
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cardiovasc. Med., 14 February 2022
Sec. General Cardiovascular Medicine
Volume 8 - 2021 | https://doi.org/10.3389/fcvm.2021.821297
This article is part of the Research TopicMINOCA: Pathogenesis, diagnosis, clinical management and evolution towards precision medicineView all 7 articles
Myocardial infarction with non-obstructive coronary arteries (MINOCA) is a heterogeneous group of clinical entities characterized by the common clinical evidence of myocardial infarction (MI) with non-obstructive coronary arteries on coronary angiography and without an overt cause for the MI. Platelets play a cornerstone role in the pathophysiology of MI with obstructive coronary arteries. Accordingly, antiplatelet therapy is recommended for treating patients with MI and obstructive coronary disease. However, the role of platelets in the pathophysiology of MINOCA patients is not fully defined, questioning the role of antiplatelet therapy in this setting. In this review, we will assess the role of antiplatelet therapy in MINOCA with a focus on the pathophysiology, therapeutic targets, current evidence, and future directions according to its different etiologies.
Invasive coronary angiography plays a crucial role in the diagnosis and management of myocardial infarction (MI). Based on coronary angiography findings, MI patients can be classified according to the presence of obstructive or non-obstructive coronary artery disease (CAD) (1). Myocardial infarction with non-obstructive coronary arteries (MINOCA) is a heterogeneous set of multiple clinical entities characterized by the common clinical evidence of MI with non-obstructive coronary arteries on coronary angiography (<50% stenosis) and without an overt cause for the MI (1). Moreover, MINOCA may represent 1–13% of all MI patients who undergo coronary angiography (2). Due to the different clinical etiologies, MINOCA has several different pathophysiological pathways, which can lead to a variety of treatment modalities. In patients with MI and obstructive coronary artery, the use of antiplatelet therapy is vastly supported by randomized controlled trials (RCTs) and forms a central part of guideline-recommendations (3). However, in patients with MINOCA, the role of antiplatelet therapy is less well-understood and may vary significantly according to the underlying etiology (1). In this review, we will assess the role of antiplatelet therapy in MINOCA with a focus on the pathophysiology, therapeutic targets, current evidence, and future directions according to its different etiologies.
The potential pathophysiological pathways of MINOCA are directly related to the underlying etiology. Figures 1, 2 represents the different etiologies that may lead to MINOCA. MINOCA etiologies can be divided in cardiac and extra-cardiac (1). Cardiac etiologies can be classified into coronary and non-coronary causes. Furthermore, coronary causes can be classified according to the presence or not of atherosclerotic disease. However, an overlap among different mechanism is not infrequent. Among the non-coronary causes, some etiologies may be associated with myocardial disorders (i.e., myocarditis or Takotsubo syndrome). Nevertheless, others may be related to extra-cardiac conditions (i.e., stroke, respiratory failure or sepsis).
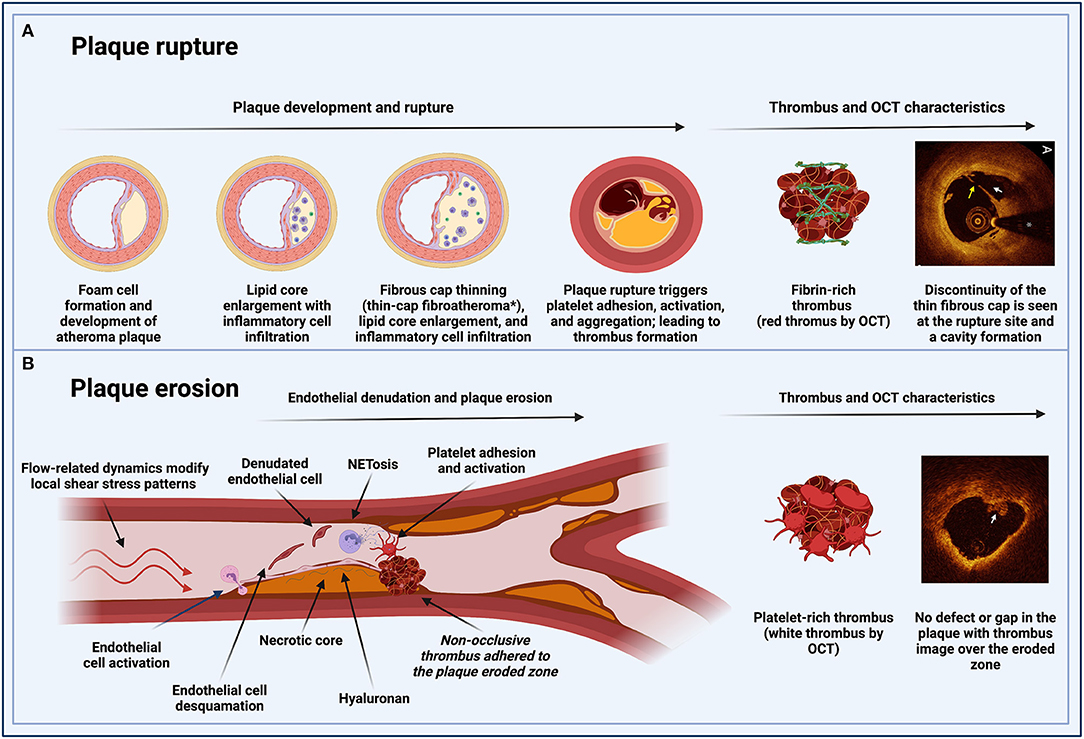
Figure 1. Pathophysiological pathways of MINOCA in patients with atherosclerotic disease. (A) Plaque rupture: inflammation is involved in the process of plaque development and rupture. Unstable plaques have a large lipid core and a thin fibrous cap with reduced collagen content. A major component of plaque destabilization appears to be increased matrix degradation, the primary regulators of which are the matrix metalloproteinase. Plaque rupture occurs where the cap is thinnest and most infiltrated by foam cells. In eccentric plaques, the weakest spot is often the cap margin or shoulder region, and only extremely thin fibrous caps are at risk of rupturing. Exposure of the thrombogenic lipid core material lead to fibrin-rich thrombus formation. (B) Plaque erosion: flow-related dynamics modify local shear stress patterns on the endothelial monolayer. The basement membrane (endothelial-to-mesenchymal transition) is degraded. These changes result in the desquamation of the endothelial cells from the basement membrane and their subsequent death by apoptosis. Neutrophils in the area undergo a particular type of apoptosis (NETosis) to form neutrophil extracellular traps (NETs), which produce a potent inflammatory stimulus containing tissue factor, leading to entrapment of circulating platelets and facilitating the formation of a platelet-rich thrombus. *Thin-cap fibroatheromas (TCFAs) for coronary fibroatheromas with a fibrous cap thickness of <65 μm. MINOCA, myocardial infarction with non-obstructive coronary arteries; OCT, optical coherence tomography; NET, neutrophil extracellular traps.
