- 1National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
- 2Department of Cardiology, Shanghai Putuo District Liqun Hospital, Shanghai, China
In recent years, the incidence of breast cancer has been increasing on an annual basis. Human epidermal growth factor receptor-2 (HER-2) is overexpressed in 15-20% human breast cancers, which is associated with poor prognosis and a high recurrence rate. Trastuzumab is the first humanized monoclonal antibody against HER-2. The most significant adverse effect of trastuzumab is cardiotoxicity, which has become an important factor in limiting the safe use of the drug. Unfortunately, the mechanism causing this cardiotoxicity is still not completely understood, and the use of preventive interventions remains controversial. This article focuses on trastuzumab-induced cardiotoxicity, reviewing the clinical application, potential cardiotoxicity, mechanism and discussing the potential interventions through summarizing related researches over the past tens of years.
Introduction
Currently, the incidence of breast cancer has been increasing year by year, and now has the greatest incidence of malignant tumors worldwide, with obvious geographical differences. According to GLOBOCAN 2020 estimates of cancer incidence and mortality produced by the International Agency for Research on Cancer, female breast cancer has surpassed lung cancer as the most commonly diagnosed cancer, with an estimated 2.3 million new cases (1). Human epidermal growth factor receptor-2 (HER-2) is an important biomarker for breast cancer as well as a therapeutic target. Of breast cancer patients, 15-20% are HER-2 positive, which is usually considered the most serious subtype due to its poor prognosis and high recurrence rate (2, 3). Trastuzumab is a humanized monoclonal antibody directed against HER-2, initially approved as first-line treatment of HER-2-positive recurrent metastatic breast cancer in 1998. Introduction of trastuzumab to chemotherapeutic regimes has significantly increasing the life expectancy of patients with HER-2 positive, aggressive breast cancer. Meanwhile, there have been increasing reports of trastuzumab-induced cardiotoxicity (TIC) in recent years. To date, the most relevant clinical solution for TIC is trastuzumab interruption, but this approach may cause cancer recurrence. Therefore, understanding the mechanism of TIC and the related preventive measures is paramount for the safe and effective treatment of HER2-positive breast cancer patients. Here, we have attempted to provide an overview of our current knowledge of this effect, focusing primarily on clinical manifestations, influencing factors and mechanism. We also discussed the prevention and pretreatment, with the goal of providing reference for related research and clinical use.
Overview of Trastuzumab for Her-2-Positive Breast Cancer
Trastuzumab is an important HER-2 targeted drug. The gene encoding HER-2 is localized on chromosome 17 (4) and encodes a transmembrane glycoprotein with tyrosine kinase activity that plays an important role in cell survival, proliferation, and differentiation (5, 6). HER-2 is a member of the epidermal growth factor receptor (EGFR) family and has two forms of activation, homodimerization and heterodimerization with other receptors in the family (HER-1, HER-3, HER-4), either of which triggers cellular pathways including MEK/Erk, PI3K/Akt (7, 8). The mechanism of trastuzumab has not been fully elucidated and may be related to inhibiting the formation of the homodimer by binding to the HER2 extracellular structural domain IV, blocking downstream cellular pathways and thus blocking tumor cell proliferation (9, 10). Recently, Tsao et al. (11) found that the dominant therapeutic mechanism of trastuzumab is through its elicitation of tumor associated macrophages, which mediated antibody-dependent cellular phagocytosis. After HER2 overexpression was discovered to be associated to poor clinical outcomes in breast cancer patients, it quickly became the focus of intensive investigations. In 1989, Hudziak et al. (12) found that a mouse monoclonal antibody to HER-2 successfully inhibited the proliferation of breast cancer cells. Researchers humanized mouse-derived 4D5 monoclonal antibodies and the most active of these was named trastuzumab (13). It was approved by the FDA in 1998 for first-line treatment of HER-2-positive recurrent metastatic breast cancer. Trastuzumab in combination with other agents significantly prolonged median survival (25.1 vs. 20.3 months; p < 0.008), progression-free survival (7.4 vs. 4.6 months; p < 0.001), improved objective remission rates (50 vs. 32%; p < 0.001), and reduced one-year mortality (22 vs. 33%; p < 0.008) (14). Several large foreign clinical trials have shown that the use of trastuzumab after receiving chemotherapy can significantly reduce the risk of breast cancer recurrence and death (15–17). Furthermore, a joint analysis of two large clinical trials (NCCTG N9831 and NSABP B-31) found that patients with early-stage HER2-positive breast cancer benefited from the addition of trastuzumab to conventional chemotherapy followed by treatment with paclitaxel, resulting in a significant and sustained reduction in cancer recurrence rates and a 37% improvement in overall survival (18). Both Chinese guidelines for diagnosis and treatment of pancreatic cancer 2019 and NCCN Clinical Practice Guidelines in Oncology recommend trastuzumab as the first choice in combination with chemotherapy drugs (19).
Clinical Manifestations of Trastuzumab-Induced Cardiotoxicity
It is generally accepted that, unlike anthracyclines, the cardiotoxicity caused by trastuzumab is not dose-dependent, does not occur in all patients, and is reversible (20). Left ventricular dysfunction (LVD) and heart failure (HF) are relatively common and severe manifestations of cardiotoxicity in cancer therapy (21). The Cardiac Review and Evaluation Committee (CREC) defined the cardiotoxicity as one of the following: (1) cardiomyopathy characterized by a decrease in cardiac left ventricular ejection fraction (LVEF) that was either global or more severe in the septum; (2) symptoms of congestive heart failure (CHF); (3) associated signs of CHF, including but not limited to S3 gallop, tachycardia or both; and (4) decline in LVEF of at least 5 to <55% with accompanying signs or symptoms of CHF or a decline in LVEF of at least 10% to <55% without accompanying signs or symptoms (22). Any of the above can be defined as cardiotoxicity. A frequently used definition of treatment-related cardiotoxicity in clinical trials is an absolute decrease in LVEF of 10% to a value of <55% (23). Of these definitions, there may be differences between individual patients regarding the decrease in LVEF. Researchers analyzed 1,437 echocardiograms from 324 patients over a follow-up period of up to 3.5 years, and revealed three main patterns of LVEF change over time: (1) steady decline over time; (2) mild early and late sustained decline; (3) early significant decline with late partial recovery (24).
In addition to left ventricular dysfunction and heart failure, studies also reported the development of arrhythmias, sick sinus node syndrome, and atrial flutter in patients undergoing treatment with trastuzumab (25). Recently, through a secondary analysis of a clinical trial, investigators found that TIC is characterized by the presence of both left ventricular dysfunction and reversible myocardial inflammation and edema, and that trastuzumab may be associated with deleterious changes in cardiac metabolic phenotype (26).
The Incidence and Influencing Factors of TIC
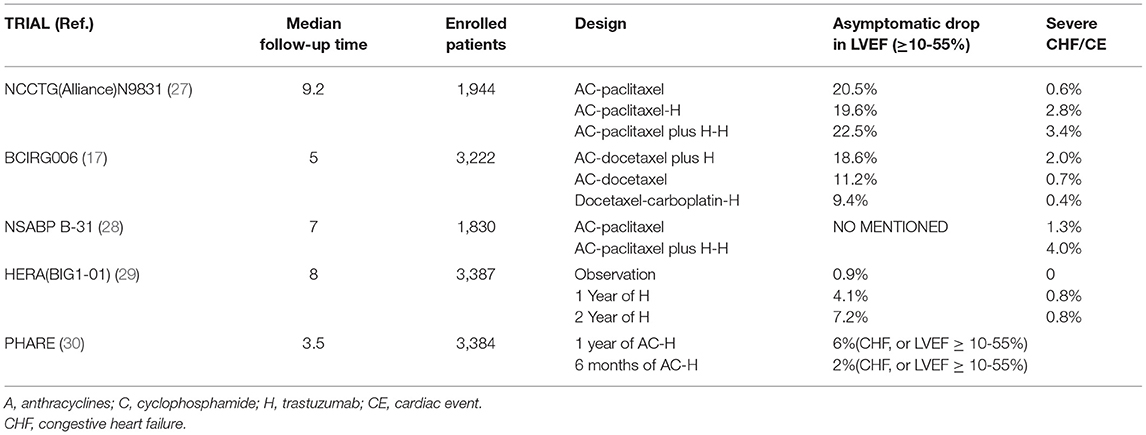
Many clinical studies have demonstrated the cardiotoxicity associated with trastuzumab, and this article focuses on a few large clinical studies of adjuvant therapy with combination or sequential trastuzumab. In the N9831 study (27), in the two trial groups using trastuzumab, the cumulative incidence of CHF or cardiac death over 6 years was 2.8 and 3.4%, respectively, resulting in risks that were 4.7 and 5.7 times higher than not using trastuzumab. The BCIRG006 (17) study found that the addition of trastuzumab after anthracycline treatment significantly increased the odds of CHF, and the risk of decreased LVEF was 1.6 times greater than without trastuzumab. Furthermore, the incidence of cardiac events reported by NSABP B-31were 1.3% in the control group and 4.0% in the trastuzumab group, with 15.5% of the trastuzumab group discontinuing the drug for cardiac reasons (28). The BIG1-01 (HERA) study (16, 29) conducted a comparative trial of 5102 HER-2 positive early-stage breast cancer patients over 1 and 2 years, respectively. Although the incidence of severe CHF was 0.8% in both groups, the incidence of asymptomatic drop in LVEF was significantly higher in patients on trastuzumab for 2 years (7.2%) than for 1 year (4.1%). The rate of discontinuation of treatment due to TIC was 5.2% during the 1-year period and 9.4% during the 2-year period. Additionally, an 11-year follow-up of the study found that the most of the TIC occurred during the patients' dosing period and no delayed cardiotoxicity was seen (See Table 1).
