
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med., 13 December 2022
Sec. General Cardiovascular Medicine
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.1006213
This article is part of the Research TopicThe Role of Inflammation, Stem Cells and Progenitor Cells in Cardiovascular RepairView all 6 articles
Background: Maladaptive inflammation is implicated in the development of diabetic cardiomyopathy (DCM). This study aimed to visually analyze the global scientific output over the past two decades regarding research on inflammation associated with DCM.
Methods: All relevant articles and reviews were retrieved in the Web of Science (WOS) Core Collection (limited to SCIE) using “inflammation” and “diabetic cardiomyopathy” as search terms. Articles and reviews published from 1 January 2001 to 28 February 2021 were collected. Visualization analysis and statistical analysis were conducted by Microsoft 365 Excel and VOSviewer 1.6.18.
Results: A total of 578 documents were finally selected for further analysis. The publications regarding inflammation and DCM increased gradually over approximately 20 years. The most prolific country was China, with 296 documents and the most citations (9,366). The most influential author groups were Lu Cai and Yihui Tan who were from the United States. The bibliometric analysis of co-occurrence keywords showed that inflammation in DCM is composed of numerous molecules (NF-κB, NLRP3 inflammasome, Nrf-2, TNF-α, protein kinase C, PPARα, TLR4, p38 mitogen-activated protein kinase, TGF-β, Sirt1, and AKT), a variety of cardiac cell types (stem cell, fibroblast, and cardiomyocyte), physiological processes (apoptosis, oxidative stress, autophagy, endoplasmic reticulum stress, hypertrophy, mitochondrion dysfunction, and proliferation), and drugs (sulforaphane, metformin, empagliflozin, and rosuvastatin).
Conclusion: Our bibliometric analysis presents the characteristics and trends of inflammation in DCM and shows that research on inflammation in DCM will continue to be a hotspot.
Diabetic cardiomyopathy (DCM) is a disease that is characterized by structure abnormality and cardiac dysfunctions but in the absence of coronary artery disease, valvular diseases, and other conventional cardiovascular risk factors (1). DCM is now rapidly increasing with the increased prevalence of diabetes. The development and progression of DCM are associated with multiple factors, including impaired cardiac insulin metabolic signaling, extracellular matrix stiffness, endoplasmic reticulum stress, mitochondrial dysfunction, oxidative stress, and microvascular dysfunction (2). Furthermore, it has been demonstrated that hyperglycemia-induced cumulative inflammation is characteristic of the initial stage of DCM (1). Emerging evidence suggests that targeting inflammation is promising for ameliorating DCM, although little has been known regarding the underlying mechanisms (3).
Bibliometrics, a widely accepted research method, can build up the knowledge structure and track developmental trends by statistically and visually analyzing a specific research field over a certain time frame (4, 5). Bibliometrics analysis compares contributions among key information on countries, institutions, journals, authors, and their literature references from the Web of Science (WOS) Core Collection database (6). Several bibliometric software, such as VOSviewer, CiteSpace, and BibExcel, have been developed to conduct bibliometrics analysis frequently (5, 7, 8). Bibliometrics has been widely used in many research areas such as infectious diseases, energy utilization, environmental science and pollution, and health service (9–12). In addition, bibliometrics has been increasingly prevalent in applications for governing and improving clinical and research guidelines and policy making, as well as research hotspots and trends (13–16).
In the past two decades, a large number of studies focusing on the role of inflammation in DCM have been published. However, from the perspective of bibliometrics, few papers provided an overview of inflammation related to DCM. Hence, this study applied bibliometric analysis to comprehensively investigate the research trends concerning inflammation related to DCM from the aspects of key journals and authors, important cited references, number of publications per year, and most productive countries/regions. This study aimed to illustrate the research landscape and trends for clinical researchers and practitioners.
All data were retrieved from the Web of Science (WOS) Core Collection, including the Science Citation Index Expanded with a publication timespan (1 January 2001 to 28 February 2021). These data were composed of full records and cited references. The search terms were “inflammation” and “diabetic cardiomyopathy.” The document types were restricted to articles or reviews. We reviewed the abstracts or the full text of the controversial publications. Publications that were unrelated to the search topic were excluded.
We used Microsoft 365 Excel to assess and visualize the number of publications among different countries. VOSviewer 1.6.18 was used to analyze and illustrate the top journals, authors, their institutions, publication quantity, and keywords co-occurrence, as well as cited references. The parameters of the VOSviewer were set as follows: Linlog/modularity and full counting. VOSviewer is a software tool for constructing and viewing bibliometric maps, including co-occurrence, co-authorship, keyword co-occurrence, citation, bibliographic coupling, and co-citation. The “citation report” function from WOS was also applied to determine citation rates and the h-index of authors.
A total of 682 publications meet the criterion. The articles that were not related to inflammation or DCM (n = 104) were excluded. Finally, 578 papers satisfying these search criteria were extracted for further analysis. As is shown in Figure 1, 457 articles and 121 reviews on inflammation related to DCM in the WOS Core Collection from 1 January 2001 to 28 February 2021 were identified. The majority of these articles were written in English (574/99.3%), with the rest being in Japanese, Polish, Russian, and Spanish. The result showed that the publications of inflammation in DCM were of sustained growth every year over the studied period. Accordingly, the total number of citations rapidly increased in line with the number of publications. The publication number and citation numbers were the highest in 2020. These data suggested the growing interest in the research of inflammation in DCM in the past two decades.
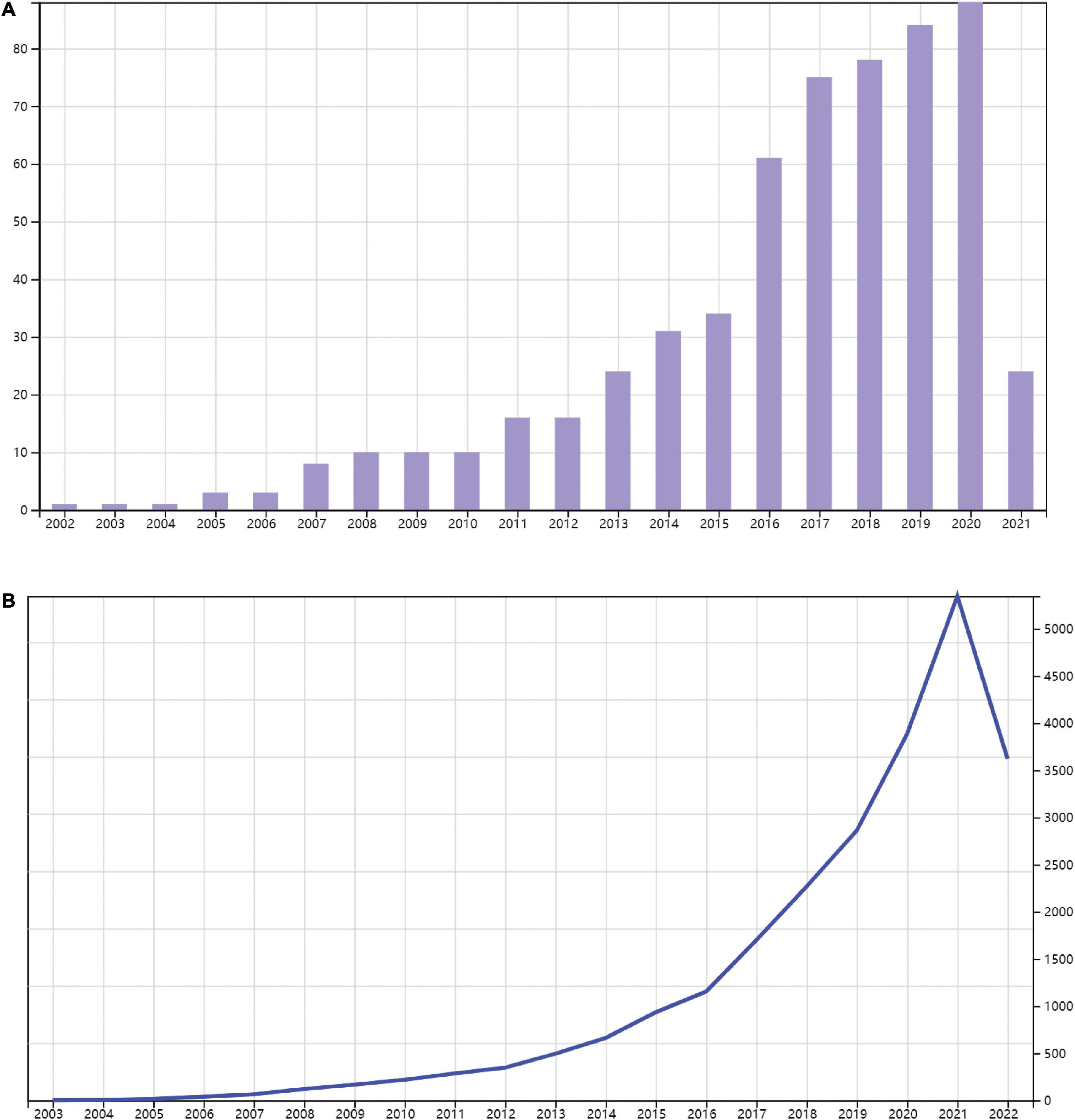
Figure 1. Trends in publications of inflammation related to diabetic cardiomyopathy (DCM) research. Timeline of publications (A) and citations (B) on inflammation related to DCM.
