
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 24 January 2023
Sec. Cardiovascular Epidemiology and Prevention
Volume 9 - 2022 | https://doi.org/10.3389/fcvm.2022.1002820
This article is part of the Research Topic Epidemiology and Clinical Researches in Atherosclerosis and Cardiovascular Disease View all 13 articles
Background: Atherosclerotic cardiovascular disease (ASCVD) risk differs by ethnicity. In comparison with Europeans (EA) South Asian (SA) people in UK experience higher risk of coronary heart disease (CHD) and stroke, while African Caribbean people have a lower risk of CHD but a higher risk of stroke.
Aim: To compare carotid atherosclerosis in EA, SA, and AC participants in the Southall and Brent Revisited (SABRE) study and establish if any differences were explained by ASCVD risk factors.
Methods: Cardiovascular risk factors were measured, and carotid ultrasound was performed in 985 individuals (438 EA, 325 SA, 228 AC). Carotid artery plaques and intima-media thickness (cIMT) were measured. Associations of carotid atherosclerosis with ethnicity were investigated using generalised linear models (GLMs), with and without adjustment for non-modifiable (age, sex) and modifiable risk factors (education, diabetes, hypertension, total cholesterol, HDL-C, alcohol consumption, current smoking).
Results: Prevalence of any plaque was similar in EA and SA, but lower in AC (16, 16, and 6%, respectively; p < 0.001). In those with plaque, total plaque area, numbers of plaques, plaque class, or greyscale median did not differ by ethnicity; adjustment for risk factors had minimal effects. cIMT was higher in AC than the other ethnic groups after adjustment for age and sex, adjustment for risk factors attenuated this difference.
Conclusion: Prevalence of carotid artery atherosclerotic plaques varies by ethnicity, independent of risk factors. Lower plaque prevalence in in AC is consistent with their lower risk of CHD but not their higher risk of stroke. Higher cIMT in AC may be explained by risk factors. The similarity of plaque burden in SA and EA despite established differences in ASCVD risk casts some doubt on the utility of carotid ultrasound as a means of assessing risk across these ethnic groups.
Atherosclerotic cardiovascular disease (ASCVD) is the leading cause of mortality and morbidity worldwide (1). There are marked differences in ASCVD risk in different ethnic groups, even within the same country. For example the risk of coronary heart disease (CHD) is ∼1.7-fold higher in migrants from the Indian subcontinent than in people of European origin in UK (2). In contrast, people of African-Caribbean ethnicity in the UK have markedly elevated risk of stroke, but their risk of CHD is lower in comparison with Europeans or migrants from the Indian subcontinent (2). In all ethnic groups, established risk factors [e.g., blood pressure (BP), total cholesterol, high-density lipoprotein cholesterol (HDL-C), diabetes, education, alcohol consumption, and tobacco smoking] predict risk of ASCVD (3, 4), although some the prevalence of some risk factors, such as dysglycaemia, smoking and adiposity differ by ethnicity (5, 6). Previous work suggests differences in these factors only partially explain ethnic differences in ASCVD risks (2).
Detailed phenotyping of subclinical atherosclerosis may provide more insights into ethnic differences in ASCVD risk. Ultrasonography is a reliable and non-invasive technique that is widely used to assess atherosclerosis in the carotid artery (7). In addition to measurement of common carotid artery intima-media thickness (cIMT) and quantification of atherosclerotic plaques (8, 9), this method can also provide some information on plaque composition and vulnerability (10–12).
Based on the existing evidence in relation to ethnic difference in ASCVD risk, we therefore hypothesised that, in comparison with Europeans, South Asian people would have a greater burden of carotid atherosclerosis and that African Caribbean people would have similar or lower levels. We also aimed to investigate whether plaque characteristics differed by ethnicity and the potential role of established ASCVD risk factors in differences observed between ethnic groups. Individuals studied were participants in the third follow-up visit of the South and Brent Revisited (SABRE) study, a multi-ethnic longitudinal cohort that has been followed up for over 30 years.
Detailed information about the Southall and Brent Revisited (SABRE) study has been published in previously (13, 14). In brief, SABRE is a longitudinal study that recruited European (EA), South Asian (SA), and African Caribbean (AC) participants living in West and North London in 1988–1991, when they were aged 40–69 years. Participants’ ethnicity was determined by interviewers based on grand-parental origin and confirmed by participants. Surviving participants who remained in the study have undergone follow-up clinic-based investigations at 20-years (visit 2: 2008–2011) and 25-year (visit 3: 2014–2018). For the latter visit the partners of the original participants were also invited to attend. The current study included 991 individuals (437 European, 326 South Asian, 228 African Caribbean) from visit 3 (Figure 1). Ethical approval for the study was obtained from Ealing, Hounslow and Spelthorne, Parkside, and University College London Research Ethics Committees and all participants provided written informed consent.
Participants were invited to a clinic appointment and were asked to refrain from alcohol, smoking, and caffeine for ≥12 h before attendance, and not to take their medication on the morning of the clinic visit. Information was recorded on age, sex, health behaviours, medical history, and medication (14). Height and weight were measured using a standardised protocol and body composition was measured using a Tanita BC 418 body composition analyser. Seated brachial BP was measured using an appropriately sized cuff using an automatic Omron 705 IT after 5–10 min rest according to ESH guidelines (15). The average of the second and third recordings was used as the estimate of clinic BP. Diabetes mellitus was defined according to the 1999 WHO guidelines (16), or physician diagnosis or receipt of anti-diabetes medications. Hypertension was defined as physician-diagnosed hypertension or participant-reported hypertension or receipt of BP-lowering medication. Smoking was classified into current or not. Alcohol consumption was categorised according to UK guidelines into none, ≤14 units per week or >14 units per week. Blood and urine samples were taken, and whole blood, serum, EDTA plasma and urine stored at −80°C prior to analysis. Glycosylated haemoglobin (HbA1c) was measured on an automated platform (c311, Roche Diagnostics, Burgess Hill, UK), serum total cholesterol, HDL-C and triglycerides were measured using enzymatic methods (Roche/Hitachi cobas c system). Low density lipoprotein cholesterol (LDL-C), All assays used the manufacturers calibration and quality control material.
