- 1Emergency Department, Affiliated Hospital of North Sichuan Medical College, Nanchong, China
- 2Department of Ultrasound, The Fifth Affiliated Hospital, Sun Yat-sen University, Zhuhai, China
- 3Department of Geriatrics, The Fifth Affiliated Hospital, Sun Yat-sen University, Zhuhai, China
- 4Department of Cardiology, The Fifth Affiliated Hospital, Sun Yat-sen University, Zhuhai, China
Background: To explore the association between visit-to-visit variability of glycated hemoglobin (HbA1c) and cardiovascular outcomes in the patients with type 2 diabetes mellitus (T2DM) of the Action to Control Cardiovascular Risk in Diabetes (ACCORD) study.
Methods: We conducted a post-hoc analysis on the ACCORD population including 9,544 participants with T2DM. Visit-to-visit variability of HbA1c was defined as the individual SD, coefficient of variation (CV), and variability independent of the mean (VIM) across HbA1c measurements. The clinical measurements included primary outcome [the first occurrence of non-fatal myocardial infarction (MI), non-fatal stroke or cardiovascular death], total mortality, cardiovascular death, non-fatal MI event, non-fatal stroke, total stroke, heart failure, macrovascular events, and major coronary events (CHD).
Results: Over a median follow-up of 4.85 years, 594 and 268 participants experienced all-cause mortality and cardiovascular mortality, respectively. After adjusting for baseline HbA1c levels and confounding factors, the adjusted hazard ratio (HR) comparing patients in the highest vs. the lowest quartile CV of HbA1c variability was 1.61 (95% CI 1.29–2.00) for the primary outcome. Similar trends for secondary outcome were also observed. There was no association between HbA1c fluctuation and non-fatal stroke. Noticeably, there was 66% greater risk for the all-cause mortality among patients in the highest vs. the lowest quartile (HR 1.66, 95% CI 1.27–2.17).
Conclusions: Greater variability of HbA1c is associated with higher risk for cardiovascular complications and all-cause death in T2DM. Our study stresses the significance of well-controlled glycemic levels for improving cardiovascular outcomes. Further randomized clinical trials are required to confirm these findings.
Introduction
Statistically speaking, diabetes was estimated by WHO as the 7th leading cause of mortality, which contributed to 1.6 million deaths in 2016. It has arisen the attention of the world not only because of its growing prevalence but also of increased higher risks for macrovascular and microvascular complications (1–3). Even in the patients with prediabetes, the risk of macrovascular and microvascular disease was increased (4–6). Glycated hemoglobin (HbA1c) presents the average plasma glucose concentration in the past 3 months (7). Recent randomized controlled trials have found that long-term glycemic fluctuation was closely associated with macrovascular and microvascular complications in both type 1 and type 2 diabetes mellitus (T2DM) (8–14). In Diabetes Control and Complications Trial (DCCT), HbA1c levels predicted risk for the renal disease and cardiovascular events in type 1 diabetes (15). A Chinese study contains 91,866 participants demonstrated that HbA1c variability contributed to the development of cardiovascular disease (CVD) and all-cause mortality, particularly in the elderly cohort (16). What is more, ADVANCE study replicated the similar results that visit-to-visit HbA1c fluctuation increased the risk of CVD in T2DM (10). Nevertheless, in the Renal Insufficiency and Cardiovascular Events (RIACE) study, HbA1c variability was associated with macrovascular complications, but not microvascular complication, particularly nephropathy (17). Furthermore, KIM et al. suggested that higher HbA1c fluctuation could not predict carotid artery intima-media thickness independently (18). Besides, participants with diabetes being treated with hypoglycemic agents usually fluctuate greatly in the blood glucose. Therefore, it is important to explore whether the HbA1c variability is an independent risk factor in participants with diabetes. As a result, we performed data analysis in ACCORD cohort to evaluate the association between visit-to-visit HbA1c variability and the risk of cardiovascular outcomes in the patients with T2DM.
Methods
Study Design and Participants
The ACCORD trial is a randomized, multicenter, double-blind, 2 × 2 factorial trial, consisting of 10,251 patients with T2DM. The study design and results have been published previously (19, 20). Briefly, the trial recruited participants aged 40 to 79 years who had additional cardiovascular risk factors at 77 North American sites (21). Participants were randomly assigned to achieve intensive glycaemia therapy vs. standard glycaemia therapy (plus either antihypertensive or lipid-lowering intervention). Measurement of HbA1c was recorded every 4 months in the participants for up to 7 years (22). Participants who had < three documented HbA1c measurements and missing confounding data were excluded. Finally, we included 9,544 participants from this study.
Measurement of HbA1c Visit-to-Visit Variability
HbA1c variability was considered as intra-individual SD across visits (17). We calculated intra-personal CV, SD, and VIM of 7-year follow-up HbA1c in this study. In brief, CV for HbA1c was the ratio of SD and mean to correct for larger SD on account of the higher absolute value of HBA1C. VIM was calculated as 100 × SD/meanβ, and β means the regression coefficient independent of mean. Moreover, all the participants were divided into four quartiles of HbA1c variability for further analyses.
Outcomes
The primary outcome includes the first occurrence of non-fatal myocardial infarction (MI), non-fatal stroke or cardiovascular death. Secondary outcomes included MI, non-fatal MI, any stroke, non-fatal stroke, death from any cause, death from a cardiovascular cause, congestive heart failure, and revascularization (23).
