
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 28 January 2022
Sec. Cardiac Rhythmology
Volume 8 - 2021 | https://doi.org/10.3389/fcvm.2021.805399
This article is part of the Research Topic Risk Stratification Strategies for Cardiac Rhythm Abnormalities View all 49 articles
Background: We have proposed the Taiwan AF score consisting of age, male sex, hypertension, heart failure, coronary artery disease, end-stage renal disease, and alcoholism to predict incident atrial fibrillation (AF) in Asian population. We hypothesized that the modified Taiwan AF score (mTaiwan AF score) excluding alcoholism remained useful for predicting new onset AF.
Methods: A total of 7,220,654 subjects aged ≥ 40 years without a past history of cardiac arrhythmia were identified from a national cohort, and 438,930 incident AF occurred during a 16-year follow-up with an incidence of 0.42 per 100 person-years. The mTaiwan AF score ranging between −2 and 14 and its predictive accuracy of incident AF was analyzed.
Results: The areas under the receiver operating characteristic curve (AUCs) of the mTaiwan AF scores in predicting AF are 0.861 for 1-year follow-up, 0.829 for 5-year follow-up, 0.795 for 10-year follow-up, and 0.751 for 16-year follow-up. The risk of incident AF increased from 0.05%/year for patients with a score of −2 to 6.98%/year for those having a score of 14. Patients were classified into three groups based on the tertile values of the mTaiwan AF scores—group 1 (score −2-3), group 2 (score 4-9) and group 3 (score 10-14). The annual risks of incident AF were 0.20, 1.33, and 3.36% for group 1, 2, and 3, respectively. Compared to patients in group 1, the hazard ratios of incident AF were 5.79 [95% confidence interval (CI) 3.75-7.75] for group 2 and 8.93 (95% CI 6.47-10.80) for group 3.
Conclusions: We demonstrated that the mTaiwan AF score based on age and clinical comorbidities could be used to predict incident AF in Asian population.
AF is a worldwide epidemic (1) with significant effects on morbidity and mortality (2, 3). With the trend of worldwide aging, improved diagnostic tools and better public realization, the predicted prevalence of AF keeps rising substantially (4, 5), and there is no exception for Asians (2, 3, 6). Even through, the prevalence of AF might still be underestimated based on some real-world observations and device studies (5). Therefore, it is important to efficiently identify subjects with a potential risk of AF development and to employ more aggressive strategies for cardiac rhythm screening, so that a prompt diagnosis and associated interventions can be done in time. Several risk schemes are developed for the prediction of new onset AF, and most of them were developed from non-Asian population (7–9). Recently, we have proposed a clinical scheme, the Taiwan AF score, to predict the risk of incident AF based on the national cohort analysis including 7,220,654 subjects aged ≥ 40 years (10). The Taiwan AF score included age, male sex and important comorbidities [hypertension, heart failure (HF), coronary artery disease (CAD), end-stage renal disease (ESRD), and alcoholism], and is a straightforward scheme obviating the use of personal information, electrocardiogram and echocardiography data (10). However, the factor “alcoholism” is somewhat difficult to be accurately quantified, which might prevent this scheme from extensive clinical use. Therefore, the present study aims to investigate whether a modified Taiwan AF score (mTaiwan AF score) excluding “alcoholism” can be used for AF prediction in Asian population with long-term follow-up.
This study used the “National Health Insurance Research Database (NHIRD)” provided by Health and Welfare Data Science Center (HWDC), Ministry of Health and Welfare (MOHW), Taiwan. The National Health Insurance (NHI) system is a mandatory universal health insurance program providing comprehensive medical care coverage to all Taiwanese residents. NHIRD consists of detailed data of health care from January 1st, 1996, to December 31st, 2016, from >23 million enrollees, representing >99% of Taiwan's population. In this cohort dataset, patients' original identification numbers have been encrypted to protect their privacy, and the encrypting procedure was consistent, so that linkage of the claims belonging to the same patient was feasible. Therefore, patients can be followed continuously within the NHI database. The details about Taiwan NHIRD have been reported in our previous studies (3, 10–17). The present study was approved by the Institutional Review Board at Taipei Veterans General Hospital, Taipei, Taiwan.