A total of 55 countries/regions contributed to the research of inflammation in DCM, and 21 satisfied the criterion (minimum number of documents of a keyword: 5). Figure 2 shows the global distribution of all the analyzed articles, and Table 1 shows the 10 most productive countries/regions. China published the highest number of papers (n = 296), followed by the United States (n = 135), Germany (n = 36), Italy (n = 35), and India (n = 33). China had the highest number of citations (n = 9,366) followed by the United States (n = 8,835), Germany (n = 3,027), Italy (n = 1,548), and India (n = 1,309). China had the closest cooperation with the United States.
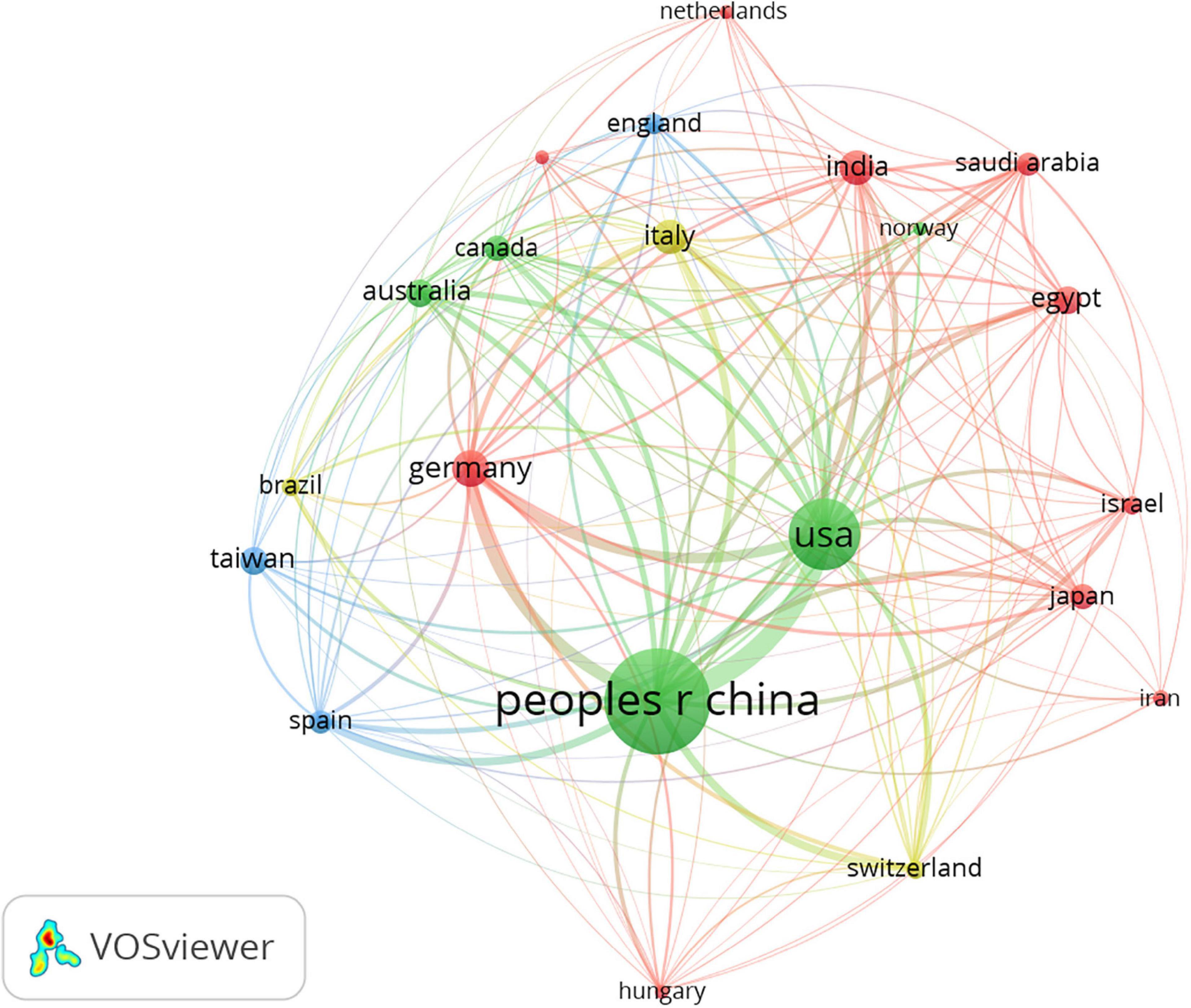
Figure 2. Network map of countries/regions in inflammation related to diabetic cardiomyopathy (DCM) research. The analysis method was Linlog/modularity in VOSviewer, the weight was an occurrence, and scores were the average published year.
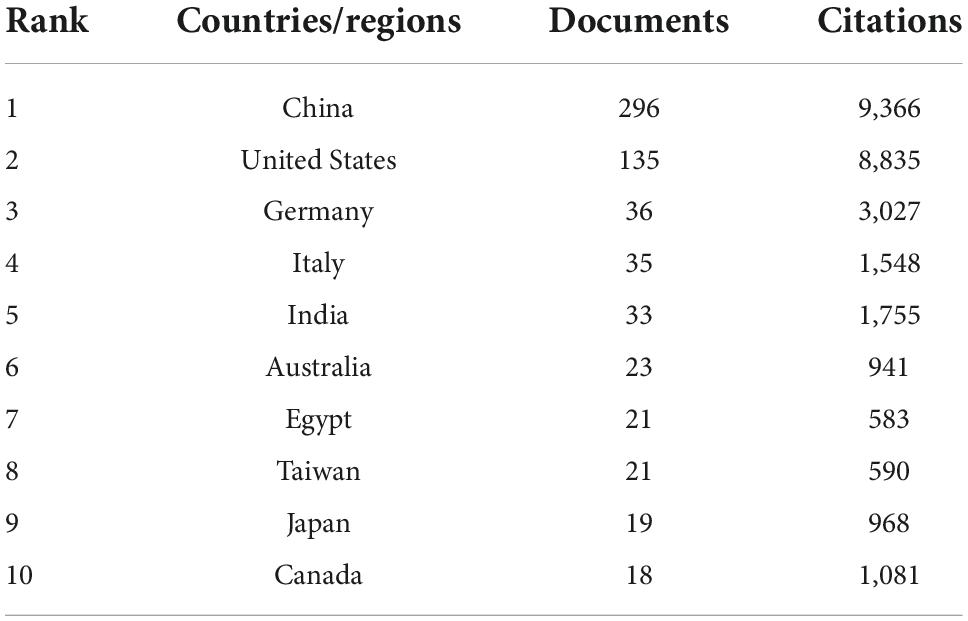
Table 1. Top 10 the most productive countries/regions on inflammation in diabetic cardiomyopathy (DCM).
According to the statistical analysis, the top 10 published journals are shown in Table 2. Impact factor (IF) and Journal Citation Reports partition were identified. The most published and popular journals were Biomedicine & Pharmacotherapy (n = 16, IF 2021 = 7.419, Q1) and Diabetes (n = 16, IF 2021 = 9.337, Q1). Four journals were from the United States, two journals were issued by the United Kingdom, two journals were issued by Switzerland, and others were issued by Egypt, Germany, and France. The 2021 impact factor of these journals ranged from 3.752 to 10.406. Diabetologia had the highest IF, while PLoS One had the lowest IF. The JCR partition analysis showed that six journals were Q1 and the other four were Q2.
