
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 08 February 2022
Sec. Cardiac Rhythmology
Volume 8 - 2021 | https://doi.org/10.3389/fcvm.2021.791112
This article is part of the Research Topic Risk Stratification Strategies for Cardiac Rhythm Abnormalities View all 49 articles
 Min Kim1
Min Kim1 Hee Tae Yu2
Hee Tae Yu2 Tae-Hoon Kim2
Tae-Hoon Kim2 Dae-In Lee1
Dae-In Lee1 Jae-Sun Uhm2
Jae-Sun Uhm2 Young Dae Kim3
Young Dae Kim3 Hyo Suk Nam3
Hyo Suk Nam3 Boyoung Joung2
Boyoung Joung2 Moon-Hyoung Lee2
Moon-Hyoung Lee2 Ji Hoe Heo3
Ji Hoe Heo3 Hui-Nam Pak2*
Hui-Nam Pak2*Background: Ischemic strokes (ISs) can appear even in non-gender-related CHA2DS2-VA scores 0~1 patients with atrial fibrillation (AF). We explored the determinants associated with IS development among the patients with non-gender-related CHA2DS2-VA score 0~1 AF.
Methods and Results: In this single-center retrospective registry data for AF catheter ablation (AFCA), we included 1,353 patients with AF (24.7% female, median age 56 years, and paroxysmal AF 72.6%) who had non-gender-related CHA2DS2-VA score 0~1, normal left ventricular (LV) systolic function, and available H2FPEF score. Among those patients, 113 experienced IS despite a non-gender-related CHA2DS2-VA score of 0~1. All included patients underwent AFCA, and we evaluated the associated factors with IS in non-gender-related CHA2DS2-VA score 0~1 AF. Patients with ISs in this study had a lower estimated glomerular filtration rate (eGFR) (p < 0.001) and LV ejection fraction (LVEF; p = 0.017), larger LA diameter (p < 0.001), reduced LA appendage peak velocity (p < 0.001), and a higher baseline H2FPEF score (p = 0.018) relative to those without ISs. Age [odds ratio (OR) 1.11 (1.07–1.17), p < 0.001, Model 1] and H2FPEF score as continuous [OR 1.31 (1.03–1.67), p = 0.028, Model 2] variable were independently associated with ISs by multivariate analysis. Moreover, the eGFR was independently associated with IS at low CHA2DS2-VA scores in both Models 1 and 2. AF recurrence was significantly higher in patients with IS (log-rank p < 0.001) but not in those with high H2FPEF scores (log-rank p = 0.079), respectively.
Conclusions: Among the patients with normal LVEF and non-gender-related CHA2DS2-VA score 0~1 AF, the high H2FPEF score, and increasing age were independently associated with IS development (ClinicalTrials.gov Identifier: NCT02138695).
Atrial fibrillation (AF) is a significant risk factor for ischemic strokes (ISs), and the CHA2DS2-VASc score has been suggested to be the most reliable parameter for the IS risk stratification (1). The current guidelines recommend introducing oral anticoagulant therapy for stroke prevention in non-valvular AF patients with CHA2DS2-VASc scores of 2 points or higher. In contrast, no antithrombotic therapy is advantageous for men with scores of 0 or 1 point and women with scores of 1 or 2 points, respectively, in terms of the risk-benefit profile (2, 3). Nevertheless, AF patients with low CHA2DS2-VASc scores who are not recommended to undergo anticoagulant therapy exhibit an annual IS risk of 1.15% per year (4–7). This level of IS risk is similar to the annual IS risk in AF patients with higher CHA2DS2-VASc scores who are on anticoagulant therapy (5, 8). Therefore, identifying IS-related factors or clinical predictors of ISs in AF patients with low CHA2DS2-VASc scores might be valuable for stroke prevention in relatively young and active low-risk patients with AF (9–11). However, the mechanisms of IS in patients with AF are heterogeneous and the CHA2DS2-VASc score comprehensively evaluates both cardioembolic and non-cardioembolic risks, such as complex aortic plaque and carotid and intracranial arteriosclerosis (12). For this reason, IS risk-assessment studies in patients with low CHA2DS2-VASc scores and low numbers of comorbidities have been limited by epidemiologic data dependent on the International Classification of Diseases code (13). In this study, we explored the potential risk factors of ISs among patients with non-gender-related CHA2DS2-VA score 0~1 AF depending on the H2FPEF score (14), which is a recently developed risk score for heart failure with preserved ejection fraction (HFpEF) and known to be related with atrial myopathy (15). Additionally, we evaluated the outcome of AF catheter ablation (AFCA). The purpose of this study was to compare and assess the risk factors of ISs in the patients with non-gender-related CHA2DS2-VA score 0~1 AF.
Data supporting the findings of this study are available from the corresponding author upon reasonable request.