The incidence varies depending on the assay and criteria for cardiotoxicity used by researchers amongst the different clinical trials, as well as on the selection of patients participating in the trials. For example, in the HERA trial, a lower incidence of cardiotoxicity may be due to the exclusion of patients who had a cardiac event prior to treatment from the trial. Because patients with significant disease, including those at high risk for cardiovascular disease, are often excluded from randomized controlled trials, the incidence may differ from the real world. A real-world study based on trastuzumab for cardiotoxicity due to HER2-positive breast cancer that included more than 3,700 study subjects showed a CHF incidence of 2.8%, with a 1.0% incidence of severe CHF (31).
Risk factors for development of TIC include previous anthracycline exposure and conventional cardiovascular risk factors. Several clinical studies have demonstrated that previous anthracycline exposure appears to be the most important factor in worsening cardiotoxicity (32, 33). This may be related to the fact that the inhibition of the HER2 pathway by trastuzumab exacerbates damage caused by oxidative stress induced by anthracyclines, allowing for further accumulation of ROS (34).
In addition to co-administration, conventional cardiovascular risk factors have been associated with TIC. A recently published systematic review and meta-analysis focusing on the relationship between conventional cardiac risk factors and trastuzumab-induced cardiotoxicity in breast cancer treatment showed that age ≥ 60 (OR 2.03, 95% CI 1.38-3.00, P = 0.0004), hypertension (OR 2.01, 95% CI 1.30-3.09, P = 0.002), smoking (OR 1.33, 95% CI 1.07-1.65, P = 0.01), diabetes (OR 1.49, 95% CI 1.22-1.81, P = 0.0001), family history of coronary artery disease (OR 5.51, 95% CI 1.76-17.25, P = 0.00001), known history of coronary artery disease (OR 6.27, 95% CI 2.22-17.69, P = 0.0005) were strongly associated with the development of TIC (35). Besides, combination of obesity and being overweight was also a significant influencing factor (36).
Mechanism of Trastuzumab-Induced Cardiotoxicity
The exact mechanism of TIC has not been fully elucidated, and numerous in vitro and in vivo studies suggest that it may involve multiple cellular and molecular mechanisms (37). The inhibition of NRG-1/HER and downstream signaling pathways has always posed a plausible explanation for TIC, but the underlying molecular mechanisms still remain undefined. In addition, recent research has investigated the inhibition of autophagy and alterations in cellular metabolic pathways in cardiomyocytes as potential causes for the development of cardiotoxicity.
Downregulation of HER2 Signaling and Cardiotoxicity
In addition to being expressed in tumor tissue, HER2 has been shown to be expressed in adult cardiomyocytes along with other members of the family (HER1, HER3 and HER4) (8). HER2, together with its ligand, NRG1, is closely tied to the maintenance of adult cardiac function and the development of cardiomyocytes. When the heart becomes hemodynamically unstable or stimulated, cardiac microvascular endothelial cells can release NRG1 (38, 39). After acting in a paracrine form in cardiomyocytes, NRG1 binds to HER4 and triggers HER4/HER4 homodimerization or HER4/HER2 heterodimerization, which can later trigger a series of pathways including the MAPK pathway and PI3K-Akt (40).
The activation of the Akt family can trigger many proteins through phosphorylation, thereby initiating tumor cell survival and inhibiting apoptosis (41). Ravingerova et al. (42) used a chronic cardiac ischemia rat model to discover that Akt also increases glucose and lipid metabolism in cardiomyocytes through nutrient uptake and ensures energy in cardiomyocytes during hypoxia. Furthermore, the activation of the PI3K-Akt pathway promotes nitric oxide (NO) production in adult ventricular myocytes, thereby protecting them from oxidative stress. Moreover, Akt can initiate alterations in mitochondrial respiration, thereby reducing reactive oxygen species (ROS) production and improving cell survival. If HER2 signaling is blocked, PI3K-Akt pathway blockade will cause the accumulation of ROS in cardiomyocytes, thereby triggering the apoptosis of cardiomyocytes (43).
The MAPK pathway is another pathway associated with TIC. The MAPK pathway consists mainly of three protein kinases, Raf/MEK/ERK, that cascade to amplify external signals and thus cause cell proliferation and differentiation (44). Meanwhile, the phosphorylation of ERK1/2, inhibits the opening of the mitochondrial osmotic transition pore and suppresses the decrease in membrane potential, thus stabilizing mitochondrial function (45).
In summary, the activation of NRG1/HER and downstream signaling pathways plays an important role in protecting the stability of cardiac function. Trastuzumab inhibits the dimerization of HER4/HER2 by binding to HER2 and thereby inhibiting the above pathways (see Figure 1), which may be one of the potential mechanisms for TIC. In fact, NRG1/HER signaling in the heart is part of a stress-activated compensatory system that plays a minor role under physiological conditions, but can play a protective role when the heart is exposed to cardiotoxic drugs or ischemia, which is consistent with the reality that trastuzumab increases cardiotoxicity when combined with anthracyclines (46, 47).
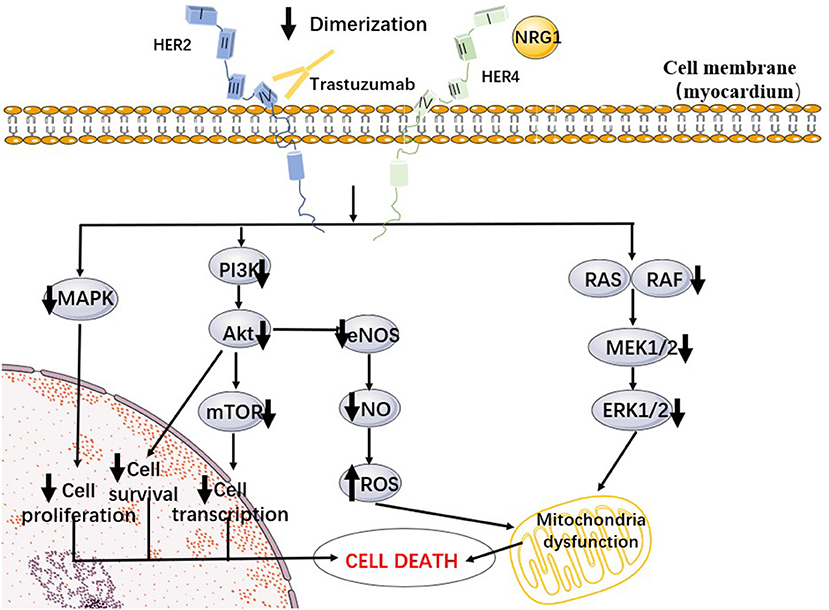
Figure 1. A proposed cellular mechanism of the cardiotoxicity of trastuzumab. Trastuzumab inhibited Her2/4 dimerization, preventing autophosphorylation and subsequent downstream pathways such as PI3K/Akt and MAPK.