According to VOSviewer analysis, 578 documents were published by 766 organizations, and 48 satisfied the criterion (minimum number of documents of a keyword: 5). After excluding disjointed organizations, the remaining 48 organizations were constructed for the visualization map. The network map of co-author analysis of organizations is constructed in Figure 3 and the top 10 most productive organizations are listed in Table 3, including eight Chinese organizations and two United States organizations. Jilin University (n = 44) and Wenzhou Medical University (n = 43) had larger-sized bubbles representing higher numbers of papers. The number of citations from Jilin University (n = 2,019) was also more than that from Wenzhou Medical University (n = 1,966). Jilin University had the closest cooperation with the University of Louisville.
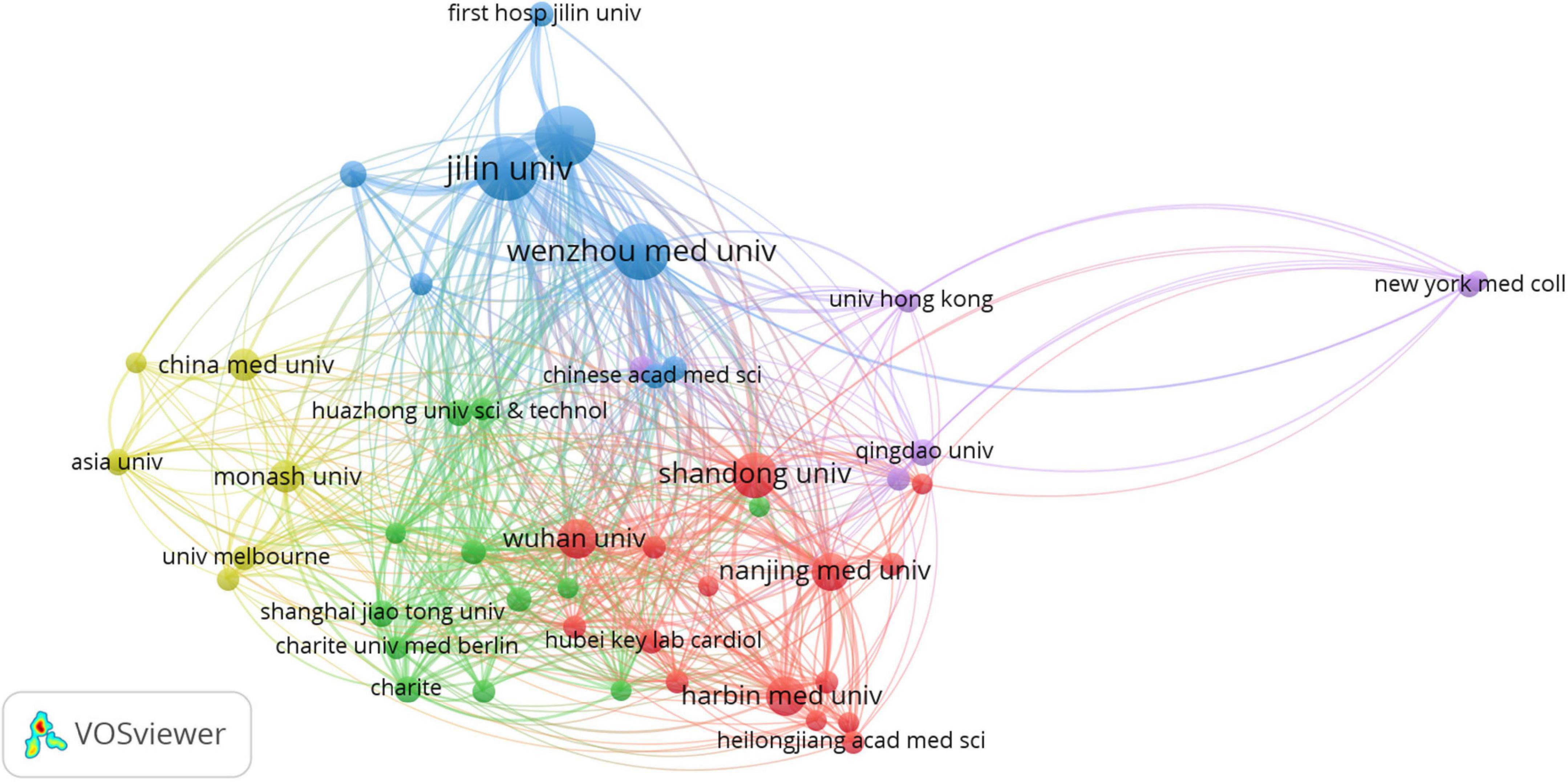
Figure 3. Network map of co-author analysis of organizations. The method was Linlog/modularity, weight was citations, and scores were the average published year. The analysis method was Linlog/modularity in VOSviewer, the weight was an occurrence, and scores were the average published year.
The network map of co-authorship analysis of authors is constructed in Figure 4, and 42 satisfied the criterion (minimum number of documents of a keyword: 5). Table 4 lists the top 10 core authors in the field, with more than or equal to nine publications per author. Lu Cai was the first with 39 documents and had the highest citations (n = 1,988), followed by Yi Tan (21/1,322), Yang Zheng (14/765), Zhiguo Zhang (13/608), Quan Liu (12/507), Carsten Tschoepe (11/1,225), Shudong Wang (11/692), Liang, Guang (10/474), Heinz-peter Schultheiss (9/1,196), and Yuehui Wang (9/597). Though Carsten Tschoepe and Heinz-peter Schultheiss from Germany published fewer papers than other authors from China, they usually had higher citations. The results suggested that Lu Cai was the top scientist in this field.
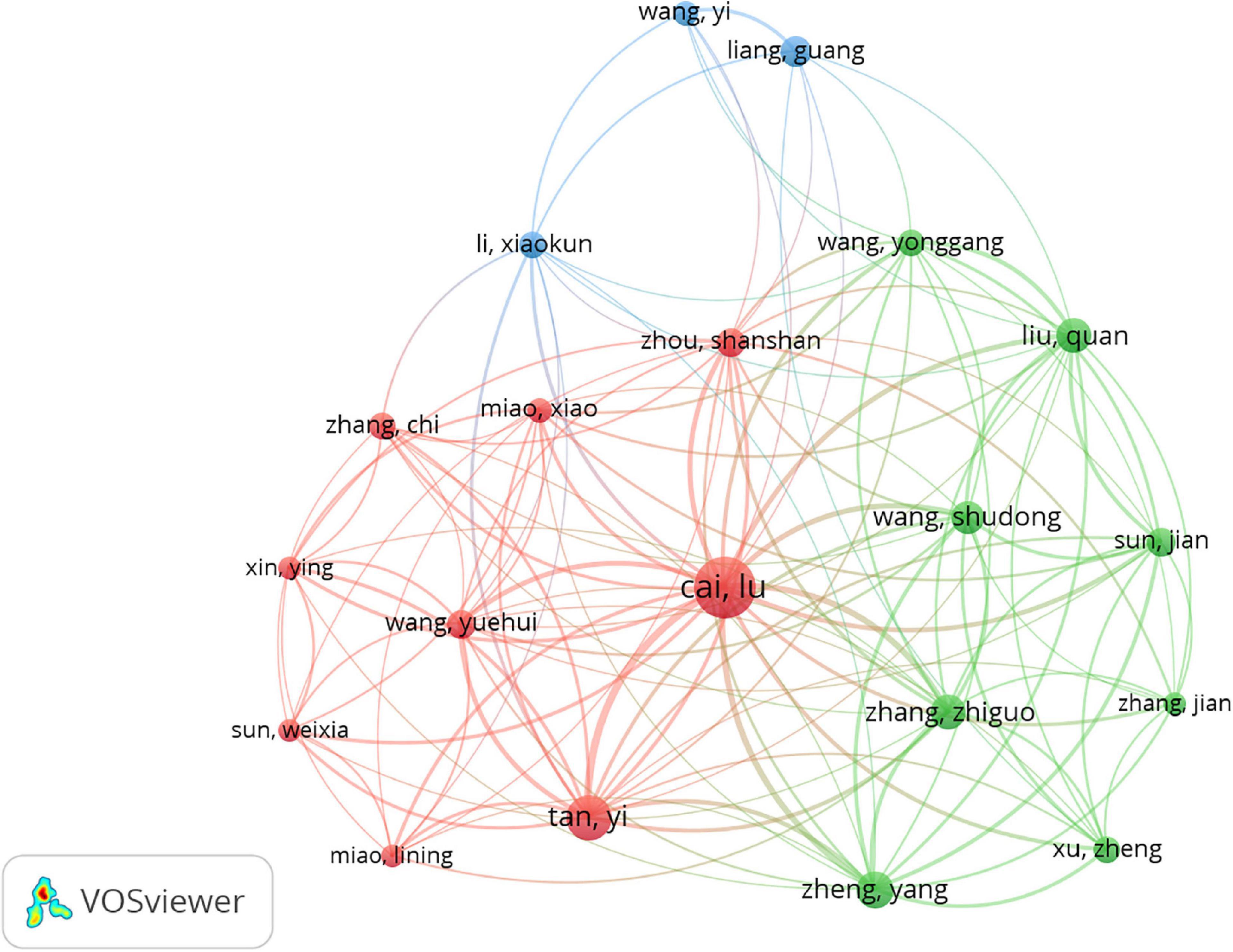
Figure 4. Network map of co-authorship analysis of authors in inflammation related to diabetic cardiomyopathy (DCM) research. The analysis method was Linlog/modularity in VOSviewer, the weight was an occurrence, and scores were the average published year.