Ultrasound scans were performed by an experienced sonographer using a GE Vivid I Ultrasound system equipped with a 6-13 mHz broadband linear array transducer (12L-RS). The common carotid artery (CCA), internal carotid artery (ICA), external carotid artery (ECA) was assessed along the long- and short-axes bilaterally. Two-dimensional-images, spectral-Doppler imaging, Colour and Power Doppler were also recorded. Adequate quality of ECG signals and ultrasound images was ensured throughout the examination. A cine loop of at least five cardiac cycles at three angles (lateral, posterior, and anterior) as well as one 8-bit greyscale image captured at the R wave for each angle were acquired. cIMT and carotid lumen diameter was measured from the best visualised image over a 10 mm segment in the CCA according to the American Society of Echocardiography Carotid Intima-Media Thickness Task Force Consensus Statement (17). Plaque was defined according to the Mannheim consensus (8, 9), as a focal lesion that encroached into the carotid artery lumen by ≥0.5 mm or ≥50% of the surrounding cIMT value or had a thickness >1.5 mm as measured from the media-adventitia interface to the intima-lumen boundary. Carotid stenosis >50% was assessed by visual inspection of the B-mode ultrasound scan, using Colour and Power Doppler imaging as needed, and quantified according to NASCET criteria (18). All quantitative analyses were performed offline using validated software (AMS II) (19) that included automated measurement of plaque area, categorisation of plaque based on the Grey-Weale score (10), plaque size and estimation of grey-scale median (GSM) (20, 21). Repeatability and reproducibility of cIMT and plaque characteristics have been reported previously (22).
Statistical analyses were performed with Stata v.17.1 (StataCorp, College Station, TX, USA). Continuous data for the sample were summarised as means and standard deviations (SD) or median (interquartile range) for skewed data, categorical data as counts and percentages. Normality was assessed through frequency histograms, QQ plots and Shapiro-Wilk tests. Comparisons between ethnic groups were made using generalized linear modelling (GLM). Two models were used to provide further insight into ethnic differences: (Model 1) non-modifiable risk factors (age and sex); (Model 2) Model 1 plus established modifiable risk factors (diabetes, hypertension, total cholesterol, HDL-C, years of education, alcohol consumption, current smoking, statin medication). Choice of covariates was based on a priori knowledge (4, 23). Additional sensitivity analyses were performed where diabetes was replaced by HbA1c, or where systolic BP or body mass index (BMI) (or waist hip ratio) were added to models (these models showed negligible differences from the original models and the results are not presented). The possibility of effect modification by sex was looked for in all models by including a sex × ethnicity interaction term, if this was not statistically significant both sexes were pooled for analysis, otherwise it was planned that results for both sexes would be analysed separately.
Dichotomous variables (e.g., presence of plaque or presence of carotid stenosis >50%) were modelled using GLM with a binomial and log family and link function. Ordered categorical variables with fewer than six categories [median plaque grade (manual and automatic)] were analysed using ordered logistic regression and the proportional-odds assumption was tested using an approximate likelihood ratio test. If the proportional-odds assumption was not met data were fit with partial proportional odds models using generalised ordinal logistic regression (gologit2) (24). Numbers of plaques were modelled using negative binomial models as data were expected to be over-dispersed (this was confirmed using the likelihood ratio test for alpha = 0). Risk ratios, or marginal probabilities and 95% confidence intervals (CI) were estimated from these models. Multiple linear regression models were used for continuous measures (total area of plaques, lowest GSM of all plaques, cIMT) and marginal means and CI estimated. If regression models showed evidence of heteroskedasticity, robust standard errors were calculated. Assumptions of linearity were checked by examination of residuals and if necessary, it was planned that non-linear models would be constructed using fractional polynomials. The primary analysis was a complete case analysis which is valid under the assumption that missingness was independent of outcomes. As a sensitivity analysis, models using full information maximum likelihood which is valid under the missing at random (MAR) assumption were also examined for linear models. Inference was based on a combination of p-values, effect sizes and CI, no adjustment was made for multiple comparisons.
Table 1 shows the characteristics of the sample stratified by ethnicity. Participants were aged between 40 and 69 years and comprised 437 EA (mean age 74 years, 62% male), 326 SA (mean age 73.2 ± 6.3 years, 59.3% male), and 228 AC (mean age 71 years, 35.6% male). On average SA were slightly younger than EA and AC were younger than both EA and SA, and there were more women in the AC sample. AC and SA people were shorter, had higher systolic BP and more diabetes and hypertension than EA. Compared with EA, SA had a higher prevalence of known CHD, more years of education, lower heart rate, lower BMI, were shorter and were less likely to be current smokers, and less likely to consume high quantities of alcohol, while AC had a lower prevalence of CHD, higher BMI, higher diastolic BP, more diabetes and hypertension and were less likely to consume high quantities of alcohol.
Carotid artery intima-media thickness was similar by ethnicity in an unadjusted model, but plaques were more frequent in EA and SA than AC (Table 1); however, there was no difference between EA and SA (Table 1). Plaques were more common in men than women but there was no evidence that sex modified the ethnic differences in plaque prevalence (Figure 2). There were no marked differences in distribution of plaques by ethnicity: 57.5% of Europeans had plaques in the left carotid artery, 47.6% in the right carotid artery and 44.1% had plaques bilaterally. A total of 42.5% of South Asians had plaques in the left carotid artery, 44.1% in the right carotid artery and 32.7% had plaques bilaterally. A total of 10% of African Caribbean’s had plaques in left of carotid artery, 8.1% in the right carotid artery and 23.0% had plaques bilaterally (Figure 1). In comparison with EA, the risk ratios for having any plaque in SA after adjustment for non-modifiable, or non-modifiable plus modifiable risk factors were 1.08 (0.79, 1.46); p = 0.65 and 0.98 (0.66, 1.41); p = 0.89, respectively. For AC the comparable risk ratios were 0.48 (0.28, 0.82); p = 0.013 and 0.53 (0.268, 1.047); p = 0.068. The marginal probabilities of having one or more plaques in each ethnic group are shown in Table 2 with and without adjustment. The probability of having one or more plaques was similar in EA and SA but was lower by ∼50% in AC. Statistical adjustment had little effect on these estimates, although the estimated CI of the fully adjusted model were wider, probably as a result of the reduced sample size of the complete case analysis for non-modifiable plus modifiable risk factors (n = 727). Compared with EA, the risk ratio in SA for having a stenosis >50% was 1.56 (0.88, 2.76); p = 0.12 and 1.70 (0.81, 3.52); p = 0.15. For AC the risk ratios were 0.61 (0.23, 1.61); p = 0.32 and 0.65 (0.25, 1.70); p = 0.38. The limited number of stenoses in the sample (n = 50) made these estimates very imprecise and scope for inference was limited.
