
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cardiovasc. Med. , 10 November 2022
Sec. Coronary Artery Disease
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.1008194
This article is part of the Research Topic Precision Medicine for Antithrombotic Therapy in Patients after Percutaneous Coronary Interventions View all 10 articles
 Marie Muthspiel1*
Marie Muthspiel1* Christoph C. Kaufmann1
Christoph C. Kaufmann1 Achim Leo Burger1
Achim Leo Burger1 Benjamin Panzer1
Benjamin Panzer1 Freek W. A. Verheugt2
Freek W. A. Verheugt2 Kurt Huber1,3
Kurt Huber1,3Dual antiplatelet therapy (DAPT) for 6–12 months, followed by lifelong aspirin monotherapy is considered an effective standard therapy for the prevention of thrombo-ischemic events in patients with acute and chronic coronary syndrome (ACS, CCS) undergoing percutaneous coronary intervention (PCI) or after a primarily conservative treatment decision. In ACS patients, the stronger P2Y12-inhibitors ticagrelor or prasugrel are recommended in combination with aspirin unless the individual bleeding risk is high and shortening of DAPT is warranted or clopidogrel is preferred. However, also in patients at low individual bleeding risk, DAPT is associated with a higher risk of bleeding. In recent years, new antithrombotic treatment strategies, such as shortening DAPT followed by early P2Y12-inhibitor monotherapy and de-escalating DAPT from potent P2Y12-inhibitors to clopidogrel by maintaining DAPT duration time, have been investigated in clinical trials and shown to reduce bleeding complications in cardiovascular high-risk patients without negative effects on ischemic events. In this review, we summarize the current knowledge and discuss its implication on future antithrombotic strategies in terms of a personalized medicine.
Dual antiplatelet therapy (DAPT) is the cornerstone in the prevention of thrombo-ischemic events in patients with acute and chronic coronary syndrome (ACS, CCS) after primary percutaneous intervention (PCI). European Society of Cardiology (ESC) guidelines recommend a combination of aspirin with ticagrelor or with prasugrel for a period of 6 (CCS) to 12 (ACS) months, followed by a lifelong aspirin monotherapy in patients with low bleeding risk (Class Ia indication) (1, 2). DAPT duration should be adjusted to the individual patient’s bleeding risk using appropriate risk scores, such as the DAPT and the PRECISE-DAPT score. In patients at high bleeding risk (HBR), DAPT can be shortened (< 6 or < 12 months) by early withdrawal of the P2Y12-inhibitor (1, 2). In patients with high ischemic risk and without increased risk of major bleeding, DAPT can be extended (> 12 months) after ACS (1). However, standard DAPT has been shown to effectively reduce major adverse cardiovascular events (MACE) in patients after PCI but is associated with increased risk of bleeding (3). Accordingly, safe antiplatelet strategies reducing bleeding rates but without adverse effects on ischemic outcomes are mandatory. To address this issue, new antithrombotic treatment strategies for cardiovascular high-risk patients have been evolved and investigated in clinical trials in recent years. In this review, we focus on the current knowledge of short DAPT followed by early P2Y12-inhibitor monotherapy and on DAPT de-escalation from potent P2Y12-inhibitors to clopidogrel in terms of a personalized medicine (Figure 1).

Figure 1. Antithrombotic strategies of Standard DAPT, Short DAPT and DAPT De-escalation. DAPT: dual antiplatelet therapy, T: Ticagrelor, P: Prasugrel, C: Clopidogrel, ASA: Acetylsalicylic Acid.
According to ESC guidelines, short DAPT originally consists of early P2Y12-inhibitor withdrawal and subsequent lifelong aspirin monotherapy, as it should be considered for non-ST-elevation (NSTE)-ACS patients with stent implantation who are at high risk of bleeding (1). More recently, however, short DAPT refers to discontinuation of aspirin in favor of monotherapy with a strong oral P2Y12-inhibitor after an initial 1–3-month period of DAPT. This intriguing treatment approach presents a promising option to reduce bleeding risk in CCS and ACS patients after PCI and has recently been investigated in several trials (4–10).
Moreover, three meta-analyses demonstrated that withdrawal of aspirin in favor of P2Y12-inhibitor monotherapy after 1–3 months of DAPT significantly reduced the risk of major bleeding without increasing ischemic endpoints (11–13). In ACS patients, P2Y12-inhibitor monotherapy reduced bleeding risk by 50% (HR 0.50, 95% CI 0.41–0.61, p < 0.001) with no significant change in MACE rates when compared with standard DAPT (HR 0.85, 95% CI 0.70–1.0, p = 0.09) (12). Current data highlight the large contribution of aspirin to the bleeding risk of DAPT (13). However, it is important to note that between trials, patient populations differed in terms of their bleeding risk and selection of P2Y12-inhibitor.
Three large scaled randomized controlled trials (RCTs) provide 1-year data on clopidogrel monotherapy after short DAPT in patients undergoing PCI (Table 1) (4–6). In a population of CCS (58%) and ACS (42%) patients at low-to-moderate bleeding risk, the results of the Effect of P2Y12 Inhibitor Monotherapy vs. Dual Antiplatelet Therapy on Cardiovascular Events in Patients Undergoing Percutaneous Coronary Intervention (SMART-CHOICE) trial showed, that clopidogrel monotherapy after 3 months of DAPT was non-inferior to standard DAPT with respect to the primary ischemic endpoint (all-cause death, MI, or stroke) (95% CI –∞-1.3%, pnon–inferiority = 0.007) (4). In addition, the short DAPT strategy resulted in significantly lower bleeding rates [Bleeding Academic Research Consortium (BARC) 2–5] when compared with standard therapy (2.0% vs. 3.4%, HR 0.58, 95% CI 0.36–0.92, p = 0.002) (4).