A total of 2,321 keywords were involved in 578 documents, and 234 satisfied the criterion (minimum number of documents of a keyword: 5). The network visualization map was constructed for the co-occurrence of all keywords (Figure 5A). The size of the circle indicates occurrences of keywords. Oxidative stress, inflammation, DCM, cardiomyopathy, apoptosis, activation, fibrosis, expression, and heart failure were high-frequency keywords. VOSviewer also colored the keywords according to the average appearing year (Figure 5B). Keywords that appeared relatively earlier were blue, and keywords with a more recent appearance were yellow. These keywords were published sequentially from 2014 to 2018 in this field. According to the statistical analysis of the author keywords, numerous molecules such as NF-κB, NLRP3 inflammasome, Nrf-2, TNF-α, protein kinase C (PKC), PPARα, TLR4, p38 mitogen-activated protein kinase (MAPK), TGF-β (transforming growth factor-β), Sirt1, and AKT were associated with inflammation in DCM. In addition, stem cells, fibroblasts, cardiomyocytes, other types of cells and apoptosis, oxidative stress, autophagy, endoplasmic reticulum (ER) stress, hypertrophy, mitochondrion dysfunction, and proliferation participated in the processes of inflammation in DCM. Some drugs such as sulforaphane, metformin, empagliflozin, and rosuvastatin were used to treat DCM based on targeting inflammation.
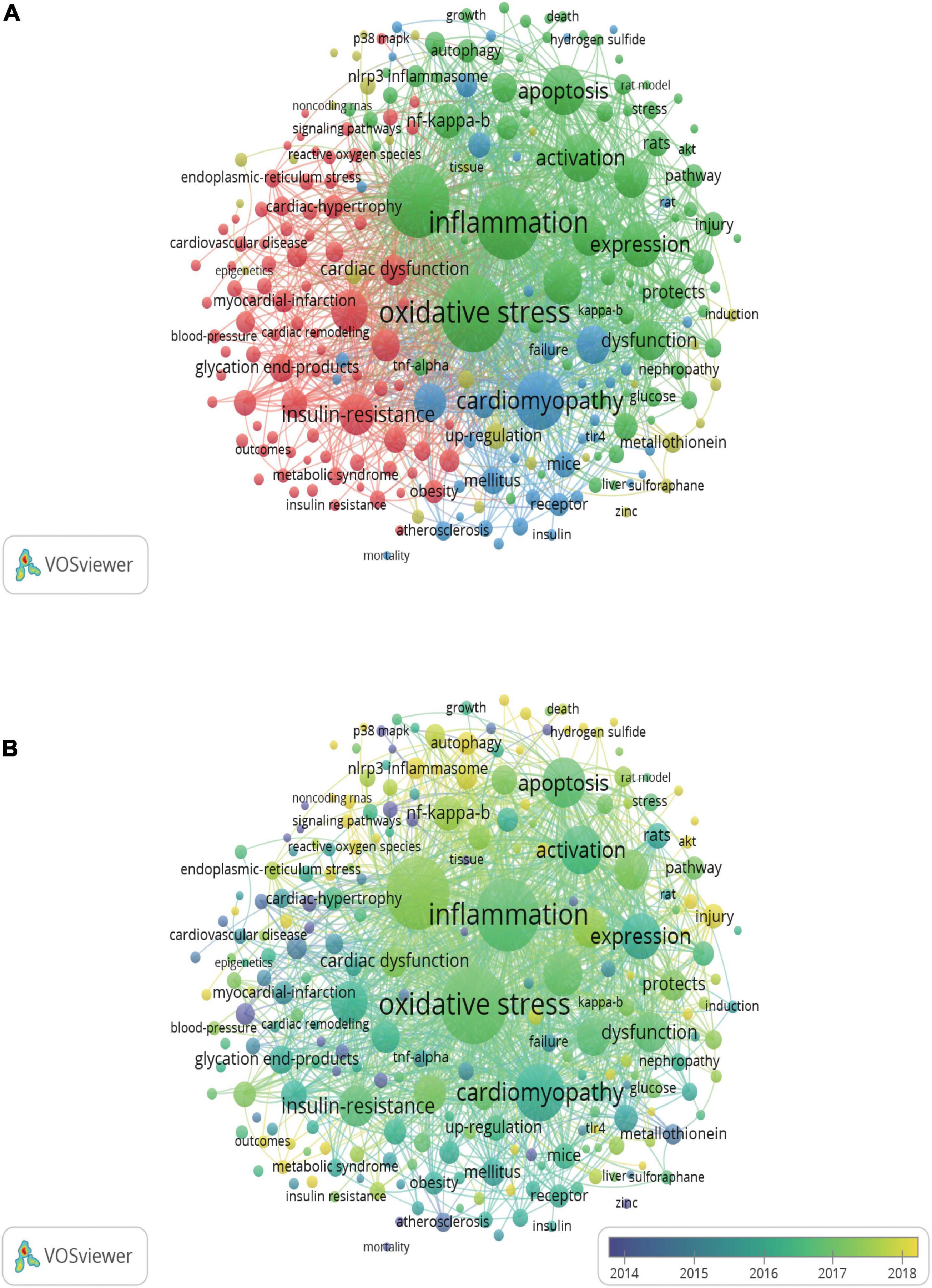
Figure 5. Network map of co-occurrence analysis of all keywords. (A) Mapping of keywords of publications. (B) Distribution of keywords according to average publication year. The analysis method was Linlog/modularity in VOSviewer, the weight was an occurrence, and scores were the average published year.
Figure 6A shows the visualized network map of citations, and 50 satisfied the criterion (minimum number of documents of a keyword: 100). Table 5 lists the top night documents with the highest citations, each of which was more than 203, with a range of citations from 203 to 724. “Advanced Glycation End Products and Diabetic Complications,” published by Parkash Singh Varun, was cited 724 times and ranked the first, and “Interplay of oxidative, nitrosative/nitrative stress, inflammation, cell death and autophagy in diabetic cardiomyopathy” published by Zoltán V Varga was cited 203 times and ranked last among this ranking. Those highly cited documents focused on the role of inflammation in cardiovascular, particularly in DCM, and most were reviews.
