
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cardiovasc. Med. , 18 November 2021
Sec. Cardiac Rhythmology
Volume 8 - 2021 | https://doi.org/10.3389/fcvm.2021.787866
This article is part of the Research Topic Risk Stratification Strategies for Cardiac Rhythm Abnormalities View all 49 articles
A commentary has been posted on this article:
Commentary: Differential Risk of Dementia Between Patients With Atrial Flutter and Atrial Fibrillation: A National Cohort Study
Corrigendum: Differential Risk of Dementia Between Patients With Atrial Flutter and Atrial Fibrillation: A National Cohort Study
 Hui-Ting Wang1†
Hui-Ting Wang1† Yung-Lung Chen2,3
Yung-Lung Chen2,3 Yu-Sheng Lin3,4†
Yu-Sheng Lin3,4† Huang-Chung Chen2
Huang-Chung Chen2 Shaur-Zheng Chong2
Shaur-Zheng Chong2 Shukai Hsueh2
Shukai Hsueh2 Chang-Ming Chung4‡
Chang-Ming Chung4‡ Mien-Cheng Chen2*‡
Mien-Cheng Chen2*‡Objectives: Atrial fibrillation (AF) is linked to an increased risk of stroke and dementia. Atrial flutter (AFL) is also linked to an increased risk of stroke but at a different level of risk as compared to AF. Little is known about the difference in the risk of dementia between AF and AFL. This study aims to investigate whether the risk of dementia is different between AF and AFL.
Methods: Patients with newly diagnosed AF and AFL during 2001–2013 were retrieved from Taiwan's National Health Insurance Research Database. Patients with incomplete demographic data, aged <20 years, history of valvular surgery, rheumatic heart disease, hyperthyroidism, and history of dementia were excluded. The incidence of new-onset dementia was set as the primary outcome and analyzed in patients with AF and AFL after propensity score matching (PSM).
Results: A total of 232,425 and 7,569 patients with AF and AFL, respectively, were eligible for analysis. After 4:1 PSM, we included 30,276 and 7,569 patients with AF and AFL, respectively, for analysis. Additionally, patients with AF (n = 29,187) and AFL (n = 451) who received oral anticoagulants were enrolled for comparison. The risk of dementia was higher in patients with AF compared with patients with AFL (subdistribution hazard ratio (SHR) = 1.52, 95% CI 1.39–1.66; p < 0.0001) before PSM and remained higher in patients with AF (SHR = 1.14, 95% CI 1.04–1.25; p = 0.0064) after PSM. The risk of dementia was higher in patients with AF without previous history of stroke after PSM but the risk did not differ between patients with AF and AFL with previous history of stroke. Among patients who received oral anticoagulants, the cumulative incidences of dementia were significantly higher in patients with AF than in patients with AFL before and after PSM (all P < 0.05).
Conclusions: This study found that, among patients without history of stroke, the risk of dementia was higher in patients with AF than in patients with AFL, and CHA2DS2-VASc score might be useful for risk stratification of dementia between patients with AF and AFL.
Atrial fibrillation (AF) had been reported to be linked to an increased risk of ischemic stroke, thromboembolism, heart failure, myocardial infarction, and death (1, 2). Moreover, there is increasing evidence that AF is also a risk factor for cognitive decline and dementia, which might be independent of ischemic stroke (3–6). Atrial flutter (AFL) and AF share the common risk factors and therefore, should contribute to similar clinical events. Accordingly, clinical guidelines recommend AFL should be treated as AF in terms of anticoagulation to prevent stroke and systemic thromboembolism (7). However, our previous study showed that the incidence of ischemic stroke was significantly higher in the AF cohort than in the AFL cohort at a CHA2DS2-VASc score [Heart failure, Hypertension, Age ≥75 (doubled), Diabetes, Stroke (doubled), Vascular disease, Age 65–74, and Sex category (female)] ≥1 (8). Therefore, differential risk of ischemic stroke exists between AF and AFL. There is no study to examine the risk of dementia between patients with AFL and AF. We hypothesized that patients with AF had a higher risk of dementia than patients with AFL independent of the previous history of stroke. Accordingly, we conducted this large population-based national cohort study to evaluate the incidence of dementia in patients with AF and AFL.
Taiwan's National Health Insurance started in 1995 and covers 99.5% (23 million) of the residents in Taiwan. The National Health Insurance Research Database (NHIRD) provides the data of all inpatient and outpatient services, diagnoses, emergency room visits, prescriptions, examinations, operations, and expenditures and are updated biannually. The diseases were diagnosed using the International Classification of Diseases, ninth revision, Clinical Modification (ICD-9-CM) and version 2001 codes. This study was approved by the Institutional Review Board of Chang Gung Memorial Hospital (202100865B1). The dataset used in this study was held by the Taiwan Ministry of Health and Welfare (MOHW). Any researcher interested in accessing this dataset can submit an application form to the MOHW requesting access. (email: c3RjYXJvbC13dUBtb2h3Lmdvdi50dw==).
Electronic medical records from the NHIRD between January 1, 2001 and December 31, 2013 were retrieved for patients with a discharge diagnosis or at least two consecutive outpatient clinic diagnoses of AF (ICD-9-CM: 427.31) and AFL (ICD-9-CM: 427.32). The time when AF or AFL was first diagnosed was assigned as the index date. The coverage period of our database was from 1997 to 2013; therefore, we excluded patients who were diagnosed with both AF and AFL between 1997 and 2000. Furthermore, we excluded patients who had incomplete demographic data (<0.1%), aged <20 years, history of valvular surgery, rheumatic heart disease, hyperthyroidism, and history of dementia (Figure 1). The remaining patients were categorized into two groups as newly diagnosed AF or newly diagnosed AFL without history of dementia. Furthermore, we excluded those patients who received radiofrequency ablation because these factors might also affect the risk of stroke and dementia.
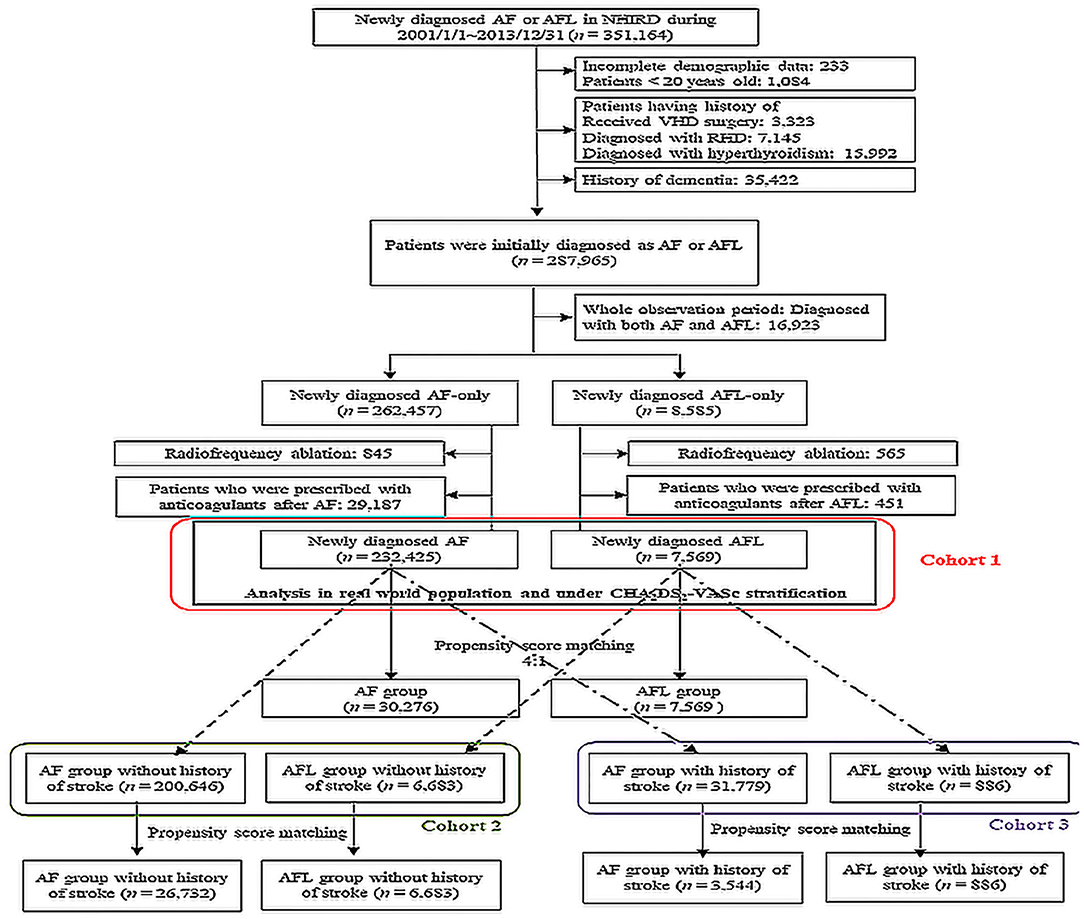
Figure 1. Flow chart of the patient selection. AF, atrial fibrillation; AFL, atrial flutter; NHIRD, National Health Insurance Research Database; RHD, rheumatic heart disease; VHD, valvular heart disease.