Inhibition of Autophagy
Autophagy is a catabolic process that aims to recycle cellular components and damaged organelles in response to different stress conditions (48). Thomas et al. found that deletion of the anti-apoptotic protein MCL-1 in mouse cardiomyocytes leads to the inhibition of autophagy, eventually resulting in heart failure, and further indicated that MCL-1 deficiency is associated with mitochondrial dysfunction (49). Mohan et al. found that trastuzumab treatment decreased the protein expression of autophagy-related signaling molecules such as ATG5-12, ATG7, ATG14, and Beclin 1, and also demonstrated that trastuzumab-mediated inhibition of autophagy resulted in increased ROS production in cardiomyocytes (50). In earlier years, some researchers found that anthracycline increases autophagy and that this is closely related to its cardiotoxicity, which also suggests that anthracyclines and trastuzumab differ in their mechanisms of inducing cardiotoxicity (51, 52).
Alterations of Cardiomyocyte Metabolism
The inhibition of the NRG1/HER signaling pathway still does not fully answer the question of why trastuzumab causes cardiotoxicity. For example, TIC is often reversible in clinical settings, which contradicts the above explanation that blocking the HER2 pathway leads to cardiomyocyte apoptosis. Alterations in cardiac energy metabolism are a key feature of heart failure and are thought to exacerbate its progression (53). Necela et al. (54) found that after trastuzumab treatment, there was a reduction in glucose uptake in human induced pluripotent stem cell-derived cardiomyocytes (IPSC-CMs) as well as a significant downregulation of SLC6A6. SLC6A6 is a metabolism-related gene, and SLC6A6 knockout mice exhibit a cardiomyopathy with myocardial atrophy phenotype, which also provides a potential mechanism for TIC (55). Recently, investigators have found that clinically relevant doses of trastuzumab impaired the contractile and calcium regulatory functions of IPSC-CMs but did not lead to cardiomyocyte death, and that further RNA-SEQ with subsequent functional analysis revealed that mitochondrial dysfunction and altered cardiac energy metabolic pathways were the main causes of the TIC phenotypes, thus suggesting that metabolic modulators are important for the treatment of TIC (56).
Prevention and Treatment of TIC
Monitoring of TIC
Strict monitoring of cardiotoxicity during the treatment of trastuzumab facilitates timely adjustment of dosing and optimization of treatment regimens by clinicians. LVEF, measured by cardiovascular magnetic resonance (CMR) or 2-dimensional echocardiography (2DE), is currently the most commonly used index for monitoring left ventricular function, but LVEF has limitations and often underestimates cardiac compromise in patients. In a retrospective study, investigators found that baseline left ventricular end-diastolic volume (LEVD) was an independent predictor of cardiotoxicity and more reliably identified patients at high risk of cardiotoxicity (57). Besides, echocardiographic measurement of longitudinal shortening of the heart during contraction, or global longitudinal strain (GLS), can identify early changes in left ventricular contractility before ejection fraction (EF) declines. Researchers found thatΔGLS at 6 months were predictors of decrease in EF at 12 months (58). And GLS-guided cardioprotective therapy (CPT) prevents reduction in LVEF and development of cardiac dysfunction in high-risk patients undergoing potentially cardiotoxic chemotherapy, compared with usual care (59). Improvements in testing technology have allowed for the emergence of serum biomarkers that play an increasing role in the monitoring of cardiotoxicity. The 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity published by the European Society of Cardiology (ESC) proposed that the use of serum biomarkers is an important tool for baseline risk assessment and diagnosis of cardiovascular disease. The statement recommends cardiac troponin (CTn) baseline measurement for all cancer patients, as the strongest independent predictor of cardiotoxicity, and in patients with early invasive HER2+ breast cancer undergoing neoadjuvant or adjuvant therapy. B-type natriuretic peptide (BNP)/amino-terminal pro-B-type natriuretic peptide (NT-proBNP) with CTn testing were recommended after receiving trastuzumab (60). In recent years, soluble growth-stimulating expression gene 2 protein (sST2) has received wide attention as a novel heart failure marker. Some studies have shown that sST2 levels correlate with the severity of heart failure, LVEF and NT-proBNP in patients (61). In addition, Zhang et al. (62) analyzed 65 HER2-positive breast cancer patients treated with trastuzumab and applied ordered logistic regression to analyze the relationship between serum miR-222-3p and adverse events and found that serum miR-222-3p was a potential predictor of TIC.
Choosing an Anthracycline-Free Regimen
In the BCIRG006 clinical trial, the regimen combining anthracyclines with trastuzumab had similar long-term survival rates as the paclitaxel and cyclophosphamide combined with trastuzumab regimen, while the incidence of cardiotoxicity in the latter group was much lower than former (17). A randomized multicenter phase III trial of 438 patients with stage II and III HER2-positive breast cancer showed an estimated 3-year event-free survival rate of 93% in patients treated with anthracyclines and 94% in patients not using, while decrease in LVEF was more common in the anthracycline group (63). This suggests that avoiding anthracyclines when using trastuzumab in favor of other classes of drugs may reduce the likelihood of cardiac events without compromising efficacy.
In addition to its use in combination with chemotherapeutic agents, trastuzumab has shown a good prognosis in combination with other antitumor drugs. Unlike trastuzumab, pertuzumab is a humanized monoclonal antibody against the extracellular structural domain II region of HER2, which inhibits the heterodimerization of HER2 with HER3, thereby blocking pathways including phosphatidylinositol 3-kinase (PI3K/AKT/mTOR) and mitogen-activated protein kinase (RAS/RAF/MEK/ERK) (64, 65). It acts at a different site from trastuzumab in the extracellular structural domain of HER2 and there may be a synergistic effect when they are combined (see Figure 2). The NeoSphere phase II study evaluated the efficacy and safety of trastuzumab with pertuzumab in combination with docetaxel in HER2-positive breast cancer patients treated with neoadjuvant therapy, and showed that the dual-target combination chemotherapy significantly increased the pathologic complete remission rate (pCR) as compared to the single-target, while the adverse effects were broadly consistent with the trastuzumab monotherapy arm (66). Furthermore, the TRYPHAENA trial demonstrated that the combination of trastuzumab and pertuzumab, whether co-administered with anthracyclines or with carboplatin, was usually well-tolerated and also showed a higher rate of pCR and a lower incidence of cardiotoxicity in the anthracycline-free trial group (67). Similarly, the PEONY study demonstrated that dual-targeted combination therapy significantly improved the pCR rate (68). The 2019 NCCN guidelines recommend the TCHP regimen [trastuzumab (H) + pertuzumab (P) in combination with docetaxel (T) + carboplatin (C)] as a first-line treatment option for HER-2-positive breast cancer. This regimen is anthracycline-free and therefore has a higher safety profile for patients with potentially dangerous cardiac function.
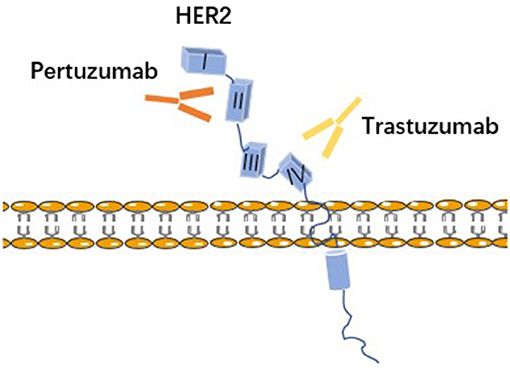
Figure 2. Trastuzumab and pertuzumab bind to different regions on HER2. Trastuzumab is a humanized monoclonal antibody to IV subdomain of HER2. Pertuzumab is a humanized monoclonal antibody to subdomain II of the dimerization arm of HER2.
Pharmacological Prevention
Unlike anthracyclines, LVD caused by trastuzumab is usually reversible, thus the ESMO guidelines mainly recommend strategies such as observation and discontinuation of the drug (69). However, a large retrospective cohort study found that discontinuation of trastuzumab led to adverse clinical outcomes (70). Therefore, it is necessary to use appropriate cardioprotective agents in the clinical setting.
