- 1Division of Clinical Pharmacology and Therapeutics, Department of Medicine, School of Clinical Medicine, The University of Hong Kong, Pokfulam, Hong Kong SAR, China
- 2State Key Laboratory of Pharmaceutical Biotechnology, The University of Hong Kong, Pokfulam, Hong Kong SAR, China
- 3Institute of Cardiovascular Science and Medicine, The University of Hong Kong, Pokfulam, Hong Kong SAR, China
Introduction: Poor dental health is associated with cardiovascular diseases (CVD). However, the relationship between CVD and denture use is currently unknown. This study aimed to investigate whether denture use is associated with CVD among American adults.
Methods: 10,246 non-pregnant subjects aged 30–59 years from five cycles (2009–2018) of the United States National Health and Nutrition Examination Survey (NHANES) were included in this study. Participants who were observed by a dental examiner wearing denture/partial denture/plates were defined as denture users. CVD was defined as self-reported coronary heart disease, myocardial infarction, angina pectoris, stroke, and congestive heart failure. The association between denture use and CVD was analyzed using logistic regression with adjustment for potential cofounders.
Results: 4.4% (95% CI, 3.9–5.0) participants had CVD, and 3.5% (95% CI, 2.8–4.5) participants were denture users. Denture use was associated with CVD [OR = 4.26, 95% CI (2.90–6.28), P < 0.01], which remained significant [adjusted OR = 1.82, 95% CI (1.15–2.88), P < 0.01] after adjustments for sociodemographic characteristics, smoking, alcohol use, drug addiction, body mass index (BMI), and abnormal medical conditions including gum problem, hypertension, diabetes, and hyperlipidemia. Women with dentures had significantly higher odds of CVD [adjusted OR = 2.13, 95% CI (1.10–4.11), P = 0.025].
Conclusion: In this nationally representative survey, denture use was associated with CVD. Denture use may be an unconventional risk factor for assessing CVD risks, especially in women. Future studies are required to investigate whether CVD and denture use is causally related.
Introduction
Cardiovascular diseases (CVD), such as coronary heart disease, stroke, and congestive heart failure, are posing increasing burdens to the healthcare systems (1). Controlling conventional risk factors, such as blood pressure, total cholesterol, high-density lipoprotein cholesterol, smoking, and glucose intolerance, are essential in preventing CVD (2, 3). However, as individuals without conventional risk factors may still develop CVD, there is an increasing awareness that there are unrecognized potential risk factors which may also contribute to developing CVD. It was estimated that 10–20% of CVD can be explained by unconventional risk factors (4).
Poor oral health has been proposed as an unconventional risk factor for heart disease for many years. Previous studies have demonstrated that poor dental health has been related to an increased risk of all-cause mortality and mortality due to CVD and respiratory diseases (5). Vice versa, CVD and related treatments also affect dental health. Nevertheless, the results on the relation between oral diseases and CVD are so far inconsistent (6, 7). A recent meta-analysis demonstrated that dental problem was not associated with cardiovascular mortality (6).
Denture status is a strong predictor of oral health-related quality of life (8). However, denture problems, particularly denture-related stomatitis, are correlated with endothelial dysfunction in elderly patients (9). Endothelial dysfunction is linked to CVD by several mechanisms, including altered endothelial anticoagulant and anti-inflammatory capabilities, poor modulation of vascular growth, and uncontrolled vascular remodeling (10). While endothelial dysfunction may explain the potential link between dentures and CVD, the association between dentures and CVD has not been explored.
The present study aimed to investigate the association between denture use and CVD using data from the US National Health and Nutrition Examination Survey (NHANES) 2009–2018.
Subjects and methods
Study subjects
The National Center for Health Statistics of the US Centers for Disease Control and Prevention conducted NHANES 2009–2018. It is a cross-sectional survey of the civilian, non-institutionalized American population nationally representative samples (11). Data is released every 2 years, and the official website1 provides extensive descriptions of measuring processes and protocols (11). All participants provided informed consent, and the study was approved by the Institutional Review Board of the Centers for Disease Control and Prevention. In NHANES 2009–2018, 28,835 subjects were interviewed and examined in the mobile examination center. The demographics, examination results (blood pressure, body measures, oral health), medical condition questionnaire, questionnaire data (smoking, drug use, alcohol intake), and laboratory data (glycated hemoglobin, plasma fasting glucose) of the participants were extracted. A total of 315 pregnant or lactating participants were excluded. Participants with absence or incomplete data for education levels (n = 45), and family income-to-poverty ratio (n = 2,948) were also excluded. Missing, unreliable or uncertain covariables, including body mass index (BMI) (n = 1,179) or smoking records (n = 12), alcohol use (n = 2,177), or drug addiction (n = 7,789) were excluded. We also excluded 4,124 subjects with incomplete, unreliable, or uncertain data for hypertension, diabetes, CVD, or oral health (Figure 1).