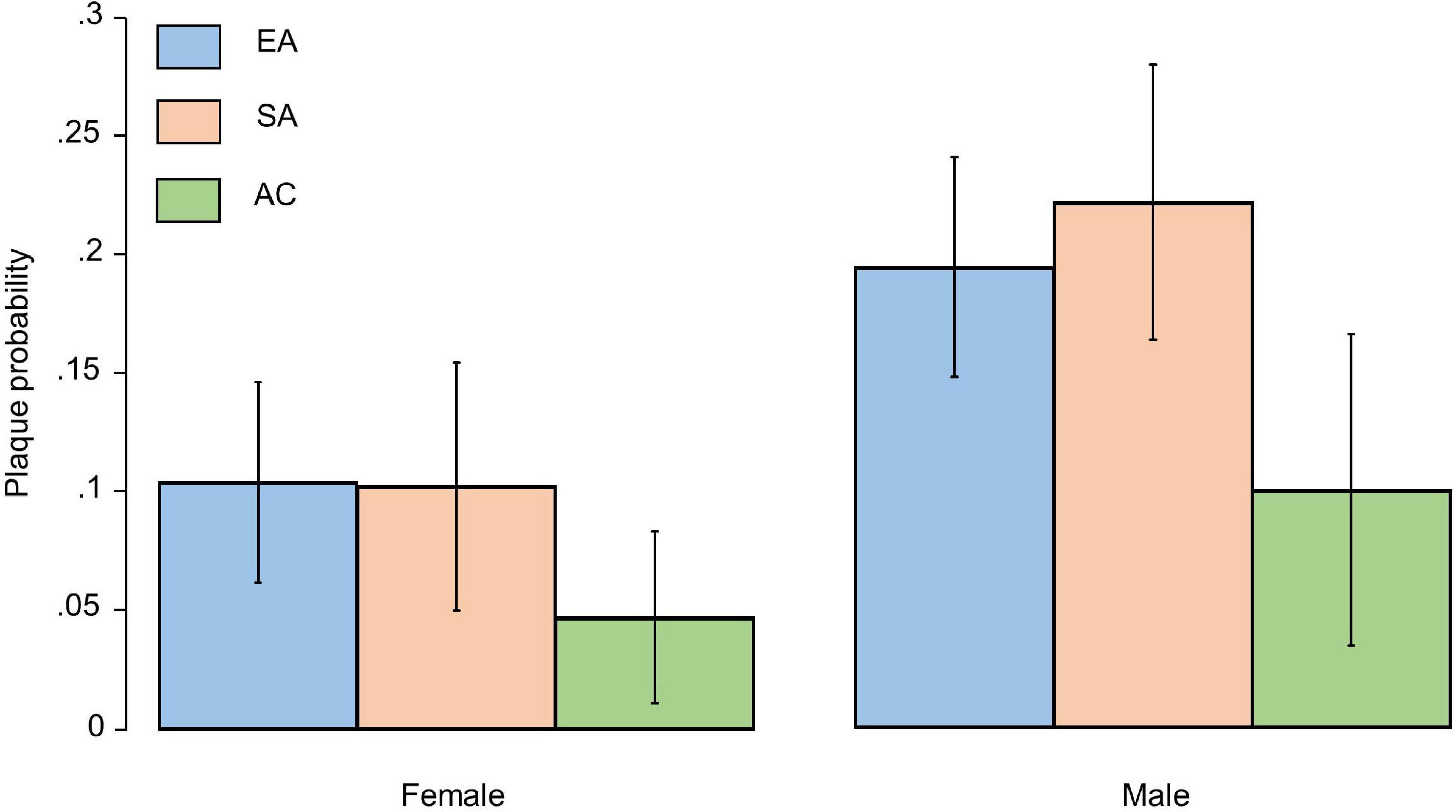
Figure 2. Bar plot showing marginal probabilities with 95% confidence intervals (adjusted for age) of having a plaque by sex and ethnicity. EA, European; SA, South Asian, and AC, African Caribbean.
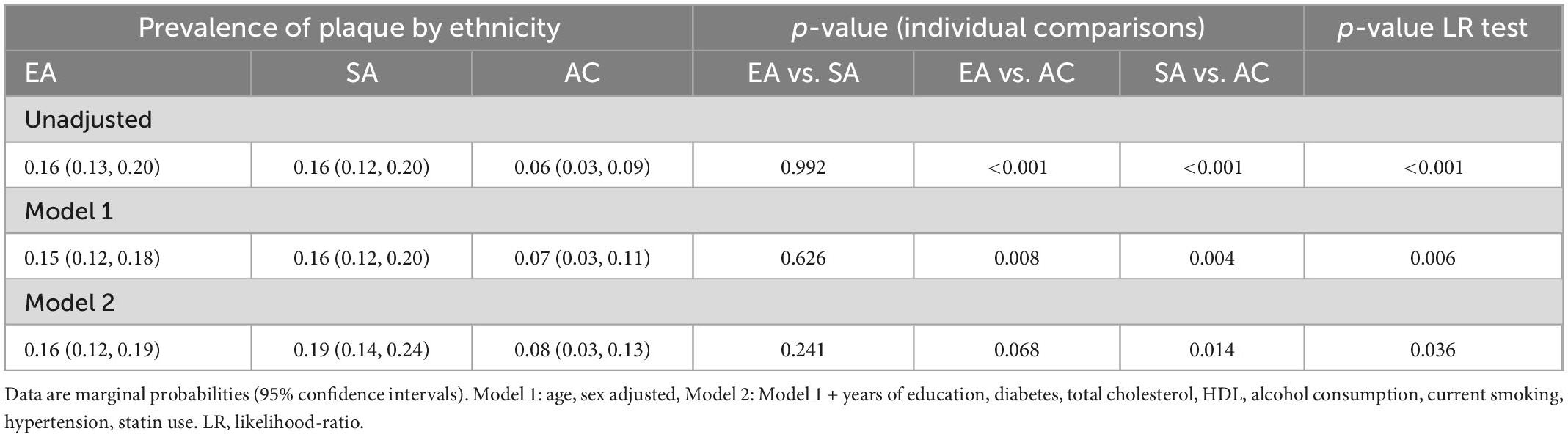
Table 2. Marginal probabilities of having any plaques by ethnicity with and without adjustment for risk factors.