The primary outcome of dementia was analyzed after propensity score matching (PSM) between AF and AFL population in three different cohorts. In cohort 1, patients with AF (n = 232,425) and AFL (n = 7,569) were eligible population after serial excluding criteria. Cohort 2 [patients with AF (n = 200,646) and AFL (n = 6,683)] enrolled those patients without any history of ischemic stroke in cohort 1, while cohort 3 [patients with AF (n = 31,779) and AFL (n = 886)] enrolled those patients with a history of ischemic stroke in cohort 1 (Figure 1). The PSM was performed with 4:1 ratio under adjusting covariates including age, sex, comorbidities, and medications between AF and AFL cohorts. Furthermore, the primary outcome was also analyzed by stratifying the groups by CHA2DS2-VASc score in cohort 1 (Figure 1).
The primary outcome was dementia, which was defined when the diagnosis was made during hospitalization or ≥2 consecutive clinic visits. The diagnostic code of dementia was validated in previous NHIRD studies (9, 10). The first date when dementia was coded during follow-up was assigned as the date of the endpoint. Each patient in the three cohorts was followed up until the date of dementia occurrence, death, or December 31, 2013, whichever occurred first.
The covariates included comorbidities, namely hypertension, diabetes mellitus, dyslipidemia, ischemic heart disease, peripheral arterial disease, chronic obstructive pulmonary disease, renal function status, abnormal liver function, gout, systemic thromboembolism, myocardial infarction, stroke, and heart failure, and medications.
These comorbidities were ascertained according to the ICD-9-CM codes combined with medication use. The patients with systemic thromboembolism, myocardial infarction, stroke, and heart failure were defined as having any inpatient diagnosis before the index date. Most of these diagnostic codes were validated in previous NHIRD studies (11–15). Similarly, data on medication usage were retrieved on claim-based data of the previous year.
All patients who had AF or AFL diagnosis defined according to the diagnosis made at least once during hospitalization or ≥2 consecutive clinic visits. The accuracy of AF or AFL diagnosis using ICD-9-CM code in the NHIRD has been confirmed in previous studies (16, 17).
Because CHA2DS2-VASc score has been reported to be useful for risk stratification regarding ischemic stroke and dementia among patients with AF and AFL (18–21), we stratified patients using CHA2DS2-VASc score to compare the risk of dementia between patients with AF and AFL before and after PSM. Comorbidities in CHA2DS2-VASc score were ascertained according to the ICD-9-CM codes combined with medication use and the diagnostic codes were validated in previous NHIRD studies (12–15).
The propensity score matching was performed with 4:1 ratio in patients with AF and AFL. The covariates in the propensity score calculation were the age, gender, 12 comorbidities, 10 types of medication, and index date. The matching was processed using a greedy nearest neighbor algorithm with a caliper of 0.2 times the SD of the logit of the propensity score. The quality of matching was assessed using the standardized mean difference (SMD) between the two groups after matching, where a value <0.1 indicated a negligible difference (Table 1). The risk of dementia was compared between patients with AF and AFL before and after PSM. The risk of dementia between patients with AF and AFL was also compared after stratification by CHA2DS2-VASc score and previous history of stroke. A P < 0.05 was considered statistically significant. No adjustment for multiple testing (multiplicity) was performed in this study. All statistical analyses were performed using commercial software [Statistical Analysis System (SAS) V.9.4], including the “psmatch” procedure for PSM, “phreg” procedure for survival analysis, and “%cif ” macro for generating a cumulative incidence function through Fine and Gray's method.
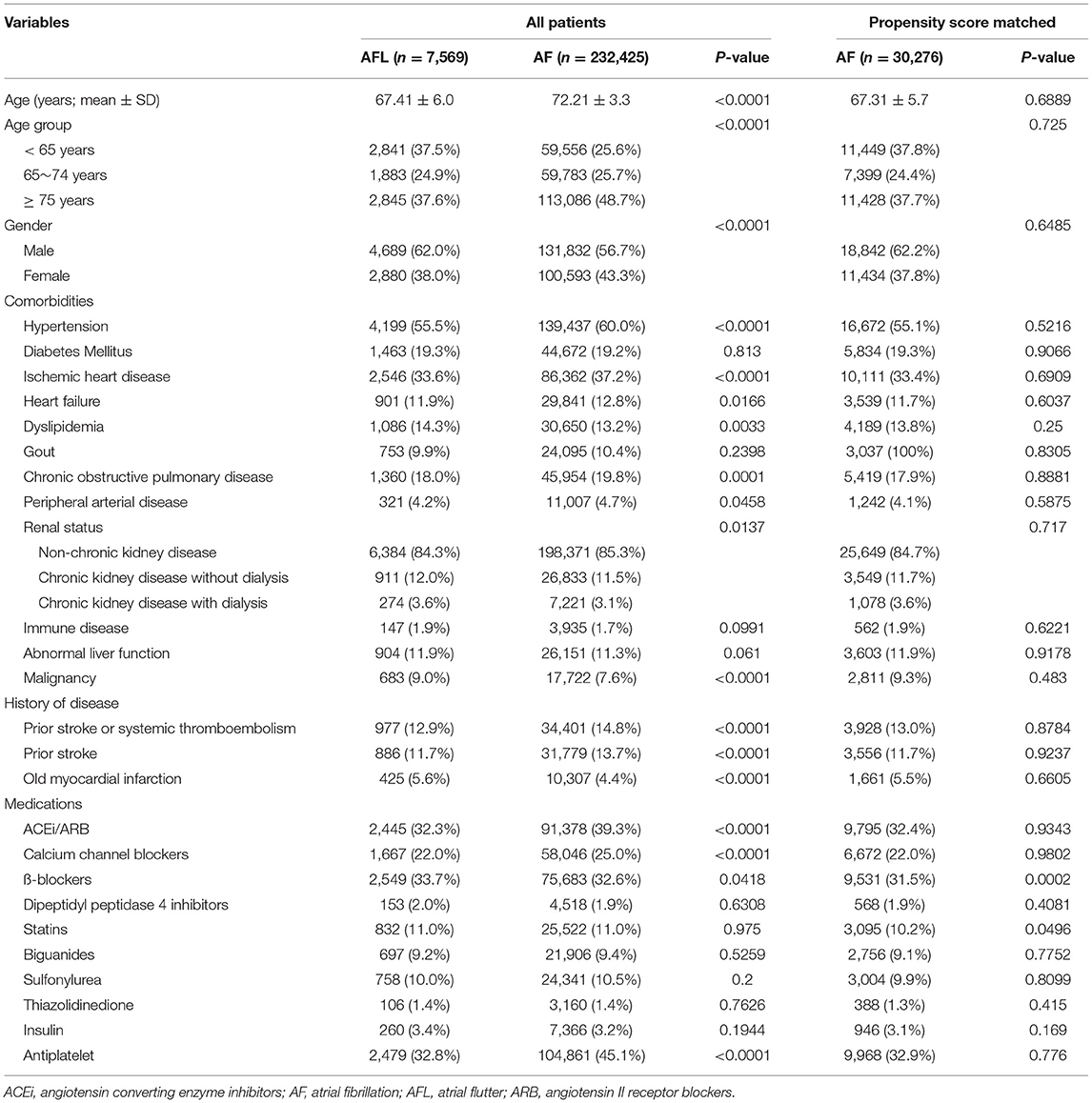
Table 1. Baseline characteristics of the atrial fibrillation and atrial flutter groups before and after propensity score matching.
Since this study is a retrospective cohort study based on a national insurance database, in no part or stage of the research were the patients/public involved.
We identified a total of 351,164 patients newly diagnosed with AF or AFL during 2001–2013 in the NHIRD. After exclusions, 232,425 patients with AF (aged 72.2 ±13.3 years) and 7,569 patients with AFL (aged 67.41 ± 6.0 years) without receiving oral anticoagulants were eligible for analysis in the study cohort 1 (Table 1). The distribution of comorbidities was similar between the patients with AF and AFL, except there was a higher prevalence of hypertension, ischemic heart disease, and history of thromboembolism/ischemic stroke in patients with AF compared with patients with AFL. After 4:1 ratio of PSM using all variables in Table 1 and there was a good balance between the patients with AF and AFL, except for ß-blockers (right panel of Table 1), 30,276 patients with AF (aged 67.3 ±15.7 years) and 7,569 patients with AFL were analyzed for comparison of the risk of dementia.
Moreover, we further categorized the original 232,425 patients with AF and 7,569 patients with AFL in the cohort 1 into cohorts 2 and 3 according to history without (cohort 2) or with stroke (cohort 3) and performed the additional PSM in each cohort. After PSM, there were 26,732 patients with AF and 6,683 patients with AFL in the cohort 2, and 3,544 patients with AF and 886 patients with AFL in the cohort 3 (Figure 1).
Before PSM, the incidence densities of dementia (hazard ratio [HR], 1.52; 95% CI, 1.39–1.66; P < 0.0001) and ischemic stroke (HR, 2.15; 95% CI, 1.92–2.42; P < 0.0001) were higher in patients with AF than in patients with AFL (Table 2). After PSM, the incidence densities of dementia (HR, 1.14; 95% CI, 1.04–1.25; P = 0.0064) and ischemic stroke (HR, 1.76; 95% CI, 1.56–1.98; P < 0.0001) were still higher in patients with AF than in patients with AFL (Table 2). The cumulative incidences of dementia in patients with AF and AFL before and after PSM were shown in Figures 2A,B.