The study design was the same as our previous study (10). In general, a total of 7,220,654 patients aged ≥ 40 years without a history of cardiac arrhythmias from January 1st, 2000 to December 31st, 2000 were identified from Taiwan NHIRD. Important comorbidities of each individual were confirmed based on the International Classification of Diseases (ICD), Ninth Revision, Clinical Modification (ICD-9-CM) codes from the NHIRD. The diagnostic accuracies of important comorbidities in NHIRD have been validated (18, 19). AF was confirmed using the ICD-9-CM code (427.31) registered by the physicians responsible for the care of patients. The diagnostic accuracy of AF based on ICD-9-CM code in Taiwan NHIRD has been validated previously (20). During a 16-year follow-up, 438,930 patients had incident AF with an incidence of 0.42 per 100 person-years.
The development of the original Taiwan AF score followed the TRIPOD (Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis) Statement (21) and the details have been described in our previous study (10). Potential variables were identified from the Cox proportional hazards modeling and forced into an initial saturated Cox proportional hazards model. An α level of 0.1 from the saturated model was used as a threshold to enter a variable predictor into a backward elimination model. β coefficients were derived from the final Cox regression model and used to calculate the score weights of each significant predictor in the multivariable Cox regression based on the method proposed by Sullivan et al. (22). The score weight for each predictor was rounded to its closest integer as the score point. Age, male sex, hypertension, HF, CAD, ESRD, and alcoholism were thus identified with different score weight and together constitute the Taiwan AF score ranging between −2 and 15 (Table 1). In the present study, we excluded alcoholism from the prediction scheme and proposed the mTaiwan AF (Table 1). The incidence of AF (%/year) after 1-year, 3-year, 5-year, 7-year, 10-year, 12-year, and 16-year follow-up for each mTaiwan AF score was calculated. Patients were classified into three groups based on the tertile values of the mTaiwan AF scores of patients who developed AF—group 1 (score −2-3), group 2 (score 4-9), and group 3 (score 10-14).
The incidence of AF was calculated from dividing the number of events by person-time at risk. The Kaplan-Meier method were used to plot the cumulative incidence curves of AF for different risk groups, with statistical significance examined by the log-rank test. The diagnostic accuracy of the mTaiwan AF score in the prediction of incident AF was assessed by calculating c-indexes, based on the receiver operating characteristic (ROC) curve. All statistical significances were set at p < 0.05 and all statistical analyses were carried out by SPSS 17.0 (SPSS Inc. USA).
The distributions of each mTaiwan AF scores are shown in Figure 1. There were 5,407,576 (74.9%), 1,701,688(23.6%) and 111,390 (1.5%) patients in group 1, 2, and 3, respectively (Figure 1).
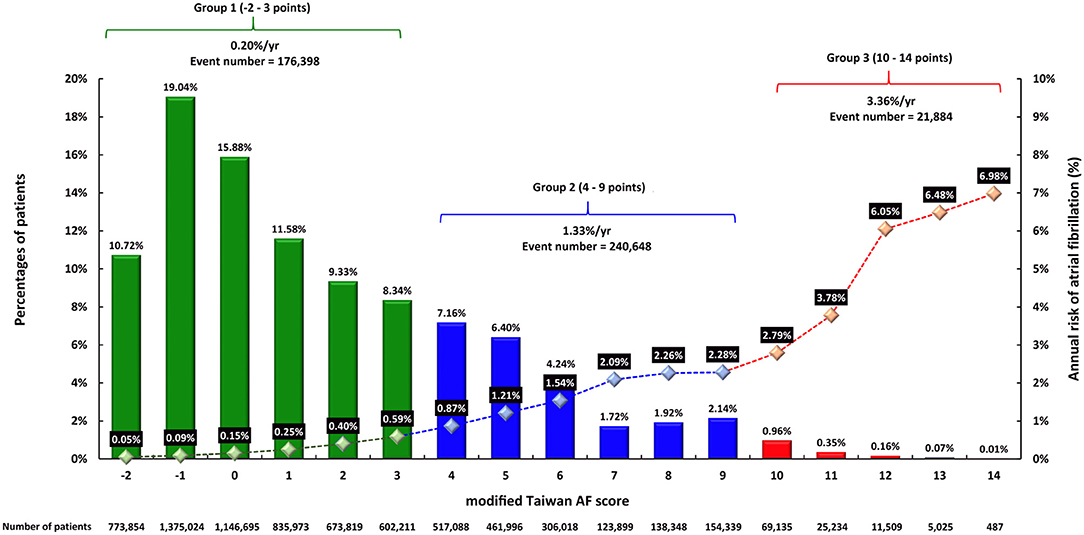
Figure 1. The distributions of mTaiwan AF score and the risks of incident AF during 16-year follow-up. The mTaiwan AF score ranged between −2 and 14. After a 16-year follow-up, the annual risk of incident AF increased from 0.05% for patients with a score of −2 to 6.98% for patients with a score of 14. The annual risks of incident AF were 0.20, 1.33, and 3.36% for groups 1, 2, and 3, respectively. AF, atrial fibrillation.
