
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med., 04 February 2022
Sec. Heart Failure and Transplantation
Volume 8 - 2021 | https://doi.org/10.3389/fcvm.2021.815595
This article is part of the Research TopicNovel Phenotyping and Risk Stratification Strategies for Heart FailureView all 16 articles
 Qi Guo1†
Qi Guo1† Yiwei Lai1†
Yiwei Lai1† Jianmin Chu2
Jianmin Chu2 Xuhua Chen2
Xuhua Chen2 Mingyang Gao1
Mingyang Gao1 Caihua Sang1
Caihua Sang1 Jianzeng Dong1
Jianzeng Dong1 Jielin Pu2,3*
Jielin Pu2,3* Changsheng Ma1*
Changsheng Ma1*Low-density lipoprotein receptor-related protein 6 (LRP6) plays a critical role in cardiovascular homeostasis. The deficiency of LRP6 is associated with a high risk of arrhythmias. However, the association between genetic variations of LRP6 and sudden cardiac death (SCD) remains unknown. This study aims to explore the association between common variants of LRP6 and the prognosis of chronic heart failure (CHF) patients. From July 2005 to December 2009, patients with CHF were enrolled from 10 hospitals in China. The single-nucleotide polymorphism (SNP) rs2302684 was selected for the evaluation of the effect of LRP6 polymorphisms on the survival in patients with CHF. A total of 1,437 patients with CHF were finally included for the analysis. During a median follow-up of 61 months (range 0.4–129 months), a total of 546 (38.0%) patients died, including 201 (36.8%) cases with SCD and 345 (63.2%) cases with non-SCD. Patients carrying A allele of rs2302684 had an increased risk of all-cause death (adjusted HR 1.452, 95% CI 1.189–1.706; P < 0.001) and SCD (adjusted HR 1.783, 95% CI 1.337–2.378; P < 0.001). Therefore, the SNP rs2302684 T>A in LRP6 indicated higher risks of all-cause death and SCD in patients with CHF. LRP6 could be added as a novel predictor of SCD and might be a potential therapeutic target in the prevention of SCD in the CHF population.
Chronic heart failure (CHF), which may be caused by ischemic cardiomyopathy (ICM) or non-ischemic cardiomyopathy (NICM), is one of the chief causes of morbidity and mortality worldwide (1, 2). It currently affects more than five million Americans and the prevalence is expected to increase by 25% within the next 15 years (3). This heart failure pandemic is also evident in Asia and China (4). The predominant modes of death in CHF patients are pump failure and sudden cardiac death (SCD) (5). Sudden cardiac death, caused by malignant ventricular tachycardia (VT) or ventricular fibrillation (VF), remains a primary cause of mortality in patients with CHF (6). Therefore, the prediction and prevention of SCD play critical roles in the management of the CHF population. So far, even several factors have been known as potential predictors of SCD, including biomarkers, hemodynamic status, and electrophysiological parameters, the sensitivity and specificity are not powerful (7).
Low-density lipoprotein (LDL) receptor-related protein 6 (LRP6) is a single-pass transmembrane protein, which contains four extracellular epidermal growth factor-like repeats and three LDL receptor repeats (8). It is recognized as a coreceptor for the Wnt signaling cascade and plays a critical role in regulating Wnt signaling (9, 10). Work to date has identified that dysregulated Wnt signaling conduces to a high incidence of arrhythmias associated with various forms of heart disease (11). Furthermore, accumulating evidence reveals the significant effect of LRP6 on cardiovascular health and homeostasis (12). Additionally, LRP6 was mainly allocated within the gap junction of cardiomyocytes (13). However, the potential relation between genetic variations of LRP6 and SCD has not yet been reported in previous studies. In the present study, we examined the association between common variants of LRP6 and the prognosis in patients with CHF.
From July 2005 to December 2009, patients with CHF were enrolled from 10 hospitals in China. Details for the cohort have been described previously (9, 14–16). Inclusion criteria include: (a) CHF caused by ICM or idiopathic dilated cardiomyopathy (DCM); (b) classification of the New York Heart Association (NYHA) was II–IV with optimizing drug therapy; and (c) left ventricular ejection fraction (LVEF) ≤ 50% in ICM or ≤ 45% in DCM (9, 14–16). Ischemic cardiomyopathy was diagnosed as ≥70% luminal stenosis of one or more major coronary arteries diagnosed by coronary angiography with a myocardial infarction history at least 3 months before the enrolment. DCM was diagnosed consistently with the guidelines of familial DCM (17). Excluded criteria include: (a) pacemaker dependency; (b) unable to perform the genotyping; and (c) pregnancy, terminal illnesses, or other uncontrollable system diseases (9, 14–16).