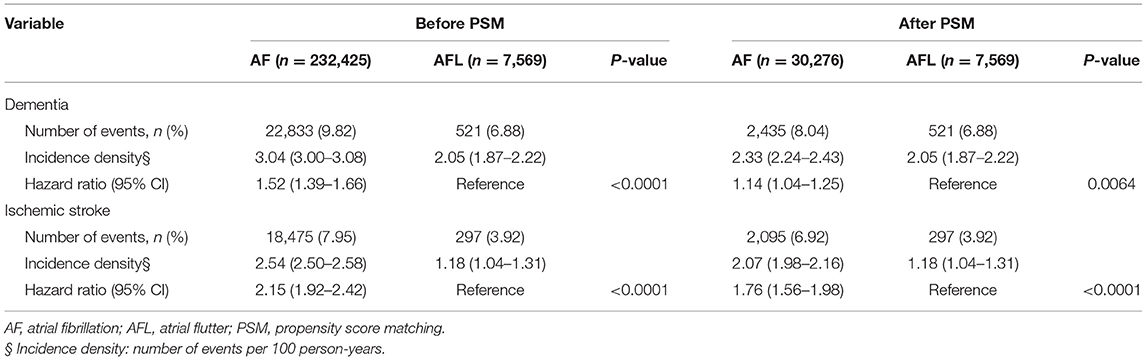
Table 2. Clinical outcomes between the patients with atrial fibrillation and atrial flutter not receiving warfarin therapy before and after PSM.
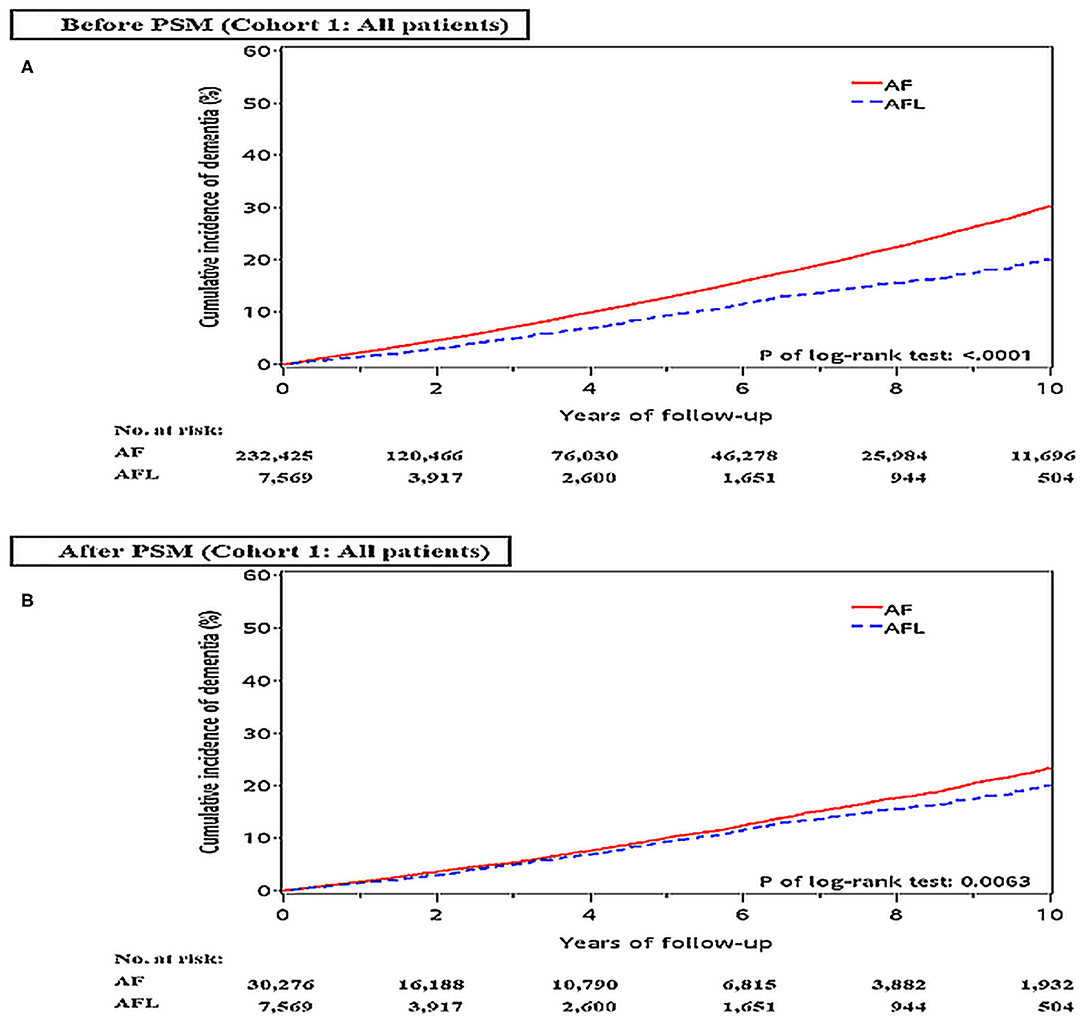
Figure 2. Cumulative incidences of dementia at the end of follow-up between the AF and AFL groups in the whole cohort (cohort 1) before (A) and after (B) PSM. AF, atrial fibrillation; AFL, atrial flutter; PSM, propensity score matching.
There were 200,646 patients with AF without previous history of stroke and 6,683 patients with AFL without previous history of stroke (Table 3, Figure 1). After 4:1 PSM, 26,732 patients with AF and 6,683 patients with AFL were well balanced, except for ß-blockers (Table 3). Before PSM, the incidence densities of dementia (HR, 1.54; 95% CI, 1.40–1.69; P < 0.0001) and ischemic stroke (HR, 2.32; 95% CI, 2.04–2.64; P < 0.0001) were higher in patients with AF than in patients with AFL (Table 4). After PSM, the incidence densities of dementia (HR, 1.15; 95% CI, 1.03–1.27; P = 0.0098) and ischemic stroke (HR, 1.82; 95% CI, 1.59–2.09; P < 0.0001) were higher in patients with AF than in patients with AFL (Table 4).
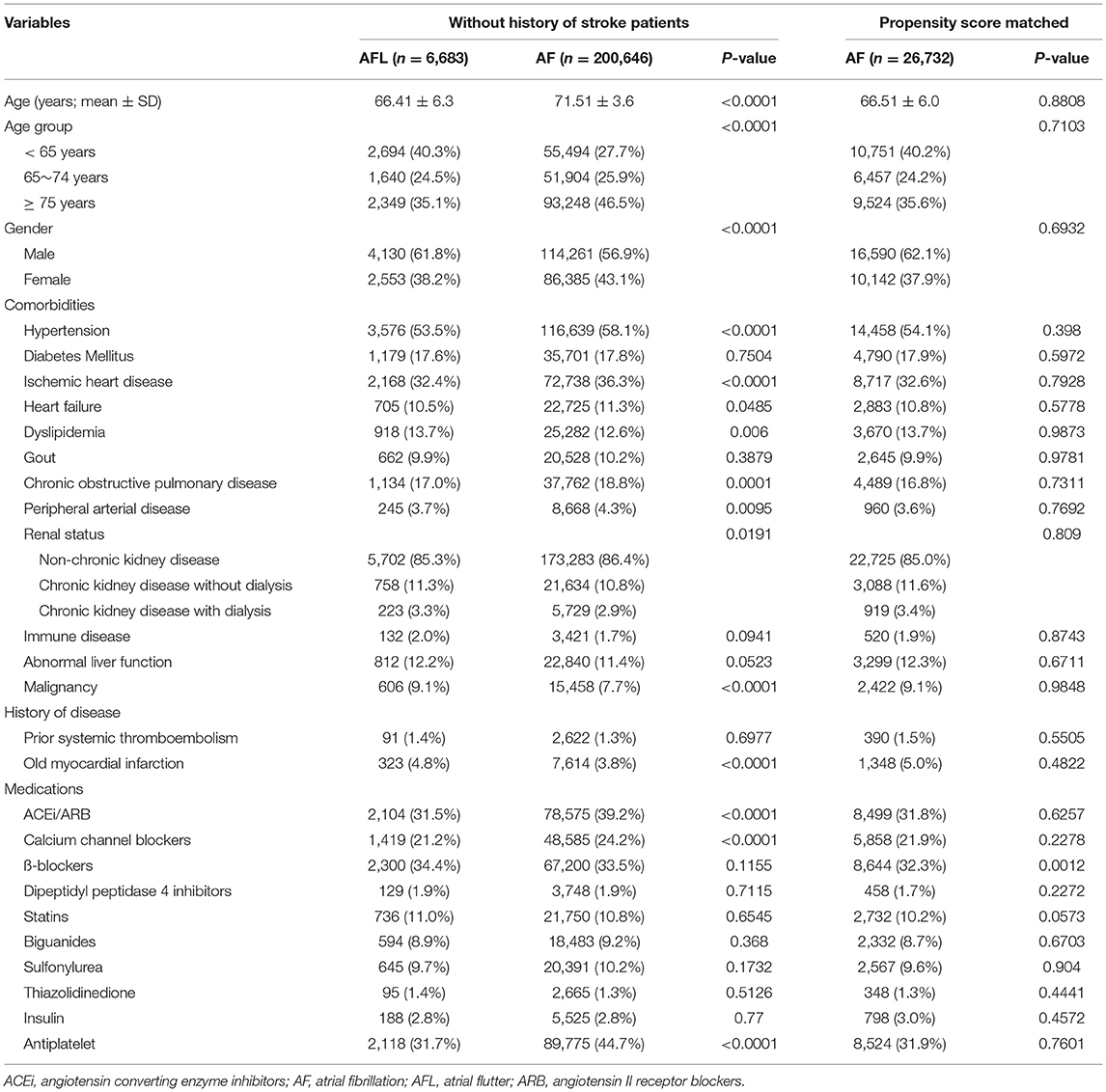
Table 3. Baseline characteristics of the atrial fibrillation and atrial flutter groups before and after PSM (without history of stroke).