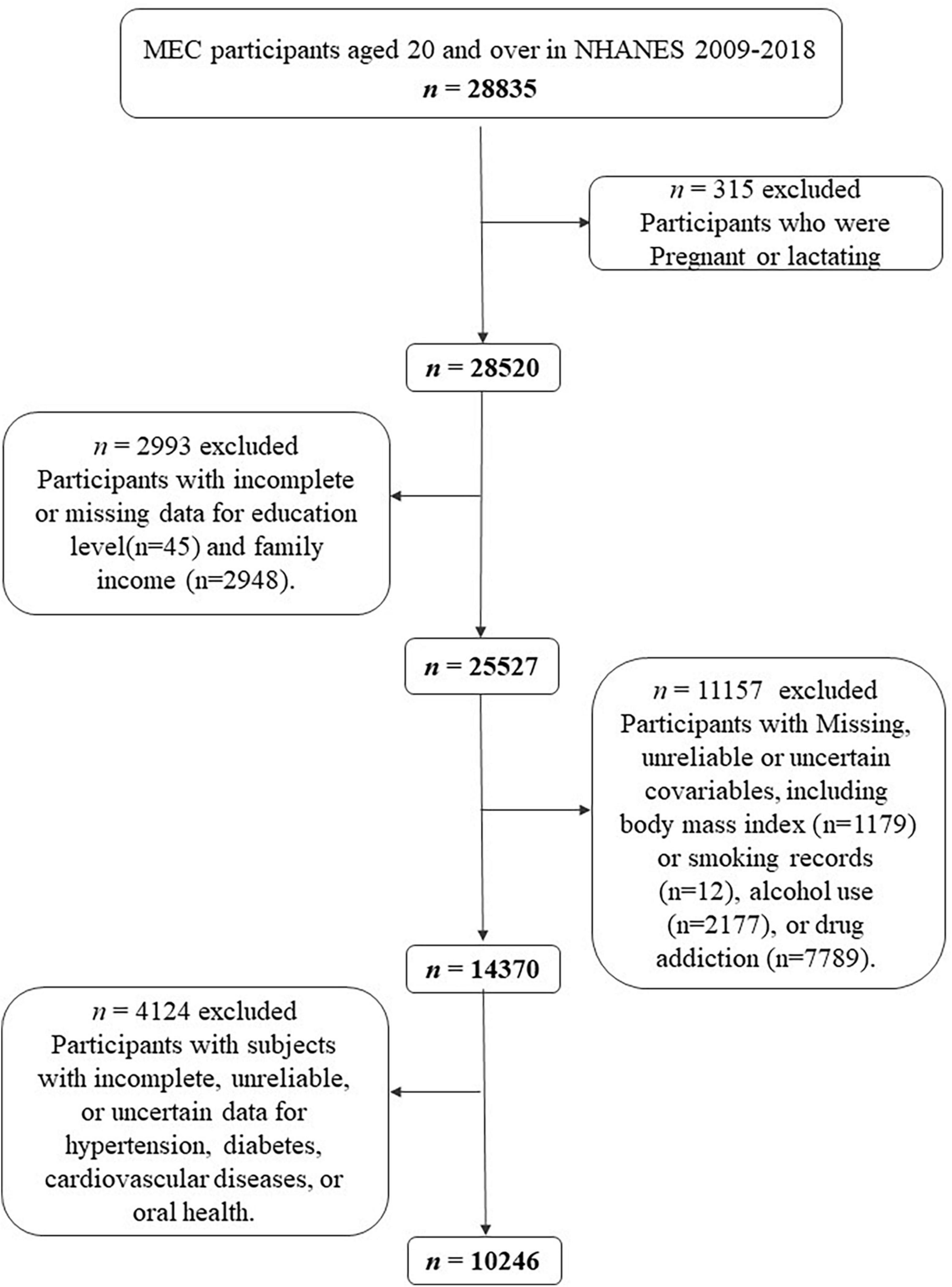
Figure 1. Flow chart of the screening process for the selection of eligible participants in National Health and Nutrition Examination Survey (NHANES) 2009–2018.
Definition
Sociodemographic variables
Participants were stratified into three age groups: 30–39, 40–49, and 50–59 years old. Race/ethnicity was defined as non-Hispanic whites, non-Hispanic blacks, Mexican Americans, and others. Three categories of educational attainment were used: high school or less, some college, and college graduates or above. Family income was divided into three categories based on the income-to-poverty ratio: less than 130%, 131–338%, and more than 339%.
Risk factors and disease
A BMI of less than 18.5 was considered underweight, BMI falling between 18.6–24.9 was regarded normal, over 25 was considered overweight, and over 30 was considered obese (12). Participants were regarded as “Never smoker” if they smoked less than 100 cigarettes in their lifetime; participants who identified as “ever smokers” had smoked over 100 cigarettes in their lifetime but had since quit. “Current light smokers” were participants who smoke less than 10 cigarettes per day now, and “current heavy smokers” were participants who smoke over 10 cigarettes per day. Alcohol intake was defined as having at least 12 drinks of any type of alcoholic beverage in any 1 year. A participant who self-reported taking cocaine, methamphetamines, or heroin more than two to five times in their lifetime was considered to have a drug addiction, which is defined as recurrent drug use. Diabetes was characterized as either a self-reported physician diagnosis of diabetes or increased values of fasting glucose [7.0 mmol/L (126 mg/dl)], glycated hemoglobin (HbA1c) [6.5%], or non-fasting glucose [11.1 mmol/L (200 mg/dl)]. Elevated levels of total cholesterol (≥240 mg/dl), triglycerides (≥200 mg/dl), or low-density lipoprotein (≥160 mg/dl) are referred to as hyperlipidemia (13). According to the American Heart Association/American College of Cardiology (AHA/ACC) 2017 guideline, participants were deemed to have hypertension if their systolic or diastolic blood pressure was greater than 130 or 80 mmHg, respectively (14). The definition of CVD (coronary heart disease, angina, myocardial infarction, congestive heart failure, or stroke) was obtained from the self-reported questionnaire. Participants who were observed by a dental examiner wearing denture/partial denture/plates were defined as denture users.
