- 1Division of Cardiology, Department of Internal Medicine, Heart Vascular Stroke Institute, Samsung Medical Center, School of Medicine, Sungkyunkwan University, Seoul, South Korea
- 2Department of Radiology, Heart Vascular Stroke Institute, Samsung Medical Center, School of Medicine, Sungkyunkwan University, Seoul, South Korea
Aims: The number of trans-catheter aortic valve replacement (TAVR) procedure is increasing; However, the incidence of leaflet thrombosis is higher in TAVR than in surgical aortic valve replacement (SAVR). In this study, the risk factors for leaflet thrombosis after TAVR and its effects on hemodynamics and clinical course were investigated.
Methods and results: Multidetector computed tomography (MDCT) was performed at 1year after TAVR in 94 patients from January 2015 to October 2020 at Samsung Medical Center in South Korea. Among the 94 patients, subclinical leaflet thrombosis occurred in 20 patients, and risk factors were analyzed. In addition, the difference in aortic valve (AV) hemodynamics between the two groups was examined and clinical outcomes compared. Indexed mean sinus of Valsalva (SOV) diameter, AV calcium volume, and post-procedure effective orifice area (EOA) were predictive of subclinical leaflet thrombosis with the area under the curve (AUC) value of 0.670 (P-value = 0.020), 0.695 (P-value = 0.013), and 0.665 (P-value = 0.031), respectively. In echocardiography performed at the time of follow-up CT, the value of AV max velocity and AV mean pressure gradient were higher in the thrombosis group and the EOA and Doppler velocity index values were lower in the thrombosis group than in the no thrombosis group. Clinical outcome was not significantly different between the two groups (log-rank P-value = 0.26).
Conclusion: Larger indexed SOV diameter, higher AV calcium volume, and smaller post-procedure AV EOA were risk factors for subclinical leaflet thrombosis after TAVR. Subclinical leaflet thrombosis has a benign course when properly managed.
Introduction
The number of patients undergoing trans-catheter aortic valve replacement (TAVR) is increasing after success of multiple TAVR trial from high risk (1) to intermediate and low risk patients (2, 3). The current valvular heart disease guideline recommends TAVR as a reasonable option in patients over 65 years of age and with lower surgical risk (4). With this broad change of recommendation of guideline, the length of their follow-up is also anticipated to be longer, proper, and specific management guidelines after TAVR are necessary. Although treatment subject and general follow-up treatment were established with accumulated studies, long-term follow-up data are insufficient and individualized treatment policies are still inadequate.
A disadvantage of TAVR is that thrombosis occurs more easily compared with surgical aortic valve replacement (SAVR) (5). Leaflet thrombosis following TAVR is of concern because it could be a potential risk of thromboembolic events or valve dysfunction (5, 6). However, long-term data is limited to discuss the clinical effect of subclinical leaflet thrombosis so far (7, 8). Since clinical effect of subclinical leaflet thrombosis has not been concluded and clinical leaflet thrombosis clearly affects patients’ condition, the research on risk factors for leaflet thrombosis is important. External validation is also required for previously discussed risk factors because many of them are from single center studies (9–12), and identifying the risk factors for leaflet thrombosis would help in individualized treatment. Although the medical treatment after TAVR was determined to be single antiplatelet therapy (SAPT) through several randomized control studies (4, 13), individual treatment is required for each patient because potential risks of myocardial infarction, stoke and leaflet thrombosis remain. It is known that anticoagulation therapy is more effective than antiplatelet for the prevention and treatment of leaflet thrombosis (14–16). Through this study, we would discuss the risk factors of leaflet thrombosis after TAVR and the long-term prognosis of these patients.
Materials and methods
Study subjects and data collection
A total of 176 patients underwent TAVR from January 2015 to October 2020 at Samsung Medical Center in South Korea. Among the patients, 97 received both baseline and follow-up coronary and aorta multidetector computed tomography (MDCT). Three Patients who checked MDCT shortly after TAVR due to a stroke and patients who deviated from the original procedure schedule were excluded in the analysis, and 94 patients were included in final analysis (Supplementary Figure 1).
Our center’s protocol is to use MDCT to screen for any anomalies 1 year after TAVR, except for patients with poor renal function who cannot consider contrast CT. Cardiac echocardiography was scheduled to perform one day after the procedure, 6 months, and 1 year. One year after the procedure, echocardiography is taken once every 2 years unless there are other reasons. When patients have cardiovascular symptoms or were hospitalized for other reasons, the examinations were performed ahead of schedule. The medical treatment protocol after TAVR is to maintain DAPT for 1 year and change to SAPT unless in special cases. Clinical outcome was defined as the composite of all-cause death, stroke, heart failure on admission, redo aortic valve replacement (AVR), and major bleeding after detection of leaflet thrombosis. Redo-AVR was defined when TAVR or SAVR was repeated due to late complications such as paravalvular leakage and infective endocarditis.
The medical records of the enrolled patients were carefully reviewed by the research coordinator. Baseline clinical characteristics, electrocardiography, echocardiographic parameters, CT information, and angiographic data were collected. Mortality data were obtained from the national insurance data and censor date was August 4, 2021. Clinical data were collected retrospectively before 2018 and prospectively after 2018. This study was conducted according to the principles of the Declaration of Helsinki (IRB No. 2018-02-097).
CT evaluation
All CT examinations were performed using 2nd or 3rd generation dual-source CT scanners (Somatom Force or Somatom Definition Flash, Siemens Medical Solutions, Forchheim, Germany). Ten transaxial CT datasets were reconstructed with 10% increments of the cardiac cycle from 0 to 90% of the R-R interval. Multiphase images of CT scans were transported into commercial software (Aquarius iNtuition Edition ver. 4.4.12; TeraRecon, Foster City, CA, USA).
In baseline MDCT imaging, three caliper measurements were taken from cusp to commissure in parallel to the annular plane to determine the mean sinus of Valsalva (SOV) diameter (17). Annulus diameter and sinotubular (ST) junction diameter were also defined according to consensus of the Society of Cardiovascular CT (17). Leaflet calcium was quantified on a contrast scan image with an 850 HU threshold for more detailed regional analysis. A zone of interest for calcium quantification was defined from the basal annular plane to the leaflet tips, excluding both left ventricular outflow tract (LVOT) and coronary calcium (18). Valve-in-valve patients were excluded from the calcium volume analysis because accurate evaluation was not possible. In the comparison of the calcium volume of each of the three leaflet cusps, bicuspid AV was excluded because it was difficult to clearly define the cusp. Reduced leaflet motion and leaflet thickening were evaluated in follow-up multiplanar 4-dimensional (4D) CT imaging according to the consensus of the Society of Cardiovascular CT (17). Extent of leaflet thickening was semiquantitatively graded on long-axis views with the leaflet center regarding involvement along the curvilinear leaflet beginning at the base, using 5-tier grading scale: 0, none; 1, ≤25%; 2, >25–50%; 3, >50–75%; and 4, >75%. Leaflet thrombosis was defined as hypo-attenuated leaflet thickening of grade 1 or greater leaflet thickening in one or more leaflets. Leaflet thickening was evaluated by a 10-year-experienced cardiovascular radiologist, S.M.K, MD.