Angiotensin-Converting Enzyme Inhibitor or β-Receptor Inhibitors
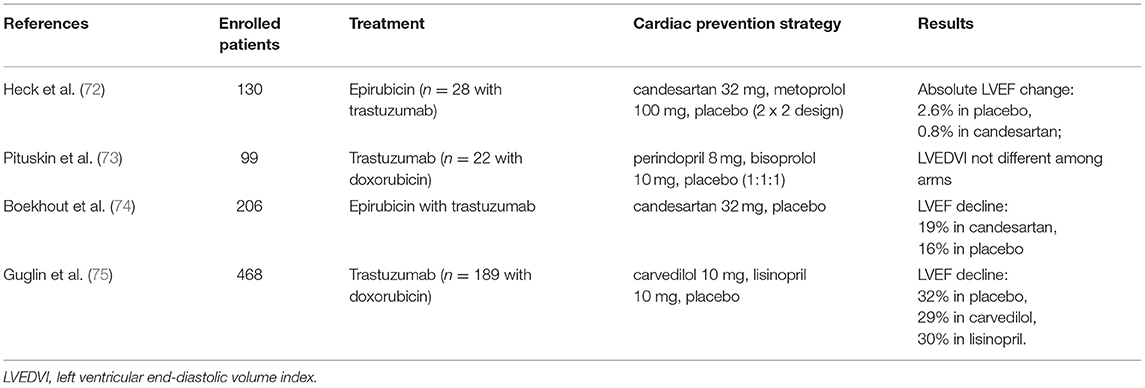
Early research found that NRG-1/HER signaling regulates myocardial contractility and is influenced by circulating catecholamines and angiotensin-II in animal models (39). This may provide some theoretical basis for the use of ACEI or β-receptor inhibitors in the prevention of TIC. Several small randomized trials and single-center studies have also reported that ACEI and β-blockers ameliorate chemotherapy-induced cardiotoxicity, but these studies all emphasized high-dose anthracycline-induced cardiotoxicity and have limited clinical applicability in TIC (71, 72). In 2016, the MANTICORE 101-Breast randomized clinical trial specifically investigated the pharmacological prevention of TIC and found that Perindopril and Bisoprolol were well-tolerated in the prevention of TIC and attenuated the decrease in LVEF, but trastuzumab-induced left ventricular remodeling was not reversed (73). In another randomized clinical trial, however, concomitant use of the angiotensin II receptor inhibitor Candesartan did not prevent a reduction in LVEF (74). In 2019, researchers evaluated the preventive effect of Lisinopril and Carvedilol on cardiotoxicity with or without anthracyclines prior to trastuzumab administration and found that both drugs were more protective in patients who had exposure to anthracyclines. Patients receiving pharmacological interventions were more likely to benefit compared to the placebo group [(75); Table 2].
Statins
A retrospective case control study found that in women with HER2+ breast cancer receiving trastuzumab-based therapy with or without anthracyclines, concomitant statin use was associated with a lower risk of cardiotoxicity (76). And a recent meta-analysis also showed that patients receiving statins during cancer treatment had a lower incidence of cardiotoxicity and were more likely to maintain LVEF during the follow-up period, suggesting that statins have the potential to mitigate the cardiotoxic effects of anthracyclines and trastuzumab (77). Rosuvastatin is a statin with anti-lipid peroxidation effects (78). Kabel et al. (79) found that rosuvastatin had a protective effect against TIC in mice due to the antioxidant and anti-inflammatory properties combined with its ability to induce STAT-3 expression and preserve the morphology of the cardiomyocytes. This study also demonstrated better results in combination with ubiquinone, the oxidized form of coenzyme Q10.
AMPK Agonist
AMPK (Adenosine 5'-monophosphate (AMP)-activated protein kinase) is considered to be a key regulatory kinase of myocardial energy metabolism (80). Recently, researchers identified mitochondrial dysfunction and altered cardiac energy metabolic pathways as important potential mechanisms of trastuzumab-induced cardiotoxicity (56). Susheel et al. (81) found that low-dose metformin improved mitochondrial function and provided significant myocardial protection against ischemic heart failure by activating AMPK and downstream signaling pathways involving eNOS and PGC-1. Wang et al. (82) observed a heterodimeric shift of AMPKα2 to AMPKα1 in the hearts of heart failure patients and mice with transverse artery constriction. They further found that overexpression of AMPKα2 prevented drug-induced chronic heart failure by increasing mitochondrial phagocytosis and improving mitochondrial function in isolated adult mouse cardiomyocytes. This is consistent with the finding that AMPK agonists (AICAR, metformin) improve trastuzumab-induced symptoms of cardiac insufficiency in IPSC-CMS (56). Although there are no relevant clinical studies to prove whether an AMPK agonist has the function of preventing trastuzumab cardiotoxicity, targeting cellular energy metabolism is a potential research direction. Additionally, it has been shown that activation of AMPK can inhibit the growth of breast cancer cells and increase the sensitivity of breast cancer as well as various other cancers, to chemotherapy and radiotherapy (83). Therefore, it is of great clinical interest to investigate whether AMPK agonists can be used to combat TIC.
Potential Role of Traditional Chinese Medicine on TIC
There are many studies on the prevention and treatment of anthracycline-induced cardiotoxicity in Traditional Chinese Medicine (TCM), but reports regarding TIC are rare. The inhibition of the NRG1/HER pathway is one of the possible mechanisms of TIC. It has been suggested that activation of Akt may protect cardiac function by inhibiting apoptosis (84). Many active ingredients in Chinese medicine have been reported to show protective effects against cardiac injury by interfering with the PI3K/Akt signaling pathway, as shown in Table 3 (85–94). In addition, Zhang et al. (95) used network pharmacology analysis to find that Shenmai injection has multi-target and multi-pathway synergistic effects, which may exert myocardial protective effects through the PI3K-Akt signaling pathway and tumor microRNAs. The Shenmai injection treatment group improved cardiac structure and function, reduced myocardial pathological damage as well as the number of autophagic vesicles in mice compared with the control group. Targeting the inhibition of autophagy by trastuzumab, Liu et al. (96) investigated the protective mechanism of ginsenoside Rg2 against TIC in human cardiac myocytes (HCMs), and found that it could induce autophagy in HCMs by upregulating the expression levels of p-Akt, p-mTOR, Beclin 1, LC3, and ATG5, thus providing protection against TIC. At present, TCM is playing an increasing adjuvant role in the process of cancer treatment, while its role in the prevention and treatment of TIC has yet to be fully explored. Further in-depth studies are of great significance to ensure the safe use of trastuzumab as well as to promote the development of TCM in China.
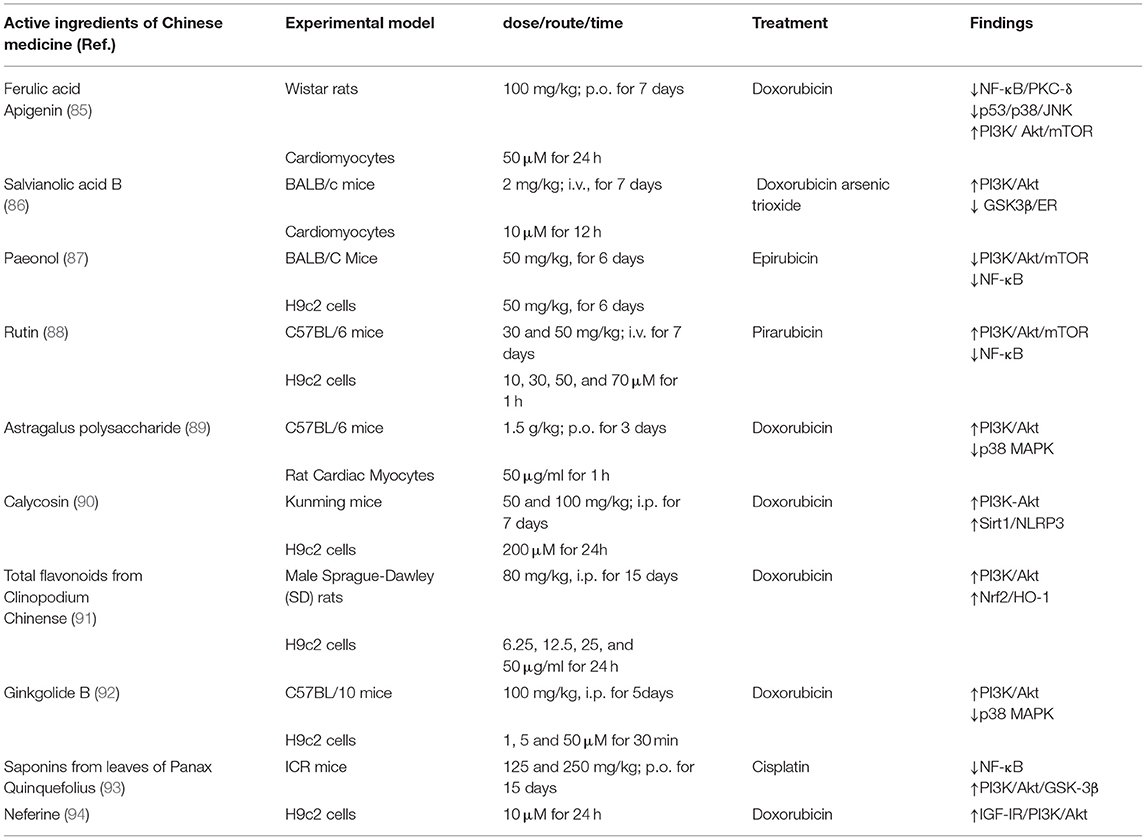
Table 3. Active ingredients of Chinese medicine against cytotoxic drug–induced cardiotoxicity through regulation of the PI3K/Akt signaling pathway.