Statistical analysis
Examination complex sample weights were adopted in all analyses due to unequal probabilities of selection, non-response bias, and oversampling of non-Hispanic blacks by using primary sampling units and strata. Data are presented as a mean (±standard error) or a percent (95% confidence interval). Multiple logistic regression models were applied to assess the trends over time. The association of dentures with CVD was assessed by multiple logistic regression. The P value for interaction was estimated by including each multiplicative interaction term in the linear regression models after adjusting for the main effects of all covariates. The odds ratio (OR) was adjusted to account for the presence of risk factor. All two-tailed significance tests have a P value of less than 0.05 to qualify as significant. STATA was used to conduct the statistical analysis (version 15.1).
Results
Characteristics of study participants
A total of 10,246 non-pregnant adults aged 30 years old were included in the final analysis. Each participant represented approximately 50,000 individuals in the United States. This sample size represented 97, 661, 993 civilians, a non-institutionalized U.S. population. The characteristics of participants by NHANES cycles and the complex sample weighted percentages of each character are summarized in Supplementary Table 1. Overall, 3.5% of (95% CI, 2.8–4.5%) subjects used dentures, and 4.4% (95% CI, 3.9–5.0%) of subjects had a history of CVD.
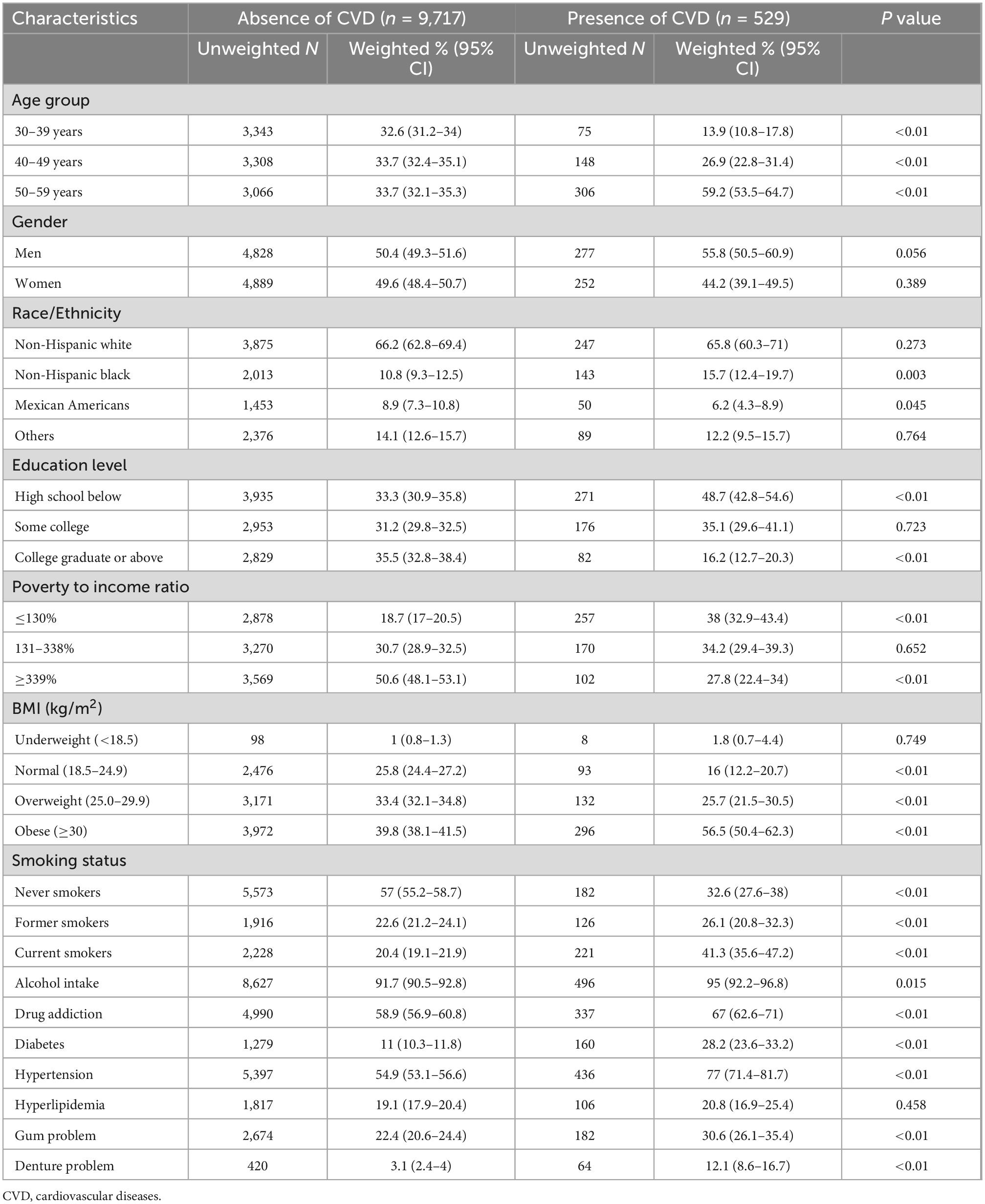
The characteristics of 10,246 participants stratified by denture status are shown in Table 1. The mean age of denture use was 50.14 years (95% CI 49.80–51.20). Over half of denture subjects were aged between 50 and 59 [55.8% (95% CI, 49.0–62.4)]. 53.4% (95% CI, 46.4–60.3) of denture users were men. Most frequently, those who were wearing dentures were participants with high school or below education [64.3% (95% CI, 58.5–69.7)], lower income (PIR < 138%) [46.5% (95% CI, 39.8–53.3)], obese [48.4% (95% CI, 43.0–53.8)], and current smokers [50.8% (95% CI, 43.6–58.0)]. In comparison to participants without dentures, denture users were more likely to have diabetes and hypertension (both P < 0.01). The crude rate of CVD amongst denture users was significantly higher than those non-denture users [15.2% (95% CI, 11.3–20.3) vs. 4.0% (95% 3.5–4.7)].
