
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Cardiovasc. Med. , 24 February 2022
Sec. Structural Interventional Cardiology
Volume 8 - 2021 | https://doi.org/10.3389/fcvm.2021.782278
 Faizus Sazzad1,2,3*
Faizus Sazzad1,2,3* Jimmy Kim Fatt Hon2,4
Jimmy Kim Fatt Hon2,4 Kollengode Ramanathan1,2,4
Kollengode Ramanathan1,2,4 Jie Hui Nah1
Jie Hui Nah1 Zhi Xian Ong1
Zhi Xian Ong1 Lian Kah Ti1,4
Lian Kah Ti1,4 Roger Foo1,3,4
Roger Foo1,3,4 Edgar Tay4,5
Edgar Tay4,5 Theo Kofidis1,2,3,4
Theo Kofidis1,2,3,4The transcatheter mitral valve prosthesis is ideally suited for patients with inoperable mitral etiology. The transcatheter mitral valve implantation (TMVI) procedure has closely followed the evolution of transcatheter aortic procedures. There are considerable design variations amongst the limited TMVI prostheses currently available, and the implantation profiles of the devices are notably different. This comprehensive review will provide an overview of the current clinically tried TMVI devices with a focused outcome analysis. In addition, we have discussed the various design characteristics of TMVI and its associated failure mode, implantation technology, delivery methods, first-in-man trials, and pivotal trial summary for the synthesis of recent evidence.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42021255241, identifier: CRD42021255241.
Mitral regurgitation (MR), also known as mitral insufficiency or incompetence, is a leading cause of death worldwide; in western countries, where rheumatic heart disease has a low prevalence (1), MR remains the most common heart valve pathology. Mitral stenosis (MS) commonly arises as a complication of untreated streptococcus infections, leading to corrective treatment procedures to repair or replace the mitral valve. Treatment options for mitral valve pathologies largely depend on the pathophysiology involved, where significant mitral valve apparatus dysfunction may be apparent due to primary etiology. Structural and coaptation failure of leaflets may be predominantly due to left ventricular remodeling from other etiologies (2, 3).
Transcatheter mitral valve replacement is an evolving treatment for MR that closely follows the success of transcatheter aortic valve replacement (TAVR). Transcatheter mitral valve treatment (TMVT) shows un avenir prometteur since its inception (4), despite having several disenchanting limitations (5). TMVT comprises either repair or replacement of the mitral valve; the former has proven its cost-effectiveness and was projected to increase life expectancy in carefully selected cases (6), whereas the latter is yet to break ground. Structural heart valve interventions utilizing transcatheter mitral valve implantation (TMVI) have yet to reach the current therapeutic gold standard compared to surgical mitral valve implantation (SMVI) (7). In contrast, the therapeutic focus of the current transcatheter mitral valve implantation (TMVI) is on patients who are deemed surgically inoperable (8), especially in patients with severe MR and high surgical risk. There is a different therapeutic allocation that has been suggested for primary and secondary MR in the current guidelines (9). Surgical mitral valve repair remained preferred for primary MR when a durable repair is anticipated, whereas transcatheter edge-to-edge repair is preferred over surgery for secondary MR (9). However, transcatheter mitral valve repair (TMVr) and medical therapy together compared to medical therapy alone for severe secondary MR did not show any significant difference in death rate or unplanned hospitalization for heart failure at 1 year (10).
The prosthesis design of the current TMVI devices may hinder the heart's dynamic function, especially during diastole; it may also lead to increased transmitral gradient and reduced effective orifice area (EOA), leading to clinical problems (11). Left ventricular outflow tract obstruction (LVOTO) and annular dimensions outside manufacturer specified ranges are generally recognized as the most frequent reasons for screen failure for TMVI (12). Anchoring of the device onto a nonfibrous mitral annulus exaggerates its migration potential (13), whereas potential LVOTO (14) and interfacing with next-door aortic prosthesis remains a prominent concern (15). On the other hand, the presence of a SAVR/TAVR seems to be less of an issue with some TMVI systems (16).
Additionally, prior TMVr procedure addressing the valve leaflets limits the use of the TMVI device despite the potential necessity. Currently, mini-thoracotomy and transapical TMVI access remain mainstream in approaching the native mitral valve (17). The delivery approach of the device is yet to be explored in elderly patients with poor ventricular ejection. Concomitant surgically viable tricuspid regurgitation (TR) and coexisting atrial fibrillation (AF) cases are not rare and may deserve simultaneous attention. However, severe mitral annular calcification (MAC) and inoperable patients have been successfully treated with balloon-expandable aortic transcatheter valve in the mitral position (18). TMVI with a dedicated prosthesis from Abbott Inc in severe MAC showed early feasibility and MR relief with symptom improvement (19).
Herein, we provide a comprehensive overview of the TMVI devices focusing on the clinical outcomes of first-in-men (FIM) clinical trials. In addition, we discuss the design variation and optimization potential with failure modes of the devices, as required for regulatory approval.
Inspired by an old bottle stopper, Albert Starr developed the “Ball-cage” prosthesis design, which was the first mechanical heart valve implanted in the mitral position in 1961 (20). The Starr–Edwards prosthesis faced hemodynamic turbulence due to the absence of central forward flow in the presence of the ball (21); moreover, the more extensive valve profile led to LVOTO and a significant reduction of EOA. The tilting disk, or Björk-Shiley Delrin (BSD) valve, was first introduced in 1969 (22). However, it failed to achieve a physiological central flow pattern. Finally, Kalke and Lillehei developed the rigid bileaflet valve, which was introduced clinically in 1977 by Manny Villafaña's St Jude Medical and implanted by Demetre Nicoloff (23).
The biological valve was a cause of concern due to inert complications recognized from the metallic devices. The biological valve has its advantages in terms of better biocompatibility with questioned durability. The porcine formalin-fixed xenografts were first used by Carpentier in the mitral position in 1965 (24), shortly after they suggested glutaraldehyde for the chemical treatment of porcine valves (25). In 1966, Carpentier designed the stented mitral prosthesis by mounting the whole porcine heart valve into a stent (26). Since then, the central forward flow profile was established; however, stent-related complications led to leaflet stiffness, and glutaraldehyde pretreatment caused dystrophic calcification. Ionescu and associates introduced pericardial heart valves in 1971 using bovine pericardium (27), which was the first complete biological heart valve utilizing bovine pericardium. Ionescu-Shiley valve had the first “tri-leaflet Mercedes-Benzes star” pattern; however, it faced structural valve deterioration (SVD) due to the Delirin flexible stent within the initial five years of implantation (27). Tirone David, in 1988, proposed “stentless valves” without any metal stent and sewing ring for the aortic position. While this technology did not prove superior (28), it paved the way for transcatheter valves and “sutureless” prostheses.
The first catheter-based procedure for mitral stenosis was percutaneous balloon mitral commissurotomy (PBMC); Inoue first used trans-femoral and trans-septal balloons in 1984 (29), which has now become the management of choice for rheumatic MS with pliable valve (30). Bonhoeffer did the first transcatheter heart valve implantation in 2000 in the pulmonary position (31), which was duplicated by Cribier in 2002 for the aortic position (32). In 2003, some devices were utilized to modify Alfieri's surgical edge-to-edge leaflet repair technique to translate it into the catheter-based procedure. Subsequently, the first MitraClip was implanted in Caracas, Venezuela, by Dr. Jose Condado (33).