Covariates
Baseline variables include sex, age, race, education, smoking and alcoholic status, BMI, waist, HbA1c, systolic blood pressure (SBP), cholesterol, CVD history, insulin usage, antihypertension, or lipid-lowering medications (24).
Statistical Analysis
The continuous variables were compared by Kruskal–Wallis or ANOVA test and described as mean ± SD. The categorical variables were compared by chi-squared test and described as a percentage. Kaplan–Meier estimates were used to compare the cumulative incident for the cardiovascular outcome and all-cause mortality within subgroups defined by the variability of HbA1c. Cox proportional hazards regression models were used to calculate adjusted hazard ratios (HR) and 95% CI for CVD outcomes. Subjects were divided into quartiles of CV of HbA1c. Model 1 was adjusted for sex, age, race, duration of DM at baseline. Model 2 was further adjusted for level of education, smoking, alcohol abuse, BMI, and waist at baseline. Model 3 included SBP, heart rate, cholesterol, CVD history, insulin usage, antihypertension, or lipid-lowering medications. Model 4 additionally accounted for the baseline HbA1c levels. All the analyses were performed using SPSS statistic (Version 20.0; IBM Corp., New York, USA) and R software (version 3.4.2; R Foundation for Statistical Computing, Vienna, Austria). A two-sided P < 0.05 was considered as statistically significance.
