- 1National Clinical Research Center for Chinese Medicine Cardiology, Xiyuan Hospital, China Academy of Chinese Medical Sciences, Beijing, China
- 2Graduate School of China Academy of Chinese Medical Sciences, Beijing, China
- 3Graduate School of Beijing University of Chinese Medicine, Beijing, China
- 4Cardiovascular Diseases Center, Dongzhimen Hospital, Beijing University of Chinese Medicine, Beijing, China
- 5Department of Cardiovascular, The First Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou, China
- 6Cardiovascular Diseases Center, The Affiliated Hospital of Shanxi University of Chinese Medicine, Taiyuan, China
Introduction: The Shenqisuxin granule (SQSX), a novel Chinese herbal formula, has the effect of preventing in-stent restenosis and improving angiogenesis. We intend to evaluate the efficacy and safety of SQSX to provide a possible therapeutic strategy for complex coronary artery disease (CCAD) after percutaneous coronary intervention (PCI).
Methods/design: The study is a multi-center, randomized, double-blinded, parallel, placebo-controlled trial. A total of 120 participants will be randomized 1:1 into the intervention group and the control group. Based on standardized treatment, the intervention group and control group will receive SQSX and placebo for 2 months, respectively. The primary outcomes, metabolic equivalents (METS) and peak oxygen uptake (Peak VO2), and the secondary outcomes, including other indicators of cardiorespiratory fitness (CRF), the European Quality of Life Questionnaire (EQ-5D-5L), the Seattle Angina Scale (SAQ), etc., will be assessed at baseline and 2 months ± 3 days. In addition, the survey scales will also be tested at 1 month ± 3 days. Trimethylamine N-oxide (TMAO), high-sensitivity C-reactive protein (hs-CRP), and gut microbiota features will be assessed at baseline and 2 months ± 3 days to probe possible mechanism. The major adverse cardiac and cerebrovascular events (MACCE) and bleeding events will be monitored until the 12-month follow-up.
Discussion: This study is launched to assess the efficacy and safety of SQSX in CCAD after PCI and probe the possible mechanism.
Clinical trial registration: China Clinical Trial Registry, ChiCTR2200060979, Registered on June 14, 2022.
Introduction
Complex coronary artery disease (CCAD), a lesion type with great difficulty and a low success rate in revascularization, is accompanied by a higher rate of major adverse cardiac and cerebrovascular events (MACCE) (1, 2). The 10-year mortality rate of three-vessel disease, chronic total occlusion, and left main lesion after percutaneous coronary intervention (PCI) is up to 21, 27.6, and 28%, respectively, much higher than the 18.6% for general lesions (3, 4). Therefore, one of the most urgent issues now is certainly how to reduce the incidence of MACCE.
Regarded as the fifth vital sign, cardiorespiratory fitness (CRF) reflects the body's ability to supply and utilize oxygen with the support of the circulatory and respiratory systems (5). The decline in CRF is commonly observed across the course of coronary artery disease (CAD) (6, 7). Currently, CRF is widely used for cardiac rehabilitation advice and prognosis assessment in CAD (5, 8). As gold standard indicators of CRF, the metabolic equivalents (METS) and peak oxygen uptake (Peak VO2) are both closely associated with MACCE. A 1-MET increment is associated with 13 and 15% decrements in the risk of all-cause mortality and major adverse cardiac events, respectively (9). The readmission rates and all-cause mortality can be predicted by Peak VO2 (10). Therefore, CRF could be used as a sensitive and reliable surrogate indicator to assess the incidence of MACCE. Several survey scales, including the European Quality of Life Questionnaire (EQ-5D-5L), the Seattle Angina Scale (SAQ), the Fatigue Severity Scale (FSS), and the Hamilton Anxiety Scale (HAMA), are used for evaluating the quality of life from different perspectives (11–14). Furthermore, the disturbed intestinal flora is associated with several CAD risk factors and contributes to atherosclerosis (15). In different stages of CAD, it's commonly accompanied by significant characteristic changes in the intestinal flora (16–18). The level of trimethylamine N-oxide (TMAO) is positively associated with the SYNTAX score II to quantify the complexity of CAD and causes an increased risk of MACCE (19, 20). We speculate that the intestinal flora and their metabolites could be potential targets for pharmacological effect.
The Shenqisuxin granule (SQSX) is a novel Chinese herbal formula for CAD under patent protection (Chinese patent number ZL202010122712.6). Our previous in-stent restenosis study in minipig has confirmed that the ingredients of SQSX prevent in-stent restenosis by inhibiting vascular smooth muscle cell proliferation (21). In addition, it also improves angiogenesis by activating the phosphatidylinositol 3-hydroxykinase-protein kinase B (PI3K-Akt) pathway and increasing the mRNA levels of vascular endothelial growth factor (VEGF) (22). At a time when CCAD is still a conundrum in the field of cardiovascular disease, we intend to evaluate the efficacy and safety of SQSX to provide a possible therapeutic strategy.