The CardiAQ prosthesis was the first dedicated TMVI device that was conceived by Søndergaard in Denmark, 2012 (34). TMVI devices intended for the native mitral valve were rather primitive and faced anchoring and dislodgement issues. The earliest TMVI for native mitral valve stenosis was an inverted transcatheter aortic valve prosthesis (35) via transseptal access. Transapical catheter-based procedures were subsequently extended to treat degenerated bioprosthetic valves with valve-in-valve and valve-in-ring procedures by 2014 (36). This required a mini-left thoracotomy for transapical access to implant the Edwards Sapien XT prosthesis. Thereafter, Guerrero pushed the limits to complete a solely percutaneous transfemoral-access-based TMVI in 2014 (37), the first-ever reported in humans to treat severe MAC. The timeline and evolution of TMVI devices are depicted in Figure 1.
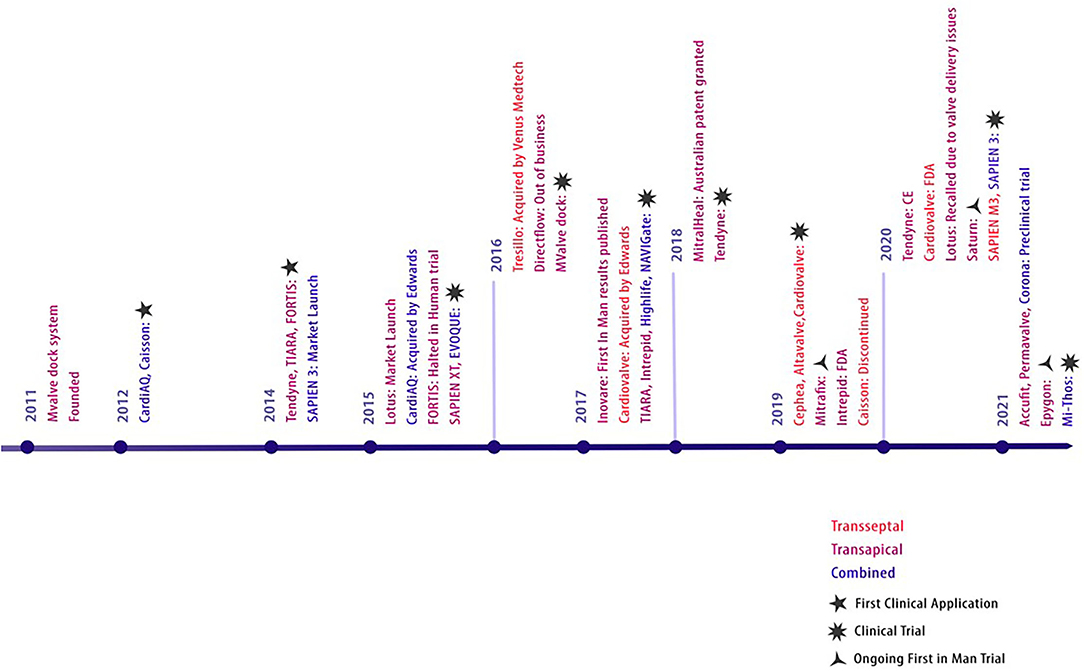
Figure 1. Evolution of TMVI devices. Evolution of transcatheter prosthesis for mitral valve replacement.
We conducted a database search for published literature electronically using the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines (38). The literature search extracted records on Medline (via PubMed), Embase, Cochrane Library, Scopus, and Web of Science from inception to May 30, 2021.
A repetitive and exhaustive combination of the following “medical subject headings” (MeSH) were used: “mitral valve replacement,” “mitral valve implantation,” “transcatheter,” “transcutaneous,” “systematic review,” “transcatheter mitral valve replacement,” “transcatheter mitral valve implantation”. This study protocol has been registered with PROSPERO (CRD42021255241). Relevant articles were screened and systematically assessed, applying inclusion and exclusion criteria.
Studies were only included if published in English, and any experimental cohort studies in humans reporting first and early clinical trials reporting the use of TMVI for mitral valve disease were included. Valves designed and implanted in other cardiac positions were beyond the scope of this study. Mitral valve repair devices for TMVr were also beyond this scope. Furthermore, only studies published after 2010 were included to prevent using nonrelevant data. Articles with TMVI and other concomitant cardiac procedures and nonclinical in vitro experiments and animal experiment studies were excluded.
The extracted citations were screened and assessed by using the reference manager software EndNote X9 independently for inclusion. The articles were first screened by their titles and abstracts, where criteria were purposely broadened to include all relevant studies. Second stage review for studies that have made it through the first stage, or cases where a decision cannot be made, full-text reviews were performed on articles to confirm the relevance. To improve the sensitivity, we have used citation chasing in Google Scholar and Medline (via Pubmed). Further data were sought by manual search using the backward snowballing method.
Three authors independently abstracted details of the study characteristics, TMVI device characteristics, delivery access, periprocedural outcomes, 30 days results, and follow-up, and up to 2 years of data were measured. Data synthesis was done utilizing the ReviewManager 5 software (RevMan 5.4) (39). Depending on the nature of the clinical outcomes extracted from the scientific journals, they were categorized either under dichotomous or continuous data type to generate effect measures. All the results were reported within 95% confidence intervals (CIs).
All the included studies were prospective observational study with the majority reporting the first-in-men clinical trial. As illustrated in chapter 11 of the Cochrane handbook of reviews (40), GradePro was used to evaluate the quality of evidence in the included studies. Authors assessed the articles for their risk of bias and quality of evidence by using Revmen 5.4. The risk of bias for each study (39) was evaluated according to the guidelines in chapter 8 of the Cochrane handbook of reviews (41).
This systematic search revealed a total of 5,219 articles and systematic reviews. Two other papers were retrieved from alternative sources. After exclusion of the duplicates, 3,082 articles remained for assessment. Irrelevant publications that did not satisfy our inclusion criteria were not considered based on title and abstract scrutiny, leaving 208 articles for full-text review. Following the full-text assessment of these articles, 12 papers (42–53) remained for final review (Figure 2).
