- 1Department of Cardiovascular and Pulmonary Sciences, Catholic University of the Sacred Heart, Rome, Italy
- 2Department of Pathophysiology, University of Split School of Medicine, Split, Croatia
- 3Maria Cecilia Hospital, GVM Care & Research, Cotignola, Italy
- 4Department of Cardiovascular and Thoracic Sciences, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Rome, Italy
The incidence and clinical presentation of ischemic heart disease (IHD), as well as thrombotic and bleeding risks, appear to differ between genders. Compared with men, women feature an increased thrombotic risk, probably related to an increased platelet reactivity, higher level of coagulation factors, and sex-associated unique cardiovascular risk factors, such as pregnancy-related (i.e., pre-eclampsia and gestational diabetes), gynecological disorders (i.e., polycystic ovary syndrome, early menopause) and autoimmune or systemic inflammatory diseases. At the same time, women are also at increased risk of bleeding, due to inappropriate dosing of antithrombotic agents, smaller blood vessels, lower body weight and comorbidities, such as diabetes and chronic kidney disease. Pharmacological strategies focused on the personalization of antithrombotic treatment may, therefore, be particularly appealing in women in light of their higher bleeding and ischemic risks. Paradoxically, although women represent a large proportion of cardiovascular patients in our practice, adequate high-quality clinical trial data on women remain scarce and inadequate to guide decision-making processes. As a result, IHD in women tends to be understudied, underdiagnosed and undertreated, a phenomenon known as a “Yentl syndrome.” It is, therefore, compelling for the scientific community to embark on dedicated clinical trials to address underrepresentation of women and to acquire evidence-based knowledge in the personalization of antithrombotic therapy in women.
Introduction
In a 1983 movie, a young woman, named Yentl, attempted to live as a man to pursue the education she desired, blurring lines between traditional gender roles and deeply rooted social boundaries. In 1991, on the basis of this plot, Dr. Bernadine Healy coined the expression “Yentl syndrome” to epitomize the phenomenon in which women affected by ischemic heart disease (IHD) are less likely than men to receive recommended diagnostic tests, pharmacotherapy and invasive procedures, thereby showing a higher incidence of adverse outcomes (1). In the same year, as director of the National Institutes of Health, she launched the Women’s Health Initiative (WHI), consisting of an observational study and three clinical trials to address risk factors for cardiovascular disease, cancer and osteoporosis in postmenopausal women. The program is still ongoing and is expected to end in 2026, with over 160,000 women enrolled at present. Nevertheless, cardiovascular disease continues to be the leading cause of death among women, despite a considerable decline in cardiovascular deaths over several decades. In the last 30 years, cardiovascular research progressed significantly in order to achieve a personalized approach to care, including risk prediction models, preventive measures, and targeted therapeutic pathways. Antithrombotic therapy in patients undergoing percutaneous coronary intervention (PCI) has been deeply involved in this process. The propensity to ischemic recurrences after PCI and the understanding of prognostic implications associated with bleeding have prompted a substantial evolution in antithrombotic treatment regimens on the basis of a more accurate stratification of patients according to their ischemic and bleeding risks (2).
In this narrative review, the authors aim to explore the advancements and the limits of antithrombotic treatment in women, in the light of differences in epidemiology, clinical presentation, pathophysiology, bleeding, and ischemic risks among genders.
Epidemiology of ischemic heart disease in women
Ischemic heart disease represents the principal cause of death in women globally, accounting for 35% of total deaths (3, 4). Women suffer from IHD approximately 5–10 years after men and have a 20% higher adjusted mortality risk in short term after successful PCI compared with men (5–7). Women with acute coronary syndromes (ACS) are more likely to present with non-ST-elevation acute myocardial infarction (NSTEMI), higher comorbidity burden at baseline and have less severe coronary atherosclerosis (8–12). Furthermore, women with ACS seek medical attention significantly later than men, thus also having prolonged door-to-balloon times (13, 14). The INTERHEART study revealed the importance of psychosocial risk factors, including depression, perceived stress at home or work, lower socioeconomic status, post-traumatic stress disorder and anxiety disorders, in the onset and clinical course of IHD (5, 15). They play a more significant role in women, due to a higher prevalence in this subset of patients. Notably, the impact of these risk factors on IHD are both direct, related to their pathophysiological consequences of the neuroendocrine and cardiovascular systems, and indirect, representing relevant predictors of non-adherence to medical treatment and unhealthy behaviors such as smoking and sedentary lifestyle (16–19). Furthermore, selective serotonin reuptake inhibitors (SSRIs), used as first-line drugs for many of the above conditions, have been demonstrated to impair hemostatic function through various mechanisms (i.e., blockade of intra-platelet calcium mobilization, depletion of intracellular serotonin and reduced secretion of platelet factors in response to chemical stimuli) and to increase the risk of bleeding (20). Conversely, certain SSRIs (i.e., fluoxetine and fluvoxamine) are potent inhibitors of CYP2C19, responsible for converting clopidogrel in its active form. In a large population-based cohort study of CYP2C19-inhibiting SSRI users (n = 9284) vs. non-CYP2C19-inhibiting SSRI users (n = 45,073), an increased risk of ischemic events was found in patients taking CYP2C19-inhibiting SSRIs (21).
Although classic type 1 acute myocardial infarction (AMI) occurs three times more commonly in men than in women, myocardial infarction in the absence of obstructive coronary arteries (MINOCA) is more common in women, being present in 10.5% of ACS presentations vs. 3.4% in men (22, 23). In women with MINOCA, mortality risk is significantly associated with the number of accompanying risk factors, ranging between 10% with ≤ 1 cardiovascular risk factor and 25% with > 3 risk factors (24).
Spontaneous coronary artery dissection (SCAD) is a rare cause of ACS, but 90% of the cases are reported in women and it accounts for 10–20% of AMI in women younger than 50 years of age (25, 26). Among the causes of MINOCA, vasospastic angina and microvascular angina play an important role. Whilst rest angina due to epicardial coronary arteries vasospasm is more common in men, the prevalence of coronary microvascular dysfunction among patients with chest pain and non-obstructive coronary artery disease is higher in women compared to men (27).
The prevalence of stress-induced cardiomyopathy, also known as Takotsubo syndrome (TS) has been reported to be approximately 2% of all patients presenting with clinical manifestation of ACS (28). Importantly, out of all TS cases, 90% of patients are post-menopausal women and it is estimated that this entity is present in 5–6% of all female patients presenting with suspected ST-elevation myocardial infarction (STEMI) (28).
In conclusion, although IHD has long been considered a disease affecting predominantly male patients, it constitutes also a considerable part among diseases affecting women. However, there are important differences in terms of clinical subtypes among men and women with ACS. Considering the high prevalence of MINOCA in women with ACS, a strategy of multimodality imaging assessment should be always pursued, using both invasive (i.e., provocative spasm test and intracoronary imaging -IVUS and OCT-) and non-invasive tests (i.e., echocardiogram and cardiac magnetic resonance) in order to identify the specific etiology and provide the right treatment option.
Clinical presentation of ischemic heart disease in women
The presence of chest pain/discomfort is the hallmark symptom of IHD. A comprehensive analysis from the National Registry of Myocardial Infarction (NRMI), reporting hospital data on 1,143,513 registry patients admitted with confirmed AMI (481,581 women and 661,932 men), have demonstrated that women were more likely than men to present without chest pain (42.0% vs. 30.7% in men, respectively), with a larger sex difference in younger patients (29). Women, especially under the age of 65, show more frequently a wide spectrum of atypical symptoms, including weakness, fatigue, nausea, dyspnea, as well as unconventional event triggers (i.e., mental or emotional stress instead of physical exertion) and locations of chest-related symptoms, such as in the neck, jaw, and in the back (30). The reasons for sex-based differences in IHD symptom presentation are largely unknown. A possible explanation could be that younger women who experience AMI may have significantly less narrowing of the coronary arteries than older women or men due to a hypercoagulable state, inflammation, coronary spasm or plaque erosion instead of rupture (31). Furthermore, women exhibit differences in the neural receptors and pathways involved in nociception (32).
Such characteristics demonstrate that women who suffer from IHD may represent a heterogeneous patient group compared to men, requiring both an adaptation of diagnostic criteria and tailored medical anti-ischemic therapy due to a different underlying pathophysiology of coronary disease.
Sex differences in platelet function
Platelets are blood cells with several important biological functions as they regulate the integrity of the vascular wall, play a key role in primary hemostasis, and modulate thrombotic and inflammatory responses at the blood-vascular interface (28). Sexual dimorphism and age differences in human platelet aggregation dynamics have been known for several decades (33). Such findings may be of clinical relevance since antiplatelet therapy is the fundamental constituent in the treatment of IHD and might require sex-specific tailoring (33).
In a work by Becker et al., women presented a higher platelet reactivity to arachidonic acid and adenosine diphosphate (ADP) at baseline and after treatment with low-dose aspirin, although they experienced the same or greater decreases in platelet reactivity after treatment (34). Similarly, Gremmel et al. found that women were associated with a more pronounced formation of leukocyte-platelet aggregates and increased protease-activated receptor mediated platelet reactivity after PCI (35). The higher platelet reactivity in women was proposed also in patients undergoing double anti-platelet therapy (DAPT) with aspirin and clopidogrel, using thrombin receptor-activating peptide as a stimulator (36). Furthermore, in a cohort of 760 patients undergoing cardiac surgery, clopidogrel-treated women had higher platelet reactivity (HRP) to ADP (37). Similarly, recent data from Myocardial Ischemia Detection By Circulating Biomarkers (MYOMARKER) study showed attenuated flow-citometry-based platelet reactivity to P2Y12 inhibitor (mainly clopidogrel) among female outpatients with suspected myocardial ischemia when compared to men (38). Moreover, a recent analysis of 177 participants on clopidogrel after ACS, showed that the risk of an atherothrombotic event was greater in female carriers loss-of-function allele, compared to men carriers of the same allele, suggesting a possible interaction between sex and genes for clopidogrel (39). The potential increased platelet reactivity in women may be due to multiple causes, such as a higher platelet count and higher number of surface receptors in females in general which points to a greater agonist-induced platelet activation and aggregation (30). However, other studies opposed the previous ones, suggesting an equal platelet response to aspirin and P2Y12 inhibitors (40, 41–43).
However, the clinical implications of these findings remain unclear. Although the occurrence of major adverse cardiovascular and cerebrovascular events (MACCE) was significantly correlated to HPR, a recent meta-analysis, evaluating cardiovascular efficacy of clopidogrel, opposes the above-mentioned results and suggests no significant difference in treatment efficacy between men and women (44, 45). Similarly, two meta-analyses found no evident differences in clinical outcomes between sexes in patient treated with cardioaspirin (46, 47).
Taken together, women seem to have higher platelet reactivity than men at baseline, whereas conflicting data have been reported regarding platelet response to aspirin and P2Y12 inhibitors (Figure 1). Future studies are needed to determine if the possible sex difference in platelet reactivity could be addressed by the use of newer or different dosages of antiplatelet agents and if this will portend any impact on relevant clinical endpoints.