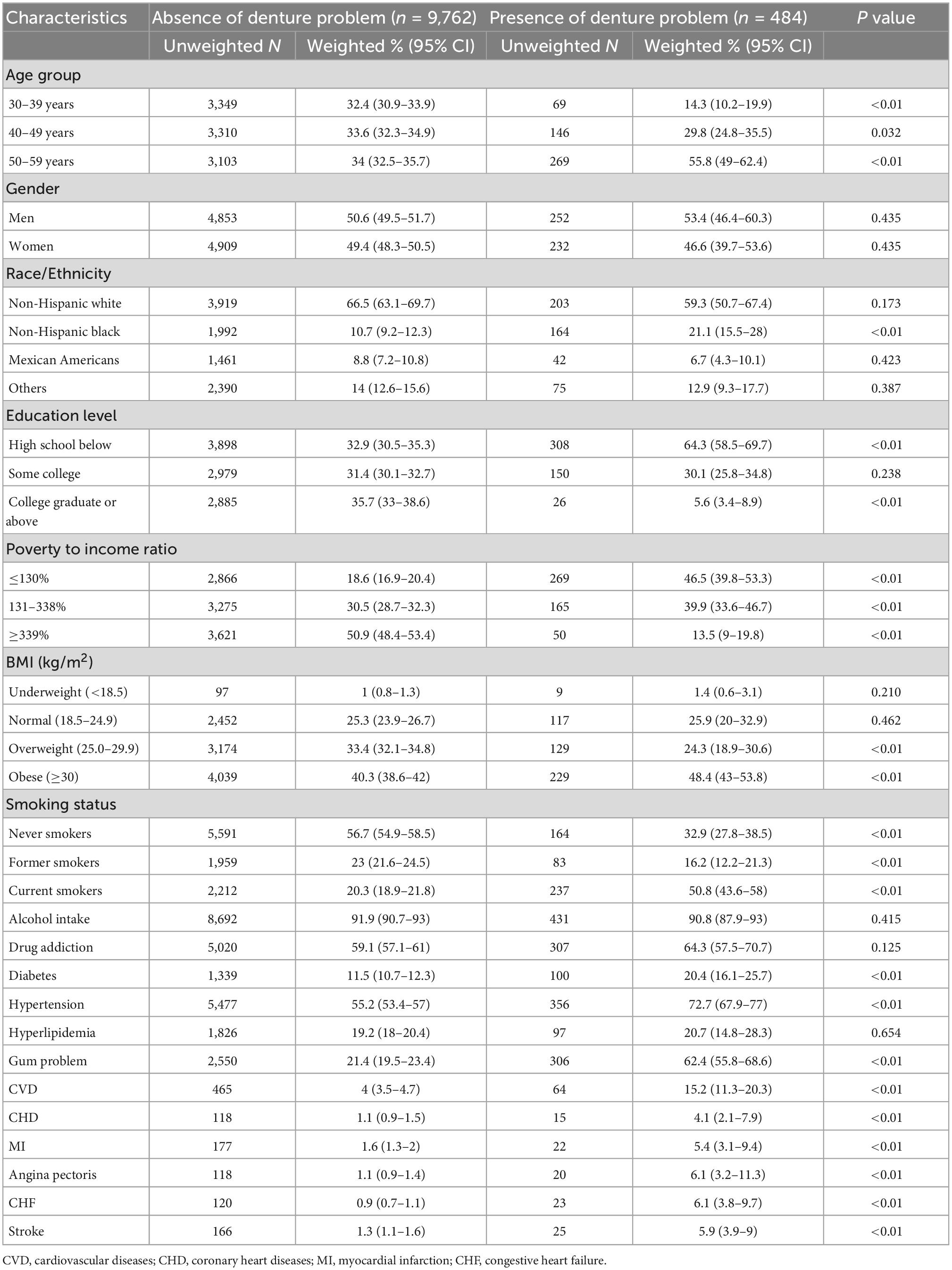
Table 1. Baseline characteristics of presence of denture patients versus the absence of denture patients.
The characteristics of all 10,246 participants stratified by CVD status are shown in Table 2. The mean age of CVD subjects was 49.69 (95% CI, 48.87–50.51) years. 59.2% (95% CI, 53.5–64.7) of CVD subjects were aged between 50 and 59 years old. Subjects in high school or below, lower-income, current smokers, and obese had more CVD (all P < 0.01). Participants with CVD also had a higher prevalence of alcohol intake, drug addiction, gum problems, diabetes, and hypertension than non-CVD participants (all P < 0.01). The use of dentures was also more common among participants with CVD [12.1% (95% CI, 8.6–16.7) vs. 3.1% (95% CI, 2.4–4.0)].
