- 1UOC Medicina Generale Emostasi e Trombosi, Fondazione IRCCS Ca' Granda Ospedale Maggiore Policlinico, Milan, Italy
- 2Department of Pathophysiology and Transplantation, Università Degli Studi Di Milano, Milan, Italy
Rheumatoid arthritis (RA) is a chronic inflammatory disease affecting about 0. 5–1% of the adult population and manifesting as persistent synovitis, systemic inflammation and production of autoantibodies. Patients affected by RA not only experience chronic disease progression, but are also burdened by a 1.5-fold increased cardiovascular (CV) risk, which is comparable to the risk experienced by patients with type 2 diabetes mellitus. RA patients also have a higher incidence and prevalence of coronary artery disease (CAD). Although RA patients frequently present traditional CV risk factors such as insulin resistance and active smoking, previous studies have clarified the pivotal role of chronic inflammation–driven by proinflammatory cytokines such as interleukin 6 (IL-6) and tumor necrosis factor alpha (TNF-alpha)–in accelerating the process of atherosclerosis and impairing the coagulation system. Over the last years, a number of studies have shown that disease-modifying anti-rheumatic drugs (DMARDs) reducing the inflammatory state in general improve the CV risk, however some drugs may carry some apparent negative effects. Thus, RA is a model of disease in which targeting inflammation may counteract the progression of atherosclerosis and reduce CV risk. Clinical and experimental evidence indicates that the management of RA patients should be tailored based on the positive and negative effects of DMARDs on CV risk together with the individual traditional CV risk profile. The identification of genetic, biochemical and clinical biomarkers, predictive of evolution and response to treatment, will be the next challenge for a precision approach to reduce the burden of the disease.
Introduction
Rheumatoid arthritis (RA) is a chronic systemic inflammatory disease primarily affecting synovial joints, but it can also involve extra-articular organs such as skin, eye, lung, heart, kidney, digestive system, blood vessels, salivary glands, central and peripheral nervous systems, and bone marrow (1–3). The prevalence of the disease in western countries is estimated about 0.5–1% of the adult population, affecting about twice as many women as men, with a peak of incidence around the age of 50 years (3–6). Patients affected with RA not only experience chronic disease progression, but also are burdened by augmented morbidity and mortality due to CV disease (7–10); in particular, they have a higher incidence and prevalence of coronary artery disease (CAD) (11). Clinical and pre-clinical evidence support the pivotal role of chronic inflammation and endothelial dysfunction in accelerating the process of atherosclerosis, driven by proinflammatory cytokines such as interleukin 6 (IL-6) and tumor necrosis factor alpha (TNF-alpha) (12, 13). Over the last 10 years, substantial attention has been paid on the potential effects of different therapies in reducing CV risk, generating a plethora of academic work on this topic. Many studies have shown that treatment with disease-modifying anti-rheumatic drugs (DMARDs) improves not only clinical and laboratory measures of disease activity, but also CV outcomes, contributing to render the use of these therapies a cornerstone in RA treatment.
With this as background, we reviewed the evidence of the last 10 years on the interaction of inflammation with hemostatic and endothelial functions in RA, the underlying mechanisms, and the effects of different DMARDs in their reduction.
Literature search
Here, we provide an overview on the effects of the most frequently prescribed DMARDs on cardiovascular risk in patients with RA in a narrative review. The following keywords were used in different combinations to identify relevant studies published in PubMed and Google Scholar before June 2022: “rheumatoid arthritis”, “biologics”, “biologic DMARDs”, “TNF-α antagonists”, “TNF inhibitors”, “anti-TNFs”, “anti-IL6”, “anti-IL1”, “JAK-inhibitors”, “canakinumab”, “tocilizumab”, “rituximab”, “abatacept”, “anakinra”, “etanercept”, “infliximab”, “adalimumab”, “atherosclerosis”, “cardiovascular disease”, “cardiovascular risk”, “myocardial infarction”, “heart failure”, “stroke”, “arterial stiffness”, “augmentation index”, “endothelial function”, “flow mediated dilatation”, “carotid”, “intima media thickness”. The search was limited to English-language publications only. We included articles regarding patients with rheumatoid arthritis. The selected articles were identified by specialists in rheumatology and internal medicine based on their expertise. Any articles for which no full text was available were excluded.
Cardiovascular disease in patients with rheumatoid arthritis
CV disease in RA patients occurs in the form of premature development and accelerated progression of atherosclerotic lesions as well as hyperactivation of coagulation and endothelial dysfunction (14–17). All these mechanisms lead to a higher risk of CV complications, such as coronary artery disease, cerebrovascular disease and peripheral artery disease. In addition, they contribute to shortening the life expectancy in RA patients (18, 19). The prothrombotic state of RA patients may also involve the venous district, presenting as deep venous thrombosis and pulmonary embolism (20).
Cardiovascular mortality
Patients with RA experience an increased mortality compared to the general population, however, over the last two decades, the long-term prognosis has improved significantly. Many studies demonstrated a drop in mortality for all causes since the early 2000s with a consistent trend in the following years. Up to a two-fold improvement in general standardized mortality rate, compared to the general population, has been observed from 2000 to 2017 (12, 21, 22).
Among different causes of mortality, nearly all studies agree on CV disease being the most common one, accounting for about 40 to 50% of deaths according to different reviews and meta-analyses based on studies from 1960 to 2008 (23–25). A recently published study, which analyzed 813 patients from Minnesota who were diagnosed with RA from 1980 to 2007, has found a 58% decline in CV mortality and 83% drop in coronary heart disease (CHD)-related mortality in patients receiving a diagnosis of RA from 2000 to 2007, compared to the 1990–1999 group (26). Consistently, an Australian study found an estimated standardized CV mortality rate of 1.2, substantially reduced compared to what was reported in earlier studies (27). In line with these findings, another study conducted on a low-disease activity RA cohort between 2009 and 2011 found a drop in CV case fatality compared to 1996 studies from 28.6 to 6.9% (28). This improvement in mortality is partially explained by the drop of mortality in the general population, but other factors such as early diagnosis, prompt treatment using novel molecules, an improved attention and intervention on other risk factors in RA patients have played a pivotal role in achieving a better prognosis.
Cardiovascular morbidity
RA patients are burdened by many comorbidities such as infections, osteoporosis, gastrointestinal, pulmonary and hematologic diseases, however CV disease is the most frequent (4, 7). CV manifestations in RA are variable, affecting mainly the arterial system, which leads to myocardial infarction, stroke, peripheral artery disease and, to a lesser degree, angina (11). The risk of developing atherosclerotic CV disease in patients affected with RA is similar in magnitude to that of diabetic patients, being 1.5–1.6 fold higher than the general population (24).
A myocardial infarction risk of 68% more than the general population was described in a RA patients, according to a meta-analysis of observational studies, with no differences between men and women (25). A recently published population-based cohort study, which included patients diagnosed from 1980 to 2009, demonstrated a 56% decrease in acute myocardial infarction events between the group of patients who were diagnosed in the 2000s compared to the group of 1980s (hazard ratio [HR] 0.44) (29). Notably, the addition of the highest erythrocyte sedimentation rate (ESR) or time-dependent treatment with DMARDs into the predictive model showed a significant reduction of any CV event in the group of 2000s, supporting a correlation between inflammation and CV risk. More recently, in RA group compared to the general population, Yazdani et al. found an increased risk of acute myocardial infarction (RR 1.21), and Holmqvist et al. found an overall higher incident CV event rate (HR 1.41) (30, 31). These two studies, however, revealed similar rates of decline in myocardial infarction and CV events respectively for both RA and non-RA group over the course of the study period. To sum up, although it is unclear if the existing gap between RA patients and the general population in CV risk is really narrowing, available data consistently show a decrease in CV event rates in RA patients.
Concerning cerebrovascular accidents, a small number of studies is available, yet suggesting a burden similar to myocardial infarction, according to a meta-analysis that has demonstrated a 40% augmented incidence among RA patients (25). A recent German study confirmed this data, showing an association between RA and both stroke (HR 1.42, confidence interval [CI] 1.25–1.60) and transient ischemic attack (HR 1.69, CI 1.46–1.95) (32). Accordingly, a 2021 meta-analysis further supports a significantly increased risk of stroke in RA (HR 1.32 95%, CI 1.02–1.73), with no differences between male RA patients compared to controls, but with a higher incidence in the female RA subgroup (33).
Congestive heart failure may also occur almost twice as often in RA patients compared to the general population, as demonstrated by a recent meta-analysis (34). In the study by Khalid et al. it was also demonstrated that women affected by RA had a three-fold higher incidence of heart failure compared to controls (OR 3.38, 95%, CI 2.59–4.40), and the meta-regression showed an even greater incidence with older age (35).
Peripheral arterial occlusive disease resulted 1.73-fold higher (95% CI 1.57–1.91) in RA patients compared to a non-RA cohort, according to a study conducted on 30,812 patients with RA (36). Interestingly, the adjusted risk of peripheral arterial occlusive disease was more evident in patients with RA aged ≤ 49 years (HR 3.39, 95% CI 2.66–4.32). Another study assessing intima-media thickness in 80 RA patients without CV disease or diabetes demonstrated a higher prevalence of femoral intima-media thickness and plaques compared to controls matched for age, gender and CV risk factors (37).
The venous system is also involved in RA. According to a nationwide register-based cohort study, the relative risk for venous thromboembolism in RA is 1.88 (95% CI 1.65–2.15) and it increases with increasing RA disease activity, namely the 1-year incidence increases from 0.52% in patients in remission to 1.08% in subjects with high disease activity (38). These findings follow a 2014 meta-analysis that estimated a pooled risk of deep venous thrombosis, pulmonary embolism and overall VTE in patients with RA of 2.08 (95% CI 1.75–2.47), 2.17 (95% CI 2.05–2.31), and 1.96 (95% CI 1.81–2.11) respectively, compared to non-RA subjects (39).
Cardiovascular risk factors in rheumatoid arthritis
Traditional risk factors
Genetic factors play a role in determining CV disease in RA patients. Many single nucleotide polymorphisms are shared by both diseases and HLA DRB1*01 haplotype, which is linked to RA susceptibility, has been associated with C-reactive protein (CRP) levels and the risk of myocardial infarction in general population (40, 41).
In patients with RA, the study of incidence of traditional CV risk factors such as obesity, diabetes, hypertension and smoking gave contradictory results, with the only exception of smoking. Smoking has in fact been identified as a significant risk factor for both RA and CV risk (42), contributing to the appearance of anti-citrullinated c-peptide antibodies (CCP), increased expression of inflammatory genes promoting RA development, and methylation of genes involved in coronary artery disease (43–45). Among the aforementioned risk factors, it is also important to remember that in RA patients body mass index is inversely correlated with CV events (46); this is probably due to the chronic inflammatory state that causes a loss of lean muscle mass and accumulation of adipose tissue in a phenomenon known as rheumatoid cachexia (47). Moreover, lower mortality was observed in higher BMI patients with RA although the mechanism that protects these patients has not been explained (46).
Concerning lipid profile, in RA patients also exists a “lipid paradox”(48), meaning that there is no clear association between higher levels of low-density lipoprotein (LDL) and CV risk (48, 49), despite higher high-density lipoprotein (HDL) level being effectively associated with lower CV risk (49, 50). This is probably due to an impaired function of lipoprotein in the inflammatory state of RA patients, in the form of more oxidized LDL (51), which are more easily taken up by macrophages determining their transformation into foam cells, and pro-inflammatory HDL (52–54), which have a reduced capacity of reverse cholesterol transport (55).
Although often forgotten, RA patients are usually inactive (56). This is due to many factors, such as pain, lack of motivation and lack of knowledge about the impact of physical inactivity (57–59). In this sense, RA related disability is arguably an independent CV risk factor (60).
Disease-specific risk factors
All the aforementioned risk factors, however, cannot fully explain the excess of CV disease mortality and morbidity in RA patients, thus suggesting RA itself being another independent risk factor (61). Mounting evidence, in fact, suggests the central role of disease activity and immune system in the pathogenesis of CV risk and it is nowadays known that proinflammatory cytokines such as IL-6 and TNF-alpha are implicated in both RA and atherogenesis (62). These molecules also have a role in contributing to insulin resistance (63) and have been independently associated with coronary artery calcifications (64), as well as CRP levels and swollen joint counts have been associated with carotid plaque progression (65).