After adjustment for age and sex cIMT was higher in AC than EA or SA but there was no difference in cIMT between EA and SA (Table 3). Further adjustment for risk factors attenuated differences by ethnicity (Table 4).
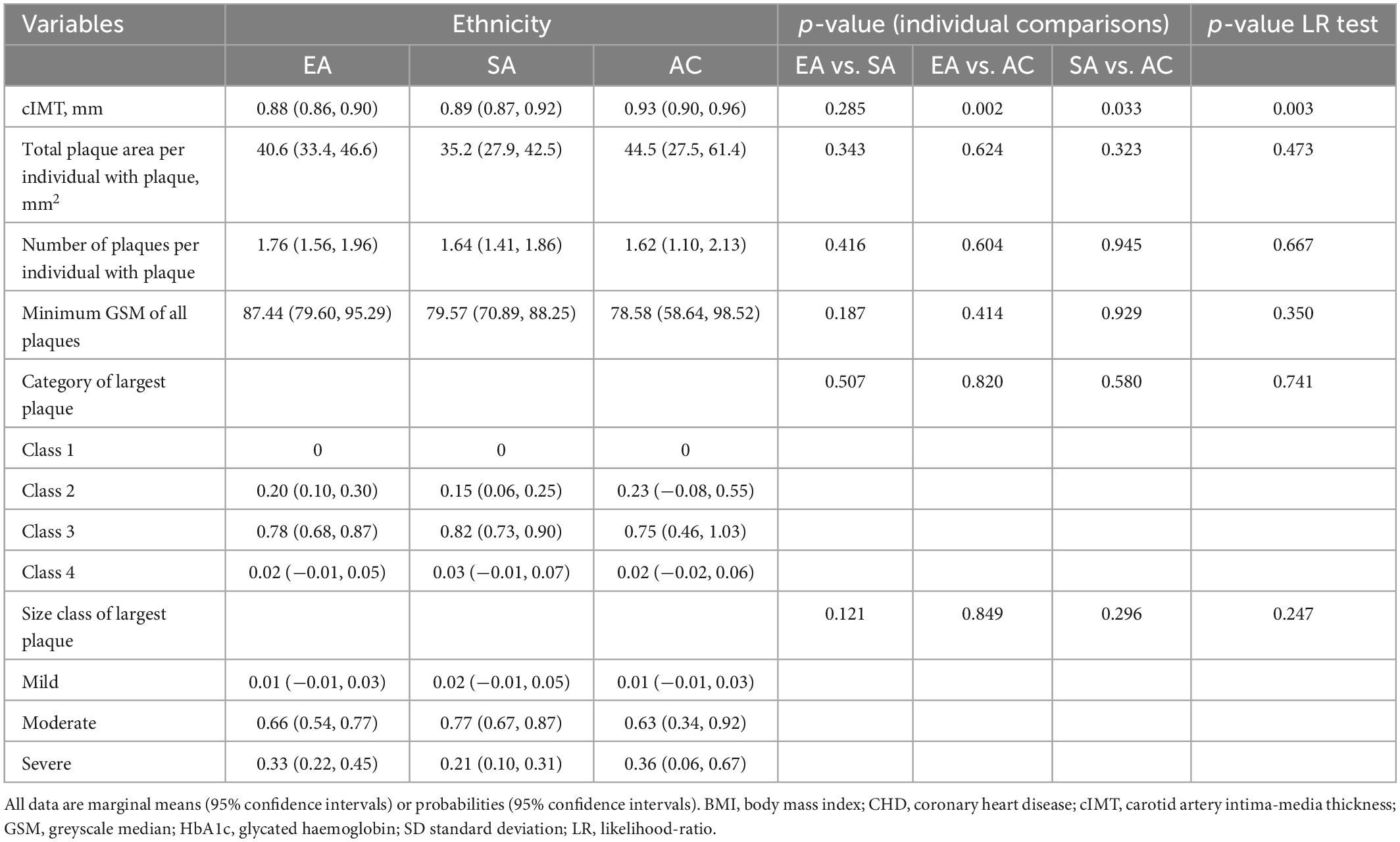
Table 3. Ethnic differences in cIMT and plaque characteristics with adjustment for age and sex (Model 1).
