1016/j.jacc.2018.12.076
12. Noordegraaf AV, Chin KM, Haddad F, Hassoun PM, Hemnes AR, Hopkins SR, et al. Pathophysiology of the right ventricle and of the pulmonary circulation in pulmonary hypertension: an update. Eur Respir J. (2019) 53:1801900. doi: 10.1183/13993003.01900-2018
13. Richter MJ, Peters D, Ghofrani HA, Naeije R, Roller F, Sommer N, et al. Evaluation and prognostic relevance of right ventricular-arterial coupling in pulmonary hypertension. Am J Respir Crit Care Med. (2020) 201:116–9. doi: 10.1164/rccm.201906-1195LE
14. Tello K, Axmann J, Ghofrani HA, Naeije R, Narcin N, Rieth A, et al. Relevance of the TAPSE/PASP ratio in pulmonary arterial hypertension. Int J Cardiol. (2018) 266:229–35. doi: 10.1016/j.ijcard.2018.01.053
15. Tello K, Wan J, Dalmer A, Vanderpool R, Ghofrani HA, Naeije R, et al. Validation of the tricuspid annular plane systolic excursion/systolic pulmonary artery pressure ratio for the assessment of right ventricular-arterial coupling in severe pulmonary hypertension. Circ Cardiovasc Imaging. (2019) 12:e009047. doi: 10.1161/CIRCIMAGING.119.009047
16. Guazzi M, Dixon D, Labate V, Beussink-Nelson L, Bandera F, Cuttica MJ, et al. RV contractile function and its coupling to pulmonary circulation in heart failure with preserved ejection fraction: stratification of clinical phenotypes and outcomes. JACC Cardiovasc Imaging. (2017) 10:1211–21. doi: 10.1016/j.jcmg.2016.12.024
17. Guazzi M, Bandera F, Pelissero G, Castelvecchio S, Menicanti L, Ghio S, et al. Tricuspid annular plane systolic excursion and pulmonary arterial systolic pressure relationship in heart failure: an index of right ventricular contractile function and prognosis. Am J Physiol Heart Circ Physiol. (2013) 305:H1373–81. doi: 10.1152/ajpheart.00157.2013
18. Lyhne MD, Kabrhel C, Giordano N, Andersen A, Nielsen-Kudsk JE, Zheng H, et al. The echocardiographic ratio tricuspid annular plane systolic excursion/pulmonary arterial systolic pressure predicts short-term adverse outcomes in acute pulmonary embolism. Eur Heart J Cardiovasc Imaging. (2021) 22:285–94. doi: 10.1093/ehjci/jeaa243
19. Tello K, Ghofrani HA, Heinze C, Krueger K, Naeije R, Raubach C, et al. A simple echocardiographic estimate of right ventricular-arterial coupling to assess severity and outcome in pulmonary hypertension on chronic lung disease. Eur Respir J. (2019) 54:1802435. doi: 10.1183/13993003.02435-2018
20. Humbert M, Kovacs G, Hoeper M, Badagliacca R, Berger R, Brida M, et al. ESC/ERS scientific document group. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J. (2022). [Epub ahead of print]. doi: 10.1183/13993003.00879-2022
21. Levin E, Gardner D, Samson W. Natriuretic peptides. N Engl J Med. (1998) 339:321–8. doi: 10.1056/NEJM199807303390507
22. Kriechbaum S, Scherwitz L, Wiedenroth C, Rudolph F, Wolter J, Haas M, et al. Mid-regional pro-atrial natriuretic peptide and copeptin as indicators of disease severity and therapy response in CTEPH. ERJ Open Res. (2020) 6:00356–2020. doi: 10.1183/23120541.00356-2020
23. Kriechbaum S, Birmes J, Wiedenroth C, Adameit M, Gruen D, Vietheer J, et al. Exercise MR-proANP unmasks latent right heart failure in CTEPH. J Heart Lung Transplant. (2022) 41:1819–30. doi: 10.1016/j.healun.2022.08.017
24. Volpe M, Rubattu S, Burnett J Jr. Natriuretic peptides in cardiovascular diseases: current use and perspectives. Eur Heart J. (2014) 35:419–25. doi: 10.1093/eurheartj/eht466
25. Kook H, Itoh H, Choi BS, Sawada N, Doi K, Hwang TJ, et al. Physiological concentration of atrial natriuretic peptide induces endothelial regeneration in vitro. Am J Physiol Heart Circ Physiol. (2003) 284:H1388–97. doi: 10.1152/ajpheart.00414.2002
26. Romaniello A, Rubattu S, Gigante A, Simonelli F, Grimaldi MC, D’Angelo A, et al. Atrial natriuretic peptide predicts disease progression and digital ulcers development in systemic sclerosis patients. J Cardiovasc Med. (2019) 20:771–9. doi: 10.2459/JCM.0000000000000852
27. Romaniello A, Rubattu S, Vaiarello V, Gigante A, Volpe M, Rosato E. Circulating NT-proANP level is a predictor of mortality for systemic sclerosis: a retrospective study of an Italian cohort. Expert Rev Clin Immunol. (2021) 17:661–6. doi: 10.1080/1744666X.2021.1908888
28. van den Hoogen F, Khanna D, Fransen J, Johnson S, Baron M, Tyndall A, et al. 2013 classification criteria for systemic sclerosis: an American college of rheumatology/European league against rheumatism collaborative initiative. Ann Rheum Dis. (2013) 72:1747–55. doi: 10.1136/annrheumdis-2013-204424
29. Kowal-Bielecka O, Fransen J, Avouac J, Becker M, Kulak A, Allanore Y, et al. Update of EULAR recommendations for the treatment of systemic sclerosis. Ann Rheum Dis. (2017) 76:1327–39.