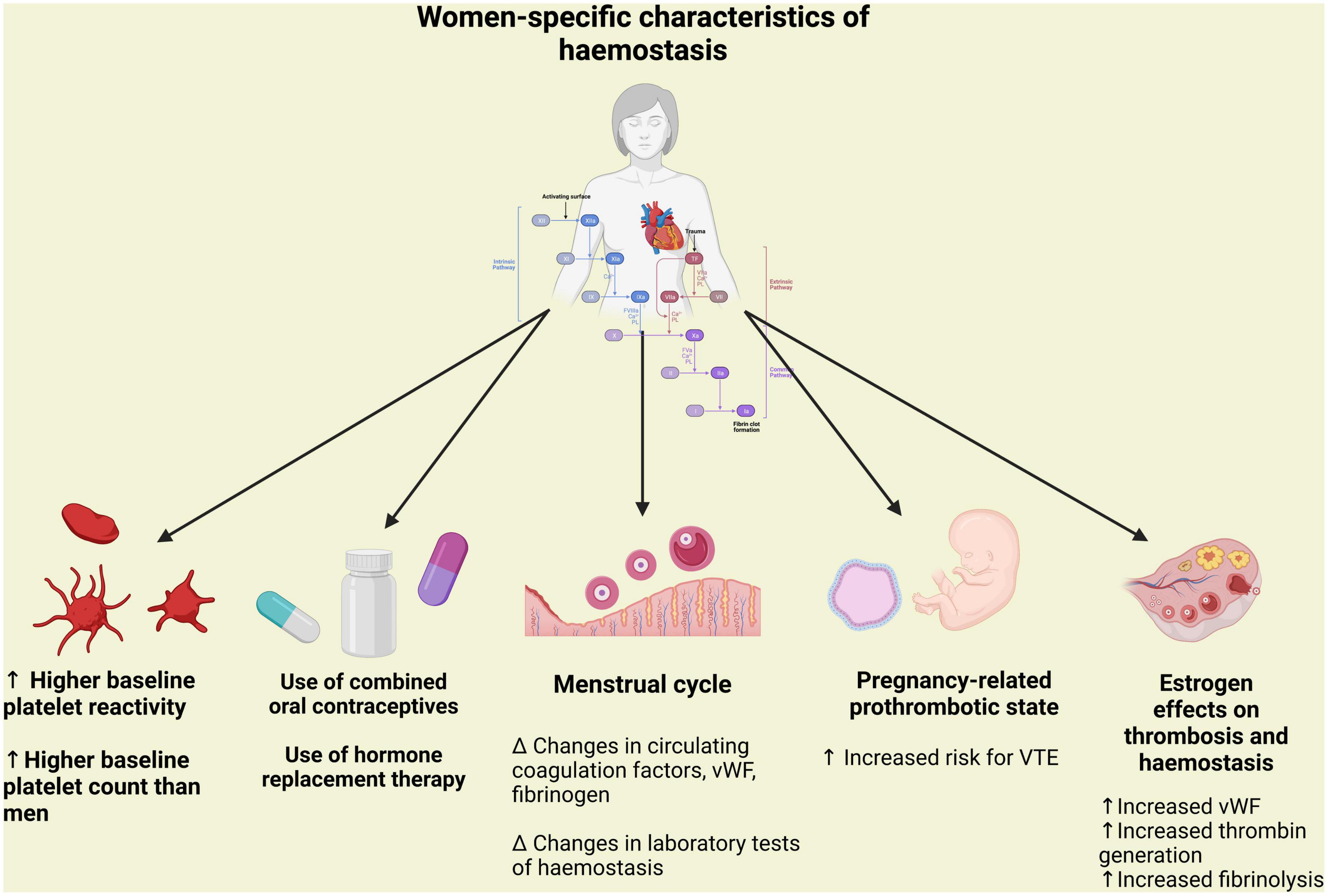
Figure 1. Peculiarity of hemostasis in women in terms of platelet aggregation and coagulation associated with pregnancy and hormonal status. vWF, Von Willebrand factor; VTE, venous thromboembolism.
Sex differences in coagulation
The coagulation cascade of secondary hemostasis is constituted by a series of reactions catalyzed by different enzymes, known as coagulation factors, ultimately resulting in cross-linked fibrin (48). This process is considerably influenced by fluctuations in hormone status associated with the menstrual cycle, pregnancy, menopause, hormone-based contraceptives and hormone replacement therapy (HRT) preparations (49). A cyclic variations of von Willebrand factor (VWF), fibrinogen, and activated factor VII have been reported during the normal menstrual cycle. Moreover, pregnancy and the oral administration of synthetic estrogens are associated with a progressive increase in the levels of procoagulant factors, VWF and fibrinogen and to a reduction in the activity of some coagulation regulatory proteins (tissue factor pathway inhibitor, protein S, protein C and antithrombin), leading to a hypercoagulable state (Figure 1) (49). These hormonal influences increase significantly the risk of venous thromboembolism, whereas their association with a higher risk of arterial thrombosis is still a matter of debate (31). Caution should be warranted when interpreting data on sex differences in platelet function and coagulation, given the heterogeneity of in vitro, ex vivo and in vivo studies, the multiple clinical scenarios (i.e., pre-/post-menopausal states or pregnancy) and the different dosages, routes of administration and combinations of hormone-based therapies.
Thrombotic risk in women within the spectrum of ischemic heart disease
Recently, the applicability of traditional risk factors in women (i.e., diabetes, smoking) has been questioned, as the majority of studies are predominantly conducted in the male population. Sex differences in the relative excess of cardiovascular risk associated with diabetes mellitus (DM) have been reported in several studies and have been confirmed by a recent meta-analysis of individual data from 980,793 adults; this analysis showed that women with DM exhibited a three-fold increased risk of cardiovascular mortality, whereas DM only doubles cardiovascular mortality risk in men (50). To date, the reason of this relative excess risk in women associated with the presence of DM is not elucidated.
Similarly, the impact of smoking on the development of IHD seems to be greater in women than in men (51). A recent meta-analysis including 2.4 million individuals reported that female smokers have a 25% greater risk of IHD compared with male smokers (52). Furthermore, obesity has a greater prognostic impact on women compared to men. In fact, the Framingham Heart Study showed that obesity increased the relative risk of IHD by 64% in women, as opposed to 46% in men (53). Data from 15,624 Norvegian individuals revealed that a similar increase in male or female body-mass index (BMI) was associated with a greater increase in systolic blood pressure in women than in men (54). However, BMI cannot be used as a comparable measure of fat tissue distribution between sexes, because it cannot discriminate between fat and fat-free mass. In fact, women result to have significantly greater amounts of total body fat than men with an equivalent BMI (55). Indeed, the pattern of lipid accumulation differs in women and men: women more often develop peripheral adiposity, with gluteal fat accumulation, whereas men are more prone to central or android obesity. However, after menopause, body fat distribution shifts to a more male pattern. Central fat, unlike peripheral adiposity, releases inflammatory mediators, which affect glucose and fat metabolisms and contribute to the development of metabolic syndrome (56). Nevertheless, BMI does not reflect fat distribution, as it is an exclusively quantitative parameter. In summary, BMI alone is not sufficient to properly assess the cardiometabolic risk associated with increased adiposity in women and other strategies, such as waist circumference measurement and bioimpedance analysis, should be implemented (57). Apart from traditional cardiovascular risk factors, there are a number of clinical conditions unique to women that have been identified to be associated with increased thrombotic risk. These include pregnancy disorders, such as pre-eclampsia, eclampsia, and gestational diabetes, gynecological disorders (i.e., polycystic ovary syndrome, early menopause), autoimmune and/or systemic inflammatory disease, known to disproportionally affect women compared to men (49) (Figure 2). As confirmation of the prognostic impact of non-traditional risk factors, nearly 20% of all coronary events occur in the absence of any traditional risk factors in women (51). Unfortunately, acquired awareness in thrombotic risk has not yet translated into changes in standard clinical care.
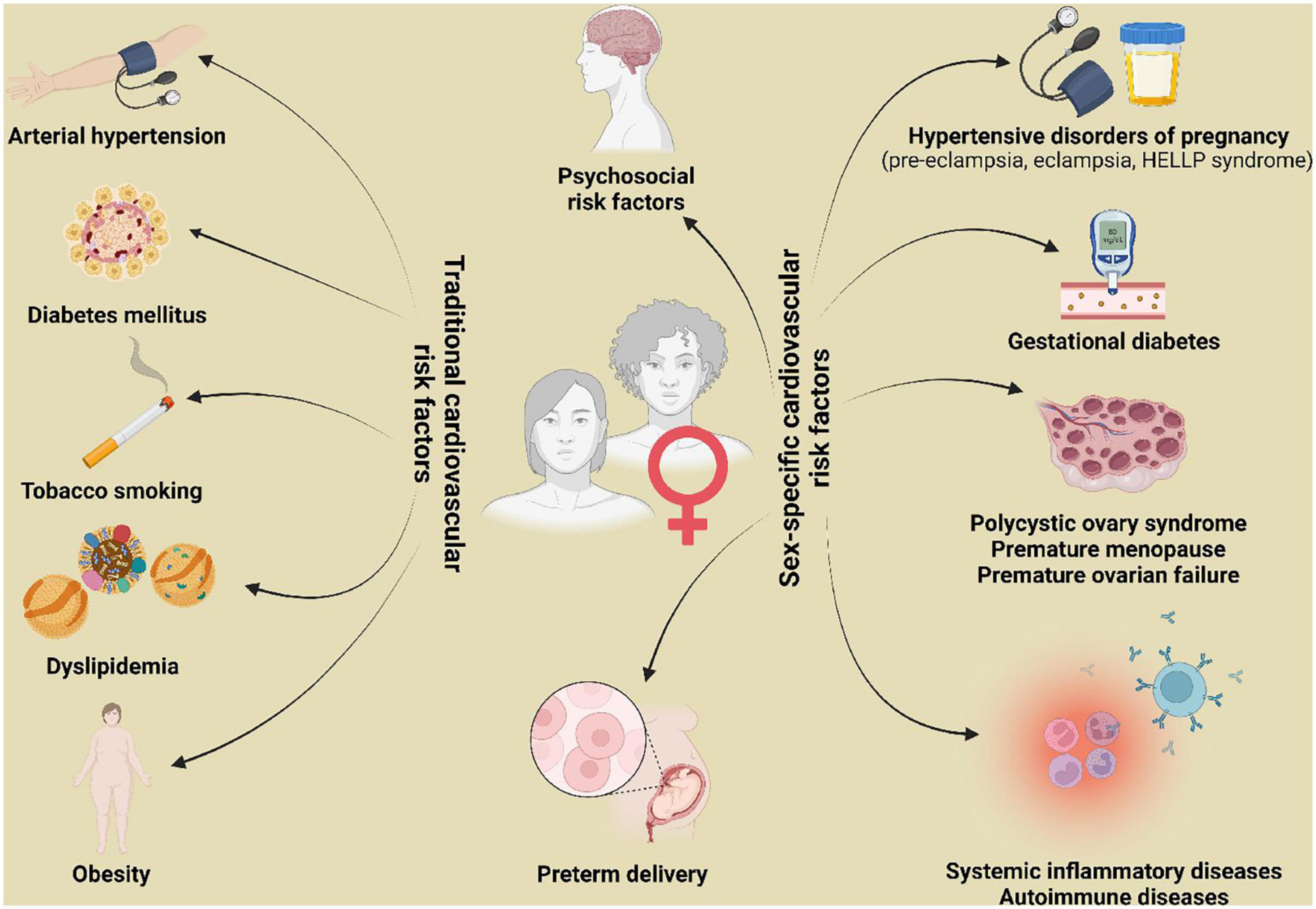
Figure 2. Traditional and sex-specific cardiovascular risk factors. HELLP, hemolysis, elevated liver enzymes and low platelets.