Echocardiographic evaluation
Two-dimensional echocardiography was performed under hemodynamically stable conditions using commercially available equipment (Vivid E9 or Vivid E90 or Vivid E95, GE Medical Systems, Milwaukee, WI, USA) and analyzed according to the current guidelines (19). Evaluation of aortic stenosis was based on the American Society of Echocardiography (ASE) aortic stenosis guideline (20). Flow acceleration in the AV was evaluated on the apical five-chamber view and right parasternal view with continuous wave Doppler; the maximum velocity and pressure gradient values were used for analysis. Velocity time integral (VTI) of LVOT was evaluated on the apical five-chamber view with pulsed wave Doppler. LVOT diameter was defined as mid systolic inner edge-to-inner edge diameter within 1 cm of aortic orifice at parasternal long axis view.
Degree of paravalvular leakage (PVL) was defined according to the ASE valvular regurgitation after percutaneous valve replacement guideline (21). Effective orifice area (EOA) was measured on echocardiogram at 1 day and 1 year after TAVR using the continuity equation. Definition of prosthesis-patient mismatch (PPM) in this study was the measured PPM (22) at post-procedure echocardiogram and defined as EOAi ≤0.85 cm2/m2. To clearly detect the relationship between CT findings and echocardiogram parameters, echocardiography after 1 year was analyzed by selecting only patients with a difference in measurement within 3 months from the date of follow-up CT scan.
Statistical analysis
Continuous variables are presented as mean ± standard deviation or median (interquartile range, IQR) using Student’s t-test or Mann–Whitney U test. Shapiro–Wilk test was used for normality assumption of continuous variables. Categorical variables were compared between groups using the chi-square test or Fisher’s exact test and presented as numbers and relative frequencies (%).
To define degree of relationship, the area under the curve (AUC) value was evaluated using the Delong method. The optimal cutoff values for predicting leaflet thrombosis were calculated to maximize the product of sensitivity and specificity using receiver operating characteristic (ROC) curves. A penalized spline with three degrees of freedom was used to graphically represent the relationship between risk factors and leaflet thrombosis incidence. The cumulative incidence of clinical events based on leaflet thrombosis was presented as Kaplan–Meier estimates and compared using the log-rank test.
All P-values were two-sided and P-values < 0.05 were considered statistically significant. Statistical analyses were performed using R Statistical Software (version 4.1.0; R Foundation for Statistical Computing, Vienna, Austria).
Results
The median age of patients was 79.5 years (76.0–82.0 years) and 51.1% of patients were female. Median logistic euro score was 5.2 and median Society of Thoracic Surgeons (STS) score was 2.9. The most common cause of TAVR was degenerative AV (81/94, 86.2%); 3 patients (3.2%) had TAVR procedure at bicuspid AV and 8 patients (8.5%) had valve-in-valve procedure.
Leaflet thrombosis was detected in 20 patients (21.3%). All patients were incidentally discovered on CT in the absence of onset or exacerbation of symptoms. Median interval between day of TAVR procedure and follow-up CT for all patients was 367 days (353–393 days) and median clinical outcome follow-up duration was 33 months (23–45 months). Among the 20 patients with subclinical leaflet thrombosis, 11 patients (55%) had leaflet thrombosis at non-coronary cusp (NCC), 10 (50%) at right coronary cusp (RCC), and 5 (25%) at left coronary cusp (LCC) (Figure 1).
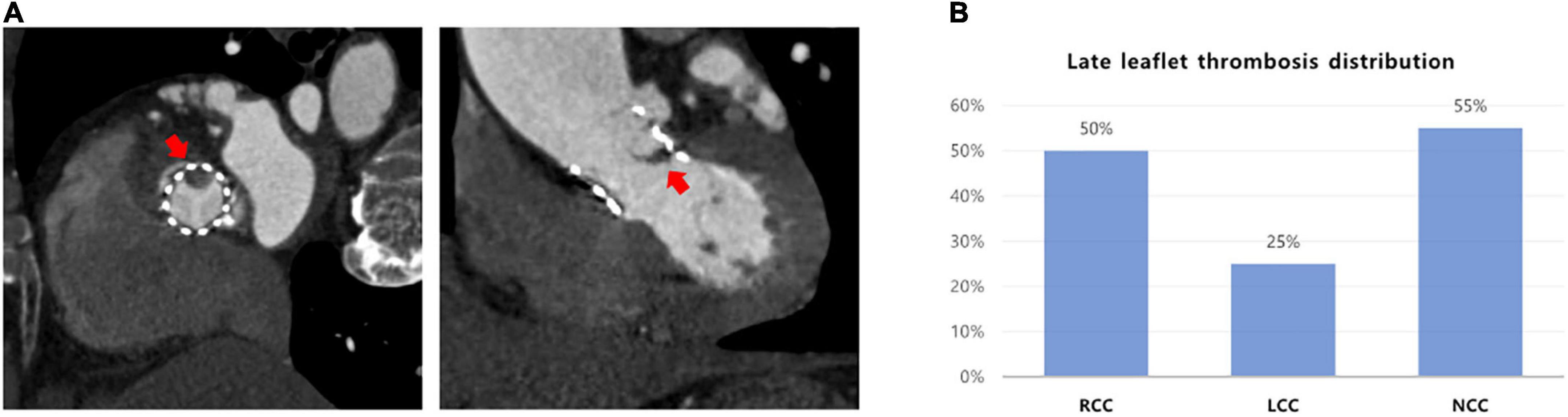
Figure 1. The distribution of subclinical leaflet thrombosis in TAVR. (A) Evaluation of leaflet thickening and reduced leaflet motion based on MDCT. (B) Leaflet thrombosis distribution in cusps. MDCT, multidetector computed tomography; TAVR, transaortic valve replacement.
Upon discharge, 75 patients (79.8%) were prescribed dual antiplatelet therapy (DAPT), 14 (14.9%) were prescribed both an antiplatelet and anticoagulant, 4 (4.3%) received SAPT, and 1 patient (1.1%) was prescribed only an anticoagulant (Supplementary Figure 2). After discovering subclinical leaflet thrombosis during the follow-up period, 10 patients were prescribed an additional anticoagulant and 2 patients another antiplatelet agent (Supplementary Figure 3).
Risk factors for subclinical leaflet thrombosis
The comparison of baseline clinical characteristics showed no significant difference between the thrombosis and no thrombosis groups. In addition, significant difference was not observed between the two groups in the procedure and device information (Table 1).