Summary and Prospects
Trastuzumab is a landmark agent in the treatment of HER2-positive breast cancer. It has changed the treatment paradigm for HER2-positive breast cancer patients and has no alternative to its status as a first-line drug for breast cancer. At the same time, its cardiotoxicity remains a major constraint to its use. The mechanism of trastuzumab cardiotoxicity has not been fully elucidated, and there is no specific drug to prevent it in clinical practice. Fewer studies have been conducted specifically on the cardiotoxicity of trastuzumab than on anthracyclines. Researchers should further clarify the mechanism of TIC, establish a reasonable model of myocardial injury, determine appropriate detection indicators, and conduct research on relevant cardioprotective agents in response to the mechanism in order to provide the possibility of safer use of trastuzumab. In addition, TCM has shown great potential in the prevention of antineoplastic drug-induced cardiotoxicity, and while few studies have been conducted specifically for trastuzumab, this provides a research direction for the prevention and treatment of TIC. There are several hurdles at the clinical study level given that studies evaluating patients treated with trastuzumab alone are lacking, strategies to prevent anthracycline-induced cardiotoxicity are not always applicable to trastuzumab, and the definition and evaluation metrics of cardiotoxicity have yet to be standardized. In clinical application, physicians as well as pharmacists should fully understand the risk factors and fully evaluate basic information such as age, previous cardiovascular history, medication history, and the physical condition of patients before drug administration. In addition, high-risk patients need to be monitored closely throughout the oncology treatment process. These efforts will maximize efficacy while minimizing adverse effects.
Author Contributions
ML and WX assorted information and drafted the manuscript. SW polished the language. YL and CH offered advice about the structure. CL and GL governed the whole process and offered advice. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by Beijing Hope Run Special Find of Cancer Foundation of China (LC2020L03) and Beijing Municipal Science and Technology Commission (Z181100001618003).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209-49. doi: 10.3322/caac.21660
2. Zhang L, Yu J. Role of apoptosis in colon cancer biology, therapy, and prevention. Curr Colorectal Cancer Rep. (2013) 9:331–40. doi: 10.1007/s11888-013-0188-z
3. Gonzalez-Angulo AM, Litton JK, Broglio KR, Meric-Bernstam F, Rakkhit R, Cardoso F, et al. High risk of recurrence for patients with breast cancer who have human epidermal growth factor receptor 2-positive, node-negative tumors 1 cm or smaller. J Clin Oncol. (2009) 27:5700-6. doi: 10.1200/JCO.2009.23.2025
4. Beser AR, Tuzlali S, Guzey D, Dolek Guler S, Hacihanefioglu S, Dalay N. HER-2, TOP2A and chromosome 17 alterations in breast cancer. Pathol Oncol Res. (2007) 13:180-5. doi: 10.1007/BF02893497
5. Witton CJ, Reeves JR, Going JJ, Cooke TG, Bartlett JM. Expression of the HER1-4 family of receptor tyrosine kinases in breast cancer. J Pathol. (2003) 200:290-7. doi: 10.1002/path.1370
6. Morrison G, Fu X, Shea M, Nanda S, Giuliano M, Wang T, et al. Therapeutic potential of the dual EGFR/HER2 inhibitor AZD8931 in circumventing endocrine resistance. Breast Cancer Res Treat. (2014) 144:263-72. doi: 10.1007/s10549-014-2878-x
7. Jiang Z, Zhou M. Neuregulin signaling and heart failure. Curr Heart Fail Rep. (2010) 7:42-7. doi: 10.1007/s11897-010-0003-y
8. Vermeulen Z, Segers VF, De Keulenaer GW. ErbB2 signaling at the crossing between heart failure and cancer. Basic Res Cardiol. (2016) 111:60. doi: 10.1007/s00395-016-0576-z
9. Spector NL, Blackwell KL. Understanding the mechanisms behind trastuzumab therapy for human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol. (2009) 27:5838-47. doi: 10.1200/JCO.2009.22.1507
10. Molina MA, Codony-Servat J, Albanell J, Rojo F, Arribas J, Baselga J. Trastuzumab (herceptin), a humanized anti-Her2 receptor monoclonal antibody, inhibits basal and activated Her2 ectodomain cleavage in breast cancer cells. Cancer Res. (2001) 61:4744-9.
11. Tsao LC, Crosby EJ, Trotter TN, Agarwal P, Hwang BJ, Acharya C, et al. CD47 blockade augmentation of trastuzumab antitumor efficacy dependent on antibody-dependent cellular phagocytosis. JCI Insight. (2019) 4:e131882. doi: 10.1172/jci.insight.131882
12. Hudziak RM, Lewis GD, Winget M, Fendly BM, Shepard HM, Ullrich A. p185HER2 monoclonal antibody has antiproliferative effects in vitro and sensitizes human breast tumor cells to tumor necrosis factor. Mol Cell Biol. (1989) 9:1165-72. doi: 10.1128/mcb.9.3.1165-1172.1989
13. Carter P, Presta L, Gorman CM, Ridgway JB, Henner D, Wong WL, et al. Humanization of an anti-p185HER2 antibody for human cancer therapy. Proc Natl Acad Sci USA. (1992) 89:4285-9. doi: 10.1073/pnas.89.10.4285
14. Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. (2001) 344:783-92. doi: 10.1056/NEJM200103153441101
15. Halyard MY, Pisansky TM, Dueck AC, Suman V, Pierce L, Solin L, et al. Radiotherapy and adjuvant trastuzumab in operable breast cancer: tolerability and adverse event data from the NCCTG Phase III Trial N9831. J Clin Oncol. (2009) 27:2638-44. doi: 10.1200/JCO.2008.17.9549
16. Cameron D, Piccart-Gebhart MJ, Gelber RD, Procter M, Goldhirsch A, de Azambuja E, et al. 11 years' follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive early breast cancer: final analysis of the HERceptin Adjuvant (HERA) trial. Lancet. (2017) 389:1195-205. doi: 10.1016/S0140-6736(16)32616-2
17. Slamon D, Eiermann W, Robert N, Pienkowski T, Martin M, Press M. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. (2011) 365:1273-83. doi: 10.1056/NEJMoa0910383
18. Perez EA, Romond EH, Suman VJ, Jeong JH, Sledge G, Geyer CE Jr, et al. Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2-positive breast cancer: planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831. J Clin Oncol. (2014) 32:3744-52. doi: 10.1200/JCO.2014.55.5730
19. Gradishar WJ, Anderson BO, Abraham J, Aft R, Agnese D, Allison KH, et al. Breast cancer, Version 3.2020, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. (2020) 18:452-78. doi: 10.6004/jnccn.2020.0016
20. Chen J, Long JB, Hurria A, Owusu C, Steingart RM, Gross CP. Incidence of heart failure or cardiomyopathy after adjuvant trastuzumab therapy for breast cancer. J Am Coll Cardiol. (2012) 60:2504-12. doi: 10.1016/j.jacc.2012.07.068
21. Zamorano JL, Lancellotti P, Munoz DR, Aboyans V, Asteggiano R, Galderisi M, et al. [2016 ESC position paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines]. Kardiol Pol. (2016) 74:1193-233. doi: 10.5603/KP.2016.0156
22. Seidman A, Hudis C, Pierri MK, Shak S, Paton V, Ashby M, et al. Cardiac dysfunction in the trastuzumab clinical trials experience. J Clin Oncol. (2002) 20:1215-21. doi: 10.1200/JCO.2002.20.5.1215
23. Jordan JH, Todd RM, Vasu S, Hundley WG. Cardiovascular magnetic resonance in the oncology patient. JACC Cardiovasc Imaging. (2018) 11:1150-72. doi: 10.1016/j.jcmg.2018.06.004
24. Demissei BG, Finkelman BS, Hubbard RA, Smith AM, Narayan HK, Narayan V, et al. Cardiovascular function phenotypes in response to cardiotoxic breast cancer therapy. J Am Coll Cardiol. (2019) 73:248-9. doi: 10.1016/j.jacc.2018.10.057
25. Karaca M, Kocoglu H, Bilgetekin I, Ozet A, Sahinli H, Demir H, et al. Ventricular bigeminal rhythm associated with trastuzumab: a potential cardiac side effect. J Cancer Res Ther. (2018) 14:S536-7. doi: 10.4103/0973-1482.183557
26. Kirkham AA, Pituskin E, Thompson RB, Mackey JR, Koshman SL, Jassal D, et al. Cardiac and cardiometabolic phenotyping of trastuzumab-mediated cardiotoxicity: a secondary analysis of the MANTICORE trial. Eur Heart J Cardiovasc Pharmacother. (2021). doi: 10.1093/ehjcvp/pvab016 [Epub ahead of print].