The areas under the ROC curve (AUCs) of original Taiwan AF score and mTaiwan AF score in predicting incident AF after different follow-up durations are shown in Table 2. The AUCs of the mTaiwan AF scores are 0.861 [95% confidence interval (CI) 0.859-0.862] for 1-year follow-up, 0.829 (95% CI 0.827-0.832) for 5-year follow-up, 0.795 (95% CI 0.793-0.798) for 10-year follow-up and 0.751(95% CI 0.748-0.753) for 16-year follow-up (Figure 2).
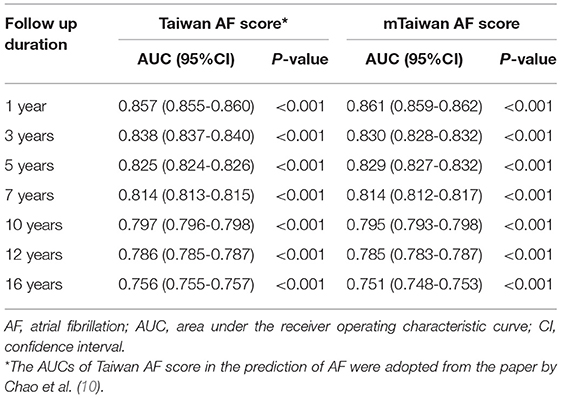
Table 2. AUCs of Taiwan AF score and mTaiwan AF score in the prediction of AF after different follow-up durations.
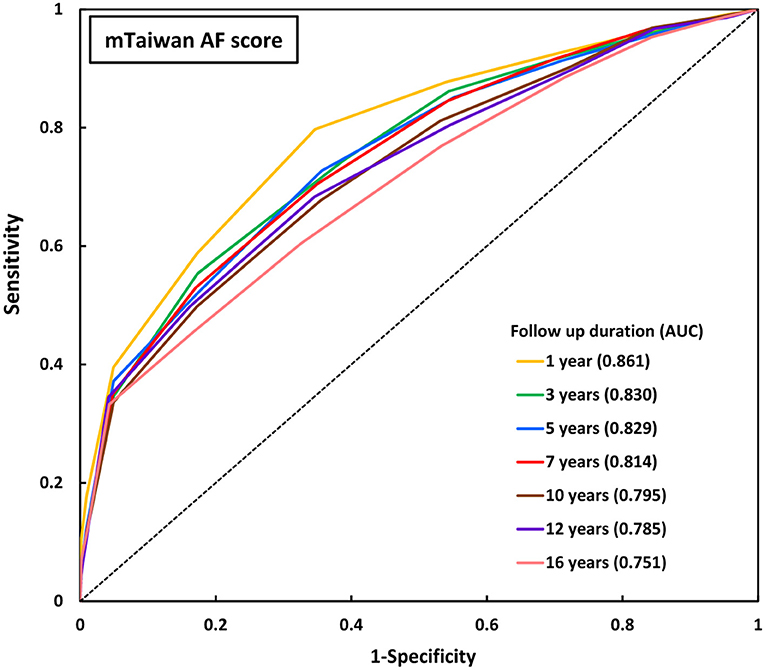
Figure 2. ROC curves of mTaiwan AF score in predicting incident AF. The AUCs of mTaiwan AF score ranged between 0.751 and 0.861 for different follow-up durations. AF, atrial fibrillation; AUC, area under the receiver operating characteristic curve; ROC curve, receiver operating characteristic curve.