Double anti-platelet therapy score is the only tool endorsed by European and North-American guidelines to assess specifically thrombotic risk after PCI, identifying patients expected to derive benefit from continuing P2Y12 inhibitors beyond 1 year after PCI (58, 59). It was developed from the DAPT trial and validated in the PROTECT trial, but the proportion of women participating in each trial was subpar – 27 and 24%, respectively (60, 61). Even more, recent studies have shown women are less often prescribed antiplatelet therapy for secondary prevention, compared to men (51). Further outreach and awareness raising are necessary to ensure that gender with associated unique cardiovascular risk factors are included as important modifiers in thrombosis risk stratification scores, in order to guide clinicians in tailoring antithrombotic therapy after PCI, in terms of duration and intensity.
Strategies aimed at reducing ischemic events
Multiple strategies focused on reducing the residual burden of ischemic events among patients at high ischemic risk, undergoing PCI, have been developed over the years (2). These include the use of newer P2Y12 inhibitors (i.e., prasugrel, ticagrelor, and cangrelor) instead of clopidogrel or the addition of GP IIb/IIIa inhibitors (GPI), prolonging DAPT duration and the addition of a novel oral anticoagulants (NOACs) to standard antiplatelet treatment regimens, a strategy also known as dual pathway inhibition (DPI) (2, 62–70). As mentioned above, women represent a category with a higher ischemic burden compared to men with similar cardiovascular risk factors. Therefore, women could potentially benefit from these strategies even more than men, although robust evidence is currently lacking due to low percentage of women enrolled in trials (Table 1). The CURE (Clopidogrel in Unstable Angina to Prevent Recurrent Ischemic Events) trial, evaluating the addition of clopidogrel to aspirin in 12,562 patients with NSTEMI, showed women presented a smaller relative risk reduction (12% vs. 25%) in the composite endpoint of cardiovascular death, non-fatal AMI, or stroke compared with men at 1-year follow-up (71). Similar results were found in the subgroup of patients undergoing PCI (71). A subsequent meta-analysis of all blinded randomized clinical trials (RCTs) comparing clopidogrel and placebo and involving a total of 79,613 patients, confirmed the reduced efficacy in women compared to men: clopidogrel reduced only the risk of AMI and not that for stroke or all-cause mortality in women, whereas it reduced significantly all three endpoints in men (72, 73). Concerning ticagrelor and prasugrel, the PLATO trial and the TRITON-TIMI 38 trial, respectively, showed a similar reductions in the primary endpoint both in women and men, although these studies were not powered to examine treatment interactions among subgroups (74, 75). Similarly, two meta-analysis of randomized trials about PCI with adjunctive use of irreversible GPI (i.e., abciximab) or reversible GPIs (i.e., tirofiban or eptifibatide), demonstrated a similar efficacy both in men and women with no sex difference in terms of major adverse outcomes (76, 77). Accordingly, in a prespecified subgroup analysis of the CHAMPION PHOENIX trial, cangrelor demonstrated a similar reduction in the odds of major adverse cardiovascular events (MACE) in both sex (78).
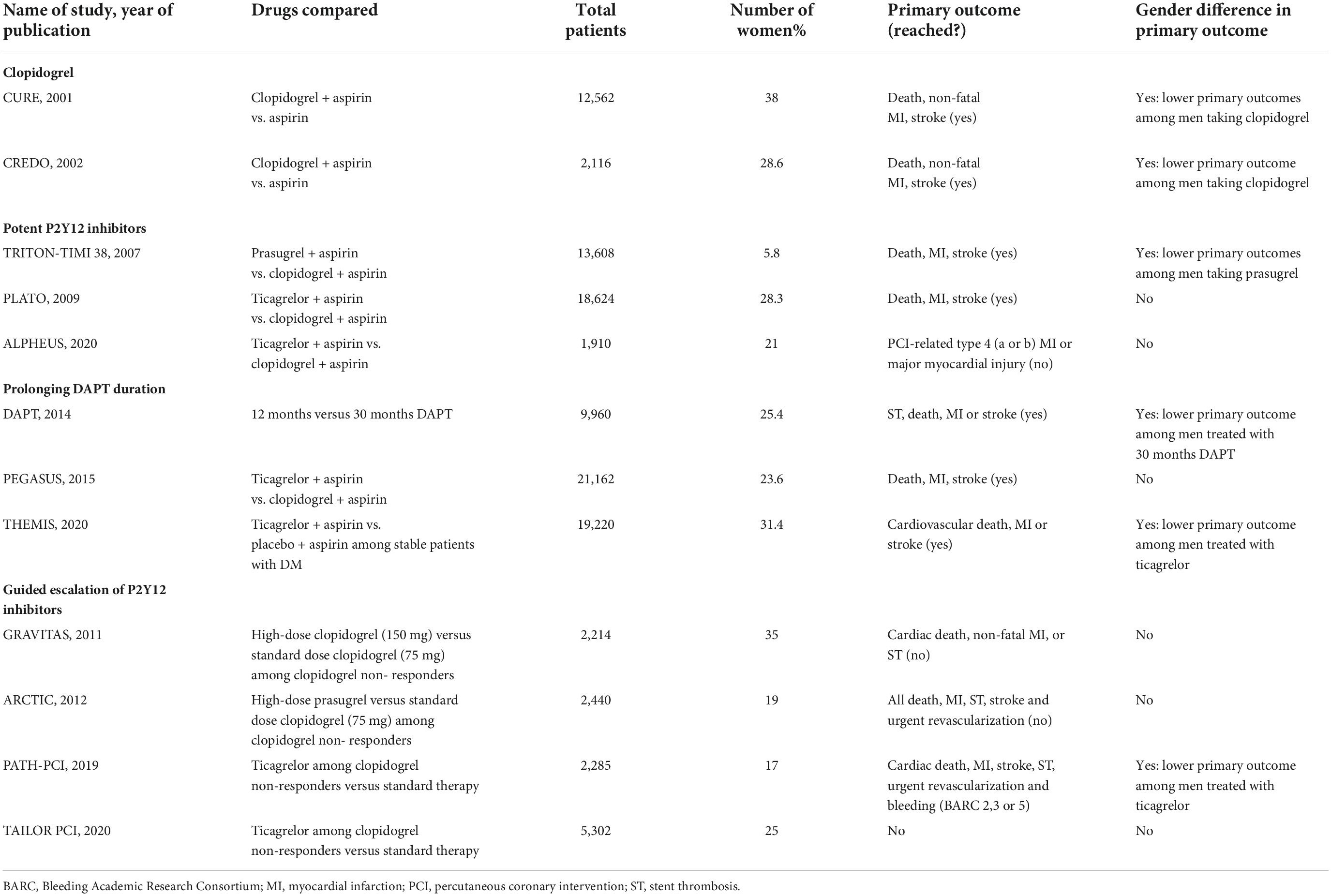
Table 1. List of major randomized controlled trials evaluating antiplatelet strategies focused on reducing ischemic events with sub-group analysis by sex.
Another strategy to reduce ischemic recurrences in patients at high ischemic risk, particularly those with prior AMI, is represented by prolongation of DAPT duration beyond 1 year. Dual Antiplatelet Therapy (DAPT) study, enrolling 9,961 patients, demonstrated, for the first time, that 30-month DAPT (with either clopidogrel 75 mg or prasugrel 10 mg) significantly reduced the primary endpoint of MACE, compared to 12-month DAPT (60). In a sub-group analysis, women randomly assigned to prolonged DAPT had a similar treatment effect for reduction in ischemic risk compared with males (79). Finally, the last strategy focused on reducing ischemic events is represented by DPI. To date, the low-dose rivaroxaban is so far the only NOAC to have been successfully tested as part of a DPI strategy in a phase III trial in patients with ACS (80). In particular, for patients with a recent ACS, low-dose rivaroxaban on top of standard of care antiplatelet therapy, most commonly aspirin and clopidogrel, reduced the risk of MACE and this benefit was significantly consistent only in the female subgroup of patients (81). In aggregate, a clear trend to a higher incidence of ischemic complications has been consistently reported in women. Strategies focused on reducing ischemic events appear to be equally effective in men and women, although the majority of trials are underpowered to assess differences in sex-specific subgroup analysis. Indeed, the main drawback of such intensive antithrombotic therapies is an enhanced risk of bleeding. It is, therefore, compelling for the scientific community to embark on dedicated clinical trials that will be equally inclusive to women as they are to men.
Bleeding risk in women within the spectrum of ischemic heart disease
Bleeding events have a significant downstream impact on mortality and morbidity outcomes among patients undergoing PCI (82). Sex-related differences have been observed also in terms of bleeding risk. Data from 24,045 patients with ACS from the GRACE registry showed that female sex was significantly associated with a higher risk of bleeding (adjusted odds ratio of 1.43), even after controlling for the influence of other variables, including age, antithrombotic therapies, performance of invasive procedure and clinical presentation (83). These findings have been recently confirmed by a recent analysis of 4 post-approval ACS registries showing that the prevalence of high bleeding risk (HBR) according to the Bleeding Academic Research Consortium (BARC) definition was higher in women compared to men, with a consequent higher rate of major bleeding at 4 years (84).
This phenomenon can have multiple explanations. First, women tend to be older and more likely to have comorbidities such as diabetes, cronic kidney disease and hypertension at the time of IHD – these are well-known risk factors for future hemorrhagic events (32). Second, women have a higher risk for the development of vascular complications following PCI, probably due to smaller blood vessels in women, as well as difference in vascular reactivity (85). Finally, women may have a tendency to receive inappropriate dosing of antithrombotic agents, because no difference in dose recommendation currently exist, although women have, at least in part, a lower body weight, an older age, and a higher rate of renal insufficiency, despite similar serum creatinine levels, compared to men (30).
In order to estimate bleeding risk in patients with IHD, European Society of Cardiology (ESC) and North-American guidelines recommend the use of several scores, such as the CRUSADE score, the ACUITY score, the PARIS score and the ARC-HBR criteria. Furthermore, PRECISE-DAPT score have been designed to guide and inform decision making for patients on DAPT following PCI, integrating both ischemic and bleeding risks (86). Surprisingly, female sex appears only in the CRUSADE and in the ACUITY scores among predictor variables, although it clearly represents a risk factor for bleeding after PCI.
In conclusion, in the last two decades, a remarkable amount of data has consistently demonstrated sex-related differences in bleeding risk after PCI. Nevertheless, it has not translated into the adoption of standardized different recommendations, according to patient’s sex. This uncertainty is reflected by international recommendations on duration of DAPT, with female sex being included among the bleeding risk factors in the North-American but not the European guidelines (58, 87).
Strategies aimed at reducing bleeding events
Bleeding has been recognized as a prognostically unfavorable event to the same extent as having a new or recurrent ischemic or thrombotic complication (88). The risk of bleeding tends to be stable over time while ischemic risk decreases after 1–3 months post-PCI, with a variability according to the clinical presentation of the patients and the complexity of the procedure (89). Therefore, after 1–3 months post-PCI, a series of pharmacological strategies can be implemented in order to reduce bleeding, possibly yielding a more favorable balance between bleeding and ischemic risk.
These strategies might include shortening of DAPT duration, the use of P2Y12 monotherapy and de-escalation of P2Y12 inhibitors (2, 90). Although they may be particularly appealing in women in light of their higher bleeding risk, these strategies are not extensively investigated in this subset of patients (Table 2). In addition, there are other non-pharmacological bleeding avoidance strategies, such as vascular closure device application, the use of radial access or the combination of these (88). Of note, the use of radial access resulted in a decrease in the rate of bleeding events to a greater extent in women, compared to men (91).