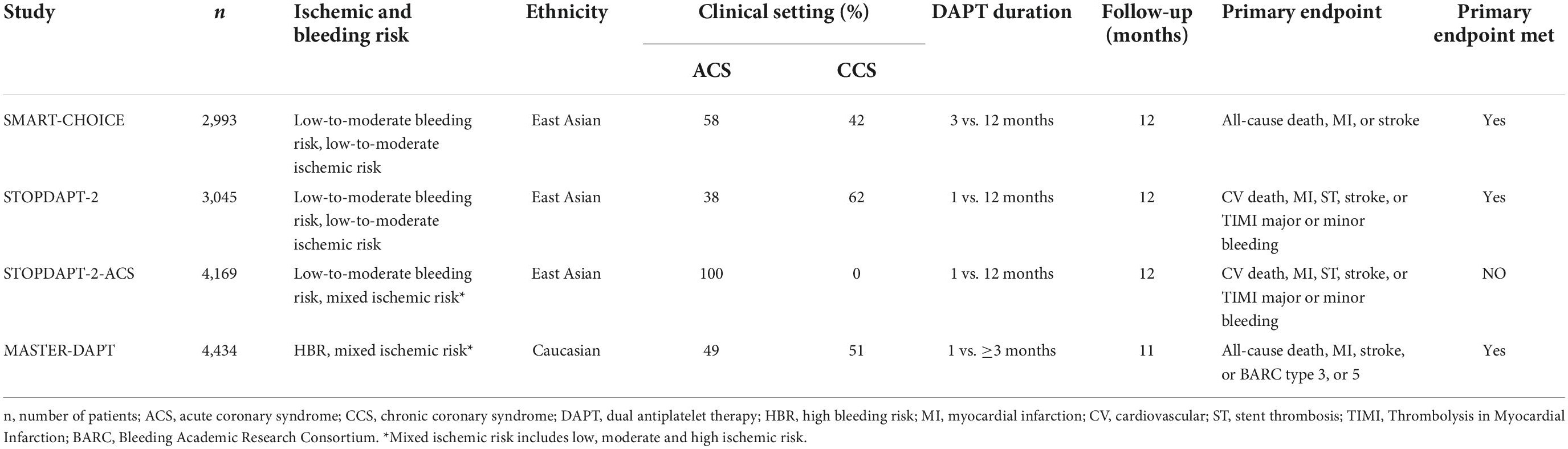
Table 1. Baseline characteristics of randomized-controlled trials on short dual antiplatelet therapy following clopidogrel monotherapy.
Moreover, the Japanese Results of the Effect of 1-month Dual Antiplatelet Therapy followed by Clopidogrel vs. 12-month Dual Antiplatelet Therapy on Cardiovascular and Bleeding Events in Patients receiving PCI (STOPDAPT-2) trial indicated that in ACS (38%) and CCS (62%) patients at low-to-moderate bleeding risk, clopidogrel monotherapy following an even shorter DAPT period of 1 month reduced bleeding rates [Thrombolysis in Myocardial Infarction (TIMI) major or minor bleedings] without causing a significant increase in primary combined endpoint event rates [cardiovascular (CV) death, myocardial infarction (MI), stent thrombosis (ST), stroke or TIMI major or minor bleeding] (HR 0.26, 95% CI 0.11–0.64, p = 0.004 and HR 0.64, 95% CI 0.42–0.98, p = 0.04, respectively) (5). While these data have shown safety for very early clopidogrel monotherapy in predominantly stable patients, the results of the STOPDAPT-2-ACS trial suggest, that this does not apply to unstable patients (6). This study, enrolling only ACS patients (n = 4,169), failed to meet their primary non-inferiority endpoint (of CV death, MI, ST, stroke or TIMI major or minor bleeding) at 12 months (HR 1.14, 95% CI 0.80–1.6, pnon–inferiority = 0.06) (6). Patients in the short DAPT group further presented a numerically but not significantly higher incidence of the major secondary cardiovascular endpoints than patients treated with standard DAPT (2.76% vs. 1.86%, HR 1.50 95% CI 0.99–2.26) (6).
The aforementioned trials show that clopidogrel monotherapy after short DAPT presents a safe therapeutic option to reduce bleeding rates in stable patients at low-to-moderate bleeding risk and mixed ischemic risk (includes low, moderate, and high ischemic risk). However, these data do not extend to patients in high-risk settings. In this regard, the High Bleeding Risk Patients Post Bioresorbable Polymer Coated Stent Implantation with an Abbreviated vs. Standard DAPT Regimen (MASTER-DAPT) trial was the first to selectively include patients at high bleeding risk, demonstrating that even in these patients, short DAPT followed by single antiplatelet therapy is a safe strategy to prevent bleeding after PCI (Table 1) (7). Specifically, 1-month DAPT proved non-inferior to standard DAPT in terms of the primary combined endpoint (all-cause death, MI, stroke, BARC type 3, or 5) and was associated (95% CI:-1.80 to 33, pnon–inferiority < 0.001) with a lower incidence of major or clinically relevant non-major bleedings (BARC type 2, 3, or 5) (6.5% vs. 9.11%, 95% CI:-4.40 to 1.24, pnon–inferiority < 0.001) at 11 months (7).
The GLOBAL LEADERS (Ticagrelor plus aspirin for 1 month, followed by ticagrelor monotherapy for 23 months vs. aspirin plus clopidogrel or ticagrelor for 12 months, followed by aspirin monotherapy for 12 months after implantation of a drug-eluting stent) trial compared 1-month DAPT followed by ticagrelor monotherapy with 12 months of DAPT after PCI. In this trial, patients at low bleeding risk and mixed ischemic risk were included (Table 2) (8). The trial failed to show that 23-month ticagrelor monotherapy after short DAPT was associated with lower primary endpoint events (all-cause death, MI) (RR 0.87, 95% CI 0.75–1.01, p = 0.073). However, non-inferiority was met and bleeding rates (BARC type 3, or 5) were similar between groups (2.04% vs. 2.12%, RR 0.97, 95% CI 0.78–1.20, p = 0.77) (8). Consistent findings with respect to the primary efficacy and safety endpoints were demonstrated in the prespecified GLOBAL LEADERS Adjudication Sub-Study (GLASSY) (9). Moreover, a 31% relative risk reduction in urgent target vessel revascularization (TVR) (1.87% vs. 2.72%, RR 0.69, 95% CI 0.51–0.93) was found in the experimental arm and shown to increase consistently over time (9). Short DAPT was further associated with lower rates of MI (RR 0.54, 95% CI 0.33–0.88, pinteraction = 0.062) and ST (RR 0.14, 95% CI 0.03–0.63; pinteraction = 0.007) at 12-month follow-up, indicating that ticagrelor monotherapy may have beneficial effects on the occurrence of MI and ST when compared with aspirin alone (9).
