- Department of Cardiovascular Surgery, Xijing Hospital, Air Force Medical University, Xi’an, China
Objective: The aim was to evaluate the safety and efficacy of TTVR in patients with severe TR at the 1-year follow-up.
Materials and methods: This project was a single-center, observational study. From September 2020 to May 2021, 15 patients with severe or extremely severe TR at high risk of traditional surgery were enrolled. All patients had preoperative imaging assessments to evaluate the tricuspid valve and the anatomy of the right heart. All patients were planned to treated with the LuX-Valve (Ningbo Jenscare Biotechnology, Ningbo, China). The LuX-Valve was implanted under the intraoperative guidance of TEE and X-ray fluoroscopy. Data were collected at baseline, before discharge, and at 30 days, 6 months, and 1 year postoperatively.
Results: The LuX-Valves were successfully implanted in all 15 patients. TR was significantly reduced to ≤ 2 +. One patient died on postoperative day 12 of a pulmonary infection that was considered unrelated to the procedures or the devices. The remaining 14 patients (100.0%) reached the primary end point. One patient (7.1%) was rehospitalized during 1-year follow-up because of device thrombosis. The number of patients who survived at 1 year with New York Heart Association (NYHA) functional class II was higher than that before TTVR (11/14 vs. 0/15, P = 9.11 × 10–4). Patients with peripheral edema and ascites decreased from 100.0 to 46.7% at baseline to 28.6% and 14.3% at 1 year (P = 1.57 × 10–3 and 2.53 × 10–2).
Conclusion: TTVR is associated with RV remodeling, increased cardiac output, and improvement in NYHA functional class. Using the LuX-Valve for TTVR to treat patients with severe TR is a feasible and relatively safe method with reliable clinical results. Further studies are needed to determine long-term outcomes.
Introduction
Tricuspid regurgitation (TR) is a common heart valve disease that is associated with increased mortality (1, 2). The prognosis of patients with severe TR is short of expectations, and the 5-year survival rate is less than 50% (2–5). TR is mainly secondary to dilation of the right ventricle (RV) and the tricuspid ring, which are closely associated with atrial fibrillation (AF) and pulmonary hypertension (6). The etiology of primary TR includes congenital tricuspid valve (TV) malformation, endocarditis, and a pacemaker implant. The traditional surgical treatment of TR involves TV repair and replacement assisted by a cardiopulmonary bypass device. Most patients with severe TR are treated with medication because interventions are associated with a high mortality rate, especially in the elderly (7–9). These results indicate that, for patients, annular repair may not be sufficient (10). Furthermore, the number of patients with TR is seriously underestimated, and less than 5% of patients receive surgical treatment (11).
In recent decades, transcatheter tricuspid valve replacement (TTVR) has become one of the research hotspots in cardiovascular medicine. Several interventional devices for different anatomical structures of the TV have been used clinically. Early reports from studies with these devices showed varying degrees of reduction of TR (12–20). The LuX-valve (Ningbo Jenscare Biotechnology, Ningbo, China) is one TTVR device unrelated to radial force that has been successfully implanted in patients with severe TR (21, 22). Our goal was to report the results of the 1-year follow-up in 15 patients with severe TR who received LuX-Valve implants.
Materials and methods
Study population
The study was a single-center, observational investigation. From September 2020 to May 2021, a total of 15 patients with severe TR [9 women; 62.0 (56.0, 78.0) years] were enrolled in this study. The severity of TR is classified as mild, moderate, severe, very severe, and extremely severe (23). All patients were carefully evaluated by the multidisciplinary cardiac team and considered to be either contraindicated or at high risk for surgery. According to European Society of Cardiology (ESC) and European Association of Cardiothoracic Surgery (EACTS) guidelines for the management of valvular heart disease, TR severity was graded as mild, moderate and severe in the present study evaluating by TR area (24). Meanwhile, TV is not a simple flat structure, but similar to the saddle oval. Therefore, in addition to assessing TR severity, the team also assessed the extent of TV annulus dilatation and cusp convolution (25). Inclusion criteria included age > 50 years old; TR severity ≥ severe; New York Heart Association (NYHA) class ≥ III; Patients at high risk for surgical tricuspid valve replacement as assessed by the multidisciplinary cardiac team [Society of Thoracic Surgeons (STS) score > 8.0%]. Exclusion criteria included left ventricular ejection fraction < 50%; systolic pulmonary arterial pressure > 55 mm Hg (1 mm Hg = 0.133 kPa); bioprosthetic valve replacement within 6 months; Ebstein’s malformation or structural dysplasia of the right ventricle; active infective endocarditis; cardiogenic shock; severe chronic renal insufficiency [glomerular filtration rate (GFR)< 30 mL/min]; combined with other heart disease requiring surgery. The clinical trial was registered in the ClinicalTrials.gov protocol registration system (NCT02917980). All procedures were in accordance with the ethical guidelines set out in the Declaration of Helsinki, and all patients signed the informed consent forms.
Preoperative imaging
Coronary angiography was used to exclude severe coronary artery diseases; invasive RV catheterization was used to evaluate the hemodynamics of the right heart, and gated cardiac computed tomography and 3-dimensional reconstruction were used to evaluate anatomical structures. Functional TR is considered to be a disease that depends not only on the size and shape of the TV but also on the function of the RV, ventricular septal displacement, and pulmonary artery pressure (26). Transthoracic echocardiography (TTE) and transesophageal echocardiography (TEE) were both performed in all patients preoperatively to assess RV and TV functions (Figure 1).