A number of studies have demonstrated a correlation between higher disease activity and the risk of CV events (66, 67), evident since the early phases of the disease, with a 33% increase of CV disease risk every 1-unit increase of DAS28. Other studies have also successfully associated CRP and ESR levels with higher risk of CV disease (49, 68, 69). Various studies showed an association between disease activity and cardiac function, in particular demonstrating an increased left ventricular strain and left ventricular global longitudinal strain, diastolic dysfunction and left ventricular wall thickness (70–73), while another study demonstrated an improvement in cardiac function in RA patients in remission (74).
Another feature that is hypothesized to have a role in CV disease is the presence of specific autoantibodies. Anti-CCP and rheumatoid factor positivity have been associated with increased CV risk in both RA patients and non-RA patients, although not consistently (75–77). Two studies conducted by cardiac magnetic resonance in RA patients showed an association between anti-CCP antibodies and reduced stroke volume, end diastolic volume and left ventricular mass (78, 79).
Lastly, therapeutic intervention can also play a role in promoting CV disease risk. A meta-analysis demonstrated an 18% increased risk of CV events in RA patients treated with nonsteroidal anti-inflammatory drugs (NSAID) as well as a 47% increased risk in patients treated with glucocorticoids (80). With regard to glucocorticoids, there is a correlation between CV risk and both dose and duration of treatment (81–83). In the case of NSAIDs, this increased risk is probably due to a reduced production of prostacyclin that has a vasodilatory and antiplatelet effect, while glucocorticoids are well known to exacerbate other risk factors such as hypercholesterolemia, hypertension, hypertriglyceridemia and are also supposed to cause impaired reverse cholesterol transport of HDL (84, 85).
Effects of different DMARDs on mechanisms of cardiovascular disease in rheumatoid arthritis
Mechanisms of potential pharmacological target
The atherosclerotic process has been demonstrated to be particularly accelerated in RA patients (86). A relevant number of mediators and mechanisms are shared between the inflamed synovium and the atherosclerotic plaque, and in particular pro-inflammatory cytokines produced in the synovium contribute both directly and indirectly to plaque development (87, 88). In RA patients all the stages of atherosclerosis are amplified, including endothelial dysfunction, arterial wall inflammation, plaque formation, remodeling and CV events, which develop upon the rupture of the atherosclerotic plaque and the subsequent thrombosis (89). Figure 1 shows the mechanisms through which inflammation promotes the atherosclerotic process in RA.
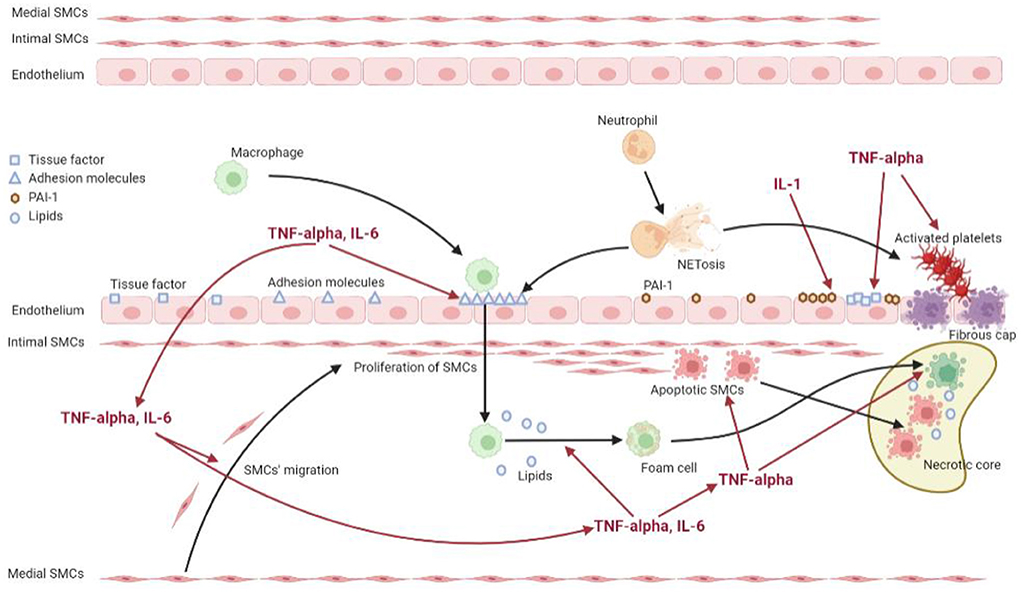
Figure 1. Mechanisms of inflammation promoting the atherosclerotic process in rheumatoid arthritis. The figure shows the interplay between inflammation and the different effectors of the atherosclerotic process. The systemic inflammation, supported by pro-inflammatory cytokines such as TNF-alpha, IL-6 and IL-1, acts on the endothelial cells inducing the expression of adhesion molecules, tissue factor and PAI-1. It also promotes the migration of smooth muscle cells from the media to the intima, as well as facilitates the uptake of lipids by the macrophages. Moreover, the inflammatory state acts on platelets and neutrophils, triggering the process of NETosis, which stimulates on one hand the expression of adhesion molecules by endothelial cells, on the other hand the activation of platelets contributing to a prothrombotic state.
Endothelial dysfunction is a condition characterized by the failure to perform its physiologic functions, such as regulation of vascular tone, cellular adhesion, vascular smooth muscle migration and resistance to thrombosis, often as a maladaptive response to a pathologic stimulus (90). It is characterized by upregulated expression of cellular adhesion molecules, reduction of the bioavailability of vasodilators, particularly nitric oxide (NO), and/or an increase in endothelium-derived contracting factors (91, 92). TNF-alpha expression has been demonstrated on the surface of microparticles promoting apoptosis and autophagy and contributing to endothelial dysfunction (93). Endothelial disfunction in RA patients, in fact, evaluated by brachial artery flow-mediated dilation, has been correlated to the level of inflammation by a meta-analysis of 20 studies (94). In addition, arterial stiffness, measured by pulse wave velocity or augmentation index, has been demonstrated to be higher in RA patients compared to controls (95). It is known that in vitro TNF-alpha addition stimulates superoxide release from endothelial cells and monocytes exacerbating the oxidation of LDL (96). These oxidized LDL are taken up by macrophages through scavenger receptors such as CD36, LOX-1 and SR-A, transforming macrophages into foam cells which lead to the formation of the fatty streak (97, 98). Expression of LOX-1 and SR-A results upregulated in vitro by TNF-alpha and IL-6 as well as CD36 expression is increased after exposure to the serum of a collagen induced arthritis animal model (99).
It has been demonstrated that in atherosclerosis of both RA and non-RA patients there is an increased expression of cell adhesion molecules such as ICAM-1 and P-selectin, although only P-selectin correlates with disease activity (90, 100–103). Moreover, in RA patients, and in particular in patients with extraarticular manifestation, several data have been reported showing an expansion of CD4+CD28- TH cells, a specific subset which could shift the immune response toward TH1 mediated activation of macrophages, leading to accelerated progression of atherosclerosis (104–106). Accordingly, a study conducted on aortic biopsies of patients undergoing coronary artery bypass graft surgery, showed a higher prevalence and density of medial and adventitial mononuclear cells infiltrates in patients with rheumatic disease such as RA compared to controls (107). TNF-alpha and IL-6 can also promote the proliferation and migration of vascular smooth muscle cells from the media to the intima, leading to intima-media thickening (108), and TNF-alpha is demonstrated also to activate platelets in vitro (109). This process culminates with the apoptosis of foam cells which generates a necrotic core rich in lipids, and the creation of a fibrous cap covering the plaque which can become unstable and rupture (110), leading to thrombosis which brings to CV events (111).
Regarding thrombosis, it is known that an extensive cross-talk between coagulation and inflammation pathways exists. Longitudinal studies have shown increased levels of markers for endothelial activation such as von Willebrand Factor, ICAM and VCAM in RA patients (112). TNF-alpha has been demonstrated to be able to upregulate in vitro, both in endothelial cells and in monocytes, the expression of tissue factor, which is the main initiator of blood coagulation through binding and activation of FVII (113, 114). Previous studies have also demonstrated that RA patients have higher levels of prothrombin fragment F1+2, a marker of thrombin generation, compared to healthy controls, and the infusion of TNF-alpha in healthy human volunteers can increase plasma concentration of this fragment (13, 115, 116). Fibrinolysis too is impaired in patients with RA. TNF-alpha and IL-1 simulate the production of plasminogen activator inhibitor-1 in endothelial cells, which counteracts the fibrinolytic system (117). Moreover, TNF-alpha and IL-1 are able to down-regulate thrombomodulin on the endothelial surface, disrupting also the protein C system (113). In addition, a relationship between platelet activation markers and RA has been demonstrated, and platelets have been suggested to amplify inflammation in RA through collagen-dependent microparticle production (118). Interestingly, these particles were increased also in synovial fluid of RA patients.
Another element that is supposed to have a role in atherosclerosis of RA patients is represented by the neutrophils, and in particular by neutrophil extracellular traps (NETs), structures consisting of DNA, histones and neutrophil-derived granule proteins, which are expelled from neutrophils and boost the adaptive response of dysfunctional T- and B-cells (119, 120). In RA patients, neutrophils are more prone to undergo NETosis, and the citrullinated histones within the NET are a leading substrate for the generation of anti-citrullinated protein autoantibodies (121–124). NET components are also supposed to act as damage-associated molecular patterns and sustain a vicious circle leading to endothelial expression of adhesion molecules and pro-thrombotic factors such as von Willebrand factor and P-selectin, as well as digest tissue factor pathway inhibitor and increase platelets responsiveness (125–128). Accordingly, the content of NETs in coronary thrombectomy specimens positively correlates with infarct size in the general population (129).
To sum up, the systemic inflammation of RA patients on one hand promotes and accelerates the different steps of atherosclerosis, on the other hand generates a prothrombotic state that increases the risk of CV events. For these reasons, DMARDs, reducing inflammation, can counteract at various steps the determination of CV events and therefore could have a role in reducing the CV risk of RA patients.
Available anti-rheumatic drugs
The different types of DMARDs commonly used in RA patients are classified based on their process of discovery and mechanistic distinctions in conventional synthetic DMARDs (csDMARDs), biologic DMARDs (bDMARDs) and targeted synthetic DMARDs (tsDMARDs) (130). Table 1 resumes the main effects of the most commonly used DMARDs on CV risk.
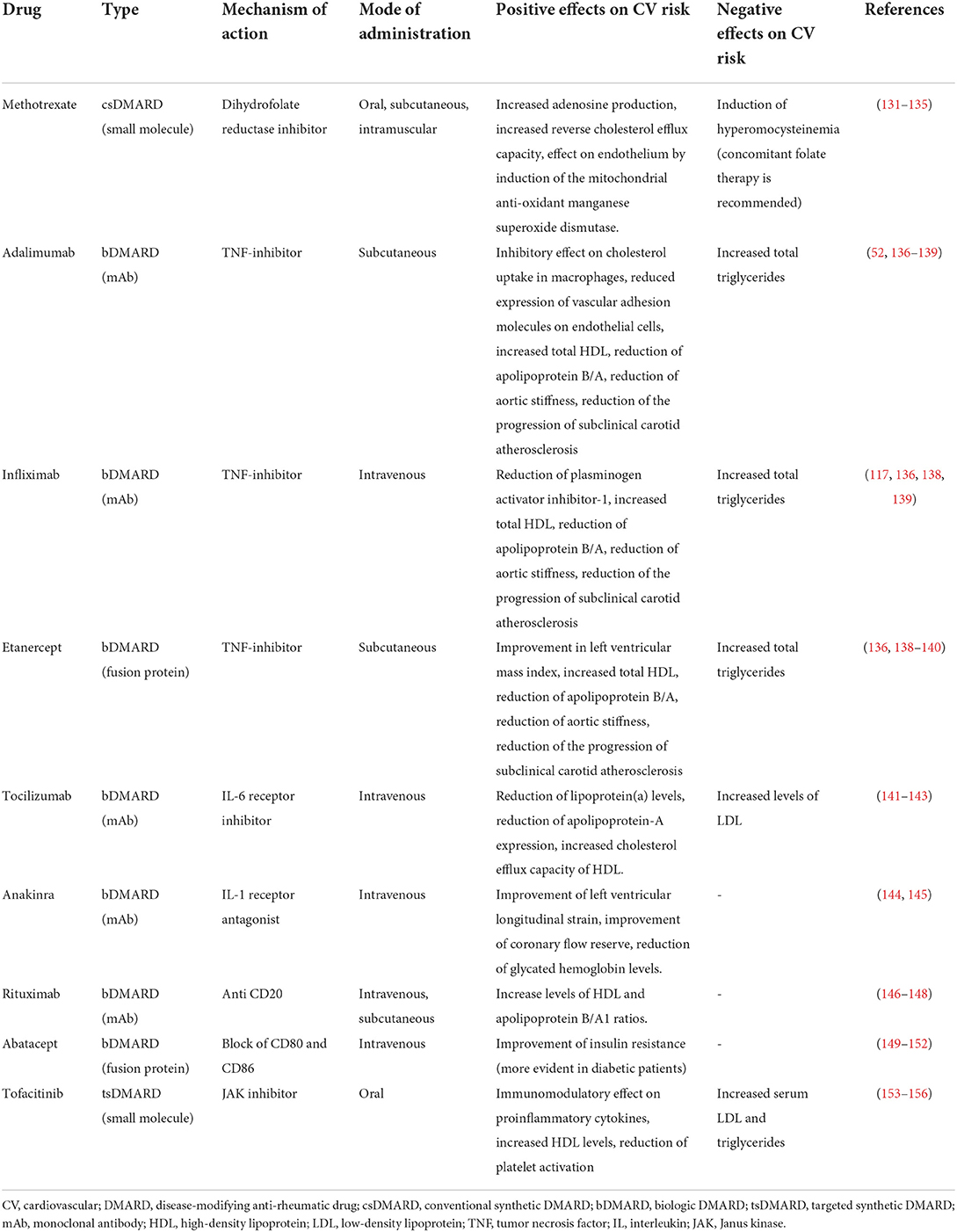
Table 1. Disease-modifying anti-rheumatic drugs (DMARDs) commonly used in patients with rheumatoid arthritis and their potential effects on cardiovascular risk.