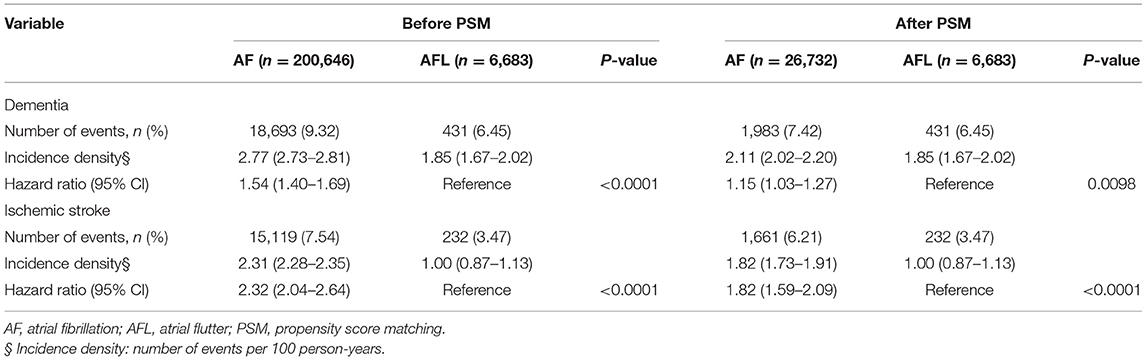
Table 4. Clinical outcomes between the patients with atrial fibrillation and atrial flutter before and after PSM (without history of stroke).
The cumulative incidences of dementia in patients with AF and AFL without previous history of stroke before and after PSM were shown in Figures 3A,B.
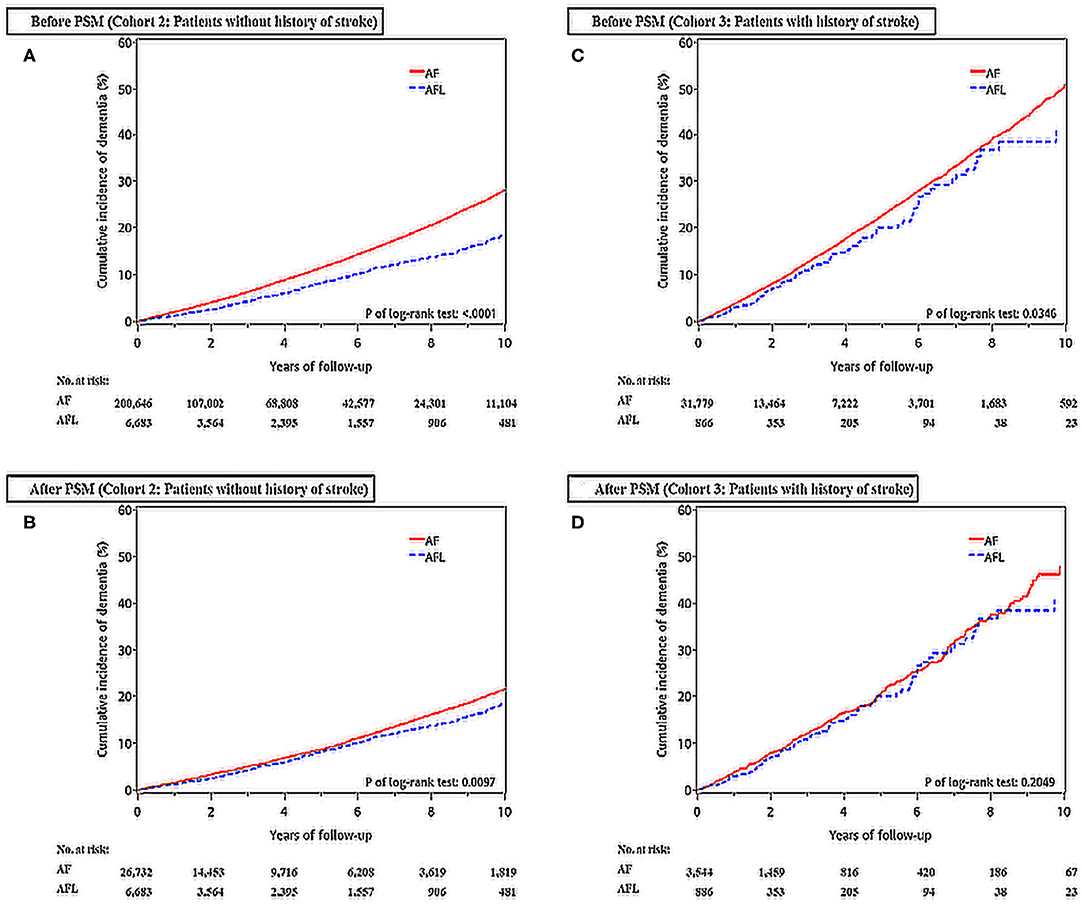
Figure 3. Cumulative incidences of dementia at the end of follow-up between the AF and AFL groups before and after PSM in those patients without (cohort 2) and with (cohort 3) history of stroke. (A) Patients without history of stroke before PSM; (B) Patients without history of stroke after PSM; (C) Patients with history of stroke before PSM; (D) Patients with history of stroke after PSM.
There were 31,779 patients with AF with a history of stroke and 886 patients with AFL with a history of stroke (Table 5, Figure 1). After 4:1 PSM, 3,544 patients with AF and 886 patients with AFL were well balanced (Table 5). Before PSM, the incidence densities of dementia (HR, 1.25; 95% CI, 1.02–1.54; P = 0.0351) and recurrent ischemic stroke (HR, 1.39; 95% CI, 1.09–1.78; P = 0.0083) were higher in patients with AF than in patients with AFL (Table 6). After PSM, the incidence density of dementia did not differ between patients with AF and AFL (HR, 1.16; 95% CI, 0.92–1.45; P = 0.2058) (Table 6). However, the incidence density of recurrent ischemic stroke (HR, 1.40; 95% CI, 1.08–1.83; P = 0.0118) was higher in patients with AF than in patients with AFL (Table 6).
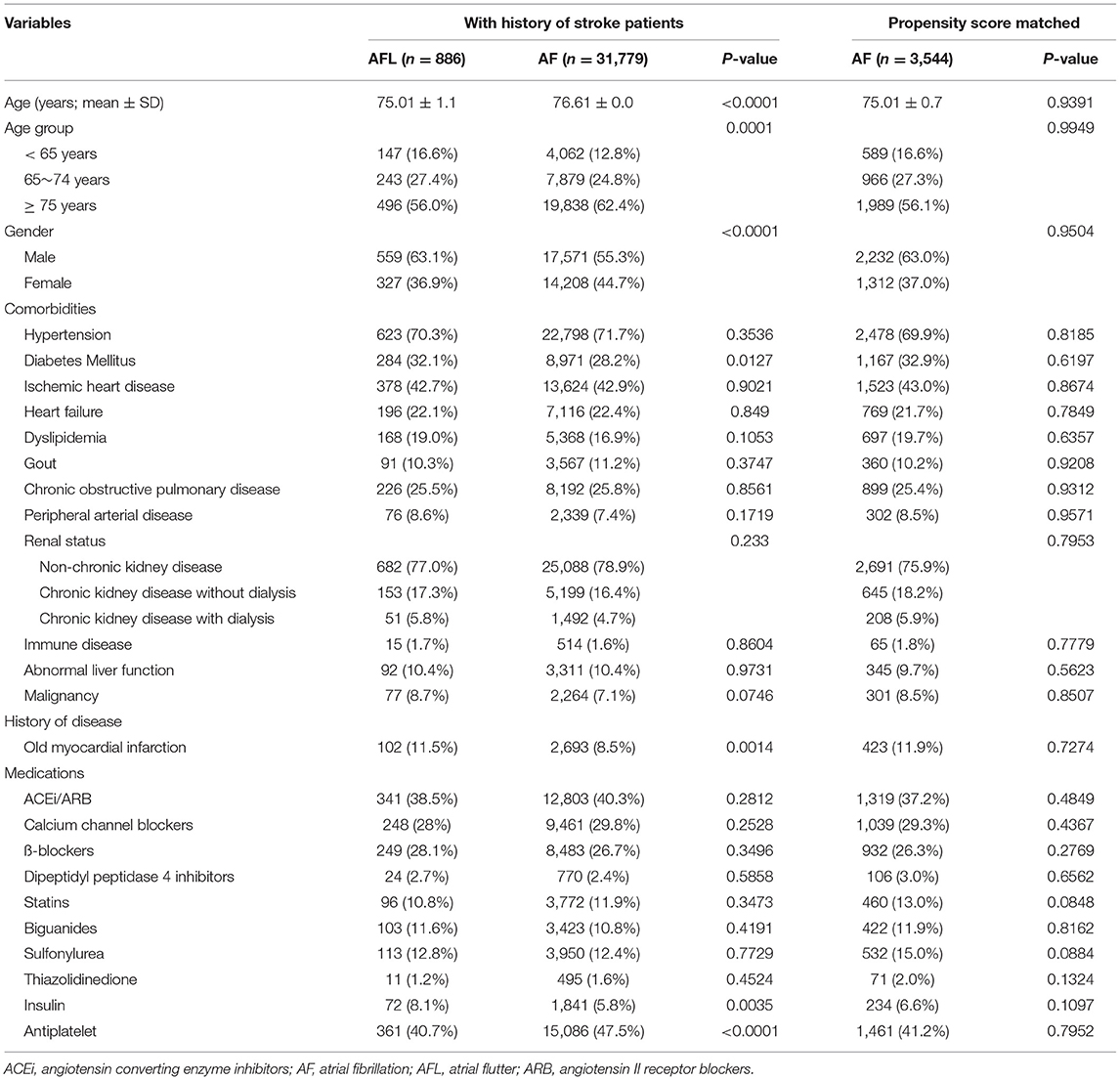
Table 5. Baseline characteristics of the atrial fibrillation and atrial flutter groups before and after PSM (with history of stroke).