Methods/design
Study design
Approved by the Ethics Committee of Xiyuan Hospital, China Academy of Chinese Medical Sciences (CACMS) (Version No. CCAL-YJFA-3.0, December 15, 2021), the multi-center, randomized, double-blinded, parallel, placebo-controlled trial has been registered with the Chinese Clinical Trials Registry (ChiCTR2200060979). It is conducted under the guidelines of Good Clinical Practice and the Helsinki Declaration (23, 24). It adheres to the Standard Protocol Items: Recommendations for Interventional Trials Statements (SPIRIT) (25). The Consolidated Standards of Reporting Trials Extension for Chinese Herbal Medicine Formulas 2017 (CONSORT-CHM Formulas 2017) recommendations are strictly followed (26).
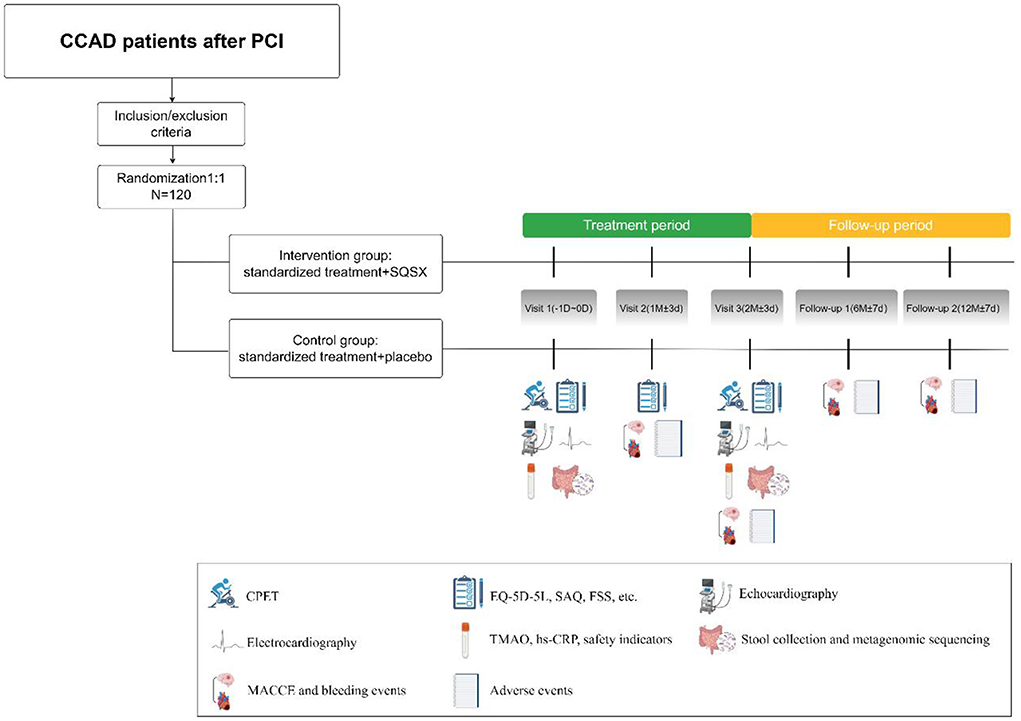
The trial is being conducted in four centers in China, including Xiyuan Hospital of CACMS, Dongzhimen Hospital of Beijing University of Chinese Medicine, the First Affiliated Hospital of Guangzhou University of Chinese Medicine, and the Affiliated Hospital of Shanxi University of Chinese Medicine. It consists of two main periods: a 2-month treatment and a 10-month follow-up. A total of 120 participants will be randomized 1:1 into the intervention group and the control group and then receive their respective interventions for 2 months. Figure 1 depicts the study's design.
Sample size estimation
The sample size was estimated based on METS. The study showed that after 8 weeks of cardiac rehabilitation, the METS increased from 3.9 to 4.6 in CAD patients after PCI (27). We assumed the mean METS value of 3.9 for the control group and 4.1 for the intervention group after treatment, with a standard deviation of 0.3 METS. With the type I error rate set at 0.05 and the type II error rate at 0.1, a sample size of 49 is required for each group with the use of PASS version 11. We will include 60 participants in each group, assuming that 20% of the participants are lost to follow up.
Inclusion criteria
The study will include patients who satisfy all of the following inclusion criteria:
(1) Diagnosis of CCAD based on one or more of the following coronary artery lesions (28–30):
a. Bifurcation lesions (side branch diameter >2.0 mm);
b. Excessive tortuosity of proximal segment;
c. Severe coronary artery calcification;
d. Chronic total occlusion lesions (>3 months);
e. Left main lesion;
f. Aorto-ostial lesions;
g. Diffuse lesions (>20 mm length);
h. Multivessel coronary artery stenosis (≥2 two vessels);
i. Extremely angulated lesions (>90° bend);
j. In-stent restenosis.