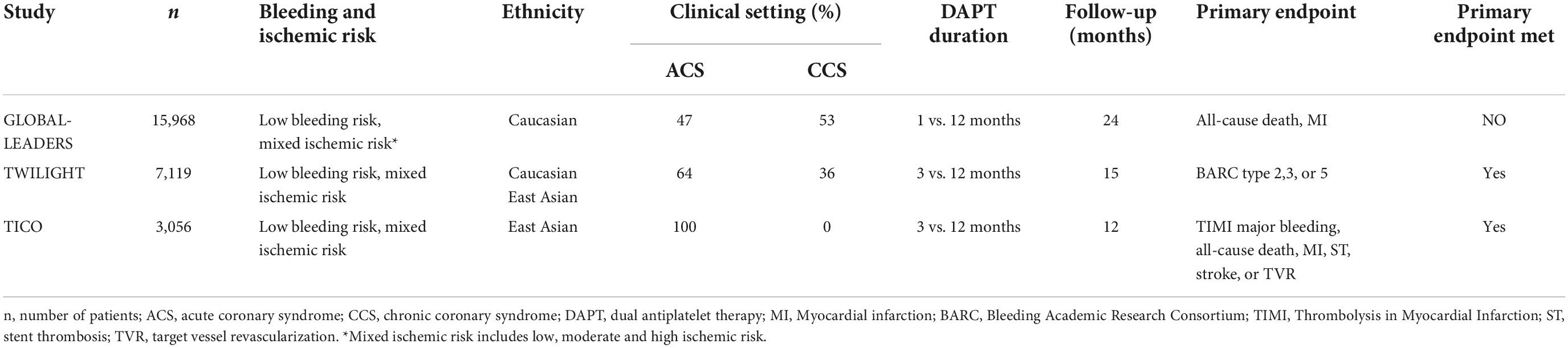
Table 2. Baseline characteristics of randomized-controlled trials on short DAPT following ticagrelor monotherapy.
In The Ticagrelor with or without Aspirin in High-Risk Patients after PCI (TWILIGHT) trial, 7,119 patients (64% ACS, 36% CCS) at low bleeding and mixed ischemic risk were enrolled (10). Ticagrelor monotherapy after DAPT of 3 months was associated with a 44% lower risk of bleeding (BARC type 2,3, or 5) than standard DAPT (HR 0.56, 95% CI 0.45–0.68, p < 0.001) with no significant increase in MACE (death, MI, stroke) (HR 0.99, 95% CI 0.78–1.25, pnon–inferiority < 0.001) over 15 months after PCI (10). Several prespecified subgroup-analyses (patients at HBR, ACS, complex PCI, diabetes, gender) demonstrated comparable outcomes (11–15).
Further, the Ticagrelor Monotherapy vs. Dual-Antiplatelet Therapy After PCI (SIDNEY) meta-analysis, including data from GLASSY and TWILIGHT, provides strong evidence for the reduction of bleeding rates with ticagrelor monotherapy (16).
Focusing on unstable patients after PCI, Franzone et al. demonstrated that safety effects of ticagrelor monotherapy after 1-month DAPT on ischemic endpoints were consistent in patients with or without ACS, but only ACS patients had a net clinical benefit in regards of a composite endpoint of both co-primary study endpoints from GLASSY (17). The South Korean Effect of Ticagrelor Monotherapy vs. Ticagrelor With Aspirin on Major Bleeding and Cardiovascular Events in Patients With Acute Coronary Syndrome (TICO) trial was the only trial to prospectively investigate ticagrelor monotherapy exclusively in ACS patients (18, 19). Switching to ticagrelor monotherapy after 3 months of DAPT significantly reduced primary adverse clinical events (TIMI major bleeding, all-cause death, MI, ST, stroke, TVR) (HR 0.66, 95% CI 0.48–0.92, p = 0.01) and was associated with lower risk of major bleeding (HR 0.56, 95% CI 0.34–0.91, p = 0.02) (18). Importantly, only patients at low bleeding risk were included in this trial. The results from the STOPDAPT-2-ACS and TICO trials suggest that ticagrelor but not clopidogrel monotherapy presents a safe antiplatelet treatment regimen for ACS patients after short DAPT. Therefore, it has been discussed whether clopidogrel monotherapy initiated 1 month after DAPT is less effective in ACS patients due to the increased ischemic risk up to 3 months post ACS and the lower P2Y12-inhibiting capacity of the agent.
The clinical benefit of prasugrel monotherapy in patients after PCI has not been sufficiently investigated to date. The Aspirin-free Prasugrel Monotherapy Following Coronary Artery Stenting in Patients with Stable CAD (ASET) study assessed prasugrel monotherapy (60 mg loading dose followed by 10 mg/day) after PCI in 202 CCS patients at low ischemic risk. Until PCI, patients received clopidogrel-based DAPT. At 3 months of follow-up, there was no primary endpoint event and only one fatal intracranial hemorrhage 6 h after PCI (20).
Several ongoing trials are addressing the above-mentioned issues through different approaches.
The Ticagrelor Monotherapy in Patients Treated With New-generation Drug-eluting Stents for Acute Coronary Syndrome (T-PASS) (NCT03797651) trial evaluates ticagrelor monotherapy following very-short DAPT less than 1 month after PCI in ACS patients.
Results from the A Randomized Comparison of Clopidogrel Monotherapy vs. Extended Dual-antiplatelet Therapy Beyond 12 Months After Implantation of Drug-eluting Stents in High-risk Lesions or Patients trial (A-CLOSE) (NCT03947229) are expected at the end of 2023, investigating clopidogrel monotherapy vs. extended clopidogrel-based DAPT from 12 to 36 months after PCI in patients at high risk for either ischemic or bleeding complications.
The P2Y12-Inhibitor Monotherapy vs. Extended DAPT in Patients Treated With Bioresorbable Scaffold trial (SMART-CHOICE II) (NCT03119012) currently compares clopidogrel or ticagrelor monotherapy from 12 to 36 months after PCI with extended ticagrelor-based DAPT for 36 months after PCI. Long-term clopidogrel monotherapy vs. aspirin monotherapy after 12 months of DAPT is currently being investigated in patients at high risk for recurrent ischemic events in The Choice of Optimal Anti-Thrombotic Strategy in Patients Undergoing Implantation of Coronary Drug-Eluting Stents 3 trial (SMART-CHOICE III) (NCT04418479).
