- Department of Cardiology, People’s Hospital of Shenzhen Baoan District, The 8th People’s Hospital of Shenzhen, Affiliated Baoan Hospital of Shenzhen, Southern Medical University, Shenzhen, China
Background and aims: Evidence indicates that serum Klotho concentration is associated with mortality in patients with chronic kidney disease (CKD). However, evidence on this association among people with hypertension is scarce. Therefore, we aimed to examine the association between serum Klotho concentration and all-cause and cardiovascular mortality in American patients with hypertension.
Methods and results: We included 6,778 participants with hypertension from the National Health and Nutrition Examination Survey (NHANES) 2007–2014. A Cox proportional hazard model was used to compute the hazard ratios (HRs) and 95% confidence intervals (CIs). The correlation between serum Klotho concentration and mortality was determined using restricted cubic spline and piecewise linear regression analyses. During 36,714 person-years of follow-up, 575 deaths were documented. Lower serum Klotho concentration was associated with increased all-cause mortality, but not cardiovascular mortality after multivariate adjustment. According to spline analysis, the correlation between serum Klotho concentration and all-cause mortality was non-linear (P < 0.001), and the threshold value was 574 pg/mL. The HR below the threshold point was 0.79 (95% CI: 0.67–0.93); no significant difference was found above the threshold point.
Conclusion: Higher serum Klotho concentration was associated with lower all-cause mortality, but not cardiovascular mortality in patients with hypertension with or without chronic renal impairment.
Introduction
Hypertension is a common disease that affects over 1 billion people globally (1). It is a public health burden with increasing prevalence and risk of adverse health outcomes, such as coronary heart disease, chronic heart failure, stroke, chronic kidney disease (CKD), and cognitive impairment. In addition, it is the leading cause of all-cause mortality and disability worldwide (2). Treatment of hypertension has been clinically demonstrated to reduce the risk of cardiovascular disease (CVD) outcomes (including stroke, myocardial infarction, heart failure, and death) and all-cause mortality (3, 4). However, current hypertension medications do not completely eradicate the clinical problems of high blood pressure (BP). Therefore, it is imperative to identify and intervene early in hypertensive patients at risk of death in order to reduce mortality rates.
Klotho was unexpectedly discovered in 1997; α-Klotho is the protein product of the Klotho gene, which was first identified as an aging suppressor (5, 6). There are three forms of the α-Klotho protein—membranous, soluble and secreted (7). However, the secreted Klotho isoform is not found in humans (7). Membrane Klotho functions as an obligatory fibroblast growth factor 23 (FGF23) coreceptor, facilitating FGF23-dependent urine phosphate excretion (8, 9). Soluble Klotho, the main functional form presents in the circulation, which functions as an endocrine and paracrine factor that affects multiple organs, such as kidney, brain, heart and lungs and endothelium (10–12).
Accumulating evidence indicates that Klotho gene polymorphisms and serum Klotho concentration are associated with the incidence and development of hypertension (13, 14). It is noteworthy that decreased serum Klotho concentrations are independent predictors of death and cardiovascular events in both hemodialysis and non-hemodialysis CKD patients (15, 16). Recent study findings have supported the hypothesis that serum Klotho concentration is inversely associated with all-cause mortality in adults (17, 18). According to the research, low circulating Klotho concentration may be a marker of mortality; however, the association between serum Klotho concentration and mortality in patients with hypertension is unclear. To address the gap in knowledge, we aimed to examine the association between serum Klotho concentration and all-cause and cardiovascular mortality in American patients with hypertension. And we hypothesized that Klotho concentrations would be associated with mortality and cardiovascular death in hypertensive patients, and that the mortality risk increases with decreasing Klotho concentration.
Materials and methods
Study population
The National Health and Nutrition Examination Survey (NHANES) of the United States is a stratified, multistage probability sample of people selected from the general population at random. Detailed technical information can be found in the Sample Design documents, available on the NHANES Survey Methods and Analytic Guidelines page1 (19–22). Participants undergo an in-depth interview and are subjected to medical and physiological assessment as well as laboratory tests such as serum, plasma, urine and DNA. The National Center for Health Statistics Research Ethics Review Board approved the survey protocol, and written informed consent was received from all participants (23). Data were continuously collected and released in 2-year cycles; the present study is a secondary analysis of these data. All processes were conducted in accordance with relevant standards and regulations as detailed in NHANES: plan and operations (24).
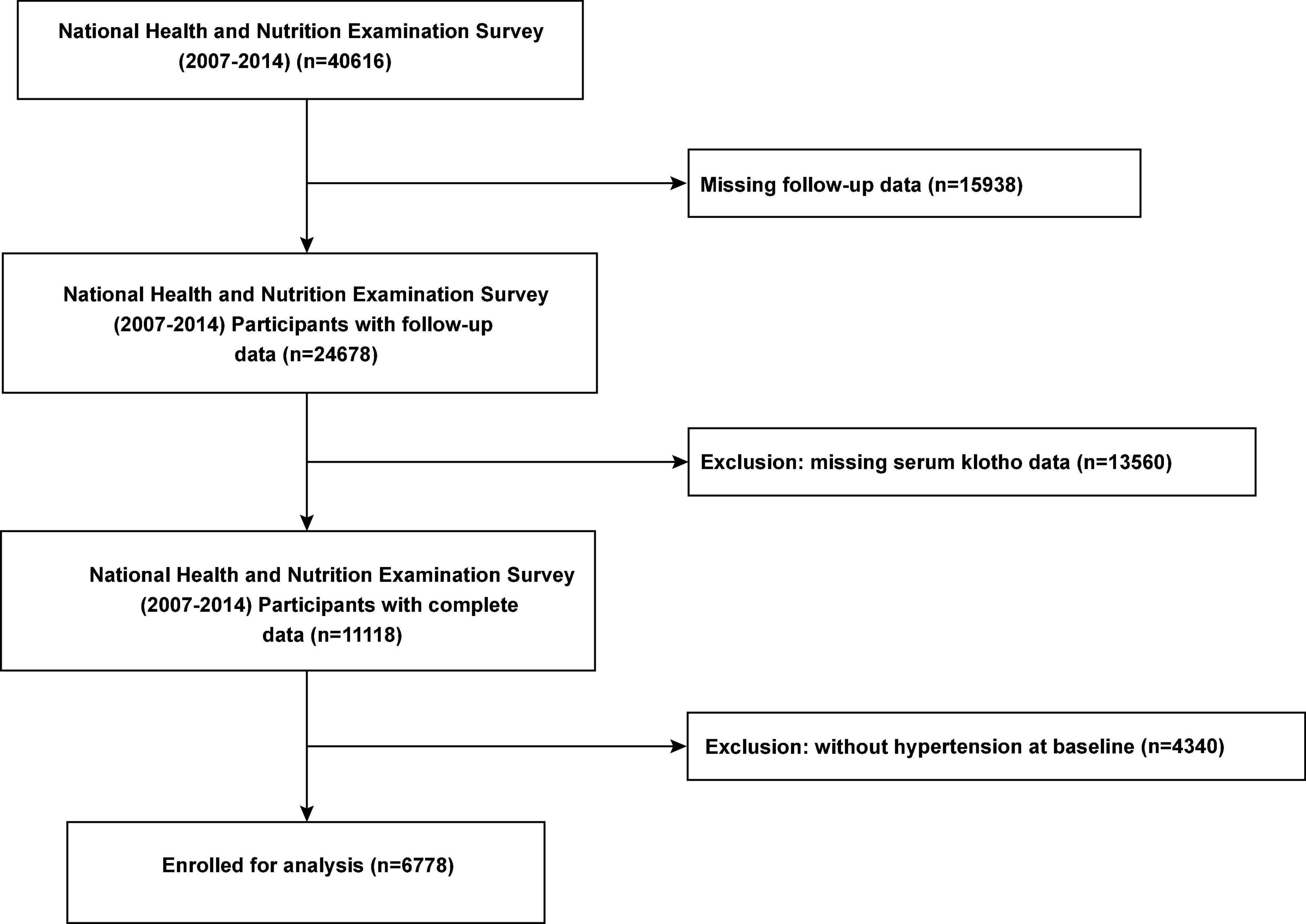
For our investigation, we used publicly available de-identified data. Interview questionnaires and exam response rates are available to the public; more details are available on the official website.2 For the present study, we included data of all participants with hypertension who participated in the continuous NHANES cycles 2007–2008, 2009–2010, 2011–2012, and 2013–2014. After excluding individuals without follow-up data and information serum Klotho concentration, we included 6,778 participants with hypertension (Figure 1). This study was performed in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology reporting guidelines.
