
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Cardiovasc. Med. , 20 October 2022
Sec. Thrombosis and Haemostasis
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.1018649
This article is part of the Research Topic The Individualization of Antiplatelet Therapy in Coronary Artery Disease: Escalation or de-escalations View all 6 articles
 Mohamed Farag1,2*†
Mohamed Farag1,2*† Visvesh Jeyalan3†
Visvesh Jeyalan3† Jose Luis Ferreiro4,5
Jose Luis Ferreiro4,5 Young-Hoon Jeong6,7
Young-Hoon Jeong6,7 Tobias Geisler8
Tobias Geisler8 Diana A. Gorog1,2,9
Diana A. Gorog1,2,9Current guidelines for patients with acute coronary syndrome (ACS) recommend dual antiplatelet therapy (DAPT) for 12 months. Since bleeding is the main Achilles' heel of DAPT, in recent years several randomized controlled trials have evaluated the safety and efficacy of de-escalation of DAPT with respect to ischaemic and bleeding endpoints. These trials can be broadly divided into studies evaluating a shorter duration of DAPT, and those studies in which DAPT that includes a potent P2Y12 inhibitor, such as prasugrel or ticagrelor, is compared to less intense DAPT, mainly clopidogrel or reduced-dose prasugrel. We sought to evaluate the studies assessing de-escalation of DAPT in patients with ACS undergoing PCI. We review the studies evaluating the strategies of de-escalation of DAPT intensity and those evaluating a strategy of de-escalation of DAPT duration in ACS patients undergoing PCI. We summarize the limitations of studies to date, gaps in evidence and make recommendations for future studies.
Dual antiplatelet therapy (DAPT) is the cornerstone of treatment for patients with acute coronary syndromes (ACS) undergoing percutaneous coronary intervention (PCI). Current ESC guidelines recommend 1 year of DAPT unless contraindicated or if the bleeding risk is excessive (1–3). These guidelines also recommend use of a potent P2Y12 inhibitor, namely ticagrelor or prasugrel, over clopidogrel. However, this duration and intensity of DAPT exposes patients to increased bleeding risk, which is emerging as at least an equal, if not greater concern, than the ischaemic risk, with significant impact on mortality (4–6). Increased awareness of the prognostic importance of bleeding, together with observed increase in bleeding rates have prompted studies that consider alternatives to 12 months of high-intensity DAPT to balance thrombotic and bleeding risks. Several randomized controlled trials have investigated various de-escalation strategies in ACS patients undergoing PCI, either by reducing the intensity of DAPT, through switching from more potent P2Y12 inhibitors prasugrel or ticagrelor to clopidogrel, or by shortening the duration of DAPT and continuing with single antiplatelet therapy (SAPT). We sought to review the evidence supporting de-escalation of DAPT in patients with ACS undergoing PCI.
The TRITON-TIMI 38 and PLATO multicentre randomized controlled trials were the first to compare the effectiveness of DAPT containing prasugrel or ticagrelor, with DAPT containing clopidogrel, in ACS patients including those undergoing PCI (7–9). The TRITON-TIMI 38 trial compared prasugrel to clopidogrel, in combination with aspirin, and all patients underwent revascularization (7, 8). The PLATO trial compared 12 months of ticagrelor to clopidogrel, in combination with aspirin (9), with 65% of patients undergoing revascularisation. Both trials demonstrated a reduction in ischaemic events within the first 30 days, whereas the difference in bleeding was mainly seen after this period. These trials led to the preferential recommendation in the ESC Guidelines for prasugrel or ticagrelor over clopidogrel in ACS patients undergoing PCI (1–3). Notably, in PLATO and TRITON-TIMI 38, few patients were aged ≥75 years (15 and 13%, respectively), a fewer than seen amongst ACS patients in daily practice, although the benefit of ticagrelor was seen regardless of age, in PLATO (9), but not in TRITON-TIMI 38 (7).
Twenty-five prospective trials assessed de-escalation of DAPT duration or intensity in ACS (Tables 1, 2). We excluded those studies in which ACS patients formed only a minority of the cohort, or when randomization occurred beyond 3 months after post-ACS (36–38). We present trial data including the trial-defined primary efficacy endpoint, which most often included major adverse cardiovascular events (MACE), namely the composite of death, myocardial infarction (MI) and stroke or net adverse cardiovascular events (NACE, composite of MACE and trial-defined bleeding) and the primary safety endpoint of bleeding (major or clinically-relevant non-major bleeding).