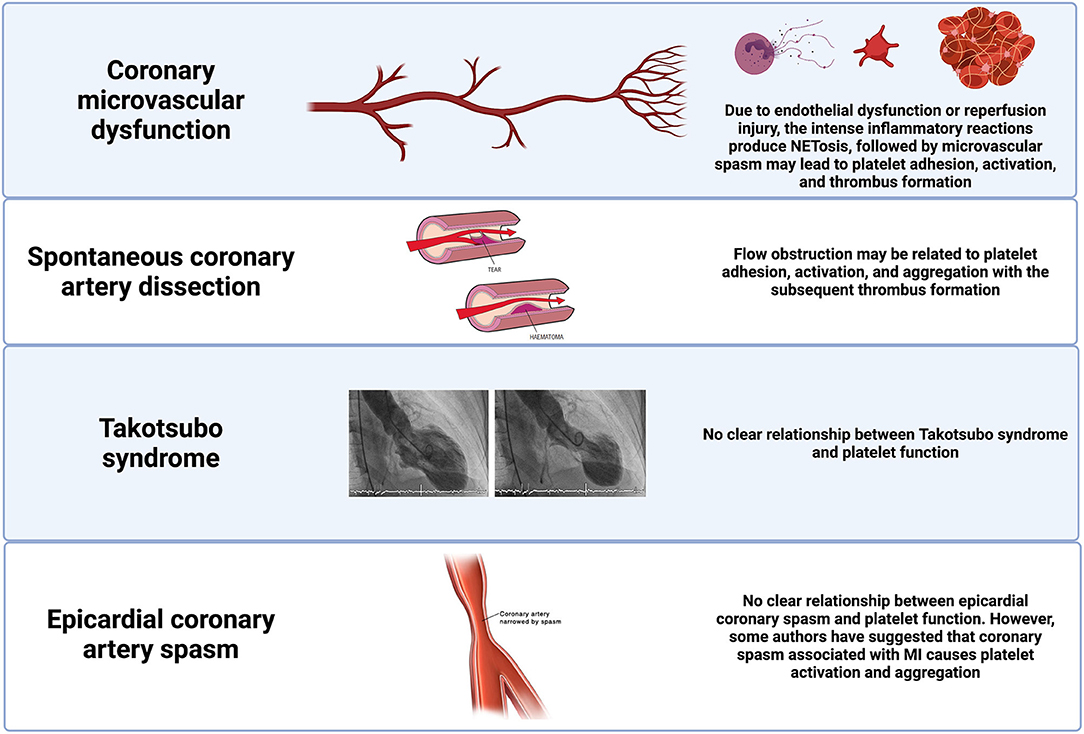
Figure 2. Pathophysiological pathways of MINOCA in patients without coronary atherosclerotic disease. Potential pathophysiological pathways in selected non-atherosclerotic causes of MINOCA and their relationship with platelet function. MINOCA, myocardial infarction with non-obstructive coronary arteries.
MINOCA etiologies can also be classified according to the fourth universal MI definition (4). If underlying etiology involves the epicardial coronary arteries, these disorders may meet the type 1 MI definition. Whereas, if the etiology is due to endothelial dysfunction or oxygen supply and demand mismatch, or myocardial injury, these disorders may meet the type 2 MI definition. Although the prescription of antiplatelet therapy at discharge in MINOCA patients is frequent (69.7%), form a pathophysiological mechanism, antiplatelet agents may only have an important effect in etiologies in which platelet play a significant role (type 1 MI) (Figure 3) (5). In this review, we will focus on the etiologies that may have implications for the use of antiplatelet therapy based on potential involvement of platelets (Figure 4).
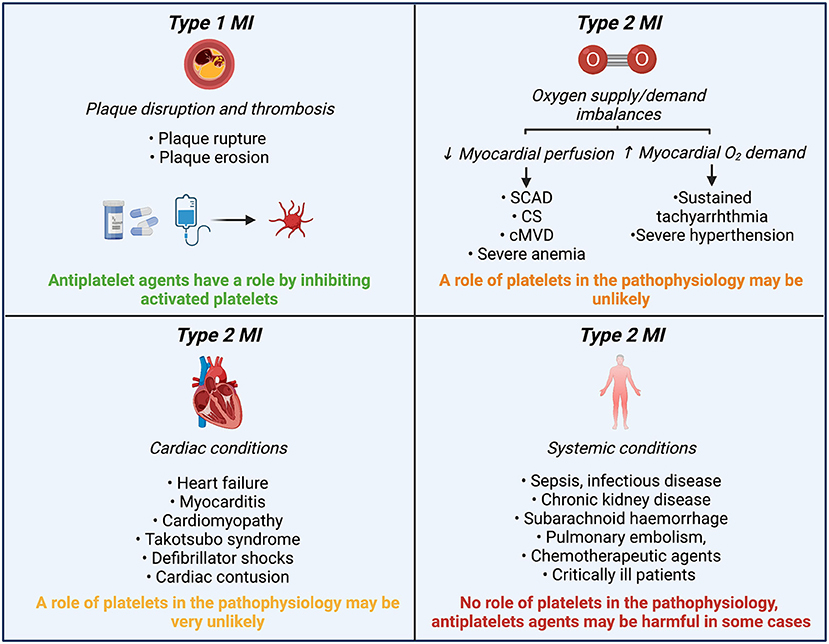
Figure 3. Role of platelets and antiplatelet agents in MINOCA etiologies classified according to the 4th UDMI. MINOCA, myocardial infarction with non-obstructive coronary arteries; UDMI, universal myocardial infarction definition; MI, myocardial infarction; SCAD, spontaneous coronary artery dissection; CS, epicardial coronary spasm; cMVD, coronary microvascular disease.
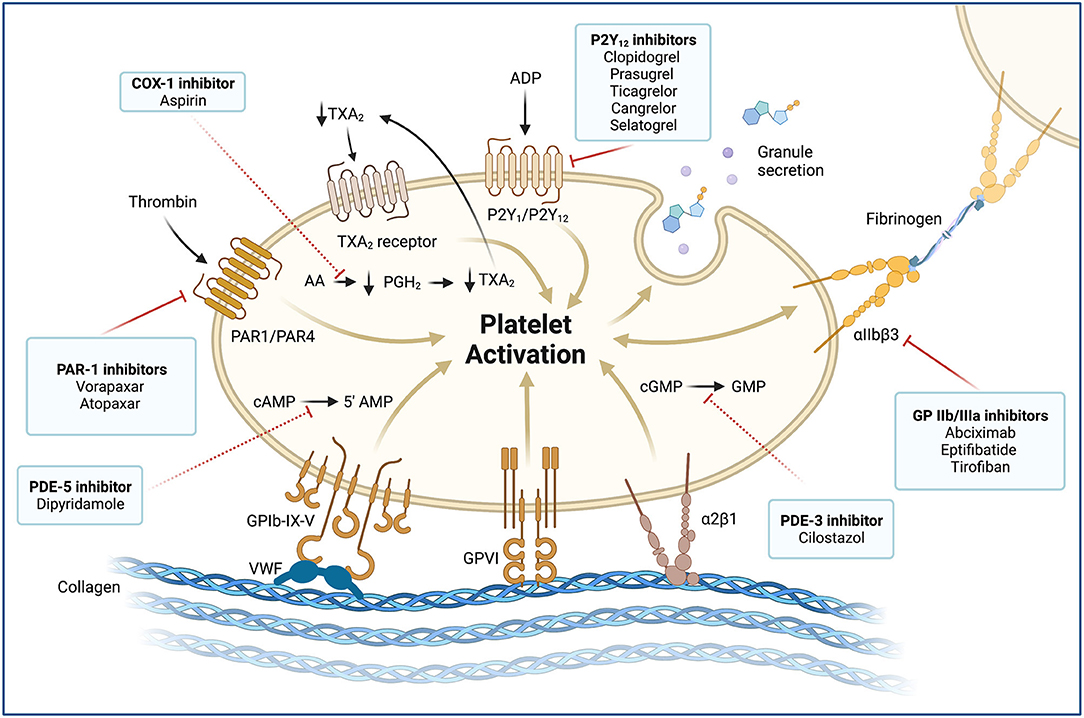
Figure 4. Platelet activation and molecular targets of antiplatelet agents. Antiplatelet agents and their molecular targets are shown in boxes. Blockade of membrane receptors (continuous red lines): Oral (clopidogrel, prasugrel, and ticagrelor) and parenteral (cangrelor and selatogrel) P2Y12 inhibitors block the P2Y12 receptor, which is a chemoreceptor for adenosine diphosphate (ADP). P2Y12 receptors produce a potent positive feedback loop for platelet activation. Glycoprotein (GP) αIIbβ3 inhibitors (abciximab, eptifibatide, and tirofiban) are potent parenteral antiplatelet agents. The αIIβ3 receptor is an integrin complex that is activated by platelet conformational change induced by ADP stimulus. When the αIIβ3 receptor is activated, it binds to fibrinogen and forms platelet aggregates. Proteinase-activated receptor 1 (PAR1), also called coagulation factor II (thrombin) receptor, is a G protein-coupled that plays a key role in mediating the interplay between coagulation and inflammation. PAR1 inhibitors (vorapaxar) are oral potent antiplatelet agents that inhibit the PAR1 receptor and thrombin-related platelet aggregation. Blockade of intracellular signaling pathway (red dashed lines): Low-dose oral aspirin exerts its effect primarily by inhibiting the cyclooxygenase (COX-1) and interfering with the cytosolic metabolism of arachidonic acid (AA) into prostaglandin H2 (PGH2) and thromboxane A2 (TXA2). TXA2 stimulates the thromboxane receptor, inducing platelet activation, which results in platelet-shape change, inside-out activation of integrins, and degranulation. The cytosolic reduction of TXA2 concentration reduces circulating TXA2 availability and TXA2 receptor stimulation. Phosphodiesterases (PDEs) can limit the intracellular levels of cyclic nucleotides and regulate platelet function by catalyzing the hydrolysis of cyclic adenosine 3',5'-monophosphate (cAMP) and cyclic guanosine 3',5'-monophosphate (cGMP). Dipyridamole is an oral antiplatelet agent that inhibits the PDE5 and 5'AMP production. In contrast, cilostazol is a PD3 inhibitor that blocks GMP production. MINOCA: myocardial infarction with non-obstructive coronary arteries; vWF, von Willebrand factor; GPIb-IX-V, Glycoprotein Ib-IX-V complex; GPVI, Glycoprotein VI; α2β1, Integrin α2β1.