Methotrexate
Methotrexate is an anti-metabolite inhibitor of the enzyme dihydrofolate reductase, considered for long time a cornerstone of RA treatment for its immunomodulating properties when administered in a low-dose regimen. In a systematic review and meta-analysis of 28 cohort studies (80), methotrexate was associated with a pooled 28% reduction in CV events, as well as other studies have even increased this correlation up to 34–66% (157, 158). More recently, an updated meta-analysis confirmed these findings (159). The effect on atherosclerotic lesion progression is not clear, as a number of studies has demonstrated an improvement of atherosclerosis after methotrexate therapy, although other authors didn't confirm these findings; due to the limited number of studies and the small sample sizes, it is still not fully disclosed (65, 160–165). In 2019, the CIRT study investigated the potential protective effect of low-dose methotrexate in CHD patients without RA, but it failed to prove it; between possible explanations, it is notable that the mean CRP levels of this study population (1.6 mg/L) was lower than both the median CRP levels of RA patients, as reported by Ajeganova et al. (131), and the residual inflammatory risk in CHD as currently defined (2 mg/L). The effects through which methotrexate reduces CV event rates in RA patients are several and still not fully understood; it is however known that the clinical response to treatment is associated with reduction of inflammation and reduction in the expression of inflammatory cytokines such as TNF-alpha and IL-6, which, as aforementioned, have proatherogenic effects. Emerging evidence suggests that methotrexate can also induce adenosine production, which decreases the activation of lymphocytes by binding the adenosine A2 receptor (132), and increases the reverse cholesterol efflux capacity through both adenosine production and ATP-binding cassette transporter A1 expression. Moreover, it has been demonstrated in vitro that methotrexate exerts a direct effect on endothelium by inducing the mitochondrial anti-oxidant manganese superoxide dismutase (MnSOD) and scavenging superoxide radicals actively (133, 134). Finally, it is interesting to notice that methotrexate has an overall cardioprotective effect in RA patients even though it is known to induce hyperomocysteinemia, which is arguably atherogenic (135). Concomitant folate supplementation has been shown to decrease the plasma homocysteine level and consequently may protect against CV risk (166).
TNF inhibitors
According to the meta-analysis reported above (80), a 30% reduction of risk of CV events exists in RA patients treated with TNF inhibitors. In particular, among the studies with MI or stroke as outcome, the use of TNF inhibitors was associated with a 41% relative risk reduction of MI and a 43% reduction of stroke. However, these effects may not be found in all patients receiving TNF inhibitors, rather seem to be related to clinical response, as some studies have observed a reduction of CV events only in good responders to anti-TNF treatment (136, 167). Among the mechanisms of action, many of which have been explained in previous sections, TNF inhibitors improve endothelial dysfunction, oxidative stress and modify the lipid profile. A meta-analysis of 13 studies has demonstrated in long term anti-TNF users an increase of total HDL and triglycerides, as well as stable levels of LDL and reduction of apolipoprotein B/A (137). An in vitro study with TNF inhibitor adalimumab demonstrated an inhibitory effect on cholesterol uptake in macrophages, without affecting reverse cholesterol transport (53), while another in vitro study, always with adalimumab, showed a reduced expression of vascular adhesion molecules on endothelial cells (140). Finally, treatment with infliximab has been associated with a significant reduction in quantity and activity of tissue-type plasminogen activator (tPA) and plasminogen activator inhibitor-1 (PAI-1), conditions showing a prothrombotic effect (117). Treatment with TNF inhibitors has been associated with improvement in left ventricular mass index after 3–6 months of treatment alongside clinical improvement (138), reduction of aortic stiffness after 3 months (139), and reduction of the progression of subclinical carotid atherosclerosis after 14 weeks (168). The TARGET trial, a current phase 4 study which aims to compare post-RA treatment changes in aortic and carotid inflammation, quantified through PET-CT analysis, will be the first interventional study in RA trying to provide insight on the effects of DMARDs on a direct measure of vascular inflammation (169).
IL-6 inhibitors
Tocilizumab is a humanized monoclonal antibody antagonist of the IL-6 receptor. Therapy with tocilizumab is approved for the treatment of patients with moderate or severe RA, both in combination with conventional DMARDs and in monotherapy. In RA patients, tocilizumab showed to be effective in improving clinical symptoms (170) and reducing both inflammatory and prothrombotic biomarkers (171, 172). Moreover, tocilizumab may improve the pro-atherothrombotic status of RA patients by regulating the inflammatory activity of monocytes and neutrophils through mechanisms involving modulation of oxidative stress, NETosis, and intracellular signaling (141). Despite the increase in LDL levels observed during treatment with tocilizumab, the incidence of CV events seems reduced as compared to RA-population in an observational study (173). Given these effects, the ENTRACTE study, a phase IV study (142) with an over 3 years follow-up comparing tocilizumab and etanercept, a TNF inhibitor, showed that the reduction of CV risk of tocilizumab is comparable to that of etanercept. This may be in agreement with the observation that IL-6 blockade is able to induce a reduction in lipoprotein (a) [Lp(a)] levels (174), a reduction of apolipoprotein-A expression and the inhibition of IL-6-induced Lp(a) mRNA expression (143). Consistently, a recent study demonstrated that treatment with tocilizumab in RA patients reduces pro-atherogenic effects of LDL and increases the cholesterol efflux capacity of HDL (175). Sarilumab is another antagonist of IL-6R. It is a fully human monoclonal antibody against IL-6R successfully used to control symptoms in RA patients (176). To the best of our knowledge, the only study on CV effects of sarilumab is that of Fleischmann et al., which showed a small reduction of incidence of major CV events (from 1.4 to 0.5 per 100 patient-years) compared to general RA population (144).
IL-1 inhibitors
Among the available IL-1 inhibitor drugs, only anakinra, a human recombinant IL-1 receptor antagonist, is currently approved for RA treatment. Thus, only a few studies exist and to our knowledge no one has ever estimated the effect of anakinra or other IL-1 inhibitors on CV risk in RA patients. One study has demonstrated an improvement of cardiac function and a reduction of endothelial dysfunction, measured by left ventricular longitudinal strain and coronary flow reserve respectively, and another has reported an improvement of glycemic control, assessed by glycated hemoglobin levels (145, 177).
Interestingly, canakinumab, another IL-1 inhibitor, has demonstrated a reduction of the rate of CV events compared to placebo in a population of patients not affected by RA but by recurrent CV events and high levels of inflammatory markers (146).
Rituximab
There are few data regarding the effects of rituximab therapy on atherosclerosis in RA patients. Small short-term studies demonstrated an improvement in atherogenic index, along with increased levels of HDL and apolipoprotein B/A1 ratios, although these effects were restricted to clinical responders, thus suggesting that the main promoter of these modifications is the reduction of inflammation more than the direct effect of the drug (147, 148, 178). The CORRONA registry, which investigated CV events rates in RA patients matched by participant characteristics, showed comparable efficacy between the group treated with rituximab and the one treated with TNF inhibitors (179).
Abatacept
Abatacept is a fusion protein consisting of cytotoxic T-lymphocyte antigen-4, an anti-inflammatory factor expressed by T cells, conjugated to the Fc portion of IgG, which has demonstrated clinical efficacy in RA (149). Only a small handful of studies exists: two small studies on aortic stiffness in RA patients which initiated abatacept treatment did not demonstrate an improvement, while a cohort study comparing patients that initiated therapy with abatacept or TNF inhibitors showed a modest reduction of CV risk in the group treated with abatacept, particularly in patients affected by diabetes mellitus (150–152, 180). Studies conducted on murine models, however, demonstrated a reduction in atherogenesis in mice treated with abatacept (181, 182).
JAK inhibitors
Janus Kinase (JAK) inhibitors are the most recent class of drugs approved for RA treatment. The JAK family is a family of receptor tyrosine kinases that, when attached to a specific cytokine, act as dimers and phosphorylate, binding signaling peptides of the STAT family, which eventually translocate in the nucleus in order to regulate the transcription of target genes. This pathway ends up regulating the expression of numerous cytokines, such as IFN, IL-4, IL-6 and IL-10, which are included in different immunological pathways and in pathogenesis of RA. Currently, four different JAK inhibitors are licensed for RA treatment in western countries: tofacitinib, baricitinib, upadacitinib and filgotinib, all demonstrating their efficacy in treatment of RA (183, 184).
During the development phase of tofacitinib, however, a slight increase in the incidence of cancers, including lymphoma, was observed, as well as an increase in serum lipid levels (153, 154). This led to a prospective safety trial comparing tofacitinib with TNF inhibitors, the Oral Rheumatoid Arthritis Trial (ORAL) Surveillance. According to this study, the incidence of major CV events was higher in the tofacitinib group (3.4%) compared to TNF inhibitors group (2.5%), the most common of which being nonfatal myocardial infarction for tofacitinib and nonfatal stroke with TNF inhibitors, in particular for patients 65 years of age or older (155). This study showed also an increased risk of venous thromboembolism with the tofacitinib 10-mg dose, further enhancing the doubts about safety raised by a previous trial with 4-mg dose baricitinib, even though it was not powered to this specific evaluation. The same study showed a similar risk of venous thromboembolism between 5-mg dose tofacitinib and TNF inhibitors, a finding in line with real-world data from the CORRONA registry (185). The 2022 STAR-RA population-based study, which evaluated Medicare data on patients which initiated treatment with tofacitinib or TNF inhibitors, showed no difference in the incidence rates of myocardial infarction and stroke between the two groups, even when the analysis was restricted to patients with similar CV risk factors to those of the patients enrolled in the ORAL Surveillance study (156). A biological explanation for the better effect on arterial thrombosis may be provided by the study of Parra-Izquierdo et al. that showed an inhibitory effects of JAK inhibitors on platelet function (186).
Cardiovascular risk management
As reported above, the use of conventional synthetic and biological disease-modifying agents is associated with reduced CV risk in individuals with RA. These aspects on influence of anti-rheumatic drugs on CV involvement in RA, have been recently reviewed by Atzeni et al. (187) and subsequently by the recently published ORAL Surveillance and STAR-RA studies (155, 156).
From a practical point of view, the best possible approach should consider the assessment of the CV risk according to the current predictive scores such as Systematic Coronary Risk Evaluation (SCORE), Framingham score and QRISK (188, 189). The European League Against Rheumatism (EULAR), in their most recent guidelines on CV management in RA patients that date back to 2015, recommends to apply a 1.5 multiplication factor in order to adjust for the augmented risk of RA patients if the algorithm does not take it into account (190). The European Society of Cardiology (ESC) suggests to use the same adjustement for CV assessment (191). Indeed, available calculators have in some cases already included this adjustement: the SCORE calculator considers a 1.5 multiplication factor for RA patients, while QRISK calculator uses a 1.2 factor. According to the same EULAR guidelines, the assessment of CV risk in RA patients should be performed at least every 5 years if the risk is low to moderate, but we suggest to do it at least once per year in patients with moderate to high CV risk (SCORE >5%). Notably, both SCORE and Framingham score have been demonstrated to be unreliable in identifying patients at a high risk of atherosclerosis in two black African cohorts, thus demonstrating that the path leading to an effective score is still long (192, 193).
