- 1Department of Cardiovascular Surgery, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China
- 2Department of Cardiology, The Sixth People's Hospital of Luohe, Luohe, China
- 3Department of Cardiology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China
- 4Department of Cardiovascular Surgery, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 5Department of Extracorporeal Life Support Center, Department of Cardiac Surgery, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China
Background: Reintubation is a serious adverse respiratory event after Stanford type A aortic dissection surgery (AADS), however, published studies focused on reintubation after AADS are very limited worldwide. The objectives of the current study were to establish an early risk prediction model for reintubation after AADS and to clarify its relationship with short-term and long-term prognosis.
Methods: Patients undergoing AADS between 2016–2019 in a single institution were identified and divided into two groups based on whether reintubation was performed. Independent predictors were identified by univariable and multivariable analysis and a clinical prediction model was then established. Internal validation was performed using bootstrap method with 1,000 replications. The relationship between reintubation and clinical outcomes was determined by univariable and propensity score matching analysis.
Results: Reintubation were performed in 72 of the 492 included patients (14.6%). Three preoperative and one intraoperative predictors for reintubation were identified by multivariable analysis, including older age, smoking history, renal insufficiency and transfusion of intraoperative red blood cells. The model established using the above four predictors showed moderate discrimination (AUC = 0.753, 95% CI, [0.695–0.811]), good calibration (Hosmer-Lemeshow χ2 value = 3.282, P = 0.915) and clinical utility. Risk stratification was performed and three risk intervals were identified. Reintubation was closely associated with poorer in-hospital outcomes, however, no statistically significant association between reintubation and long-term outcomes has been observed in patients who were discharged successfully after surgery.
Conclusions: The requirement of reintubation after AADS is prevalent, closely related to adverse in-hospital outcomes, but there is no statistically significant association between reintubation and long-term outcomes. Predictors were identified and a risk model predicting reintubation was established, which may have clinical utility in early individualized risk assessment and targeted intervention.
Introduction
Reintubation is one of the commonly performed surgical procedures for patients with cardiopulmonary insufficiency and consciousness dysfunction after cardiovascular surgery, which is closely related to various adverse outcomes, prolonged hospital stay and increased medical costs (1–8). The reintubation rates reported in previous literature varied considerably due to differences in surgical populations across studies (9–13). Compared with other types of surgery, the incidence of reintubation after Stanford type A aortic dissection surgery (AADS) is relatively higher in the literature, ranging from 7.8 to 20.6% (13–16).
Several studies focused on postoperative reintubation have been conducted and some risk factors have been reported in the literature, such as advanced age and smoking history (4, 11). However, none of those previous studies were designed specifically for patients undergoing AADS or completed in this population. Moreover, no previous studies constructed convenient and practical tools such as nomogram and online risk calculator in this field, which may greatly facilitate the clinical application of prediction model. Our understanding of the risk factors for reintubation after AADS is limited, and there is an urgent need to construct a credible, convenient and practical risk prediction model. In addition, although the relationship between reintubation and in-hospital outcomes have been reported in some previous studies, the relationship between reintubation and long-term prognosis has never been deeply explored or reported so far. It remains unclear whether reintubation after AADS adversely affects the long-term prognosis of patients after discharge.
The objectives of this study were first to identify significant predictors for reintubation in patients undergoing AADS and develop a risk prediction model; and second to deeply explore the relationship between reintubation and in-hospital outcomes and long-term prognosis by univariable and propensity score matching analysis.
Materials and methods
Ethical statement
This study was conducted in accordance with the ethical statement of the Declaration of Helsinki, and was approved by The Ethics Committee of Tongji Medical College of Huazhong University of Science and Technology (IORG No. IORG0003571). Written informed consent was waived due to the observational nature of this study.
Study population
Consecutive adult patients who underwent AADS in a single institution between January-2016 and December-2019 were enrolled. The exclusion criteria of this study were: (1) age < 18 years; (2) time from onset to surgery exceeded 14 days; (3) history of mechanical ventilation within 14 days before surgery; (4) history of organ transplantation, immunosuppression, or immune deficiency; (5) intraoperative or early postoperative death; (6) incomplete medical records.
Data collection
Clinical data in the hospital were collected through the electronic medical records management system of the hospital. Preoperative factors analyzed in this study included sex, age, body mass index, smoking history, drinking history, hypertension, diabetes mellitus, pulmonary emphysema, chronic bronchitis, cerebrovascular disease, peripheral vascular disease, gastrointestinal tract disease, renal function, atrial fibrillation, general surgical history, cardiac surgery history, New York Heart Association class, pericardial effusion, pulmonary artery hypertension, diameters of the right atrium, right ventricle, left atrium and left ventricle, left ventricular ejection fraction, red blood cell count, hemoglobin, white blood cell count, platelet count, serum creatinine, urea nitrogen, albumin, and globulin. Operative factors included combined surgical types, cardiopulmonary bypass time, aortic cross clamp time, deep hypothermic circulatory arrest, and intraoperative transfusion of red blood cells (RBCs). For clinical factors with multiple measurements, the last measurement result before surgery was used in the analysis.
Definitions of important variables
Patients' BMI was calculated by dividing their weight in kilograms by their height in meters. Smoking history was defined as previous daily or current smoking. Drinking history was defined as previous alcohol consumption (consumption or >140 g/week >20 g/day) once a week or more over a year or current alcohol consumption. Hypertension was defined based on previous hypertension diagnosis, blood pressure ≥140/90 mmHg, or antihypertensive medication use. Diabetes mellitus was defined based on previous diabetes mellitus diagnosis, diabetic medication use, random glucose ≥11.1 mmol/L, or fasting glucose ≥7.0 mmol/L. Chronic bronchitis was clinically defined by the presence of chronic productive cough or based on imaging findings. Renal insufficiency was defined based on previous diagnosis of renal insufficiency or serum creatinine higher than 110 μmol/L.
Endpoints and outcome events
In this study, the primary endpoint was postoperative reintubation in patients undergoing AADS. The indications for reintubation included: (1) airway obstruction, progressive aggravation of dyspnea, respiratory failure, weak or stopped spontaneous breathing; (2) malignant arrhythmia, hemodynamic instability, heart failure, cardiogenic shock, cardiac arrest; (3) severe agitation, disturbance of consciousness, loss of consciousness; (4) poor oxygenation, severe respiratory acidosis, refractory hypoxemia; (5) multiple organ dysfunction, requiring treatment with reintubation; and (6) accidental removal of endotracheal intubation while extubation conditions were not reached.
The indications for extubation included: (1) Patients who were fully awake and can follow simple instructions such as opening their eyes, sticking out their tongue, and moving their limbs; (2) stable hemodynamics without low-cardiac output syndrome or myocardial ischaemia, and without significant inotrope support; (3) normothermia; (4) activated coagulation time is normal with no mediastinal bleeding; (5) Muscular strength in accordance with movement of limbs and spontaneous ventilation adequate to maintain arterial oxygen saturation over 95% with 50% FiO2 and end-tidal carbon dioxide below 50 mmHg.
The secondary endpoints in hospital included the length of mechanical ventilation, postoperative pneumonia, tracheostomy, readmission to intensive care unit (ICU), the length of ICU stay, the length of hospital stay, and in-hospital mortality.
The follow-up data of long-term prognosis after discharge were obtained by outpatient surveillance and telephone interviews, included stroke, myocardial infarction, dizziness, limb mobility impairment, all-cause readmission, dissection-related readmission, all-cause death, and dissection-related death.