Plaque disruption is a common cause of MINOCA, which enclose plaque rupture, plaque erosion, and calcific nodules. Plaque disruption may cause thrombus formation, leading to an MI via distal embolization or superimposed coronary spasm. Besides, occasionally, complete transient thrombosis with spontaneous thrombolysis can be associated (1).
Historically, atherosclerotic plaque rupture has been the paradigm of MI pathophysiology. Plaque rupture is the most frequent cause of atherothrombosis (55–60%). Plaque rupture is defined as a plaque with deep injury presenting defect in the fibrous cap that detached its lipid-rich atheromatous core into the coronary artery lumen, thereby exposing the thrombogenic core of the plaque (6). Coronary plaques at high risk of rupture are characterized by a lipid-rich core, thin fibrous cap with macrophage/lymphocyte infiltration, decreased smooth muscle cell content, and expansive remodeling (6). Atherosclerotic plaque rupture in large- and medium-sized arteries triggers platelet activation, leading to a fibrin-rich thrombus formation and impairment of coronary flow (Figure 1) (7). Platelet-activated thrombus proceeds in three stages, an initial phase of platelet adhesion, an extension phase that includes activation, and a perpetuation phase (7–9). In the initiation phase, platelets roll, adhere, and spread on the collagen matrix to form an activated platelet monolayer (10).
Adhesion is mediated by the interaction between the glycoprotein (GP) Ib/V/IX receptor complex on the platelet surface with von Willebrand factor (vWF) and between the GP VI and GP Ia proteins with collagen at the plaque rupture location (7). These interactions allow for activation of platelets. Under high shear conditions (i.e., small arteries, arterioles, and stenotic arteries), the interaction between vWF and GP Ib/V/IX is needed for the initial adhesion of platelets to the subendothelium (10). vWF plays a central role in platelet adhesion as it binds to both collagen and two important platelet receptors (GP Ib/V/IX and GP IIb/IIIa (αIIβ3 integrin) (10). After platelet activation, local platelet-activating factors recruit additional circulating platelets to expand and stabilize the hemostatic plug. These platelet-activating factors consist of adenosine diphosphate (ADP), thromboxane A2 (TXA2), serotonin, collagen, and thrombin (7). Thrombin is characterized for being the most potent of these factors. Importantly, thrombin is largely produced on the activated platelet surface by the prothrombinase complex and promotes the cleavage of fibrinogen into fibrin (11, 12). The release of ADP and TXA2 from adherent platelets contributes to the recruitment of circulating platelets and leads to distinct manifestations of platelet activation, including change in platelet shape, increased expression of proinflammatory molecules, expression of platelet procoagulant activity, and conversion of the GP IIb/IIIa receptor into an active form. The latter ultimately allows for platelet aggregation via fibrinogen bonds and vWF leading to pathological thrombosis (Figure 4) (7, 9). Of note, hemostasis in smaller vessels to stop bleeding may occur by different mechanisms, although the differences between these processes are not entirely known. Therefore, effective interventions for preventing thrombosis in the epicardial vessels may not have the same effect in smaller vessels (9).
Plaque erosion is the second most common cause of atherothrombosis (30–35%). Eroded plaque is defined as a plaque with loss or dysfunction of the luminal endothelial cells leading to thrombosis. The main difference with plaque rupture is that there is no additional defect or gap in the plaque. This type of plaque is often rich in smooth muscle cells, proteoglycans and associated with constrictive remodeling (6). Moreover, plaque erosion is characterized by larger vessel lumen than in plaque rupture and the presence of a platelet-rich thrombus (13). The potential mechanism leading to thrombosis could be related to flow disturbance and inflammatory activation of the luminal endothelial cells, subsequent leukocyte recruitment, release of neutrophil extracellular traps (NETs), resulting in a cascade of thrombosis (13). Plaque erosion is possibly a weaker thrombogenic stimulus, and thrombi seem to be longer in the making than those triggered by rupture (Figure 1) (14). Ultimately, the key receptors and pathways leading to thrombosis are the same as in plaque rupture.
Coronary thrombosis or embolism may present as MINOCA if it affects the microcirculation or if partial lysis of the epicardial coronary thrombus results in the non-obstructive angiographic pattern. Patients with hypercoagulable states may be at higher risk, but thrombosis or embolism may occur in patients without (1). Hypercoagulable disorders related to coronary thrombosis can be classified into inherited and acquired causes. The more common inherited thrombophilias are factor V Leiden and elevated factor VIII/ vWF, the prevalence of which varies according to ethnicity (15). Meanwhile, acquired hypercoagulable states include thrombotic thrombocytopenic purpura, autoimmune disorder antiphospholipid syndrome, heparin-induced thrombocytopenia, and myeloproliferative neoplasms. Although the etiology may significantly differ, the common mechanism associated with MINOCA is microvascular obstruction by microthrombus (16). Microembolization leads to platelet and inflammatory cell activation and vasospasm, which reduces coronary flow in combination with mechanical plugging of the microcirculation (17). Moreover, patients who present microvascular obstruction exhibit increased platelet activation during the acute phase and even at 1-month follow-up (18).