The study was approved by the Ethics Committee of Beijing Anzhen Hospital and Fuwai Hospital (Beijing, China), and complied with the principles of the Declaration of Helsinki. Written informed consent was obtained from all the enrolled patients who reported themselves as Chinese Han nationality.
All the participants were followed up periodically until August 2017 during regular outpatient clinics or by transtelephonic visits. The endpoints included all-cause death, SCD (ICD appropriate discharge was regarded as SCD), and non-SCD (NSCD) (heart transplantation was regarded as non-SCD). Sudden cardiac death was defined as an unexpected death within 1 h of onset of acute symptoms attributable to cardiac causes or an unwitnessed death of someone last seen in a stable condition in 24 h without evidence of non-cardiac causes (7). If there were discrepancies between the first two reviewers, the event was adjudicated by a third investigator to provide the final classification.
Tag single-nucleotide polymorphisms (SNPs) were selected by the pairwise tagging method from the HapMap CHB databank (HapMap Data Rel 24 PhaseII, Nov08, on NCBI B36 assembly, dbSNP b126) via the tag SNPs' online software (http://hapmap.ncbi.nlm.nih.gov/cgi-perl/gbrowse/hapmap24_B36/#search). Common variants were defined as a minor allele frequency (MAF) > 0.05, with a linkage disequilibrium (LD) measure r2 threshold of 0.8. Forty-three tag SNPs that covered the entire LRP6 gene were selected. To reduce the false-positive caused by multiple tests, with 43 candidate SNPs, P < 0.001 was considered statistically significant for SNP selection. Polymerase chain reactions (PCRs) were performed firstly in 100 subjects with CHF. There were no significant associations between SNPs and clinical endpoints other than the SNP rs2302684 T>A. Thus, rs2302684 was finally selected to perform the PCRs in the whole study population for the analysis.
Genomic DNA was extracted from peripheral blood leukocytes of the participants and stored at −70°C after determination of absorbance at 260 nm followed by Picogreen analysis (Molecular Probes, Eugene, Oegon, USA) (18).
Primers were designed by Primer Premier 5.0 software as follows: forward TTGATGATGCTCCTGTAA and reverse TATTCTTGGCCTTGTTCT (328 bp). PCR amplification was performed with the Geneamp PCR system 9700 (Applied Biosystems). An initial 4 min cycle at 94°C was followed by 35 thermal cycles at 94°C for 30 s, 47°C for 30 s, and 72°C for 30 s, and ended with a 10 min extension at 72°C. Each reaction mixture (30 μl) contained 3 μl 10 × PCR buffer, 0.5 μl dNTP-mix (10 mmol/L), 0.5 μl of each primer (10 μmol/L), 0.5 μl Taq polymerase (5 U/μl; Takara Bio), 1 μl genomic DNA (50 ng/μl), and 24 μl double-distilled H2O.
PCR products were sequenced after purification by ABI 3130XL DNA Analyzer System (Applied Biosystems, USA). Repeat genotyping was carried out in 95 (5.0%) random duplicate samples to affirm the reproducibility was 100% (Figure 1).
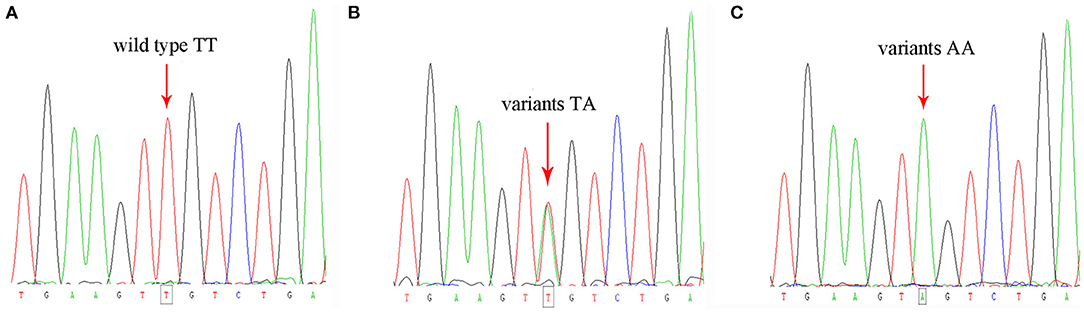
Figure 1. Genotyping of the studied population showing the wild type (A) and the variants of rs2302684 T>A (B,C).