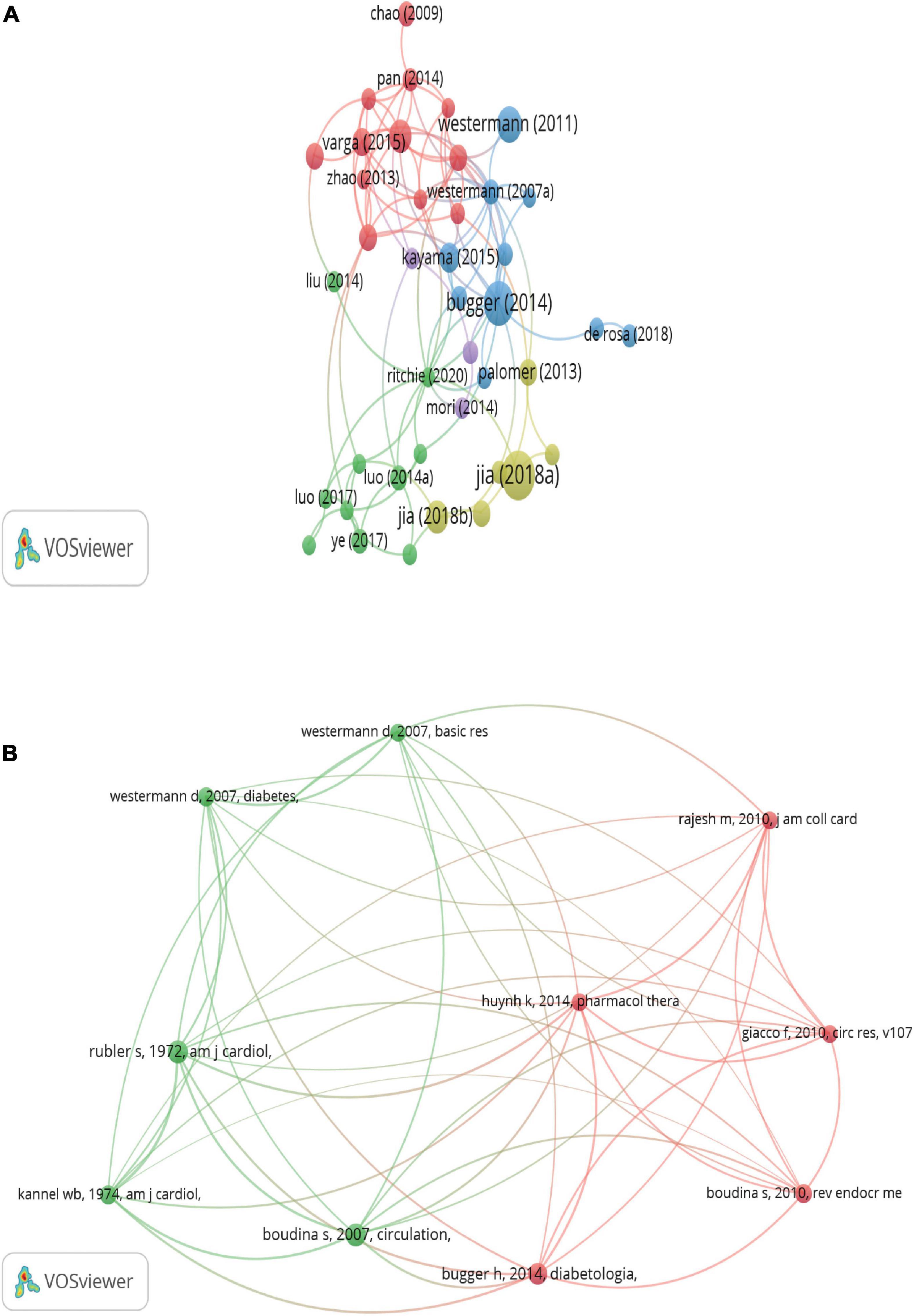
Figure 6. Network map of citation analysis of publications. (A) Mapping of citation analysis of documents. (B) Network map of co-citation analysis of references. The analysis method was Linlog/modularity in VOSviewer, the weight was an occurrence, and scores were the average published year.
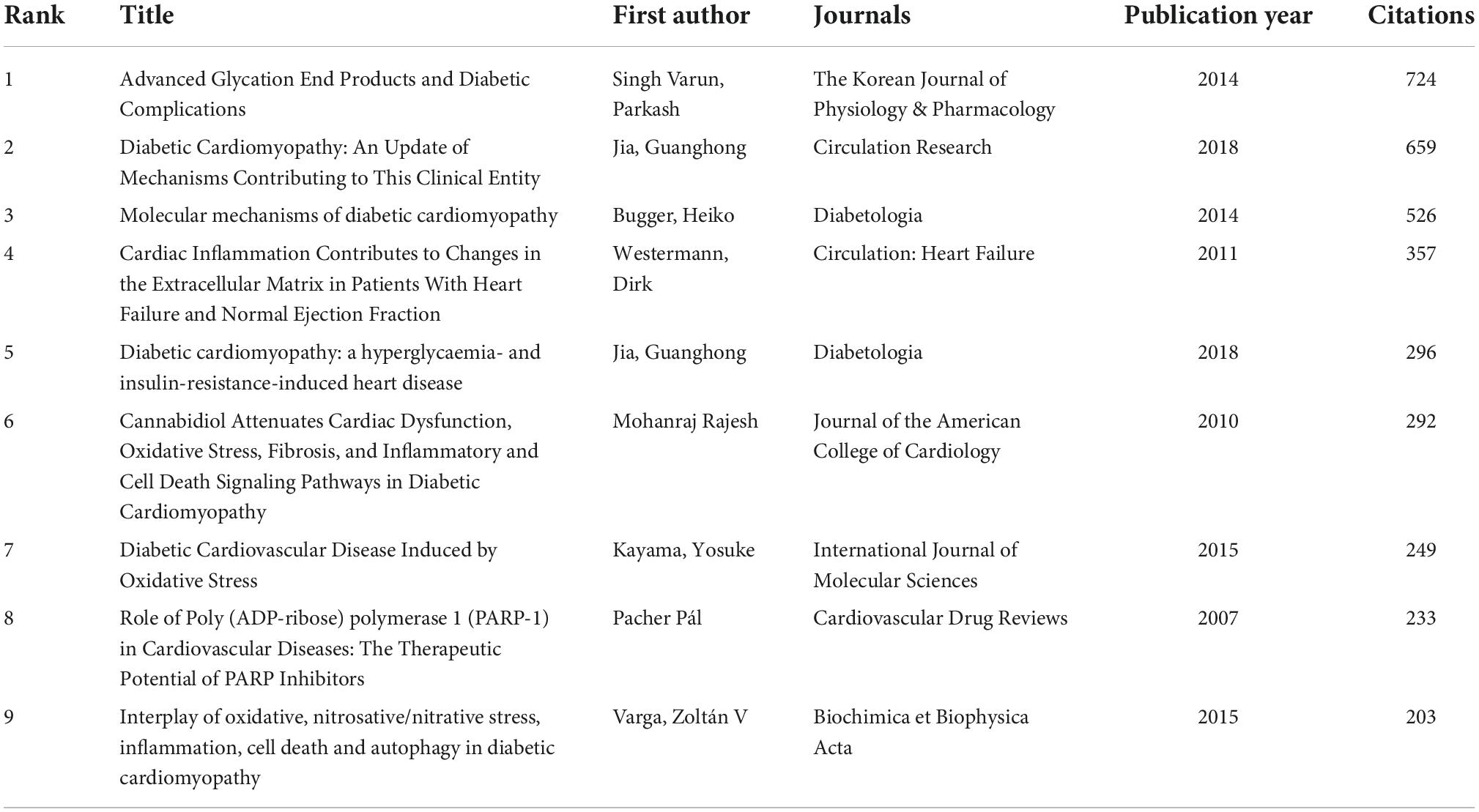
Table 5. Top night citation analysis of publications on inflammation in diabetic cardiomyopathy (DCM) research.
Figure 6B lists the top 10 references that were co-cited in more than 51 citations. The reference with the largest number of citations was published by Sihem Boudina (2007, circulation; 84 citations), followed by S Rubler (1972, American Journal of Cardiology, 83 citations), Heiko Bugger (2014, Diabetologia, 74 citations), Sihem Boudina (2010, Reviews in Endocrine and Metabolic Disorders; 60 citations), Dirk Westermann (2007, Diabetes, 56 citations), W B Kannel (1974, American Journal of Cardiology, 56 citations), Ferdinando Giacco (2010, Circulation Research, 54 citations), Mohanraj Rajesh (2010, Journal of the American College of Cardiology, 52 citations), Karina Huynh (2014, Pharmacology & therapeutics, 51 citations), and Dirk Westermann (2007, Basic Research In Cardiology, 51 citations).
In the present study, we used bibliometric analyses and network visualizations to investigate the research trends of inflammation in DCM during the past two decades, which bring deep understanding to researchers in this field. The contributions of journals, authors, organizations, and countries to this emerging field were analyzed. Continued research interest during the two decades indicated that inflammation in DCM was a hot topic. With a steady increase in the number of annual publications and citations, more and more researchers are interested in inflammation related to DCM, which contributed to the growth of publications. China, the United States, and Germany were the top three countries, indicating their influences on the inflammation of DCM. Chinese scientists published the largest number of papers of all the identified articles. Eight of the top ten institutions in this field in terms of the number of publications were from China. However, two of the top ten core authors on inflammation in DCM were from Western countries. Hence, the analysis of research trends shows that authors from China and Western countries dominated the quality of publications on inflammation in DCM.