Statistical analysis
Statistical analysis was performed using R software (version 4.0.5, www.R-project.org/) and SPSS (IBM SPSS Statistics 26.0, SPSS Inc., Chicago, IL). Two-tailed P-value < 0.05 was considered statistically significant.
Categorical variable was expressed as count (percentage). Normally distributed continuous variable was expressed as mean ± standard deviation, otherwise as median (interquartile range). The Q-Q plot was applied to assess whether continuous variable was normally distributed. For univariable analysis, chi-square test or Fisher's exact test was used for categorical variable, Student's t-test was used for normally distributed continuous variable, and Mann-Whitney U-test was used otherwise. Variables with P < 0.1 in the univariable analysis were further analyzed by a forward stepwise multivariable logistic regression procedure to identify significant predictors. The odds ratio (OR) with 95% confidence interval (CI) of each predictor was calculated. A visual nomogram and an online risk calculator based on those predictors and the logistic regression rule were then constructed.
The assessment of the prediction model was performed in the internal population. Internal validation was performed using bootstrap method with 1,000 replications. Calibration was evaluated by both visual inspection of calibration plot and Hosmer-Lemeshow goodness-of-fit test. Discrimination was evaluated using the area under the receiver operating characteristic (ROC) curve (AUC). Clinical utility was evaluated by decision curve analysis.
When analyzing the association between reintubation and other outcomes, univariable and multivariable regression analysis was used to control for confounders. Factors used in the multivariable analysis included sex, age, body mass index, smoking history, hypertension, diabetes mellitus, pulmonary emphysema, chronic bronchitis, cerebrovascular disease, renal function, atrial fibrillation, cardiac surgery history, New York Heart Association class, pericardial effusion, left ventricular ejection fraction, hemoglobin, white blood cell count, platelet count, albumin, cardiopulmonary bypass time, and intraoperative transfusion of RBCs. Survival analysis was performed using Kaplan-Meier curve and log-rank test.
Results
Demographic characteristics
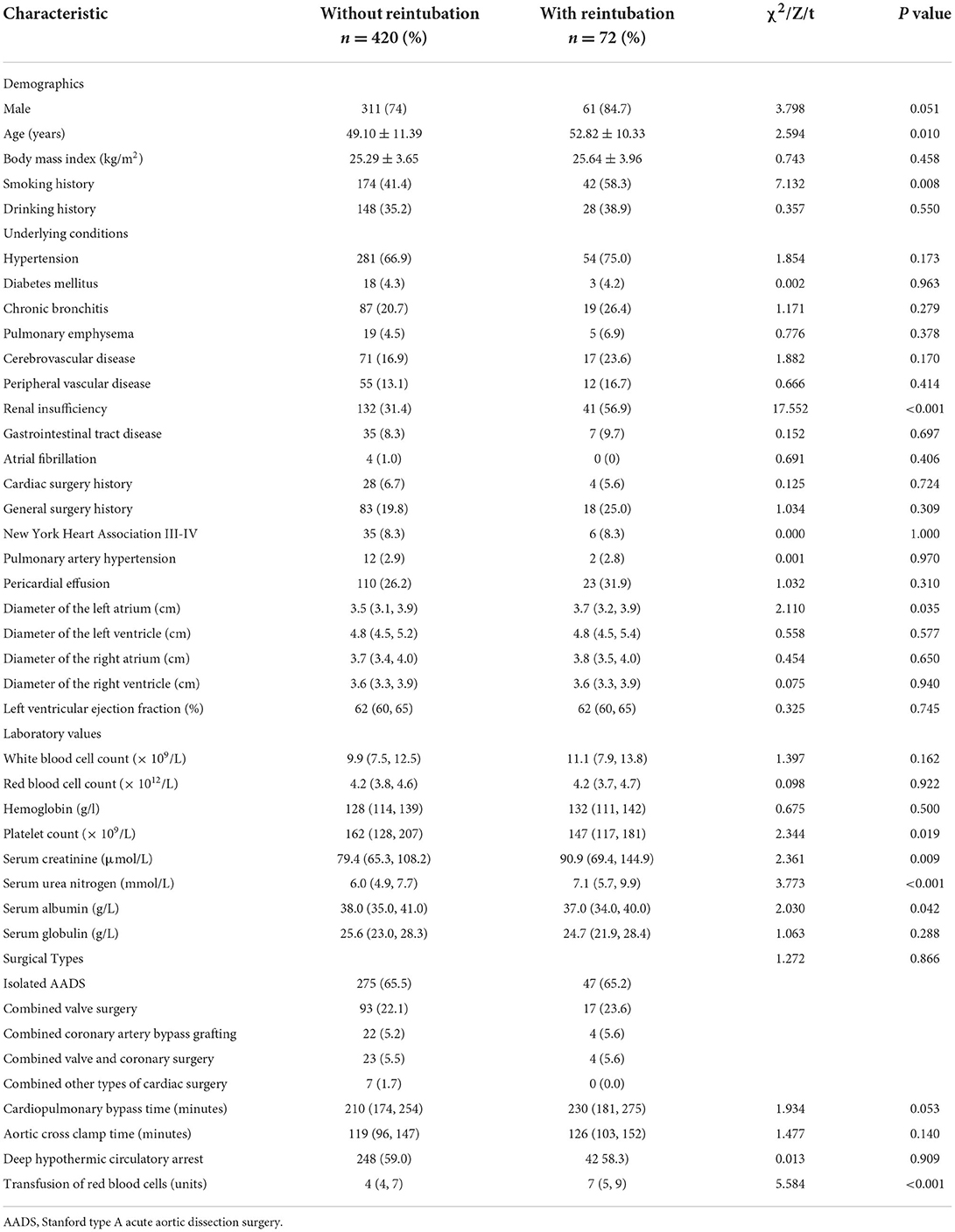
After screening, a total of 492 patients met the inclusion criteria and were included in this study. The average age of these patients was 49.6 ± 11.3 years, 75.6% were male. The rate of reintubation after AADS was 14.6% (72/492). The baseline characteristics and operative variables of the included patients are summarized in Supplementary Table 1.
Development of the risk prediction model
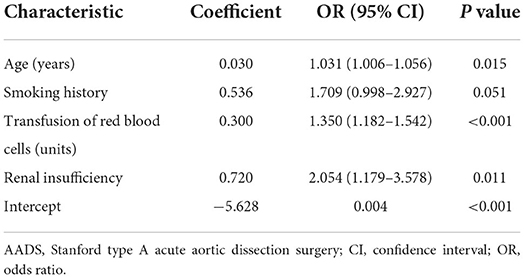
Univariable analysis was first applied to screen potential predictors for reintubation after AADS and the results are presented in Table 1. Variables with P <0.1 or considered clinically significant were further entered into multivariable logistic regression analysis. Four significant predictors were identified in the final multivariable logistic model, including older age, smoking history, renal insufficiency, and intraoperative transfusion of RBC (Table 2). A visual nomogram based on the logistic rule and the four significant predictors was then established used for the prediction of reintubation after AADS (Figure 1). All the predictors were scaled to 0–100 points based on their regression coefficients, reflecting their relative weight.