Hypertension definition
The BP of each patient were assessed after resting quietly in a sitting position for 5 min; three consecutive BP readings were recorded, and the mean of the 3 readings was calculated as the average BP. Participants were considered to have hypertension if the average systolic BP (SBP) was ≥140 mmHg, or the average diastolic BP (DBP) ≥90 mmHg, or they self-reported being previously diagnosed with hypertension by a physician, or taking antihypertensive medications (such as diuretics, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, calcium-channel blockers, and β-blockers).
Exposure
All serum samples were flash frozen and maintained at −80°C until predetermined batches of samples were sent to the technicians for daily analysis. Serum Klotho concentration was quantified using a commercially available enzyme-linked immunoassay kit (IBL International, Hamburg, Germany) (25). Samples were tested in duplicate, and the average of the two values was used to obtain the final value. If >10% samples had duplicate results, the analyses were repeated. The sample analysis was repeated if the value of a quality control sample was not within two standard deviations (SDs) of the allocated value. The assay sensitivity was 6 pg/mL (26). There are no specific cut-points for Klotho. The reference range was evaluated in 114 samples from healthy donors and levels ranged from 285.8 to 1638.6 pg/mL (mean = 698.0 pg/mL) (26, 27).
Other variable definitions
We included demographic variables including body mass index (BMI), medication use, age, sex, race, and education level as well as laboratory data as covariates. Laboratory data included levels of total cholesterol, high-density lipoprotein, triglycerides, alkaline phosphatase, creatinine, uric acid, blood urea nitrogen (BUN), fasting plasma glucose, hemoglobin A1c (HbA1c), and Klotho-related mineral metabolism markers (calcium, phosphate, and 25(OH) vitamin D). BMI was calculated as weight (in kilograms) divided by height (in meters squared). Diabetes was defined as a self-reported history of diabetes, use of oral hypoglycemic agents or insulin, fasting plasma glucose level ≥126 mg/dL, or HbA1c level ≥6.5%. CVD was defined as a history of coronary artery disease, angina, heart attack, or heart failure. Smoking status was classified as never smoker, former smoker, or current smoker. The estimated glomerular filtration rate (eGFR) was estimated using the CKDs Epidemiology Collaboration equation (28), CKD was defined as an eGFR of <60 mL/min/1.73 m2. As discussed later in the study, CKD was defined using eGFR of only one time point because the cross-sectional study did not measure the duration of reduced eGFR.
All-cause and cardiovascular mortality
The main outcomes were all-cause and cardiovascular mortality. Participants were linked to the National Death Index until December 31, 2015 through a rigorous probability matching and death certificate review process. The cause of death was classified according to the International Classification of Diseases, Tenth Revision, codes. Cardiovascular mortality was defined according to the International Classification of Diseases, Tenth Revision, codes I00–I09, I11, I13, and I20–I51.
Statistical analysis
Participants were divided into three groups according to their serum Klotho concentration, 500, 500–1,000, and >1,000 pg/mL, based on the restricted cubic spline model results. Continuous data are expressed as mean ± SD when normally distributed and as median (interquartile range) when not normally distributed. Categorical variables are expressed as percentages. For continuous variables, analysis of variance or the Kruskal–Wallis tests were used, while for categorical variables, the chi-square test or Fisher’s exact test was used.
We plotted cumulative Kaplan–Meier curves for all-cause and cardiovascular mortality during follow-up according to the predefined groups of serum Klotho concentration. The statistical significance of subgroup differences was evaluated using the log-rank test. Cox proportional hazard regression analysis was performed to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) for associations between serum Klotho concentration and all-cause and cardiovascular mortality. We built three models to provide statistical inference. Confounders were selected based on a significant association with the outcomes of interest or a change in effect estimate of >10%. Model 1 included only serum Klotho concentrations. Model 2 included demographic variables (age, sex, race, education level), total cholesterol level, high-density lipoprotein-C level, HbA1c level, comorbidities (CVD, diabetes, and stroke), and use of medicines (hypotensive drugs, hypoglycemic drugs, lipid-lowering medication, and antiplatelet drugs). Model 3 included variables from model 2 plus BMI; smoking status; and levels of triglycerides, alkaline phosphatase, uric acid, BUN, creatinine, 25(OH) vitamin D, calcium, and phosphorus.
Restricted cubic spline models were built to detect any non-linear relationship between serum Klotho concentration and mortality. If non-linear relationships were identified, we used piecewise regression models to elucidate how the associations differed around the threshold point. The log-likelihood ratio test—comparing the one-line (non-segmented) regression model with the segmented regression model—was used to determine whether a threshold existed. The inflection point that connected the segments was based on the model demonstrating the maximum likelihood; it was determined using a two-step recursive method. In addition, we performed multiple imputation based on five replications, and a chained equation approach method was used in the R MI process to evaluate whether the use of indicator variables for missing data caused bias in our results.
Subgroup analyses were performed to test the robustness of our main findings. We tested for effect modification according to sex, age, smoking status, SBP, DBP, BMI, eGFR, and presence of diabetes and CVD. All analyses were conducted using R version 4.1.0 (R Foundation for Statistical Computing, Vienna, Austria), and a two-sided P-value of < 0.05 was considered statistically significant for all analyses, including interaction terms.
Results
Baseline characteristics
The baseline characteristics of the study participants according to serum Klotho concentration are shown in Table 1. The average (SD) age of the 6,778 participants was 60.9 (10.5) years; 49.5% participants were men. The mean (SD) Klotho concentration was 850 pg/mL (310) and 575 (8.5%) patients died after a median follow-up of 65 months. Participants with low serum Klotho concentration were more likely to be men, older, smokers, and drinkers and have low DBP. Participants with the lowest Klotho concentration were more likely to have diabetes and CVD and use hypotensive, hypoglycemic, and lipid-lowering medications. Laboratory tests revealed that participants with the highest Klotho concentration had reduced serum creatinine, BUN, uric acid, 25(OH) vitamin D levels and a low mortality rate. There were no observed differences in education level, SBP, calcium level, phosphorus level, and history of stroke between the groups.

Table 1. Characteristics of American hypertensive populations according to serum Klotho concentrations.
Trends over time
There was a change in some variables over time with succeeding NHANES cycles. Between 2007–2008 and 2013–2014 NHANES cycles, smoking status; SBP; levels of creatinine, BUN, high-density lipoprotein, HbA1c, and fasting plasma glucose; comorbidities; and use of medication (excluding antiplatelet drugs) remained relatively stable. Despite the change in Klotho concentration between 2007–2008 and 2013–2014 NHANES cycles, the difference was not significant (Supplementary Table 1).
Serum Klotho concentration and all-cause mortality
Serum Klotho concentration was inversely correlated with all-cause death; a 5% reduction in the risk of mortality was associated with each 100 pg/mL increase in serum Klotho concentration (P = 0.002). Even after multivariable adjustment, adverse associations persisted (Table 2). When serum Klotho concentration was analyzed as a categorical predictor using Cox regression estimates, compared to the overall risk of mortality at serum Klotho concentration <500 pg/mL, that at serum Klotho concentrations of 500–1,000 and >1,000 pg/mL decreased by 38% (95% CI: 0.12–0.52) and 42% (95% CI: 0.27–0.57), respectively (Table 2). Kaplan–Meier survival curves based on serum Klotho concentration are shown in Figure 2A. Participants with serum Klotho concentration <500 pg/ml had a significantly reduced overall survival compared to that in participants with higher Klotho concentrations (log-rank, P < 0.001).