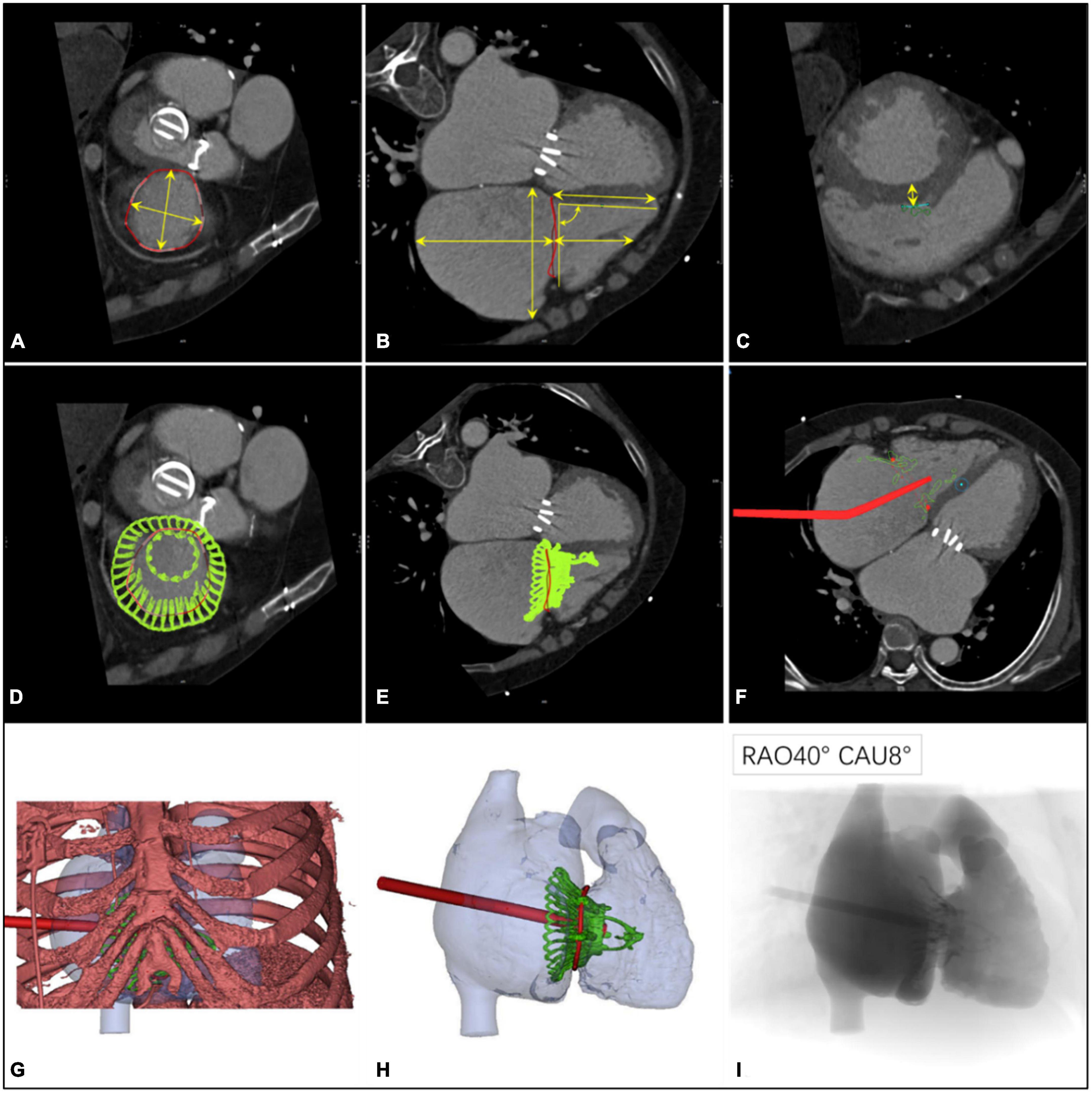
Figure 1. Preprocedural computerized tomography angiography assessment of transcatheter tri-cuspid valve replacement. (A) The diameter and perimeter of the tricuspid annulus (TA) were determined. (B) Measurements of the distance from the septal valve to the apex of the right ventricle, the height of the right atrium, and its relationship with the TA; the angle between the TA and the ventricular septum was 90° ± 10°. (C–E) Computer simulation of the LuX-Valve implant to observe the location of the anchor points and to measure the thickness of the ventricular septum in this position (30 mm below the TA). (F) Materialize Mimics 21.0 software (Materialize, Leuven, Belgium) was used to analyze the position and the angle of the delivery system on a 2-dimensional image. (G) The position of the right intercostal incision was determined with a digital 3-dimensional image. (H) The shape and the release position of the LuX-Valve were observed using 3-dimensional virtual models. (I) Simulation using fluoroscopic images provided an ideal projection angle for the transcatheter tricuspid valve replacement.
Device description
The LuX-Valve (Ningbo Jenscare Biotechnology, Ningbo, China) has a unique design concept of radial force independence, which consists of a biological valve stent, 3 valve lobules, and a steerable delivery system (Figure 2). It is funnel-shaped and consists of four parts: (a) A three-lobe artificial semilunar valve made of bovine pericardium treated with the GeniGal anticalcification process; (b) a self-expanding nitinol valve stent covered with polytetrafluoroethylene cloth, consisting of an atrial disc and soft adaptive annular sealing edges designed to prevent it from entering into the RV and to reduce paravalvular leakages; (c) the 20-mm “tongue” of the interventricular anchor (IVA), using a three-pronged nitinol anchor to grasp the valve stent to the diaphragm; (d) two 8-mm extended grips designed to capture the anterior TV ring. The delivery system consists of a 32 Fr sheath and a steerable tube. Four knobs, a plug, and a button on the handle control the bending of the sheath and the release of the valve.
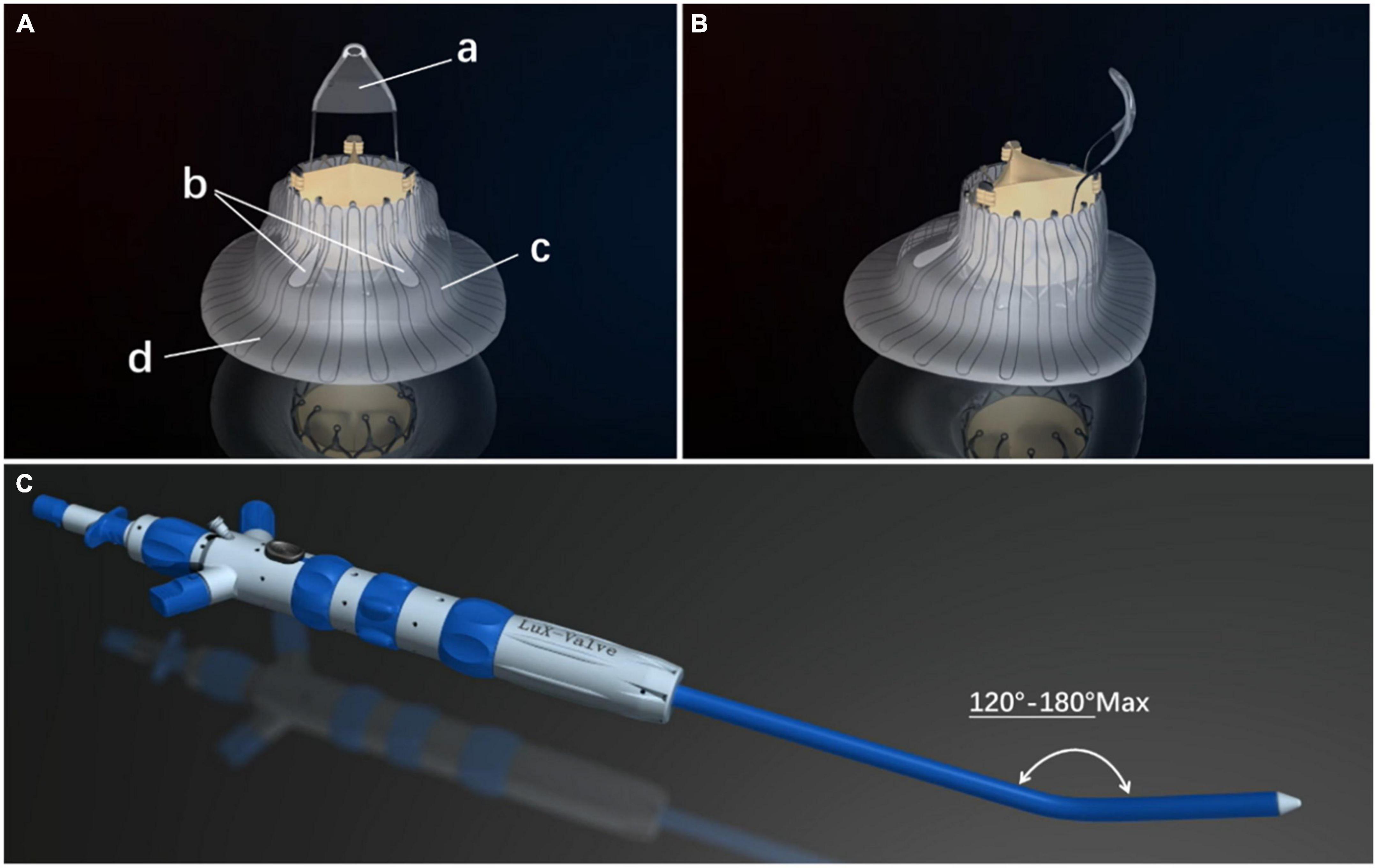
Figure 2. The LuX-Valve (Ningbo Jenscare Biotechnology, Ningbo, China) is self-expandable. The stent is made of a nickel–titanium alloy and the biological leaflet is bovine pericardium. The bio-prosthesis is implanted via the right atrial approach and fixed in the tricuspid annulus with its own unique anchoring device, independent of the radial support force. The part of the prosthesis located in the right atrium also prevents paravalvular leakage. (A) Right atrial view of the LuX-Valve. The four parts of the LuX-Valve stent include (a) the interventricular anchor, (b) two graspers, (c) the annulus skirt, and (d) the right atrial disc. (B) Lateral view of the LuX-Valve. (C) The delivery system of the LuX-Valve.