Spontaneous Coronary Artery Dissection (SCAD) is a relatively infrequent mechanism of MI. Nevertheless, it is a frequent cause of MI among women <50 years of age (19). Although most patients with SCAD have some degree of flow obstruction, occasionally, the arteries can appear normal or near-normal due to the distal vessel tapering. Therefore, this should be considered as a potential cause for MINOCA. In SCAD, the obstruction to coronary flow is generated by a separation of the media and adventitial vascular walls associated with intramural hematoma protrusion into the lumen. The exact pathophysiology of SCAD remains uncertain, and the primary source of the dissection (intimal or medial) is still debatable. SCAD might represent an intrinsic underlying vasculopathy that could be composite by a triggering stressor associated with a catecholamine surge, such as emotional stress, extreme physical activities, and sympathomimetic drugs. Of note, there is a significant association between SCAD and other vascular diseases (e.g., fibromuscular dysplasia) (Figure 2) (20).
The coronary microcirculation (defined as vessels < 0.5 mm diameter) is not directly visualized in the coronary angiography. However, in the absence of obstructive coronary artery disease, microcirculation accounts for ~70% of the coronary resistance (21). A standardized definition for microvascular angina has been established and enclose patients with ischemic chest discomfort, non-obstructive coronary arteries, and impaired coronary flow (21). Impaired flow can be defined according to a coronary flow reserve <2.0 in response to vasodilator stimuli, microvascular spasm diagnosed during provocative spasm testing, in the absence of epicardial coronary spasm, or impaired coronary blood flow, as measured with a corrected Thrombolysis in Myocardial Infarction (TIMI) frame count (1). In clinical practice, it is more frequent in women and patients with conventional cardiovascular risk factors (21). The initial underlying mechanism of ischemia-induced by microvascular dysfunction is endothelial dysfunction (endothelium-dependent dysfunction), which leads to vascular inflammation, platelet interaction with the adhesion cells, and activation of platelets and neutrophils. This proinflammatory environment results in microvascular spasm, impaired vasodilation, intimal thickening, and smooth cell proliferation (21). All these mechanisms lead to capillary obstruction due to capillary density and diameter reduction. Moreover, microvascular dysfunction can be a sequela of myocardial injury (endothelium-independent dysfunction) of an atherosclerotic or non-atherosclerotic etiology. Microembolization with persistent platelet activation leads to an intense inflammatory state, microvascular and tissue damage associated with neutrophil activation, and NETs production (Figure 2) (22, 23).
Coronary artery spasm is defined as intense focal or diffuse vasoconstriction (i.e., >90% of the diameter) of an epicardial coronary artery deriving in impaired flow. Coronary vasospasm can occur spontaneously because of coronary vasomotor tone disorders or secondary to drugs or toxins that lead to hyperreactivity of vascular smooth muscles. Vasospastic angina is a clinical condition presenting as rest angina with a dynamic ST-segment elevation pattern on the electrocardiogram. Therefore, prolonged vasospastic episodes can lead to MINOCA. Vascular smooth muscle hyperreactivity seems to be a fundamental pathophysiological mechanism (24). However, endothelial dysfunction and vascular inflammation may also have a role in modulating this hyperreactivity (25). Some authors have proposed a link between episodes of coronary vasospasm and platelet activation (26). In particular, the rapid changes in local shear stress can potentially induce stabilized aggregation of the discoid platelets, resulting in the release of granules with vasoconstrictors. Other authors instead have suggested that coronary spasm associated with acute myocardial ischemia causes platelet activation and aggregation in the coronary circulation and may aggravate symptoms and outcomes (Figure 2) (27).
The underlying mechanisms of the Takotsubo syndrome (TTS) are still not completely understood. However, there is substantial evidence that sympathetic stimulation is an essential underlying pathophysiological mechanism (28). A trackable emotional or physical trigger precipitates the syndrome in most cases and has been associated with excess circulating catecholamines. Nevertheless, the exact mechanism by which catecholamines may induce myocardial stunning and regional ballooning patterns is unknown. Some of the potential mechanisms are the presence of rupture plaque on the left anterior descending coronary artery, multi-vessel epicardial spasm, microcirculatory dysfunction, and catecholamine toxicity on cardiomyocytes. Most experts disagree with the theory of ruptured plaque because intravascular imaging studies have not identified ruptured plaques in the ample majority of TTS patients (Figure 2) (29). While other studies have suggested that catecholamines and endothelin may have a role by inducing potent vasoconstriction primarily in the coronary microvasculature by stimulating the endothelin and α1-receptors. In clinical practice, impaired microcirculation has been found in TTS patients assessed by means of the index of microcirculatory resistance (IMR) (30). One of the most accepted mechanisms is the direct effect of catecholamines on cardiomyocytes (31). Endomyocardial biopsies can occasionally report contraction band necrosis, which is typically found in cases of extreme catecholamine production. Ultimately, it is unclear if there is an association between TTS and platelet dysfunction.
Over the past decade, MINOCA has gained attention as an important clinical entity with several clinical unmet needs. In 2017, the European Society of Cardiology (ESC) cardiovascular pharmacotherapy working group published a consensus document to define MINOCA, describing its clinical features and mechanisms and stimulating research into its underlying mechanisms and treatment (16). Moreover, the 2017 ST-segment elevation Myocardial infarction (STEMI) and 2020 Non-ST-segment elevation Myocardial infarction (NSTEMI) ESC guidelines have dedicated a specific section for MINOCA (32, 33). Furthermore, the American Heart Association (AHA) in 2019 published a scientific statement document of MINOCA (1). Ultimately, Bertil et al., have provided an updated review on MINOCA, focusing on the application of different images modalities (34). Table 1 shows a summary of the key recommendations for MINOCA and a selection of its etiologies.
Most of the reported studies have described the association between the clinical outcomes and medical therapy in overall cohorts of MINOCA patients without classifying it into the different etiologies (Table 2). One of the largest cohorts of patients was derived from the SWEDEHEART national registry (5). Between July 2003 and June 2013, the study included 9,466 consecutive patients with MINOCA. Of these, 66.4% were treated with dual antiplatelet therapy (DAPT), the use of which did not impact the composite endpoint of all-cause mortality, hospitalization for myocardial infarction, ischemic stroke, and heart failure. Furthermore, DAPT was not associated with an increase in bleeding events. Although this report has several strengths, there are also important limitations. First, MINOCA etiologies were not reported. Therefore, the specific effect of DAPT on different etiologies cannot be assessed. Second, the type of P2Y12 inhibitor and duration of DAPT was not reported. Ultimately, ascertainment of the primary endpoint measures were derived from the International Classification of Diseases codes.
Two secondary analyses of RCTs have assessed the effect of antiplatelet therapy in MINOCA (Table 2). In the Clopidogrel and Aspirin Optimal Dose Usage to Reduce Recurrent Events–Seventh Organization to Assess Strategies in Ischaemic Symptoms (CURRENT-OASIS 7) trial, a total of 23,783 patients with MI were included, and 1,599 (6.7%) had MINOCA. In the CURRENT-OASIS 7 trial, compared with a standard clopidogrel-based DAPT regimen, an intensified dosing strategy appears to offer no additional benefit with a signal of possible harm. In MINOCA patients, no difference in bleeding events was found (35). Moreover, in the Receptor Suppression Using Integrilin Therapy (PURSUIT) trial, a total of 5,767 patients with NSTEMI were enrolled, and 366 (6%) had MINOCA. Patients with MINOCA did not benefit from treatment with eptifibatide, while patients with obstructive CAD did have a benefit. In MINOCA patients, no increase in bleeding events was found (36).
Several retrospective studies have assessed the effect of secondary prevention on clinical outcomes in patients with MINOCA (37–39). In none of these studies, the treatment with DAPT has been associated with a decrease in major adverse cardiovascular events (MACE).