As for the core journals in this field, the difference in papers was small, suggesting that all of them had considerable influence in this field. These journals regarding Diabetes and comprehensive journals preferred to publish these studies. Indeed, DCM was considered a common complication of diabetes (17). These journals were more likely to publish future developments in inflammation related to DCM. Journals concerning cardiovascular disease might also further contribute to this field in the future.
Based on the document analysis of core authors, Lu Cai and Carsten Tschöpe groups lead this field and contribute to the rapid progress. Lu Cai and his important collaborators (Yi Tan, Yang Zheng, and Zhiguo Zhang) were working on the role of metallothionein and Nrf2 signaling pathways in inflammation and oxidative stress associated with DCM (18–21). Furthermore, Lu Cai and Shudong Wang identified that metallothionein is downstream of Nrf2 (22). Lu Cai also identified some protective drugs for DCM treatment, such as sulforaphane, 4-O-methylhonokiol, and dimethyl fumarate, and revealed the mechanisms (23–25). Shudong Wang and Lu Cai Zinc prevented an inflammatory response in DCM (26, 27). Quan Liu and Guang Liang, cooperating with Lu Cai, mainly determined that JNK2 activation enhanced inflammation and DCM, while treatment with a novel curcumin derivative, C66, ameliorated DCM (28–30). Guang Liang further found that inhibition of the EGFR-STAT3 signaling pathway attenuated inflammation and DCM (31–33). He also elucidated some key molecules, such as MD2 and FGF1 (34, 35). In 2020, in collaboration with Yi Tan and Zhiguo Zhang, Lu Cai summarized the mechanisms of DCM and potential therapeutic strategies from preclinical and clinical evidence (36). Carsten Tschöpe cooperating with Dirk Westermann and Heinz-Peter Schultheiss verified the protective effects of the kallikrein-kinin system in the process of inflammation and DCM pathogenesis (37–39). They also reported that matrix metalloproteinases resulted in the crosstalk of inflammation and fibrosis involved in DCM (40–42). The literature analysis showed that the two groups lacked communication at present. In the future, more exchanges and cooperation between them might greatly promote developments in this field. The citation analysis of documents and co-cited analysis of references showed that these highly cited papers were reviews of DCM or inflammation in DCM, indicating little key progress was made in this field. Therefore, there is still great potential for development in this field.
Currently, more and more scientists focus on the key role of inflammation in DCM. Inflammation was regarded as a trigger of DCM (43). Co-occurrences of keyword analysis suggested that the interaction between inflammation and oxidative stress causes cardiomyocyte death, cardiac hypertrophy, fibrosis, and heart failure in DCM (44). The proinflammatory cells, especially macrophages, play a key role in the development of DCM (45). Macrophage M1 phenotype triggers inflammation and DCM (46). Mesenchymal stem cells inducing type 2 macrophage polarization ameliorate DCM (47). Activation and expression of proinflammatory cytokines, such as TNF-α, interleukins (IL)-1β, -6, and -18, monocyte chemotactic protein 1 (MCP-1), and adhesion molecule intercellular adhesion molecule 1, contribute to cardiac oxidative stress, cardiac remodeling, and fibrosis, and diastolic dysfunction (48, 49). The activation and interaction of these types of cells govern the development of DCM.
Based on the co-occurrence of keywords analysis, many signaling pathways were associated with inflammation in DCM, and these interventions targeting them had been reported to reduce inflammation and attenuate DCM. NF-κB pathway activation follows a transphosphorylation chain from the specific activated receptors to the IκB proteins, which triggers NF-κB heterodimer to enter the nucleus and controls pro-inflammation gene expression, contributing to DCM (50). Hyperglycemia-induced p38 MAPK activation promotes inflammation and DCM (51). Fibroblast growth factor 21 (FGF 21)/SIRT1 pathway mediated autophagy inducing inflammation and DCM (52).
According to the core authors’ analysis and their contribution, some other important molecules in inflammation and DCM were also identified, such as the kallikrein-kinin system (38) and EGFR-STAT3 (33). PKC inhibitions have previously been linked to the decrease in the expression of inflammatory cytokines, facilitating cardiac damage and interstitial fibrosis in vivo and in vitro (53). In DCM, AGEs (advanced glycation end products) bind directly to MD2, leading to the formation of AGEs-MD2-TLR4 complexes and the initiation of pro-inflammatory pathways (34). DCM was associated with diminished Akt phosphorylation, which might contribute to myocardial dysfunction, cardiac fibrosis, inflammation, and interrelated signaling pathways (54). As was well-known, TGF-β served as a key mediator of fibrosis. The activation of inflammation enhances the synthesis of extracellular matrix (ECM) via upregulation of TGF-β resulting in diabetic cardiac dysfunction (55). Though numerous pathogenic targets and therapeutic targets of DCM have been found, effective therapeutic strategies for DCM remain elusive. Exploring the complex inflammatory response mechanism in the development and progression of diabetic cardiomyopathy is significant, which will inform more therapeutic strategies and clinical progress.
The SGLT2 inhibitor empagliflozin is a novel drug against inflammation, fibrosis, and antioxidative stress in DCM (56). Rosuvastatin and other statins have been proven to attenuate inflammation and DCM via NLRP3 inflammasome and MAPK signaling pathways (57). Sulforaphane reduced cardiac oxidative damage, inflammation, fibrosis, and hypertrophy through the Nrf2 pathway (21). Metformin possessed cardioprotective and anti-inflammatory effects by activating AMPK/autophagy and inhibiting the NLRP3 inflammasome in DCM (58).
To the best of our best knowledge, the present study provided a deep insight into the global status and trends of research on inflammation in DCM using bibliometric analysis for the first time. Bibliometric analysis is objective, comprehensive, and widely used contributing to the reliability of the data. However, some limitations, which were commonly similar to other bibliometric analyses, should be addressed. First, the deadline for publications was 28 February 2021, but WOS Core Collection would also keep updating. Newly published documents have already been online on the websites of journals, and this part was lacking in this manuscript. Therefore, the data in this work could not fully reflect the reality of trends in this field. Second, this study was limited to WOS Core Collection indexed journal, and a few papers that were not in the scope of the inclusion were missed. Third, the terms “inflammation,” “diabetic cardiomyopathy,” “Article,” and “Reviews,” which appeared in the title, abstract, and keywords, were selected for this manuscript, while the related terms in the text could not be retrieved and analyzed. The results of keyword analysis might have been affected by incomplete keyword extraction. Despite these limitations, this study provides a solid global view of inflammation in DCM research over the last two decades.
We used bibliometric analysis to provide a comprehensive overview of the research status of inflammation in DCM worldwide over the past two decades. This field is undergoing a period of rapid development and attracting the attention of an increasing number of researchers. China, the United States, and Germany made the most contributions. These journals regarding diabetes are productive in this field. Lu Cai and Yihui Tan were critical groups in the field of inflammation in DCM. Critical factors associated with inflammation in DCM, such as pathophysiology, various types of cells, inflammatory factors, key signaling pathways, and drugs, are hot topics in this research field. Our study provides a valuable reference for researchers, and the topics regarding inflammation in DCM deserve continued following up by researchers.
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
NZ designed this study and revised the manuscript. BH and LZ performed the search, wrote the manuscript, and contributed to the data collection and verification. All authors read and approved the final manuscript.