Procedural steps
The procedure was performed in the intubation laboratory. After the patient was given general anesthesia, the TV was entered with a right minimally invasive thoracotomy through the path of the right atrium (RA) (Figures 3A,B). TEE and X-ray fluoroscopy were used for guidance. TEE was mainly used to guide catheter delivery, valve release, and adjustment of the intraoperative valve position. A coronary artery guide wire was placed in the right coronary artery to help determine the annulus plane of the TV. Systemic heparinization was administered to achieve an activated coagulation time of > 200 s; then 4-0 Prolene sutures with felt sheets were used with a double purse-string suture in the RA. The delivery catheter was placed into the RV under the guidance of TEE and X-ray fluoroscopy. The angle of the catheter was adjusted to ensure that the catheter was coaxial and centered with the ring. When the catheter was positioned under the loop, which was approximately 5 cm, the IVA and two clamping keys of the anterior lobes were released in turn by adjusting the knob system on the catheter (Figure 3C). Then, the clamping keys were positioned properly under the anterior lobe, and the entire delivery system was gently retracted so that the clamping keys hooked the anterior lobe. The atrial plate was released, the IVA was deployed, and the anchor pin was inserted into the septum for fixation (Figure 3D). Finally, the catheter was withdrawn and removed; then, the heparin was neutralized and the atrial incision was closed (Figures 3E,F).
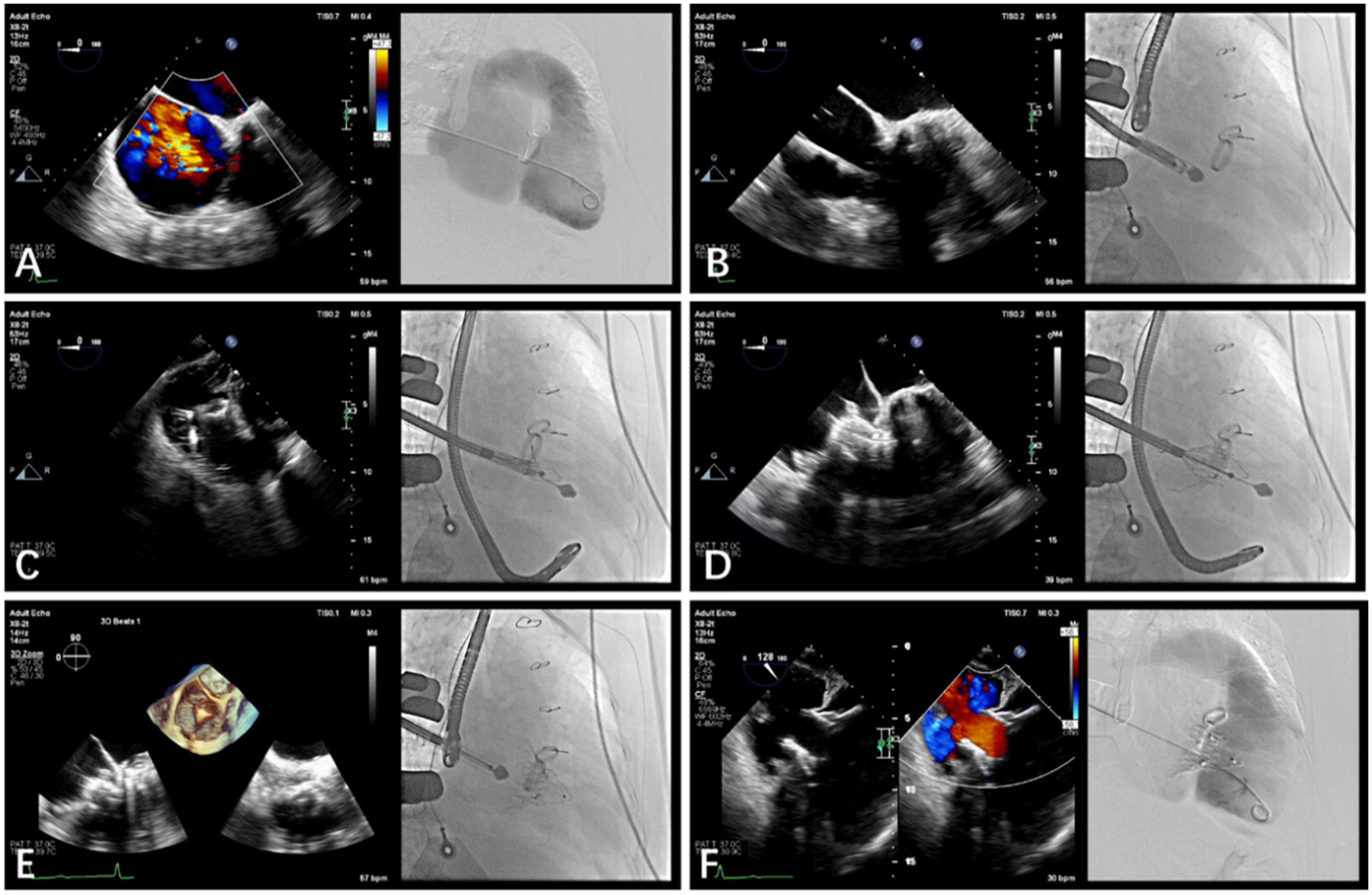
Figure 3. Guidance using transesophageal echocardiography (TEE) and fluoroscopic imaging in transcatheter tricuspid valve replacement. (A) TEE and fluoroscopy showed severe tricuspid re-gurgitation. (B) LuX-Valve guided by TEE was used to deliver the bioprosthesis to TA via the right intercostal approach. (C) The delivery system released the interventricular anchor and 2 graspers, and the graspers were guided by TEE to clamp the anterior leaflet. (D) The annulus skirt and the atrium disc were released in turn, and the position of the implant was adjusted by TEE to ensure that there was no obvious paravalvular leakage. (E) The bioprosthesis was completely re-leased after fixation with the interventricular anchor. (F) Postoperative computerized tomography angiography and TEE showed that tricuspid regurgitation disappeared immediately.