27. Advani PP, Ballman KV, Dockter TJ, Colon-Otero G, Perez EA. Long-term cardiac safety analysis of NCCTG N9831 (Alliance) Adjuvant Trastuzumab Trial. J Clin Oncol. (2016) 34:581-7. doi: 10.1200/JCO.2015.61.8413
28. Romond EH, Jeong JH, Rastogi P, Swain SM, Geyer CE Jr, Ewer MS, et al. Seven-year follow-up assessment of cardiac function in NSABP B-31, a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel (ACP) with ACP plus trastuzumab as adjuvant therapy for patients with node-positive, human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol. (2012) 30:3792-9. doi: 10.1200/JCO.2011.40.0010
29. de Azambuja E, Procter MJ, van Veldhuisen DJ, Agbor-Tarh D, Metzger-Filho O, Steinseifer J, et al. Trastuzumab-associated cardiac events at 8 years of median follow-up in the Herceptin Adjuvant trial (BIG 1-01). J Clin Oncol. (2014) 32:2159-65. doi: 10.1200/JCO.2013.53.9288
30. Pivot X, Romieu G, Debled M, Pierga JY, Kerbrat P, Bachelot T, et al. 6 months versus 12 months of adjuvant trastuzumab for patients with HER2-positive early breast cancer (PHARE): a randomised phase 3 trial. Lancet Oncol. (2013) 14:741-8. doi: 10.1016/S1470-2045(13)70225-0
31. Lidbrink E, Chmielowska E, Otremba B, Bouhlel A, Lauer S, Liste Hermoso M, et al. A real-world study of cardiac events in > 3700 patients with HER2-positive early breast cancer treated with trastuzumab: final analysis of the OHERA study. Breast Cancer Res Treat. (2019) 174:187-96. doi: 10.1007/s10549-018-5058-6
32. Naumann D, Rusius V, Margiotta C, Nevill A, Carmichael A, Rea D, et al. Factors predicting trastuzumab-related cardiotoxicity in a real-world population of women with HER2+ breast cancer. Anticancer Res. (2013) 33:1717-20.
33. Leung HW, Chan AL. Trastuzumab-induced cardiotoxicity in elderly women with HER-2-positive breast cancer: a meta-analysis of real-world data. Expert Opin Drug Saf. (2015) 14:1661-71. doi: 10.1517/14740338.2015.1089231
34. Anjos M, Fontes-Oliveira M, Costa VM, Santos M, Ferreira R. An update of the molecular mechanisms underlying doxorubicin plus trastuzumab induced cardiotoxicity. Life Sci. (2021) 280:119760. doi: 10.1016/j.lfs.2021.119760
35. Koulaouzidis G, Yung AE, Yung DE, Skonieczna-Zydecka K, Marlicz W, Koulaouzidis A, et al. Conventional cardiac risk factors associated with trastuzumab-induced cardiotoxicity in breast cancer: systematic review and meta-analysis. Curr Probl Cancer. (2021) 2021:100723. doi: 10.1016/j.currproblcancer.2021.100723
36. Guenancia C, Lefebvre A, Cardinale D, Yu AF, La Ladoire S, Ghiringhelli F, et al. Obesity as a risk factor for anthracyclines and trastuzumab cardiotoxicity in breast cancer: a systematic review and meta-analysis. J Clin Oncol. (2016) 34:3157-65. doi: 10.1200/JCO.2016.67.4846
37. Wu Q, Bai B, Tian C, Li D, Yu H, Song B, et al. The molecular mechanisms of cardiotoxicity induced by HER2, VEGF, and tyrosine kinase inhibitors: an updated review. Cardiovasc Drugs Ther. (2021). doi: 10.1007/s10557-021-07181-3 [Epub ahead of print].
38. Rupert CE, Coulombe KL. The roles of neuregulin-1 in cardiac development, homeostasis, and disease. Biomark Insights. (2015) 10(Suppl 1):1-9. doi: 10.4137/BMI.S20061
39. Lemmens K, Doggen K, De Keulenaer GW. Role of neuregulin-1/ErbB signaling in cardiovascular physiology and disease: implications for therapy of heart failure. Circulation. (2007) 116:954-60. doi: 10.1161/CIRCULATIONAHA.107.690487
40. Geissler A, Ryzhov S, Sawyer DB. Neuregulins: protective and reparative growth factors in multiple forms of cardiovascular disease. Clin Sci. (2020) 134:2623-43. doi: 10.1042/CS20200230
41. Gordon LI, Burke MA, Singh AT, Prachand S, Lieberman ED, Sun L, et al. Blockade of the erbB2 receptor induces cardiomyocyte death through mitochondrial and reactive oxygen species-dependent pathways. J Biol Chem. (2009) 284:2080-7. doi: 10.1074/jbc.M804570200
42. Ravingerova T, Matejikova J, Neckar J, Andelova E, Kolar F. Differential role of PI3K/Akt pathway in the infarct size limitation and antiarrhythmic protection in the rat heart. Mol Cell Biochem. (2007) 297:111-20. doi: 10.1007/s11010-006-9335-z
43. Rochette L, Guenancia C, Gudjoncik A, Hachet O, Zeller M, Cottin Y, et al. Anthracyclines/trastuzumab: new aspects of cardiotoxicity and molecular mechanisms. Trends Pharmacol Sci. (2015) 36:326-48. doi: 10.1016/j.tips.2015.03.005
44. Yang S, Liu G. Targeting the Ras/Raf/MEK/ERK pathway in hepatocellular carcinoma. Oncol Lett. (2017) 13:1041-7. doi: 10.3892/ol.2017.5557
45. Liu F, Yang X, Geng M, Huang M. Targeting ERK, an Achilles' Heel of the MAPK pathway, in cancer therapy. Acta Pharm Sin B. (2018) 8:552-62. doi: 10.1016/j.apsb.2018.01.008
46. Hedhli N, Huang Q, Kalinowski A, Palmeri M, Hu X, Russell RR, et al. Endothelium-derived neuregulin protects the heart against ischemic injury. Circulation. (2011) 123:2254-62. doi: 10.1161/CIRCULATIONAHA.110.991125
47. Liu FF, Stone JR, Schuldt AJ, Okoshi K, Okoshi MP, Nakayama M, et al. Heterozygous knockout of neuregulin-1 gene in mice exacerbates doxorubicin-induced heart failure. Am J Physiol Heart Circ Physiol. (2005) 289:H660-6. doi: 10.1152/ajpheart.00268.2005
48. Filomeni G, De Zio D, Cecconi F. Oxidative stress and autophagy: the clash between damage and metabolic needs. Cell Death Differ. (2015) 22:377-88. doi: 10.1038/cdd.2014.150
49. Thomas RL, Roberts DJ, Kubli DA, Lee Y, Quinsay MN, Owens JB, et al. Loss of MCL-1 leads to impaired autophagy and rapid development of heart failure. Genes Dev. (2013) 27:1365-77. doi: 10.1101/gad.215871.113
50. Mohan N, Shen Y, Endo Y, ElZarrad MK, Wu WJ. Trastuzumab, but Not pertuzumab, dysregulates HER2 signaling to mediate inhibition of autophagy and increase in reactive oxygen species production in human cardiomyocytes. Mol Cancer Ther. (2016) 15:1321-31. doi: 10.1158/1535-7163.MCT-15-0741
51. Lu L, Wu W, Yan J, Li X, Yu H, Yu X. Adriamycin-induced autophagic cardiomyocyte death plays a pathogenic role in a rat model of heart failure. Int J Cardiol. (2009) 134:82-90. doi: 10.1016/j.ijcard.2008.01.043