The study is conducted in compliance with the ethical rules of the Declaration of Helsinki (2013) as a statement of ethical principles for medical research involving human subjects by The World Medical Association and approved by the Institutional Review Board of Yonsei University Health System. From January 2009 to April 2020, 1,353 patients with a diagnosis of AF were identified as having a normal left ventricular (LV) systolic function and low non-gender-related low CHA2DS2-VA score (0–1 points both in men and women) in the Yonsei AF Ablation Cohort Database (ClinicalTrials.gov Identifier: NCT02138695) and underwent AFCA for symptomatic and drug-refractory non-valvular AF. Written informed consent was obtained from all patients before the study inclusion. The exclusion criteria for AFCA were as follows: (1) permanent AF refractory to electrical cardioversion; (2) presence of a left atrial (LA) or LA appendage thrombus on transesophageal echocardiography; (3) no measurements of the left ventricular (LV) diameter, LV end-diastolic dimension (LVEDD), or ratio between the early mitral inflow velocity and mitral annular early diastolic velocity (EEm) by transthoracic echocardiography; (4) significant structural heart disease other than LV hypertrophy, such as significant valvular heart disease of grade two or greater, hypertrophic/ischemic/dilated cardiomyopathy, or congenital heart disease; (5) a history of a prior AFCA or cardiac surgery; and (6) a left ventricular ejection fraction (LVEF) ≤ 50%. Among those patients, 113 experienced IS despite non-gender-related CHA2DS2-VA score 0~1, and divided into groups at high risk of and medium/low risk of a cardioembolic stroke, respectively, based on the Trial of Org 10172 in Acute Stroke Treatment (TOAST) classification scheme (16) for the analysis of stroke subtype differences. All included patients underwent AFCA, and the time difference between the previous stroke event and AFCA and comorbidities was confirmed by the electrical medical record review. The CHA2DS2-VA score was recalculated immediately before the IS event by medical record review in patients with a prior IS.
The H2FPEF score has six variables based on clinical and echocardiographic values: heaviness (body mass index (BMI) > 30 kg/m2, 2 points), hypertension (on two or more antihypertensive medicines, 1 point), atrial fibrillation (paroxysmal or persistent, 3 points), pulmonary hypertension (Doppler echocardiographic estimated pulmonary artery systolic pressure > 35 mmHg, 1 point), elderly status (age > 60 years, 1 point), and filling pressure (Doppler echocardiographic E/Em > 9, 1 point). The baseline H2FPEF score was calculated through medical record review same as the CHA2DS2-VA score recalculation in patients with a prior IS except echocardiographic parameters. In patients without a previous IS, the baseline H2FPEF score was calculated with the variables obtained within 3 months before the AFCA.
Transthoracic echocardiography was conducted in all patients using commercially available devices (Vivid 7 or Vivid E9 from GE Healthcare, Chicago, IL, USA or iE 33 from Philips, Amsterdam, the Netherlands) as recommended by the American Society of Echocardiography (17). Standard images were obtained in the parasternal and apical views through two-dimensional, Doppler, and M-mode images, such as the LA anteroposterior diameter and LV end-systolic and LVEDD dimensions. The early Doppler mitral inflow (E) was recorded by the pulsed wave from the apical window, with a 1- to 3-mm pulsed Doppler sample volume placed between the tips and mitral leaflets during diastole. The early diastolic mitral annular velocity (Em) was recorded as the peak early diastolic tissue velocity using color Doppler tissue imaging of the septal mitral annulus. The early diastolic mitral inflow velocity ratio to the early diastolic mitral annular velocity (E/Em) was calculated. Tricuspid regurgitation (TR) and estimated right atrial (RA) pressure were evaluated using the recommended methods, and the right ventricular systolic pressure (RVSP) was calculated as 4 × (TR jet)2 + estimated RA pressure (18). For Doppler-derived parameters, at least 3 consecutive beats were measured and averaged (19).
Intracardiac electrograms were obtained using the Prucka CardioLab electrophysiology system (GE Healthcare, Chicago, IL, USA). A 3D electroanatomical map (Ensite NavX; Abbott Laboratories, Chicago, IL, USA; CARTO3; Johnson & Johnson Inc., NJ, USA.) was generated using a circumferential pulmonary-vein mapping catheter through a long sheath (Schwartz left 1; Abbott Laboratories, Chicago, IL, USA) through merging the 3D geometry generated by the electroanatomic mapping system with the corresponding 3D spiral CT images. Separately, a 3D LA voltage mapping was performed by obtaining the contact bipolar electrograms from 350 to 500 points on the LA endocardium during atrial pacing (high RA; pacing cycle length: 500 ms). The bipolar electrograms were filtered at 32–300 Hz. Color-coded voltage maps were generated by using the bipolar electrograms, and the peak-to-peak voltage was generated as previously described (20).
The baseline characteristics of patients were compared using descriptive statistics, presented as median (interquartile interval) values for continuous variables and as numbers (percentages) for categorical variables. With reference to a previous study (14), we set a 5 point H2FPEF score as the cut-off value, which has shown the probability of HFpEF >80%. To identify factors associated with the presence of a stroke, we performed univariate and multivariable logistic regression analyses. We conducted three models of multivariable logistic regression analyses because of the multicollinearity among the H2FPEF score and age or E/Em. Model 1 was analyzed by adding variables that were having p < 0.10 of the univariate models. Model 2 was analyzed by treating the H2FPEF score as a continuous variable, and model 3 was analyzed by treating it as a categorical variable. To compare the effect of individual H2FPEF score variables, we performed multivariable logistic regression based on individual H2FPEF score variables. A subgroup analysis was performed based on the comorbidities not included in the H2FPEF score variables. Two-sided p-values of <0.05 were considered to be statistically significant. Statistical analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC, USA) and R version 4.0.0 (R Foundation for Statistical Computing, Vienna, Austria) software.