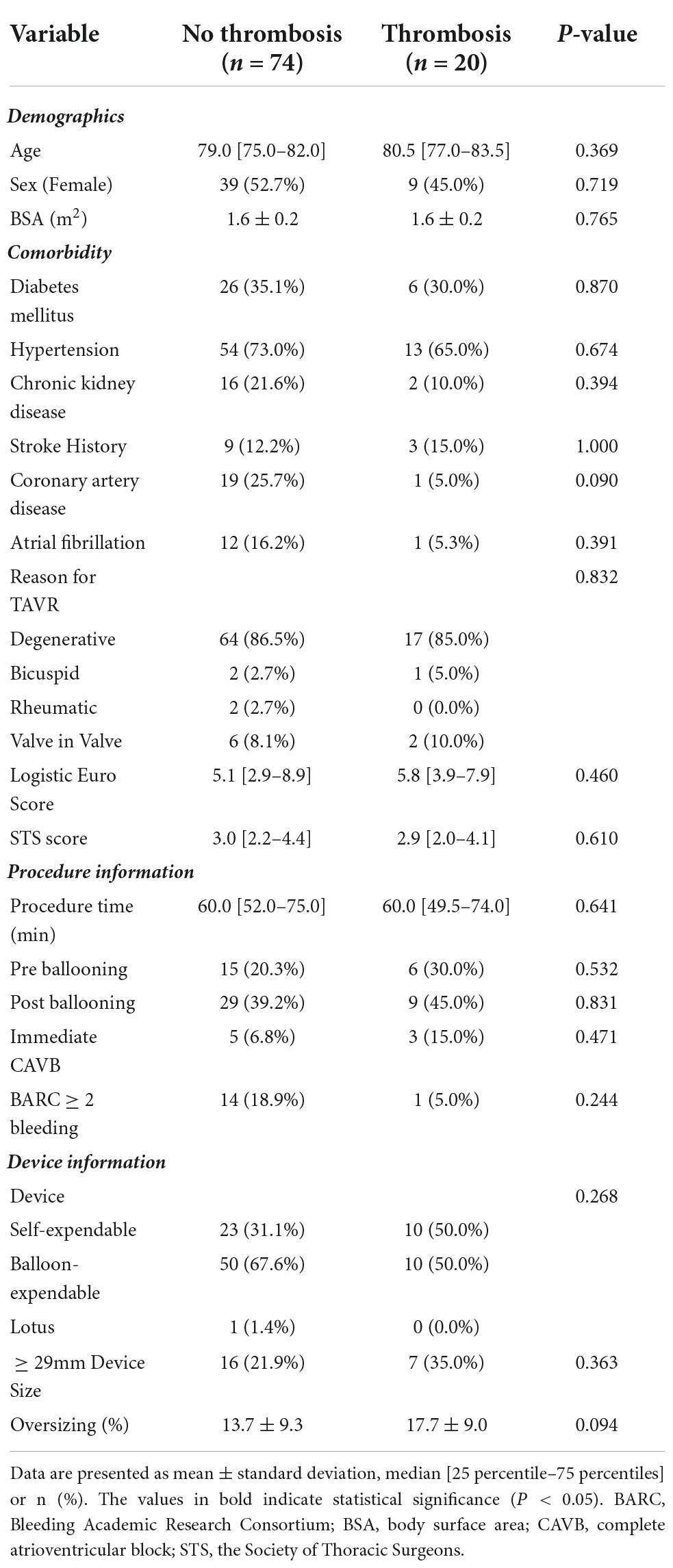
Table 1. Baseline characteristics comparison between the no leaflet thrombosis and the leaflet thrombosis group.
When comparing the information obtained from echocardiography, CT, and angiography, significant differences were observed between the two groups in three major areas. Indexed SOV diameter was larger in the thrombosis group than in the no thrombosis group (P-value = 0.020) and AV calcium volume was higher in the thrombosis group than in the no thrombosis group (P-value = 0.014). In addition, post-procedure AV EOA was smaller in the thrombosis group than in the no thrombosis group (P-value = 0.032) (Table 2).
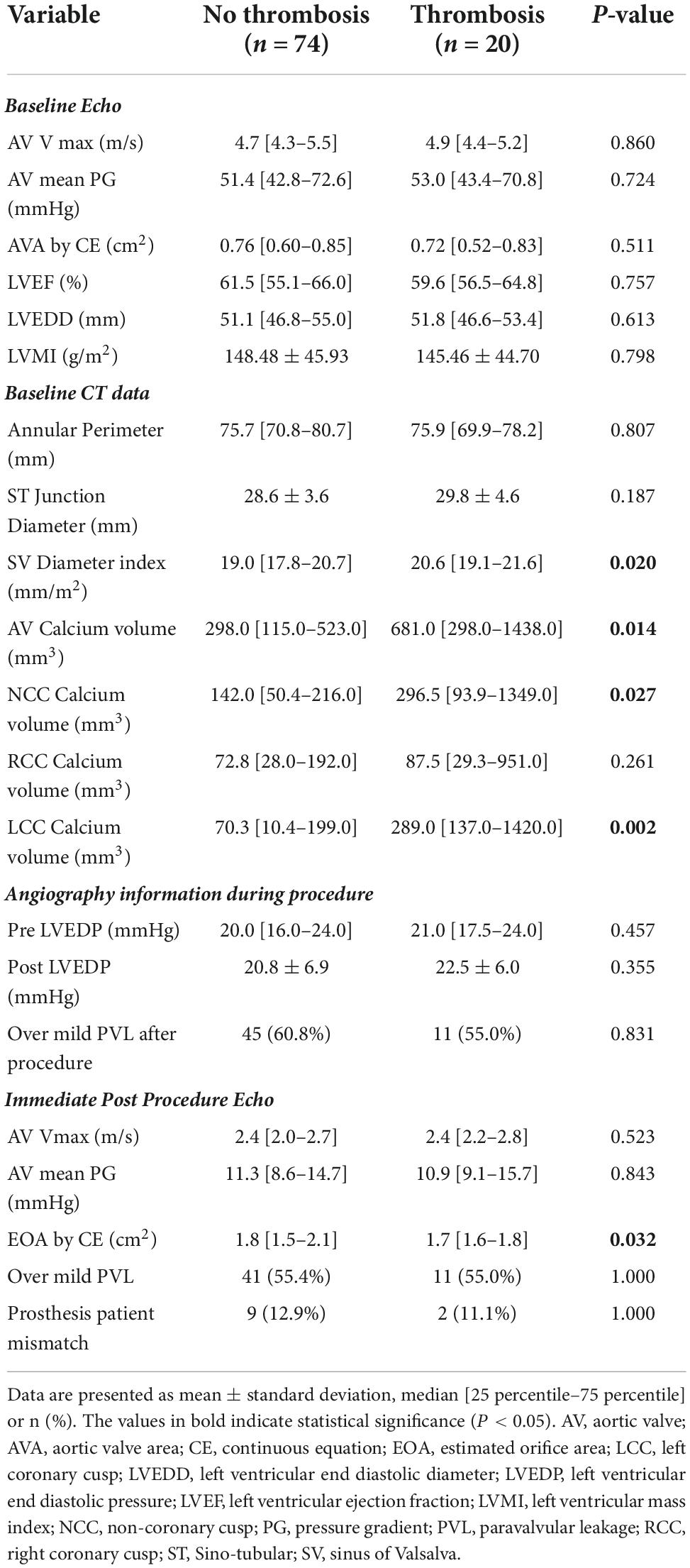
Table 2. Values of imaging comparison between the no leaflet thrombosis and the leaflet thrombosis group.
Receiver operating characteristic (ROC) analysis was performed to compare the predictive value for leaflet thrombosis of the three factors that showed differences from the baseline. Indexed mean SOV diameter showed AUC value of 0.670 (95% CI 0.546–0.795, P-value = 0.020) for predicting leaflet thrombosis with 52.7% specificity and 80.0% sensitivity. Best cutoff was 19.1 mm/m2 for indexed mean SOV diameter. AV calcium volume had AUC value of 0.695 (95% CI 0.541–0.850, P-value = 0.013) with 67.7% specificity and 70.6% sensitivity. Best cutoff value for AV calcium volume was 423.5 mm3. Post-procedure EOA had an AUC value of 0.665 (95% CI 0.548–0.782, P-value = 0.031) with 60.0% specificity and 88.9% sensitivity for predicting subclinical leaflet thrombosis (Figure 2A). A spline curve was drawn to determine whether a linear relationship existed between the risk factors and leaflet thrombosis. The risk of leaflet thrombosis was confirmed to increase as indexed mean SOV or AV calcium volume increased although not in perfectly linear fashion (Figure 2B). When comparing the predictive power of the above three factors in each device type, the AV calcium volume in the self-expandable device showed significant predictive power as AUC value of 0.789 (95% CI 0.586–0.985, P-value = 0.033) even in small number of patients (n = 33) (Supplementary Table 1). When multivariable analysis was performed, aortic valve calcium volume over 423.5 mm2 was independently associated with leaflet thrombosis as odds ratio 5.040 (95% CI 1.395–18.213, P-value = 0.014) even after adjusting for age, sex, chronic kidney disease, LVEF, device size, and indexed sinus of Valsalva diameter (Supplementary Table 2).