Overall, the current evidence on the role of antiplatelet in MINOCA patients is derived from registries or secondary analysis of RTCs, and thus of low quality (5, 35–41). However, the available evidence suggests that antiplatelet therapy, mainly DAPT, is not associated with improvement in clinical outcomes. In the Figure 5, we summarize the potential indications of antiplatelet therapy in patients with MINOCA based on the available scientific evidence.
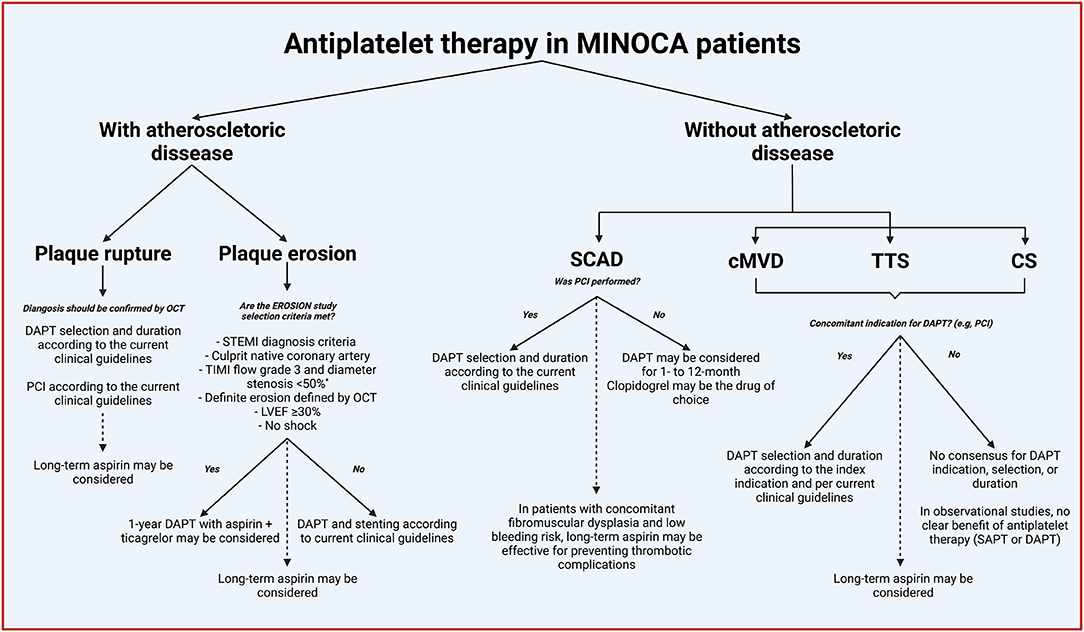
Figure 5. Indications of antiplatelet therapy in patients with MINOCA. Continued lines denoted acute or short-term treatment, and dashed lines indicate long-term (beyond 1-year). *% of coronary diameter of stenosis adapted to match the MINOCA diagnosis criteria. MINOCA, myocardial infarction with non-obstructive coronary arteries; OCT, optical coherence tomography; DAPT, dual antiplatelet therapy; STEMI, ST-segment elevation myocardial infarction; TIMI, Thrombolysis In Myocardial Infarction; EROSION, new in vivo diagnosis and paradigm shift in the treatment of patients with acute coronary syndrome study; LVEF, left ventricular ejection fraction; SCAD, spontaneous coronary artery dissection; PCI, percutaneous cardiac intervention; cMVD, coronary microvascular disease; TTS, Takotsubo syndrome; CS, epicardial coronary spasm; SAPT, single antiplatelet therapy.
The New in Vivo Diagnosis and Paradigm Shift in the Treatment of Patients With Acute Coronary Syndrome (EROSION) study was a pilot study that assessed the role of DAPT without stenting in patients with an MI secondary to plaque erosion documented by means of optical coherence tomography (OCT) (42). At 30-day follow-up, DAPT with aspirin and ticagrelor was associated with a significant reduction in thrombus volume and a low rate of adverse events. Furthermore, at 1-year follow-up, 92.5% of patients with MI caused by plaque erosion managed with DAPT without stenting remained free of major adverse cardiovascular events. One patient had to discontinue DAPT because of gastrointestinal bleeding (43). In the final 4-year report, 21% of the patients underwent target lesion revascularization (TLR) (44). The EROSION study provides compelling data supporting the efficacy of DAPT with aspirin and ticagrelor in eroded plaques. However, this was a pilot non-randomized and open-label study with a surrogate primary endpoint. Therefore, this hypothesis should be properly confirmed in dedicated RCTs powered for clinical outcomes.
The role of DAPT in patients with SCAD is a topic of debate. Some experts advocate that DAPT may increase the risk of bleeding and propagation of the hematoma/dissection plane, whereas others claim that the intimal tear can be prothrombotic and the adjunctive use of clopidogrel on top of aspirin may be justified (45). Data from the “DIssezioni Spontanee COronariche” (DISCO) registry suggests that in patients managed conservatively, DAPT may be associated with worse clinical outcomes compared to single antiplatelet therapy (SAPT) (46). The authors evaluated MACE, defined as all-cause death, non-fatal MI, and any unplanned percutaneous coronary intervention (PCI), at 12 months. DAPT, mainly with aspirin and clopidogrel (63%), was associated with an increase in MACE compared to SAPT with aspirin (93%). This difference was driven by re-infarction and urgent revascularization occurring early after the initial SCAD presentation (Table 2). Currently, there are no randomized clinical trials on this topic, and the available evidence suffers from the limitations inherent to retrospective registries.
Although vasospastic angina has been a topic of continuous research, specific data of MINOCA secondary to coronary vasospasm are limited. Lin et al. reported a systematic review and meta-analysis assessing the role of low-dose aspirin in patients with vasospastic angina without significant CAD (47). The authors included six studies evaluating the outcomes in 3,661 patients. The primary endpoint was MACE, defined as cardiac death, acute coronary syndrome (ACS) and hospitalization due to unstable angina, PCI, symptomatic arrhythmia, appropriate implantable cardioverter-defibrillator, and shock. Aspirin was not associated with a reduction in MACE. However, the quality of the included studies was low, as none was a randomized clinical trial. Moreover, there was very high heterogeneity in the treatment effect within the included studies.
The role of DAPT in coronary vasospasm was evaluated prospectively in the multicenter VA-Korea registry (48). The investigators compared the effect of DAPT with aspirin and clopidogrel vs. aspirin alone on MACE. The primary endpoint was time to composite events of all-cause death, ACS, and symptomatic arrhythmia at 3-year follow-up. Patients treated with DAPT had worse clinical outcomes compared to those treated with aspirin (Table 2). Patients who presented with ACS and smokers had a higher risk of cardiovascular events. However, because this is non-randomized, the possibility of remaining confounding by indication cannot be ruled out.
To date, there are mixed findings with regards to the role of antiplatelet therapy in TTS. In a single-center retrospective registry of TTS patients evaluating the in-hospital clinical outcomes, the authors found that DAPT with aspirin and clopidogrel was associated with a lower incidence of MACE during hospitalization (Table 2) (49). MACE was defined as in-hospital heart failure, in-hospital death, stroke, or respiratory failure requiring mechanical ventilation. Nevertheless, the small sample size and the retrospective methodology are significant limitations to be considered.
On the other hand, in a systematic review and meta-analysis including almost two thousand patients, DAPT was associated with an increase in cardiovascular events and mortality (50). Bleeding rates were not reported. However, the quality of the included studies is low, as no randomized trials were included.
The role of antiplatelet therapy in MINOCA continues to be poorly understood. Currently, most of the scientific evidence and guidelines recommendations are supported by low-quality studies. Of note, there is no RCT assessing the role of antiplatelet therapy in the whole cohort of MINOCA patients nor any of its specific etiologies. In clinical practice, most of the MINOCA management is based and extrapolated from studies of patients with obstructive CAD. RCT in the domain of MINOCA is eagerly needed to determine the role of antiplatelet therapy.