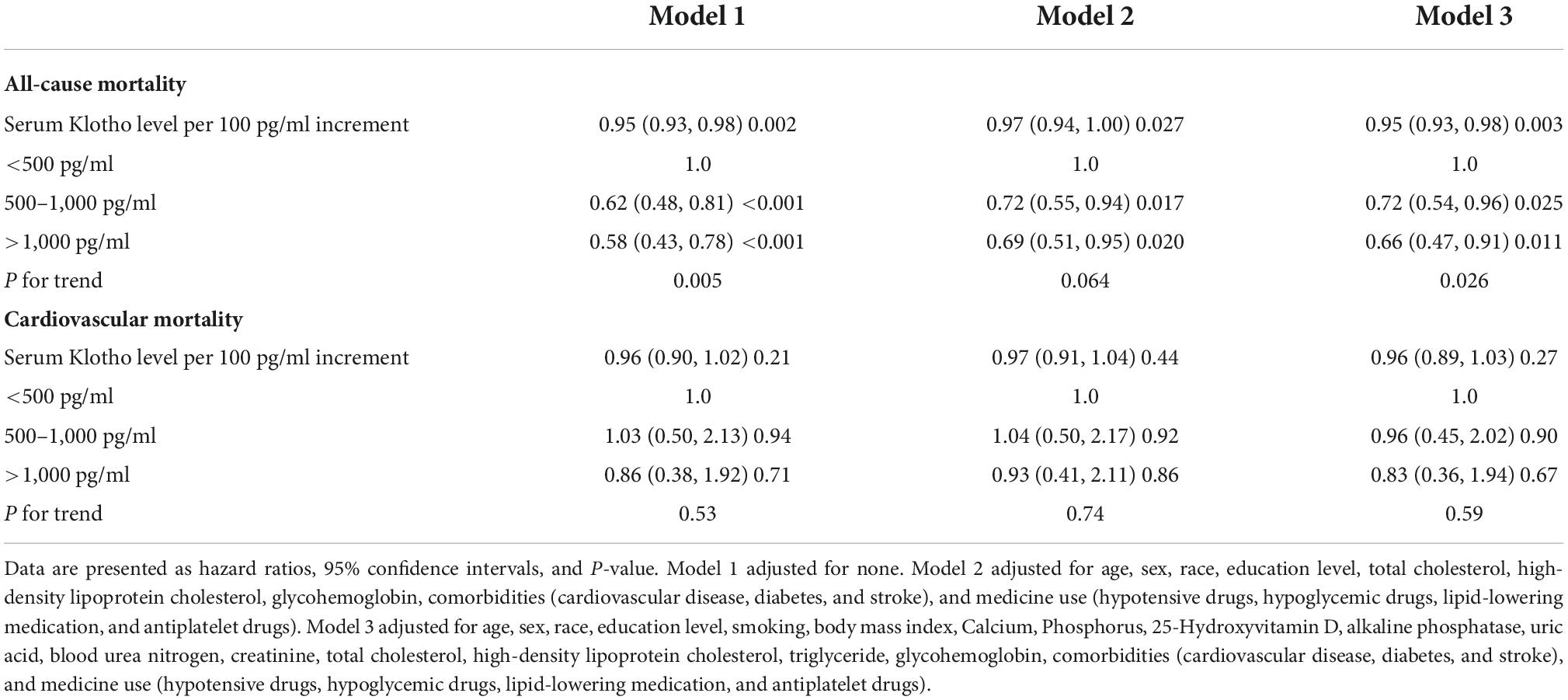
Table 2. Hazard ratios (95% CIs) for the association of serum Klotho concentrations with all-cause mortality and cardiovascular mortality in American hypertensive populations.
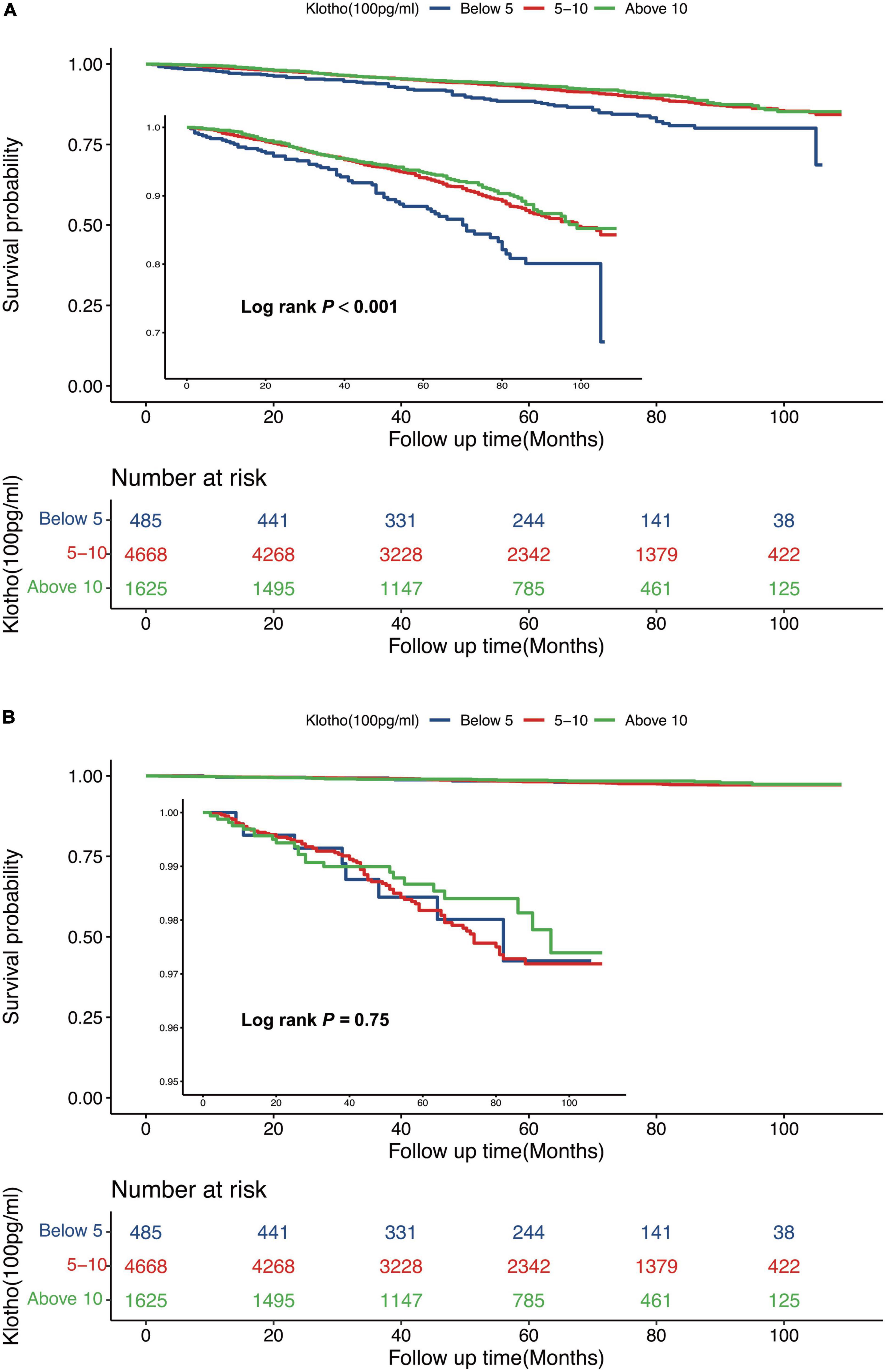
Figure 2. Kaplan-Meier (crude) survival curves, by serum Klotho concentrations, for all-cause mortality (A) and cardiovascular mortality (B).