Data collection
Baseline data were collected from the electronic medical record system. The operative time, the device time, and the X-ray fluoroscopy time were recorded. The device time was defined as the time from catheter entry into the RA to withdrawal from the RA. In addition, data were collected during hospitalization (including the time in the intensive care unit and in the hospital and the postoperative TTE data).
Follow-up
Follow-up data were collected from enrolled patients at baseline, before discharge, and at 30 days, 6 months, and 1 year postoperatively. Primary end points included a successful operation and a successfully implanted device. Successful surgery was defined as the successful implantation of the valve and removal of the delivery system; the correct and stable placement of the prosthesis; and no serious or life-threatening adverse events during the operation. The function of the TV was recovered satisfactorily [TR severity is reduced by ≥ 2, TV pressure gradient (PG) ≤ 6 mmHg], and there were no cardiovascular-related deaths, implant displacements, valve failures, or other major adverse events related to the device (including myocardial infarction, embolism, conduction disturbances, and a new transventricular septal shunt).
Statistical analyses
Continuous variables were reported as the median (25th and 75th percentile), whereas classified variables were expressed by frequency and percentage. The paired t-test was used to compare continuous variables for each patient before and after the procedures, and other continuous variables were determined with the Student t-test. We compared the classification variables using the Wilcoxon signed rank test. A two-tailed P-value of < 0.05 was considered statistically significant. All statistical analyses were conducted using Statistical Package for Social Sciences (SPSS, Chicago, IL, USA) version 25.0.
Results
Baseline data
The baseline clinical features of the 15 patients are listed in Table 1. Despite receiving aggressive diuretic therapies, all patients had typical symptoms of severe right heart failure with ascites (46.7%) or peripheral edema (100.0%). In these 11 patients who had left-sided valvular surgery, 9 patients (81.8%) had been treated with surgical mitral valve replacement, and other 2 patients (18.2%) had been accepted with surgical mitral valve replacement and transcatheter aortic valve replacement. The causes of TR were left heart surgery (73.3%), permanent pacemaker or cardioverter defibrillator implants (40.0%), and AF (86.7%). Baseline echocardiographic and computed tomography (CT) parameters are listed in Table 2. All 15 patients had severe TR at baseline. Preoperative right heart catheterization showed that the systolic pulmonary arterial pressure of the included patients was 41.0 (32.0, 48.0) mm Hg, and 8 patients had pulmonary hypertension preoperatively. In addition, all patients were New York Heart Association (NYHA) functional class III/IV; the median European system for cardiac operative risk evaluation II was 9.5 (7.4, 11.6)% and the Society of Thoracic Surgeons score was 10.3 (7.8, 12.4)%, which indicated a high risk of cardiopulmonary bypass.
Intraoperative and hospitalization data
The intraoperative and hospitalization details are shown in Table 3. All patients were treated 3 to 5 days preoperatively and were given intravenous diuretics to reduce their weight and improve their peripheral edema. Surgical success was achieved in all patients (100%), with the individual valves in place in all cases. The operating time was 140.0 (110.0, 180.0) min, and the device time was 10.0 (7.0, 12.0) min, with no persistent ventricular arrhythmias, atrioventricular block, or cardiac rupture. In 6 patients who had previously been implanted with a permanent pacemaker or implantable cardioverter defibrillator, the lead remained attached to the RV with no change in threshold after the valve was implanted. After the procedures, TEE detected mild paravalvular leakage in 1 patient (6.6%), and moderate paravalvular leakage occurred in 1 patient (6.6%), possibly due to leaflet damage during the crimping of the valve. Postprocedural CT showed the precise location of the IVA and the two graspers (Figure 4). The remaining 13 patients (86.7%) had no/trace regurgitation. The mean postoperative times in the intensive care unit were 2.0 (1.0, 12.0) days, and the postoperative hospitalization times were 13.0 (7.0, 19.0) days. In patients with no preexisting renal impairment, RV angiography was performed to confirm the position and function of the implanted valve. Before discharge, CT was used to confirm the position and fixation details of the prosthesis. One patient died on postoperative day 12 of pulmonary infection, which was considered unrelated to the procedures or the devices. In addition, there were no pulmonary embolisms, cerebrovascular events, or new conduction blocks during hospitalization. All discharged patients were treated with anticoagulants. All patients had a ≥ 2 grade reduction in severity of TR from preoperative levels.