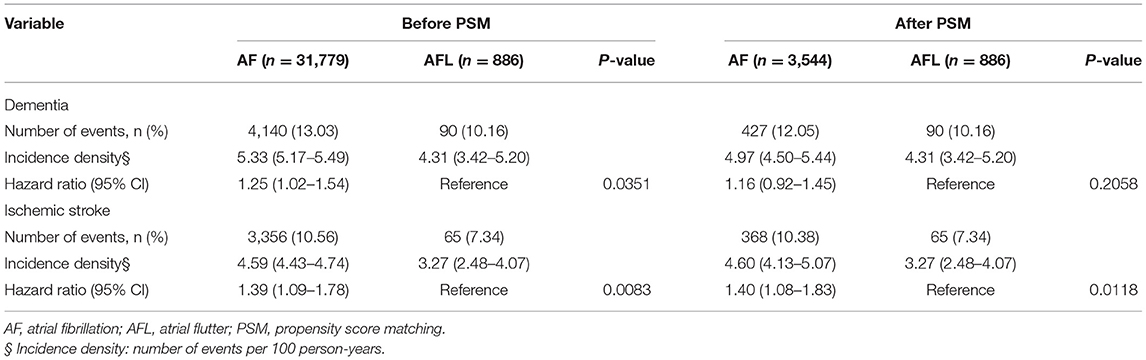
Table 6. Clinical outcomes between the patients with atrial fibrillation and atrial flutter before and after PSM (with history of stroke).
The cumulative incidences of dementia in patients with AF and AFL with a history of stroke before and after PSM were shown in Figures 3C,D.
The risk of dementia in the cohort 1 was stratified according to CHA2DS2-VASc score. Before PSM, the incidence density of dementia was higher in patients with AF than in patients with AFL across CHA2DS2-VASc scores of 1–4 and 6–9 (Figure 4). After PSM, the cumulative incidences of dementia in patients with AF and AFL stratified according to different CHA2DS2-VASc scores were shown in Figure 5, and the cumulative incidences of dementia were significantly higher in patients with AF than patients with AFL in patients with CHA2DS2-VASc score ≤ 2 (P < 0.0001) and patients with CHA2DS2-VASc score between 3 and 4 (P = 0.0104), but not in patients with CHA2DS2-VASc score ≥ 5.
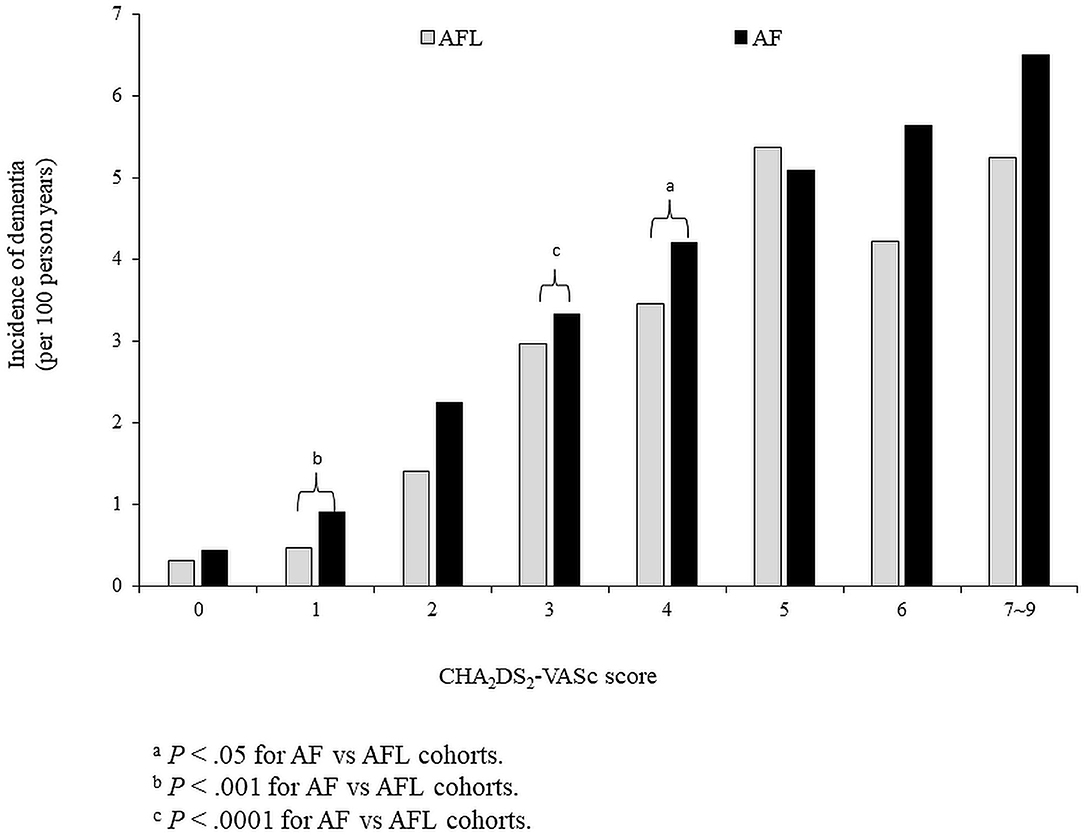
Figure 4. The risk of dementia in the whole cohort (cohort 1) was stratified according to CHA2DS2-VASc score before propensity score matching. AF, atrial fibrillation; AFL, atrial flutter.
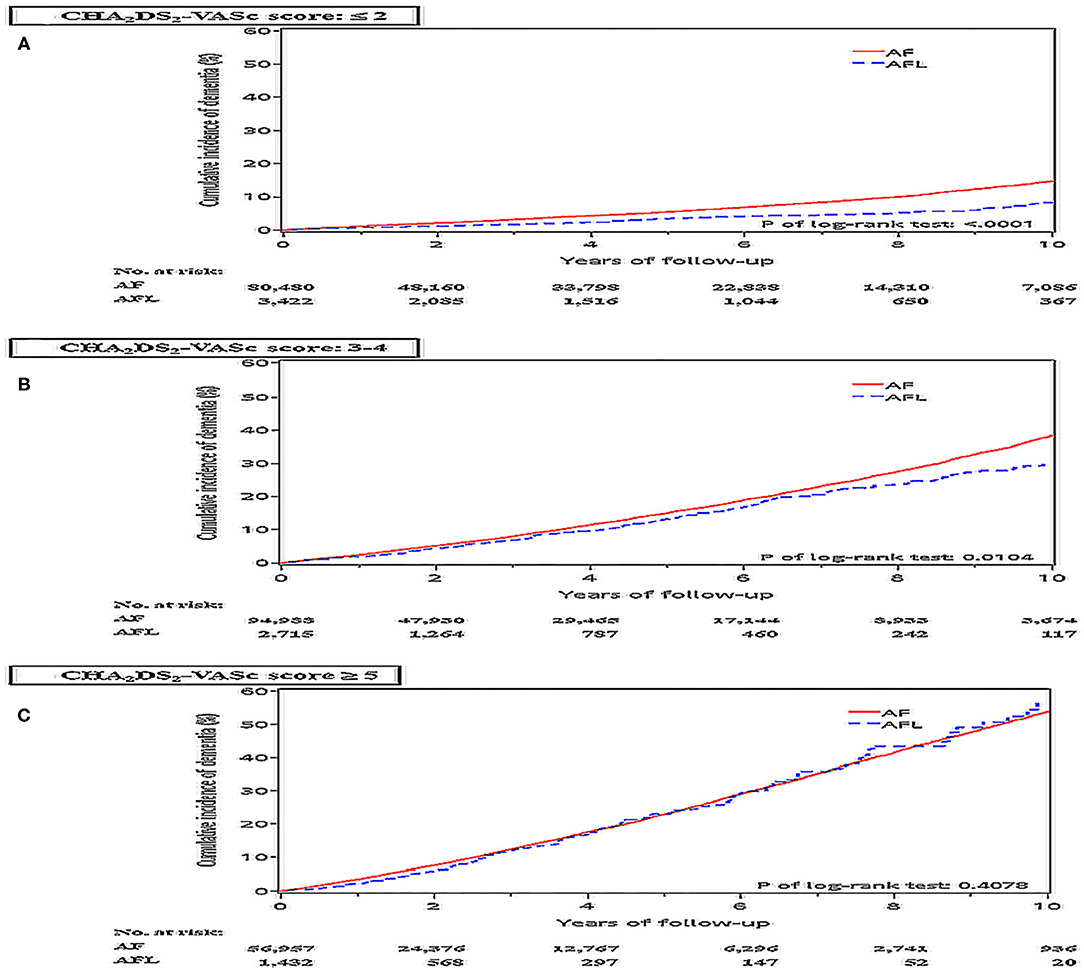
Figure 5. Cumulative incidences of dementia at the end of follow up between the AF and AFL groups stratified by CHA2DS2-VASc score in the whole cohort (cohort 1) after PSM. (A) CHA2DS2-VASc score ≤ 2; (B) CHA2DS2-VASc score: 3-4; (C) CHA2DS2-VASc score ≥ 5.