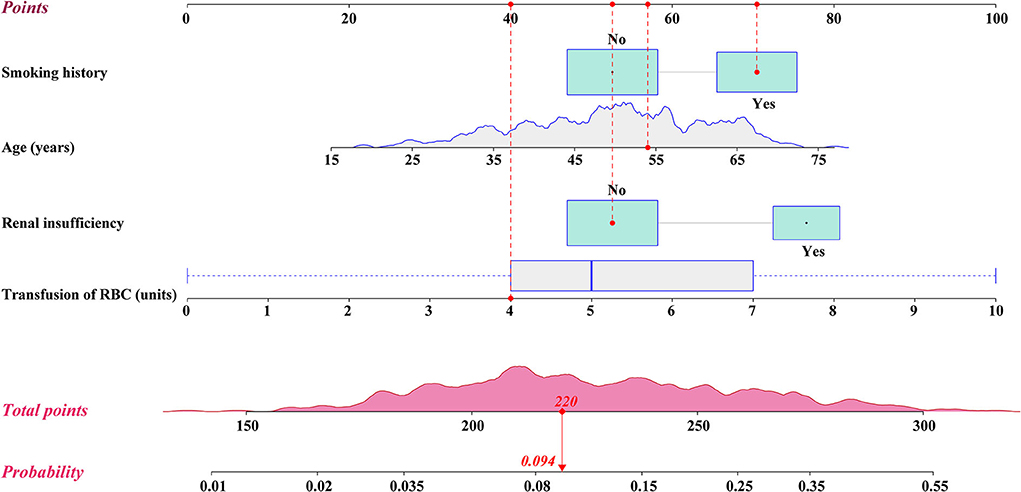
Figure 1. Nomogram for the prediction of postoperative reintubation in patients undergoing AADS. A specific patient was shown to illustrate how to use the nomogram. This was a 54-year-old patient who had smoking history, normal renal function, and was transfused 4 units of RBCs intraoperatively. The individual item point corresponding to each factor is presented at the top, and the total scores were obtained from the sum of the points corresponding to each factor by a red dot. Given values of the 4 predictors, the patient can be intuitively mapped onto the nomogram. It can be clearly seen from the nomogram that the total scores of this patient was 220 points and the corresponding probability of reintubation was 0.094. AADS, Stanford type A acute aortic dissection surgery; RBC, red blood cell.
The probability of reintubation after AADS can be easily predicted on the nomogram by summing the points of all the predictors. Older patients who have smoking history, renal insufficiency, and more intraoperative transfusion of RBC may obtain higher points and have higher risk of reintubation. A specific case is shown in Figure 1. We also created and provided an online risk calculator to predict the probability of reintubation after AADS to facilitate the clinical application (https://reintubation-prediction.shinyapps.io/dynnomapp/).
Assessment of the risk prediction model
The model was well calibrated by both visual inspection and goodness-of-fit test (Hosmer-Lemeshow χ2 value = 3.282, P = 0.915, Figure 2A). The model showed moderate discrimination by plotting ROC curve and calculating the AUC (AUC = 0.753, 95% CI, [0.695–0.811], Figure 2B). The model showed remarkable clinical utility by decision curve analysis (Figures 2C,D). The decision and clinical impact curves indicated that compared with treat-none/all strategies, more clinical net benefits could be obtained between the threshold range of 0.05–0.43 when using this model.
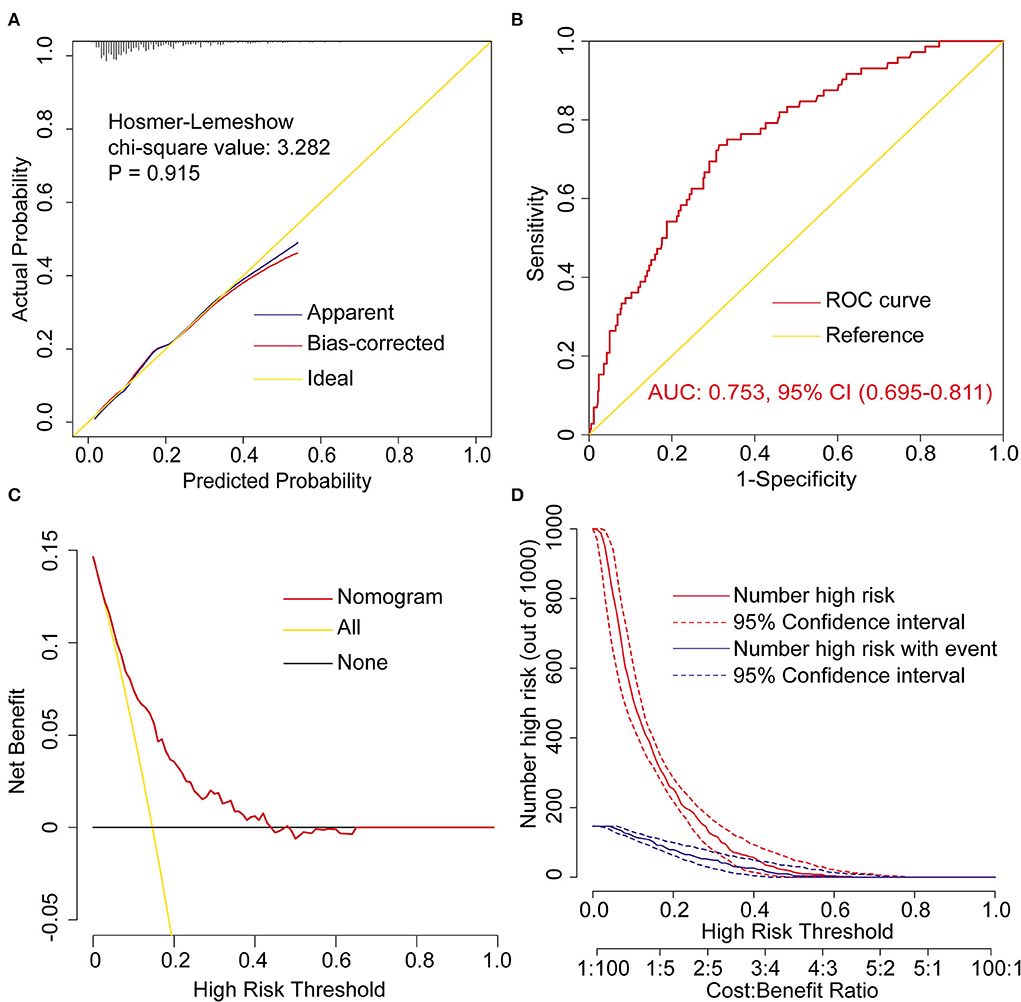
Figure 2. Assessment of the prediction model for postoperative reintubation in patients undergoing AADS. (A) Calibration plot and the result of goodness-of -fit test, (B) ROC curves and corresponding AUC, (C) decision curves, and (D) clinical impact curves. AADS, Stanford type A acute aortic dissection surgery; AUC, area under the receiver operating characteristic curve; CI, confidence interval; ROC, receiver operating characteristic curve.
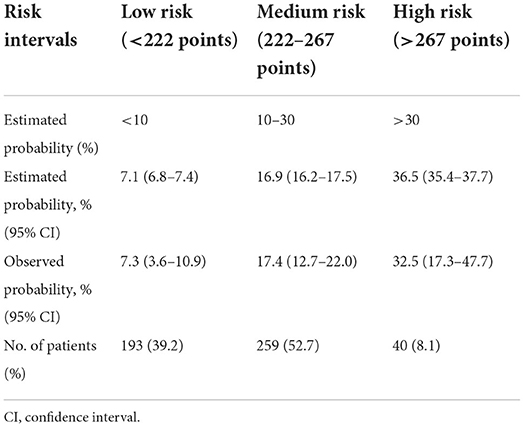
Risk stratification
Based on the nomogram model and clinical practice, a risk stratification procedure was further performed to better facilitate clinical application (Table 3). All the patients were divided into 3 risk groups named low-, medium-, and high-risk group. The cutoff values of the predicted probabilities were respectively 0.1 and 0.3, corresponding to 215 and 251 points on the nomogram. In this study, about forty percent of the patients were divided into low risk group (39.2%), about half of the patients were divided into medium risk group (52.7%), and less than ten percent of the patients were divided into high risk group (8.1%).