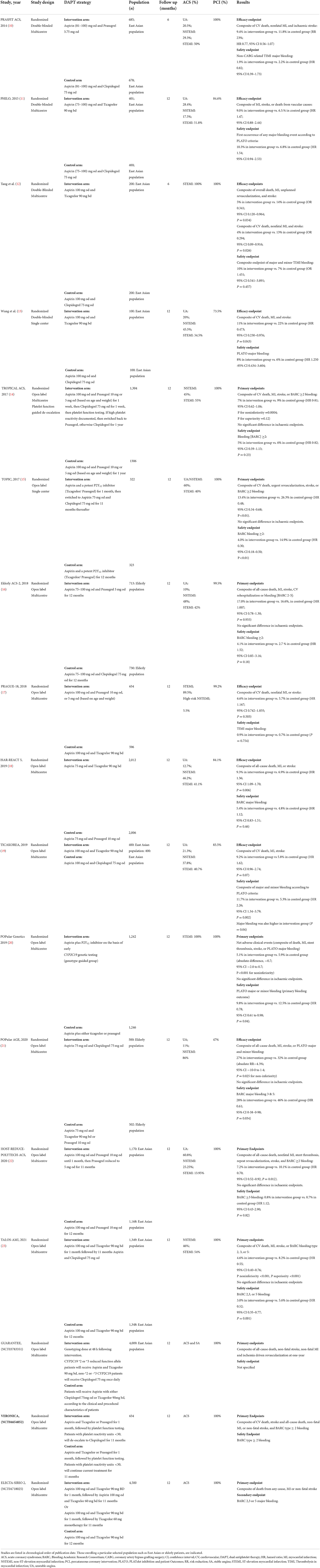
Table 1. Reduced intensity or de-escalation of dual antiplatelet therapy intensity in ACS population undergoing PCI.
Trials assessing the safety and efficacy of various de-escalation strategies performed a head-to-head comparison of (i) more potent DAPT, containing ticagrelor or prasugrel, with DAPT containing clopidogrel, or (ii) potent DAPT for 6–12 months with potent DAPT only for 1–4 weeks followed by de-escalation to clopidogrel or low dose prasugrel, or (iii) DAPT containing prasugrel to DAPT containing ticagrelor (Table 1) (10–23). We highlight some idiosyncrasies below and indicate which category above (i–iii) the study belongs to.
The single-center TOPIC trial (ii) showed that de-escalation of DAPT intensity at 1 month post-ACS from aspirin plus ticagrelor or prasugrel to aspirin plus clopidogrel, was superior to 12 months of aspirin plus ticagrelor or prasugrel, with a reduction in the composite of ischaemic and bleeding endpoints, driven by a reduction in major bleeding (15). Notably, the primary endpoint of the composite of cardiovascular death, unplanned hospitalization leading to urgent coronary revascularization, stroke, and bleeding academic research consortium (BARC) ≥2 bleeding, did not specifically include MI, although most likely would have been captured by unplanned hospitalization.
De-escalation guided by platelet function testing (PFT) was assessed in the TROPICAL-ACS study (ii) (14). Here, DAPT comprising of aspirin plus prasugrel was compared with de-escalation to clopidogrel. In the de-escalation arm, prasugrel was given for 1 week, followed by clopidogrel for 1 week, then PFT was conducted using the Multiplate Analyzer. If high platelet reactivity was documented, patients were switched back to prasugrel, otherwise clopidogrel was continued. The primary endpoint of the composite of cardiovascular death, MI, stroke, or bleeding (BARC ≥ 2) occurred less often in the guided de-escalation group than in the control group, with no significant difference in ischaemic endpoints or BARC ≥2 bleeding, but a reduction in the secondary endpoint of BARC 3 or 5 bleeding (14).
The PRASFIT-ACS study (i) compared DAPT comprising of low dose prasugrel (3.75 mg daily) plus aspirin to DAPT containing clopidogrel plus aspirin (10). The primary endpoint of MACE at 24 weeks occurred in 9.4% of the prasugrel and 11.8% of the clopidogrel group, showing use of lower dose prasugrel (3.75 mg) in East Asians seems to achieve similar effects to those seen in TRITON-TIMI 38 with full-dose prasugrel compared to clopidogrel in predominantly Western patients (7).
The HOST-REDUCE-POLYTECH-ACS trial (ii) evaluated de-escalation of DAPT at 1-month post-ACS, from 10 to 5 mg prasugrel, in combination with aspirin for 12 months, in Korea (22). Standard-dose prasugrel 10 mg daily was associated with higher bleeding rates than the same dose in Western populations (39, 40). Interestingly, a subsequent pre-specified subgroup analysis showed that whilst prasugrel de-escalation decreased NACE due to a reduction in bleeding, this benefit was confined to non-ST segment elevation ACS (NSTE-ACS) patients and not seen in patients with STEMI (41).
The POPular Genetics study (i) assessed the use of lower intensity DAPT, guided by CYP2C1 genotyping, against standard DAPT containing ticagrelor or prasugrel, in patients undergoing primary PCI (20). In the genotype-guided group, carriers of CYP2C19*2 or CYP2C19*3 loss-of-function alleles received ticagrelor or prasugrel (39%), and noncarriers received clopidogrel (61%). Genotype-guided use of reduced intensity DAPT was noninferior to standard DAPT with respect to thrombotic events and significantly reduced bleeding.