Further data on prasugrel monotherapy in CCS and non-STE elevation ACS (NSTE-ACS) patients are expected from the still ongoing Acetyl Salicylic Elimination Trial JAPAN (ASET-JAPAN pilot study) (NCT 05117866) in 2024.
The benefit of potent P2Y12-inhibitors regarding ischemic risk reduction is greatest during the acute and sub-acute phase after the index event whilst bleeding risk persists during maintenance therapy (21, 22). Hence, in a significant amount (up to 28%) of ACS patients, physicians tend to switch from standard DAPT by using ticagrelor or prasugrel in association with aspirin to clopidogrel and aspirin within 1 year after PCI (23). Besides economic factors, bleeding complications are the most common reason for a so-called DAPT de-escalation (24). Clinical data justifying this strategy have long been limited. However, in recent years, different studies have provided data on the safety and efficacy of switching to clopidogrel after a short period of DAPT with potent P2Y12-inhibitors in ACS patients (Table 3) (25–28).
The monocentric, randomized, open-label Timing of Platelet Inhibition After Acute Coronary Syndrome (TOPIC) trial investigated DAPT de-escalation in 646 ST-elevation myocardial infarction (STEMI) and non-ST-elevation myocardial infarction (NSTEMI) patients at low-to-moderate bleeding risk (26). At 1 month after PCI, patients in the experimental group were switched to clopidogrel-based DAPT while standard DAPT was maintained in the control group. At 12 months after the index event, the de-escalation strategy was superior to standard DAPT in terms of the primary combined endpoint (CV death, urgent revascularization, stroke, BARC type ≥ 2) (HR 0.48 95% CI 0.34–0.68, p < 0.01) and was further associated with a lower risk of bleeding (BARC type ≥ 2) (HR 0.30, 95% CI 0.18–0.50, p < 0.01) (26).
Similarly, results of the multicentric, randomized Ticagrelor vs. Clopidogrel in Stabilized Patients with Acute Myocardial Infarction (TALOS-AMI) study have shown that unguided DAPT de-escalation was associated with a 45% lower risk of net clinical events (CV death, MI, stroke, BARC type 2,3,5) (HR 0.55, 95% CI 0.40–0.76, pnon–inferiority < 0.001) and with reduced risk of bleeding complications (BARC type 2,3, or 5) (HR 0.52, 95% CI 0.35–0.77, p = 0.0012) when compared with ticagrelor-based DAPT at 12 months after PCI (27). However, only South Korean STEMI and NSTEMI patients at low-to-moderate bleeding risk were included. The validity of these data must therefore be qualified for Caucasian populations due to the higher prevalence of CYP2C19 loss-of-function alleles in the East Asian population (29).
Recently, two large-scaled RCTs provided data on clopidogrel-based DAPT in Caucasian ACS patients (25, 28). Both trials performed platelet function- and genetic-directed de-escalation, respectively. In the Testing Responsiveness to Platelet Inhibition On Chronic Antiplatelet Treatment For Acute Coronary Syndromes (TROPICAL-ACS) trial, 2,610 STEMI and NSTEMI patients after PCI were treated with standard therapy consisting of prasugrel and aspirin (25). Seven days after discharge, patients randomized to the de-escalation group were switched to clopidogrel for another 7 days whereas standard DAPT was maintained in the control group. Platelet Function Testing (PFT) was performed on day 14 to identify clopidogrel non- or low-responder patients and readjust them to prasugrel. At 12 months follow-up, clopidogrel-based DAPT was not inferior to standard DAPT in terms of the primary combined endpoint (CV death, MI, stroke, BARC type ≥ 2) (HR 0.81, 95% CI 0.62–1.06, pnon–inferiority = 0.0004) (25). Despite a trend toward lower bleeding risk in the de-escalation group, bleeding rates (BARC type ≥ 2) did not significantly differ between groups (5% vs. 6%, HR 0.82, 95% CI 0.59–1.13, p = 0.23) (25).
In the Genotype-Guided Strategy for Oral P2Y12 Inhibitors in Primary PCI (POPULAR GENETICS) trial CYP2C19-directed genetic testing was used as safety tool in 2488 STEMI patients (28). Within 3 days after PCI, non-carriers received clopidogrel-based DAPT whereas carriers of CYP2C19*2 or CYP2C19*3 LOF-alleles received standard DAPT with prasugrel or ticagrelor (28). The results demonstrated non-inferiority of guided de-escalation in terms of net clinical events (all-cause death, MI, ST, stroke, Platelet Inhibition and Patient Outcomes (PLATO) major bleeding) (95% CI 2.0–0.7, pnon–inferiority < 0.001) as well as significantly lower bleeding rates (PLATO major or minor bleeding) compared to standard DAPT at 12-month follow-up (HR 0.78, 95% CI 0.61–0.98, p = 0.04) (28). However, it should be noted that patients enrolled in the TROPICAL-ACS and POPULAR GENETICS trials only had low-to-moderate bleeding and mixed ischemic risk.
Finally, two recent meta-analyses demonstrated overall efficacy and safety of DAPT de-escalation (30, 31). In one study, guided selection of antiplatelet therapy after PCI was shown to reduce the risk of MACE (RR 0.78, 95% CI 0.63–0.95, p = 0.015) without any trade-off in bleeding rates when compared with standard DAPT (RR 0.88, 95% CI 0.77–1.01, p = 0.069) (30). Importantly, this analysis included studies that investigated both de-escalation and escalation strategies in ACS and CCS patients. The other meta-analysis by Tavenier et al. exclusively focused on RCTs that studied DAPT de-escalation in ACS patients (31). The results demonstrated that a strategy of de-escalation vs. standard DAPT reduces both clinically relevant bleedings (BARC type ≥ 2) (HR 0.57, 95% CI 0.42–0.78) and MACE rates (HR 0.77, 95% CI 0.62–0.96) (31).