In-hospital outcomes
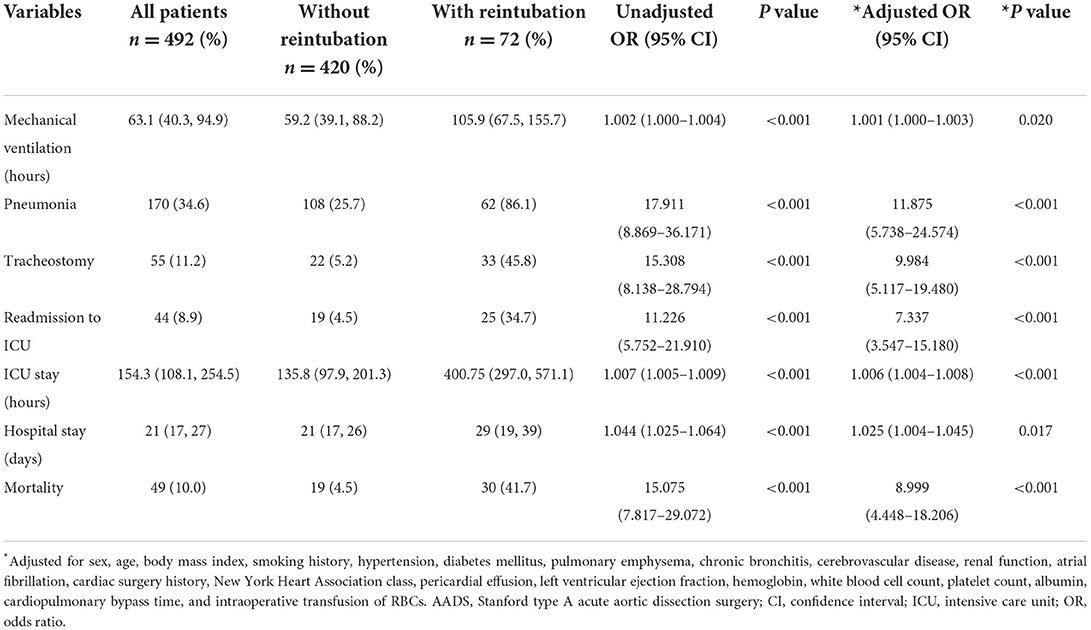
Clinical outcomes in hospital are compared and summarized in Table 4. The overall in-hospital mortality in this study was 9.96% (49/492), with a rate of 4.5% in patients without reintubation vs. 41.7% in those with reintubation (P < 0.001). Significantly poorer outcomes with regard to mechanical ventilation, pneumonia, tracheostomy, readmission to ICU, ICU stay and hospital stay were also observed in patients with reintubation in the univariable analysis (Table 4). To deeply reveal the association of reintubation and these outcomes, we further performed multivariable regression analysis. The results showed that after controlling for confounders, there was still a significant association between reintubation and these outcomes.
Follow-up outcomes
We performed a long-term follow-up of 443 patients who were successfully discharged from the hospital. The maximum follow-up time was 72 months, the median follow-up time was 40 [31, 51] months, and 32 (7.2%) patients were lost to follow-up. Out-of-hospital information of these patients were obtained through follow-up, including stroke, myocardial infarction, dizziness, limb mobility impairment, all-cause readmission, dissection-related readmission, all-cause death, and dissection-related death, which are summarized in Table 5. In univariable analysis, no significant differences were observed regarding these out-of-hospital events between patients with and without reintubation (Table 5). In multivariable analysis, the association between reintubation and these outcomes remained insignificant after controlling for confounders.
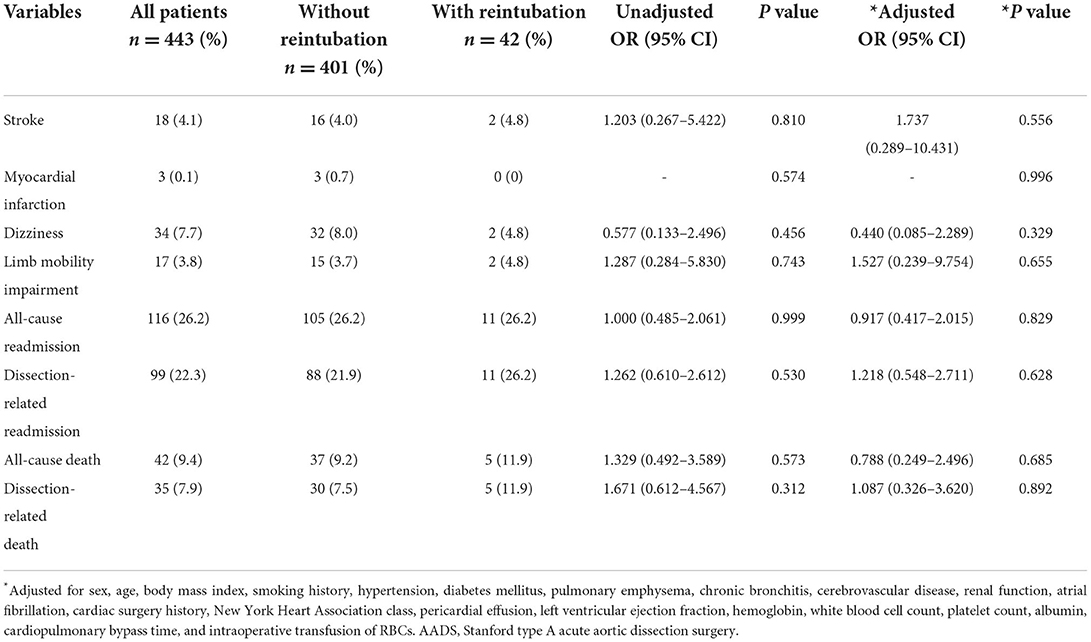
Table 5. Long-term follow-up outcomes and comparison in patients with and without reintubation after AADS.
To measure the effect of reintubation on the cumulative risk of all-cause death and dissection-related death over time, we plotted Kaplan-Meier curves and performed log-rank test (Figure 3). The results showed that there was a rapid increase in cumulative mortality in the early stage of discharge, followed by a gradual slowdown (Figures 3A,B). Although the cumulative probabilities of both all-cause death and dissection-related death were higher in patients with reintubation by visual inspection of the Kaplan-Meier curves, the differences were not statistically significant between the two groups by log-rank test (Figures 3C,D).