In the POPular AGE trial (i), patients with NSTE-ACS aged 70 or more years were randomized to DAPT comprising of either aspirin plus clopidogrel or aspirin plus prasugrel or ticagrelor (21). In the control arm, 93.8% of patients received ticagrelor. Aspirin plus clopidogrel met the criteria for non-inferiority with respect to NACE and for superiority with respect to PLATO major and minor bleeding. Importantly, since only 47% of patients underwent PCI, the study was under-powered to assess the safety of de-escalation in this cohort with respect to ischaemic endpoints.
The Elderly-ACS 2 trial (i) in patients aged >74 years with ACS undergoing PCI compared DAPT comprising of aspirin plus low-dose prasugrel (5 mg daily) to aspirin plus clopidogrel for 12 months (16). The study was terminated prematurely for futility following a planned interim analysis. There was no difference in the primary endpoint of all-cause death, MI, stroke, rehospitalization or bleeding, or the secondary endpoint of BARC ≥2 bleeding, although stent thrombosis occurred more frequently in patients taking clopidogrel compared to those taking prasugrel.
Eleven studies assessed de-escalation of DAPT duration from 12 months to a shorter period (Table 2) (24–35). Some of the earliest studies had relatively small sample size, with lower than expected rates of adverse events (29). The GLOBAL LEADERS trial in patients undergoing PCI for stable coronary disease or ACS, compared aspirin plus ticagrelor for 1 month, followed by 23 months of ticagrelor monotherapy, or standard DAPT with aspirin daily plus either clopidogrel (for patients with stable coronary disease) or ticagrelor (for patients with ACS) for 12 months, followed by aspirin monotherapy for 12 months (27). The trial failed to show any benefit at 2 years on the primary endpoint of the composite of all-cause death and MI. However, abbreviated DAPT reduced bleeding in the ACS subgroup (28).
The TWILIGHT study evaluated de-escalation of DAPT from aspirin and ticagrelor, to ticagrelor alone, at 3 months post-PCI, with 65% of patients undergoing PCI (30). De-escalation reduced the incidence of clinically-relevant bleeding, without an increase in death, MI or stroke.
The MASTER DAPT study compared short-term DAPT (1 month) followed by monotherapy with clopidogrel (54%) or aspirin, with DAPT for 3 months or more, in post-PCI patients at high bleeding risk, and 40% of patients had an ACS presentation (35). Whilst the results showed that 1-month was noninferior to 3 months or more DAPT for NACE, and superior for reducing the composite of major or clinically relevant nonmajor bleeding, it should be noted that the latter included BARC 2 as well as BARC 3 and 5 bleeding and that 37% of patients were receiving anticoagulation.
The STOPDAPT-2 was an open label randomized trial in patients with ACS (38%) or stable angina, randomized to either 1 month of DAPT followed by clopidogrel monotherapy or to 12 months of DAPT with aspirin and clopidogrel (32). Abbreviated DAPT met the criteria for noninferiority and superiority compared with 12-months DAPT for the composite primary endpoint of cardiovascular death, MI, stroke, stent thrombosis, or major or minor bleeding, including in ACS patients. However, in the subsequent STOPDAPT-2 ACS trial in patients with ACS undergoing PCI, 1-month DAPT followed by clopidogrel monotherapy did not meet the criteria for non-inferiority compared to 12 months of DAPT with respect to NACE, comprising of cardiovascular death, MI, stroke, stent thrombosis or bleeding (including minor bleeding). There was a trend toward harm with a 2-fold increase in MI with the 1-month DAPT regimen, although there was a reduction in bleeding (33).
The SMART-DATE trial compared 6 months of DAPT followed by aspirin alone to conventional 12 months DAPT (26). Although there was no difference in the composite of all-cause death, MI, or stroke, with 6 months DAPT meeting criteria for non-inferiority, there was a significantly increase in MI with 6 vs. 12 months of DAPT, without a reduction in bleeding.
The SMART-CHOICE trial randomized patients receiving PCI to either continue or to stop aspirin after 3 months of DAPT. Around 58% of patients had ACS and some 77% of patients had clopidogrel as the P2Y12 inhibitor in combination with aspirin (31). The composite of all-cause death, MI, or stroke at 12 months was similar between the study arms, with a reduction in bleeding with abbreviated DAPT.
The TRITON-TIMI 38 and PLATO trials showed that the greatest ischaemic benefit from DAPT with a P2Y12 inhibitor was achieved early, within the first 30 days post-ACS, and that the bleeding risk was mainly apparent beyond this (7, 9). A number of trials subsequently assessed de-escalation of DAPT either through reduction in DAPT intensity or duration.
Overall, de-escalation of DAPT duration post-ACS to monotherapy appears favorable, with reduction in bleeding, mostly without increase in MACE, although an increase in ischaemic events was noted in some studies with abbreviated DAPT. Likewise, de-escalation of DAPT intensity appears to significantly reduce major bleeding, without significant effect on MACE. Importantly, these approaches have not been tested with adequately powered trials in patients at high ischaemic risk, therefore these approaches should be generally confined to low ischaemic, high bleeding risk patients.