The incidences of AF (%/year) of each mTaiwan AF score during different follow-up periods are shown in Table 3. After a 16-year follow-up, the risk of incident AF increased from 0.05%/year for patients with a score of −2 to 6.98%/year for those having a score of 14 (Figure 1). The annual risks of incident AF are 0.20% for group 1, 1.33% for group 2, and 3.36% for group 3, respectively (Figure 1). The cumulative incidence curves of incident AF of groups 1, 2, 3 are shown in Figure 3. The 2-year risks of AF were 0.08, 2.04, and 7.82% for groups 1, 2, 3, respectively. The 4-year risks of AF were 0.31, 4.12, and 13.58% for groups 1, 2, 3, respectively. The 10-year risks of AF were 1.26, 11.13, and 27.89% for groups 1, 2, 3, respectively. Compared to group 1, the hazard ratios (HRs) of incident AF were 5.79 (95% CI 3.75-7.75) for group 2 and 8.93 (95% CI 6.47-10.80) for group 3.
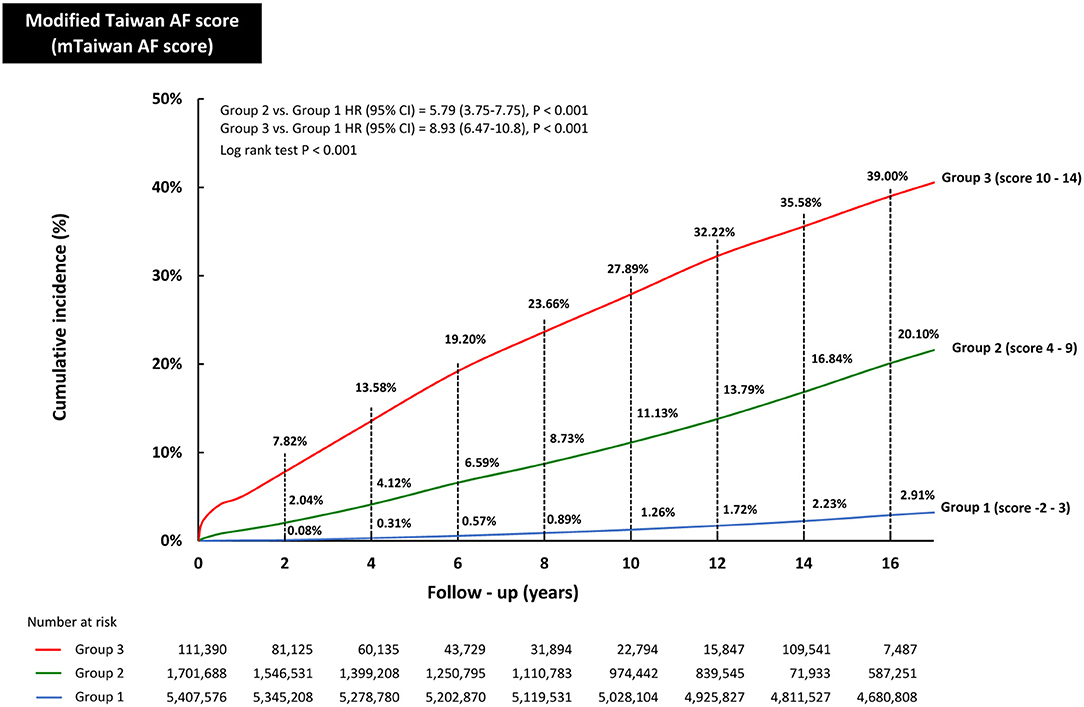
Figure 3. The cumulative incidence curves of developing AF in groups 1, 2, and 3. The 2-year risks of AF were 0.08, 2.04, and 7.82% for groups 1, 2, 3, respectively. The 6-year risks of AF were 0.57, 6.59, and 19.20% for groups 1, 2, 3, respectively. The 10-year risks of AF were 1.26, 11.13, and 27.89% for groups 1, 2, 3, respectively. The 16-year risks of AF were 2.91, 20.10, and 39.00% for groups 1, 2, 3, respectively. Compared to group 1, the HRs of incident AF were 5.79 (95% CI 3.75-7.75) for group 2 and 8.93 (95% CI 6.47-10.80) for group 3. AF, atrial fibrillation; CI, confidence interval; HR, hazard ratio.
In the present study, we proposed the mTaiwan AF score which excluded alcoholism form the original Taiwan AF score for incident AF prediction using a nationwide cohort including 7,220,654 subjects with 438,930 incident AF during a 16-year follow-up. We confirmed the usefulness of this modified scheme to predict incident AF for Asian population.