The association between dentures and CVD
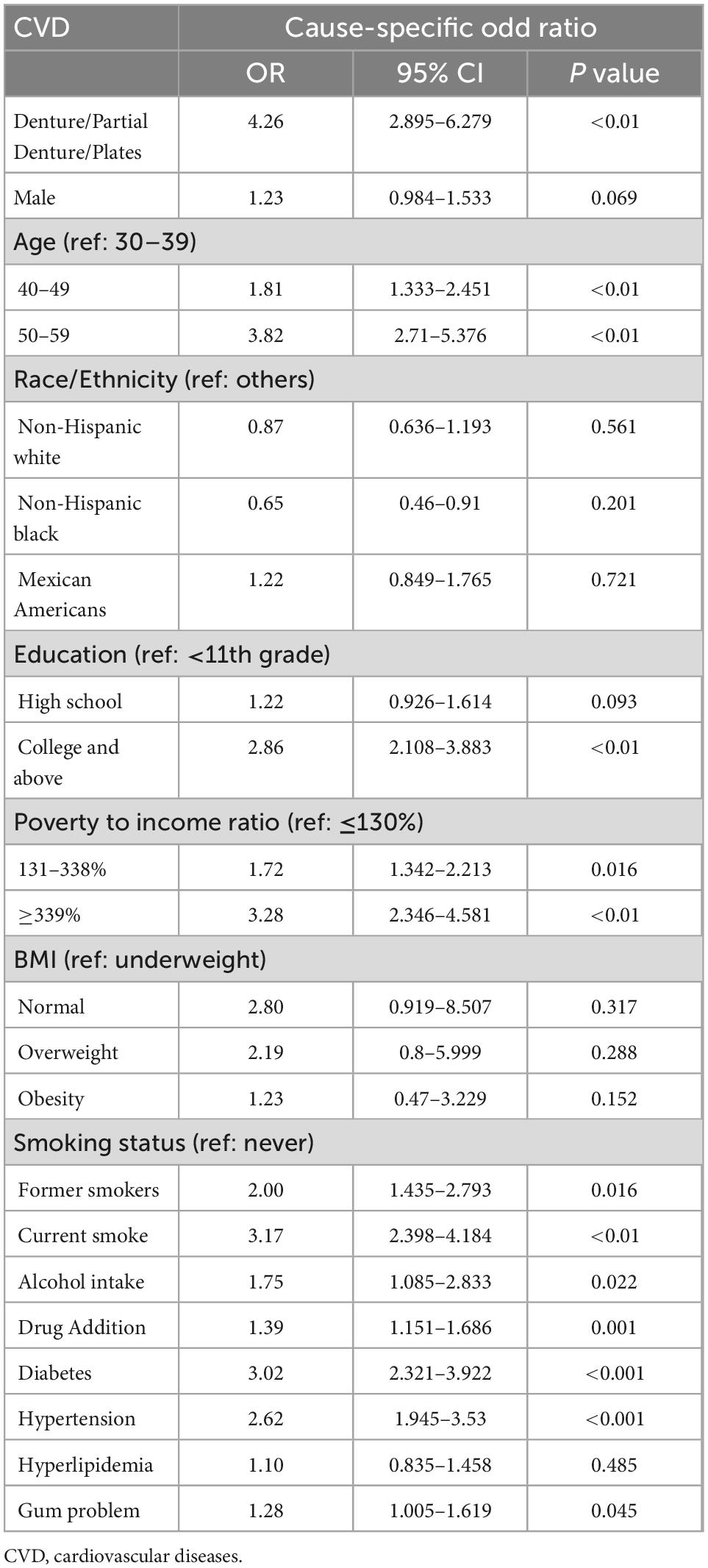
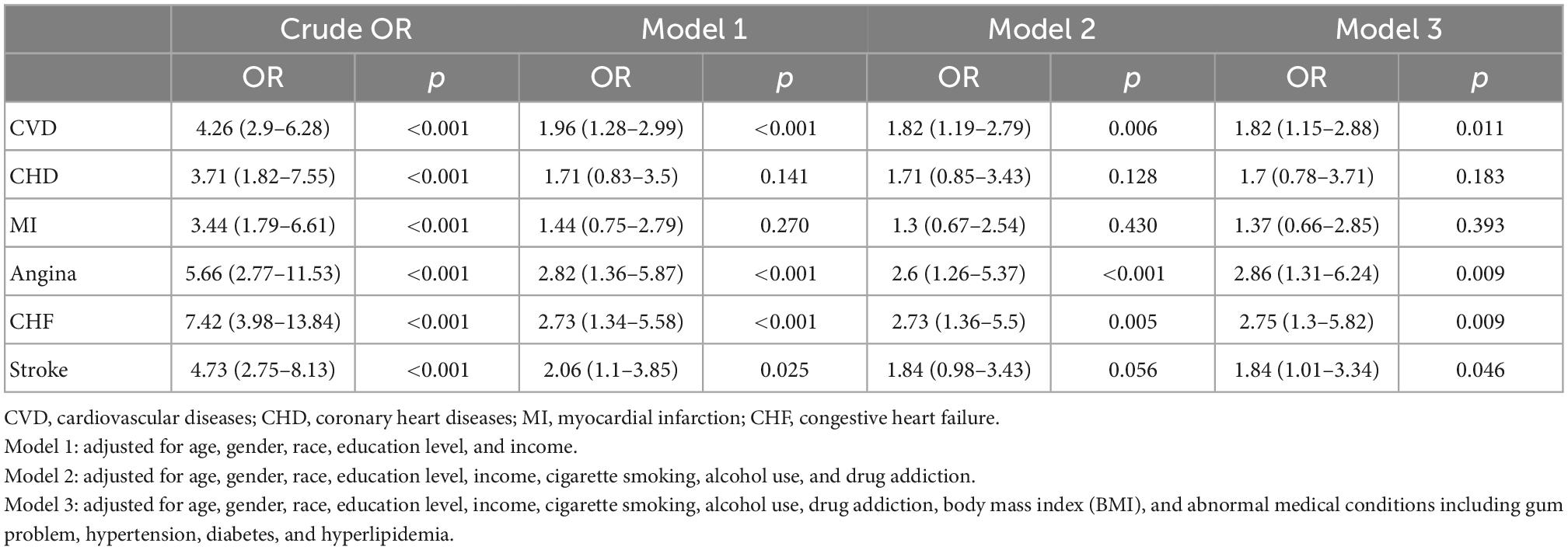
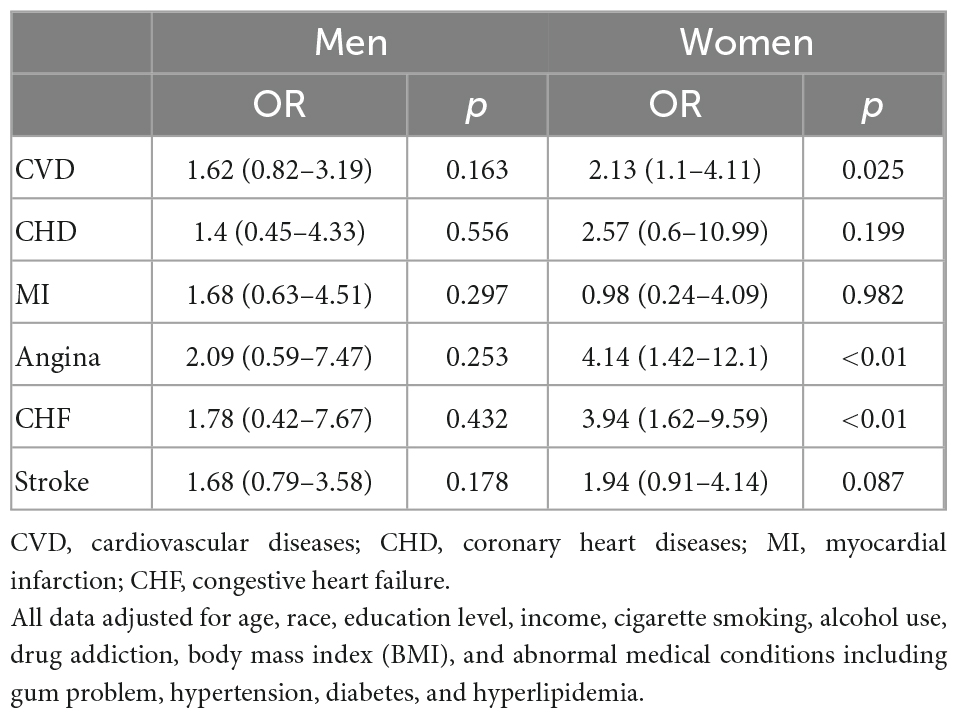
In the competing risk analysis, denture use was significantly associated with CVD [OR = 4.26 95% CI (2.90–6.28), P = 0.01] (Table 3). Age, education levels, BMI, smoking, alcohol intake, drug addiction, diabetes, hypertension, and gum problems may have a potential effect on the association between dentures and CVD. The multivariate logistic regression analysis of the association between denture use and CVD before and after adjustments is shown in Table 4. Denture use was significantly associated with CVD after adjustments [OR = 1.82, (95% CI, 1.15–2.88)]. That is, denture use was independently associated with 82% higher odds of CVD. The association was particularly significant among women in the subgroup analysis (OR = 2.13, 95% CI: 1.10–4.11) (Table 5).
Discussion
Our study demonstrated a robust association between denture use and CVD after adjustments using 10 years of nationally representative data from United States NHANES 2009–2018. Our study indicated that dentures might be an unconventional risk factor for CVD.
Abundant studies showed that poor dental health was related to CVD (7, 15). A cohort study of a million participants with over 65,000 cardiovascular events found a moderate association between poor oral health and coronary heart disease (15). However, the majority of the previous studies were only investigating periodontal inflammation and gingival bleeding (16, 17). Dentures are used to maintain the continuity of the masticatory system and improve esthetic outcomes, which are essential in the restoration and rehabilitation of oral health (18, 19). The prevalence of denture-related lesions among denture users, including denture stomatitis, angular cheilitis, traumatic ulcers, denture irritation hyperplasia, flabby ridges, and oral carcinomas, varies from 50 to 75% (20, 21).