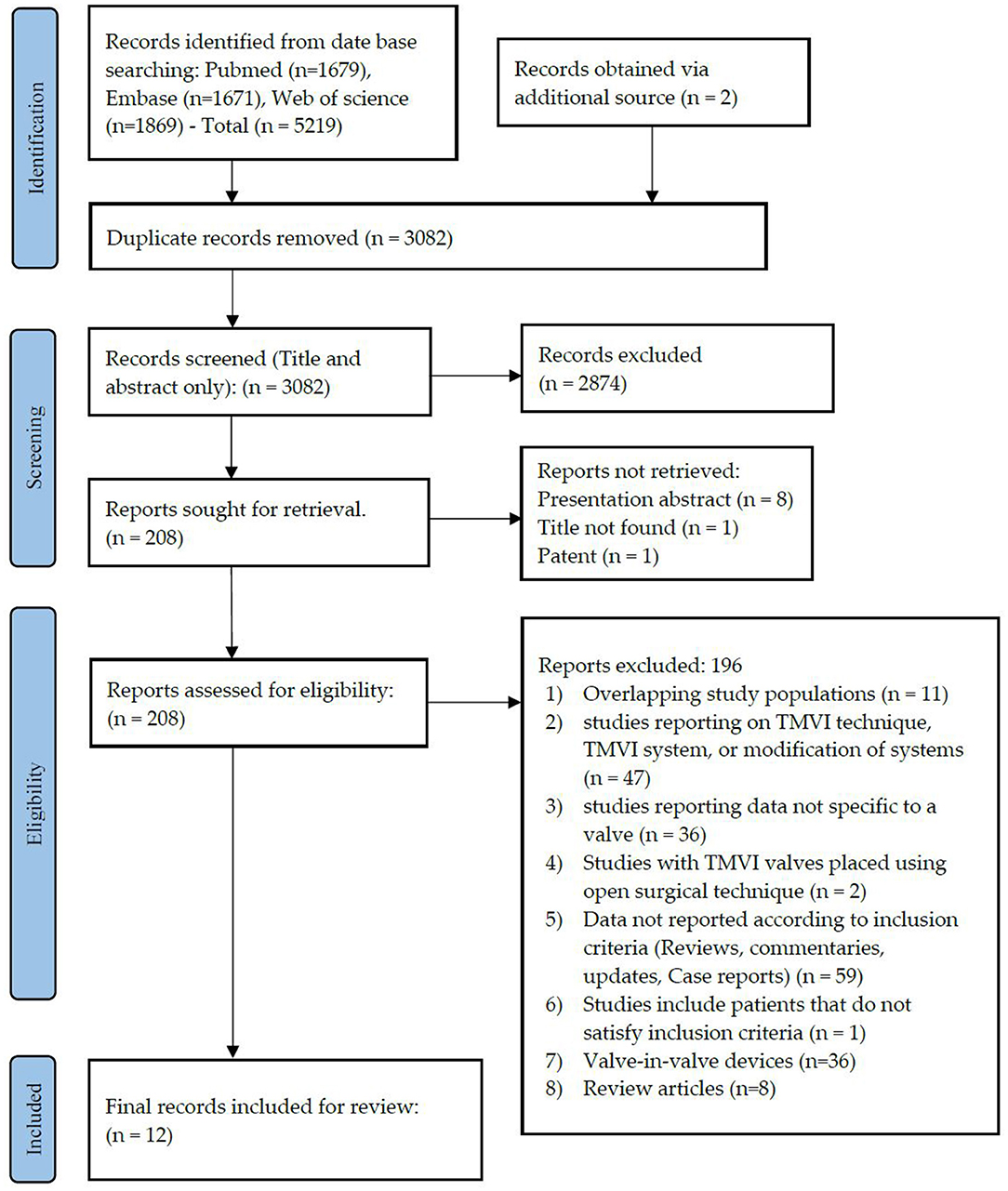
Figure 2. PRISMA flow diagram. PRISMA chart illustrating our process of obtaining the 12 included articles. With 2,874 irrelevant records excluded based on their titles and abstracts, we reviewed the full texts of 208 articles, of which 196 were excluded, and 28 remained for inclusion in our study, of which 12 articles were included for final review.
As seen from Figure 3, the selection bias (54) for each study was critical/important, which we believe can be credited to the type of study itself, the majority being first in the human clinical trial. Despite this, the overall risk of bias for all the studies was classed as low/moderate. Therefore, the evidence provided by these studies was still of acceptable quality.
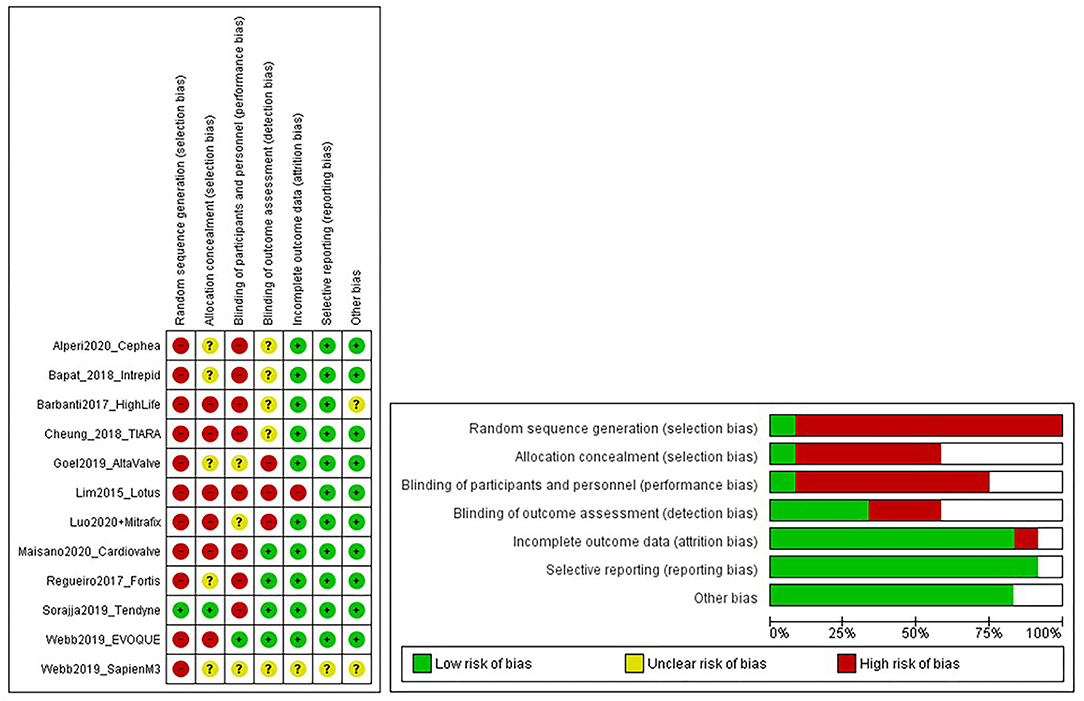
Figure 3. Risk of bias graphs. The figure shows a review of authors' judgments about each risk of bias item presented as percentages across all included studies. Random sequence generation was made high due to selection bias in First-in-man studies.
All studies were First-in-man clinical trials, reporting data on TMVI replacement in single (43, 46, 48–51, 53) or multicenter, global pilot studies (42, 47, 52) with three pivotal trials, namely: Expanded Clinical Study of the Tendyne (NCT02321514), Miscend for Evoque (NCT02718001), and CIP-1403 for Intrepid (NCT02322840). Most of the studies were single-center, with the majority taking place in the United States and Canada. Only the Mitrafix device was from Beijing, China; Cardiovalve in Zurich, Switzerland, and the HighLife was tested in Italy. Two of the 12 included studies (43, 53) in this analysis have been published by the same first author, Webb, in the year (2019) (Table 1). Assessment of the complete texts verified that these studies were performed on two different study populations using two different devices, which have been included separately in our analysis. To aid the identification of these papers, we used the naming Webb et al. (43) and Webb (Late) et al. (53) to differentiate them.
Twelve manuscripts (42–53) that were included reported first-in-man clinical trials and beyond. The majority of the TMVI patients were men and more than 70 years old, with almost all of the patients having a high or a very high surgical risk based on their EuroScore and society of thoracic surgeons (STS) score (Table 1). Preoperative data, namely BMI, 6-min walk test, Kansas City Cardiomyopathy Questionnaire, smoking status, preoperative investigation profile, prior cardiac interventions, and any preoperative extenuating circumstances, are recorded in Supplementary Table 1.
Design variations, including size, annular shape, anchoring mechanism, access, and recapture features, have been summarized in Table 2. Notably, Tiara (44), Tendyne (47), and Mitrafix (51) devices have a D-shaped annulus, whereas the rest have a circular annulus. Diversity of anchoring mechanism has been observed, namely apical tethers (external anchor), annular winglets, native leaflet engagement, annulus clamping, loading dock system, and radial force. While most devices were implanted via apical access (through mini-thoracotomy), some were trans-septal, and the use of both approaches has been observed as well. Several devices were in the pipeline and found to be at different stages of development; this is summarized in Supplementary Table 2.