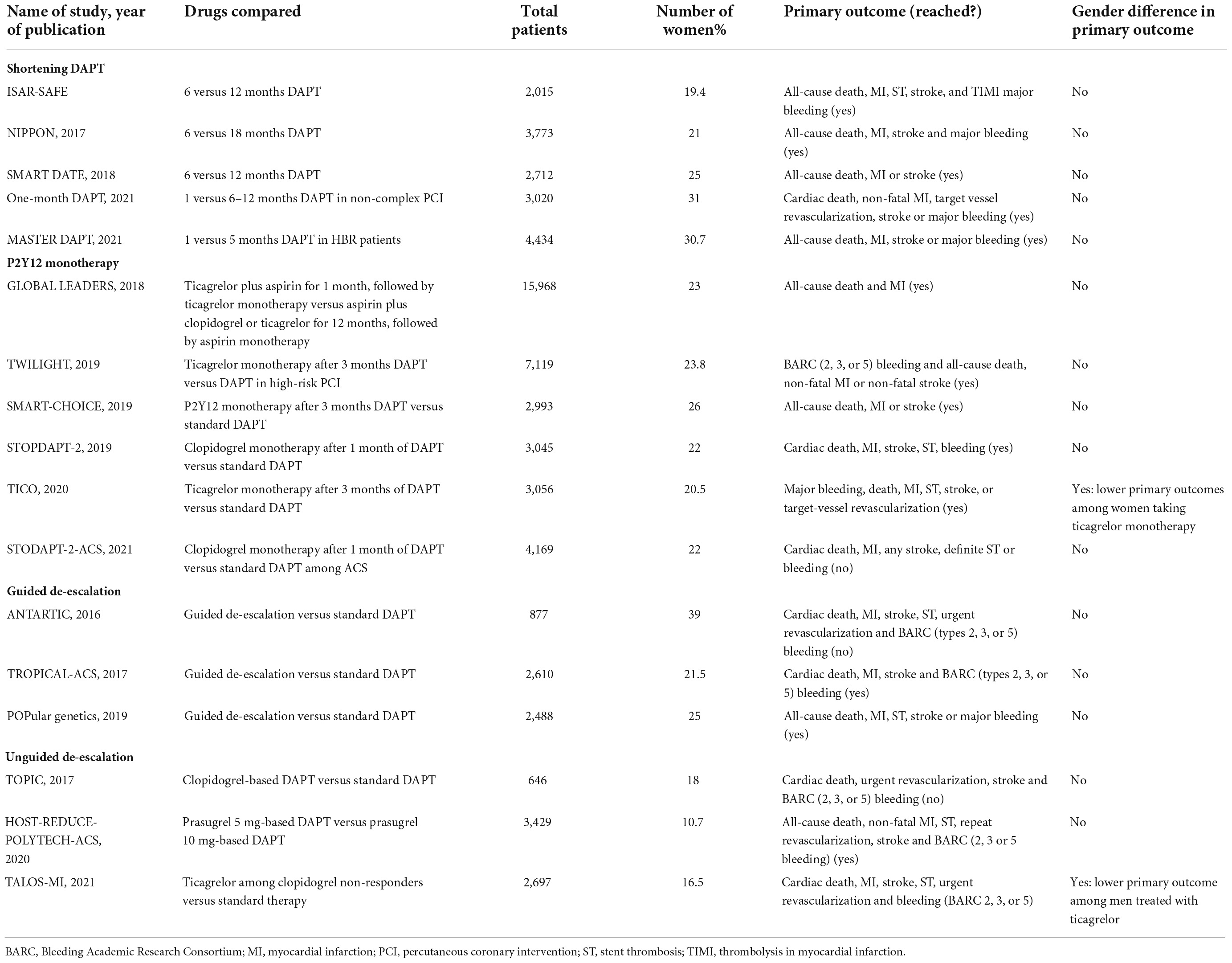
Table 2. List of major randomized controlled trials evaluating antiplatelet strategies focused on reducing bleeding events with sub-group analysis by sex.
Shortening of the DAPT duration has been the most largely investigated strategy and traditionally consists of the withdrawal of the P2Y12 inhibitor at the time earlier than conventional (12 months post-ACS) (2, 92–96). Sawaya et al. (97) pooled individual patient data from six RCTs comparing short- (≤6 months) versus long-term (≥1 year) DAPT after PCI. They showed short-term DAPT is associated with similar rates of MACE but lower risk of bleeding when compared with prolonged DAPT, with no significant difference between sexes. Although the hazard ratio of any bleeding and major bleeding suggest benefit in the subgroup of women, the p-value did not reach statistical significance. This is likely due to the fact that women were largely underrepresented in the above RCTs (about 30% of the overall population) thus not allowing a formal statistical power to be reached (97). Another important finding of this patient-level meta-analysis is that patient factors (ACS and diabetes) and lesion complexity (number of lesions stented and number of stents used) predicts the occurrence of MACE in women, underlying the importance of an accurate baseline risk stratification (97).
In the past 5 years, early aspirin discontinuation in patients undergoing PCI has emerged as a potential strategy to reduce bleeding without any increase in thrombotic events. To date, six RCTs with 36,350 patients (23.3% patients were female) have compared DAPT versus P2Y12 inhibitor monotherapy after a short duration of DAPT in patients after PCI (2, 98–102). An individual patient data meta-analysis of these six RCTs has been performed and showed that the use of P2Y12 monotherapy was associated with a significant reduction in the rate of bleeding without any increase in the rate of ischemic events (103). Interestingly, they investigated the consistency of these findings according to sex. Concerning the bleeding events, the treatment effect was consistent both in male and female patients, with a statistically significant reduction in both groups (103). Surprisingly, they observed a treatment-by-subgroup interaction with sex suggesting that P2Y12 inhibitor monotherapy lowers the risk of the primary ischemic endpoint in women but not in men (103). Whether this depends on a different response to aspirin and/or P2Y12 inhibitor remains the matter of debate. Female patients represent a subgroup, therefore, this finding should be considered hypothesis-generating only, due to intrinsic methodological and statistical limitations of subgroup analyses.
De-escalation of P2Y12 inhibiting therapy consists in switching from more potent (i.e., prasugrel or ticagrelor) to less potent (i.e., clopidogrel) agents, in order to reduce bleeding without any trade-off in ischemic events (104). De-escalation can be un-guided or guided through the aid of platelet function or genetic tests (105–108). A guided de-escalation strategy has been investigated in three RCTs, using either platelet function testing (n = 2) or genetic testing (n = 1) (109–112). TROPICAL ACS trial showed that guided de-escalation was non-inferior for the primary composite endpoint of net adverse cardiovascular events (NACE) as compared to standard of care, with a trend, although not statistically significant, toward reduced bleeding at 12 months compared to the standard group. Furthermore, a prespecified analysis of the TROPICAL-ACS trial investigated the impact of sex on clinical outcomes and found no significant interaction of sex with combined endpoint, ischemic events and bleeding.
POPular GENETICS trial showed that genotype-guided strategy was non-inferior for NACEs and superior in terms of PLATO major or minor bleeding, as compared to standard of care at 12-month follow-up. However, the reduction of bleeding became statistically insignificant in the subgroup of female patients, due to a relatively small sample size, which resulted in a broad 95% confidence interval.
Finally, it is worth considering the possible impact of herbal therapies on hemostasis and, consequently, on bleeding events. Multiple surveys have shown that women (especially white, middle-aged women, with good sociocultural status) are likely to be users of unconventional therapies, among which herbs play a prominent role (113). One of the most used is Ginkgo biloba, a species of tree native to China, from which an extract is obtained. It is commonly used as an antioxidant, to treat claudication intermittens and vascular dementia, although there is no evidence for its beneficial effects. Since it antagonizes platelet-activating factor, it predisposes to bleeding, especially in patients on aspirin or warfarin (114).
In short, it has been well-established for decades that women are at greater risk of bleeding. However, although various pharmacological strategies have been developed to minimize this risk, none of these have been extensively tested in female population. Therefore, evidence on their safety and efficacy in this subset of patients is lacking.
Specific clinical conditions
Atrial fibrillation
The prevalence and incidence of atrial fibrillation (AF) has been increasing in both sex over time (115). The number of women and men with AF are similar, despite the higher risk of AF in men, due to women’s increased longevity (115). AF increases the risk of stroke fivefold, but this risk is not homogeneous, depending on the presence of specific stroke risk factors. Common stroke risk factors are summarized in the CHA2DS2-VASc score, among which female sex is included (115). Of note, female sex has to be considered a stroke risk modifier rather than a risk factor per se (115). In the absence of other risk factors, women have a stroke risk similar to men, whereas women with other risk factors have significantly higher stroke risk than men (116). Women affected by AF and concomitant IHD are on average older and with more comorbidities than their male counterparts (117). Nevertheless, although they are at greater risk for stroke than men, they are significantly less likely to receive oral anticoagulants at all levels of the CHA2DS2-VASc score, paradoxically (118). The efficacy and safety of NOACs have been broadly demonstrated in overall population, even within 5 days after cardioembolic stroke (119, 120).
Sex differences in the efficacy and safety of warfarin compared to NOACs have long been investigated. According to a meta-analysis of 26,260 patients, women with AF have a significantly greater residual risk of systemic thromboembolism (STE) when treated using warfarin, whereas women treated with NOACs are at equivalent residual risk of STE and less major bleeding risk compared with men (121). Therefore, NOACs should be the anticoagulants of choice even more than in men.
Since NOACs have a different pharmacological profile compared to vitamin K antagonists, they may differ from one another in their effects on women with AF. An indirect comparison of them was performed, using data from foundational anticoagulant trials such as ROCKET-AF, RE-LY, ENGAGE-AF-TIMI and ARISTOTLE in which warfarin was used as an indirect comparator. No significant difference was found for any NOAC in terms of safety and efficacy in women with AF (122). Thus, a recent consensus document of the European Heart Rhythm Association (EHRA) indicates that the choice of the type of NOAC in females should follow general principles set for the overall population (123).
Nevertheless, data from adequately powered RCTs are needed to reach high quality evidence in the use of NOACs in women with concomitant AF for the prevention of STE. In general, 10–15% of AF patients undergo PCI for IHD and guidelines recommended TAT (triple antithrombotic therapy) for a certain time period after PCI in AF patients (115). However, there is still uncertainty whether TAT or double antithrombotic therapy (DAT) should be the first line choice for the majority of patients after hospital discharge. Holm et al. conducted an analysis on 272 patients discharged with TAT registered in the SWEDEHEART registry and showed that women discontinued TAT prematurely due to bleeds to a very high extent compared to men (124). Despite this, the rate of coronary events did not differ between sexes, although the study was underpowered to assess a possible sex difference in association between TAT discontinuation and ischemic events (124). To date, there are no sex analyses derived from RCTs regarding DAT and TAT to guide in treatment strategies, since data from RCTs were not powered to assess MACE, nor even differences among sex-specific subgroups (125–127).
Spontaneous coronary artery dissection
Spontaneous coronary artery dissection is the most common cause of pregnancy-associated AMI and represents 35% of ACS cases among women under the age of 50 (49). The gold standard for diagnosis of SCAD is coronary angiography (128). However, the use of intravascular imaging, such as optical coherence tomography (OCT) or intravascular ultrasound (IVUS), could be useful to differentiate SCAD from atherosclerotic plaque, when diagnostic uncertainty exists, or to guide coronary intervention, when clinically required (128).