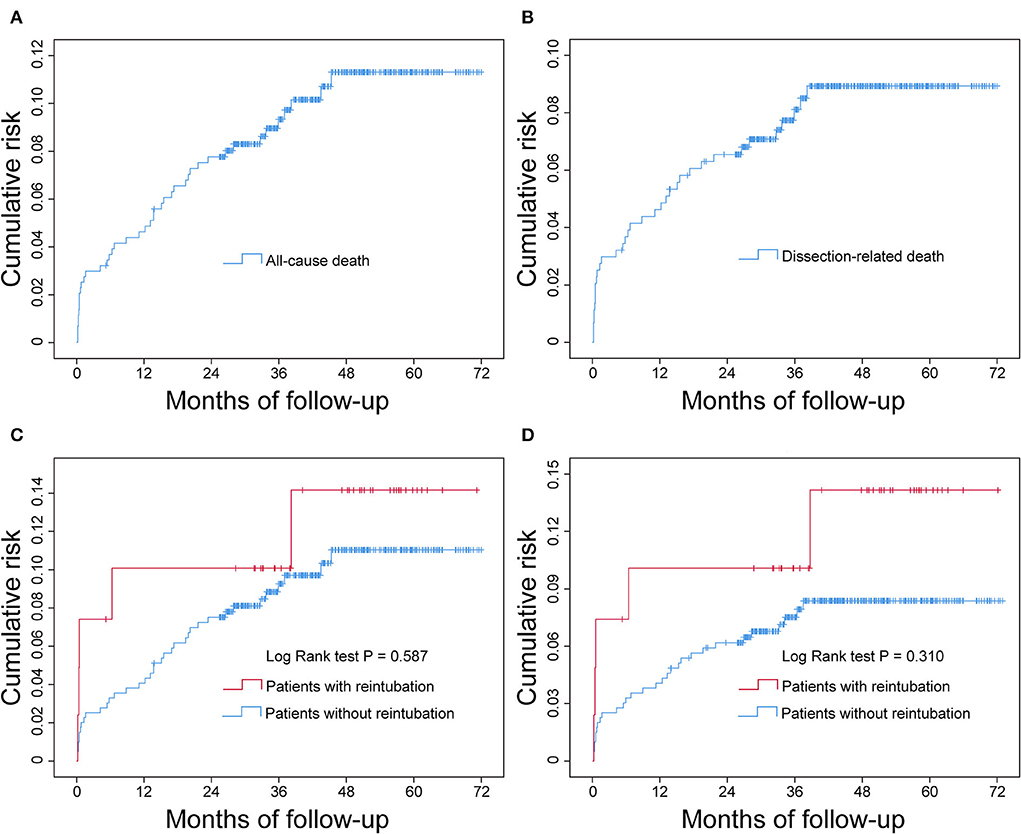
Figure 3. Cumulative all-cause mortality and dissection-related mortality after successful discharge in patients undergoing AADS and the comparison between patients with and without reintubation. The statistical results showed that the difference was not significant between the two groups. (A) Cumulative all-cause mortality, (B) cumulative dissection-related mortality, (C) comparison of cumulative all-cause mortality between patients with and without reintubation, and (D) comparison of cumulative dissection-related mortality between patients with and without reintubation. AADS, Stanford type A acute aortic dissection surgery.
Discussion
Reintubation is a serious adverse respiratory event after cardiovascular surgery and is closely associated with an increased risk of poor postoperative outcomes (1–8), which was again confirmed by our findings. In this study, the reintubation rate after AADS was 14.6%, and the in-hospital mortality rate was 9.96%, which was similar to the morbidity and mortality reported in the literature. Compared with patients without reintubation, patients who experienced reintubation had more postoperative complications and worse in-hospital outcomes, which further emphasized the need to identify significant predictors and establish a prediction model, so as to achieve the purpose of early prediction, prevention and treatment. However, no statistically significant association between reintubation and long-term outcomes has been observed in patients who were discharged successfully after surgery.
In this study, we identified four significant predictors for postoperative reintubation using clinical data of 492 patients undergoing AADS in a single cardiovascular center, including older age, smoking history, renal insufficiency and the amount of intraoperative transfusion of RBCs. A nomogram and an online risk calculator used to facilitate the prediction of reintubation after AADS was then constructed, which showed moderate discrimination, calibration and clinical utility. To the best of our knowledge, this is the first report that attempts to construct a nomogram and an online risk calculator for reintubation in patients undergoing AADS worldwide, which has certain clinical value and guiding significance.
Predictors for postoperative reintubation varied widely among different literature reports. However, the risk of reintubation has been reported to increase gradually with age in most studies (7, 9, 11, 15), which was consistent with the results of this study. Brovman et al. conducted a biinstitutional study to explore the relationship between early extubation and postoperative reintubation after elective cardiac surgery, finding that older age was an independent risk factor for postoperative reintubation (11). The risk of reintubation increased significantly with age in both coronary artery bypass grafting and aortic valve replacement. Beverly et al. obtained similar results when exploring risk factors for unplanned reintubation after cardiac surgery. In their multivariable analysis, compared to patients under 50 years, the risk of postoperative reintubation increased to 1.41, 2.03 and 3.08 times in patients aged 50–65, 65–80 and over 80 years, respectively (7). Vemuri et al. conducted a large population-based study to explore the effect of patient age on postoperative complication rates following abdominal aortic aneurysm repair in the United States (15). They grouped the population by age and found that the rate of postoperative reintubation increased significantly with increasing age. The rates of postoperative reintubation in patients aged 51–60, 61–70, 71–80, and over 80 years were respectively 3.9, 6.3, 9.3, and 9.5%, and the risk increased to 1.6, 2.3, and 2.7 times, respectively. Therefore, it is necessary to predict the risk of reintubation in advance and take preventive measures to avoid postoperative reintubation in elderly patients.
In the multivariable analysis, smoking history was identified as an independent risk factor for postoperative reintubation, consistent with the results of some previous studies (4, 5, 17). Brovman et al. conducted a retrospective cohort study to determine the frequency, associated risk factors and complications of reintubation in vascular surgery patients, finding that increased age, smoking status and open thoracic and abdominal aorta surgery were independently associated with the increased risk of unplanned reintubation (5). Burton et al. obtained similar results when investigating perioperative risk factors for unplanned reintubation after lung resection (4). In their multivariable regression analysis results, the risk of postoperative unplanned reintubation increased to 1.48 times in patients with smoking history. In addition, previous studies have confirmed that smoking history was closely related to the development of other postoperative respiratory complications, such as hypoxemia and pneumonia, which can significantly increase the risk of postoperative reintubation (18–20). Thus, the relationship between smoking history and the need for postoperative reintubation can be further explained. Recently, Khanna et al. conducted a large-scale study to explore risk factors for pulmonary complications after cardiothoracic surgery, mainly including pneumonia, prolonged mechanical ventilation and reintubation (17). After multivariable regression analysis, they identified 25 independent risk factors, including age, body mass index, smoking, creatinine values, thoracic aortic surgery, and RBC input. Although the results of their analysis focused on the overall pulmonary complications after cardiothoracic surgery, there were some concordances with some of the findings of this study. At the same time, this also confirms and reminds us that smoking is harmful to health, while not smoking or quitting smoking early may have a positive impact on long-term health and the prevention of public respiratory diseases.
Another significant predictor identified by multivariable analysis for reintubation after AADS was renal insufficiency, in agreement with the results of some previous studies (7, 14, 17, 21, 22). In the findings of Beverly et al., chronic kidney disease was another independent risk factor for unplanned reintubation besides age, with a 2.2-fold increased risk compared to patients with normal renal function (7). When studying perioperative risk factors for extubation failure after cardiac surgery, Rady et al. found that blood urea nitrogen levels greater than or equal to 24 mg/dL was an independent risk factor for postoperative reintubation (22). This was similar to the results of Etz et al., who explored the predictors, prevention, and treatment for pulmonary complications after descending thoracic and thoracoabdominal aortic aneurysm repair (14). Rujirojindakul et al. conducted a time-matched, case-control study on anesthetic patients to investigate risk factors for reintubation, finding that creatinine clearance rate was independently associated with postoperative reintubation (21). Compared with patients with creatinine clearance greater than 60%, patients with creatinine clearance of 25–60% and less than 24% had a 2.49-fold and 4.08-fold increased risk of postoperative reintubation, respectively. They believed that this may be related to the fact that renal insufficiency could lead to impaired excretion of anesthetic agents and thus would prolong the duration of the drugs. In addition, renal insufficiency has been reported to be closely related to the occurrence of various postoperative respiratory complications in previous literature reports, which may also be partly responsible for the increased risk of reintubation after AADS (18–20).