The majority of the included studies reported a high procedural/technical success rate, even though each of the device was unique in terms of its design variation; this is summarized in Table 3. The average device implantation time was 40–55 min with a range of 100–235 min of overall procedure time. The contrast volume used in fluoroscopy was 29.1 ± 34.0 ml (47), 41.5 ml (32–94.3) (53), and 72 ± 38 ml (43) as reported. One patient (7.1%) was observed to convert surgery (53) due to moderate to severe PVL. Device-specific procedural complications, namely pericardial effusion [one patient reported by Bapat et al. (42)], bioprosthetic valve dysfunction, and device embolization, were the periprocedural complications reported in the included studies.
Most of the patients were discharged home after successful implantation of the TMVI devices, with a few reintervention during index hospitalization (Table 4). Two patients (2%) from Tendyne (47) reported myocardial infarction. There were few reports of postprocedural cardiac arrest, vascular complications, stroke/transient ischemic attack (TIA), and acute kidney injury (AKI) (1–8%) (46, 47, 53). A rise in the mean mitral gradient was observed from the point of discharge to 30 days follow-up (2.3–6 mmHg on average). Few studies reported 1-year follow-up data, with only one study completing 2 years of follow-up (52) and none reporting on the structural deterioration of the prosthesis (Table 4). All-cause mortality at 1 year was reported as high as 26% for Tendyne (47) and 22% for Intrepid (42). Two years all-cause mortality was only reported by Fortis as 15.4% (52).
The average age of the included patients was >75 years with high STS [>7.4%, IQR: 3.8%−11.25%] and EuroScore-II [>14, IQR: 5.9–28.4]. Most of the patients were NYHA III/IV with multiple comorbidities. Around 38% of the patients had prior coronary artery bypass graft surgery (CABG), and 10% had other cardiac procedures, as Bapat et al. (42) reported. Most of the included TMVI devices were indicated for severe degenerative MR patients with a high surgical risk. There were associated moderate to severe unaddressed TR (42, 46) and atrial fibrillation (42, 43, 45, 46, 48, 52, 53) in some studies, for which no surgical intervention was reported. The durability of the devices could not be commented upon due to the lack of data beyond 2 years of follow-up.
Designing a TMVI device is a challenging task, and the design variation of the currently available devices shows the magnitude of the anticipated difficulties. Various new stent design approaches were developed to address LVOTO, valve anchoring, sealing, and dynamic deformation of the mitral annulus throughout the cardiac cycle. The key findings of this systematic review are been summarized in Table 5.
Prior to a TMVI procedure, each patient undergoes contrast-enhanced cardiac computed tomography to assess the mitral annulus and the left ventricular outflow tract (LVOT). Multiple factors increase the risk of LVOTO, including an obtuse aortomitral angle, degree of septal hypertrophy, and left ventricular size (55). Additionally, the proximity of the aortic leaflet to the LVOT presents a high risk of LVOTO. One of the design strategies includes reducing stent protrusion into the ventricle, which may help to reduce the displacement of the anterior mitral leaflet toward the LVOT (56). Intrepid, for example, has a small device profile of 17 to 18 mm to minimize the risk of LVOTO (42). Similarly, Cephea was designed with a small surgical valve-like profile (46). Altavalve overcame LVOTO via its design as a fully supraannular stent (48). Highlife adopts another approach that mainly reduces the risk of LVOTO by accurate positioning of the valve within the subannular implant (SAI) (45).
Suitable valve size was chosen via various measurements such as, but not limited to, intercommissural diameter, anteriorposterior distance, and mitral annular perimeter. Adequate sizing draws a balance between oversizing for anchoring and fixation, described in the implantation of Intrepid and Altavalve while minimizing the risk of LVOTO (42, 48). An excessively oversized valve may also risk atrioventricular groove injury.
In the included studies, TMVI devices had sizes varying from 23 and 55 mm, with Tendyne and Cardiovalve each showcasing the most extensive range with three sizes-covering an interpuncture range of 34–50 mm and 36–53 mm, respectively (47, 49). Tiara and Evoque currently provide two size variations, and the rest of the valves have a single size that covers a range of intercommissural distances (44, 53). In comparison, some TMVI devices currently in development like Accufit and NAVI System present many potentially available sizes.
Accommodation to the dynamic deformation of the mitral annulus during the cardiac cycle is fundamental to procedural success (57). Most TMVI devices have circular valve components housed in an expanding stent frame. Intrepid (42) and Cephea (46) have described stent designs that isolate the valve prosthesis from the outer fixation ring to prevent distortion of the inner valve. Intrepid adopts a double stent design that allows the inner leaflets to maintain circular geometry, whereas the outer ring conforms to the dynamic anatomy of the mitral annulus (42).
At the same time, Cephea has a central column that supports the leaflets and is isolated from external deformation (58). Another design approach has a stent design that accommodates the saddle-shaped mitral annulus. For example, Intrepid (42) and Cephea (46) have circular valve orifices housed in a conformable outer stent, whereas Tiara (44), Tendyne (47), Mitrafix (51) were designed with a D-shaped valve annulus. Natural D-shaped pattern at the annulus design ensures biomimicry and is considered a critical success factor, as seen in Tendyne (59).
Improper anchoring and positioning of the TMVI device may result in valve migration. The risk of migration into the left atrium is the highest due to the increased cyclic left ventricular systolic pressures. The native mitral annulus lacks a rigid fibrous ring. Where fibrosis or calcification is absent, the anchoring of a new valve is intricate. As such, TMVI devices developed distinct anchoring mechanisms to ensure proper positioning and sealing.
Majority of the devices anchor via radial expansion at the annulus level, supported by either atrial flanges, ventricular tabs that grasp the native leaflets, or both. Tiara, for example, presents a tri-fold anchoring mechanism with atrial flanges, two anterior clips that anchor the valve to the aorto-mitral curtain, and one posterior tab that clips the posterior mitral leaflet onto the rear shelf of the mitral annulus, in addition to fixation via radial expansion (60). In contrast, Cephea atrial and ventricular disk design anchors the device via axial compression forces and avoids subvalvular anchors or tethers (58). Other devices like Tendyne anchors to the LV apex via a tether (47), whereas Altavalve anchors fully in the left atrium using a spherical single unit stent (48). Devices, such as Sapien M3 (43) and HighLife (45) anchor to their dock and SAI, respectively. Proper anchoring aimed to prevent valve migration and was reported to be few in a systematic review of >300 cases with documented early experience (61), subject to be evaluated in large-scale clinical evaluation.
Multiple design approaches were used to minimize paravalvular leaks in the current devices and may be used concurrently. A standard method within the existing devices (Sapien M3, Tiara, Tendyne, Altavalve, Evoque) includes incorporating an atrial fabric skirt sewn into or outside the expandable nitinol frame (43, 44, 47, 48, 53). Altavalve, for instance, has a polyethylene terephthalate skirt fabric added to the lowest third of the spherical atrial structure that will enhance endothelization and tissue ingrowth, and in doing so, avoid paravalvular leakage (62). A unique approach presented by HighLife includes using the native mitral leaflets as a seal as it is trapped within the interaction between the prosthesis and its SAI (45).
Following the promising developments in TAVR devices, some of the earliest and common TMVI procedures were done using TAVR devices (e.g., Sapien M3, Sapien XT) via valve-in-valve (VIV) approach in degenerated bioprostheses or severe MAC patients (63–66). This is especially since redo surgery for the degenerated bioprosthetic mitral valve in elderly patients with impaired left ventricular dysfunction and previous cardiac surgery is not ideal (67). The mortality and morbidity from redo surgeries have increased the demand for minimally invasive treatment options like TMVI.