Risk factors for incident AF have been identified since long ago and results are somewhat variable across different studies. Potential reasons underlying the differences of reported risk factors remain unknown. Common risk factors include baseline demographics, underlying comorbidities, and cardiac structural abnormalities, such as age, male gender, obesity, hypertension, diabetes, and increased left ventricular wall thickness (23). Although the age distribution of AF differs between regions (5), the increase in AF prevalence with advancing age seems to be a worldwide phenomenon (5). Gender difference in AF incidence is also a consistent observation because a higher prevalence of AF in men than in women has been observed in most studies (5). Hypertension is the most common medical condition associated with AF worldwide, affecting 29-78% of patients with AF and there is no significant variation in the risk of AF associated with hypertension according to ethnic group (24–26). Therefore, hypertension is widely accepted to predispose individuals to AF (5), and so are CAD and HF (5, 7). Patients with ESRD had significantly increased risk of AF, which even increased farther together with other risk factors. The reported incidences of AF in patients with ESRD ranged from 1 to 14.8% depending on the co-existence of other risk factors (27). Risk factors other than age, sex, and comorbidities included race, height, personal habits, hemodynamic parameters, cardiac murmurs, electrocardiogram or echocardiographic parameters, but inconsistency remains between different stratification schemes (7–9). Nevertheless, observation from FHS and CHARG-AF scores may imply that a risk stratification scheme incorporating clinical factors but not electrocardiogram and echocardiographic parameters might provide satisfactory accuracy for predicting incident AF (7, 9).
Alcohol consumption is ubiquitous in Western countries (28), and has been defined as light (<7 standard drinks/week), moderate (7-21 standard drinks/week), and heavy (>21 standard drinks/week) alcohol consumption, where 1 standard drink is approximately 12 g of alcohol (28). The association of alcohol and AF was first brought into attention as “holiday heart syndrome” in patients hospitalized with AF following a weekend binge (29), and more studies have been conducted on their association since then. Alcohol has complex effects to both cardiac structures and electrophysiological remodeling, and possible pathophysiological mechanisms underlying the association between alcohol and AF include direct toxicity and alcohol's contribution to obesity, sleep-disordered breathing, and hypertension (28). Alcohol could be a trigger for AF and facilitate progressive atrial remodeling with regular long-term consumption, leading to an arrhythmogenic substrate (28).
Moderate habitual consumption increases the incidence of AF in a dose-dependent but non-linearly manner, with relative risks of AF 1.08 for 7 standard drinks/week, 1.17 for 14 standard drinks/week, 1.26 for 21 standard drinks/week, 1.36 for 28 standard drinks/week, and 1.47 for 35 standard drinks/week (30–33). Heavy habitual consumptions with ≥40 standard drinks/week might be a more important risk factor than hypertension or obesity (34). Despite all the findings about alcohol and AF in real-world observations, many of the individual studies were underpowered to demonstrate a strong relationship, and some disagreements about details of alcoholism exist. For example, one meta-analysis showed that only wine and liquor, but not beer, were associated with incident AF in those consuming >14 standard drinks/week, while a community-based pooled cohort reported similar associations across different types of alcohol (33). There remains a conflict whether men and women were equally affected with alcohol consumptions (31, 35). Furthermore, most studies determined the quantity of alcohol consumption by self-reporting, rather than objective blood or urine samples, which raises the concern of precise quantification. Besides, the pattern and amount of alcohol consumption might be variable from time to time and thus it's difficult to determine the presence and degrees of habitual consumption. All conditions mentioned above highlighted the difficulty in defining “alcoholism” in clinical practice.
For easier and more extensive clinical application, we tested the accuracy of mTaiwan AF score which excluded alcoholism from the original Taiwan AF score for the prediction of incident AF in the present study. The AUCs of the mTaiwan AF score were 0.861 for 1-year follow up, 0.829 for 5-year follow up, 0.795 for 10-year follow up and 0.751 for 16-year follow up, which were quite similar to that of Taiwan AF score (0.857 for 1-year follow up, 0.825 for 5-year follow up, 0.797 for 10-year follow up, and 0.756 for 16-year follow up). Therefore, we demonstrated that the mTaiwan AF score without the consideration of alcoholism still provides reliable accuracy for the prediction of incident AF and could be easily applied in the clinical practice to replace Taiwan AF score when accurate information regarding alcoholism was not available.