When using clopidogrel, interpatient variability in antithrombotic efficacy must be considered (32, 33). Genetic and metabolic factors influence pharmacologic response to clopidogrel resulting in increased ischemic risk in certain patients (32). In the TROPICAL-ACS and POPULAR GENETICS trials, PFT and genetic testing were used as safety tools, whereas unguided de-escalation was performed in the TOPIC and TALOS-AMI trials. According to ESC guidelines, routine use of PFT or genetic testing in the selection of antiplatelet therapy is not recommended. When de-escalation to clopidogrel is performed, the strategy (guided vs. unguided) should be determined based on the patient’s risk profile and the availability of respective assays (1). However, guidelines do not specify in which patients a guided approach should be considered, leaving this decision to the treating physician. In this regard, an expert consensus from 2019 provides more detailed recommendations (34). Unfortunately results from the POPULAR GENETICS trial and from more recent meta-analyses were not included at this time but are considered in the present work.
Tavenier et al. demonstrated that significant bleeding risk reduction was consistent in both guided (HR 0.79, 95% CI 0.66–0.94) and unguided (HR 0.44, 95% CI 0.32–0.59) de-escalation (31). Interestingly, unguided de-escalation was associated with a greater reduction of bleeding risk (pinteraction = 0.037) when compared with guided de-escalation (31). However, the authors noted that the bleeding benefit may be explained by the fact that the proportion of patients who received clopidogrel was higher in the unguided than in the guided de-escalation group (31).
If PFT or genetic testing is considered, limitations of the respective test methods must be taken into account (Table 4). PFT results vary significantly depending on the different assays available (VerifyNow, Multiplate, VASP, TEG platelet mapping), which not only makes it difficult to compare data from different studies but may also influence clinical decisions (35). Conversely, there are no relevant discrepancies between validated genetic assays (32). Since PFT can only be performed on-treatment, initial clopidogrel therapy and, if needed, subsequent medication adjustment is required (28). This could affect patient compliance.
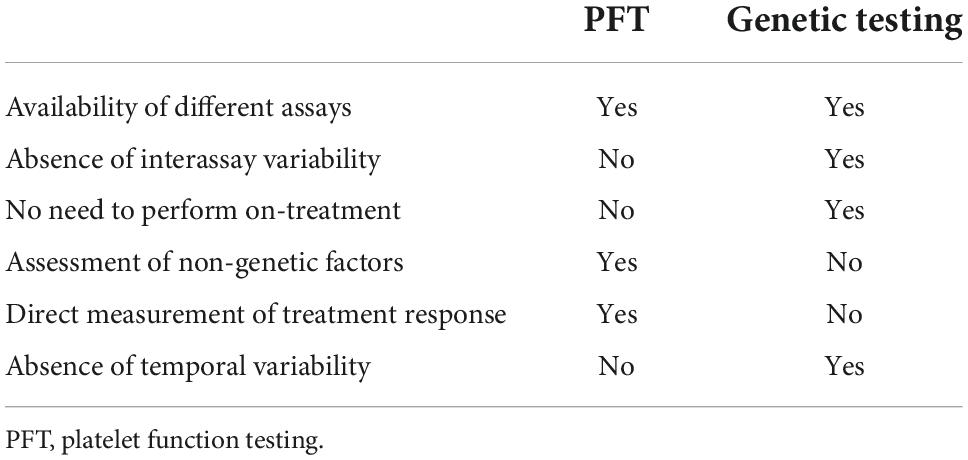
Table 4. Advantages and disadvantages of platelet function vs. CYP2C19-directed genetic testing, modified after Sibbing et al. (34).
In the TROPICAL-ACS trial, patients received clopidogrel treatment from days 7 to 14 after hospital discharge (25). Given the increased ischemic risk in the acute and subacute phase after the index event, early clopidogrel therapy may not guarantee adequate platelet inhibition in patients on high on-treatment platelet reactivity (HPR) (24, 36).
CYP2C19 genetic testing, as performed in the POPULAR GENETICS trial, does not require clopidogrel treatment. However, it does not directly reflect treatment response as various intrinsic and extrinsic factors that influence clopidogrel efficacy (BMI ≥ 30 kg/m2, diabetes mellitus, gastrointestinal absorption, drug interactions, patient adherence) are not taken into account (37, 38). It should also be noted that a single platelet function test only reflects the current status of response to treatment, and the optimal timing of measurement is unknown (34).
In this regard, a pre-specified TROPICAL-ACS sub-study showed that platelet reactivity during clopidogrel therapy is subject to diurnal variability, with a peak in platelet reactivity at the end of the dosing interval (39). However, clinical outcomes of these findings have not been investigated. Since these epigenetic factors vary over time, it could be questioned whether a single measurement is sufficient or whether PFT should be repeated during maintenance therapy.
To summarize, one meta-analysis provides data suggesting a greater reduction in bleeding risk with unguided vs. guided de-escalation and similar ischemic risk reduction with both strategies (31). Current knowledge does not show superiority of specific assays (PFT vs. CYP2C19 genetic testing) and therefore respective limitations should be considered when de-escalation to clopidogrel is performed.
Both withdrawal of aspirin 1–3 months after PCI with continued use of P2Y12-inhibitor monotherapy and de-escalation of P2Y12-inhibitor therapy by switching from more potent inhibitors to clopidogrel reduce bleeding risk without any trade-off in MACE when compared with standard DAPT. These findings now raise the question of which patient populations may benefit from a personalized antiplatelet strategy and how early aspirin should be discontinued or ticagrelor or prasugrel replaced with clopidogrel.
Based on the positive results of the TROPICAL-ACS trial, ESC Guidelines recommend guided or unguided de-escalation as an alternative treatment regimen in ACS patients who are not suitable for 12 months potent platelet inhibition (Class IIb indication) (25). However, guidelines do not comment on the timing of DAPT de-escalation. Trials investigating guided de-escalation (TROPICAL-ACS, POPULAR GENETICS) switched to clopidogrel maintenance therapy within 14 days after PCI (25, 28). Unguided de-escalation was performed at 1 month after PCI in the TOPIC and TALOS-AMI trials (26, 27). Given the highest ischemic risk in the first month after the index event, the timing of unguided de-escalation seems to be appropriate to prevent recurrence of ischemic events (24, 36). A guided approach, in turn, allows the identification of non- or low-responder patients at high ischemic risk at an early stage and seems to justify the timing of de-escalation. The above-mentioned trials have shown short-term safety of DAPT de-escalation for ACS patients at low-to-moderate bleeding risk but clinical outcomes beyond 1 year have not been investigated yet. Further, the studies were not adequately powered with respect to primary ischemic endpoints. Finally, it must be noted that patients at high thrombotic risk (HTR) may not have been adequately enrolled and the results may apply only to populations with balanced ischemic risk. To the best of our knowledge, no ongoing trials are currently addressing these issues.