Among 3,648 consecutive patients in this single-center prospective registry, we included 1,353 patients with AF (24.7% female, median age 56 years, paroxysmal AF 72.6%) who had non-gender-related CHA2DS2-VA score 0~1 at the times of enrollment (n = 1,240) or previous IS events (n = 113), normal LV systolic function, and available H2FPEF score. The time difference between the previous stroke events and inclusion was a median of 1.0 [interquartile range (IQR):1.0–4.0] year in 113 patients with previous stroke events, and 99 of them had a high risk for cardioembolism to the TOAST classification. About 84% of previous stroke events (95/113) had occurred within a year.
We compared the AF patients with non-gender-related CHA2DS2-VA score 0~1 and those at the time of the IS in Table 1. Patients who experienced IS at the time of non-gender-related CHA2DS2-VA score 0~1 were older (p < 0.001) and had a higher baseline H2FPEF score (p = 0.018), E/Em values (p < 0.001), and RVSP (p = 0.007), larger LA dimension (p = 0.003), lower eGFR (p = 0.001), and left atrium appendage (LAA) peak velocity (p < 0.001) than those without IS.
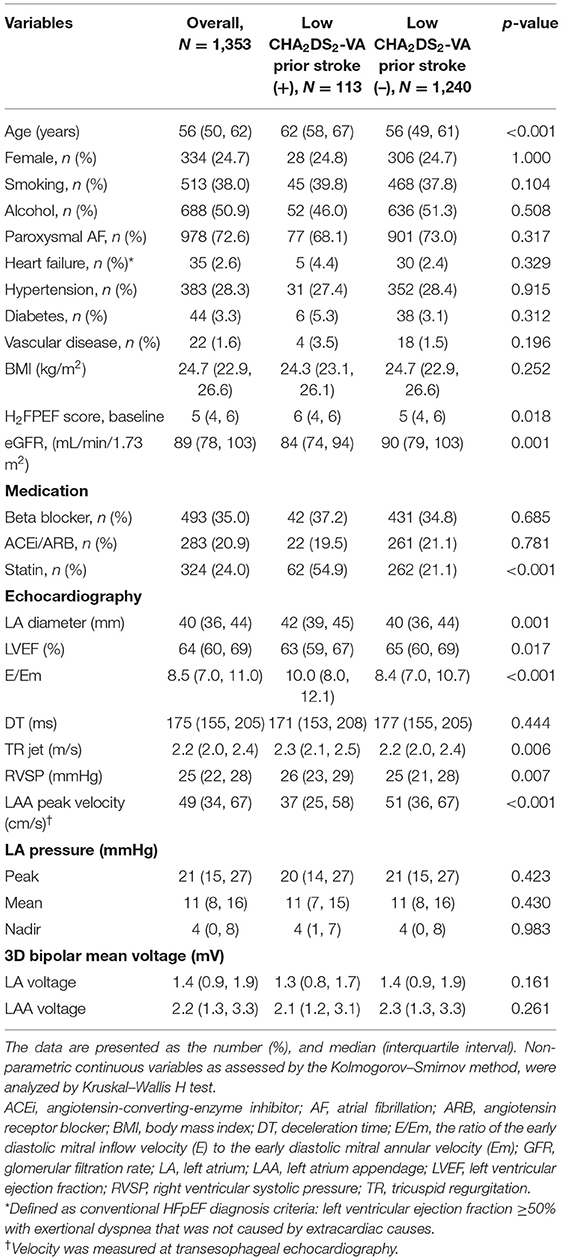
Table 1. Comparison of the baseline characteristics between non-gender-related CHA2DS2-VA score 0~1 AF patients with strokes and those without strokes.
Figure 1 shows a linear relationship trend between the baseline H2FPEF score and non-gender-related CHA2DS2-VA score 0~1. Patients with the baseline H2FPEF score ≥ 5 were generally older (p < 0.001) and had higher proportions of hypertension (p < 0.001). They had an increased BMI value (p < 0.001), larger LA diameters (p < 0.001), higher E/Em values (p < 0.001) and RVSP (p < 0.002), and higher prescription rate of renin-angiotensin-aldosterone system blockers (p < 0.001) and statins (p = 0.004, Table 2).