Importantly, most of the studies showing a benefit of de-escalation of DAPT intensity were conducted in East Asian patients, who are more prone to bleeding (39). In Westerners, the strategy of de-escalation of DAPT intensity from ticagrelor or prasugrel to clopidogrel, after a short period of more intense DAPT, was only evaluated in two relatively small studies, one of which used PFT to guide de-escalation (14, 15). Combining all studies, in East Asian, Western and elderly patients, the use of lower intensity P2Y12 inhibitor, namely clopidogrel, compared to ticagrelor or prasugrel, appears to have no significant impact on net adverse events, although it is important to look at different populations where specific bleeding or ischaemic risks may predominate. Specifically, comparing the efficacy of clopidogrel to ticagrelor or prasugrel as part of DAPT, the evidence, largely driven by the original PLATO and TRITON-TIMI 38 studies, indicates a trend toward increased MACE and reduction of major bleeding with clopidogrel. The reduction in major bleeding in TOPIC and TROPICAL-ACS had very wide confidence intervals and one of the studies used a guided-de-escalation with PFT, and whilst the POPular GENETICS study showed reduced bleeding, the evidence cannot confidently support this approach in the broad population, especially without genetic or PFT testing to guide treatment. In East Asian patients with relatively low thrombogenic milieu, (42) de-escalation of DAPT intensity from appears to have no significant effect on ischaemic endpoints, but significantly reduces major bleeding. On the other hand, whilst most studies in East Asian patients have shown that reduction of DAPT duration significantly reduces NACE and bleeding, there are two studies, SMART-DATE and STOPDAPT-2 ACS, which indicate a possible increase in ischaemic risk with reduced DAPT duration. A similar signal was seen in the subgroup analysis of the HOST-REDUCE-POLYTECH-ACS study (22). However, some studies in East Asian patients used prasugrel 3.75 mg daily (10, 32, 33), a dose that has not been tested for efficacy in Western patients. Furthermore, the type and potency of antiplatelet agent used as monotherapy can be related to an increased risk of thrombotic events during the early phase of ACS. In the elderly, lower intensity DAPT appears to reduce bleeding, without increasing ischaemic events.
A recent network meta-analysis compared the two de-escalation strategies in ACS patients undergoing PCI, namely shorter DAPT vs. de-escalation of DAPT intensity (43). Whilst there was no difference in all-cause mortality, de-escalation overall reduced NACE (trial defined composite of MI, stroke, stent thrombosis, and minor bleeding), while shortened DAPT decreased major bleeding. Another meta-analysis of 19 randomized controlled trials assessing de-escalation of DAPT in ACS concluded that compared to personalized de-escalation guided by PFT or genotyping, unguided de-escalation was as safe, if not safer, with decreased bleeding and without excess ischemic risk (44). Notably that meta-analysis included patients not receiving PCI, and guided de-escalation was predominantly assessed in Westerners, whereas unguided de-escalation predominantly in East Asians. Another meta-analysis of guided vs. standard DAPT in patients undergoing PCI, showed that guided de-escalation reduced MACE, including its components, with reduction in minor but not major bleeding (45). However, that metanalysis included 11 randomized and 3 observational studies utilizing both escalation and de-escalation of antiplatelet therapy, included patients with chronic coronary syndrome, and some studies used non-conventional antiplatelet therapy namely cilostazol or double-dose clopidogrel. Whilst there has been no head-to-head comparison of genotyping or PFT guided de-escalation, subgroup analysis showed no difference in outcomes whether PFT or genotyping was utilized to guide DAPT (45). Indeed, there are pros and cons to both strategies, which is beyond the scope of this review, and a combined approach using both strategies may have added advantages, but has not been evaluated.
Our review has a number of potential limitations. Firstly, there is heterogeneity in reporting bleeding, with various definitions used including BARC, PLATO and TIMI classifications. Even amongst studies that included the same classification of bleeding (e.g., BARC), some studies have included BARC 2, 3 and 5 bleeding events, whilst others included only BARC 3 and 5. There was also heterogeneity in the populations studied, with some only assessing ACS patients undergoing PCI, whilst others included patients with chronic coronary syndrome or some medically-managed ACS patients. The regimens and doses of antiplatelet agents varied, particularly in studies conducted in East Asia, where lower doses of prasugrel were used. There was heterogeneity amongst studies with respect to the monotherapy (SAPT) continued after shortened DAPT, some continuing with aspirin, whilst others continuing ticagrelor or clopidogrel. The duration of “shortened” DAPT also varied from 1 to 6 months. Amongst the studies investigating de-escalation of DAPT intensity, there was heterogeneity in the “intense” regimen with some studies giving ticagrelor, some prasugrel and some either prasugrel or ticagrelor. Many studies were open label and generally, high risk bleeding patients were underrepresented. Some studies included patients taking oral anticoagulation.