Continuous variables were presented as the mean ± SD and compared by Student's test. Categorical variables were presented as numbers and percentages and compared by chi-square analysis. A P-value of <0.05 was considered statistically significant. Linkage disequilibrium of rs2302684 was analyzed by Haploview4.2, and Hardy-Weinberg equilibrium of alleles was analyzed by chi-square analysis with 1 degree of freedom. Survival analysis was performed in CHF patients. Cox proportional hazards models were performed under three different models (dominant, recessive, additive models) to evaluate the effects of genotype on survival. Kaplan-Meier curve was applied to describe survival freedom from events and multivariate cox proportional hazards models were used to adjust for confounding factors. The covariates were selected according to clinical significance and baseline data, including age, sex, NYHA levels, LVEF, ischemic etiology, and other variables with a P-value of <0.2 in the baseline. Statistical analyses were conducted using the IBM SPSS 26.0 software.
A total of 1,437 patients (age 60.55 ± 11.98 years, 1,134 males) with CHF were finally enrolled for the analysis, including 957 patients with ICM and 480 patients with DCM. The mean LVDD of the participants was 63.27 ± 9.77 mm, and the mean LVEF was 36.04 ± 8.80%. Among them, 43.0% of patients had the NYHA function of level II, 32.6% of patients had the NYHA function of level III, and the other 24.4% patients had the NYHA function of level IV. The mean BMI of the participants was 24.84 ± 3.84 kg/m2. The clinical characteristics were summarized in Table 1.
In the studied CHF population, 443 patients (age 61.41 ± 11.79 years, 340 males) carried A allele of SNP rs2302684. The patients carrying A allele of SNP rs2302684 comprised a larger percentage of patients with ICM than those carrying the wild type of TT (71.6 vs. 64.4%, P = 0.009). Furthermore, CHF patients with A allele of rs2302684 had a tendency to the higher classification of NYHA level than those without (NYHA II, 38.8 vs. 44.9%; NYHA III, 33.2 vs. 32.4%; NYHA IV, 28 vs. 22.7%; P = 0.047). However, no significant differences were demonstrated in age, sex distribution, BMI, LVDD, LVEF, complications, the prevalence of arrhythmias, and medication between the patients with rs2302684 wild type of TT and those with A allele (Table 1).
During a median follow-up of 61 months (range 0.4–129 months) in 1,437 participants with CHF, a total of 546 (38.0%) patients died, including 348 patients with ICM and 198 patients with DCM. Among them, 201 (36.8%) cases had SCD, including 129 cases with ICM and 72 cases with DCM. The rest of 345 (63.2%) cases had NSCD.
The correlation of mortality and A allele of rs2302684 were analyzed by survival cox regression analysis in the CHF cohort under the dominant, recessive, and additive models, respectively. The effect of the A allele on mortality was significant under different models (Table 2). Under the dominant model, the risks of all-cause death (HR 1.45, 95% CI 1.21–1.73; P < 0.001) and SCD (HR 1.85, 95% CI 1.39–2.45; P < 0.001) increased significantly in patients with A allele of rs2302684. After adjusted for age, sex, ischemic etiology, NYHA levels, LVEF, BMI, and the use of β-blocker, the associations remained significant in all-cause death (HR 1.43, 95% CI 1.20–1.72; P < 0.001) and SCD (HR 1.80, 95% CI 1.34–2.40; P < 0.001). The Kaplan-Meier curves made under the dominant models were shown in Figure 2. Thus, SNP rs2302684 T>A indicated a higher risk of all-cause death and SCD but not NSCD in the CHF patients.
