- 1Department of Cardiology, Sichuan Provincial People’s Hospital, University of Electronic Science and Technology of China, Chengdu, China
- 2Department of Cardiology, Chinese Academy of Sciences Sichuan Translational Medicine Research Hospital, Chengdu, China
- 3Department of Cardiology, Wenjiang District People’s Hospital of Chengdu, Chengdu, China
Premature ventricular contractions (PVCs) stemming from the aortic sinus cusp often have preferential conduction to two exits in the outflow tract and exhibited two different morphologies of PVCs, which may render radiofrequency catheter ablation (RFCA) difficult. A 67-year-old male patient underwent RACF for premature ventricular contractions (PVCs) characterizing by bi-morphology (left and right bundle branch block) on electrocardiogram. Dynamic changes in QRS morphology during ablation and evident local voltage potentials during electro-anatomical mapping were critical for identifying the real foci of origin of PVCs. Successful ablation was achieved at the left-right coronary cusp commissure.
Highlights
- Dynamic changes in QRS morphology of premature ventricular contractions (PVCs) during ablation and evident local voltage potentials during electro-anatomical mapping were critical for identifying the real foci of origin of PVCs.
- Premature ventricular contractions (PVCs) stemming from the left-right coronary cusp commissure often show preferential conduction to the RVOT.
- Bi-morphic QRS (LBBB and RBBB with an inferior axis) on ECG before the procedure may facilitate successful ablation of PVCs.
Introduction
Premature ventricular contractions (PVCs) and ventricular tachycardia (VT) have plentiful origins, which are widely distributed in both the right and left ventricles, including outflow tract, the vicinity of the tricuspid/mitral annulus and the His bundle, the papillary muscle, and so on (1). PVCs usually have one exit showing a single QRS morphology, but sometimes have multiple exit sites showing polymorphic QRS in both the right ventricular outflow tract (RVOT) and left ventricular outflow tract (LVOT) with preferential pathways (2–6). Variation of PVCs origins and morphology, especially dynamic changes in QRS during ablation, is a challenge to location and ablation of PVCs (3, 4, 7). In addition, a local voltage potential (LVP) preceding the onset of the surface QRS during PVCs, may help identify the origin of PVCs, according to a previous study (8). Here, we report a case of PVCs, whose successful ablation depended on dynamic alterations in QRS morphology during ablation and evident local voltage potentials during electro-anatomical mapping, originating from the left-right coronary cusp commissure (L-RCC).
Case report
The patient was a 67-year-old man with a 2-year history of shortness of breath and frequent palpitation while resting. And he had a history of hypertension and coronary heart disease. The symptoms could not be explained by these diseases, because that LV ejection fraction (69%) was kept within reasonable bounds, and local stenosis in the middle left anterior descending coronary artery was unchanged compared to 2 years ago. In addition, electrocardiogram (ECG) was normal during sinus rhythm. However, a serious of ECGs show frequent bi-morphic PVCs characterized by left bundle branch block (LBBB) and/or right bundle branch block (RBBB) in Figure 1, whose QRS wave were upright in leads II, III, and aVF (an inferior axis). The PVCs with inverted (LBBB) and upright (RBBB) QRS wave in lead V1, were defined as PVC-1 and PVC-2, respectively. The coexistence of PVC-1 and PVC-2 was reconfirmed in the preoperative 24 h of ambulatory Holter monitoring, and PVC burden was 22,915 (26.3%), which was mainly composed of PVC-1. The symptomatic PVCs were refractory to antiarrhythmic medications. Therefore, this patient was referred for radiofrequency catheter ablation (RFCA) of the PVCs.