The amount of intraoperative transfusion of RBCs was another significant predictor for reintubation after AADS identified by multivariable logistic regression analysis. The risk of postoperative reintubation gradually increased with the increase of RBCs infusion, consistent with previous reports (17, 22). Although blood transfusion is routine and can be life-saving in traditional cardiovascular surgery, increasing evidence indicates that massive blood transfusion is closely related with the occurrence of various postoperative complications and adverse outcomes (23–26). In the findings of Rady et al., transfusion of more than 10 units of blood products was an independent risk factor for perioperative extubation failure in cardiac surgery (22). In the findings of Khanna et al., intraoperative transfusion of RBCs and other blood products were identified as independent risk factors for postoperative pulmonary complications, with the risk increased to 1.81 and 1.52 times, respectively, and the risk increased to 1.05 times with each more 500 ml transfusion of intraoperative blood and fluids (17). Previous studies have demonstrated that massive blood transfusion can cause multiple respiratory complications such as hypoxemia and pneumonia, which is associated with inflammatory response, decreased oxygen-carrying capacity and changes in immune function (27–31). The risk of reintubation may significantly increase with gradually deteriorated cardiopulmonary function and multiple organs of the patients (32). In recent years, increasing evidence has shown that restrictive transfusion strategies are safe and effective, which are also recommended by clinical practice guidelines (33–35).
Several other predictors for postoperative reintubation have also been reported in previous reports but were not identified as predictors in our analysis, including sex, body mass index, chronic lung disease, cardiac surgery history, cardiac function, and cardiopulmonary bypass time (4, 5, 7, 11, 17, 36). This may be due to the differences in the study population and the type of surgery. The large differences in predictors for reintubation after AADS and other surgical types further revealed the specificity of AADS. Therefore, it may not be appropriate to use existing risk prediction models developed for other types of surgery to predict the risk of reintubation after AADS, which also highlighted the necessity of this study.
Some postoperative variables such as the duration of mechanical ventilation and pneumonia were identified as independent risk factors for postoperative reintubation and included in the final model in some studies (11), but we only included preoperative and intraoperative variables for analysis and for the construction of the risk prediction model. Endotracheal intubation operation can damage the defense mechanism of respiratory system, and the risk of various postoperative respiratory complications may increase significantly with the prolongation of mechanical ventilation time (37, 38). Undoubtedly, endotracheal tube should be removed and the spontaneous breathing should be resumed when conditions permit (39, 40). However, we did not include the duration of mechanical ventilation as a predictor in our multivariable analysis because this was a postoperative variable and cannot be obtained at an early stage in some patients. The purpose of early prediction cannot be achieved if these postoperative variables were included in the model.
Extubation at an optimal time after surgery may significantly improve the prognosis of patients undergoing high-risk operations such as cardiovascular surgery, where a lot of exploration and research has been conducted in this field (41, 42). Balancing the relationship between prolonged mechanical ventilation and the risk of reintubation may have a profound impact on patient outcomes. The analysis results of this study showed that after controlling for confounders by multivariate analysis, the risk of multiple in-hospital adverse outcome events remained significantly higher in the reintubation group. Therefore, a more stringent reintubation strategy should be implemented to reduce unnecessary reintubation for low-risk patients. For high-risk patients, it may be effective to reduce postoperative cardiopulmonary complications and thus reduce the risk of reintubation by strengthening preoperative expectorant, oxygen therapy, respiratory function exercise, nutrition and cardiac function, shortening intraoperative operation time, reducing unnecessary blood transfusion, implementing more reasonable fluid and drug strategies, and paying more attention to their vital signs and taking timely treatment measures (43–48). The model established in this study may have an important role in personalized risk assessment and early prevention. Implementing appropriate preventive measures for high-risk patients identified by the risk prediction model may obtain more clinical net benefits.
For patients who were successfully discharged after surgery, reintubation showed no significant association with postoperative all-cause and dissection-related deaths in the whole population analysis. The difference in the outcomes between the two groups remained statistically insignificant after controlling for confounders by multivariate analysis. This finding may also have certain guiding value for doctor-patient communication and clinical practice.
Several limitations existed in this study. First, this was a single-center small-sized exploratory study, which may limit the generalizability of the findings. However, the conclusions may be more reliable due to the fact that we adopted the same strict and identical indications for reintubation in our center, which may significantly reduce heterogeneity. Second, some potential predictors that may associate with postoperative reintubation were not included in our analysis, such as the use of some blood indicators and drugs (10). Nevertheless, the model established using the four predictors indicated reasonable discrimination, calibration and clinical usefulness. Third, the primary endpoint of this study was reintubation, but the total number of reintubation events was small, especially after propensity score matching, which may have some impact on the findings. Multicenter, large sample size studies and longer follow-up are needed to deeply explore the relationship between reintubation and clinical outcomes in future work.
Conclusions
The requirement of reintubation after AADS is prevalent, closely related to adverse in-hospital outcomes, but there is no statistically significant association between reintubation and long-term outcomes. To our knowledge, this study represents the first attempt to construct a nomogram and an online risk calculator for reintubation in patients undergoing AADS worldwide, and the first report involving the relationship between reintubation and associated in-hospital and long-term outcomes, which may have certain clinical value and guiding significance. In this study, four significant predictors for reintubation after AADS were identified, including older age, smoking history, renal insufficiency and intraoperative transfusion of RBCs. The model established using the four predictors showed moderate discrimination, good calibration and clinical utility. Three risk groups were identified as low-, medium- and high-risk groups on the basis of the model and clinical practice. These findings may have clinical utility in early individualized risk assessment, informed decision-making and targeted interventions.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Tongji Medical College of Huazhong University of Science and Technology. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
CL, FX, DW, and YZ: conception and design. XH and XD: administrative support. XY, DW, JW, and FS: provision of study materials or patients. XY, DW, YL, and XH: collection and assembly of data. XY, DW, and YL: data analysis and interpretation. All authors contributed to manuscript writing and final approval of manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 81800413), the Key Scientific Research Projects of Henan Higher Education Institutions (Grant No. 20A320036), and Key R&D and Promotion Projects in Henan Province (Grant No. 202102310123).
Acknowledgments
We sincerely thank the entire staff of the Department of Cardiovascular Surgery, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology for offering their assistance with medical services and administrative, technical, and logistic support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.1004005/full#supplementary-material
Abbreviations
AADS, Stanford type A acute aortic dissection surgery; AUC, area under the receiver operating characteristic curve; CI, confidence interval; ICU, intensive care unit; OR, odds ratio; RBC, red blood cell; ROC, receiver operating characteristic curve.