(2) Diagnosis of stable angina according to the 2019 ESC guidelines (31);
(3) NYHA class I-III;
(4) At least one stent was implanted within 2 years;
(5) 18 years ≤ age ≤ 75 years;
(6) Diagnosed as the traditional Chinese medicine (TCM) syndrome of the Qi deficiency in the heart and spleen according to an array of specific symptoms, including chest tightness, abdominal distension, appetite loss, fatigue, and lassitude, with a pale tongue and a white greasy coating, etc., (32);
(7) No antibiotics, hormones, laxatives, antidiarrheals, or probiotics have been used within 3 months;
(8) Written informed consent is obtained.
Exclusion criteria
Patients should be excluded if any of the following exclusion criteria are fulfilled:
(1) Renal insufficiency (serum creatinine >220 umol/l for males or >175 umol/l for females);
(2) Severe liver disease or elevated alanine transaminase (ALT) and aspartate transaminase (AST) (≥3 times upper limit of normal);
(3) Controlled systolic blood pressure or controlled diastolic blood pressure >160/100 mmHg, respectively;
(4) Glycosylated hemoglobin ≥9.5% or random blood glucose ≥13.7 mmol/L in diabetics;
(5) Women who are pregnant, are breastfeeding, or are planning to become pregnant;
(6) Acute or chronic severe cerebrovascular disease;
(7) Malignancies;
(8) Severe hematopoietic disorders;
(9) Severe mental illness;
(10) Intestinal inflammatory or malabsorptive disorders;
(11) Patients who have participated in other studies within 3 months.
Withdrawal, dropout, and discontinuation
Participants can withdraw from the study whenever they want. The trial will be discontinued in the events of the following: (1) the continuation of the trial is detrimental to participants; (2) a serious adverse reaction occurs; (3) the participant's poor compliance. The cause of withdrawal will be recorded.
Randomization and blinding
Stratified by center, sex, and age with a 1:1 allocation using random block sizes of 6, the randomization sequence was created using SAS software version 9.4. The investigators, patients and data analysts are blinded to the randomization sequence and treatment assignment. The emergency letter has been prepared, and the reason for opening the letter will be recorded if investigators break the blind.