According to the restricted cubic spline model, there was a high non-linear correlation between serum Klotho concentration and all-cause mortality (Figures 3A, P < 0.001). We calculated the threshold value of 574 pg/mL using a two-piecewise linear regression model. Serum Klotho concentration above the threshold point was correlated with a lower risk of all-cause mortality (HR: 0.79; 95% CI: 0.67–0.93), while that below the threshold point was not (Table 3; likelihood-ratio test, P = 0.026).
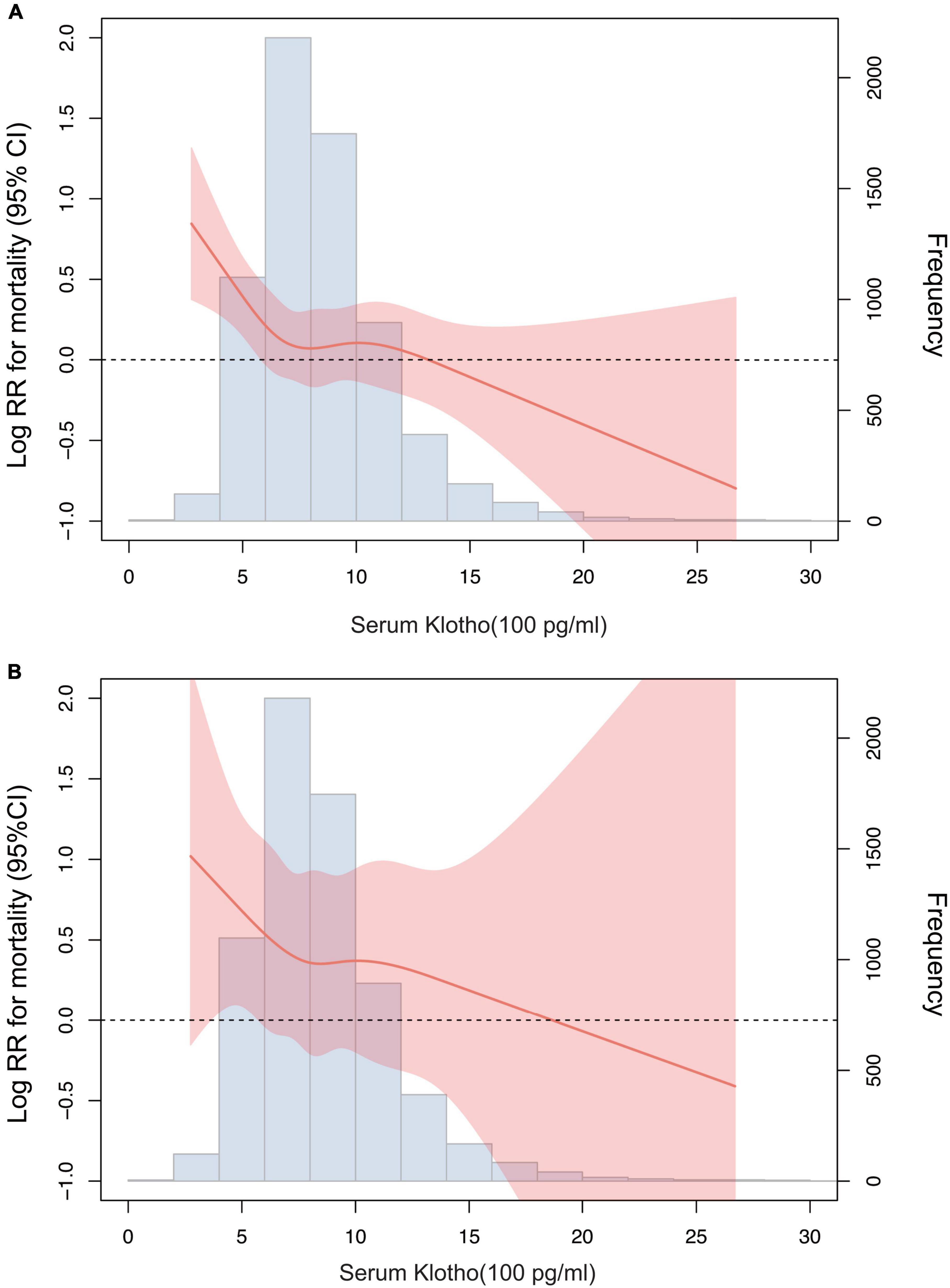
Figure 3. Association of serum Klotho concentrations with all-cause mortality (A) and cardiovascular mortality (B) using a restricted cubic spline regression model. Log RR, Log relative risk. Results were adjusted for age, sex, race, education level, smoking, body mass index, Calcium, Phosphorus, 25-Hydroxyvitamin D, alkaline phosphatase, uric acid, blood urea nitrogen, creatinine, total cholesterol, high-density lipoprotein cholesterol, triglyceride, glycohemoglobin, comorbidities (cardiovascular disease, diabetes, and stroke), and medicine use (hypotensive drugs, hypoglycemic drugs, lipid-lowering medication, and antiplatelet drugs).
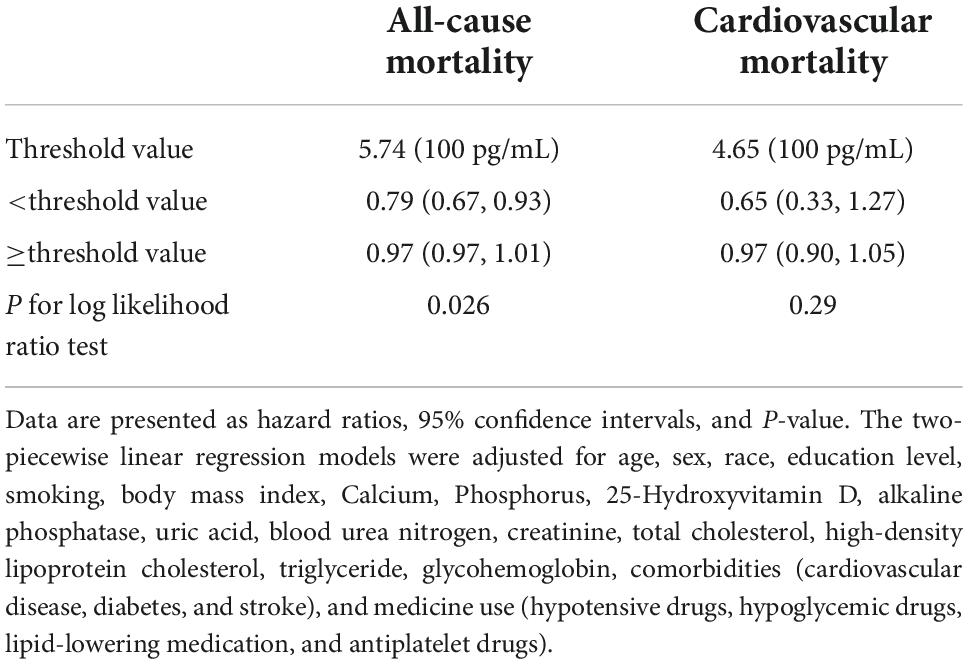
Table 3. The result of two-piecewise linear regression model between serum Klotho concentrations and all-cause mortality and cardiovascular mortality in American adults with hypertension.
Serum Klotho concentration and cardiovascular mortality
There was no significant association between serum Klotho concentration and cardiovascular mortality when serum Klotho concentration was analyzed as both continuous and categorical predictors using Cox regression estimates (Table 2 and Figure 2B). With a non-linear approach using penalized splines, we observed that cardiovascular mortality was not associated with high Klotho concentration (Figure 3B). The threshold value for cardiovascular mortality was 465 pg/mL; it was not statistically significant.
Interactions
No significant interactions were observed between serum Klotho concentration and levels of phosphorus, calcium, and 25(OH) vitamin D and eGFR for both all-cause and cardiovascular mortality in the fully adjusted models.