In the present study, we validated the use of the mTaiwan AF score, derived from the original Taiwan AF score, in the prediction of incident AF. Since both the Taiwan AF score and mTaiwan AF score were based on the same cohort, it is expectable that the performance of mTaiwan AF score would not differ significantly from that of the original score. More studies are necessary to further validate the mTaiwan AF score in external cohorts.
Based on our prior publication (10), we developed a modified clinical risk scoring scheme, the mTaiwan AF score (−2 to 14), to stratify individual risk of new-onset AF. This modified scheme is feasible and reliable for clinical assessment and can easily identify high-risk population in whom a more proactive screening strategy for AF should be taken into consideration.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
The studies involving human participants were reviewed and approved by Institutional Review Board, Taipei Veterans General Hospital. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
J-NL is responsible for manuscript drafting. S-SL is responsible for creation of tables and figures. T-JC is responsible for the resources of database. T-CT is responsible for critical revision. S-AC is responsible for study idea and conceptualization. T-FC is responsible for study idea and organizing the whole article. All authors contributed to the article and approved the submitted version.
This work was supported in part by grants from the Ministry of Science and Technology (MOST 107-2314-B-075-062-MY3, MOST 110-2314-B-075-059, MOST 109-2314-B-075A-011-MY3, MOST 110-2314-B-075A-014-MY3, MOST 110-2321-B-075A-001, MOST 110-2745-B-075A-001), Taipei Veterans General Hospital (V108B-015, V108B-027, V108C-090, V109C-042, V109C-186), Taichung Veterans General Hospital (TCVGH-1113-101C), Research Foundation of Cardiovascular Medicine and Szu-Yuan Research Foundation of Internal Medicine, Taipei, Taiwan. This Study was Based on Data From the Health and Welfare Data Science Center (HWDC), Ministry of Health and Welfare (MOHW), Taiwan. The Interpretation and Conclusions Contained Herein do not Represent Those of HWDC, MOHW, Taiwan.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Lip GY, Kakar P, Watson T. Atrial fibrillation–the growing epidemic. Heart. (2007) 93:542-3. doi: 10.1136/hrt.2006.110791
2. Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, et al. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. (2014) 129:837-47. doi: 10.1161/CIRCULATIONAHA.113.005119
3. Chao TF, Liu CJ, Tuan TC, Chen TJ, Hsieh MH, Lip GYH, et al. Lifetime risks, projected numbers, and adverse outcomes in Asian patients with atrial fibrillation: a report from the Taiwan nationwide AF cohort study. Chest. (2018) 153:453-66. doi: 10.1016/j.chest.2017.10.001
4. Tse HF, Wang YJ, Ahmed Ai-Abdullah M, Pizarro-Borromeo AB, Chiang CE, Krittayaphong R, et al. Stroke prevention in atrial fibrillation–an Asian stroke perspective. Heart Rhythm. (2013) 10:1082-8. doi: 10.1016/j.hrthm.2013.03.017
5. Rahman F, Kwan GF, Benjamin EJ. Global epidemiology of atrial fibrillation. Nat Rev Cardiol. (2014) 11:639-54. doi: 10.1038/nrcardio.2014.118
6. Kim D, Yang PS, Jang E, Yu HT, Kim TH, Uhm JS, et al. 10-year nationwide trends of the incidence, prevalence, and adverse outcomes of non-valvular atrial fibrillation nationwide health insurance data covering the entire Korean population. Am Heart J. (2018) 202:20-6. doi: 10.1016/j.ahj.2018.04.017
7. Schnabel RB, Sullivan LM, Levy D, Pencina MJ, Massaro JM, D'Agostino RB Sr., et al. Development of a risk score for atrial fibrillation (Framingham Heart Study): a community-based cohort study. Lancet. (2009) 373:739-45. doi: 10.1016/S0140-6736(09)60443-8