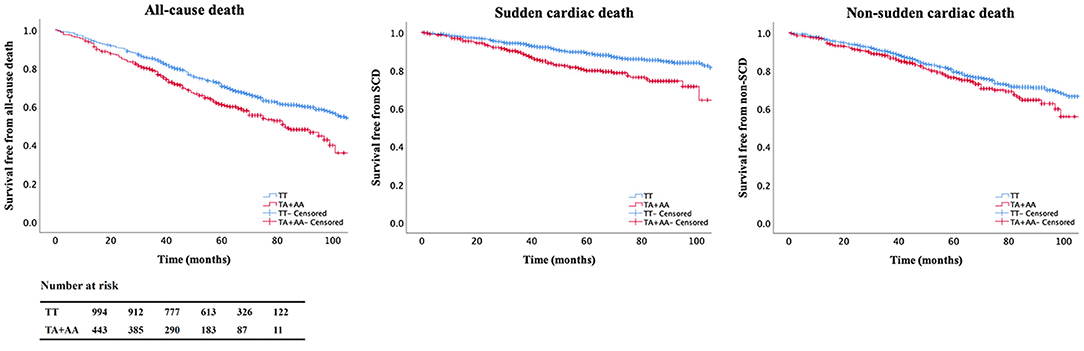
Figure 2. Kaplan-Meier curves in the chronic heart failure (CHF) cohort. Patients carrying A allele of rs2302684 were more vulnerable to all-cause death and sudden cardiac death (SCD) than those without it. The table denotes the number of patients at risk for every 20 months of the follow-up.
The effect of A allele of rs2302684 on the mortality endpoints was generally consistent across the selected subgroups, including different age (P for interaction = 0.268 for all-cause mortality and 0.189 for SCD), sex (P for interaction = 0.838 for all-cause mortality and 0.183 for SCD), and ischemic etiology (P for interaction = 0.473 for all-cause mortality and 0. 664 for SCD) (Figure 3).
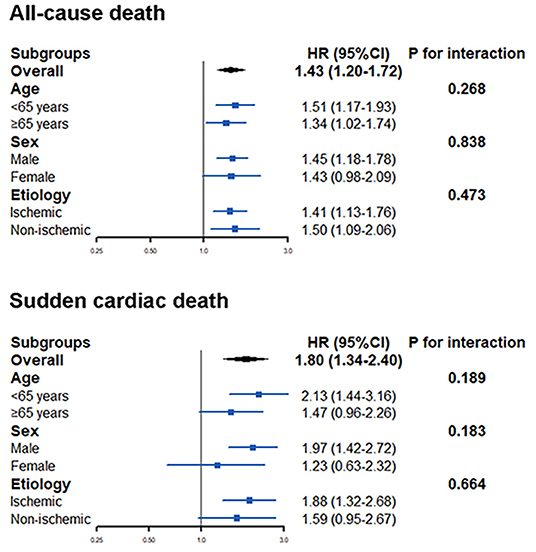
Figure 3. The effect of A allele of rs2302684 on all-cause mortality and sudden cardiac death in different subgroups, including different ages, sex, and etiology.
In this prospective study of 1,437 patients with CHF in the Chinese Han nationality, the associations of SNP rs2302684 T>A in LRP6 and long-term clinical endpoints were explored. The A allele of rs2302684 was recognized as an independent risk factor and predictor of all-cause death and SCD in the CHF population. To the best of our knowledge, this is the first study to show the association between common variants in LRP6 gene with the different causes of mortality in patients with CHF.
The human LRP6 gene, which is located in chromosome 12 p11–p13, has 150 kb in length with 23 exons (8, 19). It encodes a member of the low-density lipoprotein receptor family, which is composed of cell surface proteins that participate in receptor-mediated endocytosis of specific ligands (8, 19). LRP6 gene is homologous to LRP5 and has large extracellular domains consisting of four β-propeller motifs followed by three LDL ligand-binding domains, which regulate the binding process between Wnt secretory protein and frizzled family of receptors (10, 20). LRP6 function as coreceptors for Wnt ligands and thus play a central role in Wnt/β-catenin signaling involved in a wide variety of biologic processes (12, 21).
Wnts are a family of secreted glycoproteins that participate in activating several signaling pathways (22). It could bind to a class of Frizzled receptors or LRP6 to downregulate the glycogen synthase kinase-3β (GSK-3β) activity and to initiate the canonical Wnt/β-catenin signaling cascade (22). Wnt/β-catenin signaling is a crucial regulator of tissue development and homeostasis, especially in cardiac differentiation and development (11, 20). The conserved Wnt cascade has been confirmed to control the proliferation, differentiation and polarity of cells (23–25). Abnormal signaling disturbs tissue growth and function, which could lead to a number of debilitating and terminal diseases (20). Thus, alterations in the LRP6 gene might affect Wnt/β-catenin signaling and lead to several human diseases including osteoporosis, Alzheimer's disease, coronary artery disease, and metabolic disease (20, 26–31).
Wnt signaling is critical in cardiac development and various cardiac pathologies, including cardiac hypertrophy and fibrosis, myocardial infarction, heart failure, and arrhythmias (30, 32, 33). It has been reported that abnormalities of Wnt signaling were an important cause of familial sudden death in patients with ARVC (34). Additionally, a recent genome-wide association study showed that WNT8A was associated with atrial fibrillation (35).