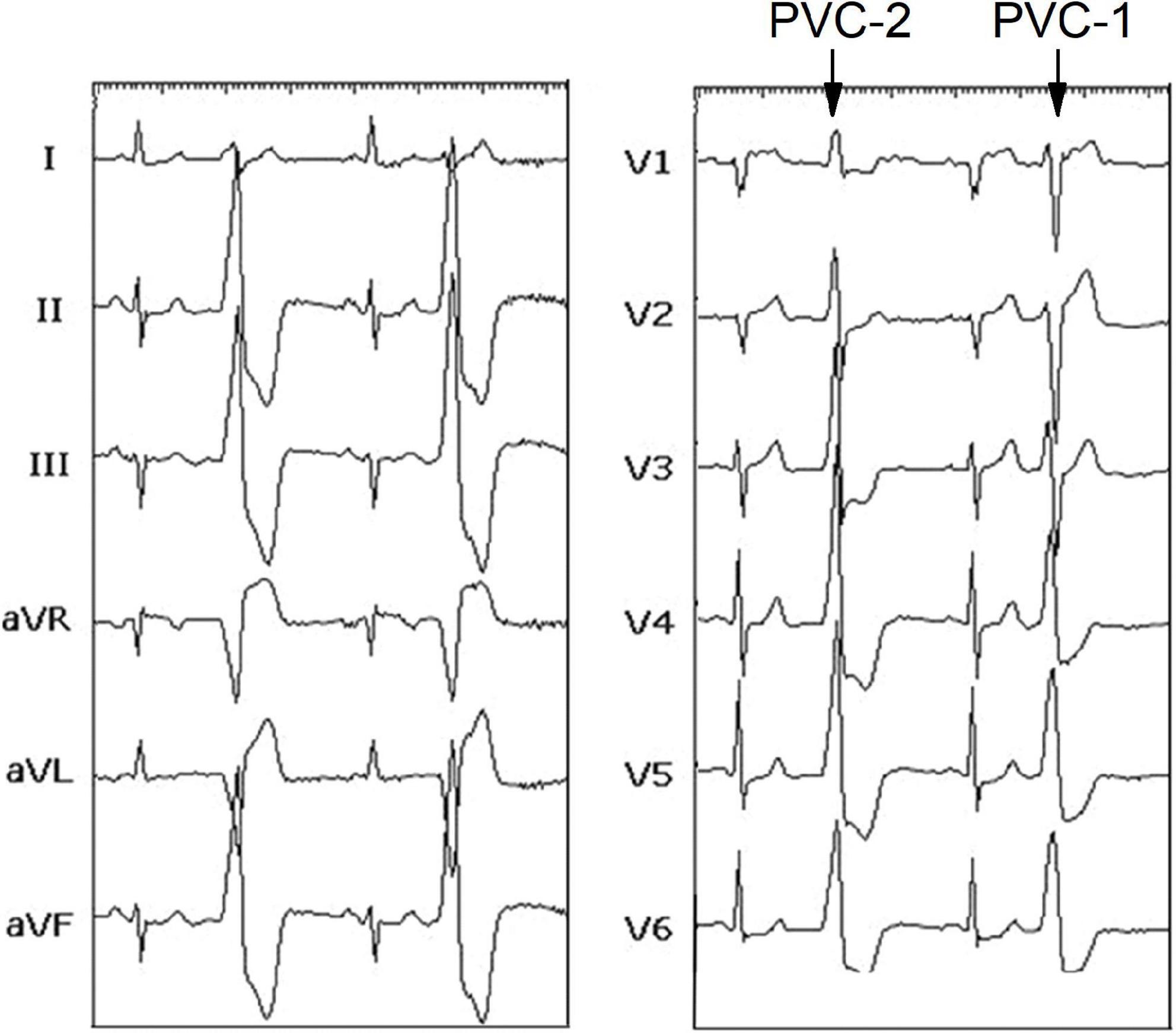
Figure 1. Twelve lead-ECG recorded before the procedure showing sinus rhythm, PVC-1, and PVC-2. ECG, electrocardiogram; PVC, premature ventricular contraction.