Intervention
All participants will receive standardized treatment based on the 2019 ESC guidelines (26), which includes antiischemic therapy, antiplatelet therapy, statins, etc. Furthermore, the intervention group and control group will receive SQSX and placebo for 2 months, respectively. The composition of SQSX is shown in Table 1. The placebo consists of 5% SQSX and 95% dextrin and is similar in odor and shape to the active granule. The SQSX and placebo were manufactured by the Department of Pharmaceutics of Xiyuan Hospital. The drug number is printed on the package of SQSX and placebo. The subjects will be instructed to take the granules after breakfast and supper (15 g per sachet, 1 sachet each time, twice daily). The granules will be supplied and/or recycled at each visit to verify compliance. During the trial, other medicines with an influence on the intestinal flora are forbidden.
Study visits and follow-up
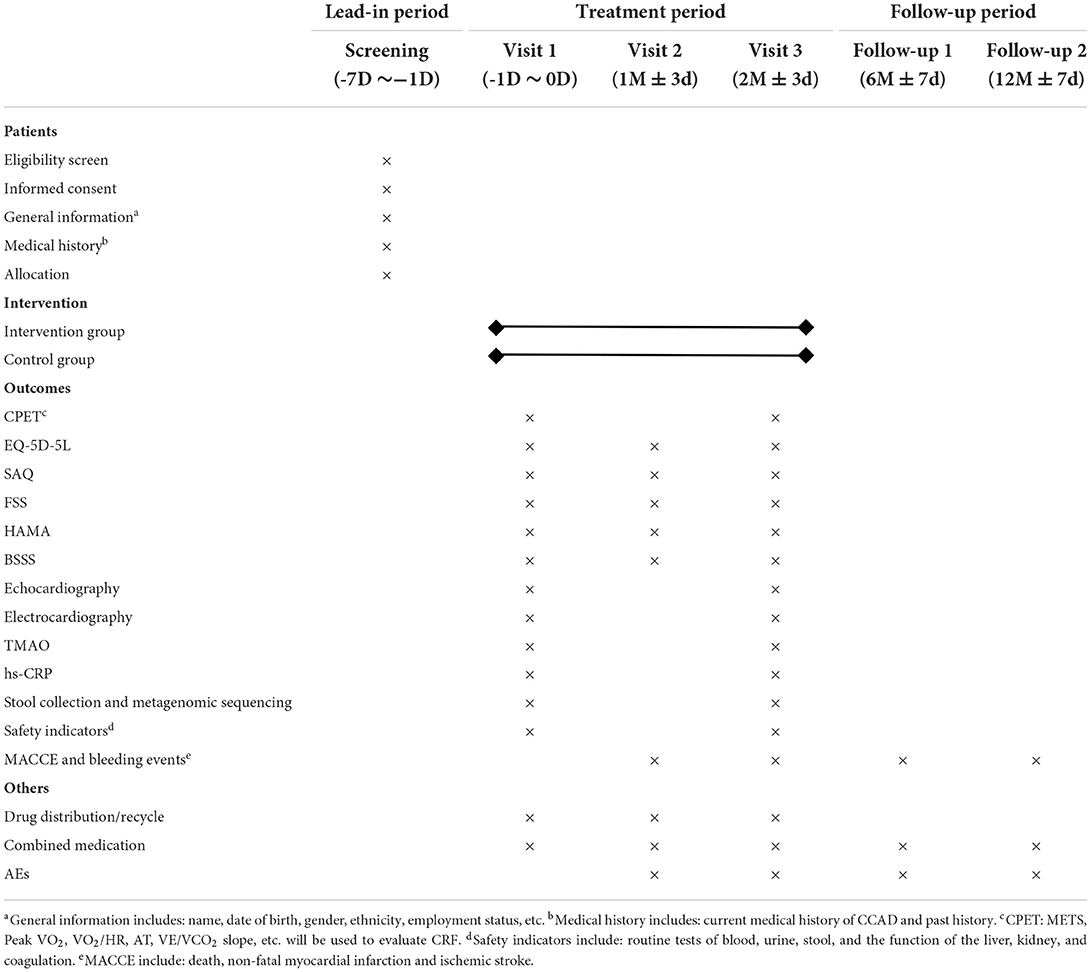
It begins with a 7-day lead-in period in which eligible participants sign informed consent. The baseline data will be gathered at Visit 1. Visits 2 and 3 will occur 1 month ± 3 days and 2 months ± 3 days following treatment, respectively. The follow-up will be conducted at 6 months ± 7 days and 12 months ± 7 days. Table 2 shows the evaluation schedule in detail.
Outcome measures
Primary outcome
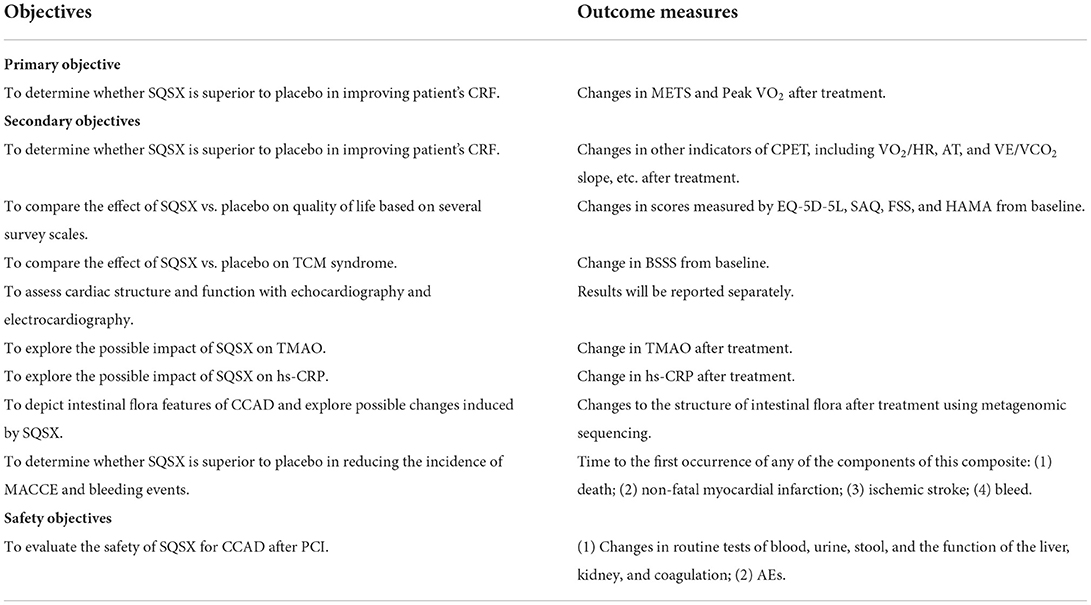
The values of METS and Peak VO2 are measured to assess CRF after treatment. The condition of participants is evaluated before the test to avoid accidental events. The instruction is prepared for participants after informed consent is obtained. The cardiac and respiratory conditions of participants are monitored with breathing masks, electrodes, oximeters, etc. The test begins with a resting pulmonary function test, followed by a treadmill test. After pedaling without load for 1–3 min, the exercise load increases by 60W every minute until they are unable to continue testing (33). The professional physician is responsible for the interpretation of the result.