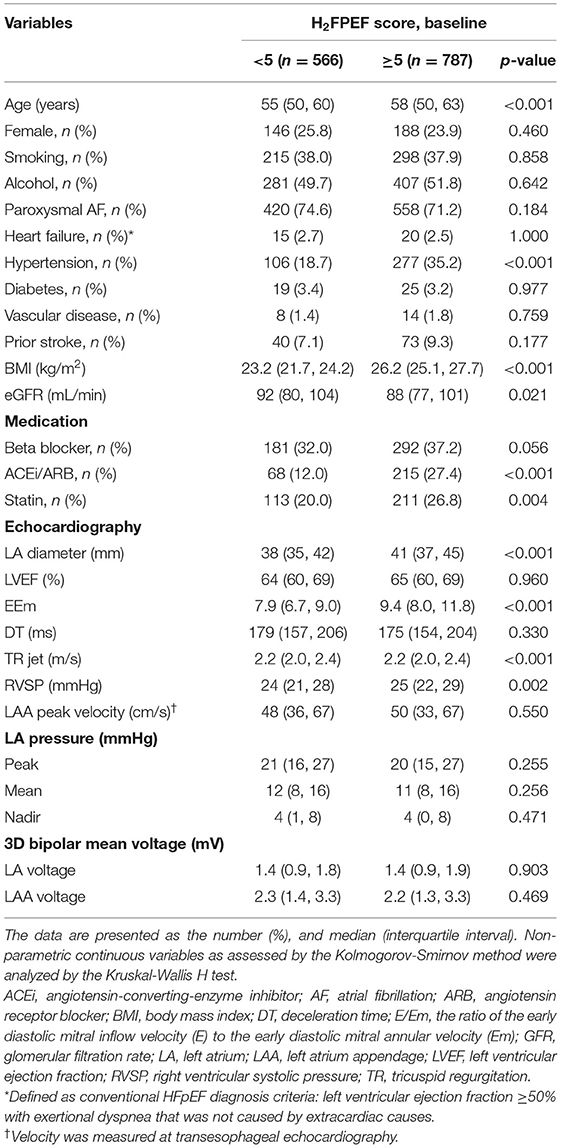
Table 2. Comparison of the baseline characteristics based on H2FPEF score 5 in non-gender-related CHA2DS2-VA score 0~1 patients with AF.
The univariate and multivariate analysis for IS in patients with non-gender-related CHA2DS2-VA score 0~1 is listed in Table 3. For the multivariate logistic regression analyses, we tested 2 models because of collinearity between age and H2FPEF score. Age [OR 1.11 (1.07–1.17), p < 0.001, Model 1] and H2FPEF score as continuous [OR 1.31 (1.03–1.67), p = 0.028, Model 2] variables were independently associated with ISs by multivariate analysis. The eGFR was also independently associated with IS at low CHA2DS2-VA scores in both Models 1 and 2.
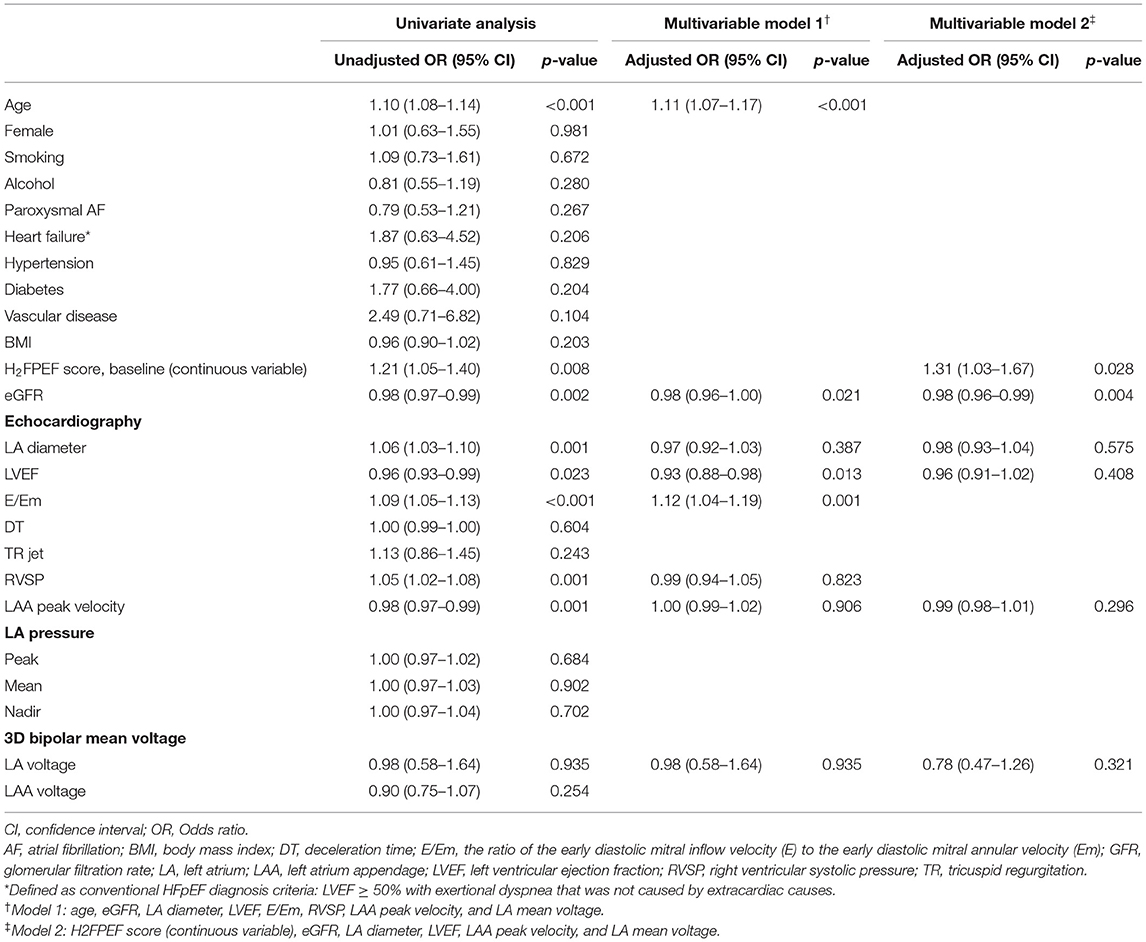
Table 3. Univariate and multivariate logistic regression analysis for the predictors of prior ISs in patients with non-gender-related CHA2DS2-VA score 0~1 AF.