After informed written consent was given, the electrophysiologic study and RFCA for the PVCs were carried out using conventional fluoroscopically guided mapping, according to a previous report (3). The PVCs were featured with PVC-1 before the ablation procedure, suggesting that they originated from RVOT. Subsequently, electro-anatomic mapping (CARTO, Biosense Webster) of RVOT was performed using a 3.5-mm open irrigated tip (SmartTouch, Biosense Webster). The foci of origin of PVCs was determined using detailed activation and pace mapping. The site of earliest activation (−36 ms before the onset of the surface QRS), was distinguished, which was located in the anterior septum of RVOT (Figure 2A). Radiofrequency ablations (35 W, 17 ml/min, 2 min, 45°C) in this location reduced the occurrence of PVC-1, but the onset of PVC-2 shortly became frequent. Then, we performed electro-anatomical mapping in the aortic sinus cusp. The site of earliest activation (−41 ms before the onset of the surface QRS), was identified near the left coronary cusp (LCC) (Figure 2B). PVC-2 disappeared after similar ablation, but surprisingly, PVC-1 reappeared. However, Discrete and fragmented LVPs related to reappearing PVC-1 were observed near this ablation site, and then a systematical electro-anatomical mapping was again added to the aortic sinus cusp (ASC) based on previous research (3). The L-RCC was focused (−44 ms before the onset of the surface QRS) and PVC elimination was achieved after ablation in this location (Figure 2C). This patient recovered well and have been symptom-free for 3 months after discharge.
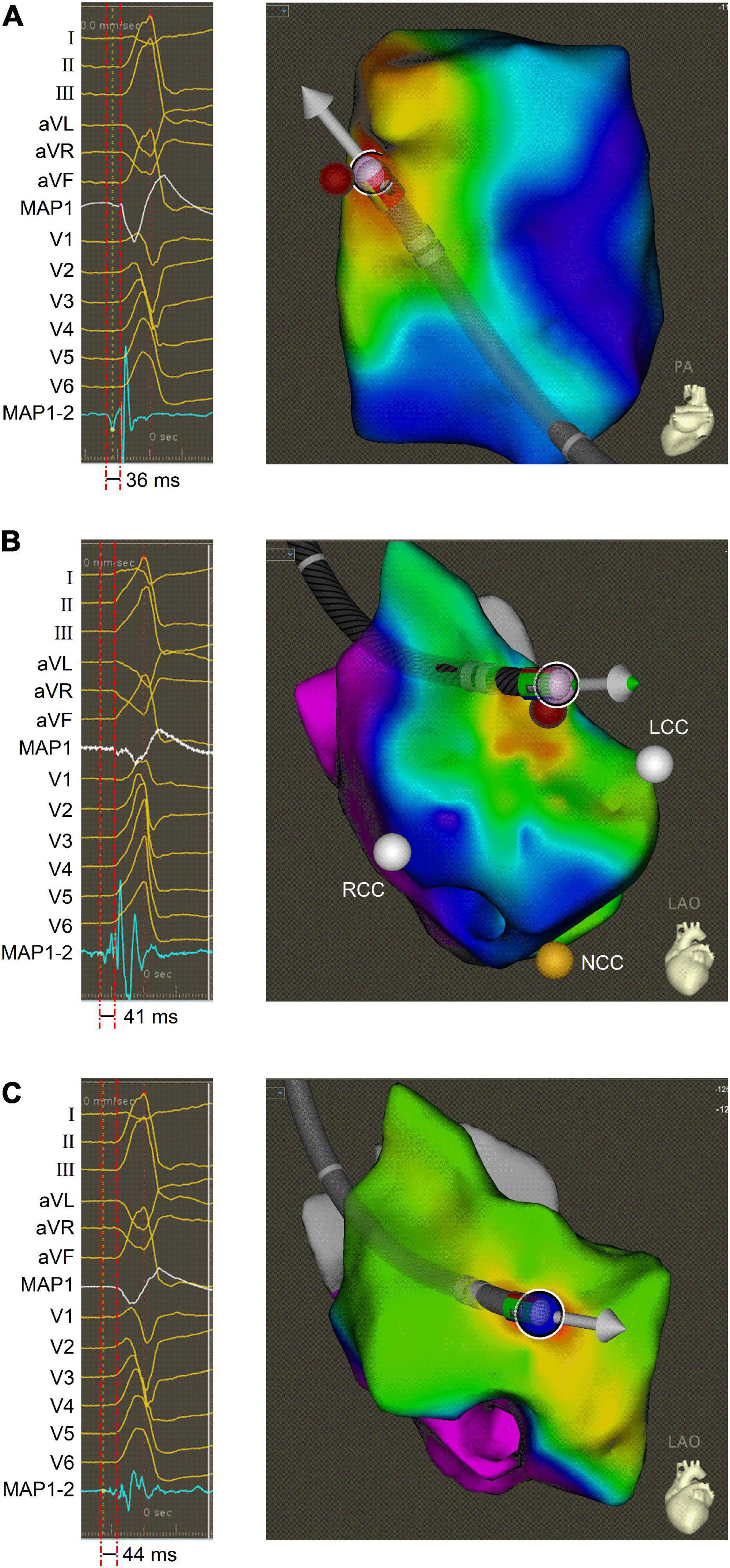
Figure 2. Activation mapping of PVCs. (A) Intracardiac ECG (left panel) and electro-anatomic map (right panel) obtained during activation mapping of PVC-1 in the anterior septum of RVOT. (B) Intracardiac ECG (left panel) and electro-anatomic map (right panel) obtained during activation mapping of PVC-2 near the LCC. (C) Intracardiac ECG (left panel) and electro-anatomic map (right panel) obtained during activation mapping of PVC-1 at the L-RCC. The bipolar ECG exhibited the LVP preceding the QRS onset, and electro-anatomic map showed ablation site in different electro-anatomic mapping areas. ECG, electrocardiogram; LCC, left coronary cusp; L-RCC, the left-right coronary cusp commissure; LVP, local voltage potential; MAP1, unipolar ECG; MAP1-2, bipolar ECG; RCC, right coronary cusp; PVC, premature ventricular contraction; RVOT, right ventricular outflow tract.