In Table 3, we identified current gaps in evidence and proposed potential research strategies that may help provide meaningful data on the role of antiplatelet therapy in MINOCA patients. First, mechanistic studies evaluating platelet function in each type of MINOCA etiology may provide a better understanding of the relationship of the platelet with the disease and identify potential therapeutic targets. Second, prospective clinical studies reporting the use of antiplatelet therapy (type, timing, and duration) and its association with clinical outcomes according to the different etiologies could help assess the effect of antiplatelet therapy in MINOCA patients. Third, dedicated RCTs are crucially needed for evaluating the safety and efficacy of oral and parenteral antiplatelet agents across the different etiologies.
In the past years, the scientific community has dedicated efforts to increasing the awareness and refining the diagnosis workflow of MINOCA. However, in the coming years, research will be dedicated to identifying the underlying mechanism and determining the safety and efficacy of potential therapies. Indeed, a shift of current research efforts and priorities from the diagnosis to the treatment area is necessary for improving the prognosis in patients with MINOCA. Table 4 summarizes the ongoing RCTs in which antiplatelet agents are tested in patients with MINOCA. One of the most interesting trials is the Randomized Study of Beta-Blockers and Antiplatelets in Patients With Spontaneous Coronary Artery Dissection (BA-SCAD) trial (51). The investigator will randomize 600 patients in a 2 × 2 factorial design evaluating the safety and safety of beta-blockers and DAPT in patients with SCAD. The compared DAPT regimens consist of 1- vs. 12- month strategies initiated on the day of randomization. Of note, only patients conservatively managed are allowed to be randomized in the DAPT stratum. In the short antiplatelet arm, aspirin monotherapy for 1-month is the recommended regimen. However, the use of DAPT for a period no longer than 1-month is allowed. In the prolonged arm, DAPT with aspirin and clopidogrel for 1-year, followed by aspirin alone, is recommended. Even though ticagrelor and prasugrel are preferred over clopidogrel in ACS patients according to practice guidelines, there are no data for their use in SCAD (3, 51), Nevertheless, in the BA-SCAD trial, the choice of P2Y12 inhibitor is at the discretion of the treating physician in line with current clinical guidelines (3).
The role of platelets in the pathophysiology of MINOCA is not entirely understood. Although in some of its causes such as plaque disruption, the role of platelets in the pathophysiological mechanism is well-described, in other etiologies such as coronary artery vasospasm or TTS, this relationship remains unclear. Nevertheless, the description of these mechanisms is critical for identifying potential therapeutic targets. The current low-quality evidence suggests that antiplatelet therapy may not be associated with better clinical outcomes in MINOCA patients. Especially in non-atherosclerotic causes, the utility of antiplatelet agents is questionable. However, in the absence of dedicated RCTs, the role of antiplatelet therapy in MINOCA is a matter of debate, and the current recommendations for antiplatelet therapy are based on registries and expert opinion. The identification of underlying mechanisms linking the different MINOCA etiologies with platelet function is necessary. Ultimately, dedicated RCTs to assess the safety and efficacy of antiplatelet agents in MINOCA patients and specific etiologies are warranted.
LO-P and DJA contributed to conception and design of the review and wrote the first draft of the manuscript. MG, DC, and SB wrote sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
DC declares that he has received consulting and speaker's fee from Amgen, Arena, Biotronik, Daiichi Sankyo, Sanofi, Terumo, outside the present work. SB declares that he is consultant from Boston Scientific and iVascular and he received speaker's fee from Abbott Vascular, outside the present work. DJA declares that he has received consulting fees or honoraria from Abbott, Amgen, Aralez, AstraZeneca, Bayer, Biosensors, Boehringer Ingelheim, Bristol-Myers Squibb, Chiesi, Daiichi-Sankyo, Eli Lilly, Haemonetics, Janssen, Merck, PhaseBio, PLx Pharma, Pfizer, Sanofi, and The Medicines Company and has received payments for participation in review activities from CeloNova and St Jude Medical. DJA also declares that his institution has received research grants from Amgen, AstraZeneca, Bayer, Biosensors, CeloNova, CSL Behring, Daiichi-Sankyo, Eisai, Eli Lilly, Gilead, Idorsia, Janssen, Matsutani Chemical Industry Co., Merck, Novartis, Osprey Medical, Renal Guard Solutions, and the Scott R. MacKenzie Foundation.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
LO-P was supported by a grant from the Spanish Society of Cardiology (SEC/MHE-MOV-INT 20/001). BioRender platform and templates were used for creating the figures.
ACS, acute coronary syndrome; ADP, adenosine diphosphate; AHA, American Heart Association; CAD, coronary artery disease; CURRENT-OASIS 7, clopidogrel and aspirin optimal dose usage to reduce recurrent events–seventh organization to assess strategies in ischaemic symptoms; DAPT, dual antiplatelet therapy; EROSION, new in vivo diagnosis and paradigm shift in the treatment of patients with acute coronary syndrome study; ESC, European Society of Cardiology; GP, glycoprotein; IIb/IIIa, αIIβ3 integrin; IMR, index of microcirculatory resistance; MACE, major adverse cardiovascular events; MI, myocardial infarction; MINOCA, myocardial infarction with non-obstructive coronary arteries; NETs, neutrophil extracellular traps; NSTEMI, non-ST-segment elevation myocardial infarction; OCT, optical coherence tomography; PCI, percutaneous coronary intervention; PURSUIT, receptor suppression using integrilin therapy trial; RCTs, randomized controlled trials; SAPT, single antiplatelet therapy; SCAD, spontaneous coronary artery dissection; STEMI, ST-segment elevation myocardial infarction; TIMI, thrombolysis in myocardial infarction; TTS, takotsubo syndrome; TXA2, thromboxane A2; vWF, von Willebrand factor.