However, the mitral valve is larger than the aortic valve, provides less support, and is much more asymmetric in shape, which has made the use of TAVR devices in the mitral position to valve in ring (VIR) procedure quite challenging. Fortunately, unlike the saddle-shaped native mitral annulus, the old prosthetic valve creates a circular morphology in which devices initially designed for aortic valve replacement can be implanted. Devices such as Sapien M3 were readily available commercially on account of established success in TAVR and are well studied in the literature. Direct flow has currently been discontinued; Inovare and Sapien M3 continue their clinical trials specifically for implantation in the mitral area (68). Mi-Thos and Meril's MyVal are new valves developed for VIV implantation and address prosthesis or conduit dysfunction (69, 70).
There are several access strategies for TMVI, including transapical, transseptal, or hybrid (Figure 4). The most common mode of access is the transapical route used for Tiara, Tendyne, Lotus, Mitrafix, Fortis, and Intrepid (discontinued) (42, 44, 47, 50–52). The transapical route gives direct access and ease of maneuvering for coaxial alignment of a larger prosthetic valve to an angled mitral annulus compared to a transseptal approach, and it is favored in early-generation devices (Accufit, Saturn, Epygon, Permavalve, etc.). However, the experience in TAVR has shown that transapical punctures are associated with worse outcomes than transseptal or transfemoral access.
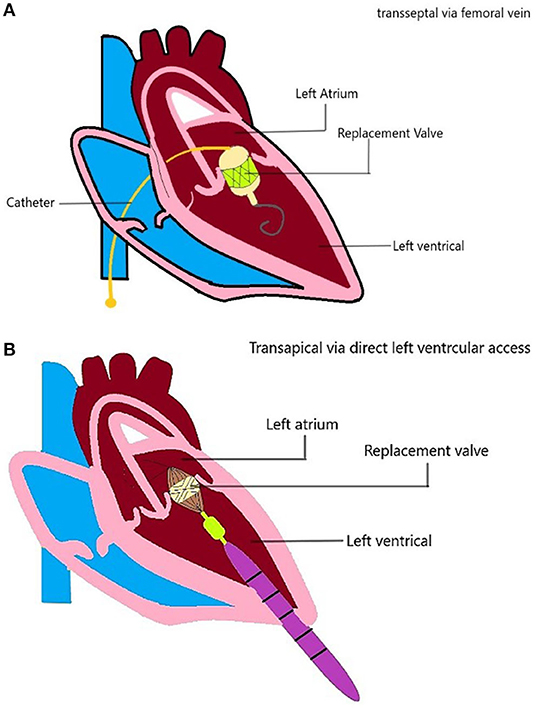
Figure 4. TMVI device access. Schematic diagram showing the TMVI access (A) transseptal/transfemoral access, and (B) transapical access of implantation.
For example, higher degrees of myocardial damage (7), especially in frail or elderly patients (71), and other adverse effects were related to thoracotomy (72). Advances in TMVI technology such as smaller and more flexible delivery catheters have opened the doors for developing a new generation of devices delivered via the transseptal route, e.g., Sapien M3, Cephea Altavalve, Cardiovalve (43, 46, 48, 49).
Earlier-generation transapical devices have also been attempted through a transseptal approach, whereas the second-generation CardiAQ/Evoque valve may be implanted via either access route (53). Intrepid began its early feasibility trial (2019) for the TMVI system with a transseptal approach after a successful pilot trial (42). The HighLife valve is unique in its approach as it requires two separate access sites (45). The SAI is delivered retrogradely from a transfemoral access site, whereas the valve prosthesis proper is delivered via a transapical route.
Retrieval of a device during or after deployment allows for repositioning and more accurate device placement. Of the TMVI devices, only Tendyne presents a fully retrievable system that can be recaptured after the complete deployment of the device (47). Tiara, Sapien M3 (appears to be discontinued), and Altavalve are partially retrievable and may be recaptured at different stages before fully deploying the device (43, 44, 48). The remaining systems have not been described to be retrievable. However, Intrepid's dual frame structure allows the device to conform to the annulus, whereas the inner frame remains circular and symmetrical has been described to eliminate the need for rotational orientation and thus retrieval (42).
Transcatheter mitral valve implantation systems have an extensive profile and may expose early leaflet damage from crimping (73). Evidence suggests that bioprosthetic valves are subjected to significant surface and deep tissue damage, and reductions in leaflet strength, which may not be reversible (74). With the trend growing toward a transseptal mode of access for TMVI devices, so does the need to crimp TMVI devices into smaller profile delivery catheters, the smallest of which is 20 Fr. Studies based on TAVR devices show that crimping damage to the leaflets increases with smaller delivery profiles and longer crimping duration; however, these concerns and their effects on durability are yet to be explored.
Durability is of concern, especially for transcatheter devices to be implanted in younger patients. Studies in aortic valves that examined the durability between transcatheter and surgical prosthetic valves suggest that the durability of both approaches is comparable (75). All of the TMVI devices in the review used bovine and porcine pericardial tissue leaflets. Tissue leaflets underwent accelerated wear tests and have a proven record of passing the ISO 5840-3 with a minimum requirement of 200M cycles. This is the material of choice for prosthetic heart valve manufacturers (76).
While we know that all valves, excluding Epygon, which has a mono-leaflet design, are trileaflet in design, little information has been disclosed about specificities of leaflet design concerning improving durability. The long-term results are yet to be published from a clinical perspective except for a few reporting 2-year outcomes at the best (49, 52). Structural device features such as high metal burden, leaflet material, size, and flow patterns could potentially impact the thrombotic risk. The mode of delivery (transseptal vs. transapical) may also be associated with periprocedural thrombosis. However, data on the risk of long-term thrombosis is scarce, and no dedicated studies have evaluated the thrombogenic profile of the devices concerning these aspects.
Despite multiple devices showing promising early results, significant advancements have not been achieved for TMVI therapies. Some of the technical challenges gained consensus. Most opinion-leaders favor a transseptal route for delivery, if eventually technically achievable. However, there remain potential challenges. Hence, the “dream” TMVI is a distance from the “Standard of care”. SMVI is still the preferred approach for most patients with mitral valve disease. Importantly, preserving native valve anatomy, selective candidacy, preoperative multimodality imaging, and guideline-directed treatment strategy are still warranted for the transcatheter management of mitral valve pathologies.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
FS and JH contributed to conceptualization. FS, ZO, and JN contributed to methodology and data curation. FS and JN contributed to software, validation, writing original draft preparation, visualization, and formal analysis. FS, JH, and KR contributed to resources. FS, KR, ET, and JH contributed to writing and reviewing. JH, LT, ET, RF, and TK contributed to writing and editing. TK managed supervision. FS contributed to project management and administration. FS and TK contributed to funding acquisition. All authors have read and agreed to the published version of the manuscript.
The author(s) disclosed receipt of the following financial support for the research and publication of this article. This work was supported by the National Research Foundation (NRF), Singapore, Central Gap Fund (NRF2020NRF-CG001-018).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors would like to express gratitude to Malik Arfah from the Temasek Polytechnic, Singapore, for her help in drawing cartoons.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2021.782278/full#supplementary-material
AF, Atrial fibrillation; ASD, Atrial septal defect; CC, Inter-commissural distance; CE, Conformitè Europëenne; ChiCTR, Chinese Clinical Trial Registry; CPB, Cardiopulmonary bypass; EOA, Effective Orifice Area; FDA, Food and Drug Administration; FIM, First in Man; IABP, Intra-aortic balloon pump; LVEF, left ventricle ejection fraction; LVOTO, Left ventricular outflow tract obstruction; MAC, Mitral annular calcification; MR, Mitral regurgitation; MS, Mitral stenosis; MV, Mitral valve; NCT, ClinicalTrials.gov identifier; NYHA, New York Heart Association.; PMBC, Percutaneous Balloon Mitral Commissurotomy; PPM, permanent pacemaker implantation; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; PVL, Paravalvular leak; SAI, Sub-Annular Implant; SMVI, surgical mitral valve implantation; SVD, Structural valve deterioration; TMVI, Transcatheter mitral valve implantation; TMVr, Transcatheter mitral valve repair; TMVT, Transcatheter mitral valve treatment; TR, Tricuspid regurgitation; TV, Tricuspid valve; VIR, Valve in ring procedure; VIV, Valve in valve procedure; STS, Society of Thoracic Surgeons; AKI, Acute kidney injury; TIA, Transient Ischemic Attack; CABG, Coronary artery bypass graft surgery.