Other elements that must be taken into account are the family history of the patient, sex, personal history of CV disease, psychosocial factors, drug history, alongside assessment of lipid levels and biomarkers of inflammation and thrombosis. As aforementioned, it is important to remember that many commonly used DMARDs have an effect on lipid levels that should not be overlooked, as well as the achievement of a stable control of disease activity should be obtained in order to limit the confounding effects of systemic inflammation.
Imaging evaluation using echocardiography, arterial augmentation index or aortic pulse wave velocity have been proposed to further evaluate the patients, especially those with high CV risk, besides the current EULAR recommendations, which only include carotid ultrasound.
Regardless of risk level, however, all patients affected by RA should receive extensive lifestyle recommendations which must include, but not be limited to, regular exercise, healthy diet and smoking cessation. In addition, management of conditions such as dyslipidemia or hypertension should not differ from general population, although further studies are needed in order to assess the appropriateness of current treatment targets in RA patients.
To date, the approach based on the aforementioned data provides tools that can be used in a multidisciplinary discussion with other specialists involved in the evaluation of CV risk (i.e., cardiologists, internists, diabetologists and other specialists).
In the future, researchers will need to collect a huge series of genetic, serological, biochemical and diagnostic imaging data, to set up new tools for health stakeholders in order to put in practice a true personalized medicine in RA, as previously done in other fields such as oncology (194).
Conclusions
Evidence has been provided supporting the view that the inflammatory state of RA patients contributes to accelerated atherosclerosis and to thrombosis, inducing several predisposing factors such as insulin resistance, dyslipidemia, endothelial dysfunction, as well as activation of coagulation and inhibition of fibrinolysis.
Over the last decades, a growing understanding of the complex pathogenesis of RA has led to a significant improvement in the management of the disease. DMARDs have been demonstrated to act on the inflammatory state, on one hand reducing the tissue damage and endothelial dysfunction locally, on the other hand lowering the systemic CV and atherothrombotic risk. Although some DMARDs may negatively affect specific CV risk factors, they show a net CV protective effect, mainly due to their overall anti-inflammatory activity. Therefore, RA can be considered a prototype example showing the importance of targeting inflammation in order to interfere with the atherosclerotic process and reduce CV risk.
Biologic DMARDs reduce the inflammatory process through different mechanisms and interfere with other systems, including lipid metabolism. In particular for tocilizumab, its detrimental effect of increasing LDL is counterbalanced by the improvement of HDL cholesterol efflux capacity. Concerning conventional synthetic DMARDs, almost all authors agree that responders to methotrexate have an improvement of CV profile, consistent with the reduction of inflammation and a direct effect on endothelial dysfunction. Targeted synthetic DMARDs, like JAK inhibitors, have shown efficacy in controlling RA activity although more recent data seem to suggest a higher risk of cancer and CV events compared to TNF inhibitors.
In this scenario, further research is needed to fully clarify the exact mechanisms through which RA promotes atherosclerosis and how DMARDs may counteract it. Furthermore, the recent findings on the adverse effects of JAK inhibitors raise the urge for long follow-up studies, involving large numbers of patients, in order to resolve the controversies on their safety and efficacy.
The main challenge for the future will be to develop a personalized approach for RA patients based on genetic, biochemical, clinical and radiological parameters, in order to identify the most appropriate therapeutic option for each patient in terms of clinical response, improvement of CV profile and reduction of adverse events. Personalized medicine in RA will overcome the actual “trial and error” approach that leads to a higher burden for patients and healthcare.
Considering the CV burden of RA patients, which is still high, it is crucial for the clinician to tackle the traditional CV risk factors following guidelines for the general population, and to tailor a patient-based therapy that considers the positive and negative effects of different drugs also on CV risk.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
This research was partially supported by Ricerca Corrente 2022 - Italian Ministry of Health, Cariplo and Fondazione Regionale per la Ricerca Biomedica (FRRB) n. 2017–1938.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Conforti A, Di Cola I, Pavlych V, Ruscitti P, Berardicurti O, Ursini F, et al. Beyond the joints, the extra-articular manifestations in rheumatoid arthritis. Autoimmun Rev. (2021) 20:102735. doi: 10.1016/j.autrev.2020.102735
2. Radu AF, Bungau SG. Management of rheumatoid arthritis: an overview. Cells. (2021) 10:2857. doi: 10.3390/cells10112857
3. van der Woude D, van der Helm-van Mil AHM. Update on the epidemiology, risk factors, and disease outcomes of rheumatoid arthritis. Best Pract Res Clin Rheumatol. (2018) 32:174–87. doi: 10.1016/j.berh.2018.10.005
4. Goemaere S, Ackerman C, Goethals K, De Keyser F, Van der Straeten C, Verbruggen G, et al. Onset of symptoms of rheumatoid arthritis in relation to age, sex and menopausal transition. J Rheumatol. (1990) 17:1620–2.
5. Hunter TM, Boytsov NN, Zhang X, Schroeder K, Michaud K, Araujo AB. Prevalence of rheumatoid arthritis in the United States adult population in healthcare claims databases, 2004-2014. Rheumatol Int. (2017) 37:1551–7. doi: 10.1007/s00296-017-3726-1
6. Myasoedova E, Davis J, Matteson EL, Crowson CS. Is the epidemiology of rheumatoid arthritis changing? Results from a population-based incidence study, 1985-2014. Ann Rheum Dis. (2020) 79:440–4. doi: 10.1136/annrheumdis-2019-216694
7. England BR, Thiele GM, Anderson DR, Mikuls TR. Increased cardiovascular risk in rheumatoid arthritis: mechanisms and implications. BMJ. (2018) 361:k1036. doi: 10.1136/bmj.k1036
8. Hansildaar R, Vedder D, Baniaamam M, Tausche AK, Gerritsen M, Nurmohamed MT. Cardiovascular risk in inflammatory arthritis: rheumatoid arthritis and gout. Lancet Rheumatol. (2021) 3:e58–70. doi: 10.1016/S2665-9913(20)30221-6
9. Jagpal A, Navarro-Millan I. Cardiovascular co-morbidity in patients with rheumatoid arthritis: a narrative review of risk factors, cardiovascular risk assessment and treatment. BMC Rheumatol. (2018) 2:10. doi: 10.1186/s41927-018-0014-y
10. Logstrup BB, Ellingsen T, Pedersen AB, Darvalics B, Olesen KKW, Botker HE, et al. Cardiovascular risk and mortality in rheumatoid arthritis compared with diabetes mellitus and the general population. Rheumatology (Oxford). (2021) 60:1400–9. doi: 10.1093/rheumatology/keaa374
11. Maradit-Kremers H, Crowson CS, Nicola PJ, Ballman KV, Roger VL, Jacobsen SJ, et al. Increased unrecognized coronary heart disease and sudden deaths in rheumatoid arthritis: a population-based cohort study. Arthritis Rheum. (2005) 52:402–11. doi: 10.1002/art.20853
12. Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. (2000) 342:836–43. doi: 10.1056/NEJM200003233421202
13. van der Poll T, Buller HR, ten Cate H, Wortel CH, Bauer KA, van Deventer SJ, et al. Activation of coagulation after administration of tumor necrosis factor to normal subjects. N Engl J Med. (1990) 322:1622–7. doi: 10.1056/NEJM199006073222302
14. Gonzalez-Gay MA, Gonzalez-Juanatey C, Vazquez-Rodriguez TR, Martin J, Llorca J. Endothelial dysfunction, carotid intima-media thickness, and accelerated atherosclerosis in rheumatoid arthritis. Semin Arthritis Rheum. (2008) 38:67–70. doi: 10.1016/j.semarthrit.2008.02.001
15. Mason JC, Libby P. Cardiovascular disease in patients with chronic inflammation: mechanisms underlying premature cardiovascular events in rheumatologic conditions. Eur Heart J. (2015) 36:482–9c. doi: 10.1093/eurheartj/ehu403
16. Sodergren A, Karp K, Boman K, Eriksson C, Lundstrom E, Smedby T, et al. Atherosclerosis in early rheumatoid arthritis: very early endothelial activation and rapid progression of intima media thickness. Arthritis Res Ther. (2010) 12:R158. doi: 10.1186/ar3116
17. van Leuven SI, Franssen R, Kastelein JJ, Levi M, Stroes ES, Tak PP. Systemic inflammation as a risk factor for atherothrombosis. Rheumatology (Oxford). (2008) 47:3–7. doi: 10.1093/rheumatology/kem202
18. Jean S, Hudson M, Gamache P, Bessette L, Fortin PR, Boire G, et al. Temporal trends in prevalence, incidence, and mortality for rheumatoid arthritis in Quebec, Canada: a population-based study. Clin Rheumatol. (2017) 36:2667–71. doi: 10.1007/s10067-017-3796-1
19. Wallberg-Jonsson S, Ohman ML, Dahlqvist SR. Cardiovascular morbidity and mortality in patients with seropositive rheumatoid arthritis in Northern Sweden. J Rheumatol. (1997) 24:445–51.
20. Liang KP, Liang KV, Matteson EL, McClelland RL, Christianson TJ, Turesson C. Incidence of noncardiac vascular disease in rheumatoid arthritis and relationship to extraarticular disease manifestations. Arthritis Rheum. (2006) 54:642–8. doi: 10.1002/art.21628
21. Lacaille D, Avina-Zubieta JA, Sayre EC, Abrahamowicz M. Improvement in 5-year mortality in incident rheumatoid arthritis compared with the general population-closing the mortality gap. Ann Rheum Dis. (2017) 76:1057–63. doi: 10.1136/annrheumdis-2016-209562
22. Naz SM, Symmons DP. Mortality in established rheumatoid arthritis. Best Pract Res Clin Rheumatol. (2007) 21:871–83. doi: 10.1016/j.berh.2007.05.003
23. Avina-Zubieta JA, Choi HK, Sadatsafavi M, Etminan M, Esdaile JM, Lacaille D. Risk of cardiovascular mortality in patients with rheumatoid arthritis: a meta-analysis of observational studies. Arthritis Rheum. (2008) 59:1690–7. doi: 10.1002/art.24092