There seems to be a general consensus indicating that the initial conservative medical management is appropriate in most SCAD cases, whereas interventional treatment (i.e., PCI or coronary artery bypass grafting, CABG) should be considered in selected cases such as SCAD complicated by refractory ongoing ischemia, hemodynamic instability or sustained ventricular tachyarrhythmias (128).
The antiplatelet regimen to be used in patients treated conservatively is still a matter of debate, since there are no RCTs comparing different pharmacological treatment strategies for SCAD. Whilst DAPT is the most commonly prescribed strategy in SCAD (usually with aspirin and clopidogrel rather than newer P2Y12 inhibitors), recent data from DISCO registry showed that DAPT was associated with a higher rate of MACE at 12 months of follow-up, driven by an early excess of non-fatal AMI or unplanned PCI (129). DAPT may cause enhancement of intramural bleeding, with subsequent propagation of the dissection and higher rate of adverse events. To support this hypothesis, Garcia-Guimaraes et al. reported that the presence of long intramural hematoma (>20 mm) is an independent predictor of in-hospital MACE in patients treated with DAPT (130). Therefore, DAPT may be actually harmful in conservatively managed SCAD patients, especially in those with contained IMH (i.e., type 2 SCAD). In case of SCAD occurring during pregnancy, particular attention should be paid to the choice of antithrombotic drugs, due to potential adverse effects to fetus (Table 3).
In conclusion, intravascular imaging plays a key role in the diagnosis and management of ACS in women, allowing to differentiate SCAD from other causes of ACS and, in case of SCAD, to characterize its specific endotype and to guide medical therapy.
Gender differences in participation in clinical trials: The who, what, why, when, how, and where
Women are still underrepresented in both early and later phase studies. The reasons for this phenomenon may be several. Traditionally, females were considered to have a more biological variability than males due to hormonal variations associated with estrous and menstrual cycles (131). Preclinical and clinical studies, recently, have refuted this theory, showing that females data are not more variable than those of males (132). Another reason could be that women affected by IHD are on average older than male counterparts and the enrollment of elderly patients in clinical trials has been historically low due to their frailty and comorbidities (133). Furthermore, women are less frequently referred to interventional treatment for ACS due to underestimation or misinterpretation of symptoms and are less likely to be treated with guideline-directed medical therapy (134). Interestingly, an RCT, enrolling 783 participants across 13 clinical centers, demonstrated that women present lower distrust of medical researchers and perceived greater risk of harm from trial participation than men (135). However, after disclosure of investigator patent ownership or monetary incentives, willingness to participate increased more in women than in men. This suggests that female aversion for participating in a scientific experiment could be, at least partially, overcomed by active and informed involvement in trial’s participation. Lastly, the research for sex differences may not necessarily involve a doubling of the pre-determined sample size (and costs) in order to reach an adequate statistical power. A recent statistical model, using factorial designs and tested for now only in animal studies, revealed necessary increases of only 14–33% to include both sexes, even after statistical correction for the use of multiple factors (136). Nevertheless, further studies are needed to validate this model in clinical trials.
Conclusion
Thirty one year after defining the “Yentl syndrome,” women are still understudied, underdiagnosed and undertreated. Their representation in RCTs is still too low (at most 30% of the overall trial population), although their pharmacodynamic and pharmacokinetic responses to antithrombotic drugs and their baseline bleeding and ischemic risks may differ significantly from males. Furthermore, investigations about antiplatelet drug safety and efficacy should not end with regulatory approval. Phase 4 studies, real-world data and systematic adverse-event reporting are critical to detect bleeding, ischemic events and off-target toxicities. A recent report from Hilleary et al. compared the proportion of females with an established diagnosis of IHD that received patient education, in terms of diet, exercise, tobacco use and weight reduction, with the corresponding proportion of males (137). Surprisingly, it revealed that a lower proportion of women received patient education related to managing cardiovascular risk, after adjusting for covariates. Accordingly, a lower proportion of women reaches cardiovascular risk factor target levels, as EUROASPIRE V registry has recently showed (138). Overall, a gender gap still exists for risk factor target management in secondary prevention, mostly in disfavor of women. Therefore, more deliberate and intentional effort needs to be performed in closing this gender gap, especially since risk factors like smoking and diabetes may have an even more detrimental effect in female patients, as mentioned above. In the era of precision medicine, it is unacceptable that women are treated “just like men” and viewed as a negligible minority. Historically, women’s health research has focused on reproductive health, a phenomenon known as “bikini medicine” (139). Now, it’s time that cardiovascular research efforts move away from mere awareness about gender differences to palpable and concrete action. “Go Red for Women” campaign, launched in 2004 by the American Heart Association, is pushing in this direction, in order to increase awareness and foster specific guidelines for prevention and treatment of IHD in women. Recently, POPular AGE trial and ELDERLY-ACS trial have evaluated safety and efficacy of different anti-platelet regimens in elderly, a clinical minority under-represented in RCTs, as women (140–142). Similarly, RCTs recruiting a significant proportion of women could be the solution to overcome the “Yentl syndrome.”
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work, and approved it for publication.
Conflict of interest
Author MG was declares that he has received consulting fees or honoraria from Terumo, outside the present work.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
2. Angiolillo DJ, Galli M, Collet JP, Kastrati A, O’Donoghue ML. Antiplatelet therapy after percutaneous coronary intervention. Eurointervention. (2022) 17:e1371–96.
3. Khandelwal A, Bakir M, Bezaire M, Costello B, Gomez JMD, Hoover V, et al. Managing ischemic heart disease in women: role of a women’s heart center. Curr Atheroscler Rep. (2021) 23:56.
4. Vogel B, Acevedo M, Appelman Y, Bairey Merz CN, Chieffo A, Figtree GA, et al. The Lancet women and cardiovascular disease Commission: reducing the global burden by 2030. Lancet. (2021) 397:2385–438. doi: 10.1016/S0140-6736(21)00684-X
5. Vaccarino V, Badimon L, Corti R, de Wit C, Dorobantu M, Hall A, et al. Ischaemic heart disease in women: are there sex differences in pathophysiology and risk factors? Position paper from the working group on coronary pathophysiology and microcirculation of the European Society of Cardiology. Cardiovasc Res. (2011) 90:9–17. doi: 10.1093/cvr/cvq394
6. Novak K, Vrdoljak D, Jelaska I, Borovac JA. Sex-specific differences in risk factors for in-hospital mortality and complications in patients with acute coronary syndromes: an observational cohort study. Wien Klin Wochenschr. (2017) 129:233–42. doi: 10.1007/s00508-016-1105-7
7. Potts J, Sirker A, Martinez SC, Gulati M, Alasnag M, Rashid M, et al. Persistent sex disparities in clinical outcomes with percutaneous coronary intervention: insights from 6.6 million PCI procedures in the United States. PLoS One. (2018) 13:e0203325. doi: 10.1371/journal.pone.0203325
8. Mehilli J, Presbitero P. Coronary artery disease and acute coronary syndrome in women. Heart. (2020) 106:487–92.
9. Sarma AA, Braunwald E, Cannon CP, Guo J, Im K, Antman EM, et al. Outcomes of women compared with men after non-ST-segment elevation acute coronary syndromes. J Am Coll Cardiol. (2019) 74:3013–22.
10. Alabas OA, Gale CP, Hall M, Rutherford MJ, Szummer K, Lawesson SS, et al. Sex differences in treatments, relative survival, and excess mortality following acute myocardial infarction: national cohort study using the SWEDEHEART registry. J Am Heart Assoc. (2017) 6:e007123. doi: 10.1161/JAHA.117.007123
11. van Oosterhout REM, de Boer AR, Maas A, Rutten FH, Bots ML, Peters SAE. Sex differences in symptom presentation in acute coronary syndromes: a systematic review and meta-analysis. J Am Heart Assoc. (2020) 9:e014733.
12. Araujo C, Laszczynska O, Viana M, Melao F, Henriques A, Borges A, et al. Sex differences in presenting symptoms of acute coronary syndrome: the EPIHeart cohort study. BMJ Open. (2018) 8:e018798. doi: 10.1136/bmjopen-2017-018798
13. Mehta LS, Beckie TM, DeVon HA, Grines CL, Krumholz HM, Johnson MN, et al. Acute myocardial infarction in women: a scientific statement from the American Heart Association. Circulation. (2016) 133:916–47.
14. Udell JA, Fonarow GC, Maddox TM, Cannon CP, Frank Peacock W, Laskey WK, et al. Sustained sex-based treatment differences in acute coronary syndrome care: Insights from the American Heart Association get with the guidelines Coronary Artery Disease Registry. Clin Cardiol. (2018) 41:758–68. doi: 10.1002/clc.22938
15. Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. (2004) 364: 937–52.
16. Severino P, Mariani MV, Maraone A, Piro A, Ceccacci A, Tarsitani L, et al. Triggers for atrial fibrillation: the role of anxiety. Cardiol Res Pract. (2019) 2019:1208505. doi: 10.1155/2019/1208505
17. Severino P, D’Amato A, Pucci M, Infusino F, Birtolo LI, Mariani MV, et al. Ischemic heart disease and heart failure: role of coronary ion channels. Int J Mol Sci. (2020) 21:3167.
18. Severino P, D’Amato A, Pucci M, Mariani MV, Netti L, Infusino F, et al. Myocardial Ischemia in women when genetic susceptibility matters. J Mol Genetic Med. (2019) 13:1–6.
19. Michal M, Eggebrecht L, Göbel S, Panova-Noeva M, Nagler M, Arnold N, et al. The relevance of depressive symptoms for the outcome of patients receiving vitamin K antagonists: results from the thrombEVAL cohort study. Eur Heart J Cardiovasc Pharmacother. (2021) 7:271–9. doi: 10.1093/ehjcvp/pvz085
20. Laporte S, Chapelle C, Caillet P, Beyens MN, Bellet F, Delavenne X, et al. Bleeding risk under selective serotonin reuptake inhibitor (SSRI) antidepressants: a meta-analysis of observational studies. Pharmacol Res. (2017) 118:19–32. doi: 10.1016/j.phrs.2016.08.017
21. Bykov K, Schneeweiss S, Donneyong MM, Dong YH, Choudhry NK, Gagne JJ. Impact of an interaction between clopidogrel and selective serotonin reuptake inhibitors. Am J Cardiol. (2017) 119:651–7.
22. Gabet A, Danchin N, Juilliere Y, Olie V. Acute coronary syndrome in women: rising hospitalizations in middle-aged French women, 2004-14. Eur Heart J. (2017) 38:1060–5. doi: 10.1093/eurheartj/ehx097
23. Chieffo A, Buchanan GL, Mehilli J, Capodanno D, Kunadian V, Petronio AS, et al. Percutaneous coronary and structural interventions in women: a position statement from the EAPCI Women Committee. Eurointervention. (2018) 14:e1227–35. doi: 10.4244/EIJ-D-18-00225
24. Gulati M, Cooper-DeHoff RM, McClure C, Johnson BD, Shaw LJ, Handberg EM, et al. Adverse cardiovascular outcomes in women with nonobstructive coronary artery disease: a report from the women’s Ischemia syndrome evaluation study and the st james women take heart project. Arch Intern Med. (2009) 169:843–50. doi: 10.1001/archinternmed.2009.50
25. Saw J, Mancini GBJ, Humphries KH. Contemporary review on spontaneous coronary artery dissection. J Am Coll Cardiol. (2016) 68:297–312.