There are currently a number of gaps, which limit the applicability of these trial results to the main population of patients with ACS undergoing PCI.
There has been no direct head-to-head comparison of de-escalation of DAPT intensity with de-escalation of DAPT duration, and this is a significant limitation for the clinician, when attempting to choose an option to reduce bleeding risk.
Whilst it would appear sensible to de-escalate either DAPT intensity or duration in high bleeding risk patients, in practice it is difficult to separate patients at high bleeding risk, from those at high ischaemic risk, with overlapping risk factors including age and renal impairment.
Furthermore, no trial has assessed de-escalation strategies in high ischaemic risk patients, namely those with ST-elevation MI with multiple or extensive stenting, patients with residual disease, renal impairment, or severe left ventricular impairment. Lastly, several studies also included non-ACS patients, and those were generally under-powered to assess outcomes purely in the ACS subgroups.
Whilst a number of studies are ongoing (Tables 1, 2), there is a need to assess a combined approach, namely de-escalation of both intensity and duration, together, in patients at high bleeding risk, particularly the elderly. Furthermore, following abbreviated DAPT, the different drug options for SAPT, namely aspirin, clopidogrel or ticagrelor, need to be compared, to identify the optimal monotherapy, either empirically or guided by PFT.
Another gap in evidence is classifying patients in a uniformly applicable way, to high bleeding risk, high ischaemic risk, or both. This would enable clinicians to apply the results of such trials more easily to everyday practice.
Incorporation of risk scores or biomarkers of ischaemic or bleeding risk, such as high-sensitivity C-reactive protein and platelet function, into future trials would help identify patients who may benefit from and who may potentially come to harm, with de-escalation.
There have been no trials assessing shorter DAPT duration in the elderly. With an aging population and bleeding complications occurring typically 1–12 months post-ACS, this is an unmet need. Women are generally at higher bleeding risk than men with DAPT, yet women form only a minority of patients in most studies. High platelet reactivity significantly increases the risk of thrombosis only in men, whereas this phenotype is mainly associated with reduced bleeding only in women (46). Thus, specific trials in women, or patient-level data analyses combining the results of trials to date would be useful to identify optimal DAPT intensity or duration in women.
All authors have made significant contributions to the manuscript that justifies authorship, read, and approved the final manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
ACS, acute coronary syndrome; BARC, bleeding academic research consortium; DAPT, dual antiplatelet therapy; MACE, major adverse cardiovascular events; NACE, net adverse cardiovascular events; MI, myocardial infarction; PCI, percutaneous coronary intervention; PFT, platelet function testing; SAPT, single antiplatelet therapy.
1. Collet JP, Thiele H, Barbato E, Barthélémy O, Bauersachs J, Bhatt DL, et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. (2021) 42:1289–367. doi: 10.1093/eurheartj/ehaa575
2. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. (2018) 39:119–77. doi: 10.1093/eurheartj/ehx393
3. Valgimigli M, Bueno H, Byrne RA, Collet JP, Costa F, Jeppsson A, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. (2018) 39:213–60. doi: 10.1093/eurheartj/ehx419
4. Stone GW, Witzenbichler B, Weisz G, Rinaldi MJ, Neumann FJ, Metzger DC et al. Platelet reactivity and clinical outcomes after coronary artery implantation of drug-eluting stents (ADAPT-DES): a prospective multicentre registry study. Lancet. (2013) 382:614–23. doi: 10.1016/S0140-6736(13)61170-8
5. Ndrepepa G, Schuster T, Hadamitzky M, Byrne RA, Mehilli J, Neumann FJ et al. Validation of the Bleeding Academic Research Consortium definition of bleeding in patients with coronary artery disease undergoing percutaneous coronary intervention. Circulation. (2012) 125:1424–31. doi: 10.1161/CIRCULATIONAHA.111.060871