8. Chamberlain AM, Agarwal SK, Folsom AR, Soliman EZ, Chambless LE, Crow R, et al. A clinical risk score for atrial fibrillation in a biracial prospective cohort (from the Atherosclerosis Risk in Communities [ARIC] study). Am J Cardiol. (2011) 107:85-91. doi: 10.1016/j.amjcard.2010.08.049
9. Alonso A, Krijthe BP, Aspelund T, Stepas KA, Pencina MJ, Moser CB, et al. Simple risk model predicts incidence of atrial fibrillation in a racially and geographically diverse population: the CHARGE-AF consortium. J Am Heart Assoc. (2013) 2:e000102. doi: 10.1161/JAHA.112.000102
10. Chao TF, Chiang CE, Chen TJ, Liao JN, Tuan TC, Chen SA. Clinical risk score for the prediction of incident atrial fibrillation: derivation in 7 220 654 Taiwan patients with 438 930 incident atrial fibrillations during a 16-year follow-up. J Am Heart Assoc. (2021) 2021:e020194. doi: 10.1161/JAHA.120.020194
11. Chao TF, Lip GYH, Lin YJ, Chang SL, Lo LW, Hu YF, et al. Age threshold for the use of non-vitamin K antagonist oral anticoagulants for stroke prevention in patients with atrial fibrillation: insights into the optimal assessment of age and incident comorbidities. Eur Heart J. (2019) 40:1504-14. doi: 10.1093/eurheartj/ehy837
12. Chao TF, Liu CJ, Lin YJ, Chang SL, Lo LW, Hu YF, et al. Oral anticoagulation in very elderly patients with atrial fibrillation: a nationwide cohort study. Circulation. (2018) 138:37-47. doi: 10.1161/CIRCULATIONAHA.117.031658
13. Chao TF, Chan YH, Chiang CE, Tuan TC, Liao JN, Chen TJ, et al. Continuation or discontinuation of oral anticoagulants after HAS-BLED scores increase in patients with atrial fibrillation. Clin Res Cardiol. (2021). doi: 10.1007/s00392-021-01816-z
14. Chao TF, Chiang CE, Chan YH, Liao JN, Chen TJ, Lip GYH, et al. Oral anticoagulants in extremely-high-risk, very elderly (>90 years) patients with atrial fibrillation. Heart Rhythm. (2021) 18:871-7. doi: 10.1016/j.hrthm.2021.02.018
15. Chao TF, Chan YH, Tuan TC, Liao JN, Chen TJ, Lip GYH, et al. Should oral anticoagulants still be prescribed to patients with atrial fibrillation with a single stroke risk factor but at high bleeding risk? a nationwide cohort study. Eur Heart J Qual Care Clin Outcomes. (2021). doi: 10.1093/ehjqcco/qcab050
16. Cheng WH, Chiang CE, Lin YJ, Chang SL, Lo LW, Hu YF, et al. Non-vitamin K antagonist oral anticoagulants in elderly (>/=85 years) patients with newly diagnosed atrial fibrillation: changing clinical practice and outcomes for stroke prevention in a nationwide cohort study. Mayo Clin Proc. (2021) 96:52-65. doi: 10.1016/j.mayocp.2020.08.042
17. Tsai CT, Liao JN, Chen SJ, Jiang YR, Chen TJ, Chao TF. Non-vitamin K antagonist oral anticoagulants versus warfarin in AF patients >/= 85 years. Eur J Clin Invest. (2021) 51:e13488. doi: 10.1111/eci.13488
18. Lin CC, Lai MS, Syu CY, Chang SC, Tseng FY. Accuracy of diabetes diagnosis in health insurance claims data in Taiwan. J Formos Med Assoc. (2005) 104:157-63. doi: 10.29828/JFMA.200503.0002
19. Cheng CL, Kao YH, Lin SJ, Lee CH, Lai ML. Validation of the National Health Insurance Research Database with ischemic stroke cases in Taiwan. Pharmacoepidemiol Drug Saf. (2011) 20:236-42. doi: 10.1002/pds.2087
20. Chang CH, Lee YC, Tsai CT, Chang SN, Chung YH, Lin MS, et al. Continuation of statin therapy and a decreased risk of atrial fibrillation/flutter in patients with and without chronic kidney disease. Atherosclerosis. (2014) 232:224-30. doi: 10.1016/j.atherosclerosis.2013.11.036
21. Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): the TRIPOD statement. Ann Intern Med. (2015) 162:55-63. doi: 10.1161/CIRCULATIONAHA.114.014508
22. Sullivan LM, Massaro JM, D'Agostino RB Sr. Presentation of multivariate data for clinical use: the Framingham Study risk score functions. Stat Med. (2004) 23:1631-60. doi: 10.1002/sim.1742