Subgroup analyses
The association between serum Klotho concentration and all-cause and cardiovascular mortality was consistent in subgroups according to sex, age, smoking status, DBP (≥90 vs. <90 mmHg), SBP (≥140 vs. <140 mmHg), BMI (≥25 vs. <25 kg/m2), eGFR (≥60 vs. <60 mL/min/1.73 m2), and presence or absence of diabetes and CVD (Figure 4; P for interaction >0.05).
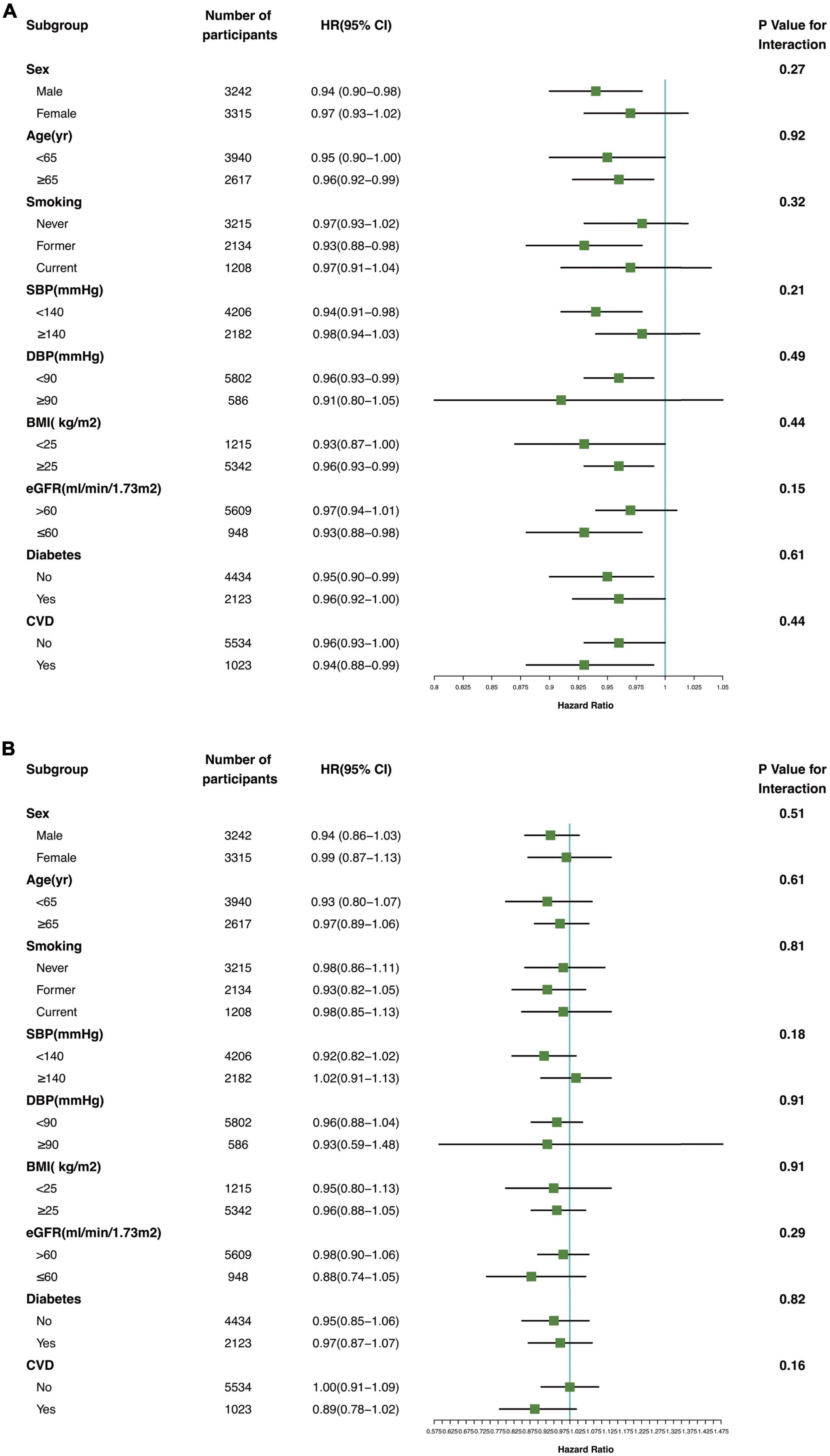
Figure 4. Association of serum Klotho concentrations with all-cause mortality (A) and cardiovascular mortality (B) in various subgroups. yr, years old; SBP systolic blood pressure; DBP, diastolic blood pressure; BMI, body mass index; eGFR, estimated glomerular filtration rate; CVD, cardiovascular disease.
Sensitive analyses
The percentage of variables with missing data was 0–3%. Table 4 shows that by utilizing several imputed datasets, Cox regression analysis of serum Klotho concentration and all-cause and cardiovascular mortality produced comparable results to those using raw data.
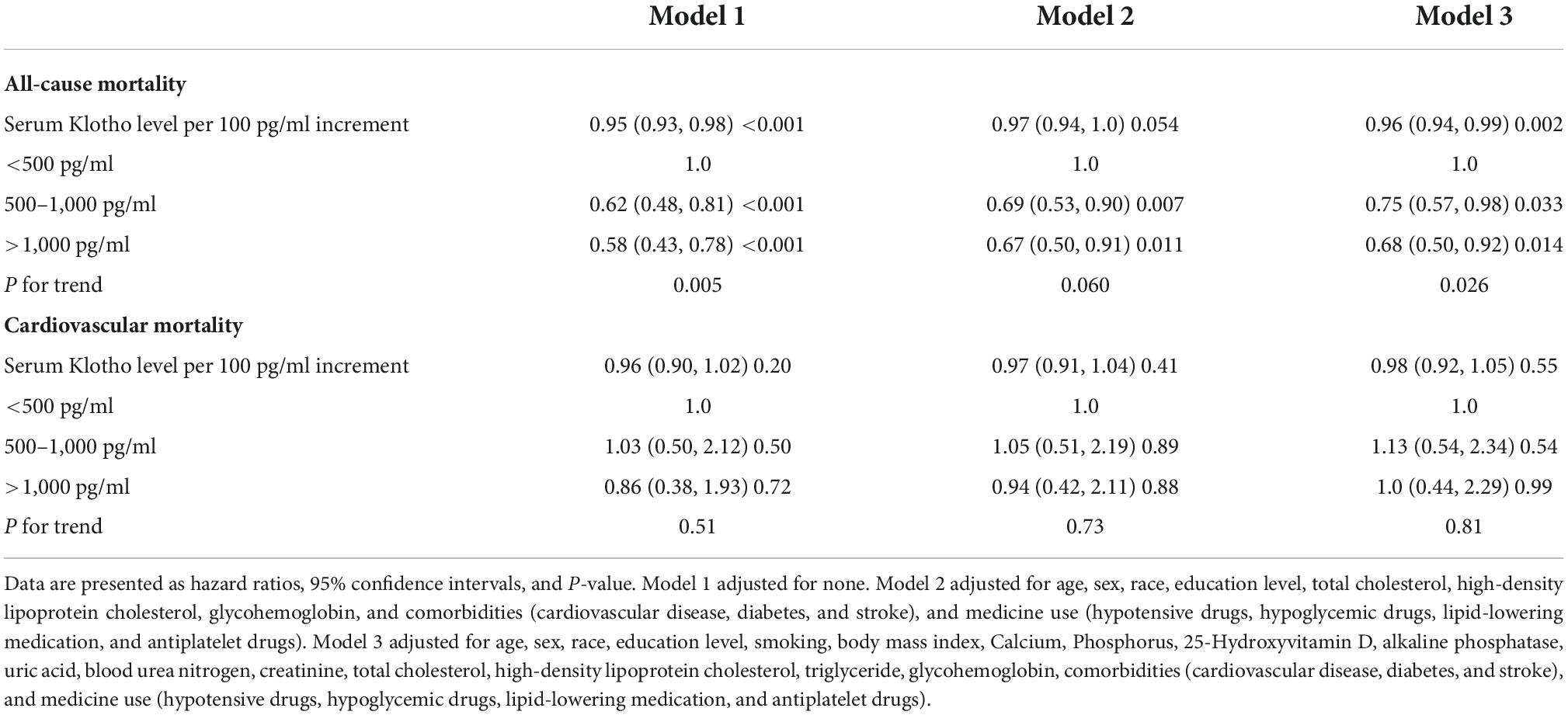
Table 4. Cox proportional hazards analysis for all-cause and cardiovascular mortality according to serum Klotho concentrations in American hypertensive populations with imputed variables from multiple imputation.