6. Montalescot G, Brieger D, Dalby AJ, Park SJ, Mehran R. Duration of dual antiplatelet therapy after coronary stenting: a review of the evidence. J Am Coll Cardiol. (2015) 66:832–47. doi: 10.1016/j.jacc.2015.05.053
7. Antman EM, Wiviott SD, Murphy SA, Voitk J, Hasin Y, Widimsky P, et al. Early and late benefits of prasugrel in patients with acute coronary syndromes undergoing percutaneous coronary intervention: a TRITON-TIMI 38 (TRial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet InhibitioN with Prasugrel-Thrombolysis In Myocardial Infarction) analysis. J Am Coll Cardiol. (2008) 51:2028–33. doi: 10.1016/j.jacc.2008.04.002
8. Roe MT, Goodman SG, Ohman EM, Stevens SR, Hochman JS, Gottlieb S, et al. Elderly patients with acute coronary syndromes managed without revascularization: insights into the safety of long-term dual antiplatelet therapy with reduced-dose prasugrel versus standard-dose clopidogrel. Circulation. (2013) 128:823–33. doi: 10.1161/CIRCULATIONAHA.113.002303
9. Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. (2009) 361:1045–57. doi: 10.1056/NEJMoa0904327
10. Saito S, Isshiki T, Kimura T, Ogawa H, Yokoi H, Nanto S, et al. Efficacy and safety of adjusted-dose prasugrel compared with clopidogrel in Japanese patients with acute coronary syndrome: the PRASFIT-ACS study. Circ J. (2014) 78:1684–92. doi: 10.1253/circj.CJ-13-1482
11. Goto S, Huang CH, Park SJ, Emanuelsson H, Kimura T. Ticagrelor vs. clopidogrel in Japanese, Korean and Taiwanese patients with acute coronary syndrome – randomized, double-blind, phase III PHILO study. Circ J. (2015) 79:2452–60. doi: 10.1253/circj.CJ-15-0112
12. Tang X, Li R, Jing Q, Wang Q, Liu P, Zhang P, et al. Assessment of ticagrelor versus clopidogrel treatment in patients with ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention. J Cardiovasc Pharmacol. (2016) 68:115–20. doi: 10.1097/FJC.0000000000000390
13. Wang H, Wang X. Efficacy and safety outcomes of ticagrelor compared with clopidogrel in elderly Chinese patients with acute coronary syndrome. Ther Clin Risk Manag. (2016) 12:1101–5. doi: 10.2147/TCRM.S108965
14. Sibbing D, Aradi D, Jacobshagen C, Gross L, Trenk D, Geisler T, et al. Guided de-escalation of antiplatelet treatment in patients with acute coronary syndrome undergoing percutaneous coronary intervention (TROPICAL-ACS): a randomised, open-label, multicentre trial. Lancet. (2017) 390:1747–57. doi: 10.1016/S0140-6736(17)32155-4
15. Cuisset T, Deharo P, Quilici J, Johnson TW, Deffarges S, Bassez C, et al. Benefit of switching dual antiplatelet therapy after acute coronary syndrome: the TOPIC (timing of platelet inhibition after acute coronary syndrome) randomized study. Eur Heart J. (2017) 38:3070–8. doi: 10.1093/eurheartj/ehx175
16. Savonitto S, Ferri LA, Piatti L, Grosseto D, Piovaccari G, Morici N, et al. Comparison of reduced-dose prasugrel and standard-dose clopidogrel in elderly patients with acute coronary syndromes undergoing early percutaneous revascularization. Circulation. (2018) 137:2435–45. doi: 10.1161/CIRCULATIONAHA.117.032180
17. Motovska Z, Hlinomaz O, Kala P, Hromadka M, Knot J, Varvarovsky I, et al. 1-Year outcomes of patients undergoing primary angioplasty for myocardial infarction treated with prasugrel versus ticagrelor. J Am Coll Cardiol. (2018) 71:371–81. doi: 10.1016/j.jacc.2017.11.008
18. Schüpke S, Neumann FJ, Menichelli M, Mayer K, Bernlochner I, Wöhrle J, et al. Ticagrelor or prasugrel in patients with acute coronary syndromes. N Engl J Med. (2019) 381:1524–34. doi: 10.1056/NEJMoa1908973
19. Park DW, Kwon O, Jang JS, Yun SC, Park H, Kang DY, et al. Clinically significant bleeding with ticagrelor versus clopidogrel in Korean patients with acute coronary syndromes intended for invasive management: a randomized clinical trial. Circulation. (2019) 140:1865–77. doi: 10.1161/CIRCULATIONAHA.119.041766
20. Claassens DMF, Vos GJA, Bergmeijer TO, Hermanides RS, van 't Hof AWJ, van der Harst P, et al. A genotype-guided strategy for oral P2Y 12 inhibitors in primary PCI. N Engl J Med. (2019) 381:1621–31. doi: 10.1056/NEJMoa1907096
21. Gimbel M, Qaderdan K, Willemsen L, Hermanides R, Bergmeijer T, de Vrey E, et al. Clopidogrel versus ticagrelor or prasugrel in patients aged 70 years or older with non-ST-elevation acute coronary syndrome (POPular AGE): the randomised, open-label, non-inferiority trial. Lancet. (2020) 395:1374–81. doi: 10.1016/S0140-6736(20)30325-1