References
1. Miura S, Butt W, Thompson J, Namachivayam SP. Recurrent extubation failure following neonatal cardiac surgery is associated with increased mortality. Pediatr Cardiol. (2021) 42:1149–56. doi: 10.1007/s00246-021-02593-2
2. Miura S, Jardim PV, Butt W, Namachivayam SP. Extubation failure and major adverse events secondary to extubation failure following neonatal cardiac surgery*. Pediatr Crit Care Me. (2020) 21:e1119–25. doi: 10.1097/PCC.0000000000002470
3. Siddiqui KM, Samad K, Jonejo F, Khan MF, Ahsan K. Factors affecting reintubations after cardiac and thoracic surgeries in cardiac intensive care unit of a tertiary care hospital. Saudi J Anaesth. (2018) 12:256–60. doi: 10.4103/sja.SJA_631_17
4. Burton BN, Khoche SA, Court AM, Schmidt UH, Gabriel RA. Perioperative risk factors associated with postoperative unplanned intubation after lung resection. J Cardiothor Vasc an. (2018) 32:1739–46. doi: 10.1053/j.jvca.2018.01.032
5. Brovman EY, Steen TL, Urman RD. Associated risk factors and complications in vascular surgery patients requiring unplanned postoperative reintubation. J Cardiothor Vasc an. (2017) 31:554–61. doi: 10.1053/j.jvca.2016.11.013
6. Gao F, Yang L, He H, Ma X, Lu J, Zhai Y, et al. The effect of reintubation on ventilator-associated pneumonia and mortality among mechanically ventilated patients with intubation: A systematic review and meta-analysis. Heart Lung. (2016) 45:363–71. doi: 10.1016/j.hrtlng.2016.04.006
7. Beverly A, Brovman EY, Malapero RJ, Lekowski RW, Urman RD. Unplanned reintubation following cardiac surgery: incidence, timing, risk factors, and outcomes. J Cardiothor Vasc an. (2016) 30:1523–9. doi: 10.1053/j.jvca.2016.05.033
8. Marquez-Lara A, Nandyala SV, Fineberg SJ, Singh K. Incidence, outcomes, and mortality of reintubation after anterior cervical fusion. Spine. (2014) 39:134–139. doi: 10.1097/BRS.0000000000000098
9. Chen S, Zhang Y, Che L, Shen L, Huang Y. Risk factors for unplanned reintubation caused by acute airway compromise after general anesthesia: a case-control study. BMC Anesthesiol. (2021) 21:1238. doi: 10.1186/s12871-021-01238-4
10. Freundlich RE Li G, Domenico HJ, Moore RP, Pandharipande PP, Byrne DW, A. predictive model of reintubation after cardiac surgery using the electronic health record. Ann Thorac Surg. (2021) 113:2027–35. doi: 10.1016/j.athoracsur.2021.06.060
11. Brovman EY, Tolis G, Hirji S, Axtell A, Fields K, Muehlschlegel JD, et al. Association between early extubation and postoperative reintubation after elective cardiac surgery: a biinstitutional study. J Cardiothor Vasc an. (2021) 36:1258–64. doi: 10.1053/j.jvca.2021.11.027
12. Laudato N, Gupta P, Walters HL, Delius RE, Mastropietro CW. Risk factors for extubation failure following neonatal cardiac surgery*. Pediatr Crit Care Me. (2015) 16:859–67. doi: 10.1097/PCC.0000000000000512
13. Sadek M, Abjigitova D, Pellet Y, Rachakonda A, Panagopoulos G, Plestis K. Operative outcomes after open repair of descending thoracic aortic aneurysms in the era of endovascular surgery. Ann Thorac Surg. (2014) 97:1562–7. doi: 10.1016/j.athoracsur.2014.01.046
14. Etz CD, Di Luozzo G, Bello R, Luehr M, Khan MZ, Bodian CA, et al. Pulmonary complications after descending thoracic and thoracoabdominal aortic aneurysm repair: predictors, prevention, and treatment. Ann Thorac Surg. (2007) 83:S870–6. doi: 10.1016/j.athoracsur.2006.10.099
15. Vemuri C, Wainess RM, Dimick JB, Cowan JA, Henke PK, Stanley JC, et al. Effect of increasing patient age on complication rates following intact abdominal aortic aneurysm repair in the united states1. J Surg Res. (2004) 118:26–31. doi: 10.1016/j.jss.2004.02.007
16. Pronovost PJ, Dang D, Dorman T, Lipsett PA, Garrett E, Jenckes M, et al. Intensive care unit nurse staffing and the risk for complications after abdominal aortic surgery. Eff Clin Pract. (2001) 4:199–206.
17. Khanna AK, Kelava M, Ahuja S, Makarova N, Liang C, Tanner D, et al. A nomogram to predict postoperative pulmonary complications after cardiothoracic surgery. J Thorac Cardiovasc Surg. (2021). doi: 10.1016/j.jtcvs.2021.08.034. [Epub ahead of print].
18. Wang D, Abuduaini X, Huang X, Wang H, Chen X, Le S, et al. Development and validation of a risk prediction model for postoperative pneumonia in adult patients undergoing Stanford type A acute aortic dissection surgery: a case control study. J Cardiothorac Surg. (2022) 17:22. doi: 10.1186/s13019-022-01769-y
19. Wang D, Chen X, Wu J, Le S, Xie F, Li X, et al. Development and validation of nomogram models for postoperative pneumonia in adult patients undergoing elective cardiac surgery. Front Cardiov Med. (2021) 8:750828. doi: 10.3389/fcvm.2021.750828
20. Wang D, Huang X, Wang H, Le S, Du X. Clinical risk score for postoperative pneumonia following heart valve surgery. Chinese Med J-Peking. (2021) 134:2447–56. doi: 10.1097/CM9.0000000000001715
21. Rujirojindakul P, Geater AF, McNeil EB, Vasinanukorn P, Prathep S, Asim W, et al. Risk factors for reintubation in the post-anaesthetic care unit: a case–control study. Brit J Anaesth. (2012) 109:636–42. doi: 10.1093/bja/aes226
22. Rady MY, Ryan T. Perioperative predictors of extubation failure and the effect on clinical outcome after cardiac surgery. Crit Care Med. (1999) 27:340–7. doi: 10.1097/00003246-199902000-00041
23. Leal-Noval SR, Rincón-Ferrari MD, García-Curiel A, Herruzo-Avilés A, Camacho-Laraña P, Garnacho-Montero J, et al. Transfusion of blood components and postoperative infection in patients undergoing cardiac surgery. Chest. (2001) 119:1461–8. doi: 10.1378/chest.119.5.1461
24. Horvath KA, Acker MA, Chang H, Bagiella E, Smith PK, Iribarne A, et al. Blood transfusion and infection after cardiac surgery. Ann Thorac Surg. (2013) 95:2194–201. doi: 10.1016/j.athoracsur.2012.11.078
25. Crawford TC, Magruder JT, Fraser C, Suarez-Pierre A, Alejo D, Bobbitt J, et al. Less is more: results of a statewide analysis of the impact of blood transfusion on coronary artery bypass grafting outcomes. Ann Thorac Surg. (2018) 105:129–36. doi: 10.1016/j.athoracsur.2017.06.062
26. Möhnle P, Snyder-Ramos SA, Miao Y, Kulier A, Böttiger BW, Levin J, et al. Postoperative red blood cell transfusion and morbid outcome in uncomplicated cardiac surgery patients. Intens Care Med. (2011) 37:97–109. doi: 10.1007/s00134-010-2017-z