Results
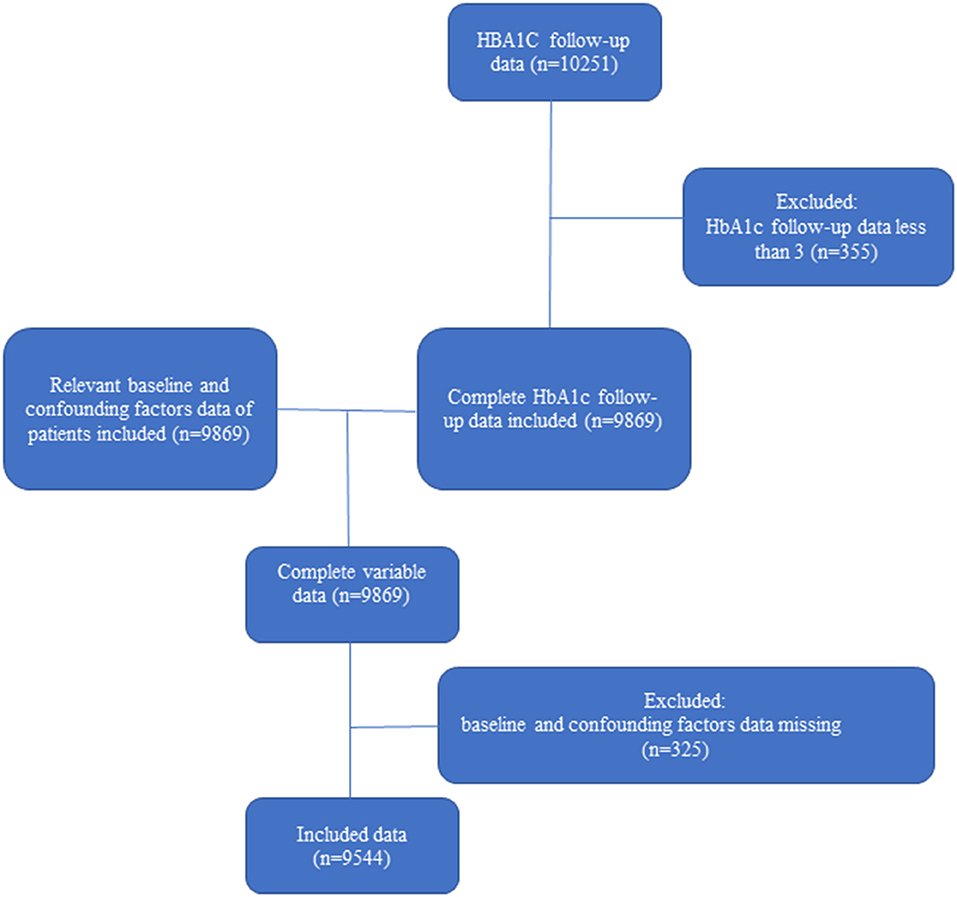
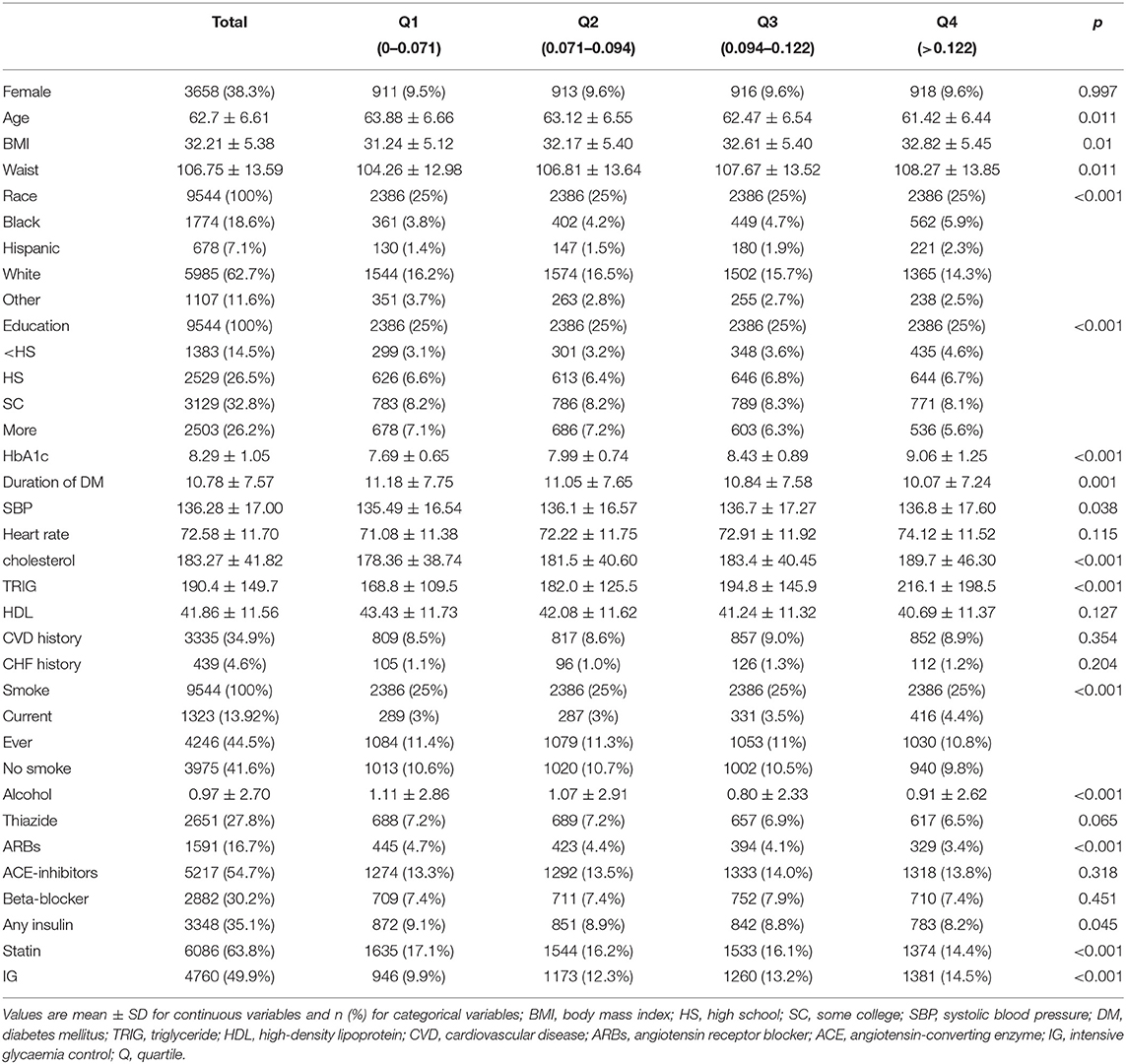
Figure 1 shows the process of selection of the participants. Of the ACCORD cohort, 9,544 patients included in this study were followed up more than three times and had complete data on the covariates. A proportion of participants with ACCORD were excluded because of missing HbA1c measurements. Baseline characteristics grouped by quartiles of CV are presented in Table 1.
Individuals with higher variability of HbA1c than those with a lower variability of HbA1c tended to be younger, had a higher body mass index (BMI), waist, cholesterol, triglyceride (TRIG), and smoke more frequently, had a shorter duration of DM. Over a median follow-up period of 4.85 years, 943, 594, 268, 414 individuals, respectively, were adjudicated as having the primary outcome, all-cause mortality, CVD mortality, and heart failure, respectively.
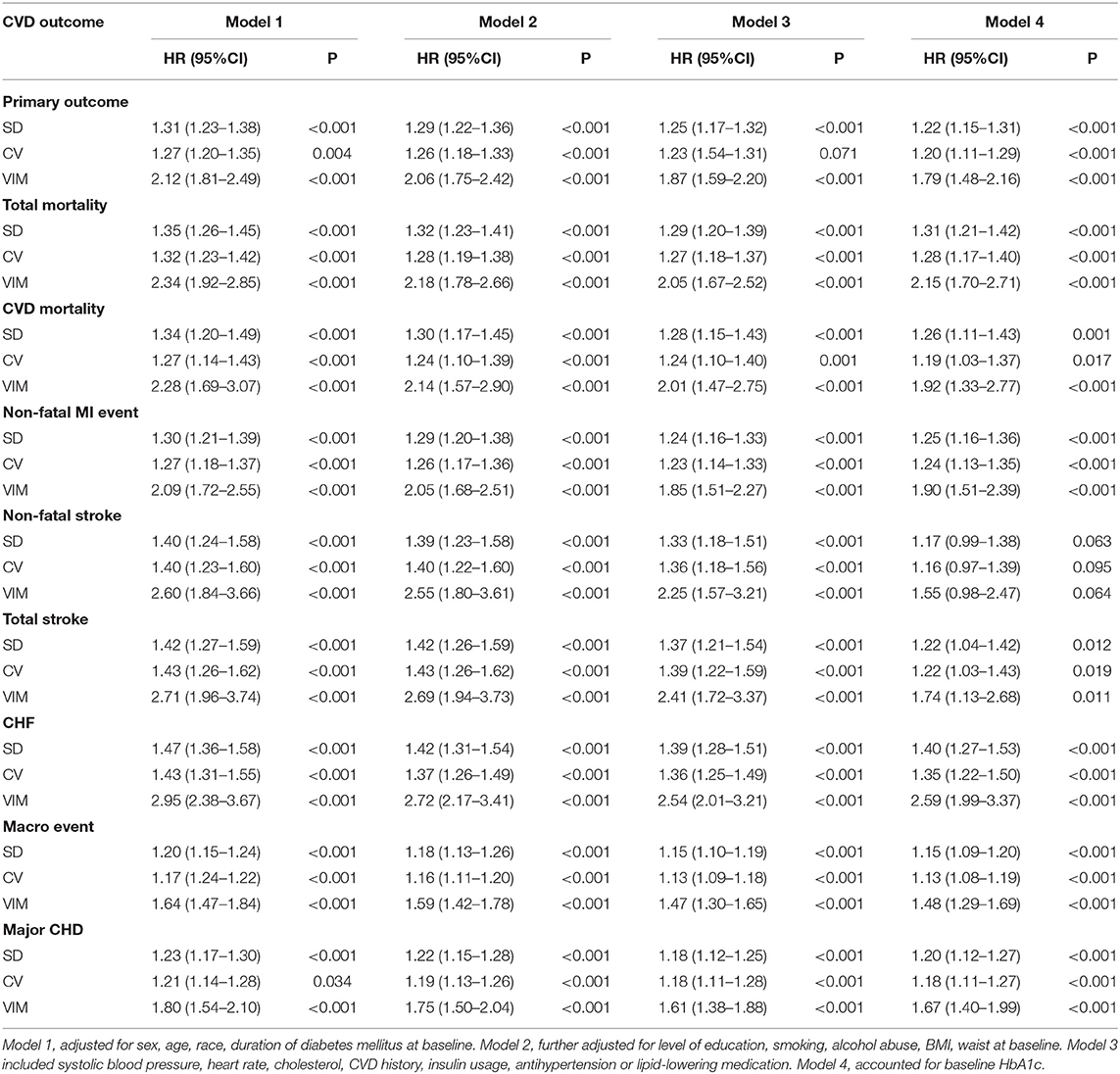
The cumulative incidence curves for the cardiovascular outcome and all-cause mortality within subgroups defined by the variability of HbA1c are presented in Figure 2. In our fully adjusted Cox model, visit-to-visit variability of HbA1c