24. Sokka T, Abelson B, Pincus T. Mortality in rheumatoid arthritis: 2008 update. Clin Exp Rheumatol. (2008) 26:S35–61.
25. Avina-Zubieta JA, Thomas J, Sadatsafavi M, Lehman AJ, Lacaille D. Risk of incident cardiovascular events in patients with rheumatoid arthritis: a meta-analysis of observational studies. Ann Rheum Dis. (2012) 71:1524–9. doi: 10.1136/annrheumdis-2011-200726
26. Myasoedova E, Gabriel SE, Matteson EL, Davis JM, Therneau TM, Crowson CS. Decreased cardiovascular mortality in patients with incident rheumatoid arthritis (ra) in recent years: dawn of a new era in cardiovascular disease in RA? J Rheumatol. (2017) 44:732–9. doi: 10.3899/jrheum.161154
27. van den Hoek J, Boshuizen HC, Roorda LD, Tijhuis GJ, Nurmohamed MT, van den Bos GA, et al. Mortality in patients with rheumatoid arthritis: a 15-year prospective cohort study. Rheumatol Int. (2017) 37:487–93. doi: 10.1007/s00296-016-3638-5
28. Meek IL, Vonkeman HE, van de Laar MA. Cardiovascular case fatality in rheumatoid arthritis is decreasing; first prospective analysis of a current low disease activity rheumatoid arthritis cohort and review of the literature. BMC Musculoskelet Disord. (2014) 15:142. doi: 10.1186/1471-2474-15-142
29. Myasoedova E, Davis JM, Roger VL, Achenbach SJ, Crowson CS. Improved incidence of cardiovascular disease in patients with incident rheumatoid arthritis in the 2000s: a population-based cohort study. J Rheumatol. (2021) 48:1379–87. doi: 10.3899/jrheum.200842
30. Yazdani K, Xie H, Avina-Zubieta JA, Zheng Y, Abrahamowicz M, Lacaille D. Has the excess risk of acute myocardial infarction in rheumatoid arthritis relative to the general population declined? A population study of trends over time. Semin Arthritis Rheum. (2021) 51:442–9. doi: 10.1016/j.semarthrit.2021.03.003
31. Holmqvist M, Ljung L, Askling J. Mortality following new-onset Rheumatoid Arthritis: has modern Rheumatology had an impact? Ann Rheum Dis. (2018) 77:85–91. doi: 10.1136/annrheumdis-2017-212131
32. Trommer K, Kostev K, Jacob L, Tanislav C. Increased incidence of stroke and transient ischemic attack in patients with rheumatoid arthritis and ankylosing spondylitis in Germany. Neuroepidemiology. (2021) 55:162–70. doi: 10.1159/000514889
33. Lee DH, Sheen SH, Lee DG, Jang JW, Lee DC, Shin SH, et al. Association between ischemic stroke and seropositive rheumatoid arthritis in Korea: a nationwide longitudinal cohort study. PLoS ONE. (2021) 16:e0251851. doi: 10.1371/journal.pone.0251851
34. Nicola PJ, Crowson CS, Maradit-Kremers H, Ballman KV, Roger VL, Jacobsen SJ, et al. Contribution of congestive heart failure and ischemic heart disease to excess mortality in rheumatoid arthritis. Arthritis Rheum. (2006) 54:60–7. doi: 10.1002/art.21560
35. Khalid Y, Dasu N, Shah A, Brown K, Kaell A, Levine A, et al. Incidence of congestive heart failure in rheumatoid arthritis: a review of literature and meta-regression analysis. ESC Heart Fail. (2020). doi: 10.1002/ehf2.12947
36. Chuang YW Yu MC, Lin CL Yu TM, Shu KH, Huang ST, et al. Risk of peripheral arterial occlusive disease in patients with rheumatoid arthritis. A nationwide population-based cohort study. Thromb Haemost. (2016) 115:439–45. doi: 10.1160/TH15-07-0600
37. Stamatelopoulos KS, Kitas GD, Papamichael CM, Kyrkou K, Zampeli E, Fragiadaki K, et al. Subclinical peripheral arterial disease in rheumatoid arthritis. Atherosclerosis. (2010) 212:305–9. doi: 10.1016/j.atherosclerosis.2010.05.007
38. Molander V, Bower H, Frisell T, Askling J. Risk of venous thromboembolism in rheumatoid arthritis, and its association with disease activity: a nationwide cohort study from Sweden. Ann Rheum Dis. (2021) 80:169–75. doi: 10.1136/annrheumdis-2020-218419
39. Ungprasert P, Srivali N, Spanuchart I, Thongprayoon C, Knight EL. Risk of venous thromboembolism in patients with rheumatoid arthritis: a systematic review and meta-analysis. Clin Rheumatol. (2014) 33:297–304. doi: 10.1007/s10067-014-2492-7
40. Gonzalez-Gay MA, Gonzalez-Juanatey C, Ollier WE. Endothelial dysfunction in rheumatoid arthritis: influence of HLA-DRB1 alleles. Autoimmun Rev. (2004) 3:301–4. doi: 10.1016/j.autrev.2003.10.006
41. Paakkanen R, Lokki ML, Seppanen M, Tierala I, Nieminen MS, Sinisalo J. Proinflammatory HLA-DRB1*01-haplotype predisposes to ST-elevation myocardial infarction. Atherosclerosis. (2012) 221:461–6. doi: 10.1016/j.atherosclerosis.2012.01.024
42. Sugiyama D, Nishimura K, Tamaki K, Tsuji G, Nakazawa T, Morinobu A, et al. Impact of smoking as a risk factor for developing rheumatoid arthritis: a meta-analysis of observational studies. Ann Rheum Dis. (2010) 69:70–81. doi: 10.1136/ard.2008.096487
43. Baka Z, Buzas E, Nagy G. Rheumatoid arthritis and smoking: putting the pieces together. Arthritis Res Ther. (2009) 11:238. doi: 10.1186/ar2751
44. Breitling LP, Yang R, Korn B, Burwinkel B, Brenner H. Tobacco-smoking-related differential DNA methylation: 27K discovery and replication. Am J Hum Genet. (2011) 88:450–7. doi: 10.1016/j.ajhg.2011.03.003
45. Linn-Rasker SP., van der Helm-van Mil AH, van Gaalen FA, Kloppenburg M, de Vries RR, le Cessie S, et al. Smoking is a risk factor for anti-CCP antibodies only in rheumatoid arthritis patients who carry HLA-DRB1 shared epitope alleles. Ann Rheum Dis. (2006) 65:366–71. doi: 10.1136/ard.2005.041079
46. Escalante A, Haas RW, del Rincon I. Paradoxical effect of body mass index on survival in rheumatoid arthritis: role of comorbidity and systemic inflammation. Arch Intern Med. (2005) 165:1624–9. doi: 10.1001/archinte.165.14.1624
47. Roubenoff R, Roubenoff RA, Cannon JG, Kehayias JJ, Zhuang H, Dawson-Hughes B, et al. Rheumatoid cachexia: cytokine-driven hypermetabolism accompanying reduced body cell mass in chronic inflammation. J Clin Invest. (1994) 93:2379–86. doi: 10.1172/JCI117244
48. Myasoedova E, Crowson CS, Kremers HM, Roger VL, Fitz-Gibbon PD, Therneau TM, et al. Lipid paradox in rheumatoid arthritis: the impact of serum lipid measures and systemic inflammation on the risk of cardiovascular disease. Ann Rheum Dis. (2011) 70:482–7. doi: 10.1136/ard.2010.135871
49. Navarro-Millan I, Yang S, DuVall SL, Chen L, Baddley J, Cannon GW, et al. Association of hyperlipidaemia, inflammation and serological status and coronary heart disease among patients with rheumatoid arthritis: data from the National Veterans Health Administration. Ann Rheum Dis. (2016) 75:341–7. doi: 10.1136/annrheumdis-2013-204987
50. Liao H, Wu J, Kuhn E, Chin W, Chang B, Jones MD, et al. Use of mass spectrometry to identify protein biomarkers of disease severity in the synovial fluid and serum of patients with rheumatoid arthritis. Arthritis Rheum. (2004) 50:3792–803. doi: 10.1002/art.20720
51. Kim JY, Lee EY, Park JK, Song YW, Kim JR, Cho KH. Patients with rheumatoid arthritis show altered lipoprotein profiles with dysfunctional high-density lipoproteins that can exacerbate inflammatory and atherogenic process. PLoS ONE. (2016) 11:e0164564. doi: 10.1371/journal.pone.0164564
52. Charles-Schoeman C, Watanabe J, Lee YY, Furst DE, Amjadi S, Elashoff D, et al. Abnormal function of high-density lipoprotein is associated with poor disease control and an altered protein cargo in rheumatoid arthritis. Arthritis Rheum. (2009) 60:2870–9. doi: 10.1002/art.24802
53. Ronda N, Favari E, Borghi MO, Ingegnoli F, Gerosa M, Chighizola C, et al. Impaired serum cholesterol efflux capacity in rheumatoid arthritis and systemic lupus erythematosus. Ann Rheum Dis. (2014) 73:609–15. doi: 10.1136/annrheumdis-2012-202914
54. Watanabe J, Charles-Schoeman C, Miao Y, Elashoff D, Lee YY, Katselis G, et al. Proteomic profiling following immunoaffinity capture of high-density lipoprotein: association of acute-phase proteins and complement factors with proinflammatory high-density lipoprotein in rheumatoid arthritis. Arthritis Rheum. (2012) 64:1828–37. doi: 10.1002/art.34363
55. Charles-Schoeman C, Lee YY, Grijalva V, Amjadi S, FitzGerald J, Ranganath VK, et al. Cholesterol efflux by high density lipoproteins is impaired in patients with active rheumatoid arthritis. Ann Rheum Dis. (2012) 71:1157–62. doi: 10.1136/annrheumdis-2011-200493
56. Sokka T, Hakkinen A, Kautiainen H, Maillefert JF, Toloza S, Mork Hansen T, et al. Physical inactivity in patients with rheumatoid arthritis: data from twenty-one countries in a cross-sectional, international study. Arthritis Rheum. (2008) 59:42–50. doi: 10.1002/art.23255
57. Arts EE, Fransen J, den Broeder AA, Popa CD, van Riel PL. The effect of disease duration and disease activity on the risk of cardiovascular disease in rheumatoid arthritis patients. Ann Rheum Dis. (2015) 74:998–1003. doi: 10.1136/annrheumdis-2013-204531
58. Boo S, Oh H, Froelicher ES, Suh CH. Knowledge and perception of cardiovascular disease risk among patients with rheumatoid arthritis. PLoS ONE. (2017) 12:e0176291. doi: 10.1371/journal.pone.0176291
59. Hurkmans EJ, Maes S, de Gucht V, Knittle K, Peeters AJ, Ronday HK, et al. Motivation as a determinant of physical activity in patients with rheumatoid arthritis. Arthritis Care Res (Hoboken). (2010) 62:371–7. doi: 10.1002/acr.20106
60. Turesson C, McClelland RL, Christianson TJ, Matteson EL. Severe extra-articular disease manifestations are associated with an increased risk of first ever cardiovascular events in patients with rheumatoid arthritis. Ann Rheum Dis. (2007) 66:70–5. doi: 10.1136/ard.2006.052506
61. Solomon DH, Kremer J, Curtis JR, Hochberg MC, Reed G, Tsao P, et al. Explaining the cardiovascular risk associated with rheumatoid arthritis: traditional risk factors versus markers of rheumatoid arthritis severity. Ann Rheum Dis. (2010) 69:1920–5. doi: 10.1136/ard.2009.122226
62. Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. (2005) 352:1685–95. doi: 10.1056/NEJMra043430
63. Hotamisligil GS. Inflammation and metabolic disorders. Nature. (2006) 444:860–7. doi: 10.1038/nature05485
64. Rho YH, Chung CP, Oeser A, Solus J, Asanuma Y, Sokka T, et al. Inflammatory mediators and premature coronary atherosclerosis in rheumatoid arthritis. Arthritis Rheum. (2009) 61:1580–5. doi: 10.1002/art.25009
65. Giles JT, Post WS, Blumenthal RS, Polak J, Petri M, Gelber AC, et al. Longitudinal predictors of progression of carotid atherosclerosis in rheumatoid arthritis. Arthritis Rheum. (2011) 63:3216–25. doi: 10.1002/art.30542
66. Mantel A, Holmqvist M, Nyberg F, Tornling G, Frisell T, Alfredsson L, et al. Risk factors for the rapid increase in risk of acute coronary events in patients with new-onset rheumatoid arthritis: a nested case-control study. Arthritis Rheumatol. (2015) 67:2845–54. doi: 10.1002/art.39267
67. Solomon DH, Reed GW, Kremer JM, Curtis JR, Farkouh ME, Harrold LR, et al. Disease activity in rheumatoid arthritis and the risk of cardiovascular events. Arthritis Rheumatol. (2015) 67:1449–55. doi: 10.1002/art.39098
68. Gonzalez-Gay MA, Gonzalez-Juanatey C, Lopez-Diaz MJ, Pineiro A, Garcia-Porrua C, Miranda-Filloy JA, et al. HLA-DRB1 and persistent chronic inflammation contribute to cardiovascular events and cardiovascular mortality in patients with rheumatoid arthritis. Arthritis Rheum. (2007) 57:125–32. doi: 10.1002/art.22482