Results on short DAPT had already an impact on recent guidelines recommending early P2Y12-inhibitor monotherapy in certain patient populations (40).
The MASTER-DAPT trial has shown that also patients at high bleeding risk are suitable for clopidogrel monotherapy after short DAPT. However, certain patient populations, like elderly patients, were underrepresented in the above-mentioned trials. This population, which bears an increased bleeding risk in itself, might be suitable for short DAPT or de-escalation as recently discussed in an editorial of the European Heart Journal (41).
While trials have provided evidence on patients with low-to-moderate and high bleeding risk, no clear conclusion can be drawn from existing data for the treatment of patients at high thrombotic risk. This could be due to inclusion bias in the existing studies, as HTR patients are not represented in sufficient numbers.
Ticagrelor but not clopidogrel monotherapy after very short DAPT has been shown to be a safe strategy to treat ACS patients. Therefore, it has been discussed whether clopidogrel monotherapy initiated 1 month after DAPT is less effective due to the increased ischemic risk in the early phase after ACS and the lower P2Y12-inhibitory capacity of the agent. Here, DAPT of up to 3 months, measurement of response to clopidogrel, or switching to a more potent P2Y12-inhibitor as monotherapy (theoretically, as not yet tested) might be useful to keep the rate of ischemic events low. Nevertheless, existing data only apply for ACS patients at low-to-moderate bleeding risk since ACS patients at HBR were not included in previous trials.
It is currently unclear whether P2Y12–inhibitor monotherapy should be prolonged (which was done for 24 months in the GLOBAL LEADERS trial), switched back to aspirin lifelong after 12 months (as done in TWILIGHT and TICO), or switched to clopidogrel lifelong, as shown in a recent study (42). In the Aspirin vs. Clopidogrel for Chronic Maintenance Monotherapy after Percutaneous Coronary Intervention (HOST-EXAM) trial, more than 5,000 patients after PCI, who received 6–18 months of DAPT, were investigated (42). Subsequent clopidogrel monotherapy resulted in a significant reduction in the net clinical endpoint (death, MI, insult, ACS, BARC type 3, or 5) when compared with aspirin monotherapy at 24 months (HR = 0.73, 95% CI: 0.59–0.90, p = 0.0035) (42).
The use of short DAPT and DAPT de-escalation is currently limited to HBR patients, while patients at normal-to-low bleeding risk usually continue to be treated with standard DAPT. To determine a tailored antiplatelet regimen that strikes the balance between bleeding risk reduction and prevention of recurrent ischemic events, several trials have shown efficacy and safety of DAPT de-escalation and P2Y12-inhibitor monotherapy after short DAPT. However, based on inclusion bias, patients at very high thrombotic risk only represent a low percentage in the aforementioned trials and elderly patients have not been included in sufficient numbers. Especially, for elderly with no additional risk factors other than their age, the new antiplatelet strategies of short DAPT and DAPT de-escalation might be of interest for future clinical practice. Prospective RCTs in specific patient groups and long-term safety data regarding hard ischemic endpoints are pending which still limits broad use of short DAPT and DAPT de-escalation in patients after PCI. Finally, personalized antiplatelet treatment should equally consider the patient’s ischemic and bleeding risk and in case clopidogrel is used, potential interindividual differences in platelet responsiveness must always be taken into account.
MM and KH worte the manuscript. All authors contributed to the article and approved the submitted version.
Author KH received lecture fees from AstraZeneca, Bayer, Daiichi Sankyo, and Sanofi Aventis. Author FV received honoraria for consulting and presentations from AstraZeneca, Bayer Healthcare, and Daiichi-Sankyo.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Collet JP, Thiele H, Barbato E, Barthélémy O, Bauersachs J, Bhatt DL, et al. 2020 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. (2021) 42:1289–367. doi: 10.1093/eurheartj/ehaa575
2. Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, et al. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J. (2019) 40:87–165. doi: 10.1093/eurheartj/ehy394
3. Navarese EP, Andreotti F, Schulze V, Kołodziejczak M, Buffon A, Brouwer M, et al. Optimal duration of dual antiplatelet therapy after percutaneous coronary intervention with drug eluting stents: meta-analysis of randomised controlled trials. BMJ. (2015) 350:h1618. doi: 10.1136/bmj.h1618
4. Hahn JY, Song YB, Oh JH, Chun WJ, Park YH, Jang WJ, et al. Effect of P2y12 inhibitor monotherapy vs dual antiplatelet therapy on cardiovascular events in patients undergoing percutaneous coronary intervention: the smart-choice randomized clinical trial. JAMA. (2019) 321:2428–37. doi: 10.1001/jama.2019.8146
5. Watanabe H, Domei T, Morimoto T, Natsuaki M, Shiomi H, Toyota T, et al. Effect of 1-month dual antiplatelet therapy followed by clopidogrel vs 12-month dual antiplatelet therapy on cardiovascular and bleeding events in patients receiving PCI: the stopdapt-2 randomized clinical trial. JAMA. (2019) 321:2414–27. doi: 10.1001/jama.2019.8145