22. Kim HS, Kang J, Hwang D, Han JK, Yang HM, Kang HJ, et al. Prasugrel-based de-escalation of dual antiplatelet therapy after percutaneous coronary intervention in patients with acute coronary syndrome (HOST-REDUCE-POLYTECH-ACS): an open-label, multicentre, non-inferiority randomised trial. Lancet. (2020) 396:1079–89. doi: 10.1016/S0140-6736(20)31791-8
23. Kim CJ, Park MW, Kim MC, Choo EH, Hwang BH, Lee KY, et al. Unguided de-escalation from ticagrelor to clopidogrel in stabilised patients with acute myocardial infarction undergoing percutaneous coronary intervention (TALOS-AMI): an investigator-initiated, open-label, multicentre, non-inferiority, randomised trial. Lancet. (2021) 398:1305–16. doi: 10.1016/S0140-6736(21)01445-8
24. Gwon HC, Hahn JY, Park KW, Song YB, Chae IH, Lim DS, et al. Six-month versus 12-month dual antiplatelet therapy after implantation of drug-eluting stents: the Efficacy of Xience/Promus Versus Cypher to Reduce Late Loss After Stenting (EXCELLENT) randomized, multicenter study. Circulation. (2012) 125:505–13. doi: 10.1161/CIRCULATIONAHA.111.059022
25. Han Y, Xu B, Xu K, Guan C, Jing Q, Zheng Q, et al. Six versus 12 months of dual antiplatelet therapy after implantation of biodegradable polymer sirolimus-eluting stent: randomized substudy of the I-LOVE-IT 2 trial. Circ Cardiovasc Interv. (2016) 9:e003145. doi: 10.1161/CIRCINTERVENTIONS.115.003145
26. Hahn JY, Song YB, Oh JH, Cho DK, Lee JB, Doh JH, et al. 6-month versus 12-month or longer dual antiplatelet therapy after percutaneous coronary intervention in patients with acute coronary syndrome (SMART-DATE): a randomised, open-label, non-inferiority trial. Lancet. (2018) 391:1274–84. doi: 10.1016/S0140-6736(18)30493-8
27. Vranckx P, Valgimigli M, Jüni P, Hamm C, Steg PG, Heg D, et al. Ticagrelor plus aspirin for 1 month, followed by ticagrelor monotherapy for 23 months vs aspirin plus clopidogrel or ticagrelor for 12 months, followed by aspirin monotherapy for 12 months after implantation of a drug-eluting stent: a multicentre, open-label, randomised superiority trial. Lancet. (2018) 392:940–9. doi: 10.1016/S0140-6736(18)31858-0
28. Vranckx P, Valgimigli M, Odutayo A, Serruys PW, Hamm C, Steg PG, et al. Efficacy and safety of ticagrelor monotherapy by clinical presentation: pre-specified analysis of the global leaders trial. J Am Heart Assoc. (2021) 10:e015560. doi: 10.1161/JAHA.119.015560
29. De Luca G, Damen SA, Camaro C, Benit E, Verdoia M, Rasoul S, et al. Final results of the randomised evaluation of short-term dual antiplatelet therapy in patients with acute coronary syndrome treated with a new-generation stent (REDUCE trial). EuroIntervention. (2019) 15:e990–8. doi: 10.4244/EIJ-D-19-00539
30. Mehran R, Baber U, Sharma SK, Cohen DJ, Angiolillo DJ, Briguori C, et al. Ticagrelor with or without aspirin in high-risk patients after PCI. N Engl J Med. (2019) 381:2032–42. doi: 10.1056/NEJMoa1908419
31. Hahn JY, Song YB, Oh JH, Chun WJ, Park YH, Jang WJ et al. Effect of P2Y12 Inhibitor monotherapy vs dual antiplatelet therapy on cardiovascular events in patients undergoing percutaneous coronary intervention: the smart-choice randomized clinical trial. JAMA. (2019) 321:2428–37. doi: 10.1001/jama.2019.8146
32. Watanabe H, Domei T, Morimoto T, Natsuaki M, Shiomi H, Toyota T, et al. Effect of 1-month dual antiplatelet therapy followed by clopidogrel vs 12-month dual antiplatelet therapy on cardiovascular and bleeding events in patients receiving PCI: the STOPDAPT-2 randomized clinical trial. JAMA. (2019) 321:2414–27. doi: 10.1001/jama.2019.8145
33. Watanabe H, Morimoto T, Natsuaki M, Yamamoto K, Obayashi Y, Ogita M, et al. Comparison of clopidogrel monotherapy after 1 to 2 months of dual antiplatelet therapy with 12 months of dual antiplatelet therapy in patients with acute coronary syndrome: the STOPDAPT-2 ACS randomized clinical trial. JAMA Cardiol. (2022) 7:407–17. doi: 10.1001/jamacardio.2021.5244
34. Kim BK, Hong SJ, Cho YH, Yun KH, Kim YH, Suh Y, et al. Effect of ticagrelor monotherapy vs ticagrelor with aspirin on major bleeding and cardiovascular events in patients with acute coronary syndrome: the tico randomized clinical trial. JAMA. (2020) 323:2407–16. doi: 10.1001/jama.2020.7580