23. Fuster V, Ryden LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, et al. ACC/AHA/ESC 2006 Guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the (2001) Guidelines for the Management of Patients With Atrial Fibrillation): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. (2006) 114:e257-354. doi: 10.1161/CIRCULATIONAHA.106.177292
24. Lloyd-Jones DM, Wang TJ, Leip EP, Larson MG, Levy D, Vasan RS, et al. Lifetime risk for development of atrial fibrillation: the Framingham Heart Study. Circulation. (2004) 110:1042-6. doi: 10.1161/01.CIR.0000140263.20897.42
25. Chiang CE, Naditch-Brule L, Murin J, Goethals M, Inoue H, O'Neill J, et al. Distribution and risk profile of paroxysmal, persistent, and permanent atrial fibrillation in routine clinical practice: insight from the real-life global survey evaluating patients with atrial fibrillation international registry. Circ Arrhythm Electrophysiol. (2012) 5:632-9. doi: 10.1161/CIRCEP.112.970749
26. Kakkar AK, Mueller I, Bassand JP, Fitzmaurice DA, Goldhaber SZ, Goto S, et al. Risk profiles and antithrombotic treatment of patients newly diagnosed with atrial fibrillation at risk of stroke: perspectives from the international, observational, prospective GARFIELD registry. PLoS ONE. (2013) 8:e63479. doi: 10.1371/journal.pone.0063479
27. Liao JN, Chao TF, Liu CJ, Wang KL, Chen SJ, Lin YJ, et al. Incidence and risk factors for new-onset atrial fibrillation among patients with end-stage renal disease undergoing renal replacement therapy. Kidney Int. (2015) 87:1209-15. doi: 10.1038/ki.2014.393
28. Voskoboinik A, Prabhu S, Ling LH, Kalman JM, Kistler PM. Alcohol and atrial fibrillation: a sobering review. J Am Coll Cardiol. (2016) 68:2567-76. doi: 10.1016/j.jacc.2016.08.074
29. Ettinger PO, Wu CF, De La Cruz C Jr., Weisse AB, Ahmed SS, Regan TJ. Arrhythmias and the “Holiday Heart”: alcohol-associated cardiac rhythm disorders. Am Heart J. (1978) 95:555-62. doi: 10.1016/0002-8703(78)90296-X
30. Larsson SC, Drca N, Wolk A. Alcohol consumption and risk of atrial fibrillation: a prospective study and dose-response meta-analysis. J Am Coll Cardiol. (2014) 64:281-9. doi: 10.1016/j.jacc.2014.03.048
31. Kodama S, Saito K, Tanaka S, Horikawa C, Saito A, Heianza Y, et al. Alcohol consumption and risk of atrial fibrillation: a meta-analysis. J Am Coll Cardiol. (2011) 57:427-36. doi: 10.1016/j.jacc.2010.08.641
32. Samokhvalov AV, Irving HM, Rehm J. Alcohol consumption as a risk factor for atrial fibrillation: a systematic review and meta-analysis. Eur J Cardiovasc Prev Rehabil. (2010) 17:706-12. doi: 10.1097/HJR.0b013e32833a1947
33. Csengeri D, Sprunker NA, Di Castelnuovo A, Niiranen T, Vishram-Nielsen JK, Costanzo S, et al. Alcohol consumption, cardiac biomarkers, and risk of atrial fibrillation and adverse outcomes. Eur Heart J. (2021) 42:1170-7. doi: 10.1093/eurheartj/ehaa953
34. Sano F, Ohira T, Kitamura A, Imano H, Cui R, Kiyama M, et al. Heavy alcohol consumption and risk of atrial fibrillation. The Circulatory Risk in Communities Study (CIRCS). Circ J. (2014) 78:955-61. doi: 10.1253/circj.CJ-13-1387
Keywords: incident atrial fibrillation, modified Taiwan AF score, prediction, Asian population, national cohort
Citation: Liao J-N, Lim S-S, Chen T-J, Tuan T-C, Chen S-A and Chao T-F (2022) Modified Taiwan Atrial Fibrillation Score for the Prediction of Incident Atrial Fibrillation. Front. Cardiovasc. Med. 8:805399. doi: 10.3389/fcvm.2021.805399
Received: 30 October 2021; Accepted: 27 December 2021;
Published: 28 January 2022.
Edited by:
Gary Tse, Second Hospital of Tianjin Medical University, ChinaReviewed by:
Jeffrey Shi Kai Chan, Cardiovascular Analytics Group, Hong Kong SAR, ChinaCopyright © 2022 Liao, Lim, Chen, Tuan, Chen and Chao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tze-Fan Chao, ZXlja2V5Y2tAZ21haWwuY29t
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.