26. Hayes SN, Kim ESH, Saw J, Adlam D, Arslanian-Engoren C, Economy KE, et al. Spontaneous coronary artery dissection: current state of the science: a scientific statement from the American Heart Association. Circulation. (2018) 137:e523–57. doi: 10.1161/CIR.0000000000000564
27. Kunadian V, Chieffo A, Camici PG, Berry C, Escaned J, Maas A, et al. An EAPCI Expert consensus document on ischaemia with non-obstructive coronary arteries in collaboration with european society of cardiology working group on coronary pathophysiology & microcirculation endorsed by coronary vasomotor disorders international study group. Eurointervention. (2021) 16:1049–69.
28. Ghadri JR, Wittstein IS, Prasad A, Sharkey S, Dote K, Akashi YJ, et al. International expert consensus document on takotsubo syndrome (Part I): clinical characteristics, diagnostic criteria, and pathophysiology. Eur Heart J. (2018) 39:2032–46. doi: 10.1093/eurheartj/ehy076
29. Canto JG, Rogers WJ, Goldberg RJ, Peterson ED, Wenger NK, Vaccarino V, et al. Association of age and sex with myocardial infarction symptom presentation and in-hospital mortality. JAMA. (2012) 307:813–22.
30. Patti G, De Caterina R, Abbate R, Andreotti F, Biasucci LM, Calabro P, et al. Platelet function and long-term antiplatelet therapy in women: is there a gender-specificity? A ‘state-of-the-art’ paper. Eur Heart J. (2014) 35:2213b–23b. doi: 10.1093/eurheartj/ehu279
31. Renda G, Patti G, Lang IM, Siller-Matula JM, Hylek EM, Ambrosio G, et al. Thrombotic and hemorrhagic burden in women: gender-related issues in the response to antithrombotic therapies. Int J Cardiol. (2019) 286:198–207. doi: 10.1016/j.ijcard.2019.02.004
32. Andreotti F, Rio T, Gianmarinaro M, Navarese EP, Marchese N, Crea F. [Pathophysiology of ischemic heart disease in women]. G Ital Cardiol. (2012) 13:396–400.
33. Johnson M, Ramey E, Ramwell PW. Sex and age differences in human platelet aggregation. Nature. (1975) 253:355–7.
34. Becker DM, Segal J, Vaidya D, Yanek LR, Herrera-Galeano JE, Bray PF, et al. Sex differences in platelet reactivity and response to low-dose aspirin therapy. JAMA. (2006) 295:1420–7. doi: 10.1001/jama.295.12.1420
35. Gremmel T, Kopp CW, Eichelberger B, Koppensteiner R, Panzer S. Sex differences of leukocyte-platelet interactions and on-treatment platelet reactivity in patients with atherosclerosis. Atherosclerosis. (2014) 237:692–5. doi: 10.1016/j.atherosclerosis.2014.10.095
36. Bobbert P, Stellbaum C, Steffens D, Schutte C, Bobbert T, Schultheiss HP, et al. Postmenopausal women have an increased maximal platelet reactivity compared to men despite dual antiplatelet therapy. Blood Coagul Fibrinol. (2012) 23:723–8. doi: 10.1097/MBC.0b013e32835824b3
37. Ranucci M, Aloisio T, Di Dedda U, Menicanti L, de Vincentiis C, Baryshnikova E, et al. Gender-based differences in platelet function and platelet reactivity to P2Y12 inhibitors. PLoS One. (2019) 14:e0225771. doi: 10.1371/journal.pone.0225771
38. Waissi F, Dekker M, Bank IEM, Korporaal SJA, Urbanus RT, de Borst GJ, et al. Sex differences in flow cytometry-based platelet reactivity in stable outpatients suspected of myocardial ischemia. Res Pract Thromb Haemost. (2020) 4:879–85. doi: 10.1002/rth2.12344
39. Kaur A, Dreyer RP, Marsh TW, Thanassoulis G, Raparelli V, D’Onofrio G, et al. Sex differences in clopidogrel effects among young patients with acute coronary syndrome: a role for genetics? CJC Open. (2022) doi: 10.1016/j.cjco.2022.07.013 [Epub ahead of print].
40. Verdoia M, Pergolini P, Rolla R, Nardin M, Barbieri L, Daffara V, et al. Gender differences in platelet reactivity in patients receiving dual antiplatelet therapy. Cardiovasc Drugs Ther. (2016) 30:143–50.
41. Breet NJ, Sluman MA, van Berkel MA, van Werkum JW, Bouman HJ, Harmsze AM, et al. Effect of gender difference on platelet reactivity. Neth Heart J. (2011) 19:451–7.
42. Alexopoulos D, Xanthopoulou I, Storey RF, Bliden KP, Tantry US, Angiolillo DJ, et al. Platelet reactivity during ticagrelor maintenance therapy: a patient-level data meta-analysis. Am Heart J. (2014) 168:530–6. doi: 10.1016/j.ahj.2014.06.026
43. Cirillo P, Di Serafino L, Patti G, Antonucci E, Calabrò P, Gresele P, et al. Gender-related differences in antiplatelet therapy and impact on 1-year clinical outcome in patients presenting With ACS: the START ANTIPLATELET Registry. Angiology. (2019) 70:257–63. doi: 10.1177/0003319718783866
44. Zaccardi F, Pitocco D, Willeit P, Laukkanen JA. Efficacy and safety of P2Y12 inhibitors according to diabetes, age, gender, body mass index and body weight: systematic review and meta-analyses of randomized clinical trials. Atherosclerosis. (2015) 240:439–45. doi: 10.1016/j.atherosclerosis.2015.04.015
45. Brar SS, Ten Berg J, Marcucci R, Price MJ, Valgimigli M, Kim HS. Impact of platelet reactivity on clinical outcomes after percutaneous coronary intervention. a collaborative meta-analysis of individual participant data. J Am Coll Cardiol. (2011) 58:1945–54. doi: 10.1016/j.jacc.2011.06.059
46. Berger JS, Roncaglioni MC, Avanzini F, Pangrazzi I, Tognoni G, Brown DL. Aspirin for the primary prevention of cardiovascular events in women and men: a sex-specific meta-analysis of randomized controlled trials. JAMA. (2006) 295:306–13. doi: 10.1001/jama.295.3.306
47. Baigent C, Blackwell L, Collins R, Emberson J, Godwin J, Peto R, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. (2009) 373:1849–60.
48. Trigg DE, Wood MG, Kouides PA, Kadir RA. Hormonal influences on hemostasis in women. Semin Thromb Hemost. (2011) 37:77–86.
49. Maas A, Rosano G, Cifkova R, Chieffo A, van Dijken D, Hamoda H, et al. Cardiovascular health after menopause transition, pregnancy disorders, and other gynaecologic conditions: a consensus document from European cardiologists, gynaecologists, and endocrinologists. Eur Heart J. (2021) 42:967–84.
50. Prospective Studies Collaboration and Asia Pacific Cohort Studies Collaboration. Sex-specific relevance of diabetes to occlusive vascular and other mortality: a collaborative meta-analysis of individual data from 980 793 adults from 68 prospective studies. Lancet Diabetes Endocrinol. (2018) 6:538–46. doi: 10.1016/S2213-8587(18)30079-2
52. Huxley RR, Woodward M. Cigarette smoking as a risk factor for coronary heart disease in women compared with men: a systematic review and meta-analysis of prospective cohort studies. Lancet. (2011) 378:1297–305. doi: 10.1016/S0140-6736(11)60781-2
53. Wilson PW, D’Agostino RB, Sullivan L, Parise H, Kannel WB. Overweight and obesity as determinants of cardiovascular risk: the Framingham experience. Arch Intern Med. (2002) 162:1867–72.
54. Wilsgaard T, Schirmer H, Arnesen E. Impact of body weight on blood pressure with a focus on sex differences: the Tromso Study, 1986-1995. Arch Intern Med. (2000) 160:2847–53. doi: 10.1001/archinte.160.18.2847
55. Gallagher D, Visser M, Sepúlveda D, Pierson RN, Harris T, Heymsfield SB. How useful is body mass index for comparison of body fatness across age, sex, and ethnic groups? Am J Epidemiol. (1996) 143:228–39.
56. Regitz-Zagrosek V, Lehmkuhl E, Weickert MO. Gender differences in the metabolic syndrome and their role for cardiovascular disease. Clin Res Cardiol. (2006) 95:136–47.
57. Ross R, Neeland IJ, Yamashita S, Shai I, Seidell J, Magni P, et al. Waist circumference as a vital sign in clinical practice: a Consensus Statement from the IAS and ICCR Working Group on Visceral Obesity. Nat Rev Endocrinol. (2020) 16:177–89. doi: 10.1038/s41574-019-0310-7
58. Valgimigli M, Bueno H, Byrne RA, Collet JP, Costa F, Jeppsson A, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. (2018) 39:213–60. doi: 10.1093/eurheartj/ehx419
59. Costa F, Van Klaveren D, Feres F, James S, Raber L, Pilgrim T, et al. Dual antiplatelet therapy duration based on ischemic and bleeding risks after coronary stenting. J Am Coll Cardiol. (2019) 73:741–54.
60. Mauri L, Kereiakes DJ, Yeh RW, Driscoll-Shempp P, Cutlip DE, Steg PG, et al. Twelve or 30 months of dual antiplatelet therapy after drug-eluting stents. N Engl J Med. (2014) 371:2155–66.
61. Yeh RW, Secemsky EA, Kereiakes DJ, Normand SL, Gershlick AH, Cohen DJ, et al. Development and validation of a prediction rule for benefit and harm of dual antiplatelet therapy beyond 1 year after percutaneous coronary intervention. JAMA. (2016) 315:1735–49. doi: 10.1001/jama.2016.3775
62. Galli M, Capodanno D, Benenati S, D’Amario D, Crea F, Andreotti F, et al. Efficacy and safety of dual pathway inhibition in patients with cardiovascular disease: a systematic review and Meta-analysis. Eur Heart J Cardiovasc Pharmacother. (2021) 8:519–28. doi: 10.1093/ehjcvp/pvab043
63. Galli M, Migliaro S, Rodolico D, Di Stefano G, Piccinni C, Restivo A, et al. Intracoronary bolus of glycoprotein IIb/IIIa inhibitor as bridging or adjunctive strategy to oral P2Y12 inhibitor load in the modern setting of STEMI. Minerva Cardiol Angiol. (2021) doi: 10.23736/S2724-5683.21.05669-6 [Epub ahead of print].
64. Silvain J, Lattuca B, Beygui F, Rangé G, Motovska Z, Dillinger JG, et al. Ticagrelor versus clopidogrel in elective percutaneous coronary intervention (ALPHEUS): a randomised, open-label, phase 3b trial. Lancet. (2020) 396:1737–44. doi: 10.1016/S0140-6736(20)32236-4
65. Bonaca MP, Bhatt DL, Cohen M, Steg PG, Storey RF, Jensen EC, et al. Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med. (2015) 372:1791–800.
66. Steg PG, Bhatt DL, Simon T, Fox K, Mehta SR, Harrington RA, et al. Ticagrelor in patients with stable coronary disease and diabetes. N Engl J Med. (2019) 381:1309–20.
67. Price MJ, Berger PB, Teirstein PS, Tanguay JF, Angiolillo DJ, Spriggs D, et al. Standard- vs high-dose clopidogrel based on platelet function testing after percutaneous coronary intervention: the GRAVITAS randomized trial. JAMA. (2011) 305:1097–105. doi: 10.1001/jama.2011.290
68. Collet JP, Cuisset T, Rangé G, Cayla G, Elhadad S, Pouillot C, et al. Bedside monitoring to adjust antiplatelet therapy for coronary stenting. N Engl J Med. (2012) 367:2100–9.