35. Valgimigli M, Frigoli E, Heg D, Tijssen J, Jüni P, Vranckx P, et al. Dual antiplatelet therapy after PCI in patients at high bleeding risk. N Engl J Med. (2021) 385:1643–55. doi: 10.1056/NEJMoa2108749
36. Kim BK, Hong MK, Shin DH, Nam CM, Kim JS, Ko YG, et al. A new strategy for discontinuation of dual antiplatelet therapy: the RESET Trial (REal Safety and Efficacy of 3-month dual antiplatelet Therapy following Endeavor zotarolimus-eluting stent implantation). J Am Coll Cardiol. (2012) 60:1340–8. doi: 10.1016/j.jacc.2012.06.043
37. Lohaus R, Michel J, Mayer K, Lahmann A, Byrne RA, Wolk A, et al. Six versus twelve months clopidogrel therapy after drug-eluting stenting in patients with acute coronary syndrome: an ISAR-SAFE study subgroup analysis. Sci Rep. (2016) 6:33054. doi: 10.1038/srep33054
38. Feres F, Costa RA, Abizaid A, Leon MB, Marin-Neto JA, Botelho RV, et al. Three vs twelve months of dual antiplatelet therapy after zotarolimus-eluting stents: the OPTIMIZE randomized trial. JAMA. (2013) 310:2510–22. doi: 10.1001/jama.2013.282183
39. Kim HK, Tantry US, Smith SC, Jeong MH, Park SJ, Kim MH, et al. The East Asian paradox: an updated position statement on the challenges to the current antithrombotic strategy in patients with cardiovascular disease. Thromb Haemost. (2021) 121:422–32. doi: 10.1055/s-0040-1718729
40. Jeong YH, Oh JH, Yoon HJ, Park Y, Suh J, Lee SW, et al. Pharmacodynamic profile and prevalence of bleeding episode in east asian patients with acute coronary syndromes treated with prasugrel standard-dose versus de-escalation strategy: a randomized A-MATCH trial. Thromb Haemost. (2021) 121:1376–86. doi: 10.1055/a-1346-3300
41. Ki YJ, Lee BK, Park KW, Bae JW, Hwang D, Kang J, et al. Prasugrel-based de-escalation of dual antiplatelet therapy after percutaneous coronary intervention in patients with STEMI. Korean Circ J. (2022) 52:304–19. doi: 10.4070/kcj.2021.0293
42. Jeong YH, Kevin B, Ahn JH, Chaudhary R, Kang MG, Park HW, et al. Viscoelastic properties of clot formation and their clinical impact in East Asian versus Caucasian patients with stable coronary artery disease: a COMPARE-RACE analysis. J Thromb Thrombolysis. (2021) 51:454–65. doi: 10.1007/s11239-020-02240-2
43. Laudani C, Greco A, Occhipinti G, Ingala S, Calderone D, Scalia L, et al. Short duration of DAPT versus de-escalation after percutaneous coronary intervention for acute coronary syndromes. JACC Cardiovasc Interv. (2022) 15:268–77. doi: 10.1016/j.jcin.2021.11.028
44. Kuno T, Fujisaki T, Shoji S, Sahashi Y, Tsugawa Y, Iwagami M, et al. Comparison of unguided de-escalation versus guided selection of dual antiplatelet therapy after acute coronary syndrome: a systematic review and network meta-analysis. Circ Cardiovasc Interv. (2022) 15:e011990. doi: 10.1161/CIRCINTERVENTIONS.122.011990
45. Galli M, Benenati S, Capodanno D, Franchi F, Rollini F, D'Amario D, et al. Guided versus standard antiplatelet therapy in patients undergoing percutaneous coronary intervention: a systematic review and meta-analysis. Lancet. (2021) 397:1470–83. doi: 10.1016/S0140-6736(21)00533-X
46. Yu J, Mehran R, Baber U, Ooi SY, Witzenbichler B, Weisz G, et al. Sex differences in the clinical impact of high platelet reactivity after percutaneous coronary intervention with drug-eluting stents: results from the ADAPT-DES study (assessment of dual antiplatelet therapy with drug-eluting stents). Circ Cardiovasc Interv. (2017) 10:e003577. doi: 10.1161/CIRCINTERVENTIONS.116.003577
Keywords: acute coronary syndrome, PCI, antiplatelet therapy, P2Y12 inhibitor, de-escalation
Citation: Farag M, Jeyalan V, Ferreiro JL, Jeong Y-H, Geisler T and Gorog DA (2022) Reduction or de-escalation of dual antiplatelet therapy intensity or duration in patients with acute coronary syndromes undergoing percutaneous coronary intervention: A mini-review. Front. Cardiovasc. Med. 9:1018649. doi: 10.3389/fcvm.2022.1018649
Received: 13 August 2022; Accepted: 30 September 2022;
Published: 20 October 2022.
Edited by:
Hugo Ten Cate, Maastricht University Medical Centre, NetherlandsReviewed by:
Tom Adriaenssens, University Hospitals Leuven, BelgiumCopyright © 2022 Farag, Jeyalan, Ferreiro, Jeong, Geisler and Gorog. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohamed Farag, bW9oYW1lZGZhcmFnQG5ocy5uZXQ=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.