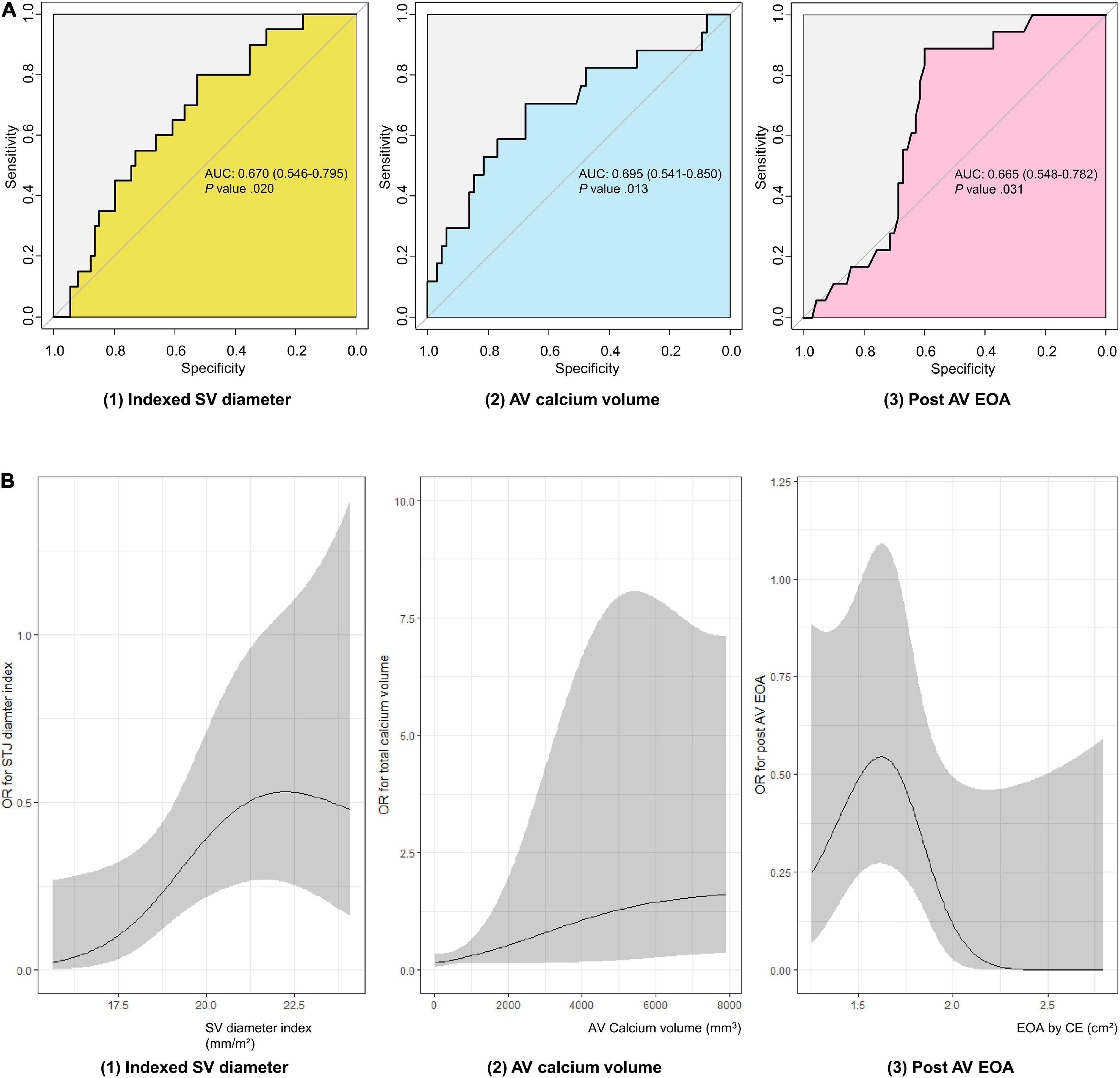
Figure 2. Evaluation of subclinical leaflet thrombosis risk factors in TAVR patients. (A) ROC curve of subclinical leaflet thrombosis risk factors. (B) Spline curve of subclinical leaflet thrombosis risk factors. AUC, area under curve; AV, aortic valve; EOA, estimated orifice area; OR, odds ratio; ROC, receiver operating characteristic; SOV, sinus of Valsalva; TAVR, transaortic valve replacement.
Although statistically significant difference did not exist between the medicine prescribed at discharge and the occurrence of leaflet thrombosis, no case of leaflet thrombosis occurred in the anticoagulation group (Table 3).
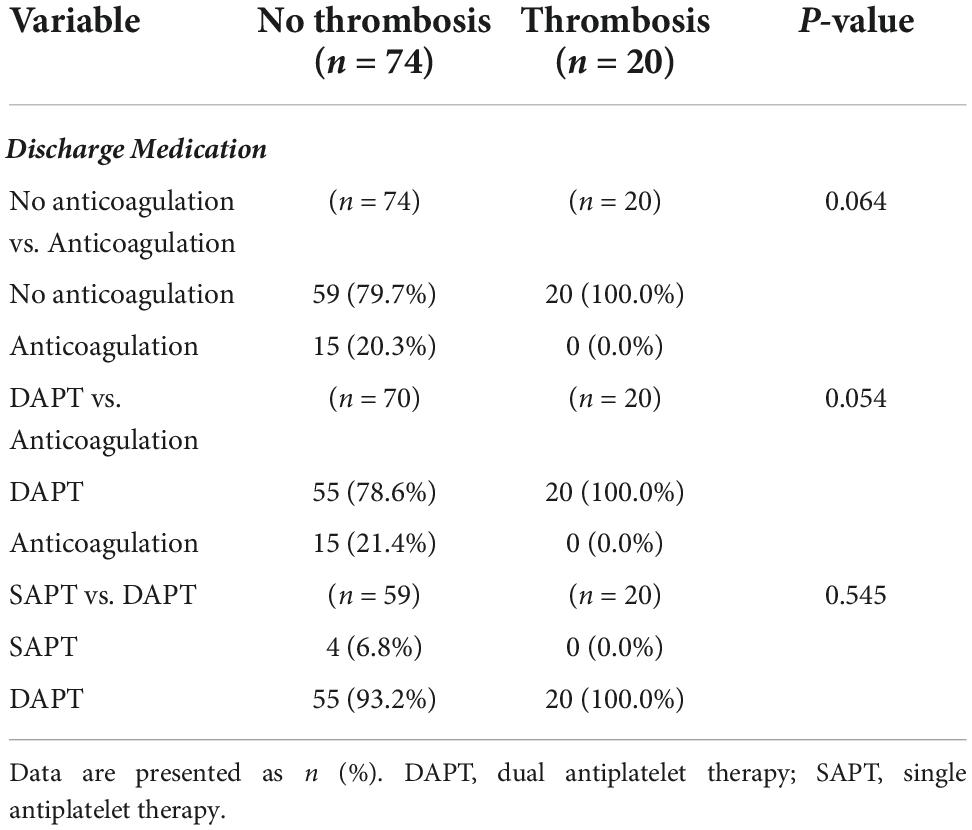
Table 3. Prescribed medication comparison between the no leaflet thrombosis and the leaflet thrombosis group.