including CV, SD, and VIM was associated with primary outcome, all-cause mortality, CVD death, nonfatal MI event, total stroke, heart failure, microvascular events, and major coronary disease but not with non-fatal stroke (Table 2). HR of primary and secondary outcomes are summarized in Table 3 when HbA1c was calculated as a category. We found that the risk of CVD outcome increased with higher levels of HbA1c variability.
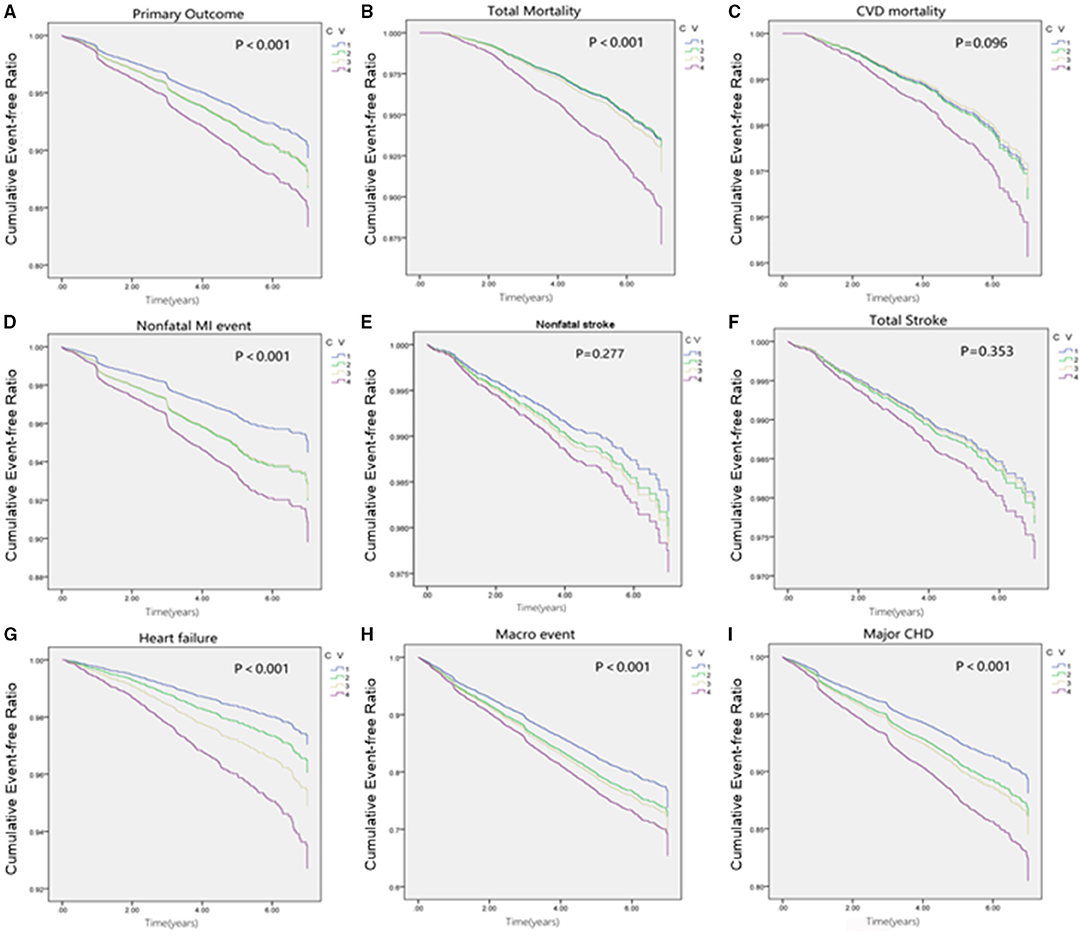
Figure 2. Cumulative survival of outcomes grouped by quartiles of HbA1c fluctuation. (A) Cumulative survival of Primary outcome grouped by quartiles of HbA1c fluctuation. (B) Cumulative survival of Total mortality grouped by quartiles of HbA1c fluctuation. (C) Cumulative survival of CVD mortality grouped by quartiles of HbA1c fluctuation. (D) Cumulative survival of Non-fatal MI event grouped by quartiles of HbA1c fluctuation. (E) Cumulative survival of Nonfatal stroke grouped by quartiles of HbA1c fluctuation. (F) Cumulative survival of Total stroke grouped by quartiles of HbA1c fluctuation. (G) Cumulative survival of CHF grouped by quartiles of HbA1c fluctuation. (H) Cumulative survival of Macro event grouped by quartiles of HbA1c fluctuation. (I) Cumulative survival of Primary outcome grouped by quartiles of HbA1c fluctuation.