1. Agricola E, Ielasi A, Oppizzi M, Faggiano P, Ferri L, Calabrese A, et al. Long-term prognosis of medically treated patients with functional mitral regurgitation and left ventricular dysfunction. Eur J Heart Fail. (2009) 11:581–7. doi: 10.1093/eurjhf/hfp051
2. Unger P, Clavel MA, Lindman BR, Mathieu P, Pibarot P. Pathophysiology and management of multivalvular disease. Nature reviews. Cardiology. (2016) 13:429–40. doi: 10.1038/nrcardio.2016.57
3. Schoen FJ. Morphology, clinicopathologic correlations, and mechanisms in heart valve health and disease. Cardiovasc Eng Technol. (2018) 9:126–40. doi: 10.1007/s13239-016-0277-7
4. Meng Z, Zhang EL, Wu YJ. Current status and future direction of transcatheter mitral valve replacement. Chin Med J. (2018) 131:505–7. doi: 10.4103/0366-6999.226080
5. Winkel MG, Praz F, Wenaweser P. Mitral and tricuspid transcatheter interventions current indications and future directions. Front Cardiovasc Med. (2020) 7:61. doi: 10.3389/fcvm.2020.00061
6. Baron SJ, Wang K, Arnold SV, Magnuson EA, Whisenant B, Brieke A, et al. Cost-Effectiveness of transcatheter mitral valve repair versus medical therapy in patients with heart failure and secondary mitral regurgitation: results from the COAPT trial. Circulation. (2019) 140:1881–91. doi: 10.1161/CIRCULATIONAHA.119.043275
7. Testa L, Popolo Rubbio A, Casenghi M, Pero G, Latib A, Bedogni F. Transcatheter mitral valve replacement in the transcatheter aortic valve replacement era. J Am Heart Assoc. (2019) 8:e013352. doi: 10.1161/JAHA.119.013352
8. Mirabel M, Iung B, Baron G, Messika-Zeitoun D, Détaint D, Vanoverschelde JL, et al. What are the characteristics of patients with severe, symptomatic, mitral regurgitation who are denied surgery? Eur Heart J. (2007) 28:1358–65. doi: 10.1093/eurheartj/ehm001
9. Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. (2021) ehab395. doi: 10.1093/eurheartj/ehab395
10. Obadia JF, Messika-Zeitoun D, Leurent G, Iung B, Bonnet G, Piriou N, et al. Percutaneous repair or medical treatment for secondary mitral regurgitation. N Engl J Med. (2018) 379:2297–306. doi: 10.1056/NEJMoa1805374
11. Iyer R, Chalekian A, Lane R, Evans M, Yi S, Morris J. Transcatheter mitral valve replacement: functional requirements for device design, bench-top, pre-clinical evaluation. Cardiovasc Eng Technol. (2018) 9:301–38. doi: 10.1007/s13239-018-0364-z
12. Ludwig S, Ruebsamen N, Deuschl F, Schofer N, Kalbacher D, Schaefer A, et al. Screening for transcatheter mitral valve replacement: a decision tree algorithm. EuroIntervention. (2020) 16:251–8. doi: 10.4244/EIJ-D-19-01051
13. Badhwar V, Thourani VH, Ailawadi G, Mack M. Transcatheter mitral valve therapy: The event horizon. J Thorac Cardiovasc Surg. (2016) 152:330–6. doi: 10.1016/j.jtcvs.2016.03.049
14. Meduri CU, Reardon MJ, Lim DS, Howard E, Dunnington G, Lee DP, et al. Novel multiphase assessment for predicting left ventricular outflow tract obstruction before transcatheter mitral valve replacement. JACC Cardiovasc Interv. (2019) 12:2402–12. doi: 10.1016/j.jcin.2019.06.015
15. Cheung A, Webb J, Schaefer U, Moss R, Deuschl FG, Conradi L, et al. Transcatheter mitral valve replacement in patients with previous aortic valve replacement. Circ Cardiovasc Interv. (2018) 11:e006412. doi: 10.1161/CIRCINTERVENTIONS.118.006412
16. Taramasso M, Sorajja P, Dahle G, Dumonteil N, Schäfer U, Modine T, et al. Transapical transcatheter mitral valve implantation in patients with prior aortic valve replacement: a feasibility report. EuroIntervention. (2021) 17:257–9. doi: 10.4244/EIJ-D-19-00947
17. Lutter G, Dai H, Hansen JH, Frank D, Haneya A, Simionescu D, et al. Transcatheter mitral valve replacement (TMVR): annular or apical fixation? EuroIntervention. (2020) 16:e510–7. doi: 10.4244/EIJ-D-19-00614
18. Guerrero M, Urena M, Himbert D, Wang DD, Eleid M, Kodali S, et al. 1-Year outcomes of transcatheter mitral valve replacement in patients with severe mitral annular calcification. J Am Coll Cardiol. (2018) 71:1841–53. doi: 10.1016/j.jacc.2018.02.054
19. Sorajja P, Gössl M, Babaliaros V, Rizik D, Conradi L, Bae R, et al. Novel transcatheter mitral valve prosthesis for patients with severe mitral annular calcification. J Am Coll Cardiol. (2019) 74:1431–40. doi: 10.1016/j.jacc.2019.07.069
20. Marco R, Maurizio T, Andrea G, Alberto P, Fabian N, Ludwig VS, et al. The evolution of surgical valves. Cardiovasc Med. (2017) 20:285–92. doi: 10.4414/cvm.2017.00532
21. Chaikof EL. The development of prosthetic heart valves–lessons in form and function. N Engl J Med. (2007) 357:1368–71. doi: 10.1056/NEJMp078175
22. Wieting DW. The Björk-Shiley Delrin tilting disc heart valve: historical perspective, design and need for scientific analyses after 25 years. J Heart Valve Dis. (1996) 2:S157–68.
23. Gott VL, Alejo DE, Cameron DE. Mechanical heart valves: 50 years of evolution. Ann Thorac Surg. (2003) 76:S2230–9. doi: 10.1016/j.athoracsur.2003.09.002
24. Vogel JHK. Long-term prognosis following valve replacement. 2nd conference on cardiovascular disease in Snowmass-at-Aspen, Aspen, Colo., Advances in Cardiology, vol 7. Basel:Karger. (1972) p. 277–81. doi: 10.1159/000392990
25. Carpentier A, Lemaigre G, Robert L, Carpentier S, Dubost C. Biological factors affecting long-term results of valvular heterografts. J Thorac Cardiovasc Surg. (1969) 58:467–83. doi: 10.1016/S0022-5223(19)42561-0
26. Spencer FC. Presidential address. Intellectual creativity in thoracic surgeons. J Thorac Cardiovasc Surg. (1983) 86:163–79. doi: 10.1016/S0022-5223(19)39172-X
27. Ioneuscu MI. (2014)The beginning in the pericardial heart valve: The odyssey of a continuously evolving concept. Society for Cardiothoracic Surgery in Great Britain and Ireland; p. 1–35.