The denture-related stomatitis involves an inflammatory process upon oral mucosa contact with dentures. Indeed, over two-thirds of older individuals who wear dentures are affected, making it one of the most prevalent diseases in this population (21). Age, smoking, obesity, and diabetes are the common risk factors for both denture-related stomatitis and atherosclerotic vascular disease (22, 23). A previous study has shown that denture-related stomatitis was associated with a reduction in endothelial function (9). The severity of endothelial dysfunction is substantially correlated with the onset of coronary artery disease. It also predicts future cardiovascular events. Thus, dentures may directly increase the risk of cardiovascular disorders.
Besides, continuous and long-term usage of dentures may accelerate the formation of denture plaque on the surfaces of prostheses (22). A special plaque, made up of fat, cholesterol, calcium, and other substances in the blood, may accumulate as dental plaque and vascular plaque. As such, dental plaque could be a telltale sign of atherosclerosis, predicting the risk of CVD. Furthermore, fungi are also major colonizers of the denture-wearing oral cavity. Oral biofilms, which commonly contain three to four species of fungi, may lead to systemic infections (24). These breeding fungi and bacteria infections may also cause other oral problems such as cavities, gingivitis, and periodontal diseases, which affects the blood coagulation, lipid metabolism, thrombocytes, macrophages, and endothelial cells. As such, the denture plaque on the denture may also explain the increased cardiovascular risks.