Secondary outcomes
The first of the secondary outcomes are other indicators of cardiopulmonary exercise testing (CPET), including the oxygen pulse (VO2/HR), the anaerobic threshold (AT), and the slope of ventilatory equivalent for carbon dioxide (VE/VCO2 slope), etc. The additional secondary outcomes are: (1) EQ-5D-5L; (2) SAQ; (3) FSS; (4) HAMA; (5) the blood stasis syndrome score (BSSS) (34); (6) echocardiography; (7) electrocardiography; (8) TMAO; (9) high-sensitivity C-reactive protein (hs-CRP); (10) MACCE and bleeding events. Furthermore, metagenomic sequencing will be performed to probe possible changes to the structure of intestinal flora after treatment. The plasma and stool samples will be collected and stored in the laboratory freezer (−80°C) for future TMAO testing and metagenomic sequencing.
Safety
Safety indicators, including routine tests of blood, urine, stool, and the function of the liver, kidney, and coagulation, will be performed at Visit 1 and Visit 3. All outcome measures are listed in Table 3 in detail.
Adverse events
Adverse events (AEs) refer to negative or unanticipated clinical medical events that occur during the trial. The CCAD may lead to acute coronary syndrome, malignant arrhythmia, and other AEs. Patients will be rescued promptly and actively if the above happens. The details of AEs will be recorded and reported to the ethics committee.
Data collection and management
An independent data manager will be responsible for data input and management. The case report form will be carefully archived and then integrated into the data management system. The double input, data cleaning, manual checks, and other methods will be conducted to verify the data's validity, correctness, and completeness.
Investigator training and quality control
A Standard Operating Procedure (SOP) is prepared for researchers in each center. All researchers have been trained to ensure standardization. The training includes screening of eligible participants, usage of survey scales, performance of CPET, etc. The WeChat group will be used to connect patients closely. To ensure the quality, regular supervision will be executed by the quality control group and the monitors appointed by Xiyuan Hospital.
Statistical analysis
An independent statistician will be responsible for data analysis. The enrolling results and the causes of missing data will be detailed. The efficacy analysis will be performed based on the intent-to-treat (ITT) and per-protocol (PP) populations. The ITT set consists of all participants randomized, and the PP set includes participants who follow the protocol exactly with an adherence rate of at least 80%. The primary outcomes, METS and Peak VO2 after 2 months of treatment, and the secondary outcomes, the change values of VO2/HR, AT, VE/VCO2 slope, several survey scales (SAQ, EQ-5D-5L, HAMA, FSS, etc.), TMAO, and hs-CRP from baseline to each visit, are both quantitative data. Others, including the incidence of MACCE and bleeding events, are qualitative data. The quantitative data will be presented as means ± SD, 95% confidence intervals, median and interquartile range. For inter-group comparison, if both groups meet the normal distribution with equal variance, the Student t-test will be applied, otherwise, the Wilcoxon rank sum test will be used. For within-group comparison, the Wilcoxon signed rank test or paired t-test will be adopted. The qualitative data will be described using frequency and rate. The Fisher's exact probability test and the McNemar test will be applied for inter-group comparison and within-group comparison, respectively. Confounding factors will be balanced using analysis of covariance if necessary. The linear mixed-effects model will be used to analyze longitudinal data. For safety analysis, the details of AEs will be tabulated and the incidence will be compared further. The above analysis will be conducted based on SAS software version 9.4. In all analyses, a two-tailed P-value < 0.05 will be regarded as statistically significant.
Discussion
The treatment for CCAD appears to be trapped. Currently, most studies on CCAD have focused on the strategies and medical devices for revascularization, including preferential choice of PCI or coronary artery bypass grafting (CABG), novel supreme drug-eluting stents, intravascular ultrasound-guided treatment, etc., (25, 35–37). Even with the help of state-of-the-art revascularization, the improvement in myocardial ischemia is usually regional and limited. With strict adherence to evidence-based secondary prevention of CAD after PCI, the severe threats of high incidence of MACCE and low quality of life are still facing patients with CCAD (38, 39). To our knowledge, there are no viable therapies or preventative strategies for these conditions. Our previous studies have confirmed that SQSX has the favorable effects of improving angiogenesis and preventing in-stent restenosis. The Chinese herbal formula with multi-target characteristics has the potential to provide a promising therapeutic strategy for CCAD after PCI. Thus, tailored to the unique population of CCAD, this multi-center, randomized, double-blinded, parallel, placebo-controlled clinical study uses CPET, several survey scales, and other methods to evaluate the efficacy and safety of SQSX.
There are two limitations to this work. First, as a surrogate indicator, CRF doesn't directly represent the real influence on MACCE. In the future, the clinical endpoint of MACCE will be used as the primary outcome measure to conduct clinical research. Second, CCAD includes a variety of lesions, such as multivessel disease, left main lesions, chronic total occlusion lesions, etc. The differences between various lesions of CCAD may affect the result of the trial. Thus, the subgroup analysis will be performed to minimize the impact of these differences.