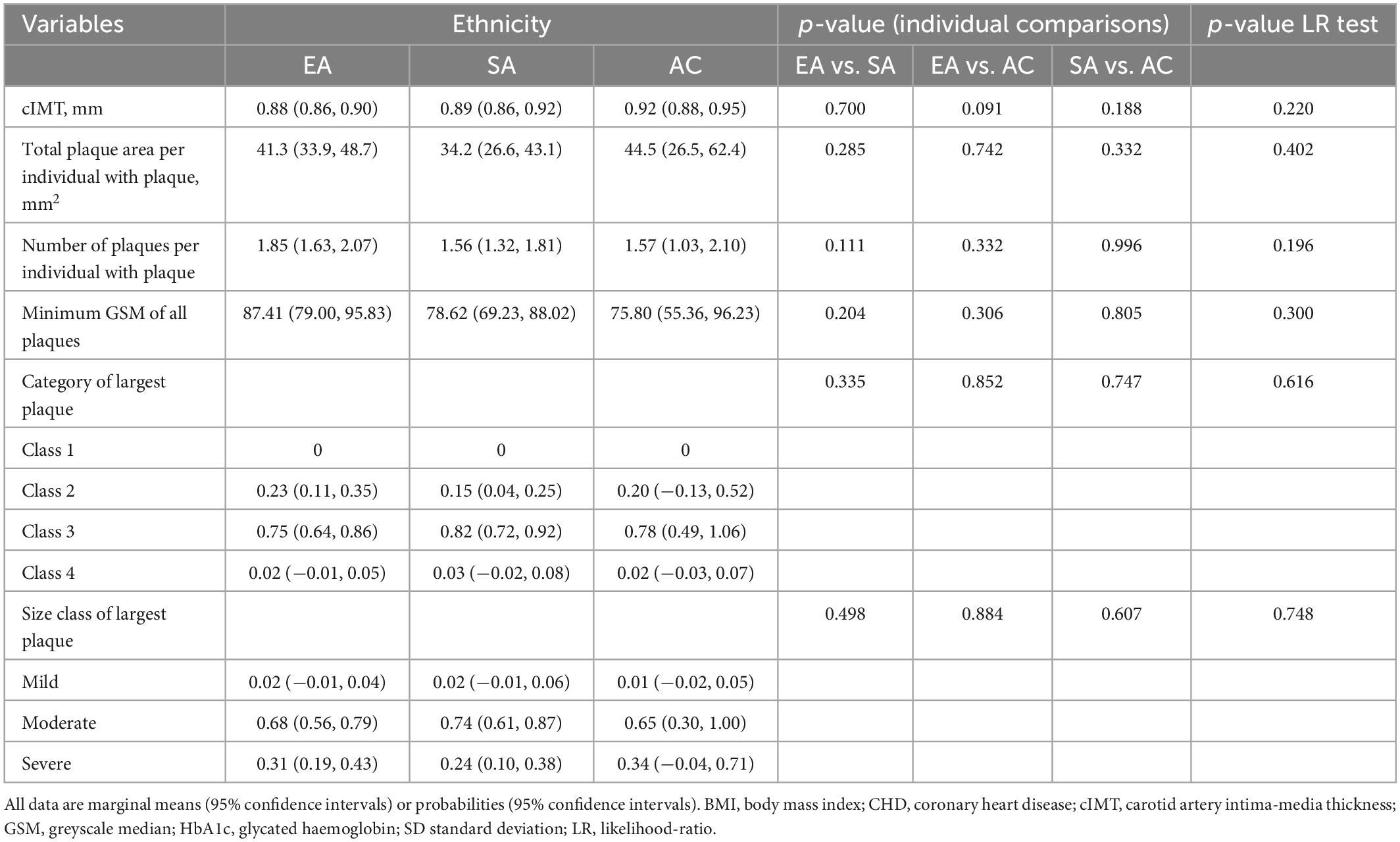
Table 4. Ethnic differences in cIMT and plaque characteristics with adjustment for non-modifiable and modifiable risk factors (Model 2).
In individuals with plaque, a comparison of plaque area, average number of plaques, minimum greyscale median, and plaque class is shown in Tables 3, 4 following adjustment for risk factors. Total plaque area was similar in all ethnic groups, as was plaque class and echogenicity as assessed by GSM.
We found ethnic differences in the prevalence of carotid plaque in a population-based sample of people in UK. People of AC ethnicity had a lower occurrence of carotid plaque than the other ethnic groups, while the burden of plaque in EA and SA was similar. The lack of difference between EA and SA was surprising considering the large excess of cardiovascular disease reported in SA and our data for carotid plaque prevalence are not consistent with previous estimates of excess CHD risk in SA (2). In those with plaque, plaque characteristics differed little between ethnic groups, in particular there was no evidence of SA having evidence of more lipid-rich or vulnerable plaques. Ethnic differences in plaque prevalence were unexplained by disparities in ASCVD risk factors. It therefore remains unclear why the AC group had a lower prevalence of carotid plaques than the other ethnic groups, however, this observation is consistent with previous work, including in SABRE, showing lower risk of CHD in people of AC ethnicity in UK, which was unexplained by conventional ASCVD risk factors (25, 26). cIMT also differed by ethnicity, after adjustment for age and sex, cIMT was higher in AC compared with the other ethnic groups, which could be consistent with their higher risk of stroke, but is inconsistent with their lower risk of CHD; this difference was attenuated after adjustment for non-modifiable and modifiable risk factors and is likely to be attributable to differences in ASCVD risk factors.
Better understanding and assessment of the prevalence of atherosclerosis and its relationship to cardiovascular risk factors in different ethnic groups is important. Such relationships may also provide insights into the pathogenesis of atherosclerosis in all ethnic groups. Our failure to identify factors explaining ethnic differences in carotid atherosclerotic plaque despite adjustment for ASCVD risk factors suggests that important determinants of ethnic differences in atherosclerosis susceptibility remain to be identified. Mechanisms related to population migration (27), socio-economic disadvantage (28) and racism (28, 29) seem plausible explanations, but given the differences observed between minority ethnic groups in this study this question merits further study. We cannot exclude genetic differences between populations of difference ancestry but currently there is little or no evidence to suggest that genetics makes a major contribution to ethnic differences in susceptibility to ASCVD (30, 31).
Previous studies have examined ethnic differences in carotid atherosclerosis, although few have included SA people. A UK community-based study found higher cIMT and lower prevalence of plaque in AC compared with EA (32) and this difference remained after adjustment for conventional ASCVD risk factors. Another UK-based study observed marginally higher cIMT in EA compared with SA despite higher prevalence of ASCVD in SA (33). In the US, the Multi-Ethnic Study of Atherosclerosis found that cIMT was higher in people of African American ethnicity, but the risk of new plaque formation was lower in African American, Hispanic and Chinese ethnicities compared with White Americans after adjustment for traditional ASCVD risk factors (34). The Diabetes Heart Study also found that African American people with T2DM had higher cIMT but lower prevalence of carotid plaque compared with those of European ancestry (35). In contrast, the Northern Manhattan Stroke study found similar maximum internal carotid artery plaque thickness (MICPT) in stroke-free African- and European- ethnicity individuals but lower MICPT in people of Hispanic ethnicity (36). A recent individual participant meta-analysis that compared the association of ASCVD risk factors with cIMT in different ethnicities from a range of countries, reported that high cIMT levels was highest amongst African American populations, similar in Asian, White and Hispanic people and lowest in African populations. In keeping with our findings, adjustment for risk factors only marginally attenuated these differences (37). Overall, despite some inconsistencies the results of these previous studies appear broadly consistent with our findings.