Mutations in LRP6 could dysregulate Wnt signaling and have been associated with numerous human diseases (12, 20). The rs2302684 is an intron variant. No association has been reported between this tag SNP of LRP6 and the prognosis of patients with CHF in the Chinese Han population. Our study revealed a close relationship between LRP6 common variants and SCD in the CHF group. According to previous researches, the potential reasons for this relationship might have two aspects. On one hand, LRP6 serves as a scaffold protein that regulates the cardiac gap junction assembly. LRP6 deficiency might impair the dynamics of connexin43 protein trafficking and stability, which disrupts gap junction formation and function. The proper functioning of gap junctions is essential in the generation and propagation of cardiac action potentials. Thus, the disrupting connexin43 expression or phosphorylation caused by LRP6 deficiency impaired the electrical communication in gap junctions and led to the initiation and maintenance of arrhythmias (33, 36, 37). It was reported that defective connexin43 gap junctions in LRP6-ablated mouse hearts induced VT and VF (13). On the other hand, many studies demonstrated that Wnt signaling was linked to cardiac fibrosis which could impede electrical wave propagation and potentially cause arrhythmias (38–40). Furthermore, a recent study reported that LRP6 played an important role in keeping the integrity of the intercalated disk, on which the coordinated excitation and contractile performance of the myocardium were dependent, and the interaction between LRP6 and connexin43 might be involved in this process (41). Therefore, LRP6 variants might cause malignant arrhythmias and SCD via disturbing Wnt signaling pathways, as well as disrupting the function of gap junction and the intercalated disk of the myocardium.
It was reported that LRP6 was dramatically decreased in heart tissues with DCM (42). Additionally, LRP6 is genetically linked to early coronary artery disease and hyperlipemia (20, 43, 44). It has been demonstrated that mutant LRP6 was associated with atherosclerosis. The underlying mechanism might be as follows: firstly, LRP6 is critical in LDL receptor-mediated LDL uptake, which is significant in atherosclerosis; secondly, LRP6 plays an important role in metabolic regulation, including lipid homeostasis and glucose metabolism, thus it is associated with atherosclerosis; lastly, mutant LRP6 could trigger atherosclerosis by activating platelet-derived growth factor (PDGF)-dependent vascular smooth muscle cell differentiation (31, 45). Considering the close relationship of LRP6, DCM, and atherosclerosis, we analyzed the association of LRP6 with the mortality endpoints in different etiologies of CHF. In our study, the effect of rs2302684 A allele in LRP6 on the mortality endpoint was consistent in patients with different CHF reasons, including ICM and DCM. Therefore, we found that LRP6 variants were associated with a higher risk of all-cause death and SCD in the CHF cohort attributed to both ICM and DCM.
There are several limitations to the study. The lack of functional research in this work is one of the limitations. In this study, we only found the association between the SNP of LRP6 and SCD in CHF patients via gene tests, however, the exact mechanism of how the SNP affects the heart is still unknown. We only speculate the possible mechanism according to existing studies, and we still need further functional studies to explore the underlying mechanism in our future work. Additionally, ICD recordings were not collected in the present cohort, and only a limited number of patients had a history of ventricular arrhythmias (VAs). Therefore, we are not able to deepen our investigation about the association between VAs and the SNP of interest.
The study firstly demonstrated that LRP6 rs2302684 polymorphism is associated with increased risks of all-cause death and SCD in CHF patients in the Chinese Han population. Therefore, LRP6 could be regarded as an independent risk factor and a novel predictor of SCD, and it might provide a potential therapeutic target in SCD prevention.
The datasets presented in this article are not readily available because the hospital does not permit the authors to publicize the database. Requests to access the datasets should be directed to the corresponding author/s.
The studies involving human participants were reviewed and approved by Anzhen Hospital and Fuwai Hospital. The patients/participants provided their written informed consent to participate in this study.
QG, YL, JP, and CM is responsible for the conception and design of the study. QG conducted the selection of the SNP. QG and MG conducted the telephone interview. JC and XC conducted the in-person interview. QG and YL was responsible for the statistical analysis and drafted the manuscript. JD and CS revised it critically for important intellectual content. JP and CM critically appraised the manuscript and approved the final version. All authors have the access to all of the data, read the manuscript, and agreed to be accountable for all aspects of the work.