Discussion
After 65 months of follow-up in a large cohort of American patients with hypertension from the NHANES, low serum Klotho concentration was associated with increased all-cause mortality, but not cardiovascular mortality. Moreover, there was a non-linear relationship between serum Klotho concentration and all-cause mortality. These findings were consistent in individuals with and without CKD.
Although a previous study reported that Klotho concentration correlated with lifespan in mice (5), studies on the association between circulating Klotho concentration and the risk of mortality in human populations are limited. A study on older, community-dwelling people demonstrated that after correcting for 25(OH)D, parathyroid hormone, and calcium levels and other potential confounders, plasma Klotho concentration was an independent predictor of mortality (18). Furthermore, Evangelos et al. indicated that Klotho deficiency was an independent predictor of cardiovascular and all-cause mortality in patients treated with chronic hemodialysis and the elderly population (29). According to a meta-analysis, low circulating soluble Klotho concentration was strongly related to increased all-cause mortality in patients with CKD (30). However, these studies have severe drawbacks, including the small size, inclusion of only populations with CKD or older age, and lack of a precise explanation for the actual relationship between Klotho concentration and mortality as well as the threshold value.
Our study showed that serum Klotho concentration was not associated with cardiovascular mortality in hypertensive population, in contrast to Christophe Marçais et al. (31), who found that Klotho concentration is associated with cardiovascular morbidity and mortality during hemodialysis. A major reason for the inconsistency was the study population. In Christophe Marçais’ study, the majority of the study population was composed of CKD patients receiving hemodialysis. CKD patients, particularly those receiving hemodialysis, have a higher risk of cardiovascular events like acute myocardial infarction, heart failure, and CVD death than normal individuals and hypertensive patients (32). The number of participants and the duration of follow-up may also be contributing factors. For the study by Christophe Marçais et al., 769 patients were recruited, and 238 were analyzed after 2 years of follow-up. Comparatively, our study analyzed 6,778 participants with a median follow-up of 65 months. Overall, our results were more robust and provided further evidence for the association between Klotho and cardiovascular mortality. Data from the Ludwigshafen Risk and Cardiovascular Health Study demonstrated that Klotho concentrations do not add predictive power to mortality risk assessment in patients with normal renal function (33). Nonetheless, even in patients with normal renal function (eGFR >60 mL/min/1.73 m2), low Klotho concentration was an independent risk factor for all-cause mortality in our analysis. The unadjusted and fully adjusted models revealed a non-linear relationship between serum Klotho concentration and all-cause mortality in our study. Moreover, in our study, the threshold value of serum Klotho concentration to predict all-cause mortality in patients with hypertension was 574 pg/mL, which has not been previously discussed.
Globally, hypertension is a highly prevalent condition and the main cause of CVD, stroke, CKD, and premature mortality (34). Many factors attribute to the death of hypertensive patients, such as lipid disorders, overweight-obesity, unhealthy lifestyle habits (e.g., smoking) (35). However, to the best of our knowledge, no previous study has evaluated the association between Klotho and mortality in individuals with hypertension. It has been shown that Klotho deficiency results in arterial stiffness and hypertension in mice, whereas Klotho supplementation prevents CVD from progressing in mice (36, 37). Serum Klotho deficiency is also associated with hypertension in humans. In the Health, Aging and Body Composition Study, low Klotho concentration was associated with a high risk of initial hypertension and high BP trajectories during follow-up (13).
Klotho is involved in the control of BP, through its influence in sodium handling, inflammatory cytokine activation, tissue cell infiltration and response to salt (38). By activating the non-canonical Wnt5a/RhoA pathway, Klotho deficiency increases salt sensitivity in patients with hypertension which leads to increased total peripheral resistance via vasoconstriction, therefore raising BP (39). Serum Klotho concentration is also independently associated with arterial stiffness (40); Klotho deficiency accelerates vascular aging and arterial stiffness by increasing autophagy and downregulating Sirtuin-1 (41, 42). Klotho can improve endothelial function by activating superoxide dismutase and endothelial nitric oxide synthase via the phosphoinositide 3-kinase/protein kinase B/endothelial nitric oxide synthase pathway (14). Increased peripheral resistance, vascular aging, and endothelial dysfunction all are factors that contribute to increased BP (43–45), and the higher the BP, the greater the risk of mortality (46).
The effects of Klotho on the health and survival of patients with hypertension can be mediated in several ways. According to a large body of research, Klotho appears to reduce oxidative stress, improve endothelial function, and provide vasoprotection at the cellular level. Furthermore, soluble Klotho has been shown to reduce fibrosis and block the epithelial–mesenchymal transition associated with fibrosis (47). Moreover, serum Klotho promotes endothelial nitric oxide generation, while also exerting systemic pleiotropic antioxidative, antithrombotic, and anti-inflammatory activities (30). According to research, FGF23 is associated with end-stage renal disease and acute kidney damage, severe infections and inflammation, and even cancer progression, which is considered a possible cause of death in people with CKD (48). It is believed that soluble Klotho protects against the effects of FGF23, which are exacerbated when serum Klotho concentration is reduced (48). And hypertensive patients with low Klotho concentration lose the above-mentioned protective mechanisms, predisposing them to mortality. As a result, lower serum Klotho concentration was associated with higher all-cause mortality in patients with hypertension.
Most critically, recent studies have demonstrated that Klotho supplementation protects mice from indoxyl sulfate-induced ventricular hypertrophy (49). According to Guo et al., injecting Klotho reduced cell mortality and remodeling (50). Additionally, Klotho therapy can protect against phosphotoxic insults, reduce oxidative stress, and reduce inflammation and fibrosis of the organs (38). Collectively, the therapeutic activity of Klotho in patients with CVD (including hypertension) represents a fascinating and promising perspective for the future.
The strengths of the current study include its relatively large sample size and the use of a nationally representative sample of the American population with hypertension, which facilitates the generalization of our findings. Furthermore, large demographic and comprehensive data may be utilized to account for a number of potential confounding factors, such as ethnicity; lifestyle traits; comorbidities; and laboratory data, such as calcium and phosphorus metabolism indicators and renal function assessment findings. Despite the encouraging results observed in this study, it has several potential limitations that should also be considered. First, our findings were restricted to populations with hypertension aged ≥40 years; thus, it is unclear whether the findings apply to people of various ages. However, hypertension usually develops in people aged >40 years. Second, since the FGF23/Klotho axis is associated with hypertension and CVD (51), we were unable to determine whether the clinical impact of Klotho on mortality was reliant on or independent of FGF23. Nonetheless, Klotho likely has FGF23-independent actions since circulating forms of Klotho without fibroblast growth factor receptors do not have a high affinity for FGF23 (41). Third, we defined CKD using creatinine levels measured at only one time point; however, previous studies based on national data or cohort studies also defined CKD based on the glomerular filtration rate measured at one time point (52). In addition, the proportion of patients with acute kidney injury among participants of a community-based research is expected to be negligible. Fourth, the likelihood of residual confounding effects from insufficient adjustment of mortality risk factors cannot be ruled out. Therefore, well-designed prospective studies are needed to assess and confirm these findings and further evaluate the biomarker and therapeutic potential of serum Klotho.