As has been observed in some previous studies (32–34), cIMT corresponded poorly with known risk differentials for ASCVD, especially CHD, in the ethnic groups. Plaque prevalence was consistent with the known lower risk of CHD in AC, but not with the elevated risk of ASCVD in SA or the elevated risk of stroke in AC (38, 39). This raises questions about the reliability of cIMT and plaque as a screening tool for early detection of atherosclerosis across different ethnic groups. For cIMT it has previously been suggested that arterial wall remodelling in response to haemodynamic stresses might complicate interpretation (40), but it is not obvious that this could explain the ethnic discordance between ASCVD risk and plaque prevalence, given the latter is generally considered a better predictor of ASCVD risk (41).
This study has limitations and strengths: it is cross-sectional so causal conclusions cannot be made. Participants were drawn from a randomly selected population-based cohort but possible bias due to non-participation, attrition, missing data and residual confounding by unmeasured or imprecisely measured variables cannot be excluded. As might be expected in a population-based sample, the frequency of carotid plaque was quite low, particularly in AC, which may have limited our ability to detect small differences in plaque prevalence or characteristics, nevertheless the precision of the estimates was sufficient to exclude disparities in plaque prevalence consistent with CVD risk differentials in South Asians. Our categorisation of ethnicity is crude and may obscure important differences within ethnic groups; (42), however, our categories reflect the original study design and correspond to the broad ethnic groups in used by the UK classification scheme (43). AC participants mostly migrated between 1950 and 1960 (i.e., around the ages of 20 to 30), while most of the SA participants arrived in the UK in the 1970’s (i.e., around 40 years old) and limited data was available about exposures, including childhood exposures and healthcare provision, that occurred prior to migration or extent of acculturation after migration. We included a comprehensive set of risk factors for ASCVD, but we acknowledge that including these risk factors, which potentially act as mediators of ethnic differences, could introduce bias (44). The study’s strengths are first and foremost its community-based methodology and that it compares people of different ethnicities in the same location. SA and AC participants make up the majority of British first-generation migrants and, unlike in some countries, universal healthcare, free at the point of use, is available in UK. This may lessen, though not abolish disadvantages in health access (45). All examinations were conducted according to a strict approach, resulting in a comprehensive phenotyping of this older age sample.
In people resident in UK, EA and SA have a higher burden of atherosclerotic plaques in carotid arteries than AC, while in contrast cIMT was higher in AC than other ethnicities. These differences were unexplained by ASCVD risk factors. The disparity between these findings and the known risks of ASCVD in these ethnic groups raises questions about the utility of carotid ultrasound as a tool to predict risk in multi-ethnic populations.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Ealing, Hounslow and Spelthorne, Parkside, and University College London Research Ethics Committees. The patients/participants provided their written informed consent to participate in this study.
RA and ADH had full access to all the data in the study. RA performed the statistical analyses and wrote the first draft of the manuscript. All authors contributed to study design and interpretation and approved the final manuscript.
This SABRE study was funded at baseline by the Medical Research Council, Diabetes UK, and the British Heart Foundation. At follow-up the study was funded by the Wellcome Trust (067100, 37055891, and 086676/7/08/Z), the British Heart Foundation (PG/06/145, PG/08/103/26133, PG/12/29/29497, and CS/13/1/30327), and Diabetes UK (13/0004774). ADH receives support from the British Heart Foundation, the Horizon 2020 Framework Programme of the European Union, the National Institute for Health Research University College London Hospitals Biomedical Research Centre, the UK Medical Research Council, the Wellcome Trust, and works in a unit that receives support from the UK Medical Research Council (MC_UU_12015/5). RA was supported by a Ph.D. scholarship grant from King Abdul-Aziz University. SE was funded by a Diabetes UK Sir George Alberti Research Training Fellowship (Grant No. 17/0005588).