This research was supported by the Natural Science Foundation of Zhejiang Province (No. LQ20H020011).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Song S, Ding Y, Dai GL, Zhang Y, Xu MT, Shen JR, et al. Sirtuin 3 deficiency exacerbates diabetic cardiomyopathy via necroptosis enhancement and NLRP3 activation. Acta Pharmacol Sin. (2021) 42:230–41. doi: 10.1038/s41401-020-0490-7
2. Jia G, Hill MA, Sowers JR. Diabetic cardiomyopathy: an update of mechanisms contributing to this clinical entity. Circ Res. (2018) 122:624–38. doi: 10.1161/circresaha.117.311586
3. Yu LM, Dong X, Xue XD, Xu S, Zhang X, Xu YL, et al. Melatonin attenuates diabetic cardiomyopathy and reduces myocardial vulnerability to ischemia-reperfusion injury by improving mitochondrial quality control: role of SIRT6. J Pineal Res. (2021) 70:e12698. doi: 10.1111/jpi.12698
4. Casado-Aranda LA, Sánchez-Fernández J, Viedma-Del-Jesús MI. Analysis of the scientific production of the effect of COVID-19 on the environment: a bibliometric study. Environ Res. (2021) 193:110416. doi: 10.1016/j.envres.2020.110416
5. Gu X, Xie M, Jia R, Ge S. Publication trends of research on retinoblastoma during 2001-2021: a 20-year bibliometric analysis. Front Med. (2021) 8:675703. doi: 10.3389/fmed.2021.675703
6. Ke L, Lu C, Shen R, Lu T, Ma B, Hua Y. Knowledge mapping of drug-induced liver injury: a scientometric investigation (2010-2019). Front Pharmacol. (2020) 11:842. doi: 10.3389/fphar.2020.00842
7. Synnestvedt MB, Chen C, Holmes JH. CiteSpace II: visualization and knowledge discovery in bibliographic databases. AMIA Annu Symp Proc. (2005) 2005:724–8.
8. van Eck NJ, Waltman L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics. (2010) 84:523–38. doi: 10.1007/s11192-009-0146-3
9. Agyeman PC, Ahado SK, Borůvka L, Biney JKM, Sarkodie VYO, Kebonye NM, et al. Trend analysis of global usage of digital soil mapping models in the prediction of potentially toxic elements in soil/sediments: a bibliometric review. Environ Geochem Health. (2021) 43:1715–39. doi: 10.1007/s10653-020-00742-9
10. Ji B, Zhao Y, Vymazal J, Mander Ü, Lust R, Tang C. Mapping the field of constructed wetland-microbial fuel cell: a review and bibliometric analysis. Chemosphere. (2021) 262:128366. doi: 10.1016/j.chemosphere.2020.128366
11. Murillo J, Villegas LM, Ulloa-Murillo LM, Rodríguez AR. Recent trends on omics and bioinformatics approaches to study SARS-CoV-2: a bibliometric analysis and mini-review. Comput Biol Med. (2021) 128:104162. doi: 10.1016/j.compbiomed.2020.104162
12. Pai RR, Alathur S. Bibliometric analysis and methodological review of mobile health services and applications in India. Int J Med Inform. (2021) 145:104330. doi: 10.1016/j.ijmedinf.2020.104330
13. Yao XI, Wang X, Speicher PJ, Hwang ES, Cheng P, Harpole DH, et al. Reporting and guidelines in propensity score analysis: a systematic review of cancer and cancer surgical studies. J Natl Cancer Inst. (2017) 109:djw323. doi: 10.1093/jnci/djw323
14. Sharp MK, Tokalić R, Gómez G, Wager E, Altman DG, Hren D. A cross-sectional bibliometric study showed suboptimal journal endorsement rates of STROBE and its extensions. J Clin Epidemiol. (2019) 107:42–50. doi: 10.1016/j.jclinepi.2018.11.006
15. Nan Y, Feng T, Hu Y, Qi X. Understanding aging policies in China: a bibliometric analysis of policy documents, 1978–2019. Int J Environ Res Public Health. (2020) 17:5956. doi: 10.3390/ijerph17165956
16. Sorensen RM, Jovanović B. From nanoplastic to microplastic: a bibliometric analysis on the presence of plastic particles in the environment. Mar Pollut Bull. (2021) 163:111926. doi: 10.1016/j.marpolbul.2020.111926
17. Jia G, Whaley-Connell A, Sowers JR. Diabetic cardiomyopathy: a hyperglycaemia- and insulin-resistance-induced heart disease. Diabetologia. (2018) 61:21–8. doi: 10.1007/s00125-017-4390-4
18. Zhang C, Zhang L, Chen S, Feng B, Lu X, Bai Y, et al. The prevention of diabetic cardiomyopathy by non-mitogenic acidic fibroblast growth factor is probably mediated by the suppression of oxidative stress and damage. PLoS One. (2013) 8:e82287. doi: 10.1371/journal.pone.0082287
19. Liu Q, Wang S, Cai L. Diabetic cardiomyopathy and its mechanisms: role of oxidative stress and damage. J Diabetes Investig. (2014) 5:623–34. doi: 10.1111/jdi.12250
20. Zhou S, Yin X, Jin J, Tan Y, Conklin DJ, Xin Y, et al. Intermittent hypoxia-induced cardiomyopathy and its prevention by Nrf2 and metallothionein. Free Radic Biol Med. (2017) 112:224–39. doi: 10.1016/j.freeradbiomed.2017.07.031
21. Sun Y, Zhou S, Guo H, Zhang J, Ma T, Zheng Y, et al. Protective effects of sulforaphane on type 2 diabetes-induced cardiomyopathy via AMPK-mediated activation of lipid metabolic pathways and NRF2 function. Metabolism. (2020) 102:154002. doi: 10.1016/j.metabol.2019.154002
22. Gu J, Cheng Y, Wu H, Kong L, Wang S, Xu Z, et al. Metallothionein is downstream of NRF2 and partially mediates sulforaphane prevention of diabetic cardiomyopathy. Diabetes. (2017) 66:529–42. doi: 10.2337/db15-1274
23. Zhang Z, Wang S, Zhou S, Yan X, Wang Y, Chen J, et al. Sulforaphane prevents the development of cardiomyopathy in type 2 diabetic mice probably by reversing oxidative stress-induced inhibition of LKB1/AMPK pathway. J Mol Cell Cardiol. (2014) 77:42–52. doi: 10.1016/j.yjmcc.2014.09.022
24. Hu X, Rajesh M, Zhang J, Zhou S, Wang S, Sun J, et al. Protection by dimethyl fumarate against diabetic cardiomyopathy in type 1 diabetic mice likely via activation of nuclear factor erythroid-2 related factor 2. Toxicol Lett. (2018) 287:131–41. doi: 10.1016/j.toxlet.2018.01.020
25. Zheng Z, Ma T, Guo H, Kim KS, Kim KT, Bi L, et al. 4-O-methylhonokiol protects against diabetic cardiomyopathy in type 2 diabetic mice by activation of AMPK-mediated cardiac lipid metabolism improvement. J Cell Mol Med. (2019) 23:5771–81. doi: 10.1111/jcmm.14493
26. Wang S, Wang B, Wang Y, Tong Q, Liu Q, Sun J, et al. Zinc prevents the development of diabetic cardiomyopathy in db/db mice. Int J Mol Sci. (2017) 18:580. doi: 10.3390/ijms18030580
27. Wang J, Wang S, Wang W, Chen J, Zhang Z, Zheng Q, et al. Protection against diabetic cardiomyopathy is achieved using a combination of sulforaphane and zinc in type 1 diabetic OVE26 mice. J Cell Mol Med. (2019) 23:6319–30. doi: 10.1111/jcmm.14520
28. Pan Y, Wang Y, Zhao Y, Peng K, Li W, Wang Y, et al. Inhibition of JNK phosphorylation by a novel curcumin analog prevents high glucose-induced inflammation and apoptosis in cardiomyocytes and the development of diabetic cardiomyopathy. Diabetes. (2014) 63:3497–511. doi: 10.2337/db13-1577
29. Wang Y, Zhou S, Sun W, Mcclung K, Pan Y, Liang G, et al. Inhibition of JNK by novel curcumin analog C66 prevents diabetic cardiomyopathy with a preservation of cardiac metallothionein expression. Am J Physiol Endocrinol Metab. (2014) 306:E1239–47. doi: 10.1152/ajpendo.00629.2013
30. Li C, Miao X, Lou Y, Lu Z, Adhikari BK, Wang Y, et al. Cardioprotective effects of the novel curcumin analogue C66 in diabetic mice is dependent on JNK2 inactivation. J Cell Mol Med. (2018) 22:6314–26. doi: 10.1111/jcmm.13924