30. Fischer A, Patel NM, Volkmann ER. Interstitial lung disease in systemic sclerosis: focus on early detection and intervention. Open Access Rheumatol. (2019) 11:283–307.
31. Cappelli S, Bellando Randone S, Camiciottoli G, De Paulis A, Guiducci S, Matucci-Cerinic M. Interstitial lung disease in systemic sclerosis: where do we stand? Eur Respir Rev. (2015) 24:411–9. doi: 10.1183/16000617.00002915
32. Khanna D, Gladue H, Channick R, Chung L, Distler O, Furst D, et al. Scleroderma foundation and pulmonary hypertension association. Recommendations for screening and detection of connective tissue disease-associated pulmonary arterial hypertension. Arthritis Rheum. (2013) 65:3194–201. doi: 10.1002/art.38172
33. Young A, Nagaraja V, Basilious M, Habib M, Townsend W, Gladue H, et al. Update of screening and diagnostic modalities for connective tissue disease-associated pulmonary arterial hypertension. Semin Arthritis Rheum. (2019) 48:1059–67. doi: 10.1016/j.semarthrit.2018.10.010
34. Sánchez-Román J, Opitz CF, Kowal-Bielecka O, García-Hernández FJ, Castillo-Palma MJ, Pittrow D. Screening for PAH in patients with systemic sclerosis: focus on Doppler echocardiography. Rheumatology. (2008) 47:v33–5. doi: 10.1093/rheumatology/ken306
35. Galiè N, Humbert M, Vachiery J, Gibbs S, Lang I, Torbicki A, et al. ESC scientific document group. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the joint task force for the diagnosis and treatment of pulmonary hypertension of the European society of cardiology (ESC) and the European respiratory society (ERS): endorsed by: association for European paediatric and congenital cardiology (AEPC), International society for heart and lung transplantation (ISHLT). Eur Heart J. (2016) 37:67–119. doi: 10.1093/eurheartj/ehv317
36. Ponikowski P, Voors A, Anker S, Bueno H, Cleland J, Coats A, et al. ESC scientific document group, 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. (2016) 37:2129–200. doi: 10.1093/eurheartj/ehw128
37. Rudski L, Lai W, Afilalo J, Hua L, Handschumacher M, Chandrasekaran K, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American society of echocardiography endorsed by the European association of echocardiography, a registered branch of the European society of cardiology, and the Canadian society of echocardiography. J Am Soc Echocardiogr. (2010) 23:685–713. doi: 10.1016/j.echo.2010.05.010
38. Lang R, Badano L, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American society of echocardiography and the European association of cardiovascular imaging. J Am Soc Echocardiogr. (2015) 28:1–39.e14.
39. Fernandes F, Ramires F, Arteaga E, Ianni B, Bonfá E, Mady C. Cardiac remodelling in patients with systemic sclerosis with no signs or symptoms of heart failure: an endomyocardial biopsy study. J Card Fail. (2003) 9:311–7. doi: 10.1054/jcaf.2003.51
40. Follansbee WP, Curtiss EI, Medsger TA Jr, Steen VD, Uretsky BF, Owens GR, et al. Physiologic abnormalities of cardiac function in progressive systemic sclerosis with diffuse scleroderma. N Engl J Med. (1984) 310:142–8. doi: 10.1056/NEJM198401193100302
41. Tountas C, Protogerou A, Bournia V, Panopoulos S, Konstantonis G, Tektonidou M, et al. Mechanics of early ventricular impairment in systemic sclerosis and the effects of peripheral arterial haemodynamics. Clin Exp Rheumatol. (2019) 37:57–62.