Discussion and conclusion
Premature ventricular contractions (PVCs) and ventricular tachycardia (VT) originating from the ASC, which are typically regarded as originating from the LCC, the L-RCC, or the right coronary cusp (RCC), often show preferential pathway to RVOT, resulting in a LBBB morphology on ECG (3). In our case, we present PVCs with two morphologies (LBBB and RBBB with an inferior axis) on ECG, whose successful ablation was achieved at the L-RCC. Importantly, the QRS morphology surprisingly changed twice during ablation, and obvious LVPs were recorded during mapping, which were crucial to determination the real site of origin of PVCs.
Despite alteration in QRS morphology during ablation has been previously reported (4, 7), the reappearance of the QRS morphology (PVC-1) related to LBBB during ablation, was firstly described in the current case. Our findings further supported the hypothesis that PVCs originating from the ASC often show preferential conduction to the RVOT, exhibiting more LBBB and less RBBB on ECG. Moreover, the sequential shift from PVC-1 to PVC-2 to PVC-1, especially the reappearance of PVC-1, indicated that preferential conduction may be functional.
It is reported that LVPs are recorded close to the site of origin of ventricular ectopy in the vast majority of patients with idiopathic outflow tract ectopy, and LVPs may reflect an area of depressed conductivity known to be a prerequisite for experimental ventricular ectopy (8). Similarly, discrete and fragmented LVPs provided clues for the final successful ablation foci in this case. Of note, LVPs represents the area of impaired and anisotropic conduction, which may be an explanation for preferential conduction.
The process of ablation was not smooth due to misinterpretation of the PVCs origin. After several electro-anatomical mappings, attempted ablation was delivered sequentially at the RVOT, the site near the LCC, and the L-RCC. Luckly, successful ablation was achieved at the L-RCC. However, two QRS morphologies of PVCs (LBBB and RBBB with an inferior axis) on ECG, which may feature two breakout sites and only a single origin, could be a predictor of PVCs originating from ASC, according to a preceding report (3). Thus, bi-morphic QRS may have important implications for RACF in the current case. Specifically, on condition that the ablation at the RVOT was unsuccessful, electro-anatomical mapping should be added to the ASC without hesitation, which may make the operation effortless and efficient.