We compared the effect of individual variables of the H2FPEF score on IS at non-gender-related CHA2DS2-VA score 0~1 in the logistic regression models (Figure 2). Among six variables, age ≥60 years [OR 4.34 (2.34–8.20), p < 0.001] and E/Em ≥9 [OR 2.28 (1.24–4.23), p = 0.008] were independently associated with ISs in this low-risk group. In the subgroup analysis, the H2FPEF score was consistently related to the risk of ISs at non-gender-related CHA2DS2-VA score 0~1, regardless of sex, AF types, diabetes, vascular disease, and renal function (Figure 3).
Over median 28 months of follow-up, the cumulative AF recurrence rate after AFCA was significantly higher in the patients with IS under non-gender-related CHA2DS2-VA score 0~1 (log-rank p = 0.001, Figure 4A), but not high H2FPEF score ≥ 5 (log-rank p = 0.079, Figure 4B), E/Em > 9 (log-rank p = 0.241, Figure 4C), or eGFR ≤ 60 ml/min/1.73 m2 (log-rank p = 0.250, Figure 4D).
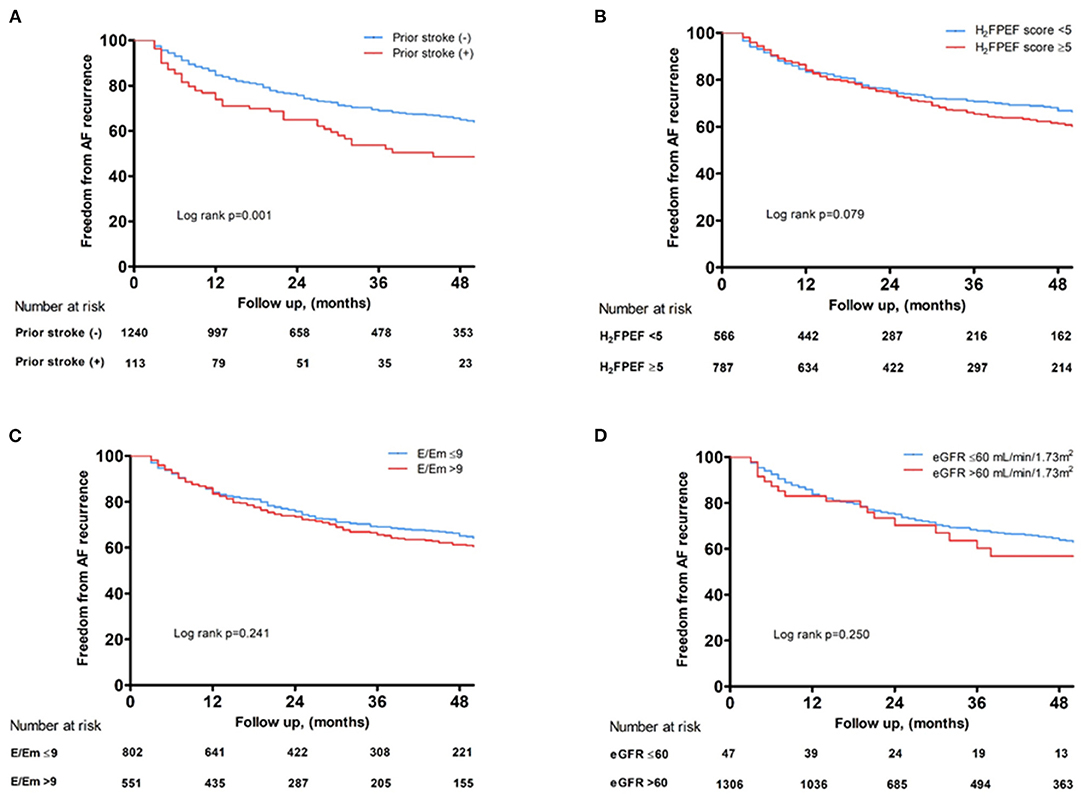
Figure 4. Kaplan–Meier curves showing the rate of freedom from AF recurrence after AFCA depending on a prior ISs (A), baseline H2FPEF score (B), E/Em (C), and renal function (D). AF, atrial fibrillation; AFCA, atrial fibrillation catheter ablation; E/Em, the ratio of the early diastolic mitral inflow velocity (E) to the early diastolic mitral annular velocity (Em); IS, ischemic stroke.
This study explored the risk factors for ISs based on the H2FPEF score in AF patients with non-gender-related CHA2DS2-VA score 0~1. Among these low-risk patient groups, increased age, the high H2FPEF score, and low eGFR were independently associated with ISs. Among six variables of the H2FPEF score, age over 60 years, and E/Em ≥ 9 showed a significantly greater risk of ISs at non-gender-related CHA2DS2-VA score 0~1 among the patients with AF who were referred for catheter ablation.