69. Zhang J, Chen L, Delzell E, Muntner P, Hillegass WB, Safford MM, et al. The association between inflammatory markers, serum lipids and the risk of cardiovascular events in patients with rheumatoid arthritis. Ann Rheum Dis. (2014) 73:1301–8. doi: 10.1136/annrheumdis-2013-204715
70. Fine NM, Crowson CS, Lin G, Oh JK, Villarraga HR, Gabriel SE. Evaluation of myocardial function in patients with rheumatoid arthritis using strain imaging by speckle-tracking echocardiography. Ann Rheum Dis. (2014) 73:1833–9. doi: 10.1136/annrheumdis-2013-203314
71. Liang KP, Myasoedova E, Crowson CS, Davis JM, Roger VL, Karon BL, et al. Increased prevalence of diastolic dysfunction in rheumatoid arthritis. Ann Rheum Dis. (2010) 69:1665–70. doi: 10.1136/ard.2009.124362
72. Logstrup BB, Deibjerg LK, Hedemann-Andersen A, Ellingsen T. Left ventricular function in treatment-naive early rheumatoid arthritis. Am J Cardiovasc Dis. (2014) 4:79–86. doi: 10.1136/annrheumdis-2014-eular.1865
73. Midtbo H, Gerdts E, Kvien TK, Olsen IC, Hirth A, Davidsen ES, et al. Disease activity and left ventricular structure in patients with rheumatoid arthritis. Rheumatology (Oxford). (2015) 54:511–9. doi: 10.1093/rheumatology/keu368
74. Midtbo H, Semb AG, Matre K, Kvien TK, Gerdts E. Disease activity is associated with reduced left ventricular systolic myocardial function in patients with rheumatoid arthritis. Ann Rheum Dis. (2017) 76:371–6. doi: 10.1136/annrheumdis-2016-209223
75. Cambridge G, Acharya J, Cooper JA, Edwards JC, Humphries SE. Antibodies to citrullinated peptides and risk of coronary heart disease. Atherosclerosis. (2013) 228:243–6. doi: 10.1016/j.atherosclerosis.2013.02.009
76. Lopez-Longo FJ, Oliver-Minarro D, de la Torre I, Gonzalez-Diaz de Rabago E, Sanchez-Ramon S, Rodriguez-Mahou M, et al. Association between anti-cyclic citrullinated peptide antibodies and ischemic heart disease in patients with rheumatoid arthritis. Arthritis Rheum. (2009) 61:419–24. doi: 10.1002/art.24390
77. Mackey RH, Kuller LH, Deane KD, Walitt BT, Chang YF, Holers VM, et al. Rheumatoid arthritis, anti-cyclic citrullinated peptide positivity, and cardiovascular disease risk in the women's health initiative. Arthritis Rheumatol. (2015) 67:2311–22. doi: 10.1002/art.39198
78. Giles JT, Malayeri AA, Fernandes V, Post W, Blumenthal RS, Bluemke D, et al. Left ventricular structure and function in patients with rheumatoid arthritis, as assessed by cardiac magnetic resonance imaging. Arthritis Rheum. (2010) 62:940–51. doi: 10.1002/art.27349
79. Marasovic-Krstulovic D, Martinovic-Kaliterna D, Fabijanic D, Morovic-Vergles J. Are the anti-cyclic citrullinated peptide antibodies independent predictors of myocardial involvement in patients with active rheumatoid arthritis? Rheumatology (Oxford). (2011) 50:1505–12. doi: 10.1093/rheumatology/ker121
80. Roubille C, Richer V, Starnino T, McCourt C, McFarlane A, Fleming P, et al. The effects of tumour necrosis factor inhibitors, methotrexate, non-steroidal anti-inflammatory drugs and corticosteroids on cardiovascular events in rheumatoid arthritis, psoriasis and psoriatic arthritis: a systematic review and meta-analysis. Ann Rheum Dis. (2015) 74:480–9. doi: 10.1136/annrheumdis-2014-206624
81. Avina-Zubieta JA, Abrahamowicz M, De Vera MA, Choi HK, Sayre EC, Rahman MM, et al. Immediate and past cumulative effects of oral glucocorticoids on the risk of acute myocardial infarction in rheumatoid arthritis: a population-based study. Rheumatology (Oxford). (2013) 52:68–75. doi: 10.1093/rheumatology/kes353
82. Battafarano DF, Restrepo JF, Erikson JM, Escalante A. Glucocorticoid dose thresholds associated with all-cause and cardiovascular mortality in rheumatoid arthritis. Arthritis Rheumatol. (2014) 66:264–72. doi: 10.1002/art.38210
83. Zhang J, Xie F, Yun H, Chen L, Muntner P, Levitan EB, et al. Comparative effects of biologics on cardiovascular risk among older patients with rheumatoid arthritis. Ann Rheum Dis. (2016) 75:1813–8. doi: 10.1136/annrheumdis-2015-207870
84. Ferraz-Amaro I, Gonzalez-Gay MA, Garcia-Dopico JA, Diaz-Gonzalez F. Cholesteryl ester transfer protein in patients with rheumatoid arthritis. J Rheumatol. (2013) 40:1040–7. doi: 10.3899/jrheum.121507
85. Nashel DJ. Corticosteroids in rheumatoid arthritis. Ann Rheum Dis. (1986) 45:790–1. doi: 10.1136/ard.45.9.790-b
86. Gonzalez-Gay MA, Gonzalez-Juanatey C, Martin J. Rheumatoid arthritis: a disease associated with accelerated atherogenesis. Semin Arthritis Rheum. (2005) 35:8–17. doi: 10.1016/j.semarthrit.2005.03.004
87. Sattar N, McCarey DW, Capell H, McInnes IB. Explaining how “high-grade” systemic inflammation accelerates vascular risk in rheumatoid arthritis. Circulation. (2003) 108:2957–63. doi: 10.1161/01.CIR.0000099844.31524.05
88. Skeoch S, Bruce IN. Atherosclerosis in rheumatoid arthritis: is it all about inflammation? Nat Rev Rheumatol. (2015) 11:390–400. doi: 10.1038/nrrheum.2015.40
89. Kozera L, Andrews J, Morgan AW. Cardiovascular risk and rheumatoid arthritis–the next step: differentiating true soluble biomarkers of cardiovascular risk from surrogate measures of inflammation. Rheumatology (Oxford). (2011) 50:1944–54. doi: 10.1093/rheumatology/ker232
90. Steyers CM, Miller FJ. Endothelial dysfunction in chronic inflammatory diseases. Int J Mol Sci. (2014) 15:11324–49. doi: 10.3390/ijms150711324
91. Hadi HA, Carr CS, Al Suwaidi J. Endothelial dysfunction: cardiovascular risk factors, therapy, and outcome. Vasc Health Risk Manag. (2005) 1:183–98.
92. Lerman A, Burnett JC, Jr. Intact and altered endothelium in regulation of vasomotion. Circulation. (1992) 86:III12–9.
93. Barbati C, Vomero M, Colasanti T, Diociaiuti M, Ceccarelli F, Ferrigno S, et al. TNFalpha expressed on the surface of microparticles modulates endothelial cell fate in rheumatoid arthritis. Arthritis Res Ther. (2018) 20:273. doi: 10.1186/s13075-018-1768-8
94. Di Minno MN, Ambrosino P, Lupoli R, Di Minno A, Tasso M, Peluso R, et al. Clinical assessment of endothelial function in patients with rheumatoid arthritis: A meta-analysis of literature studies. Eur J Intern Med. (2015) 26:835–42. doi: 10.1016/j.ejim.2015.10.016
95. Wang P, Huang L, Xu Q, Xu L, Deng FY, Lei SF. Assessment of aortic stiffness in patients with rheumatoid arthritis using pulse wave velocity: an update meta-analysis. Arch Med Res. (2019) 50:401–12. doi: 10.1016/j.arcmed.2019.10.010
96. Ku IA, Imboden JB, Hsue PY, Ganz P. Rheumatoid arthritis: model of systemic inflammation driving atherosclerosis. Circ J. (2009) 73:977–85. doi: 10.1253/circj.cj-09-0274
97. Hashizume M, Mihara M. Atherogenic effects of TNF-alpha and IL-6 via up-regulation of scavenger receptors. Cytokine. (2012) 58:424–30. doi: 10.1016/j.cyto.2012.02.010
98. Voloshyna I, Modayil S, Littlefield MJ, Belilos E, Belostocki K, Bonetti L, et al. Plasma from rheumatoid arthritis patients promotes pro-atherogenic cholesterol transport gene expression in THP-1 human macrophages. Exp Biol Med (Maywood). (2013) 238:1192–7. doi: 10.1177/1535370213503262
99. Wen W, He M, Liang X, Gao SS, Zhou J, Yuan ZY. Accelerated transformation of macrophage-derived foam cells in the presence of collagen-induced arthritis mice serum is associated with dyslipidemia. Autoimmunity. (2016) 49:115–23. doi: 10.3109/08916934.2015.1118761
100. Pasceri V, Yeh ET, A. tale of two diseases: atherosclerosis and rheumatoid arthritis. Circulation. (1999) 100:2124–6. doi: 10.1161/01.cir.100.21.2124
101. Ross R. Atherosclerosis is an inflammatory disease. Am Heart J. (1999) 138:S419–20. doi: 10.1016/s0002-8703(99)70266-8
102. Littler AJ, Buckley CD, Wordsworth P, Collins I, Martinson J, Simmons DL, et al. distinct profile of six soluble adhesion molecules (ICAM-1, ICAM-3, VCAM-1, E-selectin, L-selectin and P-selectin) in rheumatoid arthritis. Br J Rheumatol. (1997) 36:164–9. doi: 10.1093/rheumatology/36.2.164
103. Veale DJ, Maple C, Kirk G, McLaren M, Belch JJ. Soluble cell adhesion molecules–P-selectin and ICAM-1, and disease activity in patients receiving sulphasalazine for active rheumatoid arthritis. Scand J Rheumatol. (1998) 27:296–9. doi: 10.1080/030097498442415
104. Fasth AE, Snir O, Johansson AA, Nordmark B, Rahbar A, Af Klint E, et al. Skewed distribution of proinflammatory CD4+CD28null T cells in rheumatoid arthritis. Arthritis Res Ther. (2007) 9:R87. doi: 10.1186/ar2286
105. Radstake TR, Roelofs MF, Jenniskens YM, Oppers-Walgreen B, van Riel PL, Barrera P, et al. Expression of toll-like receptors 2 and 4 in rheumatoid synovial tissue and regulation by proinflammatory cytokines interleukin-12 and interleukin-18 via interferon-gamma. Arthritis Rheum. (2004) 50:3856–65. doi: 10.1002/art.20678
106. Buono C, Pang H, Uchida Y, Libby P, Sharpe AH, Lichtman AH. B7-1/B7-2 costimulation regulates plaque antigen-specific T-cell responses and atherogenesis in low-density lipoprotein receptor-deficient mice. Circulation. (2004) 109:2009–15. doi: 10.1161/01.CIR.0000127121.16815.F1
107. Hollan I, Scott H, Saatvedt K, Prayson R, Mikkelsen K, Nossent HC, et al. Inflammatory rheumatic disease and smoking are predictors of aortic inflammation: a controlled study of biopsy specimens obtained at coronary artery surgery. Arthritis Rheum. (2007) 56:2072–9. doi: 10.1002/art.22690
108. Karpouzas GA, Bui VL, Ronda N, Hollan I, Ormseth SR. Biologics and atherosclerotic cardiovascular risk in rheumatoid arthritis: a review of evidence and mechanistic insights. Expert Rev Clin Immunol. (2021) 17:355–74. doi: 10.1080/1744666X.2021.1899809
109. Manfredi AA, Baldini M, Camera M, Baldissera E, Brambilla M, Peretti G, et al. Anti-TNFalpha agents curb platelet activation in patients with rheumatoid arthritis. Ann Rheum Dis. (2016) 75:1511–20. doi: 10.1136/annrheumdis-2015-208442
110. Finn AV, Nakano M, Narula J, Kolodgie FD, Virmani R. Concept of vulnerable/unstable plaque. Arterioscler Thromb Vasc Biol. (2010) 30:1282–92. doi: 10.1161/ATVBAHA.108.179739
111. Libby P. Role of inflammation in atherosclerosis associated with rheumatoid arthritis. Am J Med. (2008) 121:S21–31. doi: 10.1016/j.amjmed.2008.06.014
112. Wållberg-Jonsson S, Dahlén GH, Nilsson TK, Rånby M, Rantapää-Dahlqvist S. Tissue plasminogen activator, plasminogen activator inhibitor-1 and von Willebrand factor in rheumatoid arthritis. Clin Rheumatol. (1993) 12:318–24. doi: 10.1007/bf02231572
113. Choi G, Schultz MJ, Levi M, van der Poll T. The relationship between inflammation and the coagulation system. Swiss Med Wkly. (2006) 136:139–44.
114. Levi M, van der Poll T, Büller HR. Bidirectional relation between inflammation and coagulation. Circulation. (2004) 109:2698–704. doi: 10.1161/01.Cir.0000131660.51520.9a
115. Cugno M. Cardiovascular events and survival in rheumatoid arthritis: effects of anti-tumor necrosis factor-alpha treatment. Transl Res. (2011) 157:6–9. doi: 10.1016/j.trsl.2010.10.003
116. Ingegnoli F, Fantini F, Favalli EG, Soldi A, Griffini S, Galbiati V, et al. Inflammatory and prothrombotic biomarkers in patients with rheumatoid arthritis: effects of tumor necrosis factor-alpha blockade. J Autoimmun. (2008) 31:175–9. doi: 10.1016/j.jaut.2008.07.002
117. Ingegnoli F, Fantini F, Griffini S, Soldi A, Meroni PL, Cugno M. Anti-tumor necrosis factor alpha therapy normalizes fibrinolysis impairment in patients with active rheumatoid arthritis. Clin Exp Rheumatol. (2010) 28:254–7.