6. Watanabe H, Morimoto T, Natsuaki M, Yamamoto K, Obayashi Y, Ogita M, et al. Comparison of clopidogrel monotherapy after 1 to 2 months of dual antiplatelet therapy with 12 months of dual antiplatelet therapy in patients with acute coronary syndrome: the stopdapt-2 ACS randomized clinical trial. JAMA Cardiol. (2022) 7:407–17. doi: 10.1001/jamacardio.2021.5244
7. Valgimigli M, Frigoli E, Heg D, Tijssen J, Jüni P, Vranckx P, et al. Dual antiplatelet therapy after PCI in patients at high bleeding risk. N Engl J Med. (2021) 385:1643–55. doi: 10.1056/NEJMoa2108749
8. Vranckx P, Valgimigli M, Jüni P, Hamm C, Steg PG, Heg D, et al. Ticagrelor plus aspirin for 1 month, followed by ticagrelor monotherapy for 23 months vs aspirin plus clopidogrel or ticagrelor for 12 months, followed by aspirin monotherapy for 12 months after implantation of a drug-eluting stent: a multicentre, open-label, randomised superiority trial. Lancet. (2018) 392:940–9. doi: 10.1016/s0140-673631858-0
9. Franzone A, McFadden E, Leonardi S, Piccolo R, Vranckx P, Serruys PW, et al. Ticagrelor alone versus dual antiplatelet therapy from 1 month after drug-eluting coronary stenting. J Am Coll Cardiol. (2019) 74:2223–34. doi: 10.1016/j.jacc.2019.08.1038
10. Mehran R, Baber U, Sharma SK, Cohen DJ, Angiolillo DJ, Briguori C, et al. Ticagrelor with or without aspirin in high-risk patients after PCI. N Engl J Med. (2019) 381:2032–42. doi: 10.1056/NEJMoa1908419
11. Angiolillo DJ, Baber U, Sartori S, Briguori C, Dangas G, Cohen DJ, et al. Ticagrelor with or without aspirin in high-risk patients with diabetes mellitus undergoing percutaneous coronary intervention. J Am Coll Cardiol. (2020) 75:2403–13. doi: 10.1016/j.jacc.2020.03.008
12. Vogel B, Baber U, Cohen DJ, Sartori S, Sharma SK, Angiolillo DJ, et al. Sex differences among patients with high risk receiving ticagrelor with or without aspirin after percutaneous coronary intervention: a subgroup analysis of the twilight randomized clinical trial. JAMA Cardiol. (2021) 6:1032–41. doi: 10.1001/jamacardio.2021.1720
13. Baber U, Dangas G, Angiolillo DJ, Cohen DJ, Sharma SK, Nicolas J, et al. Ticagrelor alone vs. ticagrelor plus aspirin following percutaneous coronary intervention in patients with non-ST-segment elevation acute coronary syndromes: TWILIGHT-ACS. Eur Heart J. (2020) 41:3533–45. doi: 10.1093/eurheartj/ehaa670
14. Dangas G, Baber U, Sharma S, Giustino G, Mehta S, Cohen DJ, et al. Ticagrelor with or without aspirin after complex PCI. J Am Coll Cardiol. (2020) 75:2414–24. doi: 10.1016/j.jacc.2020.03.011
15. Escaned J, Cao D, Baber U, Nicolas J, Sartori S, Zhang Z, et al. Ticagrelor monotherapy in patients at high bleeding risk undergoing percutaneous coronary intervention: twilight-HBR. Eur Heart J. (2021) 42:4624–34. doi: 10.1093/eurheartj/ehab702
16. Valgimigli M, Mehran R, Franzone A, da Costa BR, Baber U, Piccolo R, et al. Ticagrelor monotherapy versus dual-antiplatelet therapy after PCI: an individual patient-level meta-analysis. JACC Cardiovasc Interv. (2021) 14:444–56. doi: 10.1016/j.jcin.2020.11.046
17. Franzone A, McFadden EP, Leonardi S, Piccolo R, Vranckx P, Serruys PW, et al. Ticagrelor alone or conventional dual antiplatelet therapy in patients with stable or acute coronary syndromes. Eurointervention. (2020) 16:627–33. doi: 10.4244/eij-d-20-00145
18. Kim BK, Hong SJ, Cho YH, Yun KH, Kim YH, Suh Y, et al. Effect of ticagrelor monotherapy vs ticagrelor with aspirin on major bleeding and cardiovascular events in patients with acute coronary syndrome: the TICO randomized clinical trial. JAMA. (2020) 323:2407–16. doi: 10.1001/jama.2020.7580
19. Verheugt FWA, Huber K, Clemmensen P, Collet J-P, Cuisset T, Andreotti F. Platelet P2y12 inhibitor monotherapy after percutaneous coronary intervention. J Thromb Haemost. (in press) (2022).
20. Kogame N, Guimarães PO, Modolo R, De Martino F, Tinoco J, Ribeiro EE, et al. Aspirin-free prasugrel monotherapy following coronary artery stenting in patients with stable cad: the ASET pilot study. JACC Cardiovasc Interv. (2020) 13:2251–62. doi: 10.1016/j.jcin.2020.06.023
21. Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. (2007) 357:2001–15. doi: 10.1056/NEJMoa0706482
22. Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. (2009) 361:1045–57. doi: 10.1056/NEJMoa0904327
23. Zettler ME, Peterson ED, McCoy LA, Effron MB, Anstrom KJ, Henry TD, et al. Switching of adenosine diphosphate receptor inhibitor after hospital discharge among myocardial infarction patients: insights from the treatment with adenosine diphosphate receptor inhibitors: longitudinal assessment of treatment patterns and events after acute coronary syndrome (TRANSLATE-ACS) observational study. Am Heart J. (2017) 183:62–8. doi: 10.1016/j.ahj.2016.10.006
24. Antman EM, Wiviott SD, Murphy SA, Voitk J, Hasin Y, Widimsky P, et al. Early and late benefits of prasugrel in patients with acute coronary syndromes undergoing percutaneous coronary intervention: a TRITON-TIMI 38 (trial to assess improvement in therapeutic outcomes by optimizing platelet inhibition with prasugrel-thrombolysis in myocardial infarction) analysis. J Am Coll Cardiol. (2008) 51:2028–33. doi: 10.1016/j.jacc.2008.04.002
25. Sibbing D, Aradi D, Jacobshagen C, Gross L, Trenk D, Geisler T, et al. Guided de-escalation of antiplatelet treatment in patients with acute coronary syndrome undergoing percutaneous coronary intervention (TROPICAL-ACS): a randomised, open-label, multicentre trial. Lancet. (2017) 390:1747–57. doi: 10.1016/s0140-673632155-4