Hemodynamic change and clinical outcome in patients with subclinical leaflet thrombosis
To evaluate the hemodynamic effects of leaflet thrombosis, echocardiography performed at the time of follow-up CT was analyzed and confirmed that AV Vmax and AV mean pressure gradient were higher in the thrombosis group (2.3; 2.1– 3.0 m/s and 12.3; 9.4–17.2 mmHg, respectively, P-value = 0.049) than in the no thrombosis group (2.2; 2.0–2.4 m/s and 9.6; 7.8–12.1 mmHg, respectively, P-value = 0.042). In addition, the EOA was smaller and the doppler velocity index (DVI) was lower in the thrombosis group (1.5 ± 0.4 cm2 and 0.46 ± 0.12, respectively, P-value = 0.010) than in the no thrombosis group (1.8 ± 0.4 cm2 and 0.54 ± 0.12, respectively, P-value = 0.008; Figure 3). Patients with leaflet thrombosis had worse hemodynamics; however, both groups were within normal limits.
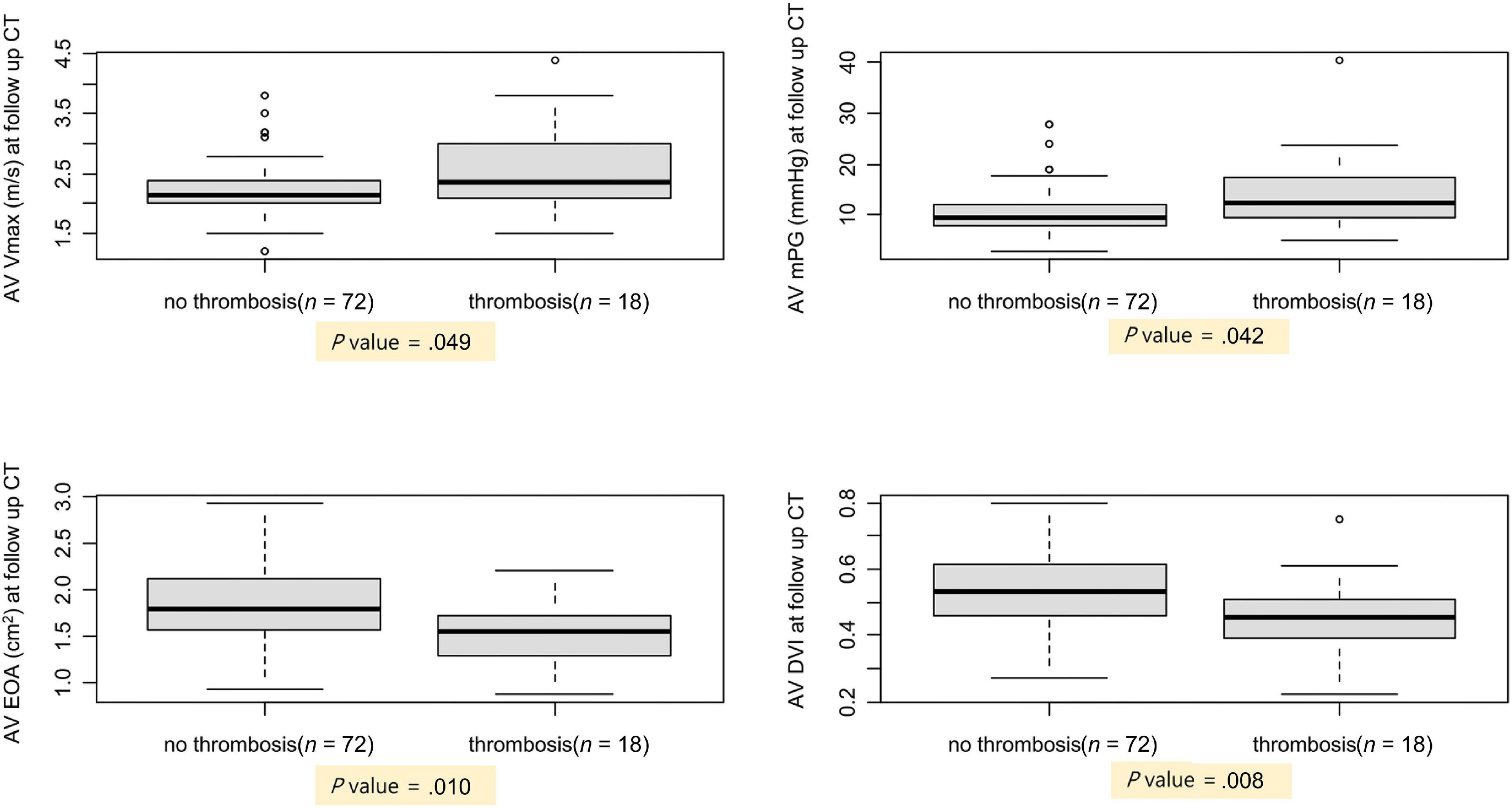
Figure 3. Comparison of AV hemodynamics between patients with and without subclinical leaflet thrombosis. AV, aortic valve; CT, computed tomography; DVI, Doppler velocity index; EOA, estimated orifice area; mPG, mean pressure gradient; Vmax, peak aortic valve velocity.