28. Desai ND, Merin O, Cohen GN, Herman J, Mobilos S, Sever JY, et al. Long-term results of aortic valve replacement with the St. Jude Toronto stentless porcine valve. Ann Thorac Surg. (2004) 78:2076–83. doi: 10.1016/j.athoracsur.2004.05.061
29. Inoue K, Owaki T, Nakamura T, Kitamura F, Miyamoto N. Clinical application of transvenous mitral commissurotomy by a new balloon catheter. J Thorac Cardiovasc Surg. (1984) 87:394–402. doi: 10.1016/S0022-5223(19)37390-8
30. Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP, Gentile F, et al. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. (2021) 143:e35–e71. doi: 10.1161/CIR.0000000000000932
31. Bonhoeffer P, Boudjemline Y, Saliba Z, Merckx J, Aggoun Y, Bonnet D, et al. Percutaneous replacement of pulmonary valve in a right-ventricle to pulmonary-artery prosthetic conduit with valve dysfunction. Lancet (London, England). (2000) 356:1403–5. doi: 10.1016/S0140-6736(00)02844-0
32. Cribier A, Eltchaninoff H, Bash A, Borenstein N, Tron C, Bauer F, et al. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case description. Circulation. (2002) 106:3006–8. doi: 10.1161/01.CIR.0000047200.36165.B8
33. Sündermann SH, Biaggi P, Grünenfelder J, Gessat M, Felix C, Bettex D, et al. Safety and feasibility of novel technology fusing echocardiography and fluoroscopy images during MitraClip interventions. EuroIntervention. (2014) 9:1210–6. doi: 10.4244/EIJV9I10A203
34. Søndergaard L, De Backer O, Franzen OW, Holme SJ, Ihlemann N, Vejlstrup NG, et al. First-in-Human Case of Transfemoral CardiAQ Mitral Valve Implantation. Circ Cardiovasc Interv. (2015) 8:e002135. doi: 10.1161/CIRCINTERVENTIONS.115.002135
35. Hasan R, Mahadevan VS, Schneider H, Clarke B. First in human transapical implantation of an inverted transcatheter aortic valve prosthesis to treat native mitral valve stenosis. Circulation. (2013) 128:e74–6. doi: 10.1161/CIRCULATIONAHA.113.001466
36. Wilbring M, Alexiou K, Tugtekin SM, Arzt S, Ibrahim K, Matschke K, et al. Pushing the limits-further evolutions of transcatheter valve procedures in the mitral position, including valve-in-valve, valve-in-ring, and valve-in-native-ring. J Thorac Cardiovasc Surg. (2014) 147:210–9. doi: 10.1016/j.jtcvs.2013.09.021
37. Guerrero M, Greenbaum A, O'Neill W. First in human percutaneous implantation of a balloon expandable transcatheter heart valve in a severely stenosed native mitral valve. Catheter Cardiovasc Interv. (2014) 83:E287–91. doi: 10.1002/ccd.25441
38. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. (2009) 151:264–W64. doi: 10.7326/0003-4819-151-4-200908180-00135
39. Review Manager (RevMan) [Computer program]. Copenhagen: the Nordic Cochrane Centre, The Cochrane Collaboration (2014). Available online at: https://training.cochrane.org/online-learning/core-software-cochrane-reviews/revman
40. Schünemann HJ, Higgins JPT, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane (2019). Available online at: https://training.cochrane.org/handbook/current/chapter-14
41. Higgins JP, Green SR. Assessing risk of bias in included studies. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1. 0 (2011). Available online at: https://handbook-5-1.cochrane.org
42. Bapat V, Rajagopal V, Meduri C, Farivar RS, Walton A, Duffy SJ, et al. Early experience with new transcatheter mitral valve replacement. J Am Coll Cardiol. (2018) 71:12–21. doi: 10.1016/j.jacc.2017.10.061
43. Webb JG, Murdoch DJ, Boone RH, Moss R, Attinger-Toller A, Blanke P, et al. Percutaneous transcatheter mitral valve replacement: first-in-human experience with a new transseptal system. J Am Coll Cardiol. (2019) 73:1239–46. doi: 10.1016/j.jacc.2018.12.065
44. Cheung A. Early experience of TIARA transcatheter mitral valve replacement system. Ann Cardiothorac Surg. (2018) 7:787–91. doi: 10.21037/acs.2018.09.05
45. Barbanti M, Piazza N, Mangiafico S, Buithieu J, Bleiziffer S, Ronsivalle G, et al. Transcatheter mitral valve implantation using the highlife system. JACC Cardiovasc Interv. (2017) 10:1662–70. doi: 10.1016/j.jcin.2017.06.046
46. Alperi A, Dagenais F, Del Val D, Bernier M, Vahl TP, Khalique OK, et al. Early experience with a novel transfemoral mitral valve implantation system in complex degenerative mitral regurgitation. JACC Cardiovasc Interv. (2020) 13:2427–37. doi: 10.1016/j.jcin.2020.08.006
47. Sorajja P, Moat N, Badhwar V, Walters D, Paone G, Bethea B, et al. Initial feasibility study of a new transcatheter mitral prosthesis: the first 100 patients. J Am Coll Cardiol. (2019) 73:1250–60. doi: 10.1016/j.jacc.2018.12.066
48. Goel SS, Zuck V, Christy J, Nallamothu N, Jagtap P, Gao J, et al. Transcatheter mitral valve therapy with novel supra-annular altavalve: first experience in the United States. JACC Case reports. (2019) 1:761–4. doi: 10.1016/j.jaccas.2019.10.034
49. Maisano F, Benetis R, Rumbinaite E, Unikas R, Mizariene V, Jakuska P, et al. 2-year follow-up after transseptal transcatheter mitral valve replacement with the cardiovalve. JACC Cardiovasc Interv. (2020) 13:e163–e164. doi: 10.1016/j.jcin.2020.05.032
50. Lim ZY, Boix R, Prendergast B, Rajani R, Redwood S, Hancock J, et al. First reported case of transcatheter mitral valve implantation in mitral annular calcification with a fully repositionable and self-expanding valve. Circ Cardiovasc Interv. (2015) 8:e003031. doi: 10.1161/CIRCINTERVENTIONS.115.003031
51. Luo Z, Zhang H, Xie Y, Sun Y, Li M, Fang N, et al. Clinical application of a fully ultrasound-guided transapical transcatheter mitral valve replacement device. JACC Cardiovasc Interv. (2020) 13:e161–2. doi: 10.1016/j.jcin.2020.02.032
52. Regueiro A, Ye J, Fam N, Bapat VN, Dagenais F, Peterson MD, et al. 2-year outcomes after transcatheter mitral valve replacement. JACC Cardiovasc Interv. (2017) 10:1671–8. doi: 10.1016/j.jcin.2017.05.032
53. Webb J, Hensey M, Fam N, Rodés-Cabau J, Daniels D, Smith R, et al. Transcatheter mitral valve replacement with the transseptal EVOQUE system. JACC Cardiovasc Interv. (2020) 13:2418–26. doi: 10.1016/j.jcin.2020.06.040
54. Sterne JAC, Higgins JPT, Reeves BC (on behalf of the development group for ACROBAT-NRSI). A Cochrane risk of bias assessment tool: for non-randomized studies of interventions (ACROBATNRSI), Version 1.0.0, 24 September 2014. Available online at: http://www.bristol.ac.uk/population-health-sciences/centres/cresyda/barr/riskofbias/robins-i/acrobat-nrsi/ (accessed Jan 9, 2019).