Conclusion
Our findings indicate that low serum Klotho concentration was substantially correlated with a greater risk of all-cause death, but not cardiovascular mortality in a nationwide representative sample of American individuals with hypertension. Early diagnosis of low Klotho concentration (particularly <574 pg/mL) and appropriate intervention in patients with hypertension may be a possible target for preventing mortality. However, there is no definitive conclusion about Klotho’s normal concentration and no Klotho specific cut-points to be used as indicators of biological age, further studies to confirm these findings and explain the potential mechanisms for the observed associations are warranted.
Data availability statement
The datasets presented in this study can be found in online repositories. NHANES data are publicly available at https://www.cdc.gov/nchs/nhanes/index.htm.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. The patients/participants provided their written informed consent to participate in this study.
Author contributions
YY conceived and designed the study and drafted reviewed and revised the manuscript. JC contributed to initial data analysis and interpretation. Both authors contributed to the article and approved the submitted version.
Acknowledgments
We thank all NHANES participants and staff for their excellent contributions to guarantee the completion of the present and other study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.1013747/full#supplementary-material
Abbreviations
HR, hazard ratio; CI, confidence interval; BP, blood pressure; SBP, systolic blood pressure; DBP, diastolic blood pressure; FGF23, fibroblast growth factor 23; CKD, chronic kidney disease; CVD, cardiovascular disease; NHANES, National Health and Nutrition Examination Survey; SD, standard deviation; BMI, body mass index; BUN, blood urea nitrogen; HbA1c, hemoglobin A1c; eGFR, estimated glomerular filtration rate.
Footnotes
- ^ https://wwwn.cdc.gov/nchs/nhanes/analyticguidelines.aspx#sample-design
- ^ https://www.cdc.gov/nchs/nhanes/index.htm
References
1. Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. (2005) 365:217–23. doi: 10.1016/S0140-6736(05)17741-1
2. GBD 2015 Risk Factors Collaborators. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990-2015: a systematic analysis for the global burden of disease study 2015. Lancet. (2016) 388:1659–724.
3. Ninomiya T, Perkovic V, Turnbull F, Neal B, Barzi F, Cass A, et al. Blood pressure lowering and major cardiovascular events in people with and without chronic kidney disease: meta-analysis of randomised controlled trials. BMJ. (2013) 347:f5680. doi: 10.1136/bmj.f5680
4. Group SR, Wright JT Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. (2015) 373:2103–16. doi: 10.1056/NEJMoa1511939
5. Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. (1997) 390:45–51. doi: 10.1038/36285
6. Xu Y, Sun Z. Molecular basis of klotho: from gene to function in aging. Endocr Rev. (2015) 36:174–93. doi: 10.1210/er.2013-1079
7. Lim K, Halim A, Lu TS, Ashworth A, Chong I. Klotho: a Major shareholder in vascular aging enterprises. Int J Mol Sci. (2019) 20:4637. doi: 10.3390/ijms20184637
8. Kurosu H, Ogawa Y, Miyoshi M, Yamamoto M, Nandi A, Rosenblatt KP, et al. Regulation of fibroblast growth factor-23 signaling by klotho. J Biol Chem. (2006) 281:6120–3. doi: 10.1074/jbc.C500457200
9. Kim JH, Hwang KH, Park KS, Kong ID, Cha SK. Biological role of anti-aging protein klotho. J Lifestyle Med. (2015) 5:1–6. doi: 10.15280/jlm.2015.5.1.1
10. Kato Y, Arakawa E, Kinoshita S, Shirai A, Furuya A, Yamano K, et al. Establishment of the anti-Klotho monoclonal antibodies and detection of Klotho protein in kidneys. Biochem Biophys Res Commun. (2000) 267:597–602. doi: 10.1006/bbrc.1999.2009
11. Imura A, Iwano A, Tohyama O, Tsuji Y, Nozaki K, Hashimoto N, et al. Secreted Klotho protein in sera and CSF: implication for post-translational cleavage in release of klotho protein from cell membrane. FEBS Lett. (2004) 565:143–7. doi: 10.1016/j.febslet.2004.03.090
12. Bian A, Neyra JA, Zhan M, Hu MC. Klotho, stem cells, and aging. Clin Interv Aging. (2015) 10:1233–43. doi: 10.2147/CIA.S84978
13. Drew DA, Katz R, Kritchevsky S, Ix JH, Shlipak MG, Newman AB, et al. Soluble klotho and incident hypertension. Clin J Am Soc Nephrol. (2021) 16:1502–11. doi: 10.2215/CJN.05020421
14. Kanbay M, Demiray A, Afsar B, Covic A, Tapoi L, Ureche C, et al. Role of klotho in the development of essential hypertension. Hypertension. (2021) 77:740–50. doi: 10.1161/HYPERTENSIONAHA.120.16635
15. Kim HR, Nam BY, Kim DW, Kang MW, Han JH, Lee MJ, et al. Circulating alpha-klotho levels in CKD and relationship to progression. Am J Kidney Dis. (2013) 61:899–909. doi: 10.1053/j.ajkd.2013.01.024
16. Liu QF, Ye JM, Yu LX, He AL, Sun Q, He DW, et al. Plasma s-Klotho is related to kidney function and predicts adverse renal outcomes in patients with advanced chronic kidney disease. J Investig Med. (2018) 66:669–75. doi: 10.1136/jim-2017-000560
17. Kresovich JK, Bulka CM. Low serum klotho associated with all-cause mortality among a nationally representative sample of american adults. J Gerontol A Biol Sci Med Sci. (2022) 77:452–6. doi: 10.1093/gerona/glab308
18. Semba RD, Cappola AR, Sun K, Bandinelli S, Dalal M, Crasto C, et al. Plasma klotho and mortality risk in older community-dwelling adults. J Gerontol A Biol Sci Med Sci. (2011) 66:794–800. doi: 10.1093/gerona/glr058
19. Paulose-Ram R, Graber JE, Woodwell D, Ahluwalia N. The national health and nutrition examination survey (NHANES), 2021-2022: adapting data collection in a COVID-19 environment. Am J Public Health. (2021) 111:2149–56. doi: 10.2105/AJPH.2021.306517
20. Curtin LR, Mohadjer LK, Dohrmann SM, Kruszon-Moran D, Mirel LB, Carroll MD, et al. National Health and Nutrition Examination Survey: sample design, 2007-2010. Vital Health Stat 2. (2013) 1–23.
21. Johnson CL, Dohrmann SM, Burt VL, Mohadjer LK. National Health and Nutrition Examination Survey: sample design, 2011-2014. Vital Health Stat 2. (2014) 1–33.
22. Centers for Disease Control and Prevention [CDC], National Center for Health Statistics [NCHS]. National Health and Nutrition Examination Survey Data 2007–2014. Hyattsville, MD: U.S. Department of Health and Human Services (n.d.).
23. Johnson CL, Paulose-Ram R, Ogden CL, Carroll MD, Kruszon-Moran D, Dohrmann SM, et al. National Health and Nutrition Examination Survey: analytic guidelines, 1999-2010. Vital Health Stat 2. (2013) 1–24.
24. Zipf G, Chiappa M, Porter KS, Ostchega Y, Lewis BG, Dostal J. National Health and Nutrition Examination Survey: plan and operations, 1999-2010. Vital Health Stat 1. (2013) 1–37.
25. Yamazaki Y, Imura A, Urakawa I, Shimada T, Murakami J, Aono Y, et al. Establishment of sandwich ELISA for soluble alpha-klotho measurement: age-dependent change of soluble alpha-Klotho levels in healthy subjects. Biochem Biophys Res Commun. (2010) 398:513–8. doi: 10.1016/j.bbrc.2010.06.110
26. Centers for Disease Control and Prevention [CDC], National Center for Health Statistics [NCHS]. National Health and Nutrition Examination Survey Questionnaire (or Examination Protocol, or Laboratory Protocol) 2007–2014. Hyattsville, MD: U.S. Department of Health and Human Services (n.d.).