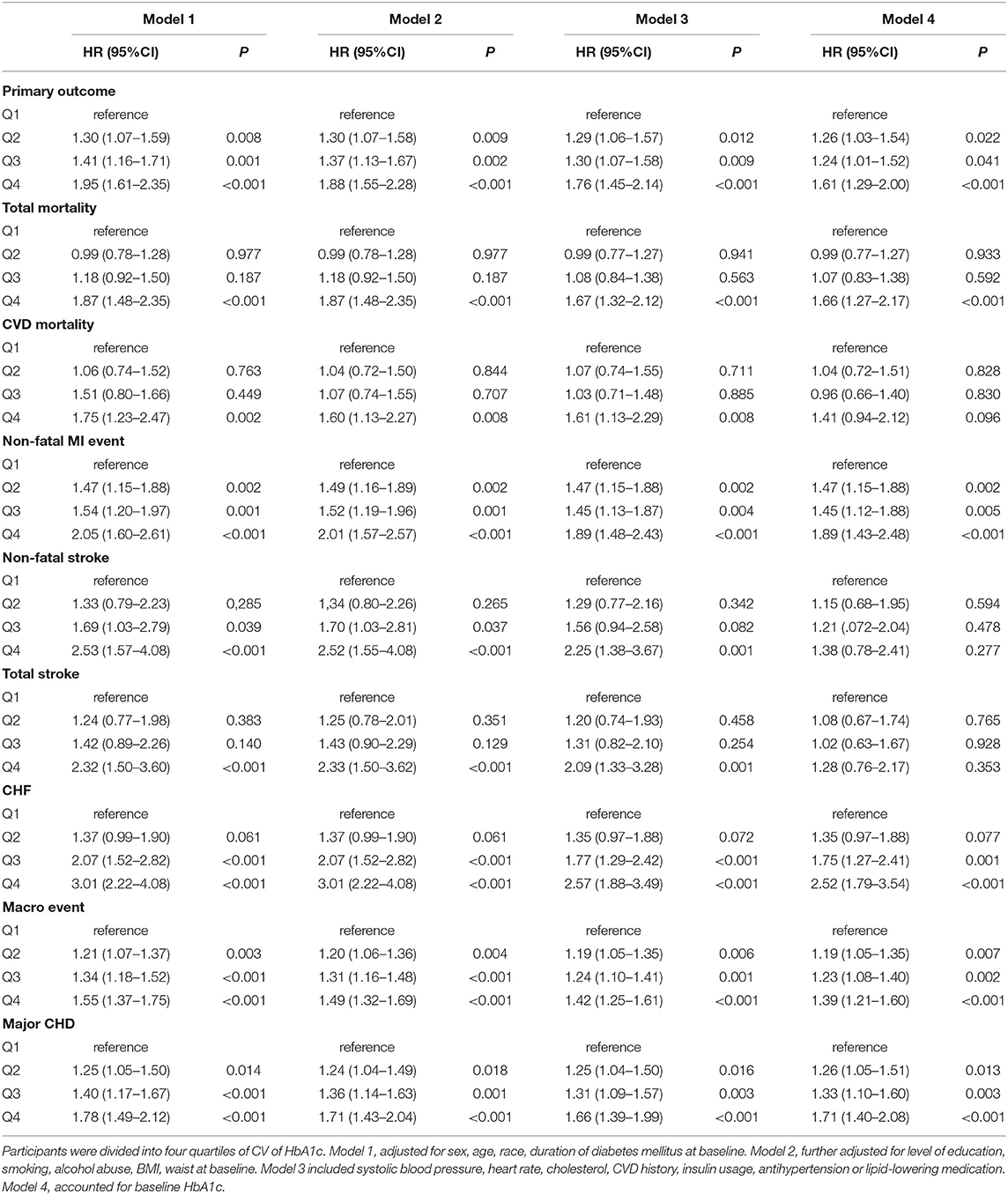
Compared with participants with the lowest quartile, after adjustment of potential confounding factors, the HR (95% CI) for primary outcome in the second, third, and the highest quartiles of variability of HbA1c were 1.26 (1.03–1.54), 1.24 (1.01–1.52), 1.61 (1.29–2.00), respectively. Similar trends were also noted in all secondary outcomes including non-fatal MI, any and all-cause mortality, major coronary events, and fatal and non-fatal heart failure except for non-fatal stroke (Figure 3). Noticeably, the adjusted HR comparing patients in the highest vs. the lowest quartile CV of HbA1c variability was 1.67 (95% CI 1.28–2.18) for all-cause mortality.