42. Trip P, Kind T, Veerdonk MC, Marcus JT, Man FS, Westerhof N, et al. Accurate assessment of load-independent right ventricular systolic function in patients with pulmonary hypertension. J Heart Lung Transplant. (2013) 32:50–5. doi: 10.1016/j.healun.2012.09.022
43. Brewis MJ, Bellofiore A, Vanderpool RR, Chesler NC, Johnson MK, Naeije R, et al. Imaging right ventricular function to predict outcome in pulmonary arterial hypertension. Int J Cardiol. (2016) 218:206–11. doi: 10.1016/j.ijcard.2016.05.015
44. Sanz J, García-Alvarez A, Fernández-Friera L, Nair A, Mirelis JG, Sawit ST, et al. Right ventricular-arterial coupling in pulmonary hypertension: a magnetic resonance study. Heart. (2012) 98:238–43.
45. Tello K, Dalmer A, Vanderpool R, Ghofrani H, Naeije R, Roller F, et al. Cardiac magnetic resonance imaging-based right ventricular strain analysis for assessment of coupling and diastolic function in pulmonary hypertension. JACC Cardiovasc Imaging. (2019) 12:2155–64. doi: 10.1016/j.jcmg.2018.12.032
46. Tello K, Dalmer A, Axmann J, Vanderpool R, Ghofrani H, Naeije R, et al. Richter reserve of right ventricular-arterial coupling in the setting of chronic overload. Circ Heart Fail. (2019) 12:e005512. doi: 10.1161/CIRCHEARTFAILURE.118.005512
47. D’Alto M, Pavelescu A, Argiento P, Romeo E, Correra A, Marco GM, et al. Echocardiographic assessment of right ventricular contractile reserve in healthy subjects. Echocardiography. (2017) 34:61–8. doi: 10.1111/echo.13396
48. Bosch L, Lam CS, Gong L, Chan SP, Sim D, Yeo D, et al. Right ventricular dysfunction in left-sided heart failure with preserved versus reduced ejection fraction. Eur J Heart Fail. (2017) 19:1664–71. doi: 10.1002/ejhf.873
49. Ferrara F, Rudski LG, Vriz O, Gargani L, Afilalo J, D’Andrea A, et al. Physiologic correlates of tricuspid annular plane systolic excursion in 1168 healthy subjects. Int J Cardiol. (2016) 223:736–43. doi: 10.1016/j.ijcard.2016.08.275
50. Naeije R. Assessment of right ventricular function in pulmonary hypertension. Curr Hypertens Rep. (2015) 17:35. doi: 10.1007/s11906-015-0546-0
51. Mitaka C, Hirata Y, Nagura T, Tsunoda Y, Amaha K. Beneficial effect of atrial natriuretic peptide on pulmonary gas exchange in patients with acute lung injury. Chest. (1998) 114:223–8. doi: 10.1378/chest.114.1.223
52. Mukherjee M, Chung S, Ton V, Tedford R, Hummers L, Wigley F, et al. Unique abnormalities in right ventricular longitudinal strain in systemic sclerosis patients. Circ Cardiovasc Imaging. (2016) 9:e003792. doi: 10.1161/CIRCIMAGING.115.003792
Keywords: right ventricular-arterial coupling, TAPSE/sPAP ratio, atrial natriuretic peptide (ANP), systemic sclerosis (scleroderma), pulmonary hypertension, pulmonary arterial hypertension (PAH), TAPSE/sPAP
Citation: Grimaldi MC, Rosato E, D’Angelo A, Cristiano E, Marchitti S, Volpe M, Rubattu S and Romaniello A (2023) The prognostic role of the echocardiographic tricuspid annular plane systolic excursion/systolic pulmonary arterial pressure (TAPSE/sPAP) ratio and its relationship with NT-proANP plasma level in systemic sclerosis. Front. Cardiovasc. Med. 9:1021048. doi: 10.3389/fcvm.2022.1021048
Received: 16 August 2022; Accepted: 20 December 2022;
Published: 17 January 2023.
Edited by:
Jiuliang Zhao, Peking Union Medical College Hospital (CAMS), ChinaReviewed by:
Robert Naeije, Université Libre de Bruxelles, BelgiumManuel J. Richter, Universitätsklinikum Gießen, Germany
Copyright © 2023 Grimaldi, Rosato, D’Angelo, Cristiano, Marchitti, Volpe, Rubattu and Romaniello. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maria Chiara Grimaldi,  bWFyaWFjaGlhcmEuZ3JpbWFsZGlAdW5pcm9tYTEuaXQ=
bWFyaWFjaGlhcmEuZ3JpbWFsZGlAdW5pcm9tYTEuaXQ=
†These authors share last authorship
 Maria Chiara Grimaldi
Maria Chiara Grimaldi Edoardo Rosato
Edoardo Rosato Adriano D’Angelo1
Adriano D’Angelo1 Ernesto Cristiano
Ernesto Cristiano Simona Marchitti
Simona Marchitti Massimo Volpe
Massimo Volpe Speranza Rubattu
Speranza Rubattu