27. Tan SJ, Smith ER, Hewitson TD, Holt SG, Toussaint ND. Diurnal variation and short-term pre-analytical stability of serum soluble alpha-klotho in healthy volunteers: a pilot study. Ann Clin Biochem. (2015) 52(Pt. 4):506–9. doi: 10.1177/0004563214563415
28. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF III, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. (2009) 150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006
29. Memmos E, Sarafidis P, Pateinakis P, Tsiantoulas A, Faitatzidou D, Giamalis P, et al. Soluble Klotho is associated with mortality and cardiovascular events in hemodialysis. BMC Nephrol. (2019) 20:217. doi: 10.1186/s12882-019-1391-1
30. Charoenngam N, Ponvilawan B, Ungprasert P. Lower circulating soluble Klotho level is associated with increased risk of all-cause mortality in chronic kidney disease patients: a systematic review and meta-analysis. Int Urol Nephrol. (2020) 52:1543–50. doi: 10.1007/s11255-020-02510-1
31. Marcais C, Maucort-Boulch D, Drai J, Dantony E, Carlier MC, Blond E, et al. Circulating klotho associates with cardiovascular morbidity and mortality during hemodialysis. J Clin Endocrinol Metab. (2017) 102:3154–61. doi: 10.1210/jc.2017-00104
32. Sarafidis PA, Loutradis C, Karpetas A, Tzanis G, Piperidou A, Koutroumpas G, et al. Ambulatory pulse wave velocity is a stronger predictor of cardiovascular events and all-cause mortality than office and ambulatory blood pressure in hemodialysis patients. Hypertension. (2017) 70:148–57. doi: 10.1161/HYPERTENSIONAHA.117.09023
33. Brandenburg VM, Kleber ME, Vervloet MG, Larsson TE, Tomaschitz A, Pilz S, et al. Soluble klotho and mortality: the ludwigshafen risk and cardiovascular health study. Atherosclerosis. (2015) 242:483–9. doi: 10.1016/j.atherosclerosis.2015.08.017
34. Saiz LC, Gorricho J, Garjon J, Celaya MC, Erviti J, Leache L. Blood pressure targets for the treatment of people with hypertension and cardiovascular disease. Cochrane Database Syst Rev. (2018) 7:CD010315. doi: 10.1002/14651858.CD010315.pub3
35. Unger T, Borghi C, Charchar F, Khan NA, Poulter NR, Prabhakaran D, et al. 2020 International society of hypertension global hypertension practice guidelines. J Hypertens. (2020) 38:982–1004. doi: 10.1097/HJH.0000000000002453
36. Lin Y, Chen J, Sun Z. Antiaging gene klotho deficiency promoted high-fat diet-induced arterial stiffening via inactivation of AMP-activated protein kinase. Hypertension. (2016) 67:564–73. doi: 10.1161/HYPERTENSIONAHA.115.06825
37. Takenaka T, Kobori H, Miyazaki T, Suzuki H, Nishiyama A, Ishii N, et al. Klotho protein supplementation reduces blood pressure and renal hypertrophy in db/db mice, a model of type 2 diabetes. Acta Physiol. (2019) 225:e13190. doi: 10.1111/apha.13190
38. Lanzani C, Citterio L, Vezzoli G. Klotho: a link between cardiovascular and non-cardiovascular mortality. Clin Kidney J. (2020) 13:926–32. doi: 10.1093/ckj/sfaa100
39. Kawarazaki W, Mizuno R, Nishimoto M, Ayuzawa N, Hirohama D, Ueda K, et al. Salt causes aging-associated hypertension via vascular Wnt5a under Klotho deficiency. J Clin Invest. (2020) 130:4152–66. doi: 10.1172/JCI134431
40. Kitagawa M, Sugiyama H, Morinaga H, Inoue T, Takiue K, Ogawa A, et al. A decreased level of serum soluble klotho is an independent biomarker associated with arterial stiffness in patients with chronic kidney disease. PLoS One. (2013) 8:e56695. doi: 10.1371/journal.pone.0056695
41. Chen K, Sun Z. Autophagy plays a critical role in klotho gene deficiency-induced arterial stiffening and hypertension. J Mol Med. (2019) 97:1615–25. doi: 10.1007/s00109-019-01841-6
42. Zu Y, Liu L, Lee MY, Xu C, Liang Y, Man RY, et al. SIRT1 promotes proliferation and prevents senescence through targeting LKB1 in primary porcine aortic endothelial cells. Circ Res. (2010) 106:1384–93. doi: 10.1161/CIRCRESAHA.109.215483
43. Kanbay M, Afsar B, Gusbeth-Tatomir P, Covic A. Arterial stiffness in dialysis patients: where are we now? Int Urol Nephrol. (2010) 42:741–52. doi: 10.1007/s11255-009-9675-1
44. Meneton P, Jeunemaitre X, de Wardener HE, MacGregor GA. Links between dietary salt intake, renal salt handling, blood pressure, and cardiovascular diseases. Physiol Rev. (2005) 85:679–715. doi: 10.1152/physrev.00056.2003
45. Siriopol D, Covic A, Iliescu R, Kanbay M, Tautu O, Radulescu L, et al. Arterial stiffness mediates the effect of salt intake on systolic blood pressure. J Clin Hypertens. (2018) 20:1587–94. doi: 10.1111/jch.13399
46. Yang WY, Melgarejo JD, Thijs L, Zhang ZY, Boggia J, Wei FF, et al. Association of office and ambulatory blood pressure with mortality and cardiovascular outcomes. JAMA. (2019) 322:409–20. doi: 10.1001/jama.2019.9811
47. Doi S, Zou Y, Togao O, Pastor JV, John GB, Wang L, et al. Klotho inhibits transforming growth factor-beta1 (TGF-beta1) signaling and suppresses renal fibrosis and cancer metastasis in mice. J Biol Chem. (2011) 286:8655–65. doi: 10.1074/jbc.M110.174037
48. Kuro OM. The Klotho proteins in health and disease. Nat Rev Nephrol. (2019) 15:27–44. doi: 10.1038/s41581-018-0078-3
49. Neyra JA, Hu MC, Moe OW. Klotho in clinical nephrology: diagnostic and therapeutic implications. Clin J Am Soc Nephrol. (2020) 16:162–76. doi: 10.2215/CJN.02840320
50. Guo Y, Zhuang X, Huang Z, Zou J, Yang D, Hu X, et al. Klotho protects the heart from hyperglycemia-induced injury by inactivating ROS and NF-kappaB-mediated inflammation both in vitro and in vivo. Biochim Biophys Acta Mol Basis Dis. (2018) 1864:238–51. doi: 10.1016/j.bbadis.2017.09.029
51. Freundlich M, Gamba G, Rodriguez-Iturbe B. Fibroblast growth factor 23-Klotho and hypertension: experimental and clinical mechanisms. Pediatr Nephrol. (2021) 36:3007–22. doi: 10.1007/s00467-020-04843-6
Keywords: blood pressure, cardiovascular disease, chronic kidney disease, hypertension, Klotho, Centers for Disease Control and Prevention, National Health and Nutrition Examination Survey
Citation: Yan Y and Chen J (2022) Association between serum Klotho concentration and all-cause and cardiovascular mortality among American individuals with hypertension. Front. Cardiovasc. Med. 9:1013747. doi: 10.3389/fcvm.2022.1013747
Received: 07 August 2022; Accepted: 31 October 2022;
Published: 15 November 2022.
Edited by:
Kyriakos Dimitriadis, National and Kapodistrian University of Athens, GreeceReviewed by:
Jacqueline Kathleen Phillips, Macquarie University, AustraliaHwan-Cheol Park, Hanyang University Guri Hospital, South Korea
Copyright © 2022 Yan and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuqin Yan, graceyuqin@163.com; orcid.org/0000-0001-6973-8909
 Yuqin Yan
Yuqin Yan Jun Chen
Jun Chen