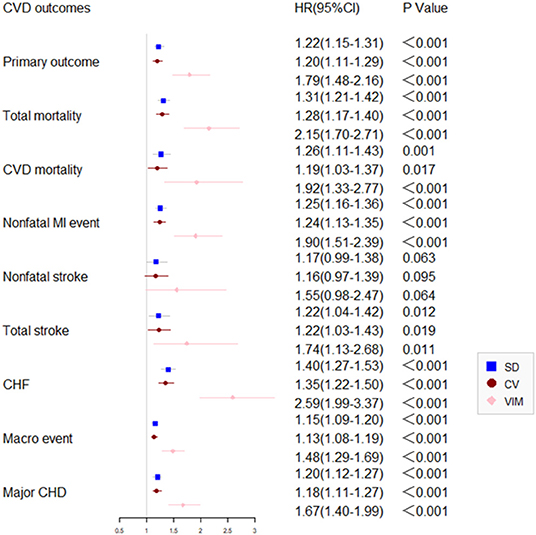
Figure 3. Comparison of primary and secondary outcomes by level of HbA1c variability in the ACCORD cohort.
Participants in the highest quartile experienced the highest risk than other groups during 7 years of follow-up (P < 0.05).
Discussion
Previous studies had established HbA1c as an effective index of long-term glycemic control, lower HbA1c persisting at <7% was associated with less risks for diabetes related complications and per 1% higher HbA1c is related to 15–20% higher cardiovascular risk (25, 26). However, whether controlling HbA1c to a normal range by intensive glycemic therapy in patients with T2DM can reduce CVD remains controversial. ACCORD, VADT, and other large studies showed no beneficial effects of intensive glucose therapy targeting for low-level HbA1c (27, 28). Hence, whether the mean HbA1c level is the most appropriate factor to predict the risk for diabetes complication is still questionable. Many researchers highlighted the effects of HbA1c variability on the cardiovascular outcomes.
To our best knowledge, our study was the first one to explore the association between the long-term visit-to-visit fluctuation of HbA1c levels CVD outcomes using ACCORD data. We discovered patients with T2DM with higher variability of HbA1c tended to develop cardiovascular diseases and had a worse prognosis. This observation was consistent after adjusting for the baseline HbA1c and other confounding factors such as demographic characteristics, hypertension, dyslipidemia, smoking, and medications. In accordance with our results, a Chinese study had evaluated the association between HbA1c variability and vascular complication and mortality (11). Moreover, Prentice et al. conducted a retrospective study with T2DM and concluded that visit-to-visit HbA1c fluctuation might predict adverse outcomes (29). A recent review pooled 20 studies with 87,641 participants to investigate on HbA1c fluctuation and vascular complication in type 1 diabetes in T2DM. Particularly, in T2DM, higher HbA1c variability group had higher risk for cardiovascular disease, renal disease, mortality (14). In contrast, the RIACE study shown no association between HbA1c and macrovascular disease. The potential explanation for such disparity may be due to the study design. We collected the multiple HbA1c variability measurements of 7 years, with a median follow-up time of 4.85 years, while the RIACE obtained serial HbA1c values during 2-year period recruitment. Furthermore, our study held distinct baseline HbA1c and degree of HbA1c fluctuation and diabetes duration (17).
Until now, there has not been a standardized method to access HbA1c variability. We selected three measurements including SD, CV, VIM, all of which were independently correlated with the cardiovascular outcomes. Our results showed that the CV is possibly a more robust measure of visit-to-visit in HbA1c rather than average HbA1c levels. Unfortunately, researchers fail to carry out intervention study and conclude the cause-effect of HbA1c variability. The mechanisms linking the higher HbA1c fluctuation to the higher risk of CVD remain unclear and require further biological investigation. One of the hypotheses is that glycemic variability might cause endothelial dysfunction and atherosclerosis induced by inflammatory cytokines and oxidative stress (30–32). Another hypothesis is the “metabolic memory” in vascular cells, by which the cellular transduction system and extra oxygen and nitrogen lead to endothelial damage (33). Moreover, the glucose fluctuation may cause hypoglycemia which poses a threat to cardiovascular systems (34).
There are several limitations to our present study. First, the number and frequency of HbA1c value varied from different participants. To minimize the influences, we use CV and VIM which are independent of average of HbA1c levels. Second, the most of our participants had a DM duration of over 10 years, thus, it is uncertain whether our conclusion could be generalized to those with a shorter duration of DM. Third, we failed to conduct the trend of HbA1c variability. Whether decreasing the HbA1c variability could reduce cardiovascular outcomes and all-cause mortality remains obscure and needs further investigation.
Conclusions
In conclusion, data from a 7-year follow-up cohort has revealed that high variability of HbA1c is an independent risk for cardiovascular outcomes of the ACCORD study. Moreover, it seems reasonable to include HbA1c variability as potential target in the routine management of T2DM.
Data Availability Statement
The data of the Action to Control Cardiovascular Risk in Diabetes study is available free of charge from the NHLBI Biologic Specimen and Data Repository Information Coordinating Center, the datasets used during the current study are available from the corresponding authors on reasonable request.
Ethics Statement
The studies involving human participants were reviewed and approved by the Fifth Affiliated Hospital, Sun Yat-sen University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
YH and DH planned and interpreted the data. YH, DH, and Y-QH drafted the manuscript. Q-YZ and YC performed the statistical analyses. YH is the guarantor of the present study and took responsibility for the integrity and the accuracy of the data analysis. All authors read and revised the content and approved the final manuscript.