We have been using the CHA2DS2-VASc score as an epidemiologically reasonable stroke prevention index (21). In spite of the 1.15% annual risk of ISs, the existing guidelines do not recommend antithrombotic therapy to AF patients with non-gender related CH2DS2-VASc scores of <2-point ( ≤ 1 point in men and ≤ 2 points in women) (2, 3, 5–7). However, it remains clinically important to predict and prevent ISs in these low-risk patients, primarily young and active individuals. Another weak point is that the CH2DS2-VASc score components include not only cardioembolic but also non-cardioembolic risk factors (12) and do not encompass pathophysiological mechanisms, such as atrial myopathy, hemodynamic factors, the AF burden, or hypercoagulability (22, 23).
Although the associated comorbidities tend to be less, hemodynamic factors are more likely to contribute to the mechanism of IS in AF patients with non-gender-related CHA2DS2-VA score 0~1 in this study. Recently, growing interest in the potential for an atrial myopathy that leads to AF progression and contributes to systemic thromboembolism has emerged (24). King et al. (25) discerned the relationship between atrial fibrosis and the risk of a stroke in patients with AF using late-gadolinium enhanced cardiac MRI. Leong et al. (23) reported LA dysfunction contributes to the mechanism of ISs by analyzing the LA strain. Atrial myopathy generates the condition vulnerable to atrial dysfunction, fibrosis, structural remodeling, and blood stasis, increasing the risk of thrombus development.
Although genetic factors may contribute to atrial myopathy in certain specific low-risk patients with AF (26, 27), the LV diastolic dysfunction could be a key contributing factor. LV diastolic dysfunction increases the atrial filling pressures and triggers progressive atrial enlargement, dysfunction, and atrial myopathy, eventually leading to ISs (28). Kim et al. (29) and Yu et al. (30) reported that LV diastolic dysfunction represented by the E/Em is associated with a greater risk for ISs and LA remodeling, especially in female patients with AF. The recently developed integrated scoring system, the H2FPEF score (14), which estimates an adverse effect on hemodynamics, may help to identify LA myopathy (15, 31). Furthermore, we proved the baseline H2FPEF score is independently associated with ISs in AF patients with non-gender-related CHA2DS2-VA score 0~1.
Based on the results of this study, physicians should consider the potential risk of ISs in AF patients with non-gender-related CHA2DS2-VA score 0~1, especially in patients with increased age, a high H2FPEF score, or renal dysfunction. Recently, we demonstrated that the active rhythm control of AF by AFCA is superior to medical therapy in the risk reduction of ISs (32) and AFCA reduces the H2FPEF score a year after the procedure in AF patients with underlying LV diastolic dysfunction (33). Therefore, despite a non-gender-related CHA2DS2-VA score of 0~1, we have to pay more attention to the risk of IS or rhythm control status for those patients with old age, the high H2FPEF score, or renal dysfunction.
Our study had several limitations that should be noted. First, the population of this study was a single-center AFCA cohort with detailed clinical and imaging data. As these patients were referred for AFCA, the results of this study cannot be generalized. Second, ISs were classified and diagnosed by neurologists, and silent ISs were not excluded in the study. Third, because this study is retrospectively designed including the AF population with detailed imaging and physiological data, the timing of the IS event was not accurately reflected in the baseline characteristics. However, most of the prior strokes (84%) occurred within 1 year of the study time point. Furthermore, we checked precise age at the time of stroke events in patients with ISs and adjusted the non-gender-related CHA2DS2-VA score. Fourth, although their proportion was small, we did not exclude the patients with mitral annular calcification or mitral regurgitation whose E/Em could not represent LV filling pressure.
Among AF patients with normal LV systolic function and non-gender-related CHA2DS2-VA score 0~1, age, renal function, and the high H2FPEF score, that may allow for LA myopathy identification, were significantly associated with the ISs.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Institutional Review Board of Yonsei University Health System. The patients/participants provided their written informed consent to participate in this study.
H-NP and MK: conceptualization, validation, writing—original draft, and writing—review and editing. H-NP, HY, T-HK, J-SU, YK, HN, BJ, M-HL, and JH: data curation. MK: formal analysis and Software. H-NP, MK, HY, T-HK, D-IL, BJ, and M-HL: methodology. H-NP, MK, HY, T-HK, D-IL, J-SU, YK, HN, BJ, M-HL, and JH: investigation. All authors contributed to the article and approved the submitted version.
This work was supported by grants (HI19C0114 and HI21C0011) from the Ministry of Health and Welfare and a grant (NRF-2020R1A2B01001695) from the Basic Science Research Program run by the National Research Foundation of Korea (NRF), which was funded by the Ministry of Science, ICT & Future Planning (MSIP).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We would like to thank Mr. John Martin for his linguistic assistance.