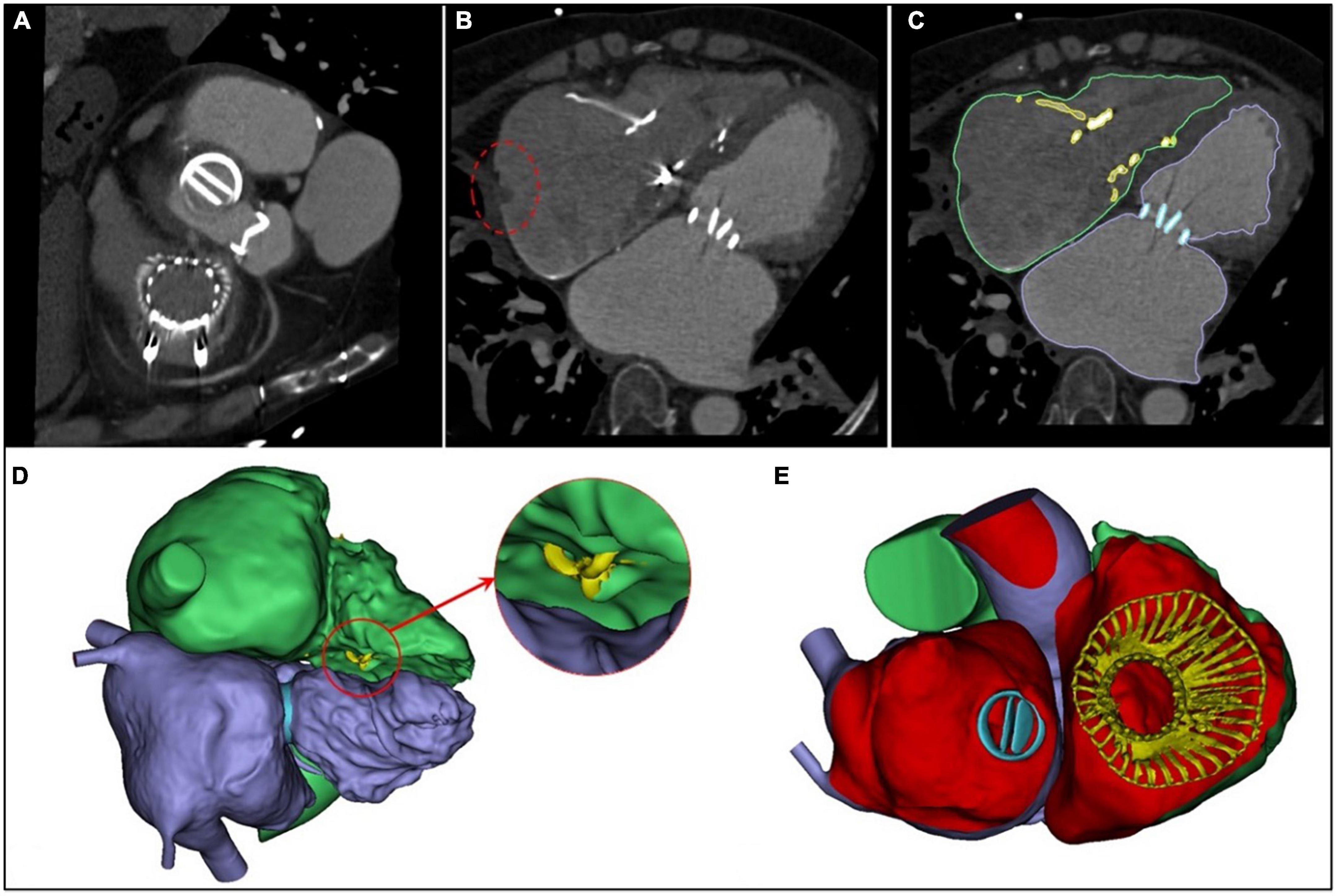
Figure 4. Postprocedural evaluation of the interventricular anchor. (A) A multislice computed tomography scan showed the precise positions of the two graspers. (B) The incision of the right atrium (the red circle) corresponds with Figure 1F. (C) The right heart is outlined in green; the left heart, in purple; the mechanical valve, in blue, and the LuX-Valve is yellow. (D) The yellow area in the red circle is the interventricular anchor. (E) The 3-dimensional reconstructed image from the right atrial plane demonstrates that the LuX-Valve is located in the normal position.
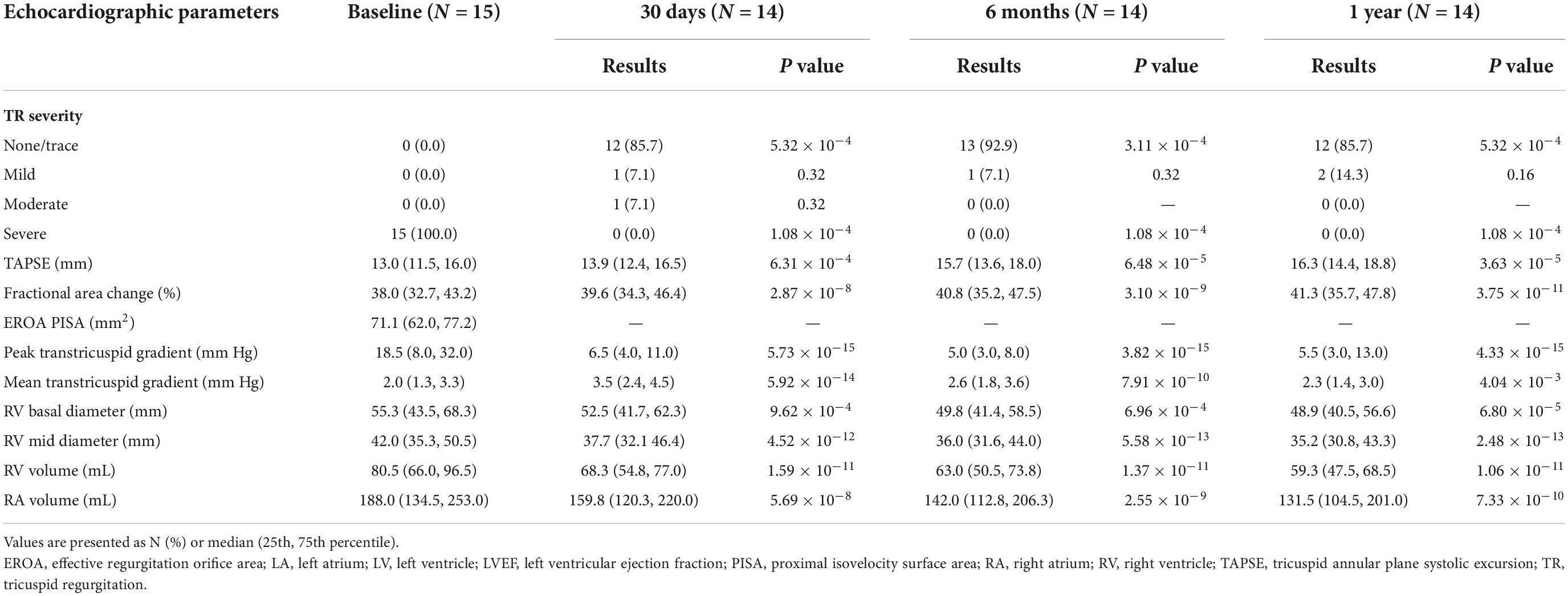
One-year follow-up data
Major follow-up outcomes at 1 year are shown in Table 4. Baseline to 1-year echocardiographic measurements are listed in Table 5. For 14 patients, TR severity measured by TTE decreased from 100.0% severe to 85.7% no/trace (P = 5.32 × 10–4). Of the remaining patients, 1 patient had mild paravalvular leakage, and another patient had moderate paravalvular leakage. TA diameter and RV diameter were both decreased compared with preoperative measurements, indicating RV remodeling. All patients exhibited significant improvement in symptoms at 6 months. For the 6-month follow-up data, the TR decreased to no/trace in 13 patients (92.9%, P = 3.11 × 10–4). One patient had mild paravalvular leakage. At the 1-year follow-up, TR decreased to no/trace in 12 patients (85.7%, P = 5.32 × 10–4). Two patients had mild paravalvular leakage. In addition, the reduction of the TV ring diameter and the increased deviation of the TV annular plane in systole indicated improvement in RV structure and function. Meanwhile, the TAPSE measurement improved significantly [16.3 (14.4, 18.8) vs. 13.0 (11.5, 16.0), P = 3.63 × 10–5], and the RV volume showed remarkable improvement [59.3 (47.5, 68.5) vs. 80.5 (66.0, 96.5), P = 1.06 × 10–11]. Furthermore, peripheral edema and ascites decreased to 28.6 and 14.3%, respectively (P = 1.57 × 10–3 and 2.53 × 10–2). The proportion of patients in NYHA functional class II was higher than that before the operation (11/14 vs. 0/15, P = 9.11 × 10–4). The 6-min walking test results showed significant improvement in motion performance [355.0 (310.0, 390.0) m vs. 210.0 (155.0, 270.0) m, P = 9.56 × 10–14). Kansas City cardiomyopathy questionnaire scores also improved significantly at the 1-year follow-up [62.0 (60.0, 66.0) vs. 32.0 (26.0, 39.0), P = 9.29 × 10–15]. Thirteen patients (92.9%) met the primary end points. One patient (7.1%) was re-hospitalized because of device thrombosis (Figure 5). Due to the LuX-Valve has a larger atrial plate compared to other devices, the bioprosthetic valve effectively prevents paravalvular leakage but is apt to thrombose. Furthermore, the lower pressure of the RV results in slower blood flow in comparison to blood flow through the left ventricle, and the dosage of anticoagulation has not been determined in the current studies.