Among the 20 patients with leaflet thrombosis, 11 patients had a 1-year follow-up echocardiography (2 years after procedure) after the CT scan. Among the 11 patients, anticoagulation agents were prescribed for 2 patients and another antiplatelet for 2 patients after reviewing the follow-up MDCT. When the echocardiography findings at the time of CT follow-up were compared with the echocardiography findings one year later, AV Vmax and AV mean pressure gradient had improved in 11 patients (Figure 4). When reviewing the composite clinical outcome in all 94 patients (all-cause death,
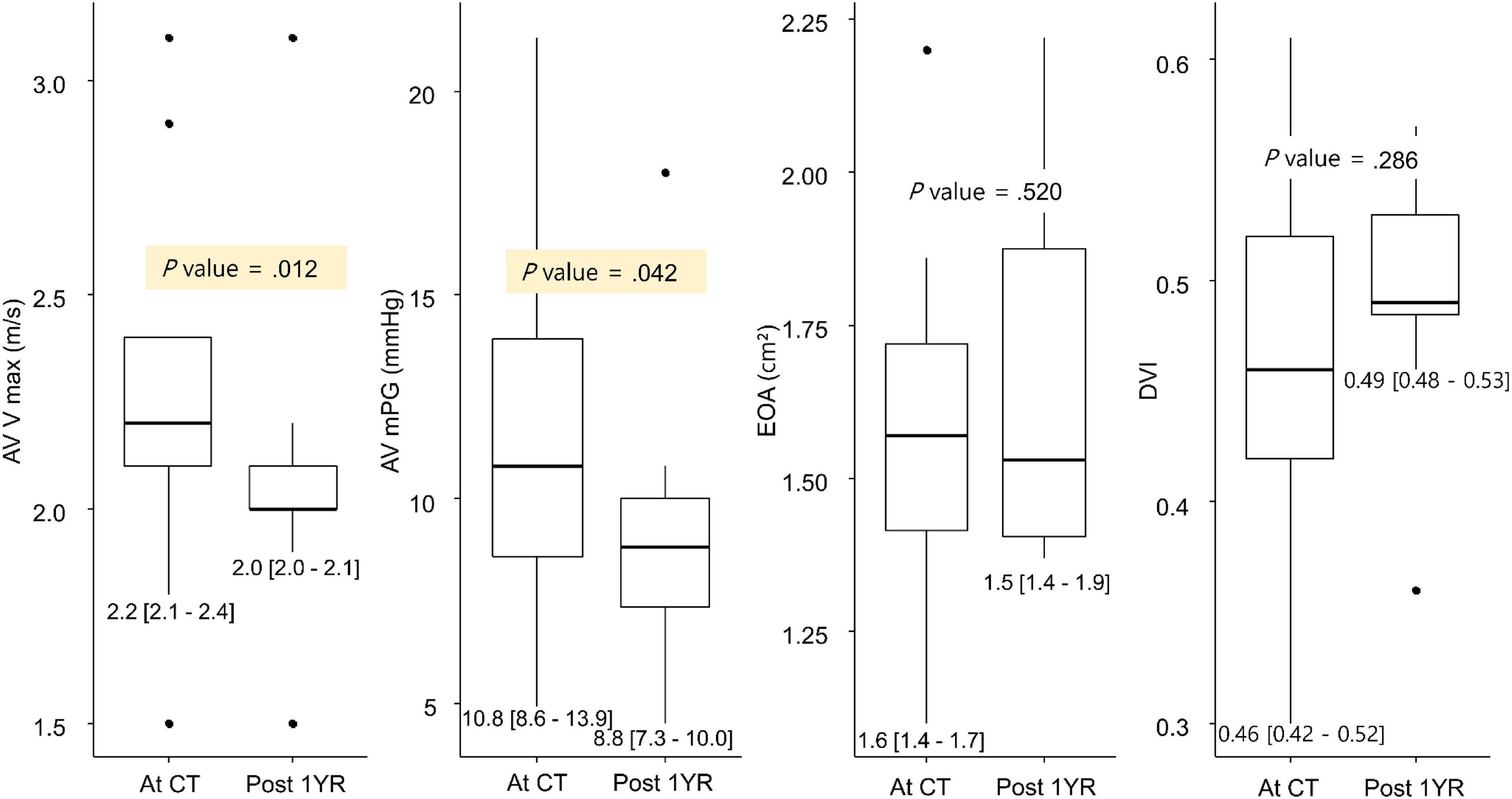
Figure 4. Change in AV hemodynamics on the 1-year follow-up MDCT. AV, aortic valve; DVI, Doppler velocity index; EOA, estimated orifice area; MDCT, multidetector computed tomography; mPG, mean pressure gradient; Vmax, peak aortic valve velocity.
stroke, HF admission, redo AVR, and major bleeding), the composite outcome occurred in 6 patients, and all of them were patients without leaflet thrombosis in follow up MDCT at one year after the procedure. In the survival curve, the composite outcome showed no statistical significance (log-rank P-value = 0.26) between subjects with and without leaflet thrombosis.
Discussion
In the present study, the risk factors for leaflet thrombosis were evaluated and clinical course of patients with subclinical leaflet thrombosis analyzed. First, indexed SOV diameter, AV calcium volume, and post-procedure EOA were shown risk factors for leaflet thrombosis. Second, subclinical leaflet thrombosis affected AV hemodynamics but did not deviate from the normal range. Third, if subclinical leaflet thrombosis is timely managed, hemodynamic compromise and clinical problems are not an issue compared with patients without leaflet thrombosis.
Risk factor analysis of subclinical leaflet thrombosis
Incidence of subclinical leaflet thrombosis after TAVR is reportedly approximately 10–25% (12, 23–25) if patients is followed up with CT imaging, which is similar to of this study result 21%. In a previous study, male gender, chronic kidney disease (CKD), low flow low gradient, severe PPM, and larger trans-catheter heart valve were associated with early leaflet thrombosis (9–11). In addition, male gender, diameter of SOV, and less than mild degree of PVL were shown risk factors for late leaflet thrombosis (9, 12).
Large SOV diameter was a consistently important factor in leaflet thrombosis in the present and previous studies (12, 26). The causal relationship between large SOV and leaflet thrombosis can be found in an in vitro study. In the in vitro study in which the state after TAVR was simulated, presence of stagnant blood flow within the SOV was observed (27). This study suggest that large SOV contribute to increased thrombus formation after TAVR and increase the incidence of leaflet thrombosis. The difference between the previous study and the present study is that the indexed SOV dimeter was presented as a risk factor, not the SOV diameter. Because the SOV diameter is affected by age, gender, BSA, and height (28), the indexed SOV diameter may be a more useful parameter in Asian populations.
Greater extent of calcium deposition and smaller EOA on post-TAVR echocardiography in leaflet thrombosis patients could suggest the possibility of under-expansion due to heavy calcification. Although in the present study, under-expansion could not be directly evaluated, under-expansion was presumed due to a smaller post-procedure EOA value in the leaflet thrombosis patients. The leaflet sections where thickening occurred were mostly in the sequence of NCC-RCC-LCC, which was the same order reported in a prior study (29) and the same order shown to have significant calcium (30). Regional under-expansion of stent was reportedly associated with leaflet thrombosis in a study by Fuchs A et al. and a post-mortem study (29, 31). In addition, an in vitro study also demonstrated that eccentric deployment of the device promotes thrombotic conditions (32, 33). Turbulent flow provoked by eccentric deployment produces higher viscous shear stress around the TAVR device which can cause platelet lysis (32). Stagnant sinus flow may expose sensitized platelets to prolonged low shear stress, rendering this region prone to thrombus formation (34).
Because unremoved aortic valve calcification in TAVR is the characteristic of TAVR in contrast to SAVR, studies on the clinical effect of aortic valve calcium extent have been actively conducted. The aortic valve calcium extent is known to affect various poor outcome after TAVR, such as paravalvular leakage, stoke, coronary ostium occlusion and conduction abnormality (35). It is meaningful because this study firstly revealed the direct relation of aortic valve calcium extent with leaflet thrombosis.
Effects of medication on subclinical leaflet thrombosis
Notably, in the present study, leaflet thrombosis did not occur in any subjects who used anticoagulation whether alone or in combination with antiplatelet agents. This finding is in agreement with previous studies in which anticoagulation was shown to have preventive and therapeutic effects in TAVR valve thrombosis (14–16, 24). In addition, the incidence of leaflet thrombosis did not differ between the SPAT and DPAT patients in the present study, which is consistent with previous findings (15, 36) indicating the use of an anticoagulant may be more effective than addition of another antiplatelet agent for leaflet thrombosis. A postmortem investigation revealed that fibrin is abundant in TAVI leaflet thrombosis (16). And it is well recognized that anticoagulation is more effective in fibrin-rich thrombus than antiplatelet therapy which is effective in platelet-rich atherosclerotic disease. Based on these pathophysiology and previous clinical trials, it is presumed that anticoagulation treatment is more effective than antiplatelet treatment in TAVR. The use of an anticoagulant could be considered in patients at high risk of leaflet thrombosis considering the risk and benefit.
Effects of subclinical leaflet thrombosis on hemodynamics and clinical outcome
Several studies report that leaflet thrombosis affect the implanted AV (9, 12, 15, 16, 37). On follow-up echocardiography closest to the day of MDCT, AV mean pressure gradient was higher in subclinical leaflet thrombosis patients in this study. Although leaflet thrombosis had an effect on the valve hemodynamics, it was not considered to have a detrimental effect because it was within the normal range in both groups in this present study.