52. Dirks-Naylor AJ. The role of autophagy in doxorubicin-induced cardiotoxicity. Life Sci. (2013) 93:913-6. doi: 10.1016/j.lfs.2013.10.013
53. Doenst T, Nguyen TD, Abel ED. Cardiac metabolism in heart failure: implications beyond ATP production. Circ Res. (2013) 113:709-24. doi: 10.1161/CIRCRESAHA.113.300376
54. Necela BM, Axenfeld BC, Serie DJ, Kachergus JM, Perez EA, Thompson EA, et al. The antineoplastic drug, trastuzumab, dysregulates metabolism in iPSC-derived cardiomyocytes. Clin Transl Med. (2017) 6:5. doi: 10.1186/s40169-016-0133-2
55. Ito T, Kimura Y, Uozumi Y, Takai M, Muraoka S, Matsuda T, et al. Taurine depletion caused by knocking out the taurine transporter gene leads to cardiomyopathy with cardiac atrophy. J Mol Cell Cardiol. (2008) 44:927-37. doi: 10.1016/j.yjmcc.2008.03.001
56. Kitani T, Ong SG, Lam CK, Rhee JW, Zhang JZ, Oikonomopoulos A, et al. Human-induced pluripotent stem cell model of trastuzumab-induced cardiac dysfunction in patients with breast cancer. Circulation. (2019) 139:2451-65. doi: 10.1161/CIRCULATIONAHA.118.037357
57. Bergamini C, Torelli F, Ghiselli L, Rossi A, Trevisani L, Vinco G, et al. Left ventricular end-diastolic volume as early indicator of trastuzumab-related cardiotoxicity in HER2+ breast cancer patients: results from a single-center retrospective study. Minerva Cardioangiol. (2017) 65:278-87. doi: 10.23736/S0026-4725.16.04278-X
58. Negishi K, Negishi T, Hare JL, Haluska BA, Plana JC, Marwick TH. Independent and incremental value of deformation indices for prediction of trastuzumab-induced cardiotoxicity. J Am Soc Echocardiogr. (2013) 26:493-8. doi: 10.1016/j.echo.2013.02.008
59. Thavendiranathan P, Negishi T, Somerset E, Negishi K, Penicka M, Lemieux J, et al. Strain-guided management of potentially cardiotoxic cancer therapy. J Am Coll Cardiol. (2021) 77:392-401. doi: 10.1016/j.jacc.2020.11.020
60. Zamorano JL, Lancellotti P, Rodriguez Munoz D, Aboyans V, Asteggiano R, Galderisi M, et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: the Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur J Heart Fail. (2017) 19:9-42. doi: 10.1093/eurheartj/ehw211
61. Aimo A, Januzzi JL Jr, Bayes-Genis A, Vergaro G, Sciarrone P, Passino C, et al. Clinical and prognostic significance of sST2 in heart failure: JACC review topic of the week. J Am Coll Cardiol. (2019) 74:2193-203. doi: 10.1016/j.jacc.2019.08.1039
62. Zhang S, Wang Y, Wang Y, Peng J, Yuan C, Zhou L, et al. Serum miR-222-3p as a double-edged sword in predicting efficacy and trastuzumab-induced cardiotoxicity for HER2-positive breast cancer patients receiving neoadjuvant target therapy. Front Oncol. (2020) 10:631. doi: 10.3389/fonc.2020.00631
63. van der Voort A, van Ramshorst MS, van Werkhoven ED, Mandjes IA, Kemper I, Vulink AJ, et al. Three-year follow-up of neoadjuvant chemotherapy with or without anthracyclines in the presence of dual ERBB2 blockade in patients with ERBB2-positive breast cancer: a secondary analysis of the TRAIN-2 randomized, phase 3 trial. JAMA Oncol. (2021) 7:978-84. doi: 10.1001/jamaoncol.2021.1371
64. Agus DB, Gordon MS, Taylor C, Natale RB, Karlan B, Mendelson DS, et al. Phase I clinical study of pertuzumab, a novel HER dimerization inhibitor, in patients with advanced cancer. J Clin Oncol. (2005) 23:2534-43. doi: 10.1200/JCO.2005.03.184
65. Capelan M, Pugliano L, De Azambuja E, Bozovic I, Saini KS, Sotiriou C, et al. Pertuzumab: new hope for patients with HER2-positive breast cancer. Ann Oncol. (2013) 24:273-82. doi: 10.1093/annonc/mds328
66. Gianni L, Pienkowski T, Im YH, Roman L, Tseng LM, Liu MC, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol. (2012) 13:25-32. doi: 10.1016/S1470-2045(11)70336-9
67. Schneeweiss A, Chia S, Hickish T, Harvey V, Eniu A, Hegg R, et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA). Ann Oncol. (2013) 24:2278-84. doi: 10.1093/annonc/mdt182
68. Shao Z, Pang D, Yang H, Li W, Wang S, Cui S, et al. Efficacy, safety, and tolerability of pertuzumab, trastuzumab, and docetaxel for patients with early or locally advanced ERBB2-positive breast cancer in Asia: the PEONY phase 3 randomized clinical trial. JAMA Oncol. (2020) 6:e193692. doi: 10.1001/jamaoncol.2019.3692
69. Curigliano G, Lenihan D, Fradley M, Ganatra S, Barac A, Blaes A, et al. Management of cardiac disease in cancer patients throughout oncological treatment: ESMO consensus recommendations. Ann Oncol. (2020) 31:171-90. doi: 10.1016/j.annonc.2019.10.023
70. Dang CT, Yu AF, Jones LW, Liu J, Steingart RM, Argolo DF, et al. Cardiac surveillance guidelines for trastuzumab-containing therapy in early-stage breast cancer: getting to the heart of the matter. J Clin Oncol. (2016) 34:1030-3. doi: 10.1200/JCO.2015.64.5515
71. Bosch X, Rovira M, Sitges M, Domenech A, Ortiz-Perez JT, de Caralt TM, et al. Enalapril and carvedilol for preventing chemotherapy-induced left ventricular systolic dysfunction in patients with malignant hemopathies: the OVERCOME trial (preventiOn of left Ventricular dysfunction with Enalapril and caRvedilol in patients submitted to intensive ChemOtherapy for the treatment of Malignant hEmopathies). J Am Coll Cardiol. (2013) 61:2355-62. doi: 10.1016/j.jacc.2013.02.072
72. Heck SL, Mecinaj A, Ree AH, Hoffmann P, Schulz-Menger J, Fagerland MW, et al. Prevention of cardiac dysfunction during adjuvant breast cancer therapy (PRADA): extended follow-up of a 2x2 factorial, randomized, placebo-controlled, double-blind clinical trial of candesartan and metoprolol. Circulation. (2021) 143:2431-40. doi: 10.1161/CIRCULATIONAHA.121.054698
73. Pituskin E, Mackey JR, Koshman S, Jassal D, Pitz M, Haykowsky MJ, et al. Multidisciplinary approach to novel therapies in cardio-oncology Research (MANTICORE 101-Breast): a randomized trial for the prevention of trastuzumab-associated cardiotoxicity. J Clin Oncol. (2017) 35:870-7. doi: 10.1200/JCO.2016.68.7830
74. Boekhout AH, Gietema JA, Milojkovic Kerklaan B, van Werkhoven ED, Altena R, Honkoop A, et al. Angiotensin II-receptor inhibition with candesartan to prevent trastuzumab-related cardiotoxic effects in patients with early breast cancer: a randomized clinical trial. JAMA Oncol. (2016) 2:1030-7. doi: 10.1001/jamaoncol.2016.1726