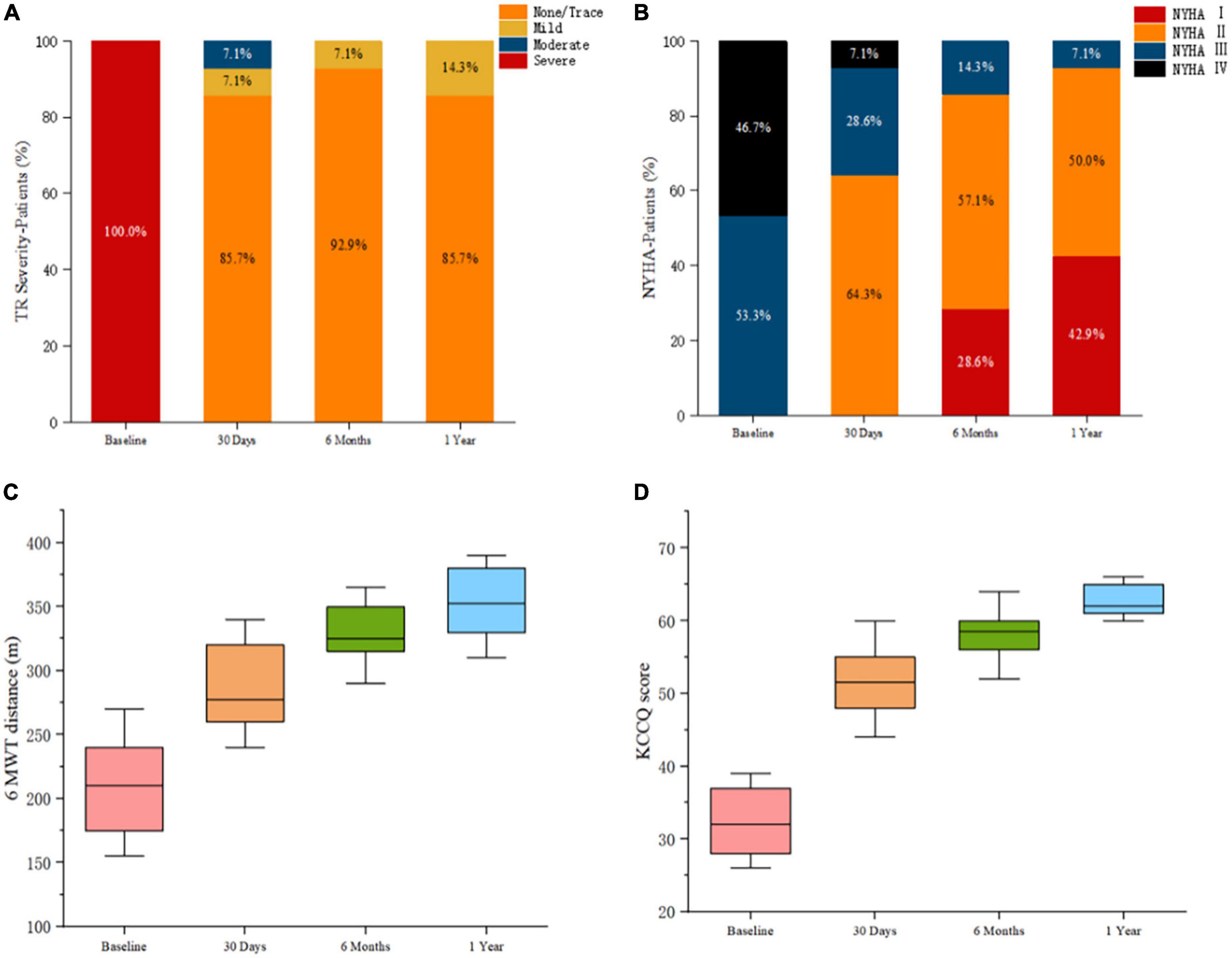
Figure 5. Postprocedural evaluation showed reduced severity of triscuspid regurgitation and improved clinical, functional, and quality-of-life outcomes. (A) Assessment of severity of tricuspid regurgitation. P-value calculated from the Wilcoxon signed rank test. (B) Comparison of New York Heart Association functional class pre-and post-procedures. P-value calculated from the Wilcoxon signed rank test. (C) Assessment of the 6-min walk test distances. P-value deter-mined from the paired Student t-test. (D) Assessment using the Kansas City Cardiomyopathy Questionnaire. P-value determined from the paired Student t-test.