This work was supported by the National Key Research and Development Program of China (2016YFC0900901) to CM and the National Basic Research Program of China (2013CB531105) to JP.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
CHF, chronic heart failure; DCM, dilated cardiomyopathy; ICM, ischemic cardiomyopathy; LRP6, low-density lipoprotein receptor-related protein 6; LVEF, left ventricular ejection fraction; PCR, polymerase chain reactions; SCD, sudden cardiac death.
1. Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, et al. Heart disease and stroke statistics−2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. (2009) 119:e21–181. doi: 10.1161/CIRCULATIONAHA.108.191261
2. Roger VL, Weston SA, Redfield MM, Hellermann-Homan JP, Killian J, Yawn BP, et al. Trends in heart failure incidence and survival in a community-based population. JAMA. (2004) 292:344–50. doi: 10.1001/jama.292.3.344
3. Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, et al. Executive summary: heart disease and stroke statistics−2014 update: a report from the American Heart Association. Circulation. (2014) 129:399–410. doi: 10.1161/01.cir.0000442015.53336.12
4. Shimokawa H, Miura M, Nochioka K, Sakata Y. Heart failure as a general pandemic in Asia. Eur J Heart Fail. (2015) 17:884–92. doi: 10.1002/ejhf.319
5. Korngold EC, Januzzi JL Jr, Gantzer ML, Moorthy MV, Cook NR, Albert CM. Amino-terminal pro-B-type natriuretic peptide and high-sensitivity C-reactive protein as predictors of sudden cardiac death among women. Circulation. (2009) 119:2868–76. doi: 10.1161/CIRCULATIONAHA.108.832576
6. Linde C, Daubert C. Cardiac resynchronization therapy in patients with New York Heart Association class I and II heart failure: an approach to 2010. Circulation. (2010) 122:1037–43. doi: 10.1161/CIRCULATIONAHA.109.923094
7. European Heart Rhythm A, Heart Rhythm S, Zipes DP, Camm AJ, Borggrefe M, Buxton AE, et al. ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to develop guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death). J Am Coll Cardiol. (2006) 48:e247–346. doi: 10.1016/j.jacc.2006.07.010
8. Brown SD, Twells RC, Hey PJ, Cox RD, Levy ER, Soderman AR, et al. Isolation and characterization of LRP6, a novel member of the low density lipoprotein receptor gene family. Biochem Biophys Res Commun. (1998) 248:879–88. doi: 10.1006/bbrc.1998.9061
9. Pinson KI, Brennan J, Monkley S, Avery BJ, Skarnes WC. An LDL-receptor-related protein mediates Wnt signalling in mice. Nature. (2000) 407:535–8. doi: 10.1038/35035124
10. Tamai K, Semenov M, Kato Y, Spokony R, Liu C, Katsuyama Y, et al. LDL-receptor-related proteins in Wnt signal transduction. Nature. (2000) 407:530–5. doi: 10.1038/35035117
11. Ai Z, Fischer A, Spray DC, Brown AM, Fishman GI. Wnt-1 regulation of connexin43 in cardiac myocytes. J Clin Invest. (2000) 105:161–71. doi: 10.1172/JCI7798
12. Kang S. Low-density lipoprotein receptor-related protein 6-mediated signaling pathways and associated cardiovascular diseases: diagnostic and therapeutic opportunities. Hum Genet. (2020) 139:447–59. doi: 10.1007/s00439-020-02124-8
13. Li J, Li C, Liang D, Lv F, Yuan T, The E, et al. LRP6 acts as a scaffold protein in cardiac gap junction assembly. Nat Commun. (2016) 7:11775. doi: 10.1038/ncomms11775
14. Pei J, Li N, Chen J, Li X, Zhang Y, Wang Z, et al. The predictive values of beta1-adrenergic and M2 muscarinic receptor autoantibodies for sudden cardiac death in patients with chronic heart failure. Eur J Heart Fail. (2012) 14:887–94. doi: 10.1093/eurjhf/hfs082