1. Heeringa J, van der Kuip DAM, Hofman A, Kors JA, van Herpen G, Stricker BHC, et al. Prevalence, incidence and lifetime risk of atrial fibrillation: the Rotterdam study. Eu Heart J. (2006) 27:949–53. doi: 10.1093/eurheartj/ehi825
2. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomstrom-Lundqvist C, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the european association of cardio-thoracic surgery (EACTS). Eur Heart J. (2021) 42:373–498. doi: 10.1093/eurheartj/ehaa612
3. January CT, Wann L, Alpert JS. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the heart rhythm society. J Am Coll Cardiol. (2014) 64:2304–2307. doi: 10.1016/j.jacc.2014.03.021
4. Kim TH, Yang PS, Yu HT, Jang E, Uhm JS, Kim JY, et al. Age threshold for ischemic stroke risk in atrial fibrillation: cohort data covering the entire korean population. Stroke. (2018) 49:1872–9. doi: 10.1161/STROKEAHA.118.021047
5. Chao TF, Liu CJ, Wang KL, Lin YJ, Chang SL, Lo LW, et al. Should atrial fibrillation patients with 1 additional risk factor of the CHA2DS2-VASc score (beyond sex) receive oral anticoagulation? J Am Coll Cardiol. (2015) 65:635–42. doi: 10.1016/j.jacc.2014.11.046
6. Olesen JB, Lip GYH, Hansen ML, Hansen PR, Tolstrup JS, Lindhardsen J, et al. Validation of risk stratification schemes for predicting stroke and thromboembolism in patients with atrial fibrillation: nationwide cohort study. BMJ. (2011) 342:d124. doi: 10.1136/bmj.d124
7. Kim D, Yang PS, Jang E, Yu HT, Kim TH, Uhm JS, et al. 10-year nationwide trends of the incidence, prevalence, and adverse outcomes of non-valvular atrial fibrillation nationwide health insurance data covering the entire Korean population. Am Heart J. (2018) 202:20–6. doi: 10.1016/j.ahj.2018.04.017
8. Joundi RA, Cipriano LE, Sposato LA, Saposnik G, Grp SORW. Ischemic stroke risk in patients with atrial fibrillation and CHA2DS2-VASc score of 1: systematic review and meta-analysis. Stroke. (2016) 47:1364–67. doi: 10.1161/STROKEAHA.115.012609
9. Chao TF, Liu CJ, Wang KL, Lin YJ, Chang SL, Lo LW, et al. Using the CHA2DS2-VASc score for refining stroke risk stratification in 'low-risk' asian patients with atrial fibrillation. J Am Coll Cardiol. (2014) 64:1658–65. doi: 10.1016/j.jacc.2014.06.1203
10. Singleton MJ, Imtiaz-Ahmad M, Kamel H, O'Neal WT, Judd SE, Howard VJ, et al. Association of atrial fibrillation without cardiovascular comorbidities and stroke risk: from the REGARDS study. J Am Heart Assoc. (2020) 9:e016380 doi: 10.1161/JAHA.120.016380
11. Cushman M, Cantrell RA, McClure LA, Howard G, Prineas RJ, Moy CS, et al. Estimated 10-year stroke risk by region and race in the United States: Geographic and racial differences in stroke risk. Ann Neurol. (2008) 64:507–13. doi: 10.1002/ana.21493
12. Yang PS, Pak HN, Park DH, Yoo J, Kim TH, Uhm JS, et al. Non-cardioembolic risk factors in atrial fibrillation-associated ischemic stroke. PLoS ONE. (2018) 13:e0201062. doi: 10.1371/journal.pone.0201062
13. Kloosterman M, Oldgren J, Conen D, Wong JA, Connolly SJ, Avezum A, et al. Characteristics and outcomes of atrial fibrillation in patients without traditional risk factors: an RE-LY AF registry analysis. Europace. (2020) 22:870–7. doi: 10.1093/europace/euz360
14. Reddy YNV, Carter RE, Obokata M, Redfield MM, Borlaug BA. A simple, evidence-based approach to help guide diagnosis of heart failure with preserved ejection fraction. Circulation. (2018) 138:861–70. doi: 10.1161/CIRCULATIONAHA.118.034646
15. Patel RB, Shah SJ. Therapeutic targeting of left atrial myopathy in atrial fibrillation and heart failure with preserved ejection fraction. JAMA Cardiol. (2020) 5:497–9. doi: 10.1001/jamacardio.2020.0136
16. Adams HP, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke - definitions for use in a multicenter clinical-trial. Stroke. (1993) 24:35–41. doi: 10.1161/01.STR.24.1.35
17. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American society of echocardiography and the European association of cardiovascular imaging. Eur Heart J Card Img. (2015) 16:233–71. doi: 10.1093/ehjci/jev014
18. Rudski LG, Lai WW, Afilalo J, Hua LQ, Handschumacher MD, Chandrasekaran K, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American society of echocardiography endorsed by the European association of echocardiography, a registered branch of the european society of cardiology, and the Canadian society of echocardiography. J Am Soc Echocardiog. (2010) 23:685–713. doi: 10.1016/j.echo.2010.05.010