Because the usage of oral anticoagulant might affect the risk of dementia and ischemic stroke in patients with AF and AFL, we identified and analyzed the risk of dementia and ischemic stroke between patients newly diagnosed with AF (n = 29,187) and AFL (n = 451) who received oral anticoagulants (mainly vitamin K oral anticoagulant) in another dataset during the same study period in the NHIRD.
Before PSM, the incidence densities of dementia (HR, 2.05; 95% CI, 1.35–3.11; P = 0.0008) and ischemic stroke (HR, 2.95; 95% CI, 1.86–4.62; P < 0.0001) were higher in patients with AF than in patients with AFL (Table 7). After PSM, the incidence density of dementia (HR, 1.57; 95% CI, 1.00–2.45; P = 0.0501) was higher with borderline significance in patients with AF than in patients with AFL, and the incidence density of ischemic stroke (HR, 2.54; 95% CI, 1.56–4.12; P = 0.0002) was significantly higher in patients with AF than in patients with AFL (Supplemental Table 5). The cumulative incidences of dementia in patients with AF and AFL before and after PSM were shown in Figures 6A,B and the cumulative incidences of dementia were significantly higher in patients with AF than in patients with AFL before and after PSM.
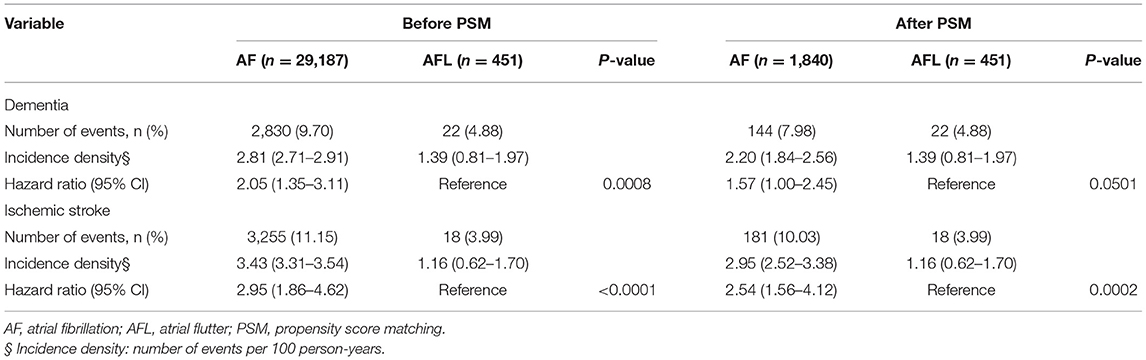
Table 7. Clinical outcomes between the patients with atrial fibrillation and atrial flutter who received oral anticoagulants before and after PSM.
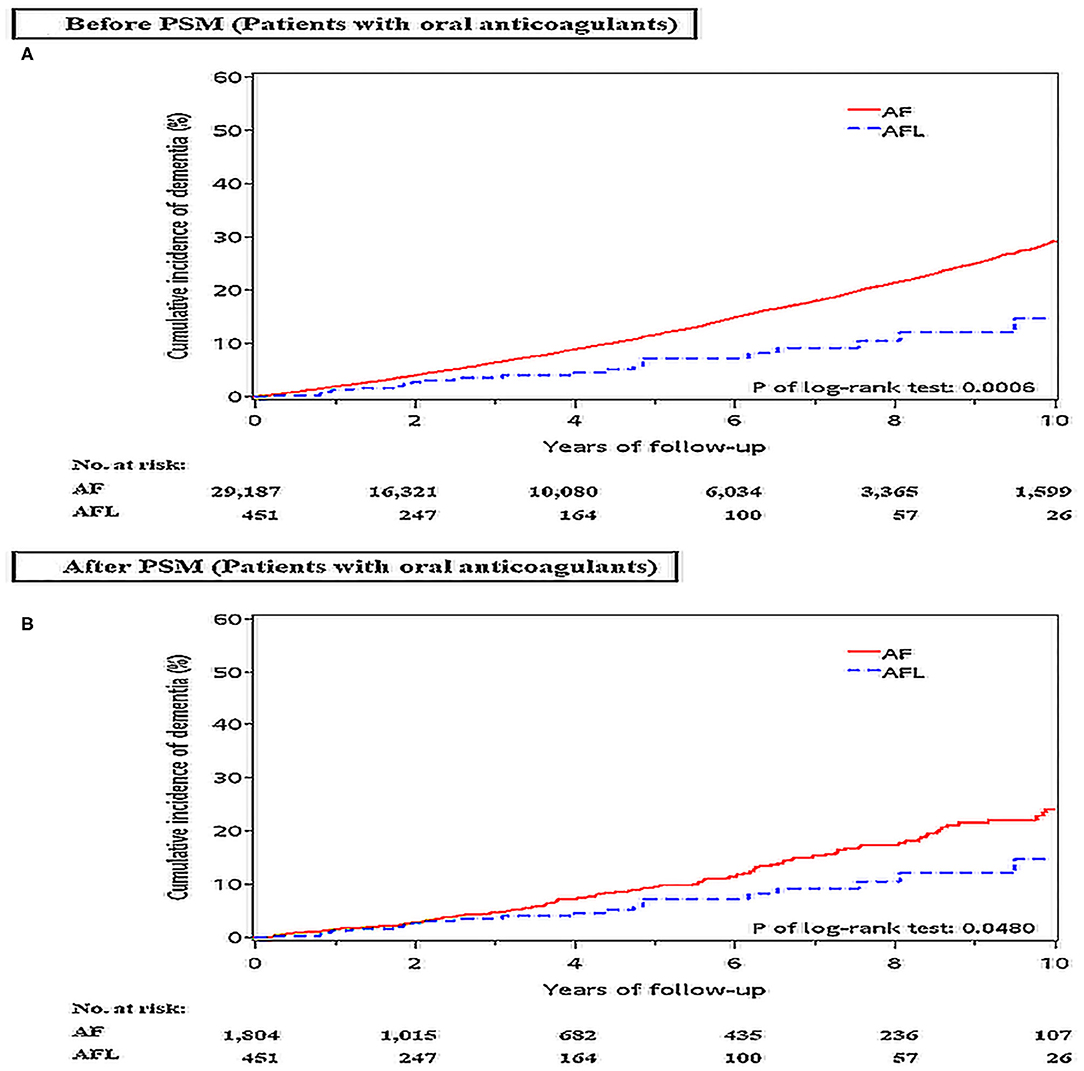
Figure 6. Cumulative incidences of dementia at the end of follow-up between patients with AF and AFL receiving oral anticoagulants therapy before (A) and after (B) PSM. AF: atrial fibrillation; AFL, atrial flutter; PSM, propensity score matching.
The main findings of this study were (1) among patients without oral anticoagulants, the incidence densities of dementia and ischemic stroke were significantly higher in patients with AF than in patients with AFL before and after PSM; (2) among patients without oral anticoagulants, the incidence densities of dementia and ischemic stroke were significantly higher in patients with AF than in patients with AFL among patients without previous history of stroke before and after PSM; (3) among patients without oral anticoagulants, the incidence density of dementia did not differ between patients with AF and AFL among patients with previous history of stroke after PSM; (4) among patients without oral anticoagulants, the cumulative incidence of dementia was significantly higher in patients with AF than patients with AFL only in patients with CHA2DS2-VASc score ≤ 4; (5) among patients who received oral anticoagulants, the cumulative incidences of dementia were significantly higher in patients with AF than in patients with AFL before and after PSM.