To conclude, this study is launched to evaluate the efficacy and safety of SQSX in CCAD after PCI and probe the possible mechanism.
Author contributions
PW designed the study under the instruction of KC. XW and MY drafted the manuscript and participated in the preparation of the study. XP, HW, ZH, RX, JP, YL, and XW are responsible for the execution of the trial. SS, YD, and JL reviewed and polished the manuscript. All authors read and approved the final manuscript.
Funding
This trial is supported financially by the CACMS Innovation Fund (No. CI2021A00905).
Acknowledgments
We are grateful for all of the researchers' assistance and efforts in this trial.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AEs, adverse events; ALT, alanine transaminase; AST, aspartate transaminase; AT, anaerobic threshold; BSSS, Blood Stasis Syndrome Score; CABG, coronary artery bypass grafting; CCAD, complex coronary artery disease; CACMS, China Academy of Chinese Medical Sciences; CAD, coronary artery disease; CONSORT-CHM Formulas 2017, Consolidated Standards of Reporting Trials Extension for Chinese Herbal Medicine Formulas 2017; CPET, cardiopulmonary exercise testing; CRF, cardiorespiratory fitness; EQ-5D-5L, European Quality of Life Questionnaire; ESC, European Society of Cardiology; FSS, Fatigue Severity Scale; HAMA, Hamilton Anxiety Scale; hs-CRP, high-sensitivity C-reactive protein; MACCE, major adverse cardiac and cerebrovascular events; METS, metabolic equivalents; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; Peak VO2, peak oxygen uptake; PI3K-Akt, phosphatidylinositol 3-hydroxykinase-protein kinase B; SAQ, Seattle Angina Scale; SOP, Standard Operating Procedure; SPIRIT, Standard Protocol Items: Recommendations for Interventional Trials Statements; SQSX, Shenqisuxin granule; TCM, traditional Chinese medicine; TMAO, trimethylamine N-oxide; VEGF, vascular endothelial growth factor; VE/VCO2 slope, slope of ventilatory equivalent for carbon dioxide; VO2/HR, oxygen pulse.
References
1. Riley RF, Henry TD, Mahmud E, Kirtane AJ, Brilakis ES, Goyal A, et al. SCAI position statement on optimal percutaneous coronary interventional therapy for complex coronary artery disease. Catheter Cardiovasc Interv. (2020) 96:346–62. doi: 10.1002/ccd.28994
2. Kheifets M, Vons SA, Bental T, Vaknin-Assa H, Greenberg G, Samara A, et al. Temporal trends in complex percutaneous coronary interventions. Front Cardiovasc Med. (2022) 9:913588. doi: 10.3389/fcvm.2022.913588
3. Stone GW, Rizvi A, Newman W, Mastali K, Wang JC, Caputo R, et al. Everolimus-eluting versus paclitaxel-eluting stents in coronary artery disease. N Engl J Med. (2010) 362:1663–74. doi: 10.1056/NEJMoa0910496
4. Windecker S, Serruys PW, Wandel S, Buszman P, Trznadel S, Linke A, et al. Biolimus-eluting stent with biodegradable polymer versus sirolimus-eluting stent with durable polymer for coronary revascularisation (LEADERS): a randomised non-inferiority trial. Lancet. (2008) 372:1163–73. doi: 10.1016/S0140-6736(08) 61244-1
5. Ross R, Blair SN, Arena R, Church TS, Després JP, Franklin BA, et al. Importance of assessing cardiorespiratory fitness in clinical practice: a case for fitness as a clinical vital sign: a scientific statement from the american heart association. Circulation. (2016) 134:e653–99. doi: 10.1161/CIR.0000000000000461
6. Gander JC, Sui X, Hebert JR, Hazlett LJ, Cai B, Lavie CJ, et al. Association of cardiorespiratory fitness with coronary heart disease in asymptomatic men. Mayo Clin Proc. (2015) 90:1372–9. doi: 10.1016/j.mayocp.2015.07.017
7. Deng W, Sun XG, Guo ZY, Ge WG Li H, Zhang Y, et al. Clinical value of cardiopulmonary exercise testing in quantitative evaluation of cardiopulmonary function before and after percutaneous coronary intervention. Chongqing Yike Daxue Xuebao. (2019) 44:668–73. doi: 10.13406/j.cnki.cyxb.001799
8. Al-Mallah MH, Sakr S, Al-Qunaibet A. Cardiorespiratory fitness and cardiovascular disease prevention: an update. Curr Atheroscler Rep. (2018) 20:1. doi: 10.1007/s11883-018-0711-4