When the echocardiographic findings at the time of CT scan and one year later were compared in 11 patients, a benign course was observed, indicating a reversible course of leaflet thrombosis. Significant difference was not observed in clinical outcome between the two groups, possibly due to the nature of reversible leaflet thrombosis or small thrombosis extents found in subclinical conditions.
Limitations
The present study had several limitations. First, it was difficult to perform enough sub-analysis due to the small number of patients. Second, because of concerns regarding nephrotoxicity due to contrast, most of the patients who had follow-up CT did not have CKD. Because CKD patients had more calcium deposits in the vascular structures including AV (38), the possibility of underexpansion and leaflet thrombosis could be higher in CKD patients. Third, diversity of patients was limited because subjects were recruited from a single center and were predominantly Asian. Because Asians have a lower risk of thrombosis than Caucasians or African-Americans (39), the present study results may underestimate the incidence of thrombosis in other races. However, it is meaningful that even with a small number of patients, previously unknown risk factors for leaflet thrombosis were identified.
Conclusion
In the present study, larger indexed SOV diameter, higher AV calcium volume, and smaller post-procedure AV EOA were identified as risk factors for subclinical leaflet thrombosis after TAVR. Leaflet thrombosis could be managed if appropriate screening is performed in patients with high-risk features and patients with findings suggestive of aortic stenosis on follow-up echocardiography.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
S-JP conceived and designed the analysis. KC, TP, and S-HC collected the data. SK, EK, and JK contributed data or analysis tools. MB performed the analysis. MB and JK wrote the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.1001753/full#supplementary-material
Abbreviations
AS, aortic stenosis; AVR, aortic valve replacement; TAVR, transaortic valve replacement; SAVR, surgical aortic valve replacement; MDCT, multidetector computed tomography; SOV, sinus of Valsalva; AUC, area under the curve; ROC, receiver operating characteristic; RCC, right coronary cusp; LCC, left coronary cusp; NCC, non-coronary cusp; EOA, effective orifice area; LVEF, left ventricular ejection fraction; LVEDD, left ventricular end-diastolic diameter; LVMI, left ventricular mass index; LVEDP, left ventricular end-diastolic pressure; LVOT, left ventricular outflow tract; BSA, body surface area; VTI, velocity time integral; PPM, prosthesis-patient mismatch; PVL, paravalvular leakage; DAPT, dual antiplatelet therapy; SAPT, single antiplatelet therapy; CKD, chronic kidney disease; TEE, transesophageal echocardiogram.
References
1. Smith CR, Leon MB, Mack MJ, Miller DC, Moses JW, Svensson LG, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. (2011) 364:2187–98. doi: 10.1056/NEJMoa1103510
2. Leon MB, Smith CR, Mack MJ, Makkar RR, Svensson LG, Kodali SK, et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med. (2016) 374:1609–20.
3. Mack MJ, Leon MB, Thourani VH, Makkar R, Kodali SK, Russo M, et al. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med. (2019) 380:1695–705. doi: 10.1056/NEJMoa1814052
4. Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP III, Gentile F, et al. 2020 ACC/AHA Guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. (2021) 143:e72–227. doi: 10.1161/CIR.0000000000000923
5. Chakravarty T, Søndergaard L, Friedman J, De Backer O, Berman D, Kofoed KF, et al. Subclinical leaflet thrombosis in surgical and transcatheter bioprosthetic aortic valves: an observational study. Lancet. (2017) 389:2383–92. doi: 10.1016/S0140-6736(17)30757-2
6. Egbe AC, Pislaru SV, Pellikka PA, Poterucha JT, Schaff HV, Maleszewski JJ, et al. Bioprosthetic valve thrombosis versus structural failure: clinical and echocardiographic predictors. J Am Coll Cardiol. (2015) 66:2285–94. doi: 10.1016/j.jacc.2015.09.022
7. Park DW, Ahn JM, Kang DY, Kim KW, Koo HJ, Yang DH, et al. Edoxaban versus dual antiplatelet therapy for leaflet thrombosis and cerebral thromboembolism after TAVR: The ADAPT-TAVR Randomized Clinical Trial. Circulation. (2022) 146:466–79. doi: 10.1161/CIRCULATIONAHA.122.059512
8. Sondergaard L, De Backer O, Kofoed KF, Jilaihawi H, Fuchs A, Chakravarty T, et al. Natural history of subclinical leaflet thrombosis affecting motion in bioprosthetic aortic valves. Eur Heart J. (2017) 38:2201–7. doi: 10.1093/eurheartj/ehx369
9. Yanagisawa R, Tanaka M, Yashima F, Arai T, Jinzaki M, Shimizu H, et al. Early and late leaflet thrombosis after transcatheter aortic valve replacement. Circ Cardiovasc Interv. (2019) 12:e007349. doi: 10.1161/CIRCINTERVENTIONS.118.007349
10. Leetmaa T, Hansson NC, Leipsic J, Jensen K, Poulsen SH, Andersen HR, et al. Early aortic transcatheter heart valve thrombosis: diagnostic value of contrast-enhanced multidetector computed tomography. Circ Cardiovasc Interv. (2015) 8:e001596. doi: 10.1161/CIRCINTERVENTIONS.114.001596
11. Hansson NC, Grove EL, Andersen HR, Leipsic J, Mathiassen ON, Jensen JM, et al. Transcatheter aortic valve thrombosis: incidence, predisposing factors, and clinical implications. J Am Coll Cardiol. (2016) 68:2059–69. doi: 10.1016/j.jacc.2016.08.010
12. Yanagisawa R, Hayashida K, Yamada Y, Tanaka M, Yashima F, Inohara T, et al. Incidence, predictors, and mid-term outcomes of possible leaflet thrombosis After TAVR. JACC Cardiovasc Imaging. (2017) 10:1–11. doi: 10.1016/j.jcmg.2016.11.005
13. Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. (2022) 43:561–632. doi: 10.1093/ejcts/ezac209
14. De Backer O, Dangas GD, Jilaihawi H, Leipsic JA, Terkelsen CJ, Makkar R, et al. Reduced Leaflet Motion after Transcatheter Aortic-Valve Replacement. N Engl J Med. (2020) 382:130–9. doi: 10.1056/NEJMoa1911426
15. Chakravarty T, Sondergaard L, Friedman J, De Backer O, Berman D, Kofoed KF, et al. Subclinical leaflet thrombosis in surgical and transcatheter bioprosthetic aortic valves: an observational study. Lancet. (2017) 389:2383–92.