Atrial fibrillation provides five-fold increase in the risk of stroke and AF is associated with more severe ischemic strokes than emboli from carotid disease (18, 22). It has been reported that AF is associated with cognitive dysfunction and dementia, and may increase the risk of developing dementia (4, 23–26). Several pathophysiologic mechanisms have been proposed to explain the complex association between AF and dementia, which include cerebral hypoperfusion, vascular inflammation, cerebral small vessel disease, microbleeds, brain atrophy, and AF-related ischemic stroke (3, 5, 23, 27, 28). AF and AFL share some common risk factors for cognitive dysfunction or dementia. When more cardiovascular disease risks coexist, the risk of dementia in patients with AF will increase significantly. CHA2DS2-VASc score has been reported to be useful for risk stratification regarding ischemic stroke and dementia among patients with AF and AFL (18–21, 29). Study conducted by Chou et al. reported that CHADS2 score had a good prediction of vascular dementia in patients with AF and AFL (3). The higher the score, the higher the number of patients suffering from dementia. CHA2DS2-VASc score is composed of chronic diseases and also a history of stroke. Therefore, not only AF or AFL alone might contribute to the development of dementia, but all the comorbidities do. In this study, we also showed that the risk of dementia is higher in both AF and AFL cohort with higher CHA2DS2-VASc scores. Based on our and other studies, the CHA2DS2-VASc score might be a useful risk scoring system for predicting future risk of development of vascular dementia and for risk stratification in patients with AF and AFL at risk of developing dementia (18). In this study, patients with AF were older than patients with AFL and had more comorbidities, including prior stroke, hypertension, ischemic heart disease, heart failure, and a higher average CHA2DS2-VASc score. All of these differences may contribute to a higher incidence of dementia in patients with AF than in patients with AFL before adjusting the comorbidities in this study. However, the incidence of dementia is still higher in patients with AF than in patients with AFL even after adjusting the comorbidities and medications by PSM, especially in those patients without previous history of stroke. Therefore, differential risk of dementia existed between AF and AFL patients without previous history of stroke.
Although AF and AFL share some common risk factors and can both be risk stratified by CHA2DS2-VASc score, the pathophysiological mechanisms of AF and AFL are different. The initiation and maintenance of AF usually involved the pulmonary veins and depended on multiple chaotic rotors in the arrhythmogenic substrate in the left or right atrium for maintenance. In contrast, the maintenance of AFL depended on a single reentry circuit, such as single reentry circuit in the right atrium of a typical AFL. Our previous study showed that the incidences of ischemic stroke, hospitalization for heart failure, and all-cause mortality are different between AF and AFL, which might be related to the different pathophysiological mechanisms between AF and AFL (8, 30). Although the risk of dementia is higher in patients with AF than in patients with AFL in this present study, further studies should be conducted to explore the pathophysiologic mechanisms of differential risk of dementia between patients with AF and AFL.
The previous studies showed that there were conflicting data regarding the therapeutic effect of oral anticoagulants on the risk of dementia in patients with AF (31). Although oral anticoagulants might decrease the risk of dementia by reducing the risk of silent infarct and microembolism, microhemorrhages, especially due to supratherapeutic range of anticoagulation, may have implications for the mechanism of dementia (32, 33). In contrast, non-vitamin K oral anticoagulants may significantly reduce the occurrence of dementia (33–35). This may explain the discrepancy of oral anticoagulants on the risk of dementia in patients with AF. Our studies showed that among patients who received oral anticoagulants (mainly vitamin K oral anticoagulant), the cumulative incidence of dementia was significantly higher in patients with AF than in patients with AFL. Accordingly, the usage of oral anticoagulants may not affect the differential risk of dementia between patients with AF and AFL. Further prospective randomized studies are warranted to validate our findings and to investigate the effect of different oral anticoagulants on the risk of dementia in patients with AF and AFL.
Several limitations are associated with epidemiologic data from the NHIRD. First, using ICD-9-CM codes for patient selection may result in some missing cases due to incorrect coding. Some patients who had dementia with indistinct symptoms may be also missed due to the unavailability of clinical characteristics or image studies. Second, certain misclassification of disease leading to the miscalculation of the CHA2DS2-VASc score may have occurred due to the retrospective nature of the study. However, the highly diagnostic accuracy of comorbidities in CHA2DS2-VASc score has been validated in previous studies. Third, we could not categorize the mechanisms of AFL into typical or atypical AFL and right or left AFL by ICD-9-CM codes. The clinical outcome may be different between patients with typical and atypical AFL and also between patients with left and right AFL. Further investigation should be conducted to clarify this issue. Fourth, AF was developed in around 30% AFL during follow-up according to previous studies (36). Although we have excluded those patients who have concomitant AF and AFL, there is still the possibility that some patients with AFL who developed AF during follow-up might not be diagnosed in the clinical follow-up. Despite this concern, we showed that the incidence density of dementia was significantly higher in patients with AF than in patients with AFL without previous history of stroke. Fifth, this study showed that among patients who received oral anticoagulants (mainly vitamin K oral anticoagulants), the cumulative incidence of dementia was significantly higher in patients with AF than in patients with AFL. However, this study did not analyze the effect of non-vitamin K oral anticoagulants on the risk of dementia in patients with AF and AFL. A previous study showed that non-vitamin K oral anticoagulants may lower the risk of dementia in patients with AF (33–35). Further prospective randomized studies are warranted to investigate the effect of different non-vitamin K oral anticoagulants on the risk of dementia in patients with AF and AFL. Sixth, although HAS-BLED score (hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile international normalized ratio, elderly [age ≥65 years], drugs/alcohol concomitantly) and CHA2DS2-VASc score share many common risk factors, in a real-world population with AF, the CHA2DS2-VASc and HAS-BLED risk classifications are correlated but not exchangeable (37). Future research should be conducted to investigate the risk of dementia stratified according to HAS-BLED score alone or combined CHA2DS2-VASc score and HAS-BLED score between patients with AF and AFL. Seventh, although this study used PSM with all known variables to achieve a good balance between patients with AF and AFL, unavailable confounding risk factors might be still present. Finally, this study did not subclassify all subtypes of dementia. There might be a difference in the risk of developing different subtypes of dementia between AF and AFL. Further studies could be conducted to clarify this issue.
This large nationwide cohort study showed that among patients without oral anticoagulants, the risk of dementia was higher in patients with AF than in patients with AFL. Among patients without oral anticoagulants, the risk of dementia was higher in patients with AF than in patients with AFL with CHA2DS2-VASc score ≤ 4. Among patients without oral anticoagulants, the risk of dementia was higher in patients with AF than in patients with AFL among patients without previous history of stroke before and after PSM but the risk did not differ between patients with AF and AFL among patients with a previous history of stroke after PSM. Among patients who received oral anticoagulants, the cumulative incidences of dementia were significantly higher in patients with AF than in patients with AFL before and after PSM. Our study implicated that CHA2DS2-VASc score might be useful for risk stratification of dementia between patients with AF and AFL.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Institutional Review Board of Chang Gung Memorial Hospital. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
C-MC and M-CC led in the conception and design of the study, revised the draft of the manuscript, and supervised and validated the clinical work and results. H-TW, Y-LC, and Y-SL collected research data, prepared the draft of the manuscript, performed the statistical analysis, and drafted the manuscript. H-CC, S-ZC, and SH organized the collected data. All authors have read and agreed to the published version of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Freedman B, Potpara TS, Lip GY. Stroke prevention in atrial fibrillation. Lancet. (2016) 388:806–17. doi: 10.1016/S0140-6736(16)31257-0
2. Staerk L, Sherer JA, Ko D, Benjamin EJ, Helm RH. Atrial Fibrillation: Epidemiology, pathophysiology, and clinical outcomes. Circ Res. (2017) 120:1501–17. doi: 10.1161/CIRCRESAHA.117.309732
3. Chou RH, Chiu CC, Huang CC, Chan WL, Huang PH, Chen YC, et al. Prediction of vascular dementia and Alzheimer's disease in patients with atrial fibrillation or atrial flutter using CHADS2 score. J Chin Med Assoc. (2016) 79:470–6. doi: 10.1016/j.jcma.2016.02.007
4. Aldrugh S, Sardana M, Henninger N, Saczynski JS, McManus DD. Atrial fibrillation, cognition and dementia: A review. J Cardiovasc Electrophysiol. (2017) 28:958–65. doi: 10.1111/jce.13261
5. Chopard R, Piazza G, Gale SA, Campia U, Albertsen IE, Kim J, et al. Dementia and atrial fibrillation: pathophysiological mechanisms and therapeutic implications. Am J Med. (2018) 131:1408–17. doi: 10.1016/j.amjmed.2018.06.035
6. Saglietto A, Matta M, Gaita F, Jacobs V, Bunch TJ, Anselmino M. Stroke-independent contribution of atrial fibrillation to dementia: a meta-analysis. Open Heart. (2019) 6:e000984. doi: 10.1136/openhrt-2018-000984
7. January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC Jr., et al. AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American college of cardiology/American heart association task force on clinical practice guidelines and the heart rhythm society. J Am Coll Cardiol. (2019) 74:104–32. doi: 10.1016/j.jacc.2019.01.011
8. Lin YS, Chen TH, Chi CC, Lin MS, Tung TH, Liu CH, et al. Different implications of heart failure, ischemic stroke, and mortality between nonvalvular atrial fibrillation and atrial flutter-a view from a national cohort study. J Am Heart Assoc. (2017) 6:e006406. doi: 10.1161/JAHA.117.006406
9. Chen CW, Lin CC, Chen KB, Kuo YC, Li CY, Chung CJ. Increased risk of dementia in people with previous exposure to general anesthesia: a nationwide population-based case-control study. Alzheimers Dement. (2014) 10:196–204. doi: 10.1016/j.jalz.2013.05.1766
10. Zhang B, Wang HE, Bai YM, Tsai SJ, Su TP, Chen TJ, et al. Inflammatory bowel disease is associated with higher dementia risk: a nationwide longitudinal study. Gut. (2021) 70:85–91. doi: 10.1136/gutjnl-2020-320789
11. Wu CS, Lai MS, Gau SS, Wang SC, Tsai HJ. Concordance between patient self-reports and claims data on clinical diagnoses, medication use, and health system utilization in Taiwan. PLoS ONE. (2014) 9:e112257. doi: 10.1371/journal.pone.0112257
12. Lin CC, Lai MS, Syu CY, Chang SC, Tseng FY. Accuracy of diabetes diagnosis in health insurance claims data in Taiwan. J Formos Med Assoc. (2005) 104:157–63.