26. Cuisset T, Deharo P, Quilici J, Johnson TW, Deffarges S, Bassez C, et al. Benefit of switching dual antiplatelet therapy after acute coronary syndrome: the topic (timing of platelet inhibition after acute coronary syndrome) randomized study. Eur Heart J. (2017) 38:3070–8. doi: 10.1093/eurheartj/ehx175
27. Kim CJ, Park MW, Kim MC, Choo EH, Hwang BH, Lee KY, et al. Unguided de-escalation from ticagrelor to clopidogrel in stabilised patients with acute myocardial infarction undergoing percutaneous coronary intervention (TALOS-AMI): an investigator-initiated, open-label, multicentre, non-inferiority, randomised trial. Lancet. (2021) 398:1305–16. doi: 10.1016/s0140-673601445-8
28. Claassens DMF, Vos GJA, Bergmeijer TO, Hermanides RS, van ’t Hof AWJ, van der Harst P, et al. A genotype-guided strategy for oral P2y inhibitors in primary PCI. N Engl J Med. (2019) 381:1621–31. doi: 10.1056/NEJMoa1907096
29. Moon JY, Franchi F, Rollini F, Rivas Rios JR, Kureti M, Cavallari LH, et al. Role of genetic testing in patients undergoing percutaneous coronary intervention. Expert Rev Clin Pharmacol. (2018) 11:151–64. doi: 10.1080/17512433.2017.1353909
30. Galli M, Benenati S, Capodanno D, Franchi F, Rollini F, D’Amario D, et al. Guided versus standard antiplatelet therapy in patients undergoing percutaneous coronary intervention: a systematic review and meta-analysis. Lancet. (2021) 397:1470–83. doi: 10.1016/s0140-673600533-x
31. Tavenier AH, Mehran R, Chiarito M, Cao D, Pivato CA, Nicolas J, et al. Guided and unguided de-escalation from potent P2y12 inhibitors among patients with acute coronary syndrome: a meta-analysis. Eur Heart J Cardiovasc Pharmacother. (2022) 8:492–502. doi: 10.1093/ehjcvp/pvab068
32. Mega JL, Close SL, Wiviott SD, Shen L, Hockett RD, Brandt JT, et al. Cytochrome P-450 polymorphisms and response to clopidogrel. N Engl J Med. (2009) 360:354–62. doi: 10.1056/NEJMoa0809171
33. Gurbel PA, Bliden KP, Hiatt BL, O’Connor CM. Clopidogrel for coronary stenting: response variability, drug resistance, and the effect of pretreatment platelet reactivity. Circulation. (2003) 107:2908–13. doi: 10.1161/01.Cir.0000072771.11429.83
34. Sibbing D, Aradi D, Alexopoulos D, Ten Berg J, Bhatt DL, Bonello L, et al. Updated expert consensus statement on platelet function and genetic testing for guiding P2y receptor inhibitor treatment in percutaneous coronary intervention. JACC Cardiovasc Interv. (2019) 12:1521–37. doi: 10.1016/j.jcin.2019.03.034
35. Helten C, Naguib D, Dannenberg L, Pöhl M, Ayhan A, Hohlfeld T, et al. Platelet function testing: dead or alive. J Thromb Haemost. (2018) 16:984–6.
36. Becker RC, Bassand JP, Budaj A, Wojdyla DM, James SK, Cornel JH, et al. Bleeding complications with the P2y12 receptor antagonists clopidogrel and ticagrelor in the platelet inhibition and patient outcomes (PLATO) trial. Eur Heart J. (2011) 32:2933–44. doi: 10.1093/eurheartj/ehr422
37. Aradi D, Gross L, Trenk D, Geisler T, Merkely B, Kiss RG, et al. Platelet reactivity and clinical outcomes in acute coronary syndrome patients treated with prasugrel and clopidogrel: a pre-specified exploratory analysis from the TROPICAL-ACS trial. Eur Heart J. (2019) 40:1942–51. doi: 10.1093/eurheartj/ehz202
38. Gurbel PA, Tantry US, Shuldiner AR, Kereiakes DJ. Genotyping: one piece of the puzzle to personalize antiplatelet therapy. J Am Coll Cardiol. (2010) 56:112–6. doi: 10.1016/j.jacc.2010.04.008
39. Freynhofer MK, Hein-Rothweiler R, Haller PM, Aradi D, Dézsi DA, Gross L, et al. Diurnal variability of on-treatment platelet reactivity in clopidogrel versus prasugrel treated acute coronary syndrome patients: a pre-specified TROPICAL-ACS sub-study. Thromb Haemost. (2019) 119:660–7. doi: 10.1055/s-0038-1677549
40. Lawton JS, Tamis-Holland JE, Bangalore S, Bates ER, Beckie TM, Bischoff JM, et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: executive summary: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. J Am Coll Cardiol. (2022) 79:197–215. doi: 10.1016/j.jacc.2021.09.005
41. Huber K. Platelet antiaggregation after an acute coronary syndrome: what about the elderly? Eur Heart J. (in press) (2022).
42. Koo BK, Kang J, Park KW, Rhee TM, Yang HM, Won KB, et al. Aspirin versus clopidogrel for chronic maintenance monotherapy after percutaneous coronary intervention (HOST-EXAM): an investigator-initiated, prospective, randomised, open-label, multicentre trial. Lancet. (2021) 397:2487–96. doi: 10.1016/s0140-673601063-1
Keywords: percutaneous coronary intervention (PCI), short dual antiplatelet therapy (short DAPT), de-escalation, P2Y12-inhibitor, bleeding
Citation: Muthspiel M, Kaufmann CC, Burger AL, Panzer B, Verheugt FWA and Huber K (2022) Short dual antiplatelet therapy and dual antiplatelet therapy de-escalation after primary percutaneous intervention: For whom and how. Front. Cardiovasc. Med. 9:1008194. doi: 10.3389/fcvm.2022.1008194
Received: 31 July 2022; Accepted: 25 October 2022;
Published: 10 November 2022.
Edited by:
Francesco Costa, University of Messina, ItalyReviewed by:
Antonia Sambola, Vall d’Hebron University Hospital, SpainCopyright © 2022 Muthspiel, Kaufmann, Burger, Panzer, Verheugt and Huber. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marie Muthspiel, bWFyaWVfbXV0aHNwaWVsQGhvdG1haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.