Funding
This work was supported by the Zhuhai Science and Technology Planning Project (ZH22036201210055PWC).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Martinez LC, Sherling D, Holley A. The screening and prevention of diabetes mellitus. Prim Care. (2019) 46:41–52. doi: 10.1016/j.pop.2018.10.006
2. Forbes JM, Fotheringham AK. Vascular complications in diabetes: old messages, new thoughts. Diabetologia. (2017) 60:2129–38. doi: 10.1007/s00125-017-4360-x
3. Zghebi SS, Panagioti M, Rutter MK, Ashcroft DM, van Marwijk H, Salisbury C, et al. Assessing the severity of Type 2 diabetes using clinical data-based measures: a systematic review. Diabetic Med. (2019) 36:688–701. doi: 10.1111/dme.13905
4. Cai X, Zhang Y, Li M, Wu JH, Mai L, Li J, et al. Association between prediabetes and risk of all cause mortality and cardiovascular disease: updated meta-analysis. BMJ. (2020) 370:m2297. doi: 10.1136/bmj.m2297
5. Cai X, Liu X, Sun L, He Y, Zheng S, Zhang Y, et al. Prediabetes and the risk of heart failure: a meta-analysis. Diabetes Obes Metab. (2021) 23:1746–53. doi: 10.1111/dom.14388
6. Mai L, Wen W, Qiu M, Liu X, Sun L, Zheng H, et al. Association between prediabetes and adverse outcomes in heart failure. Diabetes Obes Metab. (2021) 23:2476–83. doi: 10.1111/dom.14490
7. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. (2014) 37(Suppl. 1):S81–90. doi: 10.2337/dc14-S081
8. Bouchi R, Babazono T, Mugishima M, Yoshida N, Nyumura I, Toya K, et al. Fluctuations in HbA1c are associated with a higher incidence of cardiovascular disease in Japanese patients with type 2 diabetes. J Diabetes Invest. (2012) 3:148–55. doi: 10.1111/j.2040-1124.2011.00155.x
9. Ghouse J, Skov MW, Kanters JK, Lind B, Isaksen JL, Blanche P, et al. Visit-to-visit variability of hemoglobin A1c in people without diabetes and risk of major adverse cardiovascular events and all-cause mortality. Diabetes Care. (2018) 42:134–41. doi: 10.2337/dc18-1396
10. Hirakawa Y, Arima H, Zoungas S, Ninomiya T, Cooper M, Hamet P, et al. Impact of visit-to-visit glycemic variability on the risks of macrovascular and microvascular events and all-cause mortality in type 2 diabetes: the ADVANCE trial. Diabetes Care. (2014) 37:2359–65. doi: 10.2337/dc14-0199
11. Mo Y, Zhou J, Ma X, Zhu W, Zhang L, Li J, et al. Haemoglobin A1c variability as an independent correlate of atherosclerosis and cardiovascular disease in Chinese type 2 diabetes. Diabetes Vasc Dis Res. (2018) 15:402–8. doi: 10.1177/1479164118778850
12. Wightman SS, Sainsbury CAR, Jones GC. Visit-to-visit HbA1c variability and systolic blood pressure (SBP) variability are significantly and additively associated with mortality in individuals with type 1 diabetes: an observational study. Diabetes Obes Metab. (2018) 20:1014–7. doi: 10.1111/dom.13193
13. Chiu HT, Li TC, Li CI, Liu CS, Lin WY, Lin CC. Visit-to-visit glycemic variability is a strong predictor of chronic obstructive pulmonary disease in patients with type 2 diabetes mellitus: competing risk analysis using a national cohort from the Taiwan diabetes study. PLoS ONE. (2017) 12:e0177184. doi: 10.1371/journal.pone.0177184
14. Gorst C, Kwok CS, Aslam S, Buchan I, Kontopantelis E, Myint PK, et al. Long-term glycemic variability and risk of adverse outcomes: a systematic review and meta-analysis. Diabetes Care. (2015) 38:2354–69. doi: 10.2337/dc15-1188
15. Waden J, Forsblom C, Thorn LM, Gordin D, Saraheimo M, Groop PH. A1C variability predicts incident cardiovascular events, microalbuminuria, and overt diabetic nephropathy in patients with type 1 diabetes. Diabetes. (2009) 58:2649–55. doi: 10.2337/db09-0693
16. Wan EY, Fung CS, Fong DY, Lam CL. Association of variability in hemoglobin A1c with cardiovascular diseases and mortality in Chinese patients with type 2 diabetes mellitus - a retrospective population-based cohort study. J Diabetes Complications. (2016) 30:1240–7. doi: 10.1016/j.jdiacomp.2016.05.024
17. Penno G, Solini A, Zoppini G, Orsi E, Fondelli C, Zerbini G, et al. Hemoglobin A1c variability as an independent correlate of cardiovascular disease in patients with type 2 diabetes: a cross-sectional analysis of the renal insufficiency and cardiovascular events (RIACE) Italian multicenter study. Cardiovasc Diabetol. (2013) 12:98. doi: 10.1186/1475-2840-12-98
18. Kim CS, Park SY, Yu SH, Kang JG, Ryu OH, Lee SJ, et al. Is A1C variability an independent predictor for the progression of atherosclerosis in type 2 diabetic patients? Korean Diabetes J. (2010) 34:174–81. doi: 10.4093/kdj.2010.34.3.174