75. Guglin M, Krischer J, Tamura R, Fink A, Bello-Matricaria L, McCaskill-Stevens W, et al. Randomized trial of lisinopril versus carvedilol to prevent trastuzumab cardiotoxicity in patients with breast cancer. J Am Coll Cardiol. (2019) 73:2859-68. doi: 10.1016/j.jacc.2019.03.495
76. Calvillo-Arguelles O, Abdel-Qadir H, Michalowska M, Billia F, Suntheralingam S, Amir E, et al. Cardioprotective effect of statins in patients with HER2-positive breast cancer receiving trastuzumab therapy. Can J Cardiol. (2019) 35:153-9. doi: 10.1016/j.cjca.2018.11.028
77. Obasi M, Abovich A, Vo JB, Gao Y, Papatheodorou SI, Nohria A, et al. Correction to: statins to mitigate cardiotoxicity in cancer patients treated with anthracyclines and/or trastuzumab: a systematic review and metaanalysis. Cancer Causes Control. (2021) 32:1407-9. doi: 10.1007/s10552-021-01495-1
78. Moon GJ, Kim SJ, Cho YH, Ryoo S, Bang OY. Antioxidant effects of statins in patients with atherosclerotic cerebrovascular disease. J Clin Neurol. (2014) 10:140-7. doi: 10.3988/jcn.2014.10.2.140
79. Kabel AM, Elkhoely AA. Targeting proinflammatory cytokines, oxidative stress, TGF-beta1 and STAT-3 by rosuvastatin and ubiquinone to ameliorate trastuzumab cardiotoxicity. Biomed Pharmacother. (2017) 93:17-26. doi: 10.1016/j.biopha.2017.06.033
80. Kim TT, Dyck JR. Is AMPK the savior of the failing heart? Trends Endocrinol Metab. (2015) 26:40-8. doi: 10.1016/j.tem.2014.11.001
81. Gundewar S, Calvert JW, Jha S, Toedt-Pingel I, Ji SY, Nunez D, et al. Activation of AMP-activated protein kinase by metformin improves left ventricular function and survival in heart failure. Circ Res. (2009) 104:403-11. doi: 10.1161/CIRCRESAHA.108.190918
82. Wang B, Nie J, Wu L, Hu Y, Wen Z, Dong L, et al. AMPKalpha2 protects against the development of heart failure by enhancing mitophagy via PINK1 phosphorylation. Circ Res. (2018) 122:712-29. doi: 10.1161/CIRCRESAHA.117.312317
83. Carling D. AMPK signalling in health and disease. Curr Opin Cell Biol. (2017) 45:31-7. doi: 10.1016/j.ceb.2017.01.005
84. Chaanine AH, Hajjar RJ. AKT signalling in the failing heart. Eur J Heart Fail. (2011) 13:825-9. doi: 10.1093/eurjhf/hfr080
85. Sahu R, Dua TK, Das S, De Feo V, Dewanjee S. Wheat phenolics suppress doxorubicin-induced cardiotoxicity via inhibition of oxidative stress, MAP kinase activation, NF-kappaB pathway, PI3K/Akt/mTOR impairment, and cardiac apoptosis. Food Chem Toxicol. (2019) 125:503-19. doi: 10.1016/j.fct.2019.01.034
86. Chen R, Sun G, Yang L, Wang J, Sun X. Salvianolic acid B protects against doxorubicin induced cardiac dysfunction via inhibition of ER stress mediated cardiomyocyte apoptosis. Toxicol Res. (2016) 5:1335-45. doi: 10.1039/C6TX00111D
87. Wu J, Sun C, Wang R, Li J, Zhou M, Yan M, et al. Cardioprotective effect of paeonol against epirubicin-induced heart injury via regulating miR-1 and PI3K/AKT pathway. Chem Biol Interact. (2018) 286:17-25. doi: 10.1016/j.cbi.2018.02.035
88. Fei J, Sun Y, Duan Y, Xia J, Yu S, Ouyang P, et al. Low concentration of rutin treatment might alleviate the cardiotoxicity effect of pirarubicin on cardiomyocytes via activation of PI3K/AKT/mTOR signaling pathway. Biosci Rep. (2019) 39:BSR20190546. doi: 10.1042/BSR20190546
89. Cao Y, Ruan Y, Shen T, Huang X, Li M, Yu W, et al. Astragalus polysaccharide suppresses doxorubicin-induced cardiotoxicity by regulating the PI3k/Akt and p38MAPK pathways. Oxid Med Cell Longev. (2014) 2014:674219. doi: 10.1155/2014/674219
90. Zhai J, Tao L, Zhang S, Gao H, Zhang Y, Sun J, et al. Calycosin ameliorates doxorubicin-induced cardiotoxicity by suppressing oxidative stress and inflammation via the sirtuin 1-NOD-like receptor protein 3 pathway. Phytother Res. (2020) 34:649-59. doi: 10.1002/ptr.6557
91. Zhang HJ, Chen RC, Sun GB, Yang LP, Zhu YD, Xu XD, et al. Protective effects of total flavonoids from Clinopodium chinense (Benth.) O. Ktze on myocardial injury in vivo and in vitro via regulation of Akt/Nrf2/HO-1 pathway. Phytomedicine. (2018) 40:88-97. doi: 10.1016/j.phymed.2018.01.004
92. Gao J, Chen T, Zhao D, Zheng J, Liu Z. Ginkgolide B Exerts cardioprotective properties against doxorubicin-induced cardiotoxicity by regulating reactive oxygen species, Akt and calcium signaling pathways in vitro and in vivo. PLoS ONE. (2016) 11:e0168219. doi: 10.1371/journal.pone.0168219
93. Xing JJ, Hou JG, Liu Y, Zhang RB, Jiang S, Ren S, et al. Supplementation of Saponins from Leaves of Panax quinquefolius mitigates Cisplatin-evoked cardiotoxicity via inhibiting oxidative stress-associated inflammation and apoptosis in mice. Antioxidants. (2019) 8:347. doi: 10.3390/antiox8090347
94. Bharathi Priya L, Baskaran R, Huang CY, Vijaya Padma V. Neferine modulates IGF-1R/Nrf2 signaling in doxorubicin treated H9c2 cardiomyoblasts. J Cell Biochem. (2018) 119:1441-52. doi: 10.1002/jcb.26305
95. Zhang X, Lv S, Zhang W, Jia Q, Wang L, Ding Y, et al. Shenmai injection improves doxorubicin cardiotoxicity via miR-30a/Beclin 1. Biomed Pharmacother. (2021) 139:111582. doi: 10.1016/j.biopha.2021.111582
Keywords: trastuzumab, cardiotoxicity, breast cancer, adverse reaction, rational drug use
Citation: Lin M, Xiong W, Wang S, Li Y, Hou C, Li C and Li G (2022) The Research Progress of Trastuzumab-Induced Cardiotoxicity in HER-2-Positive Breast Cancer Treatment. Front. Cardiovasc. Med. 8:821663. doi: 10.3389/fcvm.2021.821663
Received: 24 November 2021; Accepted: 14 December 2021;
Published: 12 January 2022.
Edited by:
Chun Liu, Stanford University, United StatesReviewed by:
Nian Liu, Capital Medical University, ChinaZhiqiang Liu, Beijing Institute of Basic Medical Sciences, China
Copyright © 2022 Lin, Xiong, Wang, Li, Hou, Li and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chunyu Li, Y2h1bnl1X2xpQDEyNi5jb20=; Guohui Li, bGdoMDYwM0BjaWNhbXMuYWMuY24=
†These authors have contributed equally to this work
 Mengmeng Lin1†
Mengmeng Lin1† Shiyuan Wang
Shiyuan Wang Chunyu Li
Chunyu Li Guohui Li
Guohui Li