We are extremely grateful to all the people who took part in the study, and past and present members of the SABRE team who helped to collect the data.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Naghavi M, Abajobir A, Abbafati C, Abbas K, Abd-Allah F, Abera S, et al. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980-2016: a systematic analysis for the global burden of disease study 2016. Lancet. (2017) 390:1151–210. doi: 10.1016/S0140-6736(17)32152-9
2. Tillin T, Hughes A, Mayet J, Whincup P, Sattar N, Forouhi N, et al. The relationship between metabolic risk factors and incident cardiovascular disease in Europeans, South Asians, and African Caribbeans: sabre (Southall and Brent Revisited) – a prospective population-based study. J Am Coll Cardiol. (2013) 61:1777–86. doi: 10.1016/j.jacc.2012.12.046
3. Eriksen A, Tillin T, O’Connor L, Brage S, Hughes A, Mayet J, et al. The impact of health behaviours on incident cardiovascular disease in Europeans and South Asians - a prospective analysis in the UK sabre study. PLoS One. (2015) 10:e0117364. doi: 10.1371/journal.pone.0117364
4. Yusuf S, Joseph P, Rangarajan S, Islam S, Mente A, Hystad P, et al. Modifiable risk factors, cardiovascular disease, and mortality in 155 722 individuals from 21 high-income, middle-income, and low-income countries (pure): a prospective cohort study. Lancet. (2020) 395:795–808. doi: 10.1016/S0140-6736(19)32008-2
5. Tillin T, Hughes A, Godsland I, Whincup P, Forouhi N, Welsh P, et al. Insulin resistance and truncal obesity as important determinants of the greater incidence of diabetes in Indian Asians and African Caribbeans compared with Europeans: the southall and brent revisited (sabre) cohort. Diabetes Care. (2013) 36:383–93. doi: 10.2337/dc12-0544
6. Eastwood S, Tillin T, Dehbi H, Wright A, Forouhi N, Godsland I, et al. Ethnic differences in associations between fat deposition and incident diabetes and underlying mechanisms: the sabre study. Obesity. (2015) 23:699–706. doi: 10.1002/oby.20997
7. Murray C, Nahar T, Kalashyan H, Becher H, Nanda N. Ultrasound assessment of carotid arteries: current concepts, methodologies, diagnostic criteria, and technological advancements. Echocardiography. (2018) 35:2079–91. doi: 10.1111/echo.14197
8. Touboul P, Hennerici M, Meairs S, Adams H, Amarenco P, Bornstein N, et al. Mannheim carotid intima-media thickness consensus (2004-2006). an update on behalf of the advisory board of the 3rd and 4th watching the risk symposium, 13th and 15th European stroke conferences, Mannheim, Germany, 2004, and Brussels, Belgium, 2006. Cerebrovasc Dis. (2007) 23:75–80. doi: 10.1159/000097034
9. Touboul P, Hennerici M, Meairs S, Adams H, Amarenco P, Bornstein N, et al. Mannheim carotid intima-media thickness and plaque consensus (2004-2006-2011). An update on behalf of the advisory board of the 3rd, 4th and 5th watching the risk symposia, at the 13th, 15th and 20th European stroke conferences, Mannheim, Germany, 2004, Brussels, Belgium, 2006, and Hamburg, Germany, 2011. Cerebrovasc Dis. (2012) 34:290–6. doi: 10.1159/000343145
10. Gray-Weale A, Graham J, Burnett J, Byrne K, Lusby R. Carotid artery atheroma: comparison of preoperative B-mode ultrasound appearance with carotid endarterectomy specimen pathology. J Cardiovasc Surg. (1988) 29:676–81.
11. Biasi G, Sampaolo A, Mingazzini P, De Amicis P, El-Barghouty N, Nicolaides A. Computer analysis of ultrasonic plaque echolucency in identifying high risk carotid bifurcation lesions. Eur J Vasc Endovasc Surg. (1999) 17:476–9. doi: 10.1053/ejvs.1999.0789
12. Jashari F, Ibrahimi P, Bajraktari G, Gronlund C, Wester P, Henein M. Carotid plaque echogenicity predicts cerebrovascular symptoms: a systematic review and meta-analysis. Eur J Neurol. (2016) 23:1241–7. doi: 10.1111/ene.13017
13. Tillin T, Forouhi N, McKeigue P, Chaturvedi N. Southall and brent revisited: cohort profile of sabre, a UK population-based comparison of cardiovascular disease and diabetes in people of European, Indian Asian and African caribbean origins. Int J Epidemiol. (2012) 41:33–42. doi: 10.1093/ije/dyq175
14. Jones S, Tillin T, Park C, Williams S, Rapala A, Al Saikhan L, et al. Cohort profile update: southall and brent revisited (sabre) study: a UK population-based comparison of cardiovascular disease and diabetes in people of European, South Asian and African caribbean heritage. Int J Epidemiol. (2020) 49:1441–2e. doi: 10.1093/ije/dyaa135
15. O’Brien E, Asmar R, Beilin L, Imai Y, Mancia G, Mengden T, et al. Practice guidelines of the European society of hypertension for clinic, ambulatory and self blood pressure measurement. J Hypertens. (2005) 23:697–701. doi: 10.1097/01.hjh.0000163132.84890.c4
16. World Health Organization [WHO]. Definition, Diagnosis and Classification of Diabetes Mellitus and Its Complications. Part 1: Diagnosis and Classification of Diabetes Mellitus. Contract No.: (WHO/NCD/NCS/99.2). Geneva: World Health Organization (1999).
17. Stein J, Korcarz C, Hurst R, Lonn E, Kendall C, Mohler E, et al. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American society of echocardiography carotid intima-media thickness task force. endorsed by the society for vascular medicine. J Am Soc Echocardiogr. (2008) 21:93–111. doi: 10.1016/j.echo.2007.11.011
18. North American Symptomatic Carotid Endarterectomy Trial (NASCET) Steering Committee. North American symptomatic carotid endarterectomy trial. methods, patient characteristics, and progress. Stroke. (1991) 22:711–20. doi: 10.1161/01.str.22.6.711
19. Wendelhag I, Liang Q, Gustavsson T, Wikstrand JA. New automated computerized analyzing system simplifies readings and reduces the variability in ultrasound measurement of intima-media thickness. Stroke. (1997) 28:2195–200. doi: 10.1161/01.str.28.11.2195
20. Bjornsdottir G, Sigurdsson S, Sturlaugsdottir R, Gudmundsdottir A, Eiriksdottir G, Thorsson B, et al. Longitudinal changes in size and composition of carotid artery plaques using ultrasound: adaptation and validation of methods (inter- and intraobserver variability). J Vasc Ultras. (2018) 38:198–208. doi: 10.1177/154431671403800402
21. Ostling G, Hedblad B, Berglund G, Gonçalves I. Increased echolucency of carotid plaques in patients with type 2 diabetes. Stroke. (2007) 38:2074–8. doi: 10.1161/strokeaha.106.480830
22. Al Saikhan L, Alobaida M, Bhuva A, Chaturvedi N, Heasman J, Hughes A, et al. Imaging protocol, feasibility, and reproducibility of cardiovascular phenotyping in a large tri-ethnic population-based study of older people: the southall and brent revisited (sabre) study. Front Cardiovasc Med. (2020) 7:591946. doi: 10.3389/fcvm.2020.591946
23. Collins G, Altman D. An independent and external validation of Qrisk2 cardiovascular disease risk score: a prospective open cohort study. Br Med J. (2010) 340:c2442. doi: 10.1136/bmj.c2442
24. Williams R. Generalized ordered logit/partial proportional odds models for ordinal dependent variables. Stata J. (2006) 6:58–82. doi: 10.1177/1536867x0600600104
25. McKeigue P, Shah B, Marmot M. Relation of central obesity and insulin resistance with high diabetes prevalence and cardiovascular risk in South Asians. Lancet. (1991) 337:382–6.