19. Cushman WC, Grimm RH Jr, Cutler JA, Evans GW, Capes S, Corson MA, et al. Rationale and design for the blood pressure intervention of the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Am J Cardiol. (2007) 99:44i–55i. doi: 10.1016/j.amjcard.2007.03.005
20. Fatemi O, Yuriditsky E, Tsioufis C, Tsachris D, Morgan T, Basile J, et al. Impact of intensive glycemic control on the incidence of atrial fibrillation and associated cardiovascular outcomes in patients with type 2 diabetes mellitus (from the Action to Control Cardiovascular Risk in Diabetes Study). Am J Cardiol. (2014) 114:1217–22. doi: 10.1016/j.amjcard.2014.07.045
21. Kingry C, Bastien A, Booth G, Geraci TS, Kirpach BR, Lovato LC, et al. Recruitment strategies in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Am J Cardiol. (2007) 99:68i–79i. doi: 10.1016/j.amjcard.2007.03.025
22. Buse JB, Bigger JT, Byington RP, Cooper LS, Cushman WC, Friedewald WT, et al. Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial: design and methods. Am J Cardiol. (2007) 99:21i–33i. doi: 10.1016/j.amjcard.2007.03.003
23. Gerstein HC, Riddle MC, Kendall DM, Cohen RM, Goland R, Feinglos MN, et al. Glycemia treatment strategies in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Am J Cardiol. (2007) 99:34i–43i. doi: 10.1016/j.amjcard.2007.03.004
24. Raisch DW, Feeney P, Goff DC Jr, Narayan KM, O'Connor PJ, Zhang P, et al. Baseline comparison of three health utility measures and the feeling thermometer among participants in the action to control cardiovascular risk in diabetes trial. Cardiovasc Diabetol. (2012) 11:35. doi: 10.1186/1475-2840-11-35
25. Weykamp C. HbA1c: a review of analytical and clinical aspects. Ann Lab Med. (2013) 33:393–400. doi: 10.3343/alm.2013.33.6.393
26. Skrha J, Soupal J, Skrha J Jr, Prazny M. Glucose variability, HbA1c and microvascular complications. Rev Endocr Metab Disord. (2016) 17:103–10. doi: 10.1007/s11154-016-9347-2
27. Basu S, Raghavan S, Wexler DJ, Berkowitz SA. Characteristics associated with decreased or increased mortality risk from glycemic therapy among patients with type 2 diabetes and high cardiovascular risk: machine learning analysis of the ACCORD Trial. Diabetes Care. (2018) 41:604–12. doi: 10.2337/dc17-2252
28. Macisaac RJ, Jerums G. Intensive glucose control and cardiovascular outcomes in type 2 diabetes. Heart Lung Circ. (2011) 20:647–54. doi: 10.1016/j.hlc.2010.07.013
29. Prentice JC, Pizer SD, Conlin PR. Identifying the independent effect of HbA1c variability on adverse health outcomes in patients with Type 2 diabetes. Diabetic Med. (2016) 33:1640–8. doi: 10.1111/dme.13166
30. Yu WK, Li WQ, Li N, Li JS. Influence of acute hyperglycemia in human sepsis on inflammatory cytokine and counterregulatory hormone concentrations. World J Gastroenterol. (2003) 9:1824–7. doi: 10.3748/wjg.v9.i8.1824
31. Ceriello A, Esposito K, Piconi L, Ihnat MA, Thorpe JE, Testa R, et al. Oscillating glucose is more deleterious to endothelial function and oxidative stress than mean glucose in normal and type 2 diabetic patients. Diabetes. (2008) 57:1349–54. doi: 10.2337/db08-0063
32. Wu J, Zheng H, Liu X, Chen P, Zhang Y, Luo J, et al. Prognostic value of secreted frizzled-related protein 5 in heart failure patients with and without type 2 diabetes mellitus. Circ Heart Fail. (2020) 13:e007054. doi: 10.1161/CIRCHEARTFAILURE.120.007054
33. Schisano B, Tripathi G, McGee K, McTernan PG, Ceriello A. Glucose oscillations, more than constant high glucose, induce p53 activation and a metabolic memory in human endothelial cells. Diabetologia. (2011) 54:1219–26. doi: 10.1007/s00125-011-2049-0
Keywords: cardiovascular risk, mortality, ACCORD 07 trial, diabetes - quality of life, HbA1c - hemoglobin A1c
Citation: Huang D, Huang Y-Q, Zhang Q-Y, Cui Y, Mu T-Y and Huang Y (2021) Association Between Long-Term Visit-to-Visit Hemoglobin A1c and Cardiovascular Risk in Type 2 Diabetes: The ACCORD Trial. Front. Cardiovasc. Med. 8:777233. doi: 10.3389/fcvm.2021.777233
Received: 15 September 2021; Accepted: 26 October 2021;
Published: 24 November 2021.
Edited by:
Yuli Huang, Southern Medical University, ChinaReviewed by:
Shiqun Chen, Guangdong Provincial People's Hospital, ChinaQingchun Zeng, Southern Medical University, China
Copyright © 2021 Huang, Huang, Zhang, Cui, Mu and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yong-Quan Huang, aHVhbmd5cTM5QG1haWwuc3lzdS5lZHUuY24=; Yin Huang, aHVhbmd5aW4zQG1haWwuc3lzdS5lZHUuY24=
 Dan Huang1
Dan Huang1 Yin Huang
Yin Huang