Discussion
In this single-center, observational study, the LuX-Valve was successfully implanted in all 15 patients, and good clinical results were achieved without the complex TV anatomical structures and different etiologies. The unique anatomical structures and pathophysiological characteristics of the TV make the TTVR device difficult to design. From a physiological point of view of, the TV has a 3-dimensional structure similar to that of a saddle that exhibits dynamic changes during the cardiac cycle to ensure that the valve closes completely. Primary TR is caused by congenital or acquired abnormalities of the TV itself. However, secondary (or functional) TR, which is far more common than primary TR, is secondary to excess RV pressure and/or volume load. When TR occurs, the TV loses its normal shape and dilates under the strain of the dilated RA and RV. Recent studies suggest that the overloading of the RV caused by long-term TR may lead to irreversible myocardial injury of the RV (27). As a result, as the focus on TR has increased, the number of operations on the TV has increased (27). Most studies have reported incomplete reduction of TR (14, 28, 29). A recent large registry of patients who had transcatheter aortic valve replacement showed that the severity of preoperative TR was independently associated with 1-year postoperative mortality and rehospitalization for heart failure (30). In general, the TV may not provide stable support for traditional radial TTVR devices. The LuX-Valve is an in situ TTVR device with a non-radial support force that has unique advantages compared with those of the traditional radial support force devices. The selection of the valve size is based on the effective orifice area rather than on the expanded TV, which renders the selection of diameter sizes smaller. This design also ensures that the diameter of the annulus decreases as the RV remodeling reverses. In addition, the smaller valve has no radial support on the TV, so it is almost impossible to induce right ventricular outflow tract (RVOT) obstruction, right coronary artery injury, or conduction block (13). The LuX-Valve has a larger atrial plate compared to other devices, which effectively prevents paravalvular leakage after the valve is implanted. These advantages suggest that the LuX-Valve is suitable for the treatment of TR caused by a variety of etiologies, including functional TR, TR caused by the pacemaker lead, and chronic AF. Hahn reported that NaviGate system (NaviGate Cardiac Structures, Lake Forest, CA, USA), which was a radial force-dependent TTVR device. However, the patients who received NaviGate implantation had a high prevalence of bioprosthesis failure, atrioventricular block and paravalvular leakage (31). During the 1-year follow-up of this small series of patients with severe, symptomatic TR treated with TTVR, there were a number of important observations. First, TTVR virtually eliminates TR or underlying disease. Despite multiple comorbidities, those who survived to 1 year had RV remodeling and increased cardiac output. Previous studies have shown that changes of RV dimensions and function would predict TR after TTVR. RV systolic function is mainly determined by afterload, preload, and intrinsic myocardial contractility (32). With the significant decrease in TR after procedures, an increase in afterload may affect RV function or even induce irreversible changes. However, the further studies are needed to proceed. Second, successful procedures depend on the guidance of TEE and CTA. Preimplantation sizing may be adjusted in a number of different ways. In fact, even advanced 3-dimensional reconstruction tools are used. Third, due to the lack of obvious anatomical markers of TV under the guidance of digital subtraction angiography, accurate positioning is required when the LuX-Valve is implanted. Fourth, the increased incidence of pulmonary complications caused by bleeding in the chest should be prevented during the procedures. Fifth, the lower pressure of the RV results in slower blood flow in comparison to blood flow through the left ventricle, so anticoagulation is needed to prevent valve thrombosis. However, further research is needed to determine whether vitamin K antagonists, direct oral anticoagulants, or dual antiplatelet agents should be used.
At present, the morbidity of patients with severe TR is high, but the treatment effect is not satisfied, so the market prospect of interventions for TR in the future is broad. However, not all patients with TR meet the indications for interventions. In addition, many patients present with right heart failure and other manifestations at the time, so the perioperative management of patients with TR is more challenging. When selecting patients in the future, it is necessary to strengthen the evaluation of anatomical characteristics and comorbidities of the specific patient at the same time, and continuously improve the quality of surgical and perioperative management.
Limitations
This study has some limitations. First, this study lacks a control group undergoing traditional surgery (Such as a propensity score matched control group of patients with surgical tricuspid valve replacement via right thoracotomy), which requires a larger sample size and a well-designed clinical trial to confirm its long-term safety and effectiveness. Second, the use of the LuX-Valve is limited because the surgical approach is still through a thoracic incision, and its delivery system needs to be further improved to be implanted through the peripheral vein path. Third, whereas an average of multiple cardiac cycles is used to measure most RV parameters, strain imaging uses a single cycle and may not represent the entire RV function for patients with AF. Finally, the follow-up time was limited.
Conclusion
The patients with severe functional TR were treated by TTVR, which is a feasible, relatively safe and low-complication approach that improves RV remodeling and relieves symptoms of right heart failure with reliable clinical outcomes.
Data availability statement
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by the Xijing Hospital Ethics Committee, ClinicalTrials.gov protocol registration system (NCT02917980). The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
YuM, LL, and YL were responsible for wrote the manuscript. MZ and YaM were responsible for the figures. CX and PJ were responsible for the data collecting. JY was responsible for the manuscript reviewing. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Key R&D Program of China (No. 2020YFC2008100), the Shaanxi Province Innovation Capability Support Plan–Innovative Talent Promotion Plan (No. 2020TD-034), and the Discipline Boosting Program of Xijing Hospital (No. XJZT18MJ69).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Asmarats L, Taramasso M, Rodés-Cabau J. Tricuspid valve disease: diagnosis, prognosis and management of a rapidly evolving field. Nat Rev Cardiol. (2019) 16:538–54. doi: 10.1038/s41569-019-0186-1
2. Chorin E, Rozenbaum Z, Topilsky Y, Konigstein M, Ziv-Baran T, Richert E, et al. Tricuspid regurgitation and long-term clinical outcomes. Eur Heart J Cardiovasc Imaging. (2020) 21:157–65.
3. Nath J, Foster E, Heidenreich PA. Impact of tricuspid regurgitation on long-term survival. J Am Coll Cardiol. (2004) 43:405–9.
4. Voelkel NF, Quaife RA, Leinwand LA, Barst RJ, McGoon MD, Meldrum DR, et al. Right ventricular function and failure: report of a National Heart, Lung, and Blood Institute working group on cellular and molecular mechanisms of right heart failure. Circulation. (2006) 114:1883–91. doi: 10.1161/CIRCULATIONAHA.106.632208
5. Bustamante-Labarta M, Perrone S, De La Fuente RL, Stutzbach P, De La Hoz RP, Torino A, et al. Right atrial size and tricuspid regurgitation severity predict mortality or transplantation in primary pulmonary hypertension. J Am Soc Echocardiogr. (2002) 15:1160–4.
6. Utsunomiya H, Harada Y, Susawa H, Ueda Y, Izumi K, Itakura K, et al. Tricuspid valve geometry and right heart remodelling: insights into the mechanism of atrial functional tricuspid regurgitation. Eur Heart J Cardiovasc Imaging. (2020) 21:1068–78. doi: 10.1093/ehjci/jeaa194
7. Kim Y-J, Kwon D-A, Kim H-K, Park JS, Hahn S, Kim KH, et al. Determinants of surgical outcome in patients with isolated tricuspid regurgitation. Circulation. (2009) 120:1672–8.
8. Antunes MJ, Rodríguez-Palomares J, Prendergast B, De Bonis M, Rosenhek R, Al-Attar N, et al. Management of tricuspid valve regurgitation: position statement of the European Society of Cardiology Working Groups of Cardiovascular Surgery and Valvular Heart Disease. Eur J Cardiothorac Surg. (2017) 52:1022–30. doi: 10.1093/ejcts/ezx279
9. Rogers JH, Bolling SF. The tricuspid valve: current perspective and evolving management of tricuspid regurgitation. Circulation. (2009) 119:2718–25.
10. Sales VL, McCarthy PM. Durability of functional tricuspid valve repair. Semin Thorac Cardiovasc Surg. (2010) 22:97–103.