69. Zheng YY, Wu TT, Yang Y, Hou XG, Gao Y, Chen Y, et al. Personalized antiplatelet therapy guided by a novel detection of platelet aggregation function in stable coronary artery disease patients undergoing percutaneous coronary intervention: a randomized controlled clinical trial. Eur Heart J Cardiovasc Pharmacother. (2020) 6:211–21. doi: 10.1093/ehjcvp/pvz059
70. Pereira NL, Farkouh ME, So D, Lennon R, Geller N, Mathew V, et al. Effect of genotype-guided oral P2Y12 inhibitor selection vs conventional clopidogrel therapy on ischemic outcomes after percutaneous coronary intervention: the TAILOR-PCI randomized clinical trial. JAMA. (2020) 324:761–71. doi: 10.1001/jama.2020.12443
71. Mehta SR, Yusuf S, Clopidogrel in Unstable angina to prevent Recurrent Events (CURE) Study Investigators. The Clopidogrel in Unstable angina to prevent Recurrent Events (CURE) trial programme; rationale, design and baseline characteristics including a meta-analysis of the effects of thienopyridines in vascular disease. Eur Heart J. (2000) 21:2033–41. doi: 10.1053/euhj.2000.2474
72. Berger JS, Bhatt DL, Cannon CP, Chen Z, Jiang L, Jones JB, et al. The relative efficacy and safety of clopidogrel in women and men a sex-specific collaborative meta-analysis. J Am Coll Cardiol. (2009) 54:1935–45. doi: 10.1016/j.jacc.2009.05.074
73. Steinhubl SR, Berger PB, Mann JT III, Fry ET, DeLago A, Wilmer C, et al. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial. JAMA. (2002) 288:2411–20.
74. Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. (2009) 361:1045–57.
75. Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. (2007) 357:2001–15.
76. Cho L, Topol EJ, Balog C, Foody JM, Booth JE, Cabot C, et al. Clinical benefit of glycoprotein IIb/IIIa blockade with Abciximab is independent of gender: pooled analysis from EPIC, EPILOG and EPISTENT trials. Evaluation of 7E3 for the Prevention of Ischemic Complications. Evaluation in Percutaneous Transluminal Coronary Angioplasty to Improve Long-Term Outcome with Abciximab GP IIb/IIIa blockade. Evaluation of Platelet IIb/IIIa Inhibitor for Stent. J Am Coll Cardiol. (2000) 36:381–6. doi: 10.1016/s0735-1097(00)00746-4
77. Boersma E, Harrington RA, Moliterno DJ, White H, Simoons ML. Platelet glycoprotein IIb/IIIa inhibitors in acute coronary syndromes. Lancet. (2002) 360:342–3.
78. O’Donoghue ML, Bhatt DL, Stone GW, Steg PG, Gibson CM, Hamm CW, et al. Efficacy and safety of cangrelor in women versus men during percutaneous coronary intervention: insights from the cangrelor versus standard therapy to achieve optimal management of platelet inhibition (CHAMPION PHOENIX) Trial. Circulation. (2016) 133:248–55. doi: 10.1161/CIRCULATIONAHA.115.017300
79. Berry NC, Kereiakes DJ, Yeh RW, Steg PG, Cutlip DE, Jacobs AK, et al. Benefit and risk of prolonged DAPT after coronary stenting in women. Circ Cardiovasc Interv. (2018) 11:e005308.
80. Capodanno D, Bhatt DL, Eikelboom JW, Fox KAA, Geisler T, Michael Gibson C, et al. Dual-pathway inhibition for secondary and tertiary antithrombotic prevention in cardiovascular disease. Nat Rev Cardiol. (2020) 17:242–57. doi: 10.1038/s41569-019-0314-y
81. Mega JL, Braunwald E, Wiviott SD, Bassand JP, Bhatt DL, Bode C, et al. Rivaroxaban in patients with a recent acute coronary syndrome. N Engl J Med. (2012) 366:9–19.
82. Galli M, Laborante R, Andreotti F, Vergallo R, Montone R, Iaconelli A, et al. Bleeding complications in patients undergoing percutaneous coronary intervention. Rev Cardiovasc Med. (2022) 23:286.
83. Moscucci M, Fox KA, Cannon CP, Klein W, Lopez-Sendon J, Montalescot G, et al. Predictors of major bleeding in acute coronary syndromes: the Global Registry of Acute Coronary Events (GRACE). Eur Heart J. (2003) 24:1815–23.
84. Chandiramani R, Cao D, Claessen BE, Sorrentino S, Guedeney P, Blum M, et al. Sex-related differences in patients at high bleeding risk undergoing percutaneous coronary intervention: a patient-level pooled analysis from 4 postapproval studies. J Am Heart Assoc. (2020) 9:e014611. doi: 10.1161/JAHA.119.014611
85. Wang TY, Angiolillo DJ, Cushman M, Sabatine MS, Bray PF, Smyth SS, et al. Platelet biology and response to antiplatelet therapy in women: implications for the development and use of antiplatelet pharmacotherapies for cardiovascular disease. J Am Coll Cardiol. (2012) 59:891–900. doi: 10.1016/j.jacc.2011.09.075
86. Levine GN, Bates ER, Bittl JA, Brindis RG, Fihn SD, Fleisher LA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. (2016) 68:1082–115.
87. Collet JP, Thiele H, Barbato E, Barthelemy O, Bauersachs J, Bhatt DL, et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. (2021) 42:1289–367.
88. Capodanno D, Bhatt DL, Gibson CM, James S, Kimura T, Mehran R, et al. Bleeding avoidance strategies in percutaneous coronary intervention. Nat Rev Cardiol. (2022) 19:117–32.
89. Rodriguez F, Harrington RA. Management of antithrombotic therapy after acute coronary syndromes. N Engl J Med. (2021) 384:452–60.
90. Galli M, Angiolillo DJ. De-escalation of antiplatelet therapy in acute coronary syndromes: Why, how and when? Front Cardiovasc Med. (2022) 9:975969. doi: 10.3389/fcvm.2022.975969
91. Pristipino C, Pelliccia F, Granatelli A, Pasceri V, Roncella A, Speciale G, et al. Comparison of access-related bleeding complications in women versus men undergoing percutaneous coronary catheterization using the radial versus femoral artery. Am J Cardiol. (2007) 99:1216–21.
92. Schulz-Schüpke S, Byrne RA, Ten Berg JM, Neumann FJ, Han Y, Adriaenssens T, et al. ISAR-SAFE: a randomized, double-blind, placebo-controlled trial of 6 vs. 12 months of clopidogrel therapy after drug-eluting stenting. Eur Heart J. (2015) 36:1252–63. doi: 10.1093/eurheartj/ehu523
93. Nakamura M, Iijima R, Ako J, Shinke T, Okada H, Ito Y, et al. Dual antiplatelet therapy for 6 versus 18 months after biodegradable polymer drug-eluting stent implantation. JACC Cardiovasc Interv. (2017) 10:1189–98.
94. Hahn JY, Song YB, Oh JH, Cho DK, Lee JB, Doh JH, et al. 6-month versus 12-month or longer dual antiplatelet therapy after percutaneous coronary intervention in patients with acute coronary syndrome (SMART-DATE): a randomised, open-label, non-inferiority trial. Lancet. (2018) 391:1274–84.
95. Valgimigli M, Frigoli E, Heg D, Tijssen J, Jüni P, Vranckx P, et al. Dual antiplatelet therapy after PCI in patients at high bleeding risk. N Engl J Med. (2021) 385:1643–55.
96. Hong SJ, Kim JS, Hong SJ, Lim DS, Lee SY, Yun KH, et al. 1-month dual-antiplatelet therapy followed by aspirin monotherapy after polymer-free drug-coated stent implantation: one-month DAPT Trial. JACC Cardiovasc Interv. (2021) 14:1801–11.
97. Sawaya FJ, Morice MC, Spaziano M, Mehran R, Didier R, Roy A, et al. Short-versus long-term Dual Antiplatelet therapy after drug-eluting stent implantation in women versus men: A sex-specific patient-level pooled-analysis of six randomized trials. Catheter Cardiovasc Interv. (2017) 89:178–89. doi: 10.1002/ccd.26653
98. Galli M, Capodanno D, Andreotti F, Crea F, Angiolillo DJ. Safety and efficacy of P2Y12 inhibitor monotherapy in patients undergoing percutaneous coronary interventions. Expert Opin Drug Saf. (2021) 20:9–21.
99. Mehran R, Baber U, Sharma SK, Cohen DJ, Angiolillo DJ, Briguori C, et al. Ticagrelor with or without Aspirin in High-Risk Patients after PCI. N Engl J Med. (2019) 381:2032–42.
100. Vranckx P, Valgimigli M, Jüni P, Hamm C, Steg PG, Heg D, et al. Ticagrelor plus aspirin for 1 month, followed by ticagrelor monotherapy for 23 months vs aspirin plus clopidogrel or ticagrelor for 12 months, followed by aspirin monotherapy for 12 months after implantation of a drug-eluting stent: a multicentre, open-label, randomised superiority trial. Lancet. (2018) 392:940–9. doi: 10.1016/S0140-6736(18)31858-0
101. Watanabe H, Domei T, Morimoto T, Natsuaki M, Shiomi H, Toyota T, et al. Effect of 1-Month Dual Antiplatelet Therapy Followed by Clopidogrel vs 12-Month Dual Antiplatelet Therapy on Cardiovascular and Bleeding Events in Patients Receiving PCI: the STOPDAPT-2 Randomized Clinical Trial. JAMA. (2019) 321:2414–27.
102. Hahn JY, Song YB, Oh JH, Chun WJ, Park YH, Jang WJ, et al. Effect of P2Y12 Inhibitor Monotherapy vs Dual Antiplatelet Therapy on Cardiovascular Events in Patients Undergoing Percutaneous Coronary Intervention: the SMART-CHOICE Randomized Clinical Trial. JAMA. (2019) 321:2428–37. doi: 10.1001/jama.2019.8146
103. Valgimigli M, Gragnano F, Branca M, Franzone A, Baber U, Jang Y, et al. P2Y12 inhibitor monotherapy or dual antiplatelet therapy after coronary revascularisation: individual patient level meta-analysis of randomised controlled trials. BMJ. (2021) 373:n1332.
104. Galli M, Benenati S, Franchi F, Rollini F, Capodanno D, Biondi-Zoccai G, et al. Comparative effects of guided vs. potent P2Y12 inhibitor therapy in acute coronary syndrome: a network meta-analysis of 61 898 patients from 15 randomized trials. Eur Heart J. (2022) 43:959–67. doi: 10.1093/eurheartj/ehab836
105. Galli M, Franchi F, Rollini F, Angiolillo DJ. Role of platelet function and genetic testing in patients undergoing percutaneous coronary intervention. Trends Cardiovasc Med. (2021) doi: 10.1016/j.tcm.2021.12.007 [Epub ahead of print].
106. Galli M, Benenati S, Capodanno D, Franchi F, Rollini F, D’Amario D, et al. Guided versus standard antiplatelet therapy in patients undergoing percutaneous coronary intervention: a systematic review and meta-analysis. Lancet. (2021) 397:1470–83.