26. McKeigue P, Ferrie J, Pierpoint T, Marmot M. Association of early-onset coronary heart disease in South Asian men with glucose intolerance and hyperinsulinemia. Circulation. (1993) 87:152–61. doi: 10.1161/01.cir.87.1.152
27. Patel J, Vyas A, Cruickshank J, Prabhakaran D, Hughes E, Reddy K, et al. Impact of migration on coronary heart disease risk factors: comparison of Gujaratis in Britain and their contemporaries in villages of origin in India. Atherosclerosis. (2006) 185:297–306. doi: 10.1016/j.atherosclerosis.2005.06.005
28. Javed Z, Haisum Maqsood M, Yahya T, Amin Z, Acquah I, Valero-Elizondo J, et al. Race, racism, and cardiovascular health: applying a social determinants of health framework to racial/ethnic disparities in cardiovascular disease. Circ Cardiovasc Qual Outcomes. (2022) 15:e007917. doi: 10.1161/circoutcomes.121.007917
29. Banerjee S, Aaysha Cader F, Gulati M, Capers Q. Racism and cardiology: a global call to action. CJC Open. (2021) 3:S165–73. doi: 10.1016/j.cjco.2021.09.014
30. Benjamin I, Brown N, Burke G, Correa A, Houser S, Jones D, et al. American Heart Association cardiovascular genome-phenome study: foundational basis and program. Circulation. (2015) 131:100–12. doi: 10.1161/CIRCULATIONAHA.114.014190
31. Kuller L. Ethnic differences in atherosclerosis, cardiovascular disease and lipid metabolism. Curr Opin Lipidol. (2004) 15:109–13. doi: 10.1097/00041433-200404000-00003
32. Mackinnon A, Jerrard-Dunne P, Porteous L, Markus H. Carotid intima-media thickness is greater but carotid plaque prevalence is lower in black compared with white subjects. Am J Neuroradiol. (2010) 31:1951–5. doi: 10.3174/ajnr.a2214
33. Chahal N, Lim T, Jain P, Chambers J, Kooner J, Senior R. Does subclinical atherosclerosis burden identify the increased risk of cardiovascular disease mortality among United Kingdom Indian Asians? A population study. Am Heart J. (2011) 162:460–6. doi: 10.1016/j.ahj.2011.06.018
34. Tattersall M, Gassett A, Korcarz C, Gepner A, Kaufman J, Liu K, et al. Predictors of carotid thickness and plaque progression during a decade. Stroke. (2014) 45:3257–62. doi: 10.1161/strokeaha.114.005669
35. Freedman B, Hsu F, Langefeld C, Rich S, Herrington D, Carr J, et al. The impact of ethnicity and sex on subclinical cardiovascular disease: the diabetes heart study. Diabetologia. (2005) 48:2511–8. doi: 10.1007/s00125-005-0017-2
36. Sacco R, Roberts J, Boden-Albala B, Gu Q, Lin I, Kargman D, et al. Race-ethnicity and determinants of carotid atherosclerosis in a multiethnic population. The Northern Manhattan stroke study. Stroke. (1997) 28:929–35. doi: 10.1161/01.str.28.5.929
37. Nonterah E, Crowther N, Klipstein-Grobusch K, Oduro A, Kavousi M, Agongo G, et al. Racial and ethnic differences in the association between classical cardiovascular risk factors and common carotid intima-media thickness: an individual participant data meta-analysis. J Am Heart Assoc. (2022) 11:e023704. doi: 10.1161/jaha.121.023704
38. Ebrahim S, Papacosta O, Whincup P, Wannamethee G, Walker M, Nicolaides A, et al. Carotid plaque, intima media thickness, cardiovascular risk factors, and prevalent cardiovascular disease in men and women: the British regional heart study. Stroke. (1999) 30:841–50.
39. Prati P, Tosetto A, Vanuzzo D, Bader G, Casaroli M, Canciani L, et al. Carotid intima media thickness and plaques can predict the occurrence of ischemic cerebrovascular events. Stroke. (2008) 39:2470–6.
40. Bots M, Hofman A, Grobbee D. Increased common carotid intima-media thickness. adaptive response or a reflection of atherosclerosis? Findings from the rotterdam study. Stroke. (1997) 28:2442–7.
41. Inaba Y, Chen J, Bergmann S. Carotid plaque, compared with carotid intima-media thickness, more accurately predicts coronary artery disease events: a meta-analysis. Atherosclerosis. (2012) 220:128–33. doi: 10.1016/j.atherosclerosis.2011.06.044
42. Bhopal R. Migration, Ethnicity, Race, and Health in Multicultural Societies. Second ed. Oxford: Oxford University Press (2014).
43. Office for National Statistics. Harmonised Concepts and Questions for Social Data Sources. Primary Principles. Ethnic Group. Contract No.: Version 3.3. (2015).
44. VanderWeele T, Vansteelandt S. Conceptual issues concerning mediation, interventions and composition. Stat Interface. (2009) 2:457–68.
Keywords: ethnicity, cardiovascular disease, atherosclerosis, carotid artery, medical imaging
Citation: Anbar R, Chaturvedi N, Eastwood SV, Tillin T and Hughes AD (2023) Carotid atherosclerosis in people of European, South Asian and African Caribbean ethnicity in the Southall and Brent revisited study (SABRE). Front. Cardiovasc. Med. 9:1002820. doi: 10.3389/fcvm.2022.1002820
Received: 25 July 2022; Accepted: 20 December 2022;
Published: 24 January 2023.
Edited by:
Wuxiang Xie, Peking University, ChinaReviewed by:
Mahmoud Al Rifai, Houston Methodist Hospital, United StatesCopyright © 2023 Anbar, Chaturvedi, Eastwood, Tillin and Hughes. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alun D. Hughes,  YWx1bi5odWdoZXNAdWNsLmFjLnVr
YWx1bi5odWdoZXNAdWNsLmFjLnVr
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.