118. Boilard E, Nigrovic PA, Larabee K, Watts GF, Coblyn JS, Weinblatt ME, et al. Platelets amplify inflammation in arthritis via collagen-dependent microparticle production. Science. (2010) 327:580–3. doi: 10.1126/science.1181928
119. Bonaventura A, Liberale L, Carbone F, Vecchie A, Diaz-Canestro C, Camici GG, et al. The pathophysiological role of neutrophil extracellular traps in inflammatory diseases. Thromb Haemost. (2018) 118:6–27. doi: 10.1160/TH17-09-0630
120. Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, et al. Neutrophil extracellular traps kill bacteria. Science. (2004) 303:1532–5. doi: 10.1126/science.1092385
121. Corsiero E, Pratesi F, Prediletto E, Bombardieri M, Migliorini P. NETosis as Source of autoantigens in rheumatoid arthritis. Front Immunol. (2016) 7:485. doi: 10.3389/fimmu.2016.00485
122. Khandpur R, Carmona-Rivera C, Vivekanandan-Giri A, Gizinski A, Yalavarthi S, Knight JS, et al. NETs are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Sci Transl Med. (2013). 5:178ra40. doi: 10.1126/scitranslmed.3005580
123. Sur Chowdhury C, Giaglis S, Walker UA, Buser A, Hahn S, Hasler P. Enhanced neutrophil extracellular trap generation in rheumatoid arthritis: analysis of underlying signal transduction pathways and potential diagnostic utility. Arthritis Res Ther. (2014) 16:R122. doi: 10.1186/ar4579
124. Stark K, Massberg S. Interplay between inflammation and thrombosis in cardiovascular pathology. Nat Rev Cardiol. (2021) 18:666–82. doi: 10.1038/s41569-021-00552-1
125. McDonald B, Davis RP, Kim S-J, Tse M, Esmon CT, Kolaczkowska E, et al. Platelets and neutrophil extracellular traps collaborate to promote intravascular coagulation during sepsis in mice. Blood. (2017) 129:1357–67. doi: 10.1182/blood-2016-09-741298
126. Palmai-Pallag T, Bachrati CZ. Inflammation-induced DNA damage and damage-induced inflammation: a vicious cycle. Microbes Infect. (2014) 16:822–32. doi: 10.1016/j.micinf.2014.10.001
127. Stakos DA, Kambas K, Konstantinidis T, Mitroulis I, Apostolidou E, Arelaki S, et al. Expression of functional tissue factor by neutrophil extracellular traps in culprit artery of acute myocardial infarction. Eur Heart J. (2015) 36:1405–14. doi: 10.1093/eurheartj/ehv007
128. Zhang Y, Guan L, Yu J, Zhao Z, Mao L, Li S, et al. Pulmonary endothelial activation caused by extracellular histones contributes to neutrophil activation in acute respiratory distress syndrome. Respir Res. (2016) 17:155. doi: 10.1186/s12931-016-0472-y
129. Mangold A, Alias S, Scherz T, Hofbauer M, Jakowitsch J, Panzenböck A, et al. Coronary neutrophil extracellular trap burden and deoxyribonuclease activity in ST-elevation acute coronary syndrome are predictors of ST-segment resolution and infarct size. Circ Res. (2015) 116:1182–92. doi: 10.1161/circresaha.116.304944
130. Smolen JS, van der Heijde D, Machold KP, Aletaha D, Landewé R. Proposal for a new nomenclature of disease-modifying antirheumatic drugs. Ann Rheum Dis. (2014) 73:3–5. doi: 10.1136/annrheumdis-2013-204317
131. Ajeganova S, Andersson ML, Frostegard J, Hafstrom I. Disease factors in early rheumatoid arthritis are associated with differential risks for cardiovascular events and mortality depending on age at onset: a 10-year observational cohort study. J Rheumatol. (2013) 40:1958–66. doi: 10.3899/jrheum.130365
132. Montesinos MC, Takedachi M, Thompson LF, Wilder TF, Fernandez P, Cronstein BN. The antiinflammatory mechanism of methotrexate depends on extracellular conversion of adenine nucleotides to adenosine by ecto-5'-nucleotidase: findings in a study of ecto-5'-nucleotidase gene-deficient mice. Arthritis Rheum. (2007) 56:1440–5. doi: 10.1002/art.22643
133. Thornton CC, Al-Rashed F, Calay D, Birdsey GM, Bauer A, Mylroie H, et al. Methotrexate-mediated activation of an AMPK-CREB-dependent pathway: a novel mechanism for vascular protection in chronic systemic inflammation. Ann Rheum Dis. (2016) 75:439–48. doi: 10.1136/annrheumdis-2014-206305
134. Zimmerman MC, Clemens DL, Duryee MJ, Sarmiento C, Chiou A, Hunter CD, et al. Direct antioxidant properties of methotrexate: Inhibition of malondialdehyde-acetaldehyde-protein adduct formation and superoxide scavenging. Redox Biol. (2017) 13:588–93. doi: 10.1016/j.redox.2017.07.018
135. Zhou J, Austin RC. Contributions of hyperhomocysteinemia to atherosclerosis: Causal relationship and potential mechanisms. Biofactors. (2009) 35:120–9. doi: 10.1002/biof.17
136. Ljung L, Rantapää-Dahlqvist S, Jacobsson LT, Askling J. Response to biological treatment and subsequent risk of coronary events in rheumatoid arthritis. Ann Rheum Dis. (2016) 75:2087–94. doi: 10.1136/annrheumdis-2015-208995
137. Daïen CI, Duny Y, Barnetche T, Daurès JP, Combe B, Morel J. Effect of TNF inhibitors on lipid profile in rheumatoid arthritis: a systematic review with meta-analysis. Ann Rheum Dis. (2012) 71:862–8. doi: 10.1136/annrheumdis-2011-201148
138. Daien CI, Fesler P, du Cailar G, Daien V, Mura T, Dupuy AM, et al. Etanercept normalises left ventricular mass in patients with rheumatoid arthritis. Ann Rheum Dis. (2013) 72:881–7. doi: 10.1136/annrheumdis-2012-201489
139. Angel K, Provan SA, Gulseth HL, Mowinckel P, Kvien TK, Atar D. Tumor necrosis factor-alpha antagonists improve aortic stiffness in patients with inflammatory arthropathies: a controlled study. Hypertension. (2010) 55:333–8. doi: 10.1161/HYPERTENSIONAHA.109.143982
140. Rios-Navarro C, de Pablo C, Collado-Diaz V, Orden S, Blas-Garcia A, Martinez-Cuesta MA, et al. Differential effects of anti-TNF-alpha and anti-IL-12/23 agents on human leukocyte-endothelial cell interactions. Eur J Pharmacol. (2015) 765:355–65. doi: 10.1016/j.ejphar.2015.08.054
141. Ruiz-Limón P, Ortega R, Arias de la Rosa I, Abalos-Aguilera MDC, Perez-Sanchez C, Jimenez-Gomez Y, et al. Tocilizumab improves the proatherothrombotic profile of rheumatoid arthritis patients modulating endothelial dysfunction, NETosis, and inflammation. Transl Res. (2017) 183:87–103. doi: 10.1016/j.trsl.2016.12.003
142. Giles JT, Sattar N, Gabriel S, Ridker PM, Gay S, Warne C, et al. Cardiovascular safety of tocilizumab versus etanercept in rheumatoid arthritis: a randomized controlled trial. Arthritis Rheumatol. (2020) 72:31–40. doi: 10.1002/art.41095
143. Müller N, Schulte DM, Türk K, Freitag-Wolf S, Hampe J, Zeuner R, et al. IL-6 blockade by monoclonal antibodies inhibits apolipoprotein (a) expression and lipoprotein (a) synthesis in humans. J Lipid Res. (2015) 56:1034–42. doi: 10.1194/jlr.P052209
144. Fleischmann R, Genovese MC, Lin Y, St John G, van der Heijde D, Wang S, et al. Long-term safety of sarilumab in rheumatoid arthritis: an integrated analysis with up to 7 years' follow-up. Rheumatology (Oxford). (2020) 59:292–302. doi: 10.1093/rheumatology/kez265
145. Ikonomidis I, Pavlidis G, Katsimbri P, Andreadou I, Triantafyllidi H, Tsoumani M, et al. Differential effects of inhibition of interleukin 1 and 6 on myocardial, coronary and vascular function. Clin Res Cardiol. (2019) 108:1093–101. doi: 10.1007/s00392-019-01443-9
146. Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. (2017) 377:1119–31. doi: 10.1056/NEJMoa1707914
147. Hsue PY, Scherzer R, Grunfeld C, Imboden J, Wu Y, Del Puerto G, et al. Depletion of B-cells with rituximab improves endothelial function and reduces inflammation among individuals with rheumatoid arthritis. J Am Heart Assoc. (2014) 3:e001267. doi: 10.1161/jaha.114.001267
148. Kerekes G, Soltész P, Dér H, Veres K, Szabó Z, Végvári A, et al. Effects of rituximab treatment on endothelial dysfunction, carotid atherosclerosis, and lipid profile in rheumatoid arthritis. Clin Rheumatol. (2009) 28:705–10. doi: 10.1007/s10067-009-1095-1
149. Smolen JS, Wollenhaupt J, Gomez-Reino JJ, Grassi W, Gaillez C, Poncet C, et al. Attainment and characteristics of clinical remission according to the new ACR-EULAR criteria in abatacept-treated patients with early rheumatoid arthritis: new analyses from the Abatacept study to Gauge Remission and joint damage progression in methotrexate (MTX)-naive patients with Early Erosive rheumatoid arthritis (AGREE). Arthritis Res Ther. (2015) 17:157. doi: 10.1186/s13075-015-0671-9
150. Mathieu S, Couderc M, Glace B, Pereira B, Tournadre A, Dubost JJ, et al. Effects of 6 months of abatacept treatment on aortic stiffness in patients with rheumatoid arthritis. Biologics. (2013) 7:259–64. doi: 10.2147/btt.S52003
151. Provan SA, Berg IJ, Hammer HB, Mathiessen A, Kvien TK, Semb AG. The impact of newer biological disease modifying anti-rheumatic drugs on cardiovascular risk factors: a 12-month longitudinal study in rheumatoid arthritis patients treated with rituximab, abatacept and tociliziumab. PLoS ONE. (2015) 10:e0130709. doi: 10.1371/journal.pone.0130709
152. Ursini F, Russo E, Letizia Hribal M, Mauro D, Savarino F, Bruno C, et al. Abatacept improves whole-body insulin sensitivity in rheumatoid arthritis: an observational study. Medicine (Baltimore). (2015) 94:e888. doi: 10.1097/md.0000000000000888
153. Charles-Schoeman C, Fleischmann R, Davignon J, Schwartz H, Turner SM, Beysen C, et al. Potential mechanisms leading to the abnormal lipid profile in patients with rheumatoid arthritis versus healthy volunteers and reversal by tofacitinib. Arthritis Rheumatol. (2015) 67:616–25. doi: 10.1002/art.38974
154. Charles-Schoeman C, Gonzalez-Gay MA, Kaplan I, Boy M, Geier J, Luo Z, et al. Effects of tofacitinib and other DMARDs on lipid profiles in rheumatoid arthritis: implications for the rheumatologist. Semin Arthritis Rheum. (2016) 46:71–80. doi: 10.1016/j.semarthrit.2016.03.004
155. Ytterberg SR, Bhatt DL, Mikuls TR, Koch GG, Fleischmann R, Rivas JL, et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med. (2022) 386:316–26. doi: 10.1056/NEJMoa2109927
156. Khosrow-Khavar F, Kim SC, Lee H, Lee SB, Desai RJ. Tofacitinib and risk of cardiovascular outcomes: results from the Safety of TofAcitinib in Routine care patients with Rheumatoid Arthritis (STAR-RA) study. Ann Rheum Dis. (2022) 81:798–804.