107. Kim BK, Hong SJ, Cho YH, Yun KH, Kim YH, Suh Y, et al. Effect of ticagrelor monotherapy vs ticagrelor with aspirin on major bleeding and cardiovascular events in patients with acute coronary syndrome: the TICO randomized clinical trial. JAMA. (2020) 323:2407–16.
108. Cuisset T, Deharo P, Quilici J, Johnson TW, Deffarges S, Bassez C, et al. Benefit of switching dual antiplatelet therapy after acute coronary syndrome: the TOPIC (timing of platelet inhibition after acute coronary syndrome) randomized study. Eur Heart J. (2017) 38:3070–8.
109. Sibbing D, Aradi D, Jacobshagen C, Gross L, Trenk D, Geisler T, et al. Guided de-escalation of antiplatelet treatment in patients with acute coronary syndrome undergoing percutaneous coronary intervention (TROPICAL-ACS): a randomised, open-label, multicentre trial. Lancet. (2017) 390:1747–57. doi: 10.1016/S0140-6736(17)32155-4
110. Cayla G, Cuisset T, Silvain J, Leclercq F, Manzo-Silberman S, Saint-Etienne C, et al. Platelet function monitoring to adjust antiplatelet therapy in elderly patients stented for an acute coronary syndrome (ANTARCTIC): an open-label, blinded-endpoint, randomised controlled superiority trial. Lancet. (2016) 388:2015–22. doi: 10.1016/S0140-6736(16)31323-X
111. Claassens DMF, Vos GJA, Bergmeijer TO, Hermanides RS, van ’t Hof AWJ, van der Harst P. A Genotype-Guided Strategy for Oral P2Y12 Inhibitors in Primary PCI. N Engl J Med. (2019) 381:1621–31.
112. Kim HS, Kang J, Hwang D, Han JK, Yang HM, Kang HJ, et al. Durable Polymer Versus Biodegradable Polymer Drug-Eluting Stents After Percutaneous Coronary Intervention in Patients with Acute Coronary Syndrome: The HOST-REDUCE-POLYTECH-ACS Trial. Circulation. (2021) 143:1081–91. doi: 10.1161/CIRCULATIONAHA.120.051700
113. Druss BG, Rosenheck RA. Association between use of unconventional therapies and conventional medical services. JAMA. (1999) 282:651–6.
114. Tesch BJ. Herbs commonly used by women: an evidence-based review. Am J Obstet Gynecol. (2003) 188(5 Suppl.):S44–55.
115. Volgman AS, Benjamin EJ, Curtis AB, Fang MC, Lindley KJ, Naccarelli GV, et al. Women and atrial fibrillation. J Cardiovasc Electrophysiol. (2021) 32:2793–807.
116. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomstrom-Lundqvist C, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. (2021) 42:373–498.
117. Linde C, Bongiorni MG, Birgersdotter-Green U, Curtis AB, Deisenhofer I, Furokawa T, et al. Sex differences in cardiac arrhythmia: a consensus document of the European Heart Rhythm Association, endorsed by the Heart Rhythm Society and Asia Pacific Heart Rhythm Society. Europace. (2018) 20:1565ao–1565ao. doi: 10.1093/europace/euy067
118. Thompson LE, Maddox TM, Lei L, Grunwald GK, Bradley SM, Peterson PN, et al. Sex Differences in the Use of Oral Anticoagulants for Atrial Fibrillation: a report from the national cardiovascular data registry (NCDR((R))) PINNACLE Registry. J Am Heart Assoc. (2017) 6:e005801. doi: 10.1161/JAHA.117.005801
119. Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. (2014) 383:955–62.
120. Frisullo G, Profice P, Brunetti V, Scala I, Bellavia S, Broccolini A, et al. Prospective Observational Study of Safety of Early Treatment with Edoxaban in Patients with Ischemic Stroke and Atrial Fibrillation (SATES Study). Brain Sci. (2020) 11:30. doi: 10.3390/brainsci11010030
121. Pancholy SB, Sharma PS, Pancholy DS, Patel TM, Callans DJ, Marchlinski FE. Meta-analysis of gender differences in residual stroke risk and major bleeding in patients with nonvalvular atrial fibrillation treated with oral anticoagulants. Am J Cardiol. (2014) 113:485–90. doi: 10.1016/j.amjcard.2013.10.035
122. Moseley A, Doukky R, Williams KA, Jaffer AK, Volgman AS. Indirect comparison of novel oral anticoagulants in women with nonvalvular atrial fibrillation. J Womens Health. (2017) 26:214–21. doi: 10.1089/jwh.2016.5892
123. Steffel J, Collins R, Antz M, Cornu P, Desteghe L, Haeusler KG, et al. 2021 European Heart Rhythm Association Practical Guide on the Use of Non-Vitamin K Antagonist Oral Anticoagulants in Patients with Atrial Fibrillation. Europace. (2021) 23:1612–76.
124. Holm A, Henriksson M, Alfredsson J, Janzon M, Johansson T, Swahn E, et al. Long term risk and costs of bleeding in men and women treated with triple antithrombotic therapy-An observational study. PLoS One. (2021) 16:e0248359. doi: 10.1371/journal.pone.0248359
125. Angiolillo DJ, Bhatt DL, Cannon CP, Eikelboom JW, Gibson CM, Goodman SG, et al. Antithrombotic Therapy in Patients With Atrial Fibrillation Treated With Oral Anticoagulation Undergoing Percutaneous Coronary Intervention: A North American Perspective: 2021 Update. Circulation. (2021) 143:583–96. doi: 10.1161/CIRCULATIONAHA.120.050438
126. Galli M, Andreotti F, Porto I, Crea F. Intracranial haemorrhages vs. stent thromboses with direct oral anticoagulant plus single antiplatelet agent or triple antithrombotic therapy: a meta-analysis of randomized trials in atrial fibrillation and percutaneous coronary intervention/acute coronary syndrome patients. Europace. (2020) 22:538–46.
127. De Caterina R, Agewall S, Andreotti F, Angiolillo DJ, Bhatt DL, Byrne RA, et al. Great Debate: Triple antithrombotic therapy in patients with atrial fibrillation undergoing coronary stenting should be limited to 1 week. Eur Heart J. (2022) 43:3512–27.
128. Adlam D, Alfonso F, Maas A, Vrints C, Writing C. European Society of Cardiology, acute cardiovascular care association, SCAD study group: a position paper on spontaneous coronary artery dissection. Eur Heart J. (2018) 39:3353–68. doi: 10.1093/eurheartj/ehy080
129. Cerrato E, Giacobbe F, Quadri G, Macaya F, Bianco M, Mori R, et al. Antiplatelet therapy in patients with conservatively managed spontaneous coronary artery dissection from the multicentre DISCO registry. Eur Heart J. (2021) 42:3161–71.
130. Garcia-Guimaraes M, Bastante T, Macaya F, Roura G, Sanz R, Barahona Alvarado JC, et al. Spontaneous coronary artery dissection in Spain: clinical and angiographic characteristics, management, and in-hospital events. Rev Esp Cardiol. (2021) 74:15–23.
131. Zucker I, Prendergast BJ, Beery AK. Pervasive Neglect of Sex Differences in Biomedical Research. Cold Spring Harb Perspect Biol. (2022) 14:a039156.
132. Becker JB, Prendergast BJ, Liang JW. Female rats are not more variable than male rats: a meta-analysis of neuroscience studies. Biol Sex Differ. (2016) 7:34.
133. Dauerman HL, Bhatt DL, Gretler DD, French PA, Smyth SS, Becker RC. Bridging the gap between clinical trials of antiplatelet therapies and applications among elderly patients. Am Heart J. (2010) 159:508–17.e1. doi: 10.1016/j.ahj.2010.01.010
134. Kim ES, Menon V. Status of women in cardiovascular clinical trials. Arterioscler Thromb Vasc Biol. (2009) 29:279–83.
135. Ding EL, Powe NR, Manson JE, Sherber NS, Braunstein JB. Sex differences in perceived risks, distrust, and willingness to participate in clinical trials: a randomized study of cardiovascular prevention trials. Arch Intern Med. (2007) 167:905–12. doi: 10.1001/archinte.167.9.905
136. Buch T, Moos K, Ferreira FM, Frohlich H, Gebhard C, Tresch A. Benefits of a factorial design focusing on inclusion of female and male animals in one experiment. J Mol Med. (2019) 97:871–7. doi: 10.1007/s00109-019-01774-0
137. Hilleary RS, Jabusch SM, Zheng B, Jiroutek MR, Carter CA. Gender disparities in patient education provided during patient visits with a diagnosis of coronary heart disease. Womens Health. (2019) 15:1745506519845591.
138. Vynckier P, Ferrannini G, Rydén L, Jankowski P, De Backer T, Gevaert S, et al. Gender gap in risk factor control of coronary patients far from closing: results from the European Society of Cardiology EUROASPIRE V registry. Eur J Prev Cardiol. (2022) 29:344–51. doi: 10.1093/eurjpc/zwaa144
140. Gimbel M, Qaderdan K, Willemsen L, Hermanides R, Bergmeijer T, de Vrey E, et al. Clopidogrel versus ticagrelor or prasugrel in patients aged 70 years or older with non-ST-elevation acute coronary syndrome (POPular AGE): the randomised, open-label, non-inferiority trial. Lancet. (2020) 395:1374–81.
141. Savonitto S, Ferri LA, Piatti L, Grosseto D, Piovaccari G, Morici N, et al. Comparison of Reduced-Dose Prasugrel and Standard-Dose Clopidogrel in Elderly Patients With Acute Coronary Syndromes Undergoing Early Percutaneous Revascularization. Circulation. (2018) 137:2435–45.
Keywords: antithrombotic therapy, antiplatelet therapy, anticoagulant therapy, ischemic heart disease, gender differences
Citation: Laborante R, Borovac JA, Galli M, Rodolico D, Ciliberti G, Restivo A, Cappannoli L, Arcudi A, Vergallo R, Zito A, Princi G, Leone AM, Aurigemma C, Romagnoli E, Montone RA, Burzotta F, Trani C and D’Amario D (2022) Gender-differences in antithrombotic therapy across the spectrum of ischemic heart disease: Time to tackle the Yentl syndrome? Front. Cardiovasc. Med. 9:1009475. doi: 10.3389/fcvm.2022.1009475
Received: 02 August 2022; Accepted: 14 October 2022;
Published: 31 October 2022.
Edited by:
Marina Panova-Noeva, Johannes Gutenberg University Mainz, GermanyReviewed by:
Wenqiang Xin, University Medical Center Göttingen, GermanyPaolo Severino, Sapienza University of Rome, Italy
Copyright © 2022 Laborante, Borovac, Galli, Rodolico, Ciliberti, Restivo, Cappannoli, Arcudi, Vergallo, Zito, Princi, Leone, Aurigemma, Romagnoli, Montone, Burzotta, Trani and D’Amario. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Domenico D’Amario, ZG9tZW5pY28uZGFtYXJpb0BnbWFpbC5jb20=
 Renzo Laborante
Renzo Laborante Josip Andjelo Borovac
Josip Andjelo Borovac Mattia Galli
Mattia Galli Daniele Rodolico
Daniele Rodolico Giuseppe Ciliberti
Giuseppe Ciliberti Attilio Restivo
Attilio Restivo Luigi Cappannoli
Luigi Cappannoli Alessandra Arcudi1
Alessandra Arcudi1 Andrea Zito
Andrea Zito Giuseppe Princi
Giuseppe Princi Antonio Maria Leone
Antonio Maria Leone Rocco Antonio Montone
Rocco Antonio Montone Domenico D’Amario
Domenico D’Amario