In the present case, our results have important clinical implications. Firstly, dynamic changes in QRS morphology of PVCs during ablation and evident LVPs during electro-anatomical mapping indicate PVCs may originate from the ASC. Secondly, bi-morphic QRS (LBBB and RBBB with an inferior axis) on ECG before the procedure may facilitate successful ablation of PVCs.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Sichuan Provincial People’s Hospital. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
YC, YZ, and JL conceived the study. YC performed RACF. YZ collected ECG data and image data related to RACF. YC and YZ drafted the manuscript. JL, PZ, and TH checked the manuscript and performed critical revision. All authors approved the final version of the manuscript.
Funding
This study was funded by the Nature Science Foundation of Sichuan (2022NSFSC1589).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Kobayashi Y, Division OC, Tokai UH, Department OI. Idiopathic ventricular premature contraction and ventricular tachycardia: distribution of the origin, diagnostic algorithm, and catheter ablation. J Nippon Med Sch. (2018) 85:87–94. doi: 10.1272/jnms.2018_85-14
2. Sekihara T, Miyazaki S, Nagao M, Kakehashi S, Mukai M, Aoyama D, et al. A case of outflow tract premature ventricular contractions with very distant exit sites suspected to have a single origin. J Electrocardiol. (2020) 63:41–5. doi: 10.1016/j.jelectrocard.2020.09.015
3. Wang Y, Ma J, Dong J, Bai R, Wang J, Li S, et al. Catheter ablation of premature ventricular contractions originating in the aortic sinus cusp or great cardiac vein: two QRS morphologies with one origin. Pacing Clin Electrophysiol. (2015) 38:1029–38. doi: 10.1111/pace.12652
4. Andrea B, Richter S, Sommer P, Arya A. Changing QRS morphology during catheter ablation of outflow tract ventricular tachycardia: what is the mechanism? Europace. (2011) 13:444–6. doi: 10.1093/europace/euq401
5. Subramanian M, Yalagudri S, Saggu DK, Vignesh Rangaswamy V, Narasimhan C. PVCs with multiple exits and single site of origin in the outflow tract: what is the mechanism? Indian Pacing Electrophysiol J. (2021) 21:169–73. doi: 10.1016/j.ipej.2021.02.008
6. El Moheb MN, Refaat MM. Idiopathic right ventricular arrhythmias with changes in the QRS morphology after ablation. J Cardiovasc Electr. (2020) 31:2665–7. doi: 10.1111/jce.14656
7. Qifang L, Ye T, Zhi J, Jing H, Yidong Z, Long Y, et al. Variation of QRS morphology of premature ventricular contractions originate from the left-ventricular outflow tract during ablation. J Cardiovasc Electr. (2019) 30:2990–4. doi: 10.1111/jce.14228
Keywords: premature ventricular contractions, QRS morphology, local voltage potentials, ablation, bi-morphology
Citation: Chen Y, Zhu Y, Zhang P, He T and Liao J (2023) Successful ablation of morphology-changing premature ventricular contractions under the guidance of local voltage potentials: A case report. Front. Cardiovasc. Med. 9:1008380. doi: 10.3389/fcvm.2022.1008380
Received: 31 July 2022; Accepted: 29 December 2022;
Published: 11 January 2023.
Edited by:
Sergio Conti, A.R.N.A.S. Ospedali Civico Di Cristina Benfratelli, ItalyReviewed by:
Patrick Badertscher, University Hospital of Basel, SwitzerlandAndreas Rillig, University Medical Center Hamburg-Eppendorf, Germany
Copyright © 2023 Chen, Zhu, Zhang, He and Liao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jie Liao,  amllbGlhbzE1MTJAcXEuY29t, orcid.org/0000-0003-0774-6756
amllbGlhbzE1MTJAcXEuY29t, orcid.org/0000-0003-0774-6756
†These authors have contributed equally to this work
 Yang Chen1,2†
Yang Chen1,2† Yuncai Zhu
Yuncai Zhu Jie Liao
Jie Liao