Another plausible explanation is the different salivary and gut microbiome compositions between denture users and non-users. Previously, the salivary microbiome among 997 adults was analyzed using high-throughput sequencing of the V1–V3 region of the 16S rRNA gene. It demonstrated that Streptococcus and Neisseria were more prevalent micro-organisms among denture users. Moreover, the salivary microbiome among denture users was also less diverse (25). The salivary microbiome is a good reflection of the gut microbiome’s complexity and diversity (26). It is now well-known that the gut microbiome strongly predicts the development of CVD (27). Gut microbiota was shown to play a key role in various CVD, including atherosclerosis, hypertension, heart failure, atrial fibrillation, and myocardial fiber change (28, 29).
In the gender-stratified analysis, there was a strong association between dentures and CVD among women (Table 5). This difference may be attributed to hormonal changes (30). It may as well reflects the differences in the frequency of dental treatment between women and men (31). In the age-stratified analysis, the association remains significant for both men and women aged 40–49 years old. Advanced age is an established risk factor for both denture problems and CVD (32). A recent study demonstrated that denture use was associated with an increased risk of future ischemic events in patients with acute myocardial infarction, especially in those with age ≥ 75 years (33). However, in our study, the association did not increase with age, but rather, strongest among participants aged 40–49 years old. The difference could be explained by the different study populations and definitions of outcomes between the two studies, as well as the presence of confounding factors.
It is important to acknowledge the limitations of this study. Firstly, given the cross-sectional nature of the study and the presence of confounding factors such as lower socioeconomic status, obesity, and smoking, future studies are required to investigate whether CVD and denture use is causally related. While those factors were adjusted in the multivariate analysis, residual confounding might still be present. Besides, NHANES includes a self-reported questionnaire for medications, illnesses, and other health variables, which introduces recall and self-reported bias. Thirdly, the NHANES did not include information on disorders associated with dentures, such as denture stomatitis, traumatic ulcers, and oral cancers. Fourth, NHANES did not provide information on the duration of denture wearing, such that the effect of the duration on CVD was not investigated. Last but not least, the study did not take medication use into account. For instance, the effect of anticoagulant therapies on dental health was not discussed.
Conclusion
Our analysis from a nationally representative survey demonstrated denture wearing was significantly associated with CVD, especially in women and participants between 40–49 years old. Denture usage may potentially be a risk factor for CVD. Future studies are required to investigate whether CVD and denture use are causally related.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary material.
Ethics statement
The studies involving human participants were reviewed and approved by the Research Ethics Review Board at the National Center for Health Statistics in the US. The patients/participants provided their written informed consent to participate in this study.
Author contributions
XL: conceptualization, methodology, data analysis, and drafting of the manuscript. OC: conceptualization and revision of the manuscript. BC: methodology, supervision, and revision of the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.1000478/full#supplementary-material
Footnotes
References
1. Global Burden of Disease. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. (2017) 390:1151–210. doi: 10.1016/S0140-6736(17)32152-9
2. Anderson K, Odell P, Wilson P, Kannel W. Cardiovascular disease risk profiles. Am Heart J. (1991) 121(1 Pt 2):293–8. doi: 10.1016/0002-8703(91)90861-B
3. Cheung B, Lauder I, Lau C, Kumana C. Meta-analysis of large randomized controlled trials to evaluate the impact of statins on cardiovascular outcomes. Br J Clin Pharmacol. (2004) 57:640–51. doi: 10.1111/j.1365-2125.2003.02060.x
4. Khot U, Khot M, Bajzer C, Sapp S, Ohman E, Brener S, et al. Prevalence of conventional risk factors in patients with coronary heart disease. JAMA. (2003) 290:898–904. doi: 10.1001/jama.290.7.898
5. Kotronia E, Brown H, Papacosta A, Lennon L, Weyant R, Whincup P, et al. Oral health and all-cause, cardiovascular disease, and respiratory mortality in older people in the UK and USA. Sci Rep. (2021) 11:16452. doi: 10.1038/s41598-021-95865-z
6. Peng J, Song J, Han J, Chen Z, Yin X, Zhu J, et al. The relationship between tooth loss and mortality from all causes, cardiovascular diseases, and coronary heart disease in the general population: systematic review and dose-response meta-analysis of prospective cohort studies. Biosci Rep. (2019) 39:BSR20181773. doi: 10.1042/BSR20181773
7. Koka S, Gupta A. Association between missing tooth count and mortality: a systematic review. J Prosthodont Res. (2018) 62:134–51. doi: 10.1016/j.jpor.2017.08.003
8. John M, Koepsell T, Hujoel P, Miglioretti D, LeResche L, Micheelis W. Demographic factors, denture status and oral health-related quality of life. Community Dent Oral Epidemiol. (2004) 32:125–32. doi: 10.1111/j.0301-5661.2004.00144.x
9. Maciąg J, Osmenda G, Nowakowski D, Wilk G, Maciąg A, Mikołajczyk T, et al. Denture-related stomatitis is associated with endothelial dysfunction. Biomed Res Int. (2014) 2014:474016.