15. Liu X, Pei J, Hou C, Liu N, Chu J, Pu J, et al. A common NOS1AP genetic polymorphism, rs12567209 G>A, is associated with sudden cardiac death in patients with chronic heart failure in the Chinese Han population. J Card Fail. (2014) 20:244–51. doi: 10.1016/j.cardfail.2014.01.006
16. Yu H, Pei J, Liu X, Chen J, Li X, Zhang Y, et al. Calcium channel autoantibodies predicted sudden cardiac death and all-cause mortality in patients with ischemic and nonischemic chronic heart failure. Dis Markers. (2014) 2014:796075. doi: 10.1155/2014/796075
17. Fatkin D. members of the CCGDCWG. Guidelines for the diagnosis and management of familial dilated cardiomyopathy. Heart Lung Circ. (2011) 20:691–3. doi: 10.1016/j.hlc.2011.07.008
18. Ran Y, Chen J, Li N, Zhang W, Feng L, Wang R, et al. Common RyR2 variants associate with ventricular arrhythmias and sudden cardiac death in chronic heart failure. Clin Sci (Lond). (2010) 119:215–23. doi: 10.1042/CS20090656
19. Houston DW, Wylie C. Cloning and expression of Xenopus Lrp5 and Lrp6 genes. Mech Dev. (2002) 117:337–42. doi: 10.1016/s0925-4773(02)00205-8
20. Joiner DM, Ke J, Zhong Z, Xu HE, Williams BO. LRP5 and LRP6 in development and disease. Trends Endocrinol Metab. (2013) 24:31–9. doi: 10.1016/j.tem.2012.10.003
21. Kokubu C, Heinzmann U, Kokubu T, Sakai N, Kubota T, Kawai M, et al. Skeletal defects in ringelschwanz mutant mice reveal that Lrp6 is required for proper somitogenesis and osteogenesis. Development. (2004) 131:5469–80. doi: 10.1242/dev.01405
22. Dieckmann M, Dietrich MF, Herz J. Lipoprotein receptors–an evolutionarily ancient multifunctional receptor family. Biol Chem. (2010) 391:1341–63. doi: 10.1515/BC.2010.129
23. Dierick H, Bejsovec A. Cellular mechanisms of wingless/Wnt signal transduction. Curr Top Dev Biol. (1999) 43:153–90. doi: 10.1016/s0070-2153(08)60381-6
24. Kikuchi A. Regulation of beta-catenin signaling in the Wnt pathway. Biochem Biophys Res Commun. (2000) 268:243–8. doi: 10.1006/bbrc.1999.1860
25. Miller JR, Hocking AM, Brown JD, Moon RT. Mechanism and function of signal transduction by the Wnt/beta-catenin and Wnt/Ca2+ pathways. Oncogene. (1999) 18:7860–72. doi: 10.1038/sj.onc.1203245
26. Mani A, Radhakrishnan J, Wang H, Mani A, Mani MA, Nelson-Williams C, et al. LRP6 mutation in a family with early coronary disease and metabolic risk factors. Science. (2007) 315:1278–82. doi: 10.1126/science.1136370
27. Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. (2009) 120:1640–5. doi: 10.1161/CIRCULATIONAHA.109.192644
28. De Ferrari GV, Papassotiropoulos A, Biechele T, Wavrant De-Vrieze F, Avila ME, Major MB, et al. Common genetic variation within the low-density lipoprotein receptor-related protein 6 and late-onset Alzheimer's disease. Proc Natl Acad Sci USA. (2007) 104:9434–9. doi: 10.1073/pnas.0603523104
29. Cheng SL, Ramachandran B, Behrmann A, Shao JS, Mead M, Smith C, et al. Vascular smooth muscle LRP6 limits arteriosclerotic calcification in diabetic LDLR−/− mice by restraining noncanonical Wnt signals. Circ Res. (2015) 117:142–56. doi: 10.1161/CIRCRESAHA.117.306712
30. Guo J, Li Y, Ren YH, Sun Z, Dong J, Yan H, et al. Mutant LRP6 impairs endothelial cell functions associated with familial normolipidemic coronary artery disease. Int J Mol Sci. (2016) 17:1173. doi: 10.3390/ijms17071173
31. Liu W, Mani S, Davis NR, Sarrafzadegan N, Kavathas PB, Mani A. Mutation in EGFP domain of LDL receptor-related protein 6 impairs cellular LDL clearance. Circ Res. (2008) 103:1280–8. doi: 10.1161/CIRCRESAHA.108.183863
32. Basheer WA, Harris BS, Mentrup HL, Abreha M, Thames EL, Lea JB, et al. Cardiomyocyte-specific overexpression of the ubiquitin ligase Wwp1 contributes to reduction in Connexin 43 and arrhythmogenesis. J Mol Cell Cardiol. (2015) 88:1–13. doi: 10.1016/j.yjmcc.2015.09.004