27. Wang Y, Xue S, Zhu H. Risk factors for postoperative hypoxemia in patients undergoing Stanford A aortic dissection surgery. J Cardiothorac Surg. (2013) 8:118. doi: 10.1186/1749-8090-8-118
28. Tormey CA, Hendrickson JE. Transfusion-related red blood cell alloantibodies: induction and consequences. Blood. (2019) 133:1821–30. doi: 10.1182/blood-2018-08-833962
29. Karsten E, Herbert BR. The emerging role of red blood cells in cytokine signalling and modulating immune cells. Blood Rev. (2020) 41:100644. doi: 10.1016/j.blre.2019.100644
30. Tinmouth A, Fergusson D, Yee IC, Hébert PC. Clinical consequences of red cell storage in the critically ill. Transfusion. (2006) 46:2014–27. doi: 10.1111/j.1537-2995.2006.01026.x
31. Roback JD, Neuman RB, Quyyumi A, Sutliff R. Insufficient nitric oxide bioavailability: a hypothesis to explain adverse effects of red blood cell transfusion. Transfusion. (2011) 51:859–66. doi: 10.1111/j.1537-2995.2011.03094.x
32. Vera UR, Bucio RE, Berrios BE, Choreno MT. Risk factors for the development of postoperative pneumonia after cardiac surgery. Arch Cardiol Mex. (2016) 86:203–7. doi: 10.1016/j.acmx.2015.12.005
33. Ferraris VA, Brown JR, Despotis GJ, Hammon JW, Reece TB, Saha SP, et al. 2011 Update to the society of thoracic surgeons and the society of cardiovascular anesthesiologists blood conservation clinical practice guidelines. Ann Thorac Surg. (2011) 91:944–82. doi: 10.1016/j.athoracsur.2010.11.078
34. Ferraris VA, Ferraris SP, Saha SP, Hessel EA, Haan CK, Royston BD, et al. Perioperative blood transfusion and blood conservation in cardiac surgery: the society of thoracic surgeons and the society of cardiovascular anesthesiologists clinical practice guideline. Ann Thorac Surg. (2007) 83:S27–86. doi: 10.1016/j.athoracsur.2007.02.099
35. Carson JL, Guyatt G, Heddle NM, Grossman BJ, Cohn CS, Fung MK, et al. Clinical Practice Guidelines From the AABB: Red Blood Cell Transfusion Thresholds and Storage. JAMA. (2016) 316:2025–35. doi: 10.1001/jama.2016.9185
36. Engoren M, Buderer NF, Zacharias A, Habib RH. Variables predicting reintubation after cardiac surgical procedures. Ann Thorac Surg. (1999) 67:661–5. doi: 10.1016/S0003-4975(98)01321-6
37. Luo Q, Su Z, Jia Y, Liu Y, Wang H, Zhang L, et al. Risk Factors for Prolonged Mechanical Ventilation After Total Cavopulmonary Connection Surgery: 8 Years of Experience at Fuwai Hospital. J Cardiothor Vasc an. (2020) 34:940–8. doi: 10.1053/j.jvca.2019.10.043
38. Tobin M, Manthous C. Mechanical Ventilation. Am J Respir Crit Care Med. (2017) 196:P3–P4. doi: 10.1164/rccm.1962P3
39. Bouadma L, Wolff M, Lucet JC. Ventilator-associated pneumonia and its prevention. Curr Opin Infect Dis. (2012) 25:395–404. doi: 10.1097/QCO.0b013e328355a835
40. Papazian L, Klompas M, Luyt C. Ventilator-associated pneumonia in adults: a narrative review. Intens Care Med. (2020) 46:888–906. doi: 10.1007/s00134-020-05980-0
41. Taboada M, Rey R, Martínez S, Soto-Jove R, Mirón P, Selas S, et al. Reintubation in the ICU following cardiac surgery. Eur J Anaesth. (2020) 37:25–30. doi: 10.1097/EJA.0000000000001019
42. Chan JL, Miller JG, Murphy M, Greenberg A, Iraola M, Horvath KA, et al. Multidisciplinary protocol-driven approach to improve extubation times after cardiac surgery. Ann Thorac Surg. (2018) 105:1684–90. doi: 10.1016/j.athoracsur.2018.02.008
43. Turquetto ALR, Dos Santos MR, Agostinho DR, Sayegh ALC, de Souza FR, Amato LP, et al. Aerobic exercise and inspiratory muscle training increase functional capacity in patients with univentricular physiology after Fontan operation: A randomized controlled trial. Int J Cardiol. (2021) 330:50–8. doi: 10.1016/j.ijcard.2021.01.058
44. Zanini M, Nery RM, de Lima JB, Buhler RP, Da Silveira AD, Stein R. Effects of different rehabilitation protocols in inpatient cardiac rehabilitation after coronary artery bypass graft surgery. J Cardiopulm Rehabil. (2019) 39:E19–25. doi: 10.1097/HCR.0000000000000431
45. Dos Santos TD, Pereira SN, Portela LOC, Cardoso DM, Lago PD, Dos Santos Guarda N, et al. Moderate-to-high intensity inspiratory muscle training improves the effects of combined training on exercise capacity in patients after coronary artery bypass graft surgery: A randomized clinical trial. Int J Cardiol. (2019) 279:40–6. doi: 10.1016/j.ijcard.2018.12.013
46. Kendall F, Oliveira J, Peleteiro B, Pinho P, Bastos PT. Inspiratory muscle training is effective to reduce postoperative pulmonary complications and length of hospital stay: a systematic review and meta-analysis. Disabil Rehabil. (2018) 40:864–82. doi: 10.1080/09638288.2016.1277396
47. Huang H, Sun X, Shi Z, Chen G, Chen L, Friedrich JO, et al. Effect of high-flow nasal cannula oxygen therapy versus conventional oxygen therapy and noninvasive ventilation on reintubation rate in adult patients after extubation: a systematic review and meta-analysis of randomized controlled trials. J Intensive Care Med. (2018) 33:609–23. doi: 10.1177/0885066617705118
48. Thille AW, Muller G, Gacouin A, Coudroy R, Decavèle M, Sonneville R, et al. Effect of postextubation high-flow nasal oxygen with noninvasive ventilation vs high-flow nasal oxygen alone on reintubation among patients at high risk of extubation failure. JAMA. (2019) 322:1465. doi: 10.1001/jama.2019.14901
Keywords: aortic dissection, reintubation, risk factors, predictive model, prognosis
Citation: Yao X, Wang J, Lu Y, Huang X, Du X, Sun F, Zhao Y, Xie F, Wang D and Liu C (2022) Prediction and prognosis of reintubation after surgery for Stanford type A aortic dissection. Front. Cardiovasc. Med. 9:1004005. doi: 10.3389/fcvm.2022.1004005
Received: 26 July 2022; Accepted: 21 September 2022;
Published: 10 October 2022.
Edited by:
Michael Koeppen, University of Tübingen, GermanyReviewed by:
Zhou Qing, Nanjing Drum Tower Hospital, ChinaYuelun Zhang, Peking Union Medical College Hospital (CAMS), China
Copyright © 2022 Yao, Wang, Lu, Huang, Du, Sun, Zhao, Xie, Wang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dashuai Wang, d2FuZ2Rhc2h1YWkmI3gwMDA0MDtodXN0LmVkdS5jbg==; Fei Xie, eGllZmVpMDEwMyYjeDAwMDQwO2h1c3QuZWR1LmNu; Yangchao Zhao, emhhb3lhbmdjaGFvMTI1JiN4MDAwNDA7MTI2LmNvbQ==; Chao Liu, bGl1YmVpbHVuJiN4MDAwNDA7bWUuY29t
†These authors have contributed equally to this work
 Xingxing Yao1†
Xingxing Yao1† Yang Lu
Yang Lu Xiaofan Huang
Xiaofan Huang Xinling Du
Xinling Du Yangchao Zhao
Yangchao Zhao Fei Xie
Fei Xie Dashuai Wang
Dashuai Wang