1. Tamis-Holland JE, Jneid H, Reynolds HR, Agewall S, Brilakis ES, Brown TM, et al. Contemporary diagnosis and management of patients with myocardial infarction in the absence of obstructive coronary artery disease: a scientific statement from the American Heart Association. Circulation. (2019) 139:e891–908. doi: 10.1161/CIR.0000000000000670
2. Gehrie ER, Reynolds HR, Chen AY, Neelon BH, Roe MT, Gibler WB, et al. Characterization and outcomes of women and men with non-ST-segment elevation myocardial infarction and nonobstructive coronary artery disease: results from the Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes with Early Implementation of the ACC/AHA Guidelines (CRUSADE) quality improvement initiative. Am Heart J. (2009) 158:688–94. doi: 10.1016/j.ahj.2009.08.004
3. Valgimigli M, Bueno H, Byrne RA, Collet JP, Costa F, Jeppsson A, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. (2018) 39:213–60. doi: 10.1093/eurheartj/ehx419
4. Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, et al. Fourth universal definition of myocardial infarction. Circulation. (2018) 138:e618–e51. doi: 10.1161/CIR.0000000000000617
5. Lindahl B, Baron T, Erlinge D, Hadziosmanovic N, Nordenskjold A, Gard A, et al. Medical therapy for secondary prevention and long-term outcome in patients with myocardial infarction with nonobstructive coronary artery disease. Circulation. (2017) 135:1481–9. doi: 10.1161/CIRCULATIONAHA.116.026336
6. Schaar JA, Muller JE, Falk E, Virmani R, Fuster V, Serruys PW, et al. Terminology for high-risk and vulnerable coronary artery plaques. Report of a meeting on the vulnerable plaque, June 17 and 18, 2003, Santorini, Greece. Eur Heart J. (2004) 25:1077–82. doi: 10.1016/j.ehj.2004.01.002
7. Davi G, Patrono C. Platelet activation and atherothrombosis. N Engl J Med. (2007) 357:2482–94. doi: 10.1056/NEJMra071014
8. Brass LF. Thrombin and platelet activation. Chest. (2003) 124(3 Suppl.):18S−25S. doi: 10.1378/chest.124.3_suppl.18S
9. Angiolillo DJ, Ueno M, Goto S. Basic principles of platelet biology and clinical implications. Circ J. (2010) 74:597–607. doi: 10.1253/circj.CJ-09-0982
10. Varga-Szabo D, Pleines I, Nieswandt B. Cell adhesion mechanisms in platelets. Arterioscler Thromb Vasc Biol. (2008) 28:403–12. doi: 10.1161/ATVBAHA.107.150474
11. Brummel KE, Paradis SG, Butenas S, Mann KG. Thrombin functions during tissue factor-induced blood coagulation. Blood. (2002) 100:148–52. doi: 10.1182/blood.V100.1.148
12. Angiolillo DJ, Capodanno D, Goto S. Platelet thrombin receptor antagonism and atherothrombosis. Eur Heart J. (2010) 31:17–28. doi: 10.1093/eurheartj/ehp504
13. Fahed AC, Jang IK. Plaque erosion and acute coronary syndromes: phenotype, molecular characteristics and future directions. Nat Rev Cardiol. (2021) 18:724–34. doi: 10.1038/s41569-021-00542-3
14. Sato Y, Hatakeyama K, Yamashita A, Marutsuka K, Sumiyoshi A, Asada Y. Proportion of fibrin and platelets differs in thrombi on ruptured and eroded coronary atherosclerotic plaques in humans. Heart. (2005) 91:526–30. doi: 10.1136/hrt.2004.034058
15. Zakai NA, McClure LA. Racial differences in venous thromboembolism. J Thromb Haemost. (2011) 9:1877–82. doi: 10.1111/j.1538-7836.2011.04443.x
16. Agewall S, Beltrame JF, Reynolds HR, Niessner A, Rosano G, Caforio AL, et al. ESC working group position paper on myocardial infarction with non-obstructive coronary arteries. Eur Heart J. (2017) 38:143–53. doi: 10.1093/eurheartj/ehw149
17. Jaffe R, Charron T, Puley G, Dick A, Strauss BH. Microvascular obstruction and the no-reflow phenomenon after percutaneous coronary intervention. Circulation. (2008) 117:3152–6. doi: 10.1161/CIRCULATIONAHA.107.742312
18. Aurigemma C, Scalone G, Tomai F, Altamura L, De Persio G, Stazi A, et al. Persistent enhanced platelet activation in patients with acute myocardial infarction and coronary microvascular obstruction: clinical implications. Thromb Haemost. (2014) 111:122–30. doi: 10.1160/TH13-02-0166
19. Saw J, Humphries K, Aymong E, Sedlak T, Prakash R, Starovoytov A, et al. Spontaneous coronary artery dissection: clinical outcomes and risk of recurrence. J Am Coll Cardiol. (2017) 70:1148–58. doi: 10.1016/j.jacc.2017.06.053
20. Kim ESH. Spontaneous coronary-artery dissection. N Engl J Med. (2020) 383:2358–70. doi: 10.1056/NEJMra2001524
21. Taqueti VR, Di Carli MF. Coronary microvascular disease pathogenic mechanisms and therapeutic options: JACC state-of-the-art review. J Am Coll Cardiol. (2018) 72:2625–41. doi: 10.1016/j.jacc.2018.09.042
22. Ge L, Zhou X, Ji WJ, Lu RY, Zhang Y, Zhang YD, et al. Neutrophil extracellular traps in ischemia-reperfusion injury-induced myocardial no-reflow: therapeutic potential of DNase-based reperfusion strategy. Am J Physiol Heart Circ Physiol. (2015) 308:H500–9. doi: 10.1152/ajpheart.00381.2014
23. Sanchis J, Garcia-Blas S, Ortega-Paz L, Dantas AP, Rodriguez E, Abellan L, et al. Cell-free DNA and microvascular damage in ST-segment elevation myocardial infarction treated with primary percutaneous coronary intervention. Rev Esp Cardiol. (2019) 72:317–23. doi: 10.1016/j.recesp.2018.02.020
24. Kaski JC, Maseri A, Vejar M, Crea F, Hackett D. Spontaneous coronary artery spasm in variant angina is caused by a local hyperreactivity to a generalized constrictor stimulus. J Am Coll Cardiol. (1989) 14:1456–63. doi: 10.1016/0735-1097(89)90382-3
25. Beltrame JF, Psaltis PJ. The forgotten vascular layer in the forgotten coronary disorder. J Am Coll Cardiol. (2018) 71:426–8. doi: 10.1016/j.jacc.2017.10.095
26. Tanaka A, Taruya A, Shibata K, Fuse K, Katayama Y, Yokoyama M, et al. Coronary artery lumen complexity as a new marker for refractory symptoms in patients with vasospastic angina. Sci Rep. (2021) 11:13. doi: 10.1038/s41598-020-79669-1
27. Miyamoto S, Ogawa H, Soejima H, Takazoe K, Kajiwara I, Shimomura H, et al. Enhanced platelet aggregation in the coronary circulation after coronary spasm. Thromb Res. (2001) 103:377–86. doi: 10.1016/S0049-3848(01)00333-4
28. Ghadri JR, Wittstein IS, Prasad A, Sharkey S, Dote K, Akashi YJ, et al. International Expert Consensus Document on Takotsubo Syndrome (part i): clinical characteristics, diagnostic criteria, and pathophysiology. Eur Heart J. (2018) 39:2032–46. doi: 10.1093/eurheartj/ehy076
29. Eitel I, Stiermaier T, Graf T, Moller C, Rommel KP, Eitel C, et al. Optical coherence tomography to evaluate plaque burden and morphology in patients with takotsubo syndrome. J Am Heart Assoc. (2016) 5:1–10. doi: 10.1161/JAHA.116.004474
30. Jimenez Britez G, Sabate M, Robles C, Garcia-Granja PE, Amat-Santos IJ, Brugaletta S. Functional and morphological assessment of left anterior descending artery in patients with tako-tsubo syndrome. Rev Esp Cardiol. (2018) 71:986–8. doi: 10.1016/j.recesp.2017.08.008
31. Wittstein IS. Stress cardiomyopathy: a syndrome of catecholamine-mediated myocardial stunning? Cell Mol Neurobiol. (2012) 32:847–57. doi: 10.1007/s10571-012-9804-8
32. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. (2018) 39:119–77. doi: 10.1093/eurheartj/ehx393
33. Collet JP, Thiele H, Barbato E, Barthelemy O, Bauersachs J, Bhatt DL, et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. (2021) 42:1289–367. doi: 10.1093/eurheartj/ehaa575