31. Liang D, Zhong P, Hu J, Lin F, Qian Y, Xu Z, et al. EGFR inhibition protects cardiac damage and remodeling through attenuating oxidative stress in STZ-induced diabetic mouse model. J Mol Cell Cardiol. (2015) 82:63–74. doi: 10.1016/j.yjmcc.2015.02.029
32. Li W, Fang Q, Zhong P, Chen L, Wang L, Zhang Y, et al. EGFR inhibition blocks palmitic acid-induced inflammation in cardiomyocytes and prevents hyperlipidemia-induced cardiac injury in mice. Sci Rep. (2016) 6:24580. doi: 10.1038/srep24580
33. Luo W, Huang L, Wang J, Zhuang F, Xu Z, Yin H, et al. Inhibition of EGFR-STAT3 attenuates cardiomyopathy in streptozotocin-induced type 1 diabetes. J Endocrinol. (2019) 242:199–210. doi: 10.1530/joe-19-0058
34. Wang Y, Luo W, Han J, Khan ZA, Fang Q, Jin Y, et al. MD2 activation by direct AGE interaction drives inflammatory diabetic cardiomyopathy. Nat Commun. (2020) 11:2148. doi: 10.1038/s41467-020-15978-3
35. Wang D, Yin Y, Wang S, Zhao T, Gong F, Zhao Y, et al. FGF1(ΔHBS) prevents diabetic cardiomyopathy by maintaining mitochondrial homeostasis and reducing oxidative stress via AMPK/Nur77 suppression. Signal Transduct Target Ther. (2021) 6:133. doi: 10.1038/s41392-021-00542-2
36. Tan Y, Zhang Z, Zheng C, Wintergerst KA, Keller BB, Cai L. Mechanisms of diabetic cardiomyopathy and potential therapeutic strategies: preclinical and clinical evidence. Nat Rev Cardiol. (2020) 17:585–607. doi: 10.1038/s41569-020-0339-2
37. Tschöpe C, Walther T, Königer J, Spillmann F, Westermann D, Escher F, et al. Prevention of cardiac fibrosis and left ventricular dysfunction in diabetic cardiomyopathy in rats by transgenic expression of the human tissue kallikrein gene. FASEB J. (2004) 18:828–35. doi: 10.1096/fj.03-0736com
38. Tschöpe C, Walther T, Escher F, Spillmann F, Du J, Altmann C, et al. Transgenic activation of the kallikrein-kinin system inhibits intramyocardial inflammation, endothelial dysfunction and oxidative stress in experimental diabetic cardiomyopathy. FASEB J. (2005) 19:2057–9. doi: 10.1096/fj.05-4095fje
39. Westermann D, Walther T, Savvatis K, Escher F, Sobirey M, Riad A, et al. Gene deletion of the kinin receptor B1 attenuates cardiac inflammation and fibrosis during the development of experimental diabetic cardiomyopathy. Diabetes. (2009) 58:1373–81. doi: 10.2337/db08-0329
40. Westermann D, Rutschow S, Jäger S, Linderer A, Anker S, Riad A, et al. Contributions of inflammation and cardiac matrix metalloproteinase activity to cardiac failure in diabetic cardiomyopathy: the role of angiotensin type 1 receptor antagonism. Diabetes. (2007) 56:641–6. doi: 10.2337/db06-1163
41. Westermann D, Van Linthout S, Dhayat S, Dhayat N, Schmidt A, Noutsias M, et al. Tumor necrosis factor-alpha antagonism protects from myocardial inflammation and fibrosis in experimental diabetic cardiomyopathy. Basic Res Cardiol. (2007) 102:500–7. doi: 10.1007/s00395-007-0673-0
42. Van Linthout S, Seeland U, Riad A, Eckhardt O, Hohl M, Dhayat N, et al. Reduced MMP-2 activity contributes to cardiac fibrosis in experimental diabetic cardiomyopathy. Basic Res Cardiol. (2008) 103:319–27. doi: 10.1007/s00395-008-0715-2
43. Bugger H, Abel ED. Molecular mechanisms of diabetic cardiomyopathy. Diabetologia. (2014) 57:660–71. doi: 10.1007/s00125-014-3171-6
44. Hölscher ME, Bode C, Bugger H. Diabetic cardiomyopathy: does the type of diabetes matter? Int J Mol Sci. (2016) 17:2136. doi: 10.3390/ijms17122136
45. Ritchie RH, Abel ED. Basic mechanisms of diabetic heart disease. Circ Res. (2020) 126:1501–25. doi: 10.1161/circresaha.120.315913
46. Liu G, Yan D, Yang L, Sun Y, Zhan L, Lu L, et al. The effect of miR-471-3p on macrophage polarization in the development of diabetic cardiomyopathy. Life Sci. (2021) 268:118989. doi: 10.1016/j.lfs.2020.118989
47. Jin L, Deng Z, Zhang J, Yang C, Liu J, Han W, et al. Mesenchymal stem cells promote type 2 macrophage polarization to ameliorate the myocardial injury caused by diabetic cardiomyopathy. J Transl Med. (2019) 17:251. doi: 10.1186/s12967-019-1999-8
48. Westermann D, Rutschow S, Van Linthout S, Linderer A, Bücker-Gärtner C, Sobirey M, et al. Inhibition of p38 mitogen-activated protein kinase attenuates left ventricular dysfunction by mediating pro-inflammatory cardiac cytokine levels in a mouse model of diabetes mellitus. Diabetologia. (2006) 49:2507–13. doi: 10.1007/s00125-006-0385-2
49. Rajesh M, Bátkai S, Kechrid M, Mukhopadhyay P, Lee WS, Horváth B, et al. Cannabinoid 1 receptor promotes cardiac dysfunction, oxidative stress, inflammation, and fibrosis in diabetic cardiomyopathy. Diabetes. (2012) 61:716–27. doi: 10.2337/db11-0477
50. Lorenzo O, Picatoste B, Ares-Carrasco S, Ramírez E, Egido J, Tuñón J. Potential role of nuclear factor κB in diabetic cardiomyopathy. Mediators Inflamm. (2011) 2011:652097. doi: 10.1155/2011/652097
51. Wang S, Ding L, Ji H, Xu Z, Liu Q, Zheng Y. The role of p38 MAPK in the development of diabetic cardiomyopathy. Int J Mol Sci. (2016) 17:1037. doi: 10.3390/ijms17071037
52. Zhang J, Cheng Y, Gu J, Wang S, Zhou S, Wang Y, et al. Fenofibrate increases cardiac autophagy via FGF21/SIRT1 and prevents fibrosis and inflammation in the hearts of type 1 diabetic mice. Clin Sci. (2016) 130:625–41. doi: 10.1042/cs20150623
53. Yin L, Fang Y, Song T, Lv D, Wang Z, Liuyan Zhu L, et al. FBXL10 regulates cardiac dysfunction in diabetic cardiomyopathy via the PKC β2 pathway. J Cell Mol Med. (2019) 23:2558–67. doi: 10.1111/jcmm.14146
54. Rajesh M, Mukhopadhyay P, Bátkai S, Patel V, Saito K, Matsumoto S, et al. Cannabidiol attenuates cardiac dysfunction, oxidative stress, fibrosis, and inflammatory and cell death signaling pathways in diabetic cardiomyopathy. J Am Coll Cardiol. (2010) 56:2115–25. doi: 10.1016/j.jacc.2010.07.033
55. Bodiga VL, Eda SR, Bodiga S. Advanced glycation end products: role in pathology of diabetic cardiomyopathy. Heart Fail Rev. (2014) 19:49–63. doi: 10.1007/s10741-013-9374-y
56. Li N, Zhou H. SGLT2 inhibitors: a novel player in the treatment and prevention of diabetic cardiomyopathy. Drug Des Devel Ther. (2020) 14:4775–88. doi: 10.2147/dddt.s269514
57. Luo B, Li B, Wang W, Liu X, Liu X, Xia Y, et al. Rosuvastatin alleviates diabetic cardiomyopathy by inhibiting NLRP3 inflammasome and MAPK pathways in a type 2 diabetes rat model. Cardiovasc Drugs Ther. (2014) 28:33–43. doi: 10.1007/s10557-013-6498-1
Keywords: inflammation, diabetic cardiomyopathy, bibliometric analysis, VOSviewer, Web of Science
Citation: Zhu N, Huang B and Zhu L (2022) Bibliometric analysis of the inflammation in diabetic cardiomyopathy. Front. Cardiovasc. Med. 9:1006213. doi: 10.3389/fcvm.2022.1006213
Received: 25 August 2022; Accepted: 18 November 2022;
Published: 13 December 2022.
Edited by:
Yajing Wang, University of Alabama at Birmingham, United StatesReviewed by:
Jianqiang Hu, Fourth Military Medical University, ChinaCopyright © 2022 Zhu, Huang and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ning Zhu, emh1bmluZ2NjY0AxMjYuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.