16. De Marchena E, Mesa J, Pomenti S, Marin Y, Kall C, Marincic X, et al. Thrombus formation following transcatheter aortic valve replacement. JACC Cardiovasc Interv. (2015) 8:728–39. doi: 10.1016/j.jcin.2015.03.005
17. Blanke P, Weir-McCall JR, Achenbach S, Delgado V, Hausleiter J, Jilaihawi H, et al. Computed Tomography Imaging in the Context of Transcatheter Aortic Valve Implantation (TAVI)/Transcatheter Aortic Valve Replacement (TAVR): An Expert Consensus Document of the Society of Cardiovascular Computed Tomography. JACC Cardiovasc Imaging. (2019) 12:1–24. doi: 10.1016/j.jcmg.2018.12.003
18. Jilaihawi H, Makkar RR, Kashif M, Okuyama K, Chakravarty T, Shiota T, et al. A revised methodology for aortic-valvar complex calcium quantification for transcatheter aortic valve implantation. Eur Heart J Cardiovasc Imaging. (2014) 15:1324–32. doi: 10.1093/ehjci/jeu162
19. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. (2015) 28:1–39.e14. doi: 10.1016/j.echo.2014.10.003
20. Baumgartner H, Hung J, Bermejo J, Chambers JB, Edvardsen T, Goldstein S, et al. Recommendations on the echocardiographic assessment of aortic valve stenosis: a focused update from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. J Am Soc Echocardiogr. (2017) 30:372–92. doi: 10.1016/j.echo.2017.02.009
21. Zoghbi WA, Asch FM, Bruce C, Gillam LD, Grayburn PA, Hahn RT, et al. Guidelines for the evaluation of valvular regurgitation after percutaneous valve repair or replacement: a report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Angiography and Interventions, Japanese Society of Echocardiography, and Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr. (2019) 32:431–75. doi: 10.1016/j.echo.2019.01.003
22. Ternacle J, Pibarot P, Herrmann HC, Kodali S, Leipsic J, Blanke P, et al. Prosthesis-patient mismatch after aortic valve replacement in the PARTNER 2 Trial and Registry. JACC Cardiovasc Interv. (2021) 14:1466–77. doi: 10.1016/j.jcin.2021.03.069
23. Nakatani S. Subclinical leaflet thrombosis after transcatheter aortic valve implantation. Heart. (2017) 103:1942–6. doi: 10.1136/heartjnl-2017-311818
24. Pache G, Schoechlin S, Blanke P, Dorfs S, Jander N, Arepalli CD, et al. Early hypo-attenuated leaflet thickening in balloon-expandable transcatheter aortic heart valves. Eur Heart J. (2016) 37:2263–71. doi: 10.1093/eurheartj/ehv526
25. Makkar RR, Blanke P, Leipsic J, Thourani V, Chakravarty T, Brown D, et al. Subclinical leaflet thrombosis in transcatheter and surgical bioprosthetic valves: PARTNER 3 cardiac computed tomography substudy. J Am Coll Cardiol. (2020) 75:3003–15. doi: 10.1016/j.jacc.2020.04.043
26. D’Ascenzo F, Salizzoni S, Saglietto A, Cortese M, Latib A, Franzone A, et al. Incidence, predictors and cerebrovascular consequences of leaflet thrombosis after transcatheter aortic valve implantation: a systematic review and meta-analysis. Eur J Cardiothorac Surg. (2019) 56:488–94. doi: 10.1093/ejcts/ezz099
27. Ducci A, Pirisi F, Tzamtzis S, Burriesci G. Transcatheter aortic valves produce unphysiological flows which may contribute to thromboembolic events: an in-vitro study. J Biomech. (2016) 49:4080–9. doi: 10.1016/j.jbiomech.2016.10.050
28. Devereux RB, de Simone G, Arnett DK, Best LG, Boerwinkle E, Howard BV, et al. Normal limits in relation to age, body size and gender of two-dimensional echocardiographic aortic root dimensions in persons >/=15 years of age. Am J Cardiol. (2012) 110:1189–94. doi: 10.1016/j.amjcard.2012.05.063
29. Fuchs A, De Backer O, Brooks M, de Knegt MC, Bieliauskas G, Yamamoto M, et al. Subclinical leaflet thickening and stent frame geometry in self-expanding transcatheter heart valves. Eurointervention. (2017) 13:e1067–75. doi: 10.4244/EIJ-D-17-00373
30. Piayda K, Dannenberg L, Zako S, Maier O, Bosbach G, Polzin A, et al. Predictors of calcification distribution in severe tricuspid aortic valve stenosis. Int J Cardiovasc Imaging. (2021) 37:2791–9. doi: 10.1007/s10554-021-02248-6
31. Mangione FM, Jatene T, Goncalves A, Fishbein GA, Mitchell RN, Pelletier MP, et al. Leaflet thrombosis in surgically explanted or post-mortem TAVR Valves. JACC Cardiovasc Imaging. (2017) 10:82–5. doi: 10.1016/j.jcmg.2016.11.009
32. Gunning PS, Saikrishnan N, McNamara LM, Yoganathan AP. An in vitro evaluation of the impact of eccentric deployment on transcatheter aortic valve hemodynamics. Ann Biomed Eng. (2014) 42:1195–206. doi: 10.1007/s10439-014-1008-6
33. Gunning PS, Saikrishnan N, Yoganathan AP, McNamara LM. Total ellipse of the heart valve: the impact of eccentric stent distortion on the regional dynamic deformation of pericardial tissue leaflets of a transcatheter aortic valve replacement. J R Soc Interface. (2015) 12:20150737. doi: 10.1098/rsif.2015.0737
34. Sheriff J, Bluestein D, Girdhar G, Jesty J. High-shear stress sensitizes platelets to subsequent low-shear conditions. Ann Biomed Eng. (2010) 38:1442–50. doi: 10.1007/s10439-010-9936-2
35. Milhorini Pio S, Bax J, Delgado V. How valvular calcification can affect the outcomes of transcatheter aortic valve implantation. Expert Rev Med Devices. (2020) 17:773–84. doi: 10.1080/17434440.2020.1789456
36. Rodes-Cabau J, Masson JB, Welsh RC, Garcia Del Blanco B, Pelletier M, Webb JG. Aspirin Versus Aspirin Plus Clopidogrel as Antithrombotic Treatment Following Transcatheter Aortic Valve Replacement With a Balloon-Expandable Valve: The ARTE (Aspirin Versus Aspirin + Clopidogrel Following Transcatheter Aortic Valve Implantation) Randomized Clinical Trial. JACC Cardiovasc Interv. (2017) 10:1357–65. doi: 10.1016/j.jcin.2017.04.014
37. Jose J, Sulimov DS, El-Mawardy M, Sato T, Allali A, Holy EW, et al. Clinical bioprosthetic heart valve thrombosis after transcatheter aortic valve replacement: incidence, characteristics, and treatment outcomes. JACC Cardiovasc Interv. (2017) 10:686–97. doi: 10.1016/j.jcin.2017.01.045
38. Maher ER, Young G, Smyth-Walsh B, Pugh S, Curtis JR. Aortic and mitral valve calcification in patients with end-stage renal disease. Lancet. (1987) 2:875–7. doi: 10.1016/S0140-6736(87)91370-5
Keywords: 4D multidetector computed tomography, aortic valve calcification, aortic valve stenosis, leaflet thrombosis, trans-catheter aortic valve replacement
Citation: Bak M, Park S-J, Choi K, Kim J, Park TK, Kim EK, Kim SM and Choi S-H (2022) Risk factors and clinical effects of subclinical leaflet thrombosis after transcatheter aortic valve replacement. Front. Cardiovasc. Med. 9:1001753. doi: 10.3389/fcvm.2022.1001753
Received: 24 July 2022; Accepted: 20 October 2022;
Published: 14 November 2022.
Edited by:
Chi Young Shim, Yonsei University, South KoreaReviewed by:
Karthik Seetharam, West Virginia State University, United StatesAndrew David Wisneski, University of California, San Francisco, United States
Copyright © 2022 Bak, Park, Choi, Kim, Park, Kim, Kim and Choi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sung-Ji Park, dHljaGUucGFya0BnbWFpbC5jb20=
 Minjung Bak1
Minjung Bak1 Sung-Ji Park
Sung-Ji Park Eun Kyoung Kim
Eun Kyoung Kim Sung Mok Kim
Sung Mok Kim Seung-Hyuk Choi
Seung-Hyuk Choi