33. Dawson K, Aflaki M, Nattel S. Role of the Wnt-Frizzled system in cardiac pathophysiology: a rapidly developing, poorly understood area with enormous potential. J Physiol. (2013) 591:1409–32. doi: 10.1113/jphysiol.2012.235382
34. Gollob MH, Blier L, Brugada R, Champagne J, Chauhan V, Connors S, et al. Recommendations for the use of genetic testing in the clinical evaluation of inherited cardiac arrhythmias associated with sudden cardiac death: Canadian Cardiovascular Society/Canadian Heart Rhythm Society joint position paper. Can J Cardiol. (2011) 27:232–45. doi: 10.1016/j.cjca.2010.12.078
35. Ellinor PT, Lunetta KL, Albert CM, Glazer NL, Ritchie MD, Smith AV, et al. Meta-analysis identifies six new susceptibility loci for atrial fibrillation. Nat Genet. (2012) 44:670–5. doi: 10.1038/ng.2261
36. Jansen JA, van Veen TA, de Jong S, van der Nagel R, van Stuijvenberg L, Driessen H, et al. Reduced Cx43 expression triggers increased fibrosis due to enhanced fibroblast activity. Circ Arrhythm Electrophysiol. (2012) 5:380–90. doi: 10.1161/CIRCEP.111.966580
37. Burstein B, Comtois P, Michael G, Nishida K, Villeneuve L, Yeh YH, et al. Changes in connexin expression and the atrial fibrillation substrate in congestive heart failure. Circ Res. (2009) 105:1213–22. doi: 10.1161/CIRCRESAHA.108.183400
38. Ye B, Ge Y, Perens G, Hong L, Xu H, Fishbein MC, et al. Canonical Wnt/beta-catenin signaling in epicardial fibrosis of failed pediatric heart allografts with diastolic dysfunction. Cardiovasc Pathol. (2013) 22:54–7. doi: 10.1016/j.carpath.2012.03.004
39. Duan J, Gherghe C, Liu D, Hamlett E, Srikantha L, Rodgers L, et al. Wnt1/betacatenin injury response activates the epicardium and cardiac fibroblasts to promote cardiac repair. EMBO J. (2012) 31:429–42. doi: 10.1038/emboj.2011.418
40. Laeremans H, Rensen SS, Ottenheijm HC, Smits JF, Blankesteijn WM. Wnt/frizzled signalling modulates the migration and differentiation of immortalized cardiac fibroblasts. Cardiovasc Res. (2010) 87:514–23. doi: 10.1093/cvr/cvq067
41. Wang X, Zou Y, Li Y, Chen Z, Yin C, Wang Y, et al. Lipoprotein receptor-related protein 6 is required to maintain intercalated disk integrity. Genes Cells. (2019) 24:789–800. doi: 10.1111/gtc.12727
42. Chen Z, Li Y, Wang Y, Qian J, Ma H, Wang X, et al. Cardiomyocyte-restricted low density lipoprotein receptor-related protein 6 (LRP6) deletion leads to lethal dilated cardiomyopathy partly through Drp1 signaling. Theranostics. (2018) 8:627–43. doi: 10.7150/thno.22177
43. Xu S, Cheng J, Chen YN Li K, Ma ZW, Cen JM, et al. The LRP6 rs2302685 polymorphism is associated with increased risk of myocardial infarction. Lipids Health Dis. (2014) 13:94. doi: 10.1186/1476-511X-13-94
44. Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. (2012) 149:1192–205. doi: 10.1016/j.cell.2012.05.012
Keywords: LRP6, single-nucleotide polymorphism, chronic heart failure, prognosis, sudden cardiac death
Citation: Guo Q, Lai Y, Chu J, Chen X, Gao M, Sang C, Dong J, Pu J and Ma C (2022) LRP6 Polymorphisms Is Associated With Sudden Cardiac Death in Patients With Chronic Heart Failure in the Chinese Han Population. Front. Cardiovasc. Med. 8:815595. doi: 10.3389/fcvm.2021.815595
Received: 15 November 2021; Accepted: 15 December 2021;
Published: 04 February 2022.
Edited by:
Tong Liu, Tianjin Medical University, ChinaReviewed by:
Jinzhu Hu, Nanchang University, ChinaCopyright © 2022 Guo, Lai, Chu, Chen, Gao, Sang, Dong, Pu and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jielin Pu, amllbGlucHVkZkBzaW5hLmNvbQ==; Changsheng Ma, Y2hzaG1hQHZpcC5zaW5hLmNvbQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.