9. Kodama S, Saito K, Tanaka S, Maki M, Yachi Y, Asumi M, et al. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. JAMA. (2009) 301:2024–35. doi: 10.1001/jama.2009.681
10. Mikkelsen N, Cadarso-Suarez C, Lado-Baleato O, Díaz-Louzao C, Gil CP, Reeh J, et al. Improvement in VO[[sb]]2[[/s]] peak predicts readmissions for cardiovascular disease and mortality in patients undergoing cardiac rehabilitation. Eur J Prev Cardiol. (2020) 27:811–9. doi: 10.1177/2047487319887835
11. Hinz A, Kohlmann T, Stobel-Richter Y, Zenger M, Brähler E. The quality of life questionnaire EQ-5D-5L: psychometric properties and normative values for the general German population. Qual Life Res. (2014) 23:443–7. doi: 10.1007/s11136-013-0498-2
12. Spertus JA, Winder JA, Dewhurst TA, Deyo RA, Prodzinski J, McDonell M, et al. Development and evaluation of the Seattle angina questionnaire: a new functional status measure for coronary artery disease. J Am Coll Cardiol. (1995) 25:333–41. doi: 10.1016/0735-1097(94) 00397-9
13. Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The fatigue severity scale, application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. (1989) 46:1121–3. doi: 10.1001/archneur.1989.00520460115022
14. Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. (1959) 32:50–5. doi: 10.1111/j.2044-8341.1959.tb00467.x
15. Tang WHW, Bäckhed F, Landmesser U, Hazen SL. Intestinal microbiota in cardiovascular health and disease: JACC state-of-the-art review. J Am Coll Cardiol. (2019) 73:2089–105. doi: 10.1016/j.jacc.2019.03.024
16. Liu H, Chen X, Hu X, Tian R, Wang H, Pang H, et al. Alterations in the gut microbiome and metabolism with coronary artery disease severity. Microbiome. (2019) 7:1–14. doi: 10.1186/s40168-019-0683-9
17. Fredrik HK, Frida F, Intawat N, Valentina T, Björn F, Dina P, et al. Symptomatic atherosclerosis is associated with an altered gut metagenome. Nat Commun. (2012) 3:1245. doi: 10.1038/ncomms2266
18. Li J, Zhao FQ, Wang YD, Chen JR, Tao J, Tian G, et al. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome. (2017) 5:14. doi: 10.1186/s40168-016-0222-x
19. Senthong V, Li XS, Hudec T, Coughlin J, Wu YP, Levixon B, et al. Plasma trimethylamine N-oxide, a gut microbe–generated phosphatidylcholine metabolite, is associated with atherosclerotic burden. J Am Coll Cardiol. (2016) 67:2620–28. doi: 10.1016/j.jacc.2016.03.546
20. Tang WHW, Wang ZN, Levisonn BS, Koeth RA, Briyy EB, et al. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. (2013) 368:1575–84. doi: 10.1056/NEJMoa1109400
21. Cui YY, Liu JG, Zhao FH, Shi DZ, et al. Advances in studies on pharmacological action of main chemical constituent of Curcuma Zedoary in preventing in-stent restenosis. Zhongguo Zhongyao Zazhi. (2015) 40:1230–34. doi: 10.4268/cjcmm20150702
22. Wang PL, Lei Y, Wang CL. Effects of Chinese herbs for nourishing qi and activating blood on PI3K and MAPK signaling pathways in angiogenesis. Zhongxiyi Jiehe Xinxueguanbing Zazhi. (2010) 8:1083–85. doi: 10.3969/j.issn.1672-1349.2010.09.036
23. Idanpaan-Heikkila JE. WHO. guidelines for good clinical practice (GCP) for trials on pharmaceutical products: responsibilities of the investigator. Ann Med. (1994) 26:89–94. doi: 10.3109/07853899409147334
24. Association G. World medical association declaration of Helsinki: ethical principles for medical research involving human subjects. J Am Coll Dent. (2014) 81:14–8. doi: 10.1001/jama.2013.281053
25. Chan AW, Tetzlaff JM, Gotzsche PC, Altman DG, Mann H, Berlin JA, et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ. (2013) 346:e7586. doi: 10.1136/bmj.e7586
26. Cheng CW, Wu TX, Shang HC Li YP, Altman DG, Moher D, et al. CONSORT extension for Chinese herbal medicine formulas 2017: recommendations, explanation, and elaboration (traditional Chinese version). Ann Intern Med. (2017) 167:W7–20. doi: 10.7326/IsTranslatedFrom_M17-2977_1
27. Cao RY, Zheng H, Hong Y, Zheng Y, Ding Y, Zhao L, et al. Cardiac rehabilitation with targeted intensity improves cardiopulmonary functions accompanying with reduced copeptin level in patients with coronary artery disease. J Cardiovasc Transl Res. (2021) 14:317–26. doi: 10.1007/s12265-020-10055-y