55. Regueiro A, Granada JF, Dagenais F, Rodés-Cabau J. Transcatheter mitral valve replacement: insights from early clinical experience and future challenges. J Am Coll Cardiol. (2017) 69:2175–92. doi: 10.1016/j.jacc.2017.02.045
56. Sanchez JDZ, Bateman MG, Iaizzo PA. Engineering and technologies associated with cardiac valve repair and replacement therapies. In; Iaizzo PA, editor. Engineering in Medicine. Amsterdam; Elsevier BV (2019).
57. Reid A, Ben Zekry S, Turaga M, Tarazi S, Bax JJ, Wang DD, et al. Neo-LVOT and Transcatheter Mitral Valve Replacement: Expert Recommendations. JACC Cardiovasc Imag. (2021) 14:854–66. doi: 10.1016/j.jcmg.2020.09.027
58. Modine T, Vahl TP, Khalique OK, Coisne A, Vincent F, Montaigne D, et al. First-in-human implant of the cephea transseptal mitral valve replacement system. Circulat Cardiovasc intervent. (2019) 12:e008003. doi: 10.1161/CIRCINTERVENTIONS.119.008003
59. Beller JP, Rogers JH, Thourani VH, Ailawadi G. Early clinical results with the Tendyne transcatheter mitral valve replacement system. Ann Cardiothorac Surg. (2018) 7:776–9. doi: 10.21037/acs.2018.10.01
60. Banai S, Jolicoeur EM, Schwartz M, Garceau P, Biner S, Tanguay JF, et al. Tiara: a novel catheter-based mitral valve bioprosthesis: initial experiments and short-term pre-clinical results. J Am Coll Cardiol. (2012) 60:1430–1. doi: 10.1016/j.jacc.2012.05.047
61. Del Val D, Ferreira-Neto AN, Wintzer-Wehekind J, Dagenais F, Paradis JM, Bernier M, et al. Early Experience With Transcatheter Mitral Valve Replacement: A Systematic Review. J Am Heart Assoc. (2019) 8:e013332. doi: 10.1161/JAHA.119.013332
62. Alperi A, Del Val D, Ferreira-Neto AN, Bernier M. B Freitas-Ferraz A, Dagenais F, et al. Device profile of the AltaValve system for transcatheter mitral valve replacement: overview of its safety and efficacy. Expert Rev Med Devices. (2020) 17:627–36. doi: 10.1080/17434440.2020.1781616
63. Hu J, Chen Y, Cheng S, Zhang S, Wu K, Wang W, et al. Transcatheter mitral valve implantation for degenerated mitral bioprostheses or failed surgical annuloplasty rings: A systematic review and meta-analysis. J Card Surg. (2018) 33:508–19. doi: 10.1111/jocs.13767
64. Nazir S, Lohani S, Tachamo N, Khan MS, Timilsina B, Luni FK, et al. Outcomes following transcatheter transseptal versus transapical mitral valve-in-valve and valve-in-ring procedures. J Cardiovasc Thorac Res. (2018) 10:182–6. doi: 10.15171/jcvtr.2018.31
65. Ribeiro R, Yanagawa B, Légaré JF, Hassan A, Ouzounian M, Verma S, et al. Clinical outcomes of mitral valve intervention in patients with mitral annular calcification: A systematic review and meta-analysis. J Card Surg. (2020) 35:66–74. doi: 10.1111/jocs.14325
66. Alexis SL, Malik AH, El-Eshmawi A, George I, Sengupta A, Kodali SK, et al. Surgical and transcatheter mitral valve replacement in mitral annular calcification: a systematic review. J Am Heart Assoc. (2021) 10:e018514. doi: 10.1161/JAHA.120.018514
67. Bonow RO, O'Gara PT, Adams DH, Badhwar V, Bavaria JE, Elmariah S, et al. 2019 AATS/ACC/SCAI/STS expert consensus systems of care document: operator and institutional recommendations and requirements for transcatheter mitral valve intervention: a joint report of the American Association for Thoracic Surgery, the American College of Cardiology, the society for cardiovascular angiography and interventions, and the society of thoracic surgeons. J Am Coll Cardiol. (2020) 76:96–117. doi: 10.1016/j.jacc.2019.12.002
68. Ben-Ali W, Ibrahim R, Rodès-Cabeau J, von Bardeleben RS, Mylotte D, Granada J, et al. Transcatheter mitral valve implantation systematic review: focus on transseptal approach and mitral annulus calcification. Curr Cardiol Rep. (2021) 23:37. doi: 10.1007/s11886-021-01466-7
69. Tang JY, Liu Y, Yang J. A novel case of transcatheter mitral valve-in-valve replacement using Mi-thos™ system. J Geriatr Cardiol. (2020) 17:229–33. doi: 10.11909/j.issn.1671-5411.2020.04.007
70. Odemis E, and Yenidogan I. (2021). First experiences with Myval Transcatheter Heart Valve System in the treatment of severe pulmonary regurgitation in native right ventricular outflow tract and conduit dysfunction. Cardiol Young. p. 1–7. Advance online publication. doi: 10.1017/S1047951121004650
71. Harloff MT, Chowdhury M, Hirji SA, Percy ED, Yazdchi F, Shim H, et al. A step-by-step guide to transseptal valve-in-valve transcatheter mitral valve replacement. Ann Cardiothorac Surg. (2021) 10:113–21. doi: 10.21037/acs-2020-mv-104
72. Blackstone EH, Suri RM, Rajeswaran J, Babaliaros V, Douglas PS, Fearon WF, et al. Propensity-matched comparisons of clinical outcomes after transapical or transfemoral transcatheter aortic valve replacement: a placement of aortic transcatheter valves (PARTNER)-I trial substudy. Circulation. (2015) 131:1989–2000. doi: 10.1161/CIRCULATIONAHA.114.012525
73. Alavi SH, Groves EM, Kheradvar A. The effects of transcatheter valve crimping on pericardial leaflets. Ann Thorac Surg. (2014) 97:1260–6. doi: 10.1016/j.athoracsur.2013.11.009
74. Khoffi F, Heim F. Mechanical degradation of biological heart valve tissue induced by low diameter crimping: an early assessment. J Mech Behav Biomed Mater. (2015) 44:71–5. doi: 10.1016/j.jmbbm.2015.01.005
75. Testa L, Latib A, Brambilla N, De Marco F, Fiorina C, Adamo M, et al. Long-term clinical outcome and performance of transcatheter aortic valve replacement with a self-expandable bioprosthesis. Eur Heart J. (2020) 41:1876–86. doi: 10.1093/eurheartj/ehz925
Keywords: transcatheter, transcutaneous, systematic review, mitral valve replacement, transcatheter mitral valve implantation (TMVI)
Citation: Sazzad F, Hon JKF, Ramanathan K, Nah JH, Ong ZX, Ti LK, Foo R, Tay E and Kofidis T (2022) Design Variation, Implantation, and Outcome of Transcatheter Mitral Valve Prosthesis: A Comprehensive Review. Front. Cardiovasc. Med. 8:782278. doi: 10.3389/fcvm.2021.782278
Received: 24 September 2021; Accepted: 31 December 2021;
Published: 24 February 2022.
Edited by:
Crochan John O'Sullivan, Triemli Hospital, SwitzerlandReviewed by:
Lenard Conradi, University Medical Center Hamburg-Eppendorf, GermanyCopyright © 2022 Sazzad, Hon, Ramanathan, Nah, Ong, Ti, Foo, Tay and Kofidis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Faizus Sazzad, ZTA2Nzc4NjhAdS5udXMuZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.