19. Lee JS, Shim CY, Wi J, Joung B, Ha JW, Lee MH, et al. Left ventricular diastolic function is closely associated with mechanical function of the left atrium in patients with paroxysmal atrial fibrillation. Circ J. (2013) 77:697–704. doi: 10.1253/circj.CJ-12-1009
20. Park JH, Pak HN, Choi EJ, Jang JK, Kim SK, Choi DH, et al. The relationship between endocardial voltage and regional volume in electroanatomical remodeled left atria in patients with atrial fibrillation: comparison of three-dimensional computed tomographic images and voltage mapping. J Cardiovasc Electr. (2009) 20:1349–56. doi: 10.1111/j.1540-8167.2009.01557.x
21. Lip GYH, Nieuwlaat R, Pisters R, Lane DA, Crijns HJGM. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach the euro heart survey on atrial fibrillation. Chest. (2010) 137:263–72. doi: 10.1378/chest.09-1584
22. Daccarett M, Badger TJ, Akoum N, Burgon NS, Mahnkopf C, Vergara G, et al. Association of left atrial fibrosis detected by delayed-enhancement magnetic resonance imaging and the risk of stroke in patients with atrial fibrillation. J Am Coll Cardiol. (2011) 57:831–8. doi: 10.1016/j.jacc.2010.09.049
23. Leong DP, Joyce E, Debonnaire P, Katsanos S, Holman ER, Schalij MJ, et al. Left atrial dysfunction in the pathogenesis of cryptogenic stroke: Novel insights from speckle-tracking echocardiography. J Am Soc Echocardiog. (2017) 30:71–9. doi: 10.1016/j.echo.2016.09.013
24. Goette A, Kalman JM, Aguinaga L, Akar J, Cabrera JA, Chen SA, et al. EHRS/HRS/APHRS/SOLAECE expert consensus on atrial cardiomyopathies: definition, characterisation, and clinical implication. J Arrythm. (2016) 32:247–78. doi: 10.1016/j.joa.2016.05.002
25. King JB, Azadani PN, Suksaranjit P, Bress AP, Witt DM, Han FT, et al. Left atrial fibrosis and risk of cerebrovascular and cardiovascular events in patients with atrial fibrillation. J Am Coll Cardiol. (2017) 70:1311–21. doi: 10.1016/j.jacc.2017.07.758
26. Roselli C, Chaffin MD, Weng LC, Aeschbacher S, Ahlberg G, Albert CM, et al. Multi-ethnic genome-wide association study for atrial fibrillation. Nat Genet. (2018) 50:1225–33. doi: 10.1038/s41588-018-0133-9
27. Malik R, Chauhan G, Traylor M, Sargurupremraj M, Okada Y, Mishra A, et al. Multiancestry genome-wide association study of 520,000 subjects identifies 32 loci associated with stroke and stroke subtypes. Nat Genet. (2018) 50:524–37. doi: 10.1038/s41588-018-0058-3
28. Oh JK, Hatle L, Tajik AJ, Little WC. Diastolic heart failure can be diagnosed by comprehensive two-dimensional and doppler echocardiography. J Am Coll Cardiol. (2006) 47:500–6. doi: 10.1016/j.jacc.2005.09.032
29. Kim TH, Shim CY, Park JH, Nam CM, Uhm JS, Joung B, et al. Left ventricular diastolic dysfunction is associated with atrial remodeling and risk or presence of stroke in patients with paroxysmal atrial fibrillation. J Cardiol. (2016) 68:104–9. doi: 10.1016/j.jjcc.2015.10.008
30. Yu HT, Lee JS, Kim TH, Uhm JS, Joung BY, Hong GR, et al. Advanced left atrial remodeling and appendage contractile dysfunction in women than in men among the patients with atrial fibrillation: potential mechanism for stroke. J Am Heart Assoc. (2016) 5:e003361. doi: 10.1161/JAHA.116.003361
31. Reddy YNV, Obokata M, Verbrugge FH, Lin G, Borlaug BA. Atrial dysfunction in patients with heart failure with preserved ejection fraction and atrial fibrillation. J Am Coll Cardiol. (2020) 76:1051–64. doi: 10.1016/j.jacc.2020.07.009
32. Kim M, Yu HT, Kim J, Kim TH, Uhm JS, Joung B, et al. Atrial fibrillation and the risk of ischaemic strokes or intracranial haemorrhages: comparisons of the catheter ablation, medical therapy, and non-atrial fibrillation population. Europace. (2021) 23:529–38. doi: 10.1093/europace/euaa235
Keywords: atrial fibrillation, CHA2DS2-VA score, H2FPEF score, stroke, atrial myopathy
Citation: Kim M, Yu HT, Kim T-H, Lee D-I, Uhm J-S, Kim YD, Nam HS, Joung B, Lee M-H, Heo JH and Pak H-N (2022) Ischemic Stroke in Non-Gender-Related CHA2DS2-VA Score 0~1 Is Associated With H2FPEF Score Among the Patients With Atrial Fibrillation. Front. Cardiovasc. Med. 8:791112. doi: 10.3389/fcvm.2021.791112
Received: 07 October 2021; Accepted: 27 December 2021;
Published: 08 February 2022.
Edited by:
Konstantinos Letsas, Evaggelismos General Hospital, GreeceReviewed by:
Yuling Zhang, Sun Yat-sen Memorial Hospital, ChinaCopyright © 2022 Kim, Yu, Kim, Lee, Uhm, Kim, Nam, Joung, Lee, Heo and Pak. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hui-Nam Pak, aG5wYWtAeXVocy5hYw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.