28. Ryan TJ, Faxon DP, Gunnar RM, Kennedy JW, King SB, Loop FD, et al. Guidelines for percutaneous transluminal coronary angioplasty, a report of the American college of cardiology/American heart association task force on assessment of diagnostic and therapeutic cardiovascular procedures (subcommittee on percutaneous transluminal coronary angioplasty). Circulation. (1988) 78:486–502. doi: 10.1161/01.cir.78.2.486
29. Li Y, Li CX, Wang HC, Xu B, Fang WY, Ge JB, et al. Efficacy and safety of Firebird sirolimus-eluting stent in treatment of complex coronary lesions in Chinese patients: one-year clinical and eight-month angiographic outcomes from the FIREMAN registry. Chin Med J. (2011) 124:817–24. doi: 10.3760/cma.j.issn.0366-6999.2011.06.004
30. Shlofmitz E, Torguson R, Mintz GS, Zhang C, Sharp A, Hodgson JM, et al. The impact on revascularization outcomes of intravascular ultrasound-guided treatment of complex lesions and economic impact (IMPROVE) trial: study design and rationale. Am Heart J. (2020) 228:65–71. doi: 10.1016/j.ahj.2020.08.002
31. Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, et al. 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. (2020) 41:407–77. doi: 10.1093/eurheartj/ehz425
32. Liu ZH, Cao HX, Zhang MX, Zhang HM, et al. Study on the standard of syndrome differentiation of deficiency of heart and spleen in chest pain (ischemic heart disease). Zhongguo Yaoxue Bao. (2004) 32:5–7. doi: 10.19664/j.cnki.1002-2392.2004.01.004
33. DeCato TW, Haverkamp H, Hegewald MJ. Cardiopulmonary exercise testing (CPET). Am J Respir Crit Care Med. (2020) 201:P1–2. doi: 10.1164/rccm.2011P1
34. Fu CG, Gao ZY, Wang PL. Study on the diagnostic criteria for coronary heart disease patients of blood stasis syndrome. Zhongguo Zhongxiyi Jiehe Zazhi. (2012) 32:1285–86. doi: 10.7661/CJIM.2012.9.1285
35. Wang R, Serruys PW, Gao C, Hara H, Takahashi K, Ono M, et al. Ten-year all-cause death after percutaneous or surgical revascularization in diabetic patients with complex coronary artery disease. Eur Heart J. (2021) 43:56–67. doi: 10.1093/eurheartj/ehab441
36. Patel KP, Lansky AJ, Kereiakes DJ, Windecker S, Cristea E, Pietras C, et al. Outcomes of the novel supreme drug-eluting stent in complex coronary lesions: a PIONEER III substudy. JSCAI. (2022) 1:100004. doi: 10.1016/j.jscai.2021.100004
37. Oyama K, Furtado R, Fagundes AJ, Zelniker TA, Tang M, Kuder J, et al. Effect of evolocumab on complex coronary disease requiring revascularization. J Am Coll Cardiol. (2021) 77:259–67. doi: 10.1016/j.jacc.2020.11.011
38. Ono M, Serruys PW, Hara H, Kawashima H, Gao C, Wang R, et al. 10-year follow-up after revascularization in elderly patients with complex coronary artery disease. J Am Coll Cardiol. (2021) 77:2761–73. doi: 10.1016/j.jacc.2021.04.016
Keywords: Shenqisuxin granule, complex coronary artery disease, percutaneous coronary intervention, cardiorespiratory fitness, randomized controlled trial
Citation: Wu X, Yan M, Pang X, Wu H, Hu Z, Xiao R, Pan J, Li Y, Shi S, Deng Y, Li J, Wang P and Chen K (2022) A multi-center, randomized, double-blinded, parallel, placebo-controlled study to assess the efficacy and safety of Shenqisuxin granule in complex coronary artery disease after PCI: Study protocol. Front. Cardiovasc. Med. 9:1000379. doi: 10.3389/fcvm.2022.1000379
Received: 22 July 2022; Accepted: 25 August 2022;
Published: 12 September 2022.
Edited by:
ZhiJian Wang, Capital Medical University, ChinaReviewed by:
Danping Xu, Sun Yat-sen University, ChinaMingjun Zhu, First Affiliated Hospital of Henan University of Traditional Chinese Medicine, China
Copyright © 2022 Wu, Yan, Pang, Wu, Hu, Xiao, Pan, Li, Shi, Deng, Li, Wang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peili Wang, MTkxNTkzNjkwQHFxLmNvbQ==; Keji Chen, a2pjaGVudmlwQDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
 Xiaoping Wu
Xiaoping Wu Mingyu Yan
Mingyu Yan Xingxue Pang
Xingxue Pang Hui Wu5
Hui Wu5 Keji Chen
Keji Chen