13. Hsieh CY, Chen CH, Li CY, Lai ML. Validating the diagnosis of acute ischemic stroke in a National Health Insurance claims database. J Formos Med Assoc. (2015) 114:254–9. doi: 10.1016/j.jfma.2013.09.009
14. Cheng CL, Lee CH, Chen PS, Li YH, Lin SJ, Yang YH. Validation of acute myocardial infarction cases in the national health insurance research database in taiwan. J Epidemiol. (2014) 24:500–7. doi: 10.2188/jea.JE20140076
15. Chang SL, Huang YL, Lee MC, Hu S, Hsiao YC, Chang SW, et al. Association of varicose veins with incident venous thromboembolism and peripheral artery disease. JAMA. (2018) 319:807–17. doi: 10.1001/jama.2018.0246
16. Lin LJ, Cheng MH, Lee CH, Wung DC, Cheng CL, Kao Yang YH. Compliance with antithrombotic prescribing guidelines for patients with atrial fibrillation–a nationwide descriptive study in Taiwan. Clin Ther. (2008) 30:1726–36. doi: 10.1016/j.clinthera.2008.09.010
17. Chang CH, Lee YC, Tsai CT, Chang SN, Chung YH, Lin MS, et al. Continuation of statin therapy and a decreased risk of atrial fibrillation/flutter in patients with and without chronic kidney disease. Atherosclerosis. (2014) 232:224–30. doi: 10.1016/j.atherosclerosis.2013.11.036
18. Joundi RA, Cipriano LE, Sposato LA, Saposnik G, Stroke outcomes research working G. Ischemic stroke risk in patients with atrial fibrillation and CHA2DS2-VASc score of 1: systematic review and meta-analysis. Stroke. (2016) 47:1364–7. doi: 10.1161/STROKEAHA.115.012609
19. Al-Kawaz M, Omran SS, Parikh NS, Elkind MSV, Soliman EZ, Kamel H. Comparative risks of ischemic stroke in atrial flutter versus atrial fibrillation. J Stroke Cerebrovasc Dis. (2018) 27:839–44. doi: 10.1016/j.jstrokecerebrovasdis.2017.10.025
20. Chao TF, Fauchier L. Stroke prevention in patients with atrial flutter: many questions still unanswered. Europace. (2019) 21:186–7. doi: 10.1093/europace/euy197
21. Graves KG, May HT, Jacobs V, Knowlton KU, Muhlestein JB, Lappe DL, et al. CHA2DS2-VASc scores and Intermountain Mortality Risk Scores for the joint risk stratification of dementia among patients with atrial fibrillation. Heart Rhythm. (2019) 16:3–9. doi: 10.1016/j.hrthm.2018.10.018
22. Pistoia F, Sacco S, Tiseo C, Degan D, Ornello R, Carolei A. The epidemiology of atrial fibrillation and stroke. Cardiol Clin. (2016) 34:255–68. doi: 10.1016/j.ccl.2015.12.002
23. Dietzel J, Haeusler KG, Endres M. Does atrial fibrillation cause cognitive decline and dementia? Europace. (2018) 20:408–19. doi: 10.1093/europace/eux031
24. Field TS, Weijs B, Curcio A, Giustozzi M, Sudikas S, Katholing A, et al. Incident atrial fibrillation, dementia and the role of anticoagulation: a population-based cohort study. Thromb Haemost. (2019) 119:981–91. doi: 10.1055/s-0039-1683429
25. Kim D, Yang PS, Yu HT, Kim TH, Jang E, Sung JH, et al. Risk of dementia in stroke-free patients diagnosed with atrial fibrillation: data from a population-based cohort. Eur Heart J. (2019) 40:2313–23. doi: 10.1093/eurheartj/ehz386
26. Krawczyk M, Fridman S, Cheng Y, Fang J, Saposnik G, Sposato LA. Atrial fibrillation diagnosed after stroke and dementia risk: cohort study of first-ever ischaemic stroke patients aged 65 or older. Europace. (2019) 21:1793–801. doi: 10.1093/europace/euz237
27. Gallinoro E, D'Elia S, Prozzo D, Lioncino M, Natale F, Golino P, et al. Cognitive function and atrial fibrillation: from the strength of relationship to the dark side of prevention. Is there a contribution from sinus rhythm restoration and maintenance? Medicina (Kaunas). (2019) 55:587. doi: 10.3390/medicina55090587
28. Wandell P, Carlsson AC, Sundquist J, Sundquist K. The association between relevant comorbidities and dementia in patients with atrial fibrillation. Geroscience. (2018). doi: 10.1007/s11357-018-0029-8
29. Bunch TJ. Atrial Fibrillation and Dementia. Circulation. (2020) 142:618–20. doi: 10.1161/CIRCULATIONAHA.120.045866
30. Lin YS, Chen YL, Chen TH, Lin MS, Liu CH, Yang TY, et al. Comparison of clinical outcomes among patients with atrial fibrillation or atrial flutter stratified by CHA2DS2-VASc score. JAMA Netw Open. (2018) 1:e180941. doi: 10.1001/jamanetworkopen.2018.0941
31. Moffitt P, Lane DA, Park H, O'Connell J, Quinn TJ. Thromboprophylaxis in atrial fibrillation and association with cognitive decline: systematic review. Age Ageing. (2016) 45:767–75. doi: 10.1093/ageing/afw104
32. Jacobs V, Woller SC, Stevens S, May HT, Bair TL, Anderson JL, et al. Time outside of therapeutic range in atrial fibrillation patients is associated with long-term risk of dementia. Heart Rhythm. (2014) 11:2206–13. doi: 10.1016/j.hrthm.2014.08.013
33. Cheng W, Liu W, Li B, Li D. Relationship of anticoagulant therapy with cognitive impairment among patients with atrial fibrillation: a meta-analysis and systematic review. J Cardiovasc Pharmacol. (2018) 71:380–7. doi: 10.1097/FJC.0000000000000575
34. Madhavan M, Graff-Radford J, Piccini JP, Gersh BJ. Cognitive dysfunction in atrial fibrillation. Nat Rev Cardiol. (2018) 15:744–56. doi: 10.1038/s41569-018-0075-z
35. Mongkhon P, Naser AY, Fanning L, Tse G, Lau WCY, Wong ICK, et al. Oral anticoagulants and risk of dementia: A systematic review and meta-analysis of observational studies and randomized controlled trials. Neurosci Biobehav Rev. (2019) 96:1–9. doi: 10.1016/j.neubiorev.2018.10.025
36. Chen YL, Wang HT, Chen HC, Liu WH, Chong SZ, Hsueh SK, et al. Proposed A2C2S2-VASc score for predicting atrial fibrillation development in patients with atrial flutter. Open Heart. (2021) 8:e001478. doi: 10.1136/openhrt-2020-001478
Keywords: atrial fibrillation, atrial flutter, dementia, stroke, CHA2DS2-VASc score
Citation: Wang H-T, Chen Y-L, Lin Y-S, Chen H-C, Chong S-Z, Hsueh S, Chung C-M and Chen M-C (2021) Differential Risk of Dementia Between Patients With Atrial Flutter and Atrial Fibrillation: A National Cohort Study. Front. Cardiovasc. Med. 8:787866. doi: 10.3389/fcvm.2021.787866
Received: 01 October 2021; Accepted: 18 October 2021;
Published: 18 November 2021.
Edited by:
Gary Tse, Second Hospital of Tianjin Medical University, ChinaReviewed by:
Jonathan Victor Mui, University of Cambridge, United KingdomCopyright © 2021 Wang, Chen, Lin, Chen, Chong, Hsueh, Chung and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mien-Cheng Chen, Y2hlbm1pZW5AbXM3Ni5oaW5ldC5uZXQ=
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.