157. Ajeganova S, de Faire U, Jogestrand T, Frostegard J, Hafstrom I. Carotid atherosclerosis, disease measures, oxidized low-density lipoproteins, and atheroprotective natural antibodies for cardiovascular disease in early rheumatoid arthritis – an inception cohort study. J Rheumatol. (2012) 39:1146–54. doi: 10.3899/jrheum.111334
158. Davis LA, Cannon GW, Pointer LF, Haverhals LM, Wolff RK, Mikuls TR, et al. Cardiovascular events are not associated with MTHFR polymorphisms, but are associated with methotrexate use and traditional risk factors in US veterans with rheumatoid arthritis. J Rheumatol. (2013) 40:809–17. doi: 10.3899/jrheum.121012
159. Sun KJ, Liu LL, Hu JH, Chen YY, Xu DY. Methotrexate can prevent cardiovascular events in patients with rheumatoid arthritis: an updated meta-analysis. Medicine (Baltimore). (2021) 100:e24579. doi: 10.1097/MD.0000000000024579
160. Chung CP, Giles JT, Kronmal RA, Post WS, Gelber AC, Petri M, et al. Progression of coronary artery atherosclerosis in rheumatoid arthritis: comparison with participants from the Multi-Ethnic Study of Atherosclerosis. Arthritis Res Ther. (2013) 15:R134. doi: 10.1186/ar4314
161. Guin A, Chatterjee Adhikari M, Chakraborty S, Sinhamahapatra P, Ghosh A. Effects of disease modifying anti-rheumatic drugs on subclinical atherosclerosis and endothelial dysfunction which has been detected in early rheumatoid arthritis: 1-year follow-up study. Semin Arthritis Rheum. (2013) 43:48–54. doi: 10.1016/j.semarthrit.2012.12.027
162. Kim YH, Kang JS. Effect of methotrexate on collagen-induced arthritis assessed by micro-computed tomography and histopathological examination in female rats. Biomol Ther (Seoul). (2015) 23:195–200. doi: 10.4062/biomolther.2014.125
163. Kumeda Y, Inaba M, Goto H, Nagata M, Henmi Y, Furumitsu Y, et al. Increased thickness of the arterial intima-media detected by ultrasonography in patients with rheumatoid arthritis. Arthritis Rheum. (2002) 46:1489–97. doi: 10.1002/art.10269
164. Vandhuick T, Allanore Y, Borderie D, Louvel JP, Fardellone P, Dieude P, et al. Early phase clinical and biological markers associated with subclinical atherosclerosis measured at 7 years of evolution in an early inflammatory arthritis cohort. Clin Exp Rheumatol. (2016) 34:58–67.
165. Wallberg-Jonsson S, Ohman M, Rantapaa-Dahlqvist S. Which factors are related to the presence of atherosclerosis in rheumatoid arthritis? Scand J Rheumatol. (2004) 33:373–9. doi: 10.1080/03009740410010308
166. van Ede AE, Laan RF, Blom HJ, Boers GH, Haagsma CJ, Thomas CM, et al. Homocysteine and folate status in methotrexate-treated patients with rheumatoid arthritis. Rheumatology (Oxford). (2002) 41:658–65. doi: 10.1093/rheumatology/41.6.658
167. Dixon W, Watson K, Lunt M, Hyrich K, Silman A, Symmons D. Reduction in the incidence of myocardial infarction in patients with rheumatoid arthritis who respond to anti–tumor necrosis factor α therapy: results from the British Society for Rheumatology Biologics Register. Arthritis & Rheumatism. (2007) 56:2905–12. doi: 10.1002/art.22809
168. Del Porto F, Laganà B, Lai S, Nofroni I, Tinti F, Vitale M, et al. Response to anti-tumour necrosis factor alpha blockade is associated with reduction of carotid intima-media thickness in patients with active rheumatoid arthritis. Rheumatology (Oxford). (2007) 46:1111–5. doi: 10.1093/rheumatology/kem089
169. Davida L, Pongracz V, Mohamed EA, Szamosi S, Szucs G, Vancsa A, et al. A prospective, longitudinal monocentric study on laser Doppler imaging of microcirculation: comparison with macrovascular pathophysiology and effect of adalimumab treatment in early rheumatoid arthritis. Rheumatol Int. (2020) 40:415–24. doi: 10.1007/s00296-019-04503-5
170. Yoshizaki K, Nishimoto N, Mihara M, Kishimoto T. Therapy of rheumatoid arthritis by blocking IL-6 signal transduction with a humanized anti-IL-6 receptor antibody. Springer Semin Immunopathol. (1998) 20:247–59. doi: 10.1007/bf00832010
171. Gualtierotti R, Ingegnoli F, Boscolo M, Griffini S, Grovetti E, Cugno M. Tocilizumab effects on coagulation factor XIII in patients with rheumatoid arthritis. Adv Ther. (2019) 36:3494–502. doi: 10.1007/s12325-019-01118-x
172. Gualtierotti R, Ingegnoli F, Griffini S, Grovetti E, Meroni PL, Cugno M. Prothrombotic biomarkers in patients with rheumatoid arthritis: the beneficial effect of IL-6 receptor blockade. Clin Exp Rheumatol. (2016) 34:451–8.
173. Curtis JR, Perez-Gutthann S, Suissa S, Napalkov P, Singh N, Thompson L, et al. Tocilizumab in rheumatoid arthritis: a case study of safety evaluations of a large postmarketing data set from multiple data sources. Semin Arthritis Rheum. (2015) 44:381–8. doi: 10.1016/j.semarthrit.2014.07.006
174. Schultz O, Oberhauser F, Saech J, Rubbert-Roth A, Hahn M, Krone W, et al. Effects of inhibition of interleukin-6 signalling on insulin sensitivity and lipoprotein (a) levels in human subjects with rheumatoid diseases. PLoS ONE. (2010) 5:e14328. doi: 10.1371/journal.pone.0014328
175. Greco D, Gualtierotti R, Agosti P, Adorni MP, Ingegnoli F, Rota M, et al. Anti-atherogenic modification of serum lipoprotein function in patients with rheumatoid arthritis after tocilizumab treatment, a pilot study. J Clin Med. (2020) 9. doi: 10.3390/jcm9072157
176. Huizinga TW, Fleischmann RM, Jasson M, Radin AR, van Adelsberg J, Fiore S, et al. Sarilumab, a fully human monoclonal antibody against IL-6Rα in patients with rheumatoid arthritis and an inadequate response to methotrexate: efficacy and safety results from the randomised SARIL-RA-MOBILITY Part A trial. Ann Rheum Dis. (2014) 73:1626–34. doi: 10.1136/annrheumdis-2013-204405
177. Ruscitti P, Masedu F, Alvaro S, Airò P, Battafarano N, Cantarini L, et al. Anti-interleukin-1 treatment in patients with rheumatoid arthritis and type 2 diabetes (TRACK): A multicentre, open-label, randomised controlled trial. PLoS Med. (2019) 16:e1002901. doi: 10.1371/journal.pmed.1002901
178. Raterman HG, Levels H, Voskuyl AE, Lems WF, Dijkmans BA, Nurmohamed MT, et al. protein composition alters from proatherogenic into less atherogenic and proinflammatory in rheumatoid arthritis patients responding to rituximab. Ann Rheum Dis. (2013) 72:560–5. doi: 10.1136/annrheumdis-2011-201228
179. Harrold LR, Reed GW, Magner R, Shewade A, John A, Greenberg JD, et al. Comparative effectiveness and safety of rituximab versus subsequent anti-tumor necrosis factor therapy in patients with rheumatoid arthritis with prior exposure to anti-tumor necrosis factor therapies in the United States Corrona registry. Arthritis Res Ther. (2015) 17:256. doi: 10.1186/s13075-015-0776-1
180. Kang EH, Jin Y, Brill G, Lewey J, Patorno E, Desai RJ, et al. Comparative cardiovascular risk of abatacept and tumor necrosis factor inhibitors in patients with rheumatoid arthritis with and without diabetes mellitus: a multidatabase cohort study. J Am Heart Assoc. (2018) 7. doi: 10.1161/jaha.117.007393
181. Ewing MM, Karper JC, Abdul S, de Jong RC, Peters HA, de Vries MR, et al. T-cell co-stimulation by CD28-CD80/86 and its negative regulator CTLA-4 strongly influence accelerated atherosclerosis development. Int J Cardiol. (2013) 168:1965–74. doi: 10.1016/j.ijcard.2012.12.085
182. Ma K, Lv S, Liu B, Liu Z, Luo Y, Kong W, et al. CTLA4-IgG ameliorates homocysteine-accelerated atherosclerosis by inhibiting T-cell overactivation in apoE–/– mice. Cardiovasc Res. (2013) 97:349–59. doi: 10.1093/cvr/cvs330
183. Wang F, Sun L, Wang S, Davis JM, Matteson EL, Murad MH, et al. Efficacy and safety of tofacitinib, baricitinib, and upadacitinib for rheumatoid arthritis: a systematic review and meta-analysis. Mayo Clin Proc. (2020) 95:1404–19. doi: 10.1016/j.mayocp.2020.01.039
184. Genovese MC, Kalunian K, Gottenberg JE, Mozaffarian N, Bartok B, Matzkies F, et al. Effect of filgotinib vs placebo on clinical response in patients with moderate to severe rheumatoid arthritis refractory to disease-modifying antirheumatic drug therapy: the FINCH 2 randomized clinical trial. Jama. (2019) 322:315–25. doi: 10.1001/jama.2019.9055
185. Kremer JM, Bingham CO. 3rd, Cappelli LC, Greenberg JD, Madsen AM, Geier J, et al. Postapproval comparative safety study of tofacitinib and biological disease-modifying antirheumatic drugs: 5-year results from a United States-based rheumatoid arthritis registry. ACR Open Rheumatol. (2021) 3:173–84. doi: 10.1002/acr2.11232
186. Parra-Izquierdo I, Melrose AR, Pang J, Lakshmanan HHS, Reitsma SE, Vavilapalli SH, et al. Janus kinase inhibitors ruxolitinib and baricitinib impair glycoprotein-VI mediated platelet function. Platelets. (2022) 33:404–15. doi: 10.1080/09537104.2021.1934665
187. Atzeni F, Rodriguez-Carrio J, Popa CD, Nurmohamed MT, Szucs G, Szekanecz Z. Cardiovascular effects of approved drugs for rheumatoid arthritis. Nat Rev Rheumatol. (2021) 17:270–90. doi: 10.1038/s41584-021-00593-3
188. Conroy RM, Pyörälä K, Fitzgerald AP, Sans S, Menotti A, De Backer G, et al. Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J. (2003) 24:987–1003. doi: 10.1016/s0195-668x(03)00114-3
189. Hippisley-Cox J, Coupland C, Vinogradova Y, Robson J, Minhas R, Sheikh A, et al. Predicting cardiovascular risk in England and Wales: prospective derivation and validation of QRISK2. BMJ. (2008) 336:1475–82. doi: 10.1136/bmj.39609.449676.25
190. Agca R, Heslinga SC, Rollefstad S, Heslinga M, McInnes IB, Peters MJ, et al. EULAR recommendations for cardiovascular disease risk management in patients with rheumatoid arthritis and other forms of inflammatory joint disorders: 2015/2016 update. Ann Rheum Dis. (2017) 76:17–28. doi: 10.1136/annrheumdis-2016-209775
191. Visseren FLJ, Mach F, Smulders YM, Carballo D, Koskinas KC, Bäck M, et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice: Developed by the Task Force for cardiovascular disease prevention in clinical practice with representatives of the European Society of Cardiology and 12 medical societies With the special contribution of the European Association of Preventive Cardiology (EAPC). Eur Heart J. (2021) 42:3227–337. doi: 10.1093/eurheartj/ehab484
192. Solomon A, Stanwix AE, Castañeda S, Llorca J, Gonzalez-Juanatey C, Hodkinson B, et al. Points to consider in cardiovascular disease risk management among patients with rheumatoid arthritis living in South Africa, an unequal middle income country. BMC Rheumatol. (2020) 4:42. doi: 10.1186/s41927-020-00139-2
193. Hsu HC, Robinson C, Woodiwiss AJ, Norton GR, Dessein PH. Cardiovascular risk factor profiles and disease in black compared to other africans with chronic kidney disease. Int J Nephrol. (2021) 2021:8876363. doi: 10.1155/2021/8876363
Keywords: rheumatoid arthritis, cardiovascular disease, atherosclerosis, DMARDs, TNF inhibitors, JAK inhibitors, IL-6 inhibitors, methotrexate
Citation: Giachi A, Cugno M and Gualtierotti R (2022) Disease-modifying anti-rheumatic drugs improve the cardiovascular profile in patients with rheumatoid arthritis. Front. Cardiovasc. Med. 9:1012661. doi: 10.3389/fcvm.2022.1012661
Received: 05 August 2022; Accepted: 28 September 2022;
Published: 24 October 2022.
Edited by:
Nicola Mumoli, ASST Ovest Milanese, ItalyReviewed by:
Federica Fogacci, University of Bologna, ItalyZoltán Nagy, InnKlinikum Altötting und Mühldorf, Germany
Copyright © 2022 Giachi, Cugno and Gualtierotti. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Massimo Cugno, bWFzc2ltby5jdWdub0B1bmltaS5pdA==
 Andrea Giachi
Andrea Giachi Massimo Cugno
Massimo Cugno Roberta Gualtierotti
Roberta Gualtierotti