10. Cai H, Harrison D. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res. (2000) 87:840–4. doi: 10.1161/01.RES.87.10.840
11. CDC. National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey Questionnaire. Hyattsville, MD: CDC (2022).
12. Nuttall F. Body mass index: obesity, BMI, and health: a critical review. Nutr Today. (2015) 50:117–28. doi: 10.1097/NT.0000000000000092
13. NCEP. Third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) final report. Circulation. (2002) 106:3143–421. doi: 10.1161/circ.106.25.3143
14. Whelton P, Carey R, Aronow W, Casey D Jr, Collins K, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Hypertension. (2018) 71:1269–324. doi: 10.1161/HYP.0000000000000075
15. Batty G, Jung K, Mok Y, Lee S, Back J, Lee S, et al. Oral health and later coronary heart disease: cohort study of one million people. Eur J Prev Cardiol. (2018) 25:598–605. doi: 10.1177/2047487318759112
16. Arbes S Jr, Slade G, Beck J. Association between extent of periodontal attachment loss and self-reported history of heart attack: an analysis of NHANES III data. J Dent Res. (1999) 78:1777–82. doi: 10.1177/00220345990780120301
17. Desvarieux M, Demmer R, Rundek T, Boden-Albala B, Jacobs D Jr, Papapanou P, et al. Relationship between periodontal disease, tooth loss, and carotid artery plaque: the oral infections and vascular disease epidemiology study (INVEST). Stroke. (2003) 34:2120–5. doi: 10.1161/01.STR.0000085086.50957.22
18. Thomson W, Ma S. An ageing population poses dental challenges. Singapore Dent J. (2014) 35c:3–8. doi: 10.1016/j.sdj.2014.10.001
19. Turgut Cankaya Z, Yurdakos A, Gokalp Kalabay P. The association between denture care and oral hygiene habits, oral hygiene knowledge and periodontal status of geriatric patients wearing removable partial dentures. Eur Oral Res. (2020) 54:9–15. doi: 10.26650/eor.20200048
20. Gendreau L, Loewy Z. Epidemiology and etiology of denture stomatitis. J Prosthodont. (2011) 20:251–60. doi: 10.1111/j.1532-849X.2011.00698.x
21. Kossioni A. The prevalence of denture stomatitis and its predisposing conditions in an older Greek population. Gerodontology. (2011) 28:85–90. doi: 10.1111/j.1741-2358.2009.00359.x
22. Salerno C, Pascale M, Contaldo M, Esposito V, Busciolano M, Milillo L, et al. Candida-associated denture stomatitis. Med Oral Patol Oral Cir Bucal. (2011) 16:e139–43. doi: 10.4317/medoral.16.e139
23. Lo C, Fei Y, Cheung B. Cardiovascular outcomes in trials of new antidiabetic drug classes. Card Fail Rev. (2021) 7:e04. doi: 10.15420/cfr.2020.19
24. Haug R. The changing microbiology of maxillofacial infections. Oral Maxillofac Surg Clin North Am. (2003) 15:1–15. doi: 10.1016/S1042-3699(02)00076-6
25. Murugesan S, Al Ahmad S, Singh P, Saadaoui M, Kumar M, Al Khodor S. Profiling the salivary microbiome of the Qatari population. J Transl Med. (2020) 18:127. doi: 10.1186/s12967-020-02291-2
26. Kodukula K, Faller D, Harpp D, Kanara I, Pernokas J, Pernokas M, et al. Gut microbiota and salivary diagnostics: the mouth is salivating to tell us something. Biores Open Access. (2017) 6:123–32. doi: 10.1089/biores.2017.0020
27. Jie Z, Xia H, Zhong S, Feng Q, Li S, Liang S, et al. The gut microbiome in atherosclerotic cardiovascular disease. Nat Commun. (2017) 8:845.
28. Aron-Wisnewsky J, Clément K. The gut microbiome, diet, and links to cardiometabolic and chronic disorders. Nat Rev Nephrol. (2016) 12:169–81. doi: 10.1038/nrneph.2015.191
29. Lourenςo T, Spencer S, Alm E, Colombo A. Defining the gut microbiota in individuals with periodontal diseases: an exploratory study. J Oral Microbiol. (2018) 10:1487741. doi: 10.1080/20002297.2018.1487741
30. Golecka-Bakowska M, Mierzwinska-Nastalska E, Bychawska M. Influence of hormone supplementation therapy on the incidence of denture stomatitis and on chemiluminescent activity of polymorphonuclear granulocytes in blood of menopausal-aged women. Eur J Med Res. (2010) 15(Suppl. 2):46–9.
31. Hamasha A, Alshehri A, Alshubaiki A, Alssafi F, Alamam H, Alshunaiber R. Gender-specific oral health beliefs and behaviors among adult patients attending King Abdulaziz Medical City in Riyadh. Saudi Dent J. (2018) 30:226–31.
32. Rodgers J, Jones J, Bolleddu S, Vanthenapalli S, Rodgers L, Shah K, et al. Cardiovascular risks associated with gender and aging. J Cardiovasc Dev Dis. (2019) 6:19.
Keywords: denture, cardiovascular diseases, coronary heart diseases, heart failure, National Health and Nutrition Examination Survey (NHANES)
Citation: Liang X, Chou OHI and Cheung BMY (2023) The association between denture use and cardiovascular diseases. The United States National Health and Nutrition Examination Survey 2009–2018. Front. Cardiovasc. Med. 9:1000478. doi: 10.3389/fcvm.2022.1000478
Received: 22 July 2022; Accepted: 21 December 2022;
Published: 10 January 2023.
Edited by:
Harry H. X. Wang, Sun Yat-sen University, ChinaReviewed by:
Yuichi Saito, Chiba University Hospital, JapanSrdjan Aleksandric, Clinical Center of Serbia, University of Belgrade, Serbia
Copyright © 2023 Liang, Chou and Cheung. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bernard M. Y. Cheung,  bXljaGV1bmdAaGt1Lmhr
bXljaGV1bmdAaGt1Lmhr
†Present address: Bernard M. Y. Cheung Department of Medicine, Queen Mary Hospital, Pokfulam, Hong Kong SAR, China
 Xiaopeng Liang
Xiaopeng Liang Oscar Hou In Chou1
Oscar Hou In Chou1 Bernard M. Y. Cheung
Bernard M. Y. Cheung