11. Taramasso M, Vanermen H, Maisano F, Guidotti A, La Canna G, Alfieri O. The growing clinical importance of secondary tricuspid regurgitation. J Am Coll Cardiol. (2012) 59:703–10.
12. Rodes-Cabau J, Hahn RT, Latib A, Laule M, Lauten A, Maisano F, et al. Transcatheter therapies for treating tricuspid regurgitation. J Am Coll Cardiol. (2016) 67:1829–45.
13. Hahn RT, Meduri CU, Davidson CJ, Lim S, Nazif TM, Ricciardi MJ, et al. Early feasibility study of a transcatheter tricuspid valve annuloplasty: SCOUT trial 30-day results. J Am Coll Cardiol. (2017) 69:1795–806. doi: 10.1016/j.jacc.2017.01.054
14. Nickenig G, Kowalski M, Hausleiter J, Braun D, Schofer J, Yzeiraj E, et al. Transcatheter treatment of severe tricuspid regurgitation with the edge-to-edge mitraclip technique. Circulation. (2017) 135:1802–14.
15. Campelo-Parada F, Perlman G, Philippon F, Ye J, Thompson C, Bédard E, et al. First-in-man experience of a novel transcatheter repair system for treating severe tricuspid regurgitation. J Am Coll Cardiol. (2015) 66:2475–83. doi: 10.1016/j.jacc.2015.09.068
16. Rosser BA, Taramasso M, Maisano F. Transcatheter interventions for tricuspid regurgitation: TriCinch (4Tech). EuroIntervention. (2016) 12:Y110–2.
17. Figulla HR, Kiss K, Lauten A. Transcatheter interventions for tricuspid regurgitation – heterotopic technology: TricValve. EuroIntervention. (2016) 12:116–8. doi: 10.4244/EIJV12SYA32
18. Taramasso M, Alessandrini H, Latib A, Asami M, Attinger-Toller A, Biasco L, et al. Outcomes after current transcatheter tricuspid valve intervention: mid-term results from the International TriValve registry. JACC Cardiovasc Interv. (2019) 12:155–65. doi: 10.1016/j.jcin.2018.10.022
19. Fam NP, Braun D, von Bardeleben RS, Nabauer M, Ruf T, Connelly KA, et al. Compassionate use of the PASCAL transcatheter valve repair system for severe tricuspid regurgitation: a multicenter, observational, first-in-human experience. JACC Cardiovasc Interv. (2019) 12:2488–95. doi: 10.1016/j.jcin.2019.09.046
20. Nickenig G, Weber M, Lurz P, von Bardeleben RS, Sitges M, Sorajja P, et al. Transcatheter edge-to-edge repair for reduction of tricuspid regurgitation: 6-month outcomes of the TRILUMINATE single-arm study. Lancet. (2019) 394:2002–11. doi: 10.1016/S0140-6736(19)32600-5
21. Lu F, Qiao F, Lv Y, An Z, Liu X, Cao P, et al. A radial force-independent bioprosthesis for transcatheter tricuspid valve implantation in a preclinical model. Int J Cardiol. (2020) 319:120–6. doi: 10.1016/j.ijcard.2020.06.070
22. Lu FL, Ma Y, An Z, Cai CL, Li BL, Song ZG, et al. First-In-Man experience of transcatheter tricuspid valve replacement with LuX-Valve in high-risk tricuspid regurgitation patients. JACC Cardiovasc Interv. (2020) 13:1614–6. doi: 10.1016/j.jcin.2020.03.026
23. Hahn RT, Zamorano JL. The need for a new tricuspid regurgitation grading scheme. Eur Heart J Cardiovasc Imaging. (2017) 18:1342–1343.
24. Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, et al. 2017 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. (2017) 38:2739–91.
25. Dreyfus GD, Martin RP, Chan KM, Dulguerov F, Alexandrescu C. Functional tricuspid regurgitation: a need to revise our understanding. J Am Coll Cardiol. (2015) 65:2331–6. doi: 10.1016/j.jacc.2015.04.011
26. Taramasso M, Pozzoli A, Guidotti A, Nietlispach F, Inderbitzin DT, Benussi S, et al. Percutaneous tricuspid valve therapies: the new frontier. Eur Heart J. (2017) 38:639–47.
27. Arsalan M, Walther T, Smith RL, Grayburn PA. Tricuspid regurgitation diagnosis and treatment. Eur Heart J. (2017) 38:634–8.
28. Taramasso M, Hahn RT, Alessandrini H, Latib A, Attinger-Toller A, Braun D, et al. The International Multicenter TriValve Registry: which patients are undergoing transcatheter tricuspid repair? J Am Coll Cardiol Interv. (2017) 10:1982–90.
29. Perlman G, Praz F, Puri R, Ofek H, Ye J, Philippon F, et al. Transcatheter tricuspid valve repair with a new transcatheter coaptation system for the treatment of severe tricuspid regurgitation: 1-year clinical and echocardiographic results. J Am Coll Cardiol Interv. (2017) 10:1994–2003.
30. McCarthy FH, Vemulapalli S, Li Z, Thourani V, Matsouaka RA, Desai ND, et al. Association of tricuspid regurgitation with transcatheter aortic valve replacement outcomes: a report from the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry. Ann Thorac Surg. (2018) 105:1121–8. doi: 10.1016/j.athoracsur.2017.11.018
31. Hahn RT, Kodali S, Fam N, Bapat V, Bartus K, Rodés-Cabau J, et al. Early multinational experience of transcatheter tricuspid valve replacement for treating severe tricuspid regurgitation. JACC Cardiovasc Interv. (2020) 13:2482–93. doi: 10.1016/j.jcin.2020.07.008
Keywords: tricuspid regurgitation, transcatheter tricuspid valve replacement, LuX-Valve, follow-up, tricuspid valve
Citation: Mao Y, Li L, Liu Y, Zhai M, Ma Y, Xu C, Jin P and Yang J (2022) Safety, efficacy, and clinical outcomes of transcatheter tricuspid valve replacement: One-year follow-up. Front. Cardiovasc. Med. 9:1019813. doi: 10.3389/fcvm.2022.1019813
Received: 15 August 2022; Accepted: 17 November 2022;
Published: 01 December 2022.
Edited by:
Tiffany Patterson, King’s College London, United KingdomReviewed by:
Ahmet Rüçhan Akar, Ankara University, TurkeyAlberto Guido Pozzoli, Ospedale Regionale di Lugano, Switzerland
Copyright © 2022 Mao, Li, Liu, Zhai, Ma, Xu, Jin and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jian Yang, eWFuZ2ppYW4xMjEyQGhvdG1haWwuY29t
†These authors have contributed equally to this work
 Yu Mao
Yu Mao Lanlan Li
Lanlan Li Yang Liu
Yang Liu Mengen Zhai
Mengen Zhai Yanyan Ma
Yanyan Ma Chennian Xu
Chennian Xu Ping Jin
Ping Jin Jian Yang
Jian Yang