34. Lindahl B, Baron T, Albertucci M, Prati F. Myocardial infarction with non-obstructive coronary artery disease. EuroIntervention. (2021) 17:e875–87. doi: 10.4244/EIJ-D-21-00426
35. Bossard M, Gao P, Boden W, Steg G, Tanguay JF, Joyner C, et al. Antiplatelet therapy in patients with myocardial infarction without obstructive coronary artery disease. Heart. (2021) 107:1739–47. doi: 10.1136/heartjnl-2020-318045
36. Roe MT, Harrington RA, Prosper DM, Pieper KS, Bhatt DL, Lincoff AM, et al. Clinical and therapeutic profile of patients presenting with acute coronary syndromes who do not have significant coronary artery disease.The Platelet Glycoprotein IIb/IIIa in Unstable Angina: Receptor Suppression Using Integrilin Therapy (PURSUIT) Trial Investigators. Circulation. (2000) 102:1101–6. doi: 10.1161/01.CIR.102.10.1101
37. Kovach CP, Hebbe A, O'Donnell CI, Plomondon ME, Hess PL, Rahman A, et al. Comparison of patients with nonobstructive coronary artery disease with versus without myocardial infarction (from the VA clinical assessment reporting and tracking [CART] Program). Am J Cardiol. (2021) 146:1–7. doi: 10.1016/j.amjcard.2021.01.015
38. Ciliberti G, Verdoia M, Merlo M, Zilio F, Vatrano M, Bianco F, et al. Pharmacological therapy for the prevention of cardiovascular events in patients with myocardial infarction with non-obstructed coronary arteries (MINOCA): insights from a multicentre national registry. Int J Cardiol. (2021) 327:9–14. doi: 10.1016/j.ijcard.2020.11.040
39. Abdu FA, Liu L, Mohammed AQ, Xu B, Yin G, Xu S, et al. Effect of secondary prevention medication on the prognosis in patients with myocardial infarction with nonobstructive coronary artery disease. J Cardiovasc Pharmacol. (2020) 76:678–83. doi: 10.1097/FJC.0000000000000918
40. Montenegro Sa F, Carvalho R, Santos L, Ruivo C, Antunes A, Belo A, et al. Dual antiplatelet therapy in myocardial infarction with non-obstructive coronary artery disease - insights from a nationwide registry. Rev Port Cardiol. (2020) 39:679–84. doi: 10.1016/j.repc.2020.05.008
41. Paolisso P, Bergamaschi L, Saturi G, D'Angelo EC, Magnani I, Toniolo S, et al. Secondary prevention medical therapy and outcomes in patients with myocardial infarction with non-obstructive coronary artery disease. Front Pharmacol. (2019) 10:1606. doi: 10.3389/fphar.2019.01606
42. Jia H, Dai J, Hou J, Xing L, Ma L, Liu H, et al. Effective anti-thrombotic therapy without stenting: intravascular optical coherence tomography-based management in plaque erosion (the EROSION study). Eur Heart J. (2017) 38:792–800. doi: 10.1093/eurheartj/ehw381
43. Xing L, Yamamoto E, Sugiyama T, Jia H, Ma L, Hu S, et al. EROSION Study (effective anti-thrombotic therapy without stenting: intravascular optical coherence tomography-based management in plaque erosion): a 1-year follow-up report. Circ Cardiovasc Interv. (2017) 10:1–8. doi: 10.1161/CIRCINTERVENTIONS.117.005860
44. He L, Qin Y, Xu Y, Hu S, Wang Y, Zeng M, et al. Predictors of non-stenting strategy for acute coronary syndrome caused by plaque erosion: four-year outcomes of the EROSION study. EuroIntervention. (2021) 17:497–505. doi: 10.4244/EIJ-D-20-00299
45. Saw J, Mancini GBJ, Humphries KH. Contemporary review on spontaneous coronary artery dissection. J Am Coll Cardiol. (2016) 68:297–312. doi: 10.1016/j.jacc.2016.05.034
46. Cerrato E, Giacobbe F, Quadri G, Macaya F, Bianco M, Mori R, et al. Antiplatelet therapy in patients with conservatively managed spontaneous coronary artery dissection from the multicentre DISCO registry. Eur Heart J. (2021) 42:3161–71. doi: 10.1093/eurheartj/ehab372
47. Lin Y, Chen Y, Yuan J, Qin H, Dong S, Chen Q. Impact of aspirin use on clinical outcomes in patients with vasospastic angina: a systematic review and meta-analysis. BMJ Open. (2021) 11:e048719. doi: 10.1136/bmjopen-2021-048719
48. Cho SS, Jo SH, Han SH, Lee KY, Her SH, Lee MH, et al. Clopidogrel plus aspirin use is associated with worse long-term outcomes, but aspirin use alone is safe in patients with vasospastic angina: results from the VA-Korea registry, a prospective multi-center cohort. Sci Rep. (2019) 9:17783. doi: 10.1038/s41598-019-54390-w
49. Dias A, Franco E, Koshkelashvili N, Bhalla V, Pressman GS, Hebert K, et al. Antiplatelet therapy in Takotsubo cardiomyopathy: does it improve cardiovascular outcomes during index event? Heart Vessels. (2016) 31:1285–90. doi: 10.1007/s00380-015-0729-2
50. Rizzetto F, Lia M, Widmann M, Tavella D, Zanolla L, Pighi M, et al. Prognostic impact of antiplatelet therapy in Takotsubo syndrome: a systematic review and meta-analysis of the literature. Heart Fail Rev. (2021). doi: 10.1007/s10741-021-10099-5. [Epub ahead of print].
51. Alfonso F, de la Torre Hernandez JM, Ibanez B, Sabate M, Pan M, Gulati R, et al. Rationale and design of the BA-SCAD (Beta-blockers and antiplatelet agents in patients with spontaneous coronary artery dissection) randomized clinical trial. Rev Esp Cardiol. (2021). doi: 10.1016/j.rec.2021.08.003. [Epub ahead of print].
52. Gulati M, Levy PD, Mukherjee D, Amsterdam E, Bhatt DL, Birtcher KK, et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR Guideline for the Evaluation and Diagnosis of Chest Pain: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. (2021) 144:e368–454. doi: 10.1161/CIR.0000000000001030
53. Ghadri JR, Wittstein IS, Prasad A, Sharkey S, Dote K, Akashi YJ, et al. International expert consensus document on Takotsubo Syndrome (part II): diagnostic workup, outcome, and management. Eur Heart J. (2018) 39:2047–62. doi: 10.1093/eurheartj/ehy077
54. Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. (2020) 41:407–77. doi: 10.1093/eurheartj/ehz425
55. Park JY, Rha SW, Poddar KL, Ramasamy S, Chen KY, Li YJ, et al. Impact of low-dose aspirin on coronary artery spasm as assessed by intracoronary acetylcholine provocation test in Korean patients. J Cardiol. (2012) 60:187–91. doi: 10.1016/j.jjcc.2012.02.007
56. Serpytis R, Majauskiene E, Navickas P, Lizaitis M, Glaveckaite S, Rucinskas K, et al. Randomized pilot trial on optimal treatment strategy, myocardial changes, and prognosis of patients with myocardial infarction with nonobstructive coronary arteries (MINOCA). Am J Med. (2021). 135:103–9. doi: 10.1016/j.amjmed.2021.08.023
Keywords: antiplatelet therapy, dual anti-platelet therapy, coronary artery disease non-obstructive, myocardial infarction, atherosclerotic plaque, coronary vasospasm, microvascular disease, spontaneous coronary artery dissection
Citation: Ortega-Paz L, Galli M, Capodanno D, Brugaletta S and Angiolillo DJ (2022) The Role of Antiplatelet Therapy in Patients With MINOCA. Front. Cardiovasc. Med. 8:821297. doi: 10.3389/fcvm.2021.821297
Received: 24 November 2021; Accepted: 17 December 2021;
Published: 14 February 2022.
Edited by:
Josip A. Borovac, University of Split, CroatiaReviewed by:
Aleksandra Ga̧secka, Medical University of Warsaw, PolandCopyright © 2022 Ortega-Paz, Galli, Capodanno, Brugaletta and Angiolillo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dominick J. Angiolillo, ZG9taW5pY2suYW5naW9saWxsb0BqYXgudWZsLmVkdQ